Abstract
Ischemic heart diseases (IHD) cause millions of deaths around the world annually. While surgical and pharmacological interventions are commonly used to treat patients with IHD, their efficacy varies from patient to patient and is limited by the severity of the disease. One promising, at least theoretically, approach for treating IHD is induction of coronary collateral growth (CCG). Coronary collaterals are arteriole-to-arteriole anastomoses that can undergo expansion and remodeling in the setting of coronary disease when the disease elicits myocardial ischemia and creates a pressure difference across the collateral vessel that creates unidirectional flow. Well-developed collaterals can restore blood flow in the ischemic area of the myocardium and protect the myocardium at risk. Moreover, such collaterals are correlated to reduced mortality and infarct size and better cardiac function during occlusion of coronary arteries. Therefore, understanding the process of CCG is highly important as a potentially viable treatment of IHD. While there are several excellent review articles on this topic, this review will provide a unified overview of the various aspects related to CCG as well as an update of the advancements in the field. We also call for more detailed studies with an interdisciplinary approach to advance our knowledge of CCG. In this review, we will describe growth of coronary collaterals, the various factors that contribute to CCG, animal models used to study CCG, and the cardioprotective effects of coronary collaterals during ischemia. We will also discuss the impairment of CCG in metabolic syndrome and the therapeutic potentials of CCG in IHD.
Keywords: cardioprotection, coronary circulation, coronary collateral growth, ischemia
INTRODUCTION
Cardiovascular disease remains the top underlying cause of death worldwide, with 17.3 of 54 million deaths attributed to it in 2013 (3). It is further estimated that 16.5 million Americans over the age of 20 yr old have coronary heart disease (CHD) and ~790,000 individuals suffer myocardial infarctions (MIs) each year (3). The consequences of such can be severe: in 2014, 114,019 deaths were attributed to MI, and ~36% of the people who experienced a coronary event perished (3). Even aside from possible mortality, acute coronary syndrome events (i.e., MI) caused by heart disease and atherosclerotic plaque ruptures can lead to tissue ischemia and successive tissue damage. A sobering statistic of these acute coronary events is that they are often the first indicator that a patient has ischemic heart disease (IHD). Accordingly, preserving the viability and contractility of the myocardium after MI are two of the objectives of treating patients with IHD. The conundrum of this treatment lies in the timely restoration of blood flow to the area at risk, but even timely reperfusion can induce further damage to the tissue, a phenomenon known as reperfusion injury (26). Common procedures to reestablish blood flow in the setting of severe coronary disease, such as stent implantation and angioplasty, can lead to complications including reperfusion injury as well as restenosis or rupture followed by additional episodes of coronary ischemia, MI, stroke, and death, especially in the elderly (3, 13).
Because reperfusion injury plays a significant role in a patient’s outcome, a great deal of focus has been directed toward cardioprotection by ischemic pre-, per-, and postconditioning, yielding disparate results in terms of potential therapies. Although pharmacological approaches have not translated into therapies (26) and most postconditioning trials have not produced positive outcomes (12, 32), some recent trials using perconditioning strategies have shown promise (44).
The coronary collateral circulation is a network of arterial-arterial anastomotic connections present in the heart between vascular branches from different regions (16). In their native state, these vessels cannot offer much protection against ischemia due to their small caliber, which renders high resistance and poor ability to conduct flow. In ideal circumstances, when a major coronary artery is obstructed, native coronary collaterals will undergo arteriogenesis or abluminal expansion into natural bypasses to compensate for the deficient blood supply to the myocardial regions distal to stenotic lesion. Stimulation of CCG is therefore a potential therapy for patients with severe angina pectoris who have contraindications for coronary artery bypass grafting or percutaneous coronary intervention (17, 53, 69). Furthermore, when patients with IHD with preexisting coronary collaterals suffer a MI, the well-developed collaterals can provide sufficient blood flow to limit the extent of ischemic injury to the myocardium (22).
In this review, we will discuss the cardioprotective effect of a well-developed coronary collateral circulation as well as underlying mechanisms for this adaptive process and how to stimulate the CCG in IHD.
CORONARY COLLATERALS AND CCG
Native Coronary Collaterals
The term “collaterals” encompasses both collateral arteries and microvascular collaterals. Microvascular collaterals are arteriole-to-arteriole anastomoses found in the crowns of adjacent arterial trees (16). Microvascular collaterals are distinguished from collateral arteries, which are artery-to-artery anastomoses and present in other systemic arterial territories such as the superior ulnar collateral artery, palmar and plantar arch collaterals, etc. These microvascular collateral vessels connect an extremely small percentage of arterioles. In the absence of occlusion (and therefore a pressure gradient), these native collaterals experience almost no net flow between the adjacent trees, although there appears to be both anterograde and retrograde flow, which keeps the vessel patent and prevents any clots from forming (66). In their native, unstimulated state, the diameter of these vessels is small as they have not yet undergone remodeling and expansion. Intratree anastomoses, such as those within left anterior descending coronary artery (LAD), also function similar to collaterals but cover a much smaller area.
Native collaterals are typically microvascular arterial-to-arterial anastomoses that are present in healthy tissues without any arterial obstructions. Native coronary collaterals can be present at birth but show wide variation in their functional capacity. In most species, microvascular collateral vessels are present in healthy organs under normal physiological conditions (no occlusion of arteries). However, the presence of native collaterals can be difficult to identify by conventional angiography or even more advanced techniques, such as laser speckle contrast, until obstruction is induced (16). Studies to better image the coronary collaterals will help to address the presence and extent of native collaterals. It is important to mention that there is a species to species variation in native coronary collaterals. It is well documented that humans have native coronary collaterals (80), although there may be substantial variations in the extent of this native circulation. Guinea pigs have the most abundant native coronary collaterals. Maxwell et al. (33) reported that the coronary collaterals in guinea pigs could compensate blood flow in the whole heart during occlusion of a major coronary artery. Also, canines and felines have well-developed coronary collaterals. In contrast, the native coronary collateral circulation of rats, ferrets, baboons, rabbits, and pigs appears to be less developed than the dog and cat. Interestingly, we (unpublished observations) have shown that in murine hearts, native collaterals are absent by microcomputed topography scanning with high resolution (4 μm), which is consistent with a report by Zhang and Faber (77).
CGG (Arteriogenesis)
The native coronary collateral circuit exists as artery-artery anastomotic connections that can function as an alternative source of blood flow supply during upstream coronary occlusion. Upon stimulation by coronary occlusion, these vessels can undergo remodeling to a larger caliber with diameters expanding 5- to 10-fold in humans, which greatly reduces their resistance to blood flow and can alleviate ischemia (11, 50, 70). Fulton (18) demonstrated that the diameter of the anastomoses in the absence of coronary artery disease (CAD) ranges from 10 to 200 µm compared with 100–800 µm in the presence of CAD. The process of abluminal expansion of collateral vessels is known as CCG or coronary arteriogenesis. CCG has been shown to reduce infarct size and prevent sudden death during coronary occlusions (4, 10).
During arteriogenesis, collaterals undergo abluminal expansion and wall thickening so as to transform into wider, corkscrew-like vessels. Collaterals exhibit significant tortuosity, which distinguishes them from other vessels of similar diameter. Unlike arteries, collaterals also grow lengthwise during their remodeling, reducing axial tension, which explains the tortuous path they take to connect one arteriole to another through the myocardium (54). However, one or more collaterals could regress after remodeling as part of their pruning process, whereas other collaterals become dominant and persist.
Growth of collateral vessels (also termed arteriogenesis) and angiogenesis are frequently used as synonyms; however, they are different processes. Angiogenesis is defined as new capillaries that stem from the budding of preexisting capillary vessels (16). Arteriogenesis, as previously defined, pertains to the remodeling of preexisting arterial vessels through the “anatomic increase in lumen area and wall thickness.” CCG in adult animals is thought to develop by expansion of a preexisting collateral network. However, there are not systemic studies to exclude the growth of new vessels. Zhang and Faber (77) reported that new collaterals formed in response to a very distal occlusion of coronary artery in the apex in murine hearts. In the same study, new anastomoses were found to connect the branches of LAD to the right coronary artery and the septal artery. However, it is not clear that whether these connections occurred from preexisting vessels or the formation of new vessels. More detailed studies such as lineage tracing would be beneficial in addressing where these connections are coming from.
The mechanisms of CCG are also incompletely understood. The factors causing arteriogenesis are likely a combination of mechanical (shear stress) and chemical factors (related to ischemia and genes activated by ischemia), whereas angiogenesis is thought to be related to tissue hypoxia and the chemical factors (16). The outward remodeling and increase in diameter of native collaterals in the presence of CAD was inferred in the study by Fulton (18) and has been demonstrated in a rabbit hindlimb ischemia model by Scholz et al. (55) along with Schaper and Scholz (54). Schaper and Scholz (54) explored fluid shear stress as the main trigger of arteriogenesis. However, CCG has also been shown to occur solely in the presence of ischemia with the absence of shear stress. It is likely that the process of CCG requires regulation from both the ischemic signals and shear stress but at different time points, e.g., shear stress being more important in the final stages of CCG and ischemia being most important for initiation (10, 11, 67). Other factors that have been implicated are cytokines, monocytes, growth factors, and stem cells (8, 11, 51, 65, 75). However, a complete understanding of this process has not been attained.
In humans, coronary collateral growth is most often reported in patients with CAD. Again, it is challenging to distinguish between new collateral formation and outward remodeling of preexisting collaterals in clinical setting. For this reason, de novo collateral formation in humans has been questioned. Zoll et al. (80) examined 1,271 human hearts postmortem by injecting differently colored (red or blue) radiopaque lead-agar masses into right coronary arteries (RCAs) and left coronary arteries (LCAs) at constant pressure. Three categories of evidence of anastomoses were accepted: 1) the presence of a continuous, colored mass-filled, endothelial cell-lined channel between two coronary arteries, 2) the presence of a lead-agar mass of any color distal to the coronary occlusion, and 3) visible mixing of colors in the injection mass. Anastomoses were found to be of varying diameters, with smaller ones ranging from 60 to 70 μm and the larger, dissectible ones in the range from 200 to 500 μm. The incidence of interarterial coronary anastomoses was 9% in hearts of normal weight from nonanemic patients and 39% in normal hearts from anemic patients, suggesting that systemic hypoxemia and perhaps lower myocardial oxygenation can stimulate CCG. Interestingly, the incidence of interarterial coronary anastomoses was 95% in nonhypertrophic hearts with coronary artery occlusion. Seventeen percent of hearts with a slight narrowing of a coronary artery had interarterial anastomoses, whereas, those with moderate and marked narrowing of a coronary artery, this percentage rose to 25% and 63%, respectively. Anastomoses were present in 25% of hypertrophic hearts without any coronary or valvular disease. The factor of relative cardiac hypoxia in all these conditions appears to be a common underlying stimulus for the development of interarterial coronary anastomoses (80). The collaterals protect the “at-risk” myocardium, and the extent of CCG is associated with improved outcomes in patients with IHD (79).
CLINICAL STUDIES IN THE CORONARY COLLATERAL CIRCULATION
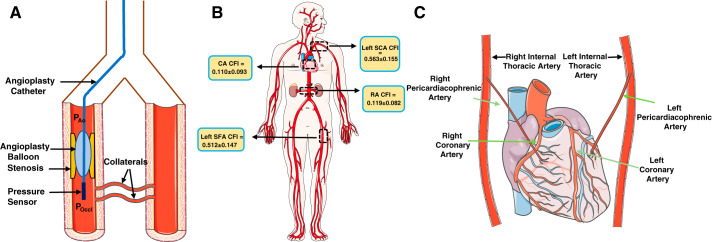
There are three prevalent clinical methods used to measure the extent of coronary collaterals: the Rentrop score, collateral flow index (CFI), and intracoronary electrocardiogram. The Rentrop score is assessed during coronary angiography as a visual assessment, with the score ranging from grade 0 to 3, where 0 indicates no visible filling of collaterals and 3 signifies complete collateral filling of the vessel being dilated (48). One limitation is that the scores can be influenced by systemic and coronary hemodynamics (24). Currently, the most well-accepted method is CFI, which was first calculated by Seiler et al. (56). The procedure for measuring CFI involves the occlusion of the stenosis by an angioplasty catheter and measurement of aortic pressure, distal coronary artery pressure, central venous pressure, and flow velocity (Fig. 1A). By measurement of the intracoronary occlusive pressure distal to the stenosis, collateral flow can be expressed relative to the normal flow through the same vessel. CFI can be calculated as follows:
where CFIp is pressure-derived CFI, Poccl is distal intracoronary occlusive pressure, Pao is mean aortic pressure, and CVP is central venous pressure (40, 72). Although this index has untested assumptions, such as the assumption that the vascular resistances distal to the occlusion are equivalent, it is a reasonable estimate of collateral growth. Stoller and Steiler (63) measured CFI in different arterial regions within the same individual (Fig. 1B) and found that in the human heart, the left and right internal thoracic arteries [formerly referred to as the internal mammary arteries (IMAs)] form anastomoses with the LCAs and RCAs, respectively. Occlusion of the distal left IMA and LCA simultaneously resulted in an increase in CFI (61). Simultaneous occlusion of the right IMA (RIMA) and RCA also increased CFI. They also showed that permanent closure of the RIMA helps to improve blood flow to the respective myocardial region via ipsilateral connections between the RIMA and RCA resulting in anti-ischemic benefits (Fig. 1C) (62). CFI also increases with prevalence of atherosclerotic lesions, given that the lesions are in hemodynamically appropriate regions (59). Patients with chronic complete coronary occlusion also have higher CFI values than those lacking it (39). It would appear that collateral growth is directly stimulated in the setting of occlusive coronary disease. It is well known that coronary collaterals occur in patients with overt CAD (59), suggesting some degree of compensation for the developing coronary lesions. Another interesting feature of patients with IHD with increased CFI is the increased prevalence of larger diameter anastomoses, whereas smaller anastomoses are “pruned” and tend to regress (59).
Fig. 1.
Clinical aspect of coronary collaterals. A: schematic showing calculation of the collateral flow index (CFI). Pao, mean aortic pressure; Poccl, distal intracoronary occlusive pressure. CFI can be calculated as follows: (Poccl – CVP)/(Pao – CVP), where CVP is central venous pressure. B: CFI as calculated by Stoller et al. in different systemic arterial territories. CA, coronary artery; left SCA, left subclavian artery; RA, renal artery; left SFA, left superficial femoral artery. C: schematic showing the orientation of the right and left internal thoracic (mammary) arteries with respect to the heart. The internal thoracic arteries give rise to the pericardiacophrenic arteries, which supply blood to the heart. Stoller et al. (63) reported increased CFI (in the right CA) in patients whose distal right internal thoracic arteries had been closed permanently. This figure was created by modifying graphic elements freely available from Servier Medical Art (https://smart.servier.com/) under a Creative Commons Attribution 3.0 Unported License.
Because reperfusion of the ischemic myocardium is critical to minimize ischemic injury (26) and collaterals can restore the blood flow, increasing CFI has been targeted as a potential therapy for patients with IHD. In the “EXCITE” study (exercise induced the formation of collaterals), CFI increased in patients with chronic stable CAD (40). While the coronary collateral circulation is related to poor outcomes in patients with CAD, the collateral circulation itself contributes beneficially by promoting reperfusion post-MI (59). The coronary microvasculature is perfused mostly during diastole, so increasing the diastolic duration by exercise improves the coronary blood flow. Therefore, a pharmacological agent capable of reducing heart rate without any adverse effects could be used to improve coronary blood flow and collateral function. Gloekler et al. (19) reported that CFI of patients with chronic stable CAD increased after 6 mo of treatment with ivabradine, whereas CFI of patients in the placebo-treated group decreased. Patients treated with ivabradine also exhibited a significant decrease in heart rate. This study corroborated the results of a previous study in a canine model of CAD (31), where bradycardia during gradual coronary occlusion stimulated arteriolar and collateral growth, improved myocardial perfusion, and upregulated VEGF and Tie-2, which are involved in angiogenesis. Another beneficial effect of ivabradine-induced diastolic prolongation is an improvement in coronary flow reserve (60, 64), which is the ratio of coronary blood flow that can be achieved under maximum vasodilation to the blood flow at baseline under normal physiological conditions. Diastolic prolongation has also been associated with improved collateral growth. Patel et al. (43) have shown that a significantly larger proportion (97%) of patients with obstructive CAD with a heart rate of 50 beats/min or lower had developed collaterals compared with patients with a heart rate of 60 beats/min or higher (55%).
CARDIOPROTECTIVE EFFECTS OF MICROVASCULAR CORONARY COLLATERALS
Development of collaterals in response to occlusion of the coronary artery is a compensatory mechanism to restore blood flow in the ischemic areas. A case was reported where the patient had complete occlusion of the main LCA and 80–90% stenosis in the RCA. Interestingly, the patient had no angina symptoms and only mild symptoms with exertion. Collaterals provided the entire blood supply to the heart, bypassing the lesion to supply the RCA area and also the LAD and left circumflex areas (34). This patient benefited from coronary collaterals. However, this line of thinking has not always been the consensus. The presence of a highly developed coronary collateral network in patients with CAD has also been shown to be positively correlated to adverse cardiovascular outcomes (52). As previously mentioned, the growth of collaterals is likely a response to the progression of coronary disease; thus, the collateral circulation should not be viewed as causative, rather as a consequence of IHD. Hence, CAD is responsible for both collateral growth as well as an unfavorable prognosis for the patient.
Collaterals show beneficial effects on cardiovascular outcomes in chronic as well as acute ischemia. Collaterals start to restore blood flow in cases of chronic total occlusion after ~12 wk postocclusion (71). A slower progression of CAD allows more time for the collaterals to remodel and develop. In cases of progressive coronary disease leading to total occlusion, normal left ventricular function has been observed as a result of collaterals restoring blood flow to the myocardium at risk. There is also evidence that left ventricular function correlates with collateral flow in acute ischemia. Seiler et al. (57) observed that patients’ CFI correlated significantly with systolic and diastolic ventricular function during myocardial ischemia. Similarly, Werner et al. (71) showed that in patients with chronic total coronary occlusion and normal regional function, collateral flow was better compared with those with impaired regional function. Moreover, Habib et al. (22) showed that in humans the presence of coronary collaterals before MI occurs leads to a smaller sized infarct as well as better left ventricular ejection fraction. On the other hand, in patients with a poor collateral network, acute MI may lead to cardiogenic shock, if the infarct is large. A well-developed coronary collateral circulation has been shown to have a beneficial effect on QT prolongation caused by ischemia, again suggesting a salubrious effect of collaterals in the setting of ischemia (36).
The presence of coronary collaterals might be also a predictor of prognosis in patients of CAD. Meier et al. (38) conducted a meta-analysis of 12 separate studies of over 6,000 patients with stable or acute CAD. Most of the studies included in this meta-analysis used Rentrop scoring to estimate coronary collateral conductance, although one used CFI (37). Subjects were grouped into two categories: high or low collateralization based on either Rentrop scores or CFI. The results indicated that patients with a higher degree of collateralization had significantly lower risk of mortality (42). Regieli et al. (47) also reported that 2-yr event-free survival was 84% and 92% in patients without and with coronary collaterals, respectively. The beneficial effect of coronary collaterals was not modified by the extent of vascular disease. The authors concluded that angiographically visible coronary collaterals are predictive of a good prognosis in patients with CHD (9, 47).
IMPAIRED CCG IN METABOLIC SYNDROME
As important as it is to fully understand the process of CCG under normal metabolic conditions, it is also important to study CCG within the context of disorders of metabolism. CCG is impaired in type II diabetes and metabolic syndrome, and patients with metabolism disorders are likely to have more severe CAD or mortality (70). The hostile environment of diabetes and metabolic syndrome affects factors that stimulate CCG, including proangiogenic growth factors, endothelial cell and smooth muscle cell function, signaling pathways, inflammatory cells and stem cells, and the redox state of the coronary circulation (70).
There is also growing appreciation of CCG in the prognosis of CHD in patients with metabolic syndrome, a condition characterized by abnormal obesity, hypertriglyceridemia, insulin resistance, and hyperinsulinemia (14, 45). Metabolic syndrome confers a two- to-fourfold increased risk for cardiovascular disease (30). Overall, patients with metabolic syndrome have a higher risk of IHDs, and nearly 30–40% of these patients have little to no growth of the coronary collateral circulation (1, 49, 74). In contrast, patients with well-defined coronary collaterals show better outcomes from a MI than those with a poorly developed collateral circulation (45). We and others have shown that CCG is impaired in preclinical models of metabolic syndrome (15, 21, 28, 29, 46). There are some reports suggesting the possible causes. In an animal model of metabolic syndrome, elevations in 20-hydroxyeicosatetraenoic acid are linked to impaired CCG via excessive infiltration of neutrophils (29). Oxidative stress in metabolic syndrome could also cause progenitor cell dysfunction and impair CCG (23, 45, 46). MicroRNAs like miR-21 and miR-145 were shown to be involved in the impaired CCG in rats with metabolic syndrome (27). Increased generation of endostatin and angiostatin via metalloproteinase activation inhibited late-stage collateral remodeling (15). Overall, the underlying mechanisms that impede collateral growth in these models of disease are not well understood, due in part to the lack of a well-developed mouse model that allows for genetic modification. Further studies using genetically modified rodents will lead to better understanding of the mechanisms underlying the inhibitory influences on collateral growth.
STIMULATION OF CCG
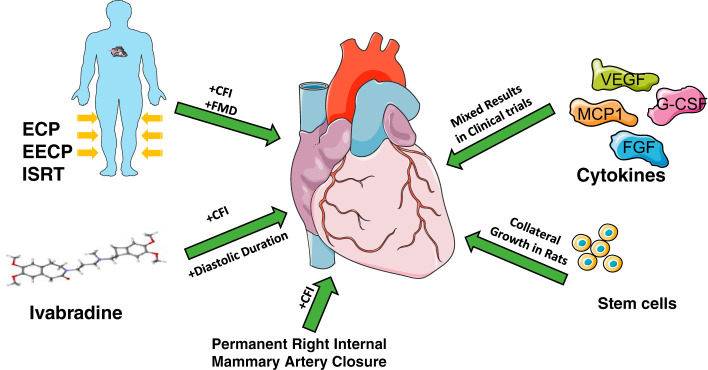
The association of a high degree of coronary collateralization with better cardiovascular outcomes led to a number of studies in which cytokines, stem cells, or physical means were used to stimulate CCG (see Fig. 3 for a summary; Refs. 8, 11, 51, 65, and 75). VEGF and FGF were two growth factors that were tested on patients with IHD in the VIVA (25) and AGENT (20) trials, respectively. In both studies, the treatment group showed no significant benefits over the control group. To specifically target arteriogenesis to promote collateral growth, Seiler et al. (58) used granulocyte macrophage-colony-stimulating factor on patients with CAD. The results of this small-scale study were promising, as the treatment group was found to have higher CFI compared with the control group. Due to adverse effects of granulocyte monocyte-colony-stimulating factor on some of the patients in a later study (76), the focus shifted to granulocyte colony-stimulating factor (35). Monocyte chemoattractant protein 1 is a proinflammatory cytokine that also showed promise as a therapeutic agent. Monocyte chemoattractant protein-1 administration resulted in the formation of collaterals in a murine model but also resulted in plaque progression at the same time (68), so it is not clear the benefit was entirely from CCG. Overall, attempts to use cytokines to stimulate arteriogenesis have not yielded significant results.
Fig. 3.
Summary of the various clinical and experimental approaches that have been used to promote coronary collateral-mediated cardioprotection in ischemic heart disease. CFI, collateral flow index; ECP, external counterpulsation; EECP, enhanced external counterpulsation; ISRT, individual shear rate therapy; FMD, flow-mediated dilation; VEGF, vascular endothelial growth factor; G-CSF, granulocyte colony-stimulating factor; MCP1, monocyte chemoattractant protein-1; FGF, fibroblast growth factor. This figure was created by modifying graphic elements freely available from Servier Medical Art (https://smart.servier.com/) under a Creative Commons Attribution 3.0 Unported License.
Mechanical phenomena also play a role in arteriogenesis, and fluid shear stress is one likely contributing factor (54). According to Newtonian fluid dynamics, shear stress (τ) can be calculated as follows:
where η is viscosity, Q is flow rate, and a is the internal radius of the vessel. It must be noted here, however, that blood is a non-Newtonian fluid. Although it is likely that shear stress contributes to collateral growth, we believe many results have been overinterpreted, especially those implying that fluid shear is the singular cause of collateral growth. For example, augmenting blood flow using physical exercise was found to have a positive impact on collateral growth in clinical studies (41, 73). This was interpreted as an effect of shear stress, but exercise would also exacerbate ischemia in the setting of a coronary stenosis. Our previous data revealed that ischemia initiates CCG (10), but if the effects of shear stress were eliminated, the final growth of collaterals is minimal. We opine that it is an oversimplification to believe CCG is caused by a single factor, and undoubtedly more factors, in addition to shear stress, inflammation, and ischemia, will be discovered as being critical to this adaptive process.
One application of mechanical stimuli for CCG is external counterpulsation, which has been used to facilitate growth of coronary collaterals through increases in flow and shear stress in the coronary circulation. In this method, pressure cuffs are applied to lower limbs and are connected to and triggered by the subjects’ ECG. During systole, these cuffs remain deflated. During diastole, however, the cuffs are inflated with air. When applied with pressures up to 300 mmHg, this procedure is referred to as enhanced external counterpulsation (EECP). Application of this pressure increases coronary driving pressure and coronary blood flow, which causes an increase in shear stress on the endothelial wall of native collaterals. This appears to facilitate the remodeling process. A study by Zhang et al. (78) showed that EECP on high-cholesterol diet-fed pigs significantly increased shear stress on the right brachial artery wall and induced shear stress-responsive gene expression changes that led to vascular remodeling in the treated animals: intimal hyperplasia was inhibited, proliferation of vascular smooth muscle cells into the intima was reduced, and endothelial nitric oxide sythase expression was rescued. In the Multicenter Study of Enhanced External Counterpulsation (MUST-EECP), use of EECP resulted in an increase in the coronary flow reserve (2). Buschmann et al. (6) also showed that external counterpulsation improves fractional flow reserve and CFIp in patients with stable CAD. The researchers calculated a novel parameter to assess blood flow velocity and shear rate, relative pulse slope index, which was significantly higher in arteries than in veins (7). They then used the relative pulse slope index to modify EECP into individual shear rate therapy by inflating the cuff with an individualized treatment pressure that achieved optimal peripheral perfusion in patients with peripheral artery disease (5). Flow-mediated dilation in the brachial artery in patients after 30 h of individual shear rate therapy was significantly increased compared with baseline measurements.
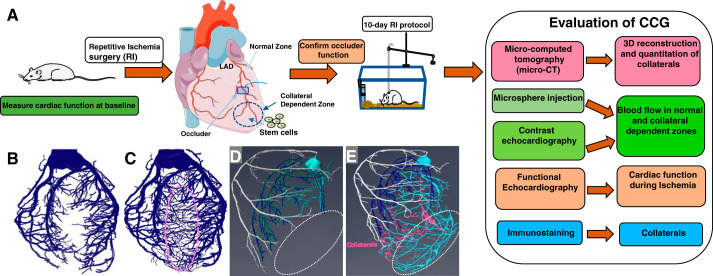
Stem cells are also promising in their ability to induce CCG, although this is an area of incomplete study. Recently, we used induced vascular progenitor cells partially reprogrammed from vascular endothelial cells to stimulate CCG in a rat model of repetitive ischemia (Fig. 2A). Induced vascular progenitor cells successfully engrafted into blood vessels and improved blood flow during ischemia better than mesenchymal stem cells, pluripotent stem cells, or endothelial cells. It suggested that stem cells might be a novel approach in stimulating CCG (75), but the potential treatment modality for the purpose of increasing collateral flow is in its infancy.
Fig. 2.
Stimulation of coronary collateral growth. A: the work flow of experimental stimulation of coronary collateral growth (CCG). B–D: three-dimensional reconstruction of microcomputed tomography images of the vasculature in the rodent heart. B: vascular tree of a heart without the stimulation of repetitive ischemia (RI). C: artist’s rendering of vascular tree of a heart with the stimulation of RI. D: vascular tree of a heart without the stimulation during the ligation of the left anterior descending coronary artery (LAD). Note the lack of vascular perfusion in the collateral dependent zone. E: artist’s rendering of vascular tree of a heart with the stimulation during ligation of the LAD. Note repetitive ischemia stimulated growth of coronary collaterals (pink) and provides an alternate supply route, bypassing the site of LAD ligation.
FUTURE DIRECTIONS
Despite the clinical importance of coronary collaterals in IHDs, factors that stimulate coronary collateral development and growth in physiological conditions, as well as those that impair CCG under pathological conditions, are still incompletely understood. The growth of coronary collaterals is a conundrum. Their significance is indisputable: a well-developed coronary collateral circulation ameliorates the consequences of CHD, reducing the incidence of sudden death and infarct sizes after coronary occlusion. However, the conundrum is how collateral growth can be stimulated in patients with CHD, as clinical trials directed at stimulating CCG have failed. Although the reasons for these failures are unresolved, one criticism of the preclinical studies (used as a basis for the therapy) was the use of young healthy animals, which does not mimic aged humans with CHD risk factors. The trials also used growth factor therapies without even establishing if the growth factors were requisite for CCG. Despite these shortcomings, there is no question that if CCG could be stimulated, it would offer a prophylactic treatment that could ameliorate the consequences of CHD. Although many approaches have been attempted to stimulate CCG, as shown in Fig. 3, to date there have been at best only modest beneficial results. However, with the implementation of new models that can better mimic the risk factors that inhibit collateral growth in patients, there is hope that these approaches could lead to new effective therapeutic strategies (79).
Restoration of CCG in metabolic syndrome is a promising approach for the treatment of IHD. Currently, our understanding of CCG is based on studies in animal models of CCG in pigs, dogs, and rats, in which certain inhibitors are administered to reduce CCG. A limitation of such “loss of function” studies is the cellular “target” of the inhibitor is unknown. The inhibitor could be acting on endothelial cells, smooth muscle cells, cardiac myocytes, inflammatory cells, and/or fibroblasts. There is no way to decipher the cell-based mechanisms of coronary blood vessel growth in the available animal models. Moreover, pharmacological inhibitors suffer from the problem of nonspecificity. To overcome these deficiencies, a murine model of CCG would enable us to use genetically modified mice to investigate many questions regarding the roles of specific genes and cell types involved in the process of CCG. While there are some reports of murine models of CCG, these models previously lacked validation and quantitation of the collateral growth, as detailed in Fig. 2A. In our preliminary study, high-resolution microcomputed topography was used to differentiate CCG from angiogenesis, and vascular tree analysis was able to quantify the CCG (Fig. 2B). We also used contrast echocardiography to measure coronary blood flow in vivo at different time points to maximize CCG (70). Tissue-specific transgenic or knockout mice can help determine the mechanisms and regulators behind CCG, and inducible transgenic mice will help study the temporal and spatial control of CCG. Better understanding the interaction of cell signaling that regulates CCG will eventually lead to new therapies designed to help patients with IHD, restoring blood flow and providing cardioprotection in ischemic hearts.
GRANTS
The work was funded by National Heart, Lung, and Blood Institute Grants 1-R01-HL-135110-01 (to W. M. Chilian and L. Yin), 1-R01-HL-137008-01A1 (to L. Yin), and 1-R15-HL-115540-01 (to L. Yin) and American Heart Association Grant 14BGIA18770028.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J., C.J., F.D., and L.Y. drafted manuscript; A.J., C.J., F.D., D.C., M.E., W.M.C., and L.Y. edited and revised manuscript; L.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Laura Zhang for her contribution to the illustrations.
Reference
- 1.Abacı A, Oğuzhan A, Kahraman S, Eryol NK, Unal S, Arinç H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 99: 2239–2242, 1999. doi: 10.1161/01.CIR.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 2.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol 33: 1833–1840, 1999. doi: 10.1016/S0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics−2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. [Corrections in Circulation 135: e646 and 136: e196, 2017.] doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry C, Balachandran KP, L’Allier PL, Lespérance J, Bonan R, Oldroyd KG. Importance of collateral circulation in coronary heart disease. Eur Heart J 28: 278–291, 2007. doi: 10.1093/eurheartj/ehl446. [DOI] [PubMed] [Google Scholar]
- 5.Buschmann EE, Brix M, Li L, Doreen J, Zietzer A, Li M, Buschmann I, Hillmeister P. Adaptation of external counterpulsation based on individual shear rate therapy improves endothelial function and claudication distance in peripheral artery disease. Vasa 45: 317–324, 2016. doi: 10.1024/0301-1526/a000544. [DOI] [PubMed] [Google Scholar]
- 6.Buschmann EE, Utz W, Pagonas N, Schulz-Menger J, Busjahn A, Monti J, Maerz W, le Noble F, Thierfelder L, Dietz R, Klauss V, Gross M, Buschmann IR; Arteriogenesis Network (Art. Net.) . Improvement of fractional flow reserve and collateral flow by treatment with external counterpulsation (Art.Net.-2 Trial). Eur J Clin Invest 39: 866–875, 2009. doi: 10.1111/j.1365-2362.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 7.Buschmann I, Pries A, Styp-Rekowska B, Hillmeister P, Loufrani L, Henrion D, Shi Y, Duelsner A, Hoefer I, Gatzke N, Wang H, Lehmann K, Ulm L, Ritter Z, Hauff P, Hlushchuk R, Djonov V, van Veen T, le Noble F. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development 137: 2187–2196, 2010. doi: 10.1242/dev.045351. [DOI] [PubMed] [Google Scholar]
- 8.Carrão AC, Chilian WM, Yun J, Kolz C, Rocic P, Lehmann K, van den Wijngaard JP, van Horssen P, Spaan JA, Ohanyan V, Pung YF, Buschmann I. Stimulation of coronary collateral growth by granulocyte stimulating factor: role of reactive oxygen species. Arterioscler Thromb Vasc Biol 29: 1817–1822, 2009. doi: 10.1161/ATVBAHA.109.186445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celik T, Iyisoy A, Yuksel CU, Celik M, Isik E. The prognostic significance of coronary collaterals in patients with ischemic heart disease: an essential response to ischemia. Int J Cardiol 138: 101–103, 2010. doi: 10.1016/j.ijcard.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Chilian WM, Mass HJ, Williams SE, Layne SM, Smith EE, Scheel KW. Microvascular occlusions promote coronary collateral growth. Am J Physiol 258: H1103–H1111, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Chilian WM, Penn MS, Pung YF, Dong F, Mayorga M, Ohanyan V, Logan S, Yin L. Coronary collateral growth–back to the future. J Mol Cell Cardiol 52: 905–911, 2012. doi: 10.1016/j.yjmcc.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho YJ, Lee EH, Lee K, Kim TK, Hong DM, Chin JH, Choi DK, Bahk JH, Sim JY, Choi IC, Jeon Y. Long-term clinical outcomes of Remote Ischemic Preconditioning and Postconditioning Outcome (RISPO) trial in patients undergoing cardiac surgery. Int J Cardiol 231: 84–89, 2017. doi: 10.1016/j.ijcard.2016.12.146. [DOI] [PubMed] [Google Scholar]
- 13.Dangas G, Kuepper F. Cardiology patient page. Restenosis: repeat narrowing of a coronary artery: prevention and treatment. Circulation 105: 2586–2587, 2002. doi: 10.1161/01.CIR.0000019122.00032.DF. [DOI] [PubMed] [Google Scholar]
- 14.Devaraj S, Jialal I. Dysfunctional endothelial progenitor cells in metabolic syndrome. Exp Diabetes Res 2012: 585018, 2012. doi: 10.1155/2012/585018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodd T, Wiggins L, Hutcheson R, Smith E, Musiyenko A, Hysell B, Russell JC, Rocic P. Impaired coronary collateral growth in the metabolic syndrome is in part mediated by matrix metalloproteinase 12-dependent production of endostatin and angiostatin. Arterioscler Thromb Vasc Biol 33: 1339–1349, 2013. doi: 10.1161/ATVBAHA.113.301533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faber JE, Chilian WM, Deindl E, van Royen N, Simons M. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol 34: 1854–1859, 2014. doi: 10.1161/ATVBAHA.114.303929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita M, Sasayama S. Reappraisal of functional importance of coronary collateral circulation. Cardiology 117: 246–252, 2010. doi: 10.1159/000323499. [DOI] [PubMed] [Google Scholar]
- 18.Fulton WF. Arterial anastomoses in the coronary circulation. I. Anatomical features in normal and diseased hearts demonstrated by stereoarteriography. Scott Med J 8: 420–434, 1963. doi: 10.1177/003693306300801102. [DOI] [PubMed] [Google Scholar]
- 19.Gloekler S, Traupe T, Stoller M, Schild D, Steck H, Khattab A, Vogel R, Seiler C. The effect of heart rate reduction by ivabradine on collateral function in patients with chronic stable coronary artery disease. Heart 100: 160–166, 2014. doi: 10.1136/heartjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 20.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, West A, Rade JJ, Marrott P, Hammond HK, Engler RL. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation 105: 1291–1297, 2002. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 21.Guarini G, Kiyooka T, Ohanyan V, Pung YF, Marzilli M, Chen YR, Chen CL, Kang PT, Hardwick JP, Kolz CL, Yin L, Wilson GL, Shokolenko I, Dobson JG Jr, Fenton R, Chilian WM. Impaired coronary metabolic dilation in the metabolic syndrome is linked to mitochondrial dysfunction and mitochondrial DNA damage. Basic Res Cardiol 111: 29, 2016. doi: 10.1007/s00395-016-0547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habib GB, Heibig J, Forman SA, Brown BG, Roberts R, Terrin ML, Bolli R; The TIMI Investigators . Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. Circulation 83: 739–746, 1991. doi: 10.1161/01.CIR.83.3.739. [DOI] [PubMed] [Google Scholar]
- 23.Hattan N, Chilian WM, Park F, Rocic P. Restoration of coronary collateral growth in the Zucker obese rat: impact of VEGF and ecSOD. Basic Res Cardiol 102: 217–223, 2007. doi: 10.1007/s00395-007-0646-3. [DOI] [PubMed] [Google Scholar]
- 24.Helfant RH, Vokonas PS, Gorlin R. Functional importance of the human coronary collateral circulation. N Engl J Med 284: 1277–1281, 1971. doi: 10.1056/NEJM197106102842301. [DOI] [PubMed] [Google Scholar]
- 25.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER; VIVA Investigators . The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 107: 1359–1365, 2003. doi: 10.1161/01.CIR.0000061911.47710.8A. [DOI] [PubMed] [Google Scholar]
- 26.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116: 674–699, 2015. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 27.Hutcheson R, Chaplin J, Hutcheson B, Borthwick F, Proctor S, Gebb S, Jadhav R, Smith E, Russell JC, Rocic P. miR-21 normalizes vascular smooth muscle proliferation and improves coronary collateral growth in metabolic syndrome. FASEB J 28: 4088–4099, 2014. doi: 10.1096/fj.14-251223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jadhav R, Dodd T, Smith E, Bailey E, Delucia AL, Russell JC, Madison R, Potter B, Walsh K, Jo H, Rocic P. Angiotensin type I receptor blockade in conjunction with enhanced Akt activation restores coronary collateral growth in the metabolic syndrome. Am J Physiol Heart Circ Physiol 300: H1938–H1949, 2011. doi: 10.1152/ajpheart.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph G, Soler A, Hutcheson R, Hunter I, Bradford C, Hutcheson B, Gotlinger KH, Jiang H, Falck JR, Proctor S, Schwartzman ML, Rocic P. Elevated 20-HETE impairs coronary collateral growth in metabolic syndrome via endothelial dysfunction. Am J Physiol Heart Circ Physiol 312: H528–H540, 2017. doi: 10.1152/ajpheart.00561.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288: 2709–2716, 2002. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 31.Lamping KG, Zheng W, Xing D, Christensen LP, Martins J, Tomanek RJ. Bradycardia stimulates vascular growth during gradual coronary occlusion. Arterioscler Thromb Vasc Biol 25: 2122–2127, 2005. doi: 10.1161/01.ATV.0000179598.57819.77. [DOI] [PubMed] [Google Scholar]
- 32.Lavi S, Abu-Romeh N, Wall S, Alemayehu M, Lavi R. Long-term outcome following remote ischemic postconditioning during percutaneous coronary interventions-the RIP-PCI trial long-term follow-up. Clin Cardiol 40: 268–274, 2017. doi: 10.1002/clc.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res 21: 737–746, 1987. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 34.Meier P. The sword of Damocles: an illustrative example of the life-saving effect of the collateral circulation. J Invasive Cardiol 23: E47–E48, 2011. [PubMed] [Google Scholar]
- 35.Meier P, Gloekler S, de Marchi SF, Indermuehle A, Rutz T, Traupe T, Steck H, Vogel R, Seiler C. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease: a controlled randomized trial. Circulation 120: 1355–1363, 2009. doi: 10.1161/CIRCULATIONAHA.109.866269. [DOI] [PubMed] [Google Scholar]
- 36.Meier P, Gloekler S, de Marchi SF, Zbinden R, Delacrétaz E, Seiler C. An indicator of sudden cardiac death during brief coronary occlusion: electrocardiogram QT time and the role of collaterals. Eur Heart J 31: 1197–1204, 2010. doi: 10.1093/eurheartj/ehp576. [DOI] [PubMed] [Google Scholar]
- 37.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation 116: 975–983, 2007. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 38.Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J 33: 614–621, 2012. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 39.Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C. The collateral circulation of the heart. BMC Med 11: 143, 2013. doi: 10.1186/1741-7015-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Möbius-Winkler S, Uhlemann M, Adams V, Sandri M, Erbs S, Lenk K, Mangner N, Mueller U, Adam J, Grunze M, Brunner S, Hilberg T, Mende M, Linke AP, Schuler G. Coronary collateral growth induced by physical exercise: results of the impact of intensive exercise training on coronary collateral circulation in patients with stable coronary artery disease (EXCITE) Trial. Circulation 133: 1438–1448, 2016. doi: 10.1161/CIRCULATIONAHA.115.016442. [DOI] [PubMed] [Google Scholar]
- 41.Naylor LH, O’Driscoll G, Fitzsimons M, Arnolda LF, Green DJ. Effects of training resumption on conduit arterial diameter in elite rowers. Med Sci Sports Exerc 38: 86–92, 2006. doi: 10.1249/01.mss.0000181220.03855.1c. [DOI] [PubMed] [Google Scholar]
- 42.Nicolau JC, Nogueira PR, Pinto MA, Serrano CV Jr, Garzon SA. Early infarct artery collateral flow does not improve long-term survival following thrombolytic therapy for acute myocardial infarction. Am J Cardiol 83: 21–26, 1999. doi: 10.1016/S0002-9149(98)00776-0. [DOI] [PubMed] [Google Scholar]
- 43.Patel SR, Breall JA, Diver DJ, Gersh BJ, Levy AP. Bradycardia is associated with development of coronary collateral vessels in humans. Coron Artery Dis 11: 467–472, 2000. doi: 10.1097/00019501-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Prunier F, Angoulvant D, Saint Etienne C, Vermes E, Gilard M, Piot C, Roubille F, Elbaz M, Ovize M, Bière L, Jeanneteau J, Delépine S, Benard T, Abi-Khalil W, Furber A. The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol 109: 400, 2014. doi: 10.1007/s00395-013-0400-y. [DOI] [PubMed] [Google Scholar]
- 45.Pung YF, Chilian WM. Corruption of coronary collateral growth in metabolic syndrome: Role of oxidative stress. World J Cardiol 2: 421–427, 2010. doi: 10.4330/wjc.v2.i12.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pung YF, Rocic P, Murphy MP, Smith RA, Hafemeister J, Ohanyan V, Guarini G, Yin L, Chilian WM. Resolution of mitochondrial oxidative stress rescues coronary collateral growth in Zucker obese fatty rats. Arterioscler Thromb Vasc Biol 32: 325–334, 2012. doi: 10.1161/ATVBAHA.111.241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regieli JJ, Jukema JW, Nathoe HM, Zwinderman AH, Ng S, Grobbee DE, van der Graaf Y, Doevendans PA. Coronary collaterals improve prognosis in patients with ischemic heart disease. Int J Cardiol 132: 257–262, 2009. doi: 10.1016/j.ijcard.2007.11.100. [DOI] [PubMed] [Google Scholar]
- 48.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 5: 587–592, 1985. doi: 10.1016/S0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 49.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation 99: 111–120, 1999. doi: 10.1161/01.CIR.99.1.111. [DOI] [PubMed] [Google Scholar]
- 50.Rocic P. Why is coronary collateral growth impaired in type II diabetes and the metabolic syndrome? Vascul Pharmacol 57: 179–186, 2012. doi: 10.1016/j.vph.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736, 2007. doi: 10.1152/ajpheart.01330.2006. [DOI] [PubMed] [Google Scholar]
- 52.Schaper W. Collateral vessels reduce mortality. Eur Heart J 33: 564–566, 2012. doi: 10.1093/eurheartj/ehr385. [DOI] [PubMed] [Google Scholar]
- 53.Schaper W, Görge G, Winkler B, Schaper J. The collateral circulation of the heart. Prog Cardiovasc Dis 31: 57–77, 1988. doi: 10.1016/0033-0620(88)90011-4. [DOI] [PubMed] [Google Scholar]
- 54.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol 23: 1143–1151, 2003. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 55.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis). Virchows Arch 436: 257–270, 2000. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- 56.Seiler C, Fleisch M, Garachemani A, Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol 32: 1272–1279, 1998. doi: 10.1016/S0735-1097(98)00384-2. [DOI] [PubMed] [Google Scholar]
- 57.Seiler C, Pohl T, Lipp E, Hutter D, Meier B. Regional left ventricular function during transient coronary occlusion: relation with coronary collateral flow. Heart 88: 35–42, 2002. doi: 10.1136/heart.88.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seiler C, Pohl T, Wustmann K, Hutter D, Nicolet PA, Windecker S, Eberli FR, Meier B. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation 104: 2012–2017, 2001. doi: 10.1161/hc4201.097835. [DOI] [PubMed] [Google Scholar]
- 59.Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J 34: 2674–2682, 2013. doi: 10.1093/eurheartj/eht195. [DOI] [PubMed] [Google Scholar]
- 60.Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis 215: 160–165, 2011. doi: 10.1016/j.atherosclerosis.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 61.Stoller M, de Marchi SF, Seiler C. Function of natural internal mammary-to-coronary artery bypasses and its effect on myocardial ischemia. Circulation 129: 2645–2652, 2014. doi: 10.1161/CIRCULATIONAHA.114.008898. [DOI] [PubMed] [Google Scholar]
- 62.Stoller M, Seiler C. Effect of permanent right internal mammary artery closure on coronary collateral function and myocardial ischemia. Circ Cardiovasc Interv 10: e004990, 2017. doi: 10.1161/CIRCINTERVENTIONS.116.004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoller M, Seiler C. Intraindividual variability and association of human collateral supply to different arterial regions. Am J Cardiol 117: 685–690, 2016. doi: 10.1016/j.amjcard.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 64.Tagliamonte E, Cirillo T, Rigo F, Astarita C, Coppola A, Romano C, Capuano N. Ivabradine and bisoprolol on doppler-derived coronary flow velocity reserve in patients with stable coronary artery disease: beyond the heart rate. Adv Ther 32: 757–767, 2015. doi: 10.1007/s12325-015-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O’Malley P, Rocic P, Focardi M, Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation 112: 2108–2113, 2005. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 66.Trzeciakowski J, Chilian WM. Chaotic behavior of the coronary circulation. Med Biol Eng Comput 46: 433–442, 2008. doi: 10.1007/s11517-008-0329-8. [DOI] [PubMed] [Google Scholar]
- 67.van den Wijngaard JP, Schulten H, van Horssen P, Ter Wee RD, Siebes M, Post MJ, Spaan JA. Porcine coronary collateral formation in the absence of a pressure gradient remote of the ischemic border zone. Am J Physiol Heart Circ Physiol 300: H1930–H1937, 2011. doi: 10.1152/ajpheart.00403.2010. [DOI] [PubMed] [Google Scholar]
- 68.van Royen N, Hoefer I, Böttinger M, Hua J, Grundmann S, Voskuil M, Bode C, Schaper W, Buschmann I, Piek JJ. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res 92: 218–225, 2003. doi: 10.1161/01.RES.0000052313.23087.3F. [DOI] [PubMed] [Google Scholar]
- 69.van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research. J Am Coll Cardiol 55: 17–25, 2009. doi: 10.1016/j.jacc.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 70.Wan W, Chinchilla S, Zhang C, Jamaiyar A, Enrick M, Nikolov Z, Kolz C, Ohanyan V, Chilian W, Yin L. A mouse model for coronary collateral growth validated via micro-CT and echo-contrast (Abstract). Circulation 136: A19191, 2018. [Google Scholar]
- 71.Werner GS, Ferrari M, Betge S, Gastmann O, Richartz BM, Figulla HR. Collateral function in chronic total coronary occlusions is related to regional myocardial function and duration of occlusion. Circulation 104: 2784–2790, 2001. doi: 10.1161/hc4801.100352. [DOI] [PubMed] [Google Scholar]
- 72.Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation 107: 2213–2220, 2003. doi: 10.1161/01.CIR.0000066321.03474.DA. [DOI] [PubMed] [Google Scholar]
- 73.Yang HT, Prior BM, Lloyd PG, Taylor JC, Li Z, Laughlin MH, Terjung RL. Training-induced vascular adaptations to ischemic muscle. J Physiol Pharmacol 59, Suppl 7: 57–70, 2008. [PMC free article] [PubMed] [Google Scholar]
- 74.Yilmaz MB, Biyikoglu SF, Akin Y, Guray U, Kisacik HL, Korkmaz S. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord 27: 1541–1545, 2003. doi: 10.1038/sj.ijo.0802474. [DOI] [PubMed] [Google Scholar]
- 75.Yin L, Ohanyan V, Pung YF, Delucia A, Bailey E, Enrick M, Stevanov K, Kolz CL, Guarini G, Chilian WM. Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth. Circ Res 110: 241–252, 2012. doi: 10.1161/CIRCRESAHA.111.250126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol 46: 1636–1642, 2005. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H, Faber JE. De-novo collateral formation following acute myocardial infarction: Dependence on CCR2+ bone marrow cells. J Mol Cell Cardiol 87: 4–16, 2015. doi: 10.1016/j.yjmcc.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, He X, Chen X, Ma H, Liu D, Luo J, Du Z, Jin Y, Xiong Y, He J, Fang D, Wang K, Lawson WE, Hui JC, Zheng Z, Wu G. Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation 116: 526–534, 2007. doi: 10.1161/CIRCULATIONAHA.106.647248. [DOI] [PubMed] [Google Scholar]
- 79.Zimarino M, D’Andreamatteo M, Waksman R, Epstein SE, De Caterina R. The dynamics of the coronary collateral circulation. Nat Rev Cardiol 11: 191–197, 2014. doi: 10.1038/nrcardio.2013.207. [DOI] [PubMed] [Google Scholar]
- 80.Zoll PM, Wessler S, Schlesinger MJ. Interarterial coronary anastomoses in the human heart, with particular reference to anemia and relative cardiac anoxia. Circulation 4: 797–815, 1951. doi: 10.1161/01.CIR.4.6.797. [DOI] [PubMed] [Google Scholar]