Abstract
The interleukin-1 system (IL-1) is a prominent pro-inflammatory pathway responsible for the initation and regulation of immune responses. Human genetic and preclinical studies suggest a critical role for IL-1β signaling in ethanol drinking and dependence, but little is known about the effects of chronic ethanol on the IL-1 system in addiction-related brain regions such as the central amygdala (CeA). In this study, we generated naïve, non-dependent (Non-Dep) and dependent (Dep) male mice using a paradigm of chronic-intermittent ethanol vapor exposure interspersed with two-bottle choice to examine 1) the expression of IL-1β, 2) the role of the IL-1 system on GABAergic transmission, and 3) the potential interaction with the acute effects of ethanol in the CeA. Immunohistochemistry with confocal microscopy was used to assess expression of IL-1β in microglia and neurons in the CeA, and whole-cell patch clamp recordings were obtained from CeA neurons to measure the effects of IL-1β (50 ng/ml) or the endogenous IL-1 receptor antagonist (IL-1ra; 100 ng/ml) on action potential-dependent spontaneous inhibitory postsynaptic currents (sIPSCs). Overall, we found that IL-1β expression is significantly increased in microglia and neurons of Dep compared to Non-Dep and naïve mice, IL-1β and IL-1ra bi-directionally modulate GABA transmission through both pre- and postsynaptic mechanisms in all three groups, and IL-1β and IL-1ra do not alter the facilitation of GABA release induced by acute ethanol. These data suggest that while ethanol dependence induces a neuroimmune response in the CeA, as indicated by increased IL-1β expression, this does not significantly alter the neuromodulatory role of IL-1β on synaptic transmission.
Keywords: IL-1β, CeA, ethanol dependence, GABA, alcohol
Introduction
The interleukin-1 system (IL-1) is a prominent pro-inflammatory pathway responsible for the initation and regulation of immune responses, but it has also received much attention for its pleiotropic neuromodulatory effects under physiological and pathophysiological conditions. In particular, its main cytokine ligand, IL-1β, has emerged as a key regulator of the ethanol-induced neuroimmune response, contributing to ethanol drinking and the development of ethanol dependence. Human genetic studies have found polymorphisms in genes encoding components of the IL-1β signaling pathway (i.e. IL-1ra (Il1rn) and IL-1β (Il1b)) associated with increased susceptibility to alcoholism1, and IL-1β levels are elevated in the periphery and brain of alcoholic patients2–4. Therefore, elucidating the mechanistic role of IL-1β in ethanol drinking and dependence is critical for understanding disease progression, as well as for the identification of novel therapeutic targets.
IL-1β is produced by caspase-1 cleavage of the inactive IL-1β precursor in response to specific immune receptor activation including: TLR4/IL-1R1, purinergic receptor or inflammasome complex5–9. IL-1β exerts its effects by binding to its cognate IL-1R1 receptor, which subsequently associates with the IL-1R1 accessory protein (IL-1RAcP) to activate intracellular signaling through multiple effectors including: MyD88, PI3K/AKT, p38 MAPK, and NF-κB10–12. Of note, IL-1R1 can also bind a second ligand, IL-1α. IL-1β/IL-1R1 signaling can be negatively regulated by other members of the IL-1 system, including an endogenous receptor antagonist (IL-1ra) and a second IL-1 receptor (IL-1R2). Specifically, IL-1R2 lacks an intracellular domain and does not initiate downstream signaling when activated by IL-1β13, but rather promotes receptor internalization14 and thus, effectively serves as a decoy receptor.Additionally, IL-1ra, a homologue of IL-1β, prevents IL-1β signaling, as it binds IL-1R1 with a similar affinity as IL-1β, but does not induce IL-1R1 signaling12. Therefore, IL-1β activity is under the control of multiple intrinsic regulatory mechanisms to ensure tight coordination of its signaling.
Preclinical studies using animal models of ethanol drinking and dependence provide strong support for the link between the IL-1β system and acute and chronic ethanol-induced changes in the brain8,15–19. Specifically, IL-1ra administration prevents ethanol-induced neuroinflammation in the cerebellum of mice given five weeks of ethanol20. Moreover, brain expression of several genes encoding components of the IL-1R1 pathway are altered in mice with a genetic predisposition to ethanol consumption21. Nevertheless, the contribution of individual components of the IL-1 system to ethanol drinking and preference can vary. For example, mice deficient in IL-1ra (i.e. IL-1rn knockout mice) have reduced ethanol preference and drinking17. In contrast, IL-1R1 (IL1r1) knockout mice display unchanged ethanol preference and drinking22, but have altered ethanol-induced sedation and withdrawal severity22. In addition, infusion of IL-1ra into the basolateral amygdala reduces binge ethanol consumption in naïve mice23 and intracerebroventricular injection of IL-1β potentiates ethanol withdrawal-induced anxiety24. While these data provide compelling evidence for the role of IL-1β signaling in ethanol dependence, little is known regarding the mechanisms by which this system modulates critical brain regions associated with ethanol drinking and the development of dependence.
The central nucleus of the amygdala (CeA), a predominantly GABAergic region, is implicated in both the escalation of ethanol drinking in dependent animals and withdrawal-associated anxiety-like behaviors25,26.We have demonstrated that neuroadaptations in GABAA-mediated transmission in the CeA are implicated in the development of ethanol dependence27–29. IL-1R1 is expressed in the CeA30 and it has been shown that excitotoxic stimuli or systemic immune challenge induces IL-1β and IL-1ra expression in the CeA 31,32. We also reported that IL-1β and IL-1ra bi-directionally modulate (both increase or decrease) electrically-evoked and action potential-independent GABAergic transmission (mIPSCs) in the CeA33,34 of ethanol naïve mice. Moreover, in neurons where IL-1β decreased action potential-independent GABA release, acute ethanol’s ability to facilitate GABA release was abolished. A similar loss in acute ethanol’s effects on action potential-independent GABA transmission was observed in CeA neurons of IL-1ra knockout mice33,34. However, IL-1β did not alter the facilitatory actions of ethanol on evoked GABA responses33, suggesting that this interaction may be less robust when measured across the CeA synaptic network. Therefore, in the present study we hypothesized that the IL-1 system in mouse CeA is dysregulated by ethanol drinking and dependence. Specifically, we investigated the role of IL-1β signaling on action potential-dependent GABA transmission (sIPSCs) and its interaction with acute ethanol in the mouse CeA, and whether these neuromodulatory actions are altered with ethanol dependence. Additionally, we hypothesized that ethanol dependence would induce a peripheral immune response characterized by increased pro- (i.e. IL-1β) and decreased anti-inflammatory cytokines and increased central IL-1β in the CeA.
Methods
Animals
Male C57BL/6J (n = 111; 9–10 weeks) mice were obtained from The Jackson Laboratory (ME) and group-housed in a temperature and humidity-controlled vivarium on a 12 hour light/dark cycle with food and water available ad libitum. All protocols involving the use of experimental animals in this study were approved by The Scripps Research Institute (TSRI) Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chronic-intermittent ethanol two-bottle choice paradigm
To induce ethanol dependence, we used the chronic intermittent ethanol two bottle choice (CIE-2BC) paradigm. This method consistently produces ethanol dependence in C57BL/6J mice35–37, as exhibited by their increased ethanol drinking, anxiety-like behavior and reward deficits. We exposed 78 mice to a limited access ethanol (15% w/v) two-bottle choice (2BC) paradigm followed by either chronic intermittent ethanol (CIE) exposure in vapor chambers (La Jolla Alcohol Research, La Jolla, CA), to induce ethanol dependence (Dep, n =38), or air exposure in identical chambers (Non-Dep, n = 40). The remaining 33 mice were ethanol naïve and were not exposed to any ethanol either by drinking or vapor exposure.
To establish baseline drinking in the ethanol-exposed mice, we performed 2BC testing 5 days per week for 4 consecutive weeks. Mice were singly housed 30 minutes before the lights were turned off and given limited access (2 hours) to two drinking tubes containing either 15% ethanol or water. Following this baseline phase, the mice were divided into two balanced groups with equal ethanol and water consumption (Figure 1). The Dep and Non-Dep groups were exposed to CIE vapor and air, respectively. Mice in the Dep group were i.p. injected with 1.75 g/kg ethanol + 68.1 mg/kg pyrazole (alcohol dehydrogenase inhibitor) and placed in vapor chambers for 4 days (16 hours vapor on, 8 hours off). Naïve and Non-Dep mice were injected with 68.1 mg/kg pyrazole in saline. After pyrazole injection, naïve mice were placed back in their home cages, while Non-Dep mice were transferred into air chambers for the same intermittent period as the Dep group. The vapor/air exposure was followed by 72 hours of abstinence and 5 days of 2BC testing. This regimen was repeated 2–3 additional times for a total of 3–4 full rounds. On the third or fourth day of vapor exposure tail blood was collected to determine blood ethanol levels (BELs). Ethanol drip rates in the vapor chambers were altered such that BELs progressively increased over the vapor rounds to a final target of 200–250 mg/dL. Before euthanasia, Dep mice were exposed to a single ethanol vapor exposure (16 hours) (Figure 1A), and tail blood was collected to determine terminal BELs.
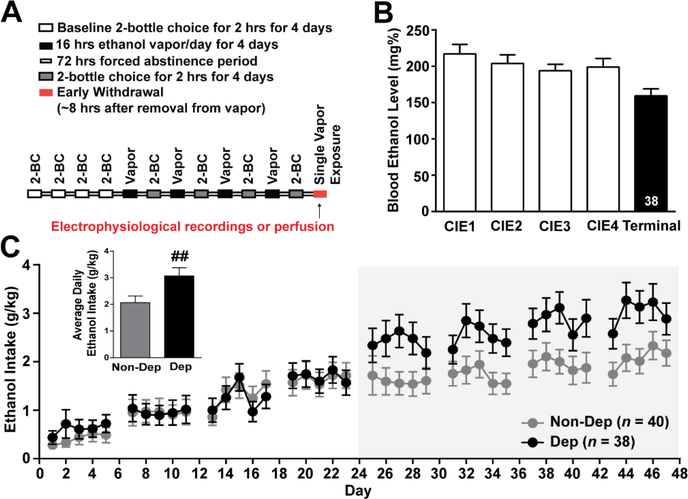
Figure 1. Chronic-intermittent ethanol two-bottle choice paradigm.
A. Schematic illustrating the chronic-intermittent ethanol two-bottle choice behavioral paradigm used to induce ethanol dependence in C57BL/6J mice. B. Average blood ethanol levels (BEL) achieved during weeks of chronic intermittent ethanol vapor exposure and terminal BEL for Dep mice. C. Ethanol intake during two-bottle choice testing, demonstrating a significant escalation in the average ethanol intake in dependent (Dep, n = 38) compared to non-dependent (Non-Dep, n = 40) mice during the last week of 2-bottle choice (inset). ##, p < 0.01 by one-tailed t-test.
Immunohistochemistry and confocal microscopy
We perfused mice (n = 4 naïve, n = 3 Non-Dep and n = 3 Dep) with ice-cold PBS followed with Z-Fix fixation solution (Anatech Ltd., Battle Creek, MI). After perfusion, brains were postfixed in Z-Fix at 4˚C for 72 hours, cryoprotected with 30% sucrose at 4˚C for ~72 hours (until brains sank), flash frozen with 2-methylbutane on dry ice, and stored at −80˚C. From each mouse, sequential coronal sections (35 μm) containing the CeA, starting at approximately Bregma −1.20 mm to Bregma −1.55mm, were cut on a cryostat and free-floating brain sections were stored in cryoprotective solution (50% v/v phosphate buffer, 30% w/v sucrose, 1% w/v polyvinylpyrrolidone, 30% v/v ethylene glycol) at −20˚C until staining. Every third section collected was subsequently used for immunostaining. Brain sections were blocked in 10% normal donkey serum in PBS, incubated in an unconjugated Fab fragment donkey anti-mouse to block endogenous mouse IgG for 2 hours at room temperature, and subsequently incubated in mouse anti-NeuN (1:500, MAB377, Millipore, MA) overnight at 4°C. The following day, sections were incubated in biotin conjugated-fab fragment donkey anti-mouse secondary followed by Alexa Fluor 568-conjugated streptavidin. Then, sections were incubated in rabbit anti-IL-1β (1:500, ab9787, Abcam, MA) and guinea pig anti-Iba-1 (1:500, ab107159, Abcam) antibodies overnight at 4°C followed by incubation in a secondary antibody solution containing Alexa Fluor 488-conjugated donkey anti-rabbit and Alexa Fluor 647-conjugated donkey anti-goat and Hoechst 33342 (1:1000) for 1 hour at room temperature. Antibody specificity for IL-1β was confirmed by preabsorption with recombinant mouse IL-1β, which blocked further staining with the IL-1β antibody. This antibody has been previously used for immunohistochemistry in mouse38,39 and detects both the inactive, precursor and active form of IL-1β40. The Iba-1 antibody41,42 and NeuN antibody43,44 have been extensively used in mice.
We used the Zeiss LSM 780 and Zeiss LSM 880 Airyscan Confocal microscope (Carl Zeiss Microscopy, NY USA) for image acquisition and Imaris (Bitplane, MA USA) and Image Pro Premier (Media Cybernetics, MD, USA) software for image analyses. Analysis was conducted in a treatment/group blind manner and applied consistently across all treatment groups. All images were acquired as z stacks with both a 40x (1.4 na) and 63x (1.4 na) objective, at 0.4 μm and 0.3 μm step sizes, respectively. Using a module within the Zeiss Zen software, z stacks [obtained with a 40x objective] of image panels of the entire CeA were tiled and auto-stitched into a 3D mega image. The tiled mega images were imported as maximum intensity projections, which were used to define and outline the CeA based on fiducial markers and Allen Mouse Brain Atlas45 (as seen in Figure 2A). Specifically, the medial boundary was limited by the optic tract/substantia innominate and the lateral boundary by the external capsule which is directly adjacent to the basolateral amygdala (BLA). The dorsal and ventral boarders were determined by the striations of the caudo-putamen and stria terminalis, respectively.
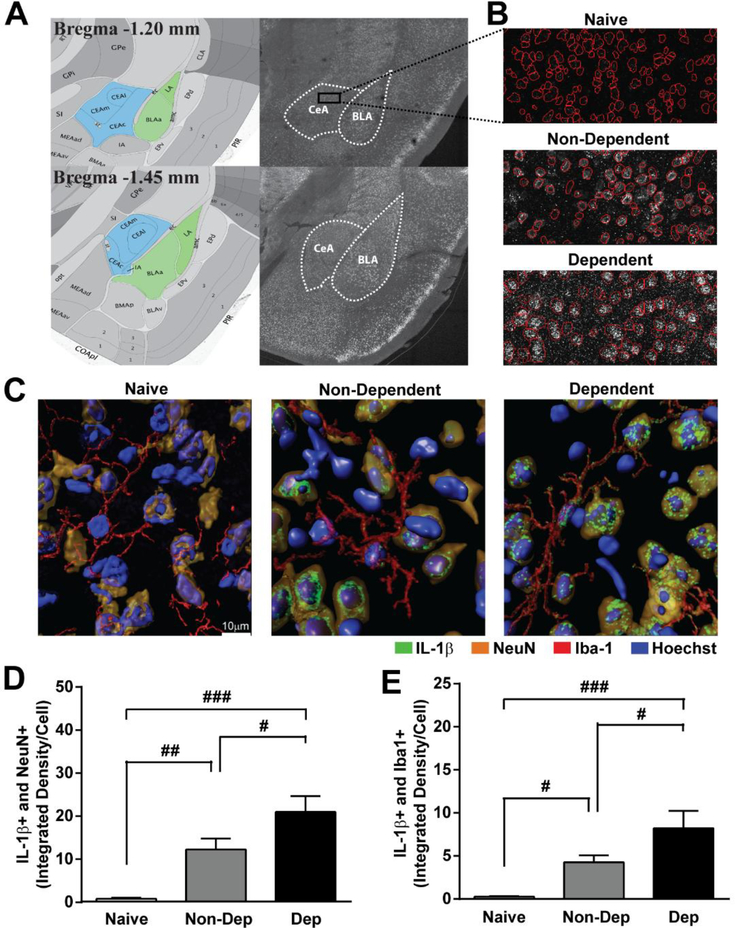
Figure 2. IL-1β expression in neurons and microglia in the CeA of naïve, non-dependent and dependent mice.
A. Allen Mouse Brain Altas images and corresponding serial tiled and stitched confocal image depicting the amygdala including the central nucleus of the amygdala (CeA, blue) and basolateral amygdala (BLA, green). B. 40X magnified panel from outlined CeA showing IL-1β staining and auto-traced cell body outlines (red) from naïve (top), Non-Dep (middle) and Dep (bottom) mice. C. Representative 3D rendered 63X confocal images of IL-1β (green) co-labeling with NeuN (orange) and Iba-1 (red) in the CeA of naïve (left), Non-Dep (middle), and Dep (right) mice. D and E. Quantification of IL-1β co-labeling with NeuN and Iba1, respectively in naïve, Non-Dep, and Dep mice. ###, p < 0.001; ##, p < 0.01; #, p < 0.05 by one-way ANOVA with Tukey post-hoc test for multiple comparisons.
Prior to acquisition of all the sections, a sample section from naïve, Non-Dep and Dep mice were pre-screened blindly to determine which group had roughly the greatest IL-1β signal. The group with the greatest signal (visual intensity) was used to set and determine the power (P) and gain (G) setting to be used by the confocal system to acquire a full signal dynamic range of 0.256 (signal intensity) per each 8-bit image. Those power and gain setting were incorporated into a method of acquisition in the ZEN software that was used for all of the other groups in the study Settings were as follows: [Blue P=1, G=600; Green P=3, G=750; Red P=2, G=800; FR P=3, G=800], which was used consistently across all samples.
Sample size was based on previous immunohistochemical studies conducted in the CeA36,46. Using Image Pro Premier (IPP), cell bodies of neurons (based on NeuN and DAPI signals outlined and dilated in IPP) and microglia (based on dilated Iba-1 and DAPI signal outlined and dilated in IPP) were auto-traced within the defined CeA (Figure 2B). Auto-traced outlines of the cells were used as regions of interest from which the total signal above background was extracted for the IL-1β signal. The total cellular area and mean fluorescence intensity of IL-1β in each cell was scored and plotted as integrated density (ID) (average area × mean fluorescence intensity/100), which was estimated separately for approximately 800 neurons and 200 microglial cells per section The average ID/cell per section for 3–4 sections from each mouse, containing both the left and right hemisphere CeA, was used for statistical analysis and graphing47–50.
Slice Preparation
Mice were anesthetized with 3–5% isofluorane, decapitated, and the brains were quickly removed and placed in ice-cold oxygenated (95% O2 and 5% CO2) high-sucrose cutting solution containing (in mM): 206 sucrose, 2.5 KCl, 2.5 CaCl2, 7 MgCl2, 1.2 NaH2PO4, 26 NaHCO3, 5 glucose, 5 HEPES; pH 7.3. Coronal slices (300 μM) containing the CeA were cut using a Leica 1200S vibratome (Leica Microsystems, Buffalo Grove, IL) and incubated in artificial cerebrospinal fluid (ACSF) containing (in mM): 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 1.5 MgSO4, 2 CaCl2, 24 NaHCO3, 10 glucose; pH 7.3 at 37°C for 30 minutes and then at room temperature for at least 30 minutes before use.
Whole-cell patch clamp recordings
We recorded from 148 neurons in the medial subdivision of the CeA that were visualized with infrared differential interference contrast (IR-DIC) optics. First, whole-cell current-clamp recordings were used to determine firing properties of the cell using a step protocol consisting of incremental hyperpolarizing to depolarizing current injections as previously described51,52. Then, whole-cell patch clamp recordings were obtained in the sample cells using a voltage-clamp, gap-free acquisition mode using Multiclamp 700B amplifier, Digidata 1440A and pClamp 10 software (Molecular Devices, Sunnyvale, CA). Glass pipettes were pulled to a resistance of 3–6 MΩ and filled with an internal solution containing (in mM):135 KCl, 5 EGTA, 2 MgCl2, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP; 290–300 mOsms; pH 7.2–7.3. GABAA-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) were pharmacologically isolated with glutamatergic transmission blockers (20μM 6,7-dinitroquinoxaline-2,3-dione, DNQX and 30 μM DL-2-amino5-phosphonovalerate, AP-5) and a GABAB receptor blocker (1 μM CGP55845A). CeA neurons were voltage-clamped at −60 mV. Series resistance was not compensated, and cells with a series resistance > 20 MΩ or with a > 20% change during the recording, as monitored by 10 mV pulses, or with a holding current ≥ 100 pA were excluded.
Frequency, amplitude and kinetics of sIPSCs were analyzed using Mini Analysis (Synaptosoft Inc., Fort Lee, NJ), and each sIPSC was visually confirmed. Three minute time intervals were binned together to obtain average sIPSC characteristics. The maximum effect during minute 6 through 15 of drug application was taken, and changes greater or equal to 15% were considered as an increase or decrease. Data were analyzed and graphed in Prism 6.01 (GraphPad, San Diego, CA). Data are presented as mean ± standard error (SEM), and n represents sample number of cells or mice as indicated. Slices from each mouse were used for different experiments to increase biological diversity in each experimental group.
Multiplex assay of the peripheral cytokine/chemokine levels.
Trunk blood was collected, during isolation of the brain for electrophysiological experiments, and used to assay peripheral cytokine levels. Briefly, blood was allowed to coagulate for 10–15 minutes at room temerature, centrifuged at 1,000g for 10 minutes at 4°C, and collected sera were stored at −80°C. Duplicates of each sample were tested using MAP Mouse Cytokine/Chemokine Magnetic Bead Panel kit (MCYTOMAG-70K; MilliporeSigma, Burlington, MA) and the Luminex 200 System (Luminex Coorporation, Austin, TX). The assay was conducted by the Metabolic Core at The Scripps Research Institute Florida (Jupiter, FL) following the manufacturer’s protocol. Results are presented as mean ± SEM (pg/ml) and n represents the number of samples.
Drugs
We purchased CGP55845A, DNQX, and AP-5 from Tocris Bioscience (Ellisville, MI), recombinant mouse IL-1β from Biolegend (San Diego, CA), recombinant human IL-1ra from Peprotech (Rocky Hill, NJ), and ethanol from Remet (La Mirada, CA). Drugs were dissolved in ACSF by adding a known concentration of the stock solutions. Drug concentrations for IL-1β, IL-1ra, and ethanol were selected based on our previous reports33,53.
Results
Ethanol dependence dysregulates peripheral immune cytokine levels.
We used the chronic intermittent ethanol two bottle choice (CIE-2BC) paradigm to generate ethanol dependent mice for this study (Figure 1). This method consistently produces ethanol dependence in C57BL/6J mice35–37, as exhibited by their increased ethanol drinking, anxiety-like behavior and reward deficits. All experiments in this study were conducted across three groups: 1) naïve mice (n = 33) that did not receive any ethanol, 2) Non-Dep mice (n = 40) that received only two-bottle choice to control for the vapor exposure, and 3) Dep mice (n = 38) that received two-bottle choice and chronic-intermitent ethanol vapor exposure. Dep mice achieved an average BEL across the four rounds of CIE of 203.60 ± 5.79 mg% (Figure 1B). We observed a significant escalation (p < 0.01, one-tailed t-test) in the average daily ethanol intake during the last round of 2BC in Dep (3.07 ± 0.30 g/kg) compared to Non-Dep (2.07 ± 0.25 g/kg) mice (Figure 1C, inset) indicative of a dependence-like phenotype. Additionally, Dep mice (26.90 ± 0.04 g) had a significantly (F(2,83) = 0.07; p < 0.001; one-way ANOVA) decreased terminal weight compared to naïve (31.12 ± 0.51 g) and Non-Dep (29.87 ± 0.44 g) mice. To assess changes in peripheral immune activation in this model of ethanol dependence, we measured levels of an array of cytokines including IL-1 related elements in blood serum. Several cytokine levels were altered in Non-Dep and Dep mice compared to naïve mice (Table 1). In particular, we found that the peripheral IL-1 system was dysregulated; specifically, IL-1α levels in serum of Dep (224.20 ± 26.74 pg/mL, n = 22) compared to naïve (380.40 ± 44.39 pg/mL, n = 20) and Non-Dep (406.30 ± 45.43, n = 17) mice were significantly (F(2,56) = 1.33; p < 0.01; one-way ANOVA) decreased (Table 1). Although overall no changes in peripheral IL-1β levels among the groups were observed, likely due to greater variability within the Dep group (Table 1), other pro-inflammatory cytokines including interferon gamma-induced protein 10 (IP-10) and tumer necrosis factor alpha (TNF-α) levels significantly and trended towards an increase compared to naïve, respectively. Anti-inflammatory cytokines including IL-4, IL-10, IL-13, leukemia inhibitory factor (LIF) decreased with chronic ethanol exposure compared to naive, while IL-5 trended towards an increase compared to naïve. Thus, our CIE-2BC paradigm effectively generates ethanol-dependent mice and induces peripherial alterations in pro-inflammatory and anti-inflammatory cytokines.
Table 1.
Cytokine levels (pg/mL) in terminal blood serum collected from naïve, Non-Dep and Dep mice.
| pg/ml | Naïve | Non-Dependent | Dependent |
|---|---|---|---|
| IL-1α | 380.4 ± 44.39 | 406.3 ± 46.43 | 224.2 ± 26.74*,## |
| IL-1β | 3.39 ± 0.62 | 1.94 ± 0.41 | 4.79 ± 2.61 |
| G-CSF | 583.7 ± 204.7 | 581.4 ± 294.2 | 310.5 ± 76.4 |
| Eotaxin | 577.7 ± 41.8 | 451.4 ± 33.0* | 381.2 ± 28.1** |
| GM-CSF | 4.03 ± 1.2 | 0.7 ± 0.5 | 7.1 ± 4.1 |
| IFNγ | 0.08 ± 0.05 | 0.02 ± 0.02 | 0.8 ± 0.5 |
| IL-2 | 0.72 ± 0.4 | 0.01 ± 0.01 | n.d. |
| IL-3 | 0.36 ± 0.2 | 0.007 ± 0.007 | 0.52 ± 0.5 |
| IL-4 | 0.98 ± 0.03 | 0.86 ± 0.01* | 0.80 ± 0.03*** |
| IL-5 | 3.03 ± 0.43 | 2.48 ± 0.70 | 25.58 ± 17.67 |
| IL-6 | 17.8 ± 8.0 | 24.2 ± 13.5 | 9.66 ± 2.82 |
| IL-7 | 2.92 ± 2.47 | 7.02 ± 6.54 | 0.65 ± 0.63 |
| IL-9 | 150.3 ± 26.0 | 197.8 ± 48.8 | 121.1 ± 14.3 |
| IL-10 | 2.01 ± 0.38 | 0.97 ± 0.20* | 0.61 ± 0.25** |
| IL-12 (p40) | 9.48 ± 3.98 | 2.45 ± 0.58 | 5.55 ± 4.32*** |
| IL-12 (p70) | 3.53 ± 1.06 | 0.08 ± 0.08** | 1.29 ± 0.93** |
| LIF | 0.93 ± 0.21 | 0.93 ± 0.13### | 0.45 ± 0.11** |
| IL-13 | 37.9 ± 5.01 | 31.0 ± 3.09 | 23.5 ± 2.47* |
| LIX | 4369 ± 351.6 | 4663 ± 245.2 | 3271 ± 273.6*,## |
| IL-15 | 59.05 ± 4.63 | 51.6 ± 6.00 | 19.8 ± 6.35***,### |
| IL-17 | 0.91 ± 0.21 | 0.02 ± 0.01*** | 0.38 ± 0.19** |
| IP-10 | 126.1 ± 11.0 | 103.6 ± 8.17 | 258.1 ± 45.0# |
| KC | 145.9 ± 51.6 | 225.4 ± 85.4 | 88.1 ± 25.1 |
| MCP1 | 19.2 ± 8.90 | 11.7 ± 5.1 | 13.2 ± 5.27 |
| MIP-1a | 40.3 ± 3.33 | 29.2 ± 3.92 | 13.8 ± 3.56***,# |
| MIP-1b | 19.5 ± 2.63 | 14.5 ± 2.26 | 26.1 ± 4.53 |
| M-CSF | 111.9 ± 80.5 | 208.5 ± 102.8 | 289.3 ± 115.7 |
| MIP-2 | 111.9 ± 11.6 | 78.3 ± 4.03 | 50.5 ± 12.6***,# |
| MIG | 39.0 ± 3.28 | 45.2 ± 5.29 | 107.9 ± 28.1 |
| RANTES | 20.1 ± 2.28 | 16.7 ± 2.78 | 11.4 ± 2.13* |
| VGF | 2.46 ± 0.32 | 2.796 ± 0.46 | 2.28 ± 0.33 |
| TNFα | 0.41 ± 0.20 | 0.11 ± 0.07 | 1.71 ± 1.10 |
p < 0.05
p < 0.01
p < 0.001 compared to Naïve
p < 0.05
p < 0.01
p < 0.001 for Non-Dep versus Dep by one-way ANOVA and Tukey posthoc test; n = 17 – 22 mice.
Ethanol dependence increases IL-1β expression in CeA neurons and microglia.
We first asked whether neurons and microglia in the CeA produce IL-1βand if chronic ethanol exposure alters the expression of IL-1βusing immunohistochemistry and confocal microscopy. We found that IL-1β co-localizes with neuronal (NeuN) and microglial (Iba-1) markers (Figure 2), suggesting that both cell-types produce IL-1βin the CeA. IL-1β staining was increased in neurons of Non-Dep (12.28 ± 2.55 ID/cell; n = 9 sections), and to a greater degree in Dep (20.98 ± 3.70 ID/cell; n = 9 sections) mice (Figure 2C, D) compared to naïve, which showed very low expression of IL-1β in neuronal cell bodies (0.81 ± 0.24 ID/cell; n = 15 sections). One-way ANOVA showed a significant effect of chronic ethanol treatment on IL-1β staining in CeA neurons (F(2,30) = 13.30; p < 0.0001; η2 = 0.62) and Tukey posthoc test determined significant differences among all three treatment groups. In microglia, we observed a similar pattern of IL-1β staining, with low IL-1β expression in naïve (0.27 ± 0.08 ID/cell), higher levels in Non-Dep (4.27 ± 0.81 ID/cell), and the highest levels in Dep (8.21 ± 2.03 ID/cell) mice (Figure 2E). One-way ANOVA also showed a significant effect of chronic ethanol treatment on IL-1β staining in CeA microglia (F(2,32) = 9.54; p < 0.0001; η2 = 0.52) and Tukey posthoc test determined significant differences among all three treatment groups. These findings suggest that IL-1β levels in CeA neurons and microglia are sensitive to varying degrees of ethanol exposure and thus may be a critical neuroadaptation contributing to the development of ethanol dependence.
Ethanol dependence does not alter the dual effects of IL-1β on spontaneous action potential-dependent GABA transmission in the CeA.
First, we compared baseline sIPSC characteristics between naïve, Non-Dep and Dep mice. We found that the sIPSC amplitude was significantly (p < 0.05) decreased in Non-Dep compared to naïve and Dep mice, and sIPSC rise time was significantly increased in Non-Dep compared to naïve mice (Table 2). No significant differences were observed in the frequency and decay time of sIPSCs across groups. We next asked whether the increased expression of IL-1β observed with chronic ethanol exposure at the cellular level is associated with alterations in the neuromodulatory role of IL-1β on GABAergic transmission in the CeA. In previous work from our lab, we demonstrated that CeA neurons from naïve B6129SF2/J mice have cell-specific pre- and postsynaptic responses to exogenous IL-1β characterized by mixed effects on mIPSC frequency and amplitude, respectively33. In line with those findings, here we found that IL-1β (50 ng/ml33) had mixed effects on action potential-dependent sIPSC frequency in naïve C57BL/6J mice (Figure 3A, C-E). IL-1β significantly increased (p < 0.001; one sample t-test; 51.7 ± 7.3%) sIPSC frequency in 42% (10 of 24 cells) and decreased (p < 0.001; one sample t-test; 28.7 ± 1.74%) it in 42% (10 of 24 cells) of neurons in naïve mice, suggesting that IL-1β can have dual effects on GABA release probabilities54. Of note, we examined and compared sIPSC kinetics (rise and decay time) in all experiments in this study; but overall, while a few significant changes were observed, no obvious conclusions could be made and therefore are not reported.
Table 2.
Baseline sIPSC characteristics in Naïve, Non-dependent and Dependent mice.
| Frequency | Amplitude | Rise Time | Decay Time | |
|---|---|---|---|---|
| Naïve | 1.28 ± 0.13 | 67.70 ± 3.27 | 2.35 ± 0.07 | 7.94 ± 0.42 |
| Non-Dep | 1.43 ± 0.16 | 55.13 ± 2.13* | 2.67 ± 0.08* | 7.93 ± 0.42 |
| Dep | 1.58 ± 0.21 | 65.88 ± 3.4# | 2.51 ± 0.46 | 7.96 ± 0.46 |
p < 0.05 compared to Naïve
p < 0.05; for Non-Dep versus Dep by one-way ANOVA and Tukey posthoc test; n = 53 – 60 cells.
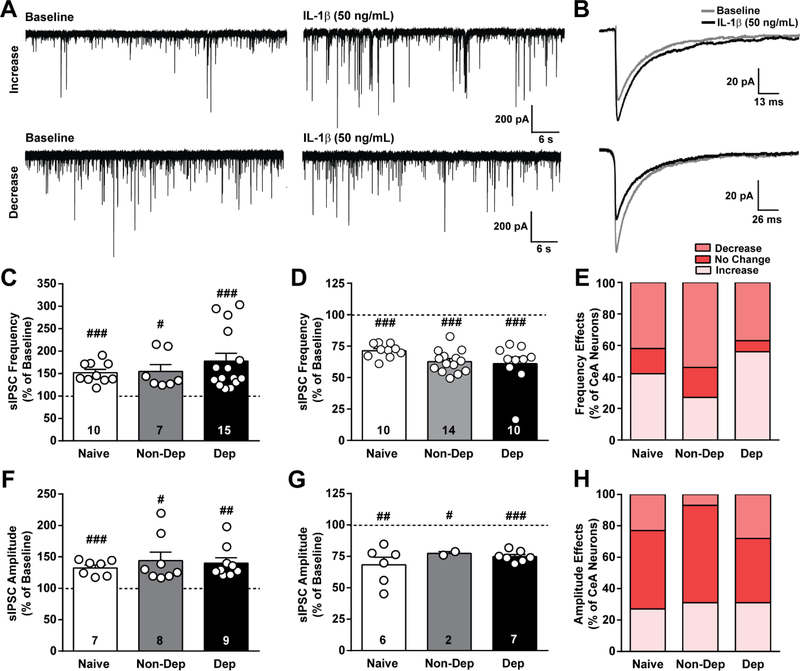
Figure 3. IL-1β effects on GABAergic transmission in the CeA of naïve, non-dependent, and dependent mice.
A. Representative traces of spontaneous action potential-dependent inhibitory postsynaptic currents (sIPSCs) during baseline and during application of IL-1β (50 ng/mL) from CeA neurons of naïve mice showing an increase (top row) and decrease (bottom row) in sIPSCs induced by IL-1β. B. Average sIPSC from a single naïve cell showing either an increase (top) or decrease (bottom) in amplitude induced by IL-1β. C and D. IL-1β-induced increase and decrease, respectively, in sIPSC frequency plotted as a percent of baseline for naïve, Non-Dep, and Dep mice. E. Percent of CeA neurons responding to IL-1β with an increase, decrease or no change in sIPSC frequency. F and G. IL-1β-induced increase and decrease, respectively, in sIPSC amplitude plotted as a percent of baseline. H. Percent of CeA neurons reponding to IL-1β with an increase, decrease or no change in sIPSC amplitude. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 by one-sample t-test; n = 24 – 27 cells from 14 – 15 mice per group, and the exact number of cells in each condition is reported in the bars.
Similar to naïve mice, in Non-Dep mice, IL-1βsignificantly increased (p < 0.05; one sample t-test; 54.7 ± 15.2%) and decreased (p < 0.001; one sample t-test; 37.3 ± 2.4%) sIPSC frequency in 27% (7 of 26 cells) and 54% (14 of 26 cells) of neurons, respectively. IL-1βalso significantly increased (p < 0.001; one sample t-test; 77.7 ± 17.4%) and decreased (p < 0.001; one sample ttest; 39.0 ± 5.4 %) sIPSC frequency in 56% (15 of 27 cells) and 37% (10 of 27 cells) of Dep neurons, respectively. To compare the magnitude of IL-1β’s effect on sIPSC frequency among the treatment groups, we used an one-way ANOVA and found no significant differences in either increases or decreases (F(2,31) = 1.07; p > 0.05). Additionally, we used the Chi-square test to determine if there was a significant difference in the distribution of neuronal responses to IL-1β, and we did not observe any significant differences (p > 0.05) among the three treatment groups. Collectively, these data demonstrate that ethanol dependence does not affect IL-1β’s regulation of CeA GABA release at the synaptic and cellular population level.
We also examined the effect of IL-1β on sIPSC amplitude, independent of frequency effects, and observed dual effects in naïve, Non-Dep and Dep mice (Figure 3B, F-H). In naïve mice, IL-1βsignificantly increased (p < 0.001; one sample t-test; 32.5 ± 4.6%) and decreased (p < 0.01; one sample t-test; 31.7 ± 6.1%) sIPSC amplitude in 29% (7 of 24 cells) and 25% (6 of 24 cells) of neurons, respectively. In Non-Dep mice, IL-1βsignificantly increased (p < 0.05; one sample t-test; 44.1 ± 13.4%) in 31% (8 of 26 cells) and decreased (p < 0.05; one samplpe t-test; 22.6 ± 1.4%) sIPSC amplitude in 7% (2 of 26 cells) of neurons. In Dep mice, IL-1β also significantly increased (p < 0.01; one sample t-test; 40.2 ± 8.5%) and decreased (p < 0.001; one sample t-test; 25.1 ± 1.6%) sIPSC amplitude in 33% (9 of 27 cells) and 26% (7 of 27 cells) of neurons, respectively. Changes in amplitude did not correlate with changes in frequency in any of the groups. Overall, there were no significant differences in the magnitude of IL-1β’s effect on amplitude in the distribution of responses to IL-1βacross the treatment groups. Additionally, we found no significant correlation between IL-1β’s effect on sIPSC frequency or amplitude and the cell type, assessed by firing properties. Therefore, IL-1β can bi-directionally modulate CeA GABA transmission through independent pre- and postsynaptic mechanisms, and chronic ethanol exposure does not significantly alter the neuromodulatory role of IL-1βon GABA transmission in this region.
Ethanol dependence does not alter the dual effects of IL-1ra on spontaneous action potential-dependent GABA transmission in the CeA.
To assess changes in basal regulation of spontaneous GABAergic transmission by the IL-1 system with chronic ethanol, we used IL-1ra, an endogenous antagonist of IL-1R1. We found that IL-1ra (100 ng/ml33) had mixed effects on sIPSC frequency in the CeA of naïve mice (Figure 4A, C-E). Specifically, IL-1ra increased sIPSC frequency (p > 0.05; one sample t-test; 51.5 ± 21.0% ) in 29% (4 of 14 of cells) but did not reach statistical significance, and significantly decreased (p < 0.001; one sample t-test; 25.4 ± 2.1%) sIPSC frequency in 50% (7 of 14 cells) of neurons, similar to the IL-1ra-induced dual effects on mIPSC frequency we observed in naïve B6129SF2/J mice33. Notably, here, the percentage of cells that responded to IL-1ra (antagonist) with a decreased sIPSC frequency in naïve mice was similar to the percentage of cells that responded to IL-1β (agonist) with an increased frequency, and vice versa (Figure 3E, 4E).
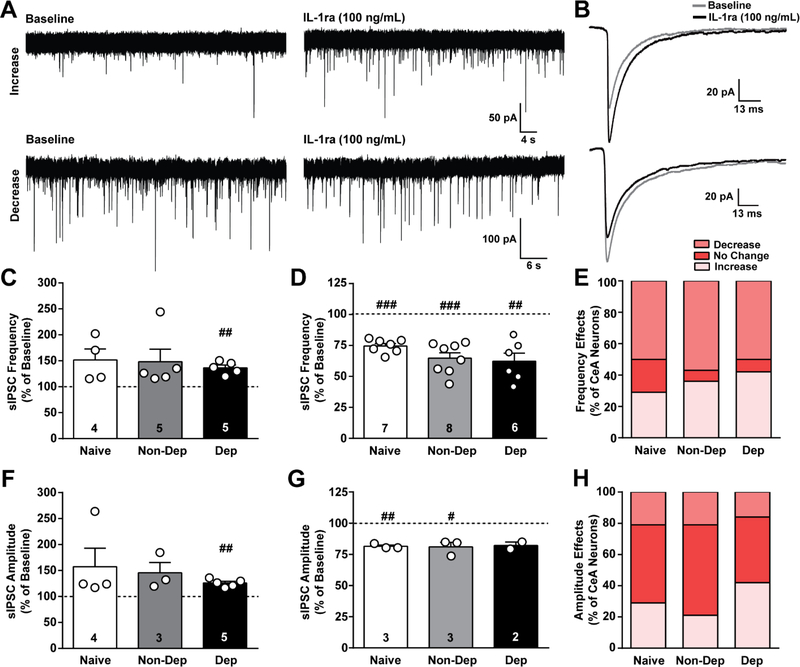
Figure 4. IL-1ra effects on GABAergic transmission in the CeA of naïve, non-dependent, and dependent mice.
A. Representative traces of spontaneous action potential-dependent inhibitory postsynaptic currents (sIPSCs) during baseline and during application of IL-1ra (100 ng/mL) from naïve mice showing an increase (top row) and decrease (bottom row) in sIPSCs induced by IL-1ra. B. Average sIPSC from a single naïve cell showing either an increase (top) or decrease (bottom) in amplitude induced by IL-1ra. C and D. IL-1ra-induced increase and decrease, respectively, in sIPSC frequency plotted as a percent of baseline for naïve, Non-Dep, and Dep mice. E. Percent of CeA neurons reponding to IL-1ra with an increase, decrease or no change in sIPSC frequency. F and G. IL-1ra-induced increase and decrease, respectively, in sIPSC amplitude plotted as a percent of baseline. H. Percent of CeA neurons reponding to IL-1ra with an increase, decrease or no change in sIPSC amplitude. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 by one-sample t-test; n = 12 – 14 cells from 8 – 10 mice, and the exact number of cells in each condition is reported in the bars.
Similar to naïve, IL-1ra increased sIPSC frequency (p > 0.05; one sample t-test; 48.1 ± 24.2) in 36% (5 of 14 cells) but did not reach statistical significance, and significantly decreased (p < 0.001; one sample t-test; 35.3 ± 4.4%) sIPSC frequency in 57% (8 of 14 cells) of neurons in Non-Dep mice (Figure 4A, C-E). Finally, in Dep mice, IL-1ra significantly increased (p < 0.01; one sample t-test; 36.4 ± 5.4%) sIPSC frequency in 42% (5 of 12 cells) and decreased (p < 0.01; one sample t-test; 37.8 ± 6.6%) it in 50% (6 of 12 cells) of neurons. We did not observe corresponding changes in amplitude of sIPSCs. One-way ANOVA and Chi-square test (p > 0.05) determined no significant differences in the magnitude of IL-1ra’s effect on sIPSC frequency and the distribution of cellular responses to IL-1ra among the treatment groups. Overall, these data suggest that tonic IL-1R1 activity regulates CeA GABA transmission in naïve mice, and chronic ethanol exposure does not alter these neuromodulatory effects, consistent with the IL-1β results (Fig. 3).
We also examined the effect of IL-1ra on sIPSC amplitude, independent of frequency effects, and found that IL-1ra had dual effects on sIPSC amplitude in naïve, Non-Dep, and Dep mice (Figure 4B, F-H). In naïve mice, IL-1ra increased sIPSC amplitude (57.3 ± 35.6%) in 29% (4 of 14 cells) but did not reach statistical significance, and significantly decreased (p < 0.01; one sample t-test; 18.5 ± 1.1%) sIPSC amplitude in 21% (3 of 14 cells) of neurons. Similarly, IL-1ra increased sIPSC amplitude (p > 0.05; one sample t-test; 45.5 ± 19.9%) in 21% (3 of 14 cells) but did not reach statistical significance, and significantly decreased (p < 0.05; one sample t-test; 19.0 ± 3.6%) sIPSC amplitude in 21% (3 of 14 cells) of neurons in Non-Dep mice. In Dep mice, IL-1ra significantly increased (p < 0.01; one sample t-test; 25.8 ± 3.3%) sIPSC amplitude in 42% (5 of 12 cells) and decreased (17.9 ± 2.8%) it in 16% (2 of 12 cells) of neurons but did not reach significance. Changes in amplitude did not correlate with changes in frequency.The magnitude of IL-1ra’s effects on amplitude or the distribution of cellular responses to IL-1ra did not differ among the treatment groups (tested by one-way ANOVA and Chi-square (p > 0.05) tests). No significant correlation between IL-1ra’s effect on sIPSC frequency or amplitude and the cell type, assessed by firing properties, were observed. Overall, IL-1ra bi-directionally modulates sIPSCs, suggesting that the IL-1 system modulates GABAergic transmission in the CeA under basal conditions through pre- and post-synaptic mechanisms, and chronic ethanol exposure does not significantly alter this basal regulation.
IL-1 system and acute ethanol signal through distinct mechanisms to regulate CeA spontaneous GABA release, and this persists with ethanol dependence.
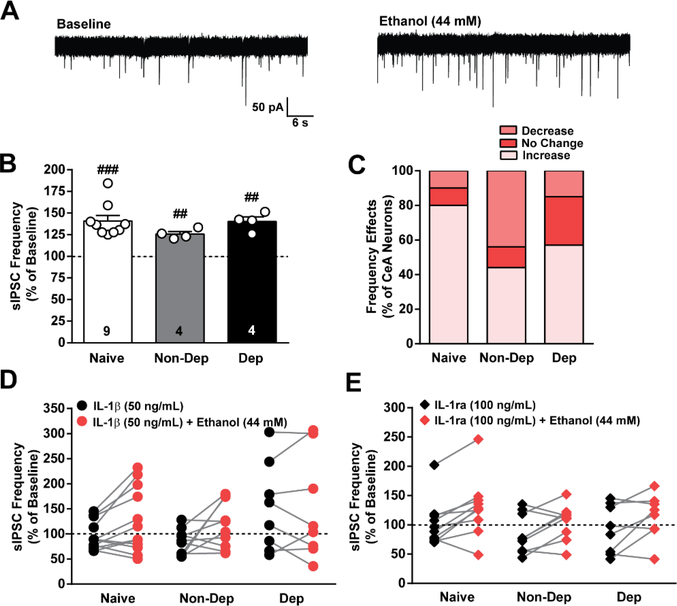
We and others have shown that acute ethanol facilitates GABA release in the CeA of naïve and ethanol-dependent rodents33,34,53,55,56, and this increased local inhibition drives both ethanol drinking and dependence-induced behaviors27,28. Therefore, we lastly asked whether the IL-1 system modulates the acute ethanol sensitivity of CeA GABAergic synapses and if this changes with CIE-2BC. Acute application of ethanol (44 mM53) significantly increased CeA sIPSC frequency in naïve (p < 0.001; one sample t-test; 40.9 ± 6.4%) in 75% (9 of 12 cells), Non-Dep (p < 0.01; one sample t-test; 25.8 ± 2.9%) in 40% ( 4 of 10 cells), and Dep (p < 0.01; one sample t-test; 40.3 ± 5.3%) mice in 44% (4 of 9 cells) of neurons (Figure 5). Overall, there were no statistical differences in the magnitude of ethanol’s effect on sIPSCs frequency as assessed by one-way ANOVA. There was also no statistical difference in the number of cells responding to acute ethanol with an increase in sIPSC frequency using the Chi-square test, confirming that there is a lack of tolerance to acute ethanol’s facilitation of GABA release in CeA neurons following chronic ethanol exposure with the CIE-2BC paradigm.
Figure 5. IL-1β and IL-1ra do not alter acute ethanol’s effect on GABA release in the CeA of naïve, non-dependent, and dependent mice.
A. Representative traces of spontaneous action potential-dependent inhibitory postsynaptic currents (sIPSCs) during baseline and after application of ethanol (44 mM) from naïve mice. B. Ethanol-induced effect on sIPSC frequency plotted as a percent of baseline for naïve, Non-Dep, and Dep mice. C. Percent of CeA neurons reponding to ethanol with an increase, decrease or no change in sIPSC frequency. D. Frequency of sIPSCs plotted as a percent of baseline during IL-1β (50 ng/mL) application (black circles, n = 9–12 cells) and during IL-1β co-application with ethanol (red circles) for each cell in naïve, nondep and dep mice. E. Frequency of sIPSCs plotted as a percent of baseline during IL-1ra (100 ng/mL) application (black diamonds, n = 7–9 cells) and during IL-1ra co-application with ethanol (red diamonds) for each cell. ##, p < 0.01; ###, p < 0.001; n = 8 – 12 cells from 8 – 11 mice, and the exact number of cells in each condition is reported in the bars.
With regards to a potential interaction between the IL-1 system and ethanol, we previously reported mixed results in the CeA of naïve mice that appeared to be dependent on the mode of neurotransmission studied (action potential-independent vs. electrically-evoked). Specifically, IL-1β occludes ethanol’s facilitation of GABA release in CeA neurons that displayed a decrease in mIPSC frequency in response to IL-1β; however, IL-1β did not alter the ethanol-induced increase of evoked GABA responses33. Thus, here we applied either IL-1β or IL-1ra, and then co-applied ethanol (44 mM) to investigate the effects on action potential-dependent neurotransmission with the synaptic network intact (Figure 5D, E). This serial experimental design allowed us to first measure each neuron’s response to either IL-1β or IL-1ra and subsequently determine the same neuron’s response to co-application of acute ethanol. We found no correlation between a neuron’s response (including increase, decrease, or no change) to either IL-1β or IL-1ra and ethanol co-application, such that ethanol can still increase GABA transmission despite IL-1R1 manipulation. Overall, these data show that the acute ethanol sensitivity of GABAergic synapses in the CeA is not modulated by IL-1β or IL-1ra in naïve, Non-Dep or Dep mice, suggesting that IL-1β/IL-1ra and ethanol signal through distinct downstream effectors to modulate spontaneous action potential-dependent GABA release.
Discussion
In this study, we examined whether the CIE-2BC paradigm alters peripheral cytokines, impacts IL-1β expression, and modulates IL-1β’s effect on GABAergic transmission, and its interaction with ethanol-induced facilitation of spontaneous GABA release in the CeA. We found that CIE-2BC alters peripheral cytokine levels. In the CeA, IL-1β expression in neurons and microglia is sensitive to ethanol exposure, such that dependent mice displayed a significantly greater expression of IL-1β compared to naïve and non-dependent mice. Baseline sIPSC frequency was not significantly altered with CIE-2BC, but a significant decrease in sIPSC amplitude was observed in non-dependent mice compared to naïve and dependent mice. Additionally, IL-1β and IL-1ra bi-directionally modulate spontaneous GABA transmission through both pre- and postsynaptic mechanims in all groups. Moreover, IL-1β and IL-1ra do not affect the facilitation of GABA release by acute ethanol. These electrophysiological findings highlight the basal regulation of spontaneous GABAergic transmission in the CeA by IL-1R1, and the persistence of IL-1β’s regulation of spontaneous GABAergic transmission following chronic ethanol, suggesting a possible divergence in the ethanol sensitivity of IL-1β’s immunological versus neuromodulatory role in this brain region.
To our knowledge, this is the first study to report a parallel determination of changes in multiple serum cytokines including elements of the IL-1 system in the periphery, and a functional characterization of the IL-1 system in the amygdala (IHC and electrophysiology) in the same animals generated using the CIE-2BC paradigm. We found that the peripheral IL-1 system is dysregulated with increased IL-1β, although not significant due to a great degree of variance, in ethanol dependent mice, while IL-1α levels were significantly decreased compared to naive mice. Additionally, peripheral TNF-a levels were increased, although not significant due to a great degree of variance, in ethanol dependent mice, and IL-10 levels were decreased, in agreement with a general inflammatory state induced by CIE-2BC. Notably, similar changes in peripheral circulating levels of IL-1β, TNFα and IL-10 have been observed in human alcoholics 57–59. While the simultaneous quantification of multiple cytokines using Luminex can provide detailed insight into changes in the peripheral immune response, results can vary depending on collection and processing of samples60, which is an important limitation of this technique. Of note, we did not observe any significant differences in serum protein concentration across groups using the CIE-2BC paradigm (data not shown). Moreover, changes in peripheral cytokine levels do not necessarily correlate to changes in the CNS, where cytokines are locally produced and regulated. IL-1β has several non-immunological, neuromodulatory roles in the CNS including: neurogenesis, induction of neurotrophic factors, and synaptic33,34,61and circuit plasticity62. Here, we show that antagonizing IL-1R1 using IL-1ra bi-directionally modulates sIPSCs, indicating that the receptor plays an important tonic role in regulating spontaneous GABAergic transmission in the CeA through pre- and postsynaptic mechanisms. Indeed, active IL-1β can be detected under basal conditions in the brain63, and there is evidence for a physiological role of IL-1β in modulating excitability and neurotransmission to regulate memory formation and sleep64–68. Additionally, IL-1β signaling is associated with anxiety and depression69–72, and it is possible that changes in IL-1R1’s modulation of basal neurotransmission in the CeA could contribute to the expression of these disorders.
Interestingly, these neuromodulatory effects of IL-1R1 agonism (by IL-1β) and antagonism (by IL-1ra) appear to be synapse-specific, as both compounds elicited mixed sIPSC frequency and amplitude responses, with no correlation between the two effects. It is known that IL-1β has brain region-specific effects, either increasing or decreasing GABAergic transmission in other brain regions61,69,70,73. This is likely due, in part, to expression of IL-1β and IL-1R1 on many different cellular populations including astrocytes, microglia, and neurons. Therefore, IL-1β could be working directly through neuronal IL-1R1 to alter synaptic release and GABAA receptor function or indirectly through IL-1β-induced release of other cytokines/neuromodulators from neurons or glial cells. We speculate that the overall effect of IL-1R1 signaling on synaptic transmission may reflect a complex combination of several cellular effects. Moreover, it is possible that there are differences in the expression and/or coupling of downstream effectors of IL-1R1 within these different cellular populations. However, since there is minimal expression of IL-1R1 on glial cells under basal conditions74,75, and IL-1β activates neurons at concentrations a thousand-fold lower than necessary for non-neuronal cells; we hypothesize that IL-1β is predominantly acting through neuronal IL-1R1 to modulate spontanous GABAergic transmission in the CeA of naïve mice, though this was not directly tested in these studies.
IL-1β expression is increased in the brain following various insults and neurological conditions and, in some cases, blocking IL-1β and/or IL-1R1 signaling improves outcomes and recovery76–78, directly implicating it in the pathogenesis of these diseases. Consistent with this, we found a significant increase in IL-1β expression, including both active and inactive precursor forms of IL-1β, with ethanol exposure in the CeA in non-dependent and to a greater degree in dependent mice. Of note, increased expression of IL-1β in ethanol dependent mice compared to naïve and non-dependent mice is likely due to a combination of the greater ethanol intake and vapor exposure in these mice, which cannot be parsed out as escalated ethanol intake reflects a dependence phenotype. Increased expression of IL-1β in microglia, the resident immune cells in the brain and a major producer of IL-1β74, support their increased function and role in the ethanol dependence- induced neuroimmune response. Increased IL-1β expression in microglia and microglial activation has been associated with numerous psychiatric disorders79,80. However, the extent of activation of microglia is determined by concurrent changes in their morphology and expression of other microglial markers (e.g., CD11b, CD45, CD68, etc.), which we did not directly examine in this study. The importance of activated microglia in the development of ethanol dependence has been supported by studies showing the presence of activated microglia in the human alcoholics81 and animals exposed to ethanol19,79,82–87. Specifically, in animal studies inhibition of microglial activation prevented the development of ethanol-related behaviors87–92. Interestingly, the CeA is also involved in feeding behaviors93,94, and neuroinflammation in this region could contribute to feeding. Surprisingly, despite the increased expression of IL-1β in neurons, we did not observe any significant ethanol dependence-induced changes in IL-1β’s regulation of spontaneous GABAergic signaling in the CeA. Although, we did not find any correlation between the effects of IL-1β/IL-1ra and neuronal cell type as defined by their firing characteristics, we cannot rule out that the same neuronal populations responded to IL-1β in the same way across all three groups. Critically, IL-1β signaling is tightly regulated by various IL-1 system components (e.g. endogenous antagonist IL-1ra and decoy receptor IL-1R2) and so chronic ethanol-induced concurrent changes in negative regulators of IL-1β signaling could compensate for the elevated IL-1β expression. Future studies will assess potential changes in IL1R1 expression, localization (i.e. pre- and postsynaptic compartments) and activated intracellular pathways. Of note, a limitation of this study is that only one concentration of IL-1β was used to determine its synaptic effects. It is possible that other concentrations may reveal changes in the sensitivity of IL-1R1 (e.g. alterations in receptor function and expression) and subsequently associated changes in synaptic responses following chronic alcohol. Moreover, given the pleiotropic effects of IL-1β, we speculate that IL-1β may also simultaneously regulate several signaling systems with opposing functions, leading to an overall maintenance of IL-1R1’s function and regulatory tone at CeA GABAergic synapses. For example, IL-1β can induce the release of corticotropin-releasing factor95,96 and interact with the endocannabinoid system97–100, and we have previously shown that these two signaling systems modulate CeA GABAergic transmission in opposing directions and are sensitive to chronic ethanol exposure29,101,102. Conceivably, IL-1β’s interaction with these specific targets and other neuromodulatory systems could result in no net change in sIPSCs across the treatment groups. Overall, these findings highlight the sensitivity of IL-1β expression to ethanol exposure, as well as the critical functional role this system plays in maintaining homeostasis at CeA GABA synapses after chronic exposure.
A major hallmark of the ethanol dependent-state is the persistent CeA sensitivity to the acute effects of ethanol33,34,53. Specifically, acute ethanol increases CeA GABA release to a similar extent in naïve and ethanol-dependent rodents (as shown here), and this local inhibition has been directly implicated in ethanol drinking and dependence-induced behaviors27–29. We have previously shown in the CeA of B6129SF2/J mice, that neurons that responded to IL-1β with a decrease in action potential-independent GABA release (pharmacologically isolated synapses) no longer display an acute ethanol-induced increase in vesicular GABA release in the presence of IL-1β. Notably, in the same animals, we found that IL-1β significantly decreased electrically-evoked GABAergic responses (action potential-dependent network driven transmission) and did not alter the acute ethanol-induced increase in evoked GABA responses. These findings suggest a role for IL-1R1 in differentially modulating acute ethanol effects at pharmacologically-isolated GABA synapses versus across the CeA synaptic network. To gain further mechanistic insight into the contribution of network activity to the interaction between the IL-1 system and acute ethanol, here we examined spontaneous action potential-dependent GABAergic transmission wherein the network activity is intact. We found that IL-1β and IL-1ra do not modulate the sensitivity of spontaneous action potential-dependent GABAergic transmission to acute ethanol in naïve, non-dependent and dependent mice. This is consistent with the previously mentioned evoked network-dependent GABA responses103 and the potentially distinct downstream signaling effectors of IL-1β and ethanol. Indeed, we previously demonstrated that acute ethanol’s facilitation of GABA release is mediated through distinct calcium channels for different modes of neurotransmission, that is P/Q-type calcium channels for mIPSCs and L-type calcium channels for sIPSCs104,105. In addition to calcium entry and intracellular concentration rise, spontaneous action potential-dependent and -independent neurotransmission likely results from distinct presynaptic neurotransmitter release mechanisms (e.g., vesicle fusion machinery, spatial segregation of the vesicles and/or vesicle populations, synaptic vesicle pool dynamics)106,107. Moreover, presynaptic molecular pathways are critical in mediating GABA release and in our case, acute ethanol facilitates GABA release through PKA and PKC mechanisms108–111, while IL-1β canonically signals through PI3K/AKT and p38 MAPK, which have been shown to modulate GABAergic transmission in other brain regions112–115. Previously in other models of CIE vapor exposure55,104, we have observed increased baseline vesicular GABA release in Dep compared to naïve. However, in this study with CIE-2BC we did not observe this baseline difference between Naïve, Non-Dep and Dep. Although this was unexpected, it is not surprising given differences in the model and potential cell-type specific effects of ethanol dependence52. Overall, our findings highlight the complex, reciprocal interaction between the neuroimmune reponse and acute and chronic ethanol.
Conclusion
While the prospect of targeting the IL-1β system to treat alcohol use disorders is encouraging, it is tempered by the fact that the IL-1 system has an important physiological role in regulating cellular function, in addition to its pathological role in the immune response. Our findings highlight the significantly increased IL-1β expression in the CeA after chronic to ethanol, the complex basal regulation of GABAergic transmission by IL-1R1 and how the neuromodulatory effects of IL-1β on synaptic transmission persist after chronic ethanol, suggesting a possible divergence in the sensitivity of IL-1β’s immunological versus neuromodulatory role to ethanol in this brain region.
Highlights.
Chronic ethanol exposure increased IL-1β expression in CeA neurons and microglia.
IL-1β bi-directionally modulated GABAergic transmission through pre- and postsynaptic mechanisms in naïve, non-dependent and dependent mice.
IL-1R1 regulation of basal GABAergic transmission was unaltered by chronic ethanol.
IL-1β and IL-1ra did not modulate acute ethanol’s facilitation of GABA release.
Acknowledgements
We would like to thank Dr. Kazantzis from the Metabolic Core at Scripps Research Institute (Jupiter, Florida) for conducting the Luminex assay. This work was supported by National Institute of Alcohol Abuse and Alcoholism grants INIA-Neuroimmune U01 AA03498, F32 AA026865, AA01566, AA021491, AA06420, AA017447, T32 AA007456, and K99/R00 AA025408 and the Austrian Science Fund FWF-J-3942. The authors declare no conflict of interest. This is Scripps manuscript number 29721.
Abbreviations
- IL-1β
interleukin-1β
- IL-1R1
IL-1 receptor 1
- IL-1ra
IL-1 receptor antagonist
- IL-1RAcP
IL-1R1 accessory protein
- CeA
central nucleus of the amygdala
- Non-Dep
non-dependent
- Dep
dependent
- CIE-2BC
chronic-intermittent ethanol two-bottle choice
- mIPSC
miniature inhibitory postsynaptic current
- sIPSC
spontaneous inhibitory postsynaptic current
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pastor IJ, Laso FJ, Romero A & Gonzalez-Sarmiento R Interleukin-1 gene cluster polymorphisms and alcoholism in Spanish men. Alcohol Alcohol 40, 181–186 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Coleman LG Jr., Zou J, Qin L & Crews FT HMGB1/IL-1beta complexes regulate neuroimmune responses in alcoholism. Brain Behav Immun 72, 61–77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou J & Crews FT Inflammasome-IL-1beta Signaling Mediates Ethanol Inhibition of Hippocampal Neurogenesis. Front Neurosci 6, 77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achur RN, Freeman WM & Vrana KE Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol 5, 83–91 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangan MSJ, et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov (2018). [DOI] [PubMed] [Google Scholar]
- 6.Mehta N, et al. Purinergic receptor P2X(7): a novel target for anti-inflammatory therapy. Bioorg Med Chem 22, 54–88 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Dubyak GR P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol 14, 1697–1706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montesinos J, Alfonso-Loeches S & Guerri C Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res 40, 2260–2270 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Alfonso-Loeches S, Urena-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J & Guerri C Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci 8, 216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill LA & Greene C Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol 63, 650–657 (1998). [PubMed] [Google Scholar]
- 11.Garlanda C, Dinarello CA & Mantovani A The interleukin-1 family: back to the future. Immunity 39, 1003–1018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumm B, Xiang Y & Deng J Structural biology of the IL-1 superfamily: key cytokines in the regulation of immune and inflammatory responses. Protein Sci 23, 526–538 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang D, et al. The type II IL-1 receptor interacts with the IL-1 receptor accessory protein: a novel mechanism of regulation of IL-1 responsiveness. J Immunol 161, 6871–6877 (1998). [PubMed] [Google Scholar]
- 14.Malinowsky D, Lundkvist J, Laye S & Bartfai T Interleukin-1 receptor accessory protein interacts with the type II interleukin-1 receptor. FEBS Lett 429, 299–302 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Crews FT & Vetreno RP Addiction, adolescence, and innate immune gene induction. Front Psychiatry 2, 19 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crews FT, Zou J & Qin L Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25 Suppl 1, S4–S12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blednov YA, et al. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol 17, 108–120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabo G & Lippai D Converging actions of alcohol on liver and brain immune signaling. Int Rev Neurobiol 118, 359–380 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I & Guerri C Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30, 82858295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippai D, et al. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol 94, 171–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulligan MK, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A 103, 6368–6373 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blednov YA, Benavidez JM, Black M, Mayfield J & Harris RA Role of interleukin-1 receptor signaling in the behavioral effects of ethanol and benzodiazepines. Neuropharmacology 95, 309–320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall SA, et al. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun 51, 258–267 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breese GR, et al. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology 33, 867–876 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilpin NW & Roberto M Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev 36, 873–888 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koob GF & Volkow ND Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberto M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry 67, 831–839 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyytia P & Koob GF GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol 283, 151–159 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Roberto M, Gilpin NW & Siggins GR The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med 2, a012195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frost P, Barrientos RM, Makino S, Wong ML & Sternberg EM IL-1 receptor type I gene expression in the amygdala of inflammatory susceptible Lewis and inflammatory resistant Fischer rats. J Neuroimmunol 121, 32–39 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Eriksson C, Tehranian R, Iverfeldt K, Winblad B & Schultzberg M Increased expression of mRNA encoding interleukin-1beta and caspase-1, and the secreted isoform of interleukin-1 receptor antagonist in the rat brain following systemic kainic acid administration. J Neurosci Res 60, 266–279 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Konsman JP, et al. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci 28, 2499–2510 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Bajo M, et al. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front Pharmacol 6, 49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajo M, et al. Role of the IL-1 receptor antagonist in ethanol-induced regulation of GABAergic transmission in the central amygdala. Brain Behav Immun 45, 189–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker HC & Lopez MF Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28, 1829–1838 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Bajo M, et al. Evaluation of TLR4 Inhibitor, T5342126, in Modulation of Ethanol-Drinking Behavior in Alcohol-Dependent Mice. Alcohol Alcohol 51, 541–548 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huitron-Resendiz S, et al. Effects of Withdrawal from Chronic Intermittent Ethanol Exposure on Sleep Characteristics of Female and Male Mice. Alcohol Clin Exp Res 42, 540–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim C, et al. Hypoestoxide reduces neuroinflammation and alpha-synuclein accumulation in a mouse model of Parkinson’s disease. J Neuroinflammation 12, 236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin JW, et al. alpha-Asarone Ameliorates Memory Deficit in Lipopolysaccharide-Treated Mice via Suppression of Pro-Inflammatory Cytokines and Microglial Activation. Biomol Ther (Seoul) 22, 17–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang MX, Liu YL, Yang Y, Zhang DM & Kong LD Nuciferine restores potassium oxonateinduced hyperuricemia and kidney inflammation in mice. Eur J Pharmacol 747, 59–70 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Evonuk KS, et al. Inhibition of System Xc(−) Transporter Attenuates Autoimmune Inflammatory Demyelination. J Immunol 195, 450–463 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinnhardt B, et al. Multimodal imaging reveals temporal and spatial microglia and matrix metalloproteinase activity after experimental stroke. J Cereb Blood Flow Metab 35, 1711–1721 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuo L, et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31, 85–94 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Rakela B, Brehm P & Mandel G Astrocytic modulation of excitatory synaptic signaling in a mouse model of Rett syndrome. Elife 7(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Funk CK, O’Dell LE, Crawford EF & Koob GF Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26, 11324–11332 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teijaro JR, et al. S1PR1-mediated IFNAR1 degradation modulates plasmacytoid dendritic cell interferon-alpha autoamplification. Proc Natl Acad Sci U S A 113, 1351–1356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carames B, et al. Glucosamine activates autophagy in vitro and in vivo. Arthritis Rheum 65, 1843–1852 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carames B, Olmer M, Kiosses WB & Lotz MK The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol 67, 1568–1576 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alirezaei M, et al. Short-term fasting induces profound neuronal autophagy. Autophagy 6, 702710 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herman MA, Contet C, Justice NJ, Vale W & Roberto M Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci 33, 3284–3298 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herman MA, Contet C & Roberto M A Functional Switch in Tonic GABA Currents Alters the Output of Central Amygdala Corticotropin Releasing Factor Receptor-1 Neurons Following Chronic Ethanol Exposure. J Neurosci 36, 10729–10741 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberto M, Madamba SG, Moore SD, Tallent MK & Siggins GR Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A 100, 2053–2058 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otis TS, De Koninck Y & Mody I Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A 91, 7698–7702 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberto M, Madamba SG, Stouffer DG, Parsons LH & Siggins GR Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci 24, 10159–10166 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nie Z, et al. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science 303, 1512–1514 (2004). [DOI] [PubMed] [Google Scholar]
- 57.McClain CJ & Cohen DA Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology 9, 349–351 (1989). [DOI] [PubMed] [Google Scholar]
- 58.McClain CJ, Song Z, Barve SS, Hill DB & Deaciuc I Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 287, G497–502 (2004). [DOI] [PubMed] [Google Scholar]
- 59.McClain CJ, Barve S, Deaciuc I, Kugelmas M & Hill D Cytokines in alcoholic liver disease. Semin Liver Dis 19, 205–219 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Leng SX, et al. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci 63, 879–884 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pringle AK, Gardner CR & Walker RJ Reduction of cerebellar GABAA responses by interleukin-1 (IL-1) through an indomethacin insensitive mechanism. Neuropharmacology 35, 147–152 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Schneider H, et al. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A 95, 7778–7783 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quan N, Zhang Z, Emery M, Bonsall R & Weiss JM Detection of interleukin-1 bioactivity in various brain regions of normal healthy rats. Neuroimmunomodulation 3, 47–55 (1996). [DOI] [PubMed] [Google Scholar]
- 64.Krueger JM, et al. Sleep. A physiologic role for IL-1 beta and TNF-alpha. Ann N Y Acad Sci 856, 148–159 (1998). [DOI] [PubMed] [Google Scholar]
- 65.Hallett H, Churchill L, Taishi P, De A & Krueger JM Whisker stimulation increases expression of nerve growth factor- and interleukin-1beta-immunoreactivity in the rat somatosensory cortex. Brain Res 1333, 48–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.del Rey A, Balschun D, Wetzel W, Randolf A & Besedovsky HO A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immun 33, 15–23 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Depino AM, et al. Learning modulation by endogenous hippocampal IL-1: blockade of endogenous IL-1 facilitates memory formation. Hippocampus 14, 526–535 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Vitkovic L, Bockaert J & Jacque C “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem 74, 457–471 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Yu B & Shinnick-Gallagher P Interleukin-1 beta inhibits synaptic transmission and induces membrane hyperpolarization in amygdala neurons. J Pharmacol Exp Ther 271, 590–600 (1994). [PubMed] [Google Scholar]
- 70.Miller LG, Galpern WR, Dunlap K, Dinarello CA & Turner TJ Interleukin-1 augments gamma-aminobutyric acidA receptor function in brain. Mol Pharmacol 39, 105–108 (1991). [PubMed] [Google Scholar]
- 71.Huang Y, Smith DE, Ibanez-Sandoval O, Sims JE & Friedman WJ Neuron-specific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci 31, 18048–18059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maes M, Song C & Yirmiya R Targeting IL-1 in depression. Expert Opin Ther Targets 16, 10971112 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Allan SM & Rothwell NJ Cytokines and acute neurodegeneration. Nat Rev Neurosci 2, 734744 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Liu X & Quan N Microglia and CNS Interleukin-1: Beyond Immunological Concepts. Front Neurol 9, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.French RA, et al. Expression and localization of p80 and p68 interleukin-1 receptor proteins in the brain of adult mice. J Neuroimmunol 93, 194–202 (1999). [DOI] [PubMed] [Google Scholar]
- 76.Pinteaux E, Rothwell NJ & Boutin H Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia 53, 551–556 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Relton JK & Rothwell NJ Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull 29, 243–246 (1992). [DOI] [PubMed] [Google Scholar]
- 78.Betz AL, Yang GY & Davidson BL Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J Cereb Blood Flow Metab 15, 547–551 (1995). [DOI] [PubMed] [Google Scholar]
- 79.Pradier B, Erxlebe E, Markert A & Racz I Microglial IL-1beta progressively increases with duration of alcohol consumption. Naunyn Schmiedebergs Arch Pharmacol 391, 455–461 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Mendiola AS & Cardona AE The IL-1beta phenomena in neuroinflammatory diseases. J Neural Transm (Vienna) 125, 781–795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He J & Crews FT Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol 210, 349–358 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marshall SA, Geil CR & Nixon K Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain Sci 6(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marshall SA, et al. Microglial activation is not equivalent to neuroinflammation in alcoholinduced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis 54, 239–251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McClain JA, et al. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun 25 Suppl 1, S120–128 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kane CJ, et al. Effects of ethanol on immune response in the brain: region-specific changes in aged mice. J Neuroinflammation 10, 66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blanco AM & Guerri C Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci 12, 2616–2630 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Fernandez-Lizarbe S, Pascual M & Guerri C Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol 183, 4733–4744 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Drew PD, Johnson JW, Douglas JC, Phelan KD & Kane CJ Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39, 445–454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kane CJ, et al. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain Behav Immun 25 Suppl 1, S137–145 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Y, et al. Attenuation of microglial and IL-1 signaling protects mice from acute alcoholinduced sedation and/or motor impairment. Brain Behav Immun 25 Suppl 1, S155–164 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Montesinos J, Gil A & Guerri C Nalmefene Prevents Alcohol-Induced Neuroinflammation and Alcohol Drinking Preference in Adolescent Female Mice: Role of TLR4. Alcohol Clin Exp Res 41, 1257–1270 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Walter TJ & Crews FT Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J Neuroinflammation 14, 86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Douglass AM, et al. Central amygdala circuits modulate food consumption through a positivevalence mechanism. Nat Neurosci 20, 1384–1394 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Fadok JP, Markovic M, Tovote P & Luthi A New perspectives on central amygdala function. Curr Opin Neurobiol 49, 141–147 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Saphier D & Ovadia H Selective facilitation of putative corticotropin-releasing factor-secreting neurones by interleukin-1. Neurosci Lett 114, 283–288 (1990). [DOI] [PubMed] [Google Scholar]
- 96.Barbanel G, Ixart G, Szafarczyk A, Malaval F & Assenmacher I Intrahypothalamic infusion of interleukin-1 beta increases the release of corticotropin-releasing hormone (CRH 41) and adrenocorticotropic hormone (ACTH) in free-moving rats bearing a push-pull cannula in the median eminence. Brain Res 516, 31–36 (1990). [DOI] [PubMed] [Google Scholar]
- 97.Rossi S, et al. Interleukin-1beta causes anxiety by interacting with the endocannabinoid system. J Neurosci 32, 13896–13905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Chiara V, et al. Interleukin-1beta alters the sensitivity of cannabinoid CB1 receptors controlling glutamate transmission in the striatum. Neuroscience 250, 232–239 (2013). [DOI] [PubMed] [Google Scholar]
- 99.Gentile A, et al. Interaction between interleukin-1beta and type-1 cannabinoid receptor is involved in anxiety-like behavior in experimental autoimmune encephalomyelitis. J Neuroinflammation 13, 231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rossi S, et al. Inflammation inhibits GABA transmission in multiple sclerosis. Mult Scler 18, 1633–1635 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Varodayan FP, et al. Chronic ethanol exposure decreases CB1 receptor function at GABAergic synapses in the rat central amygdala. Addict Biol 21, 788–801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberto M, et al. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology 35, 1962–1972 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roberto M, Patel RR & Bajo M Ethanol and Cytokines in the Central Nervous System. Handb Exp Pharmacol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varodayan FP, de Guglielmo G, Logrip ML, George O & Roberto M Alcohol Dependence Disrupts Amygdalar L-Type Voltage-Gated Calcium Channel Mechanisms. J Neurosci 37, 45934603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varodayan FP, Logrip ML & Roberto M P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses. Neuropharmacology 125, 197–206 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Atasoy D, et al. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci 28, 10151–10166 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chanaday NL & Kavalali ET Presynaptic origins of distinct modes of neurotransmitter release. Curr Opin Neurobiol 51, 119–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelm MK, Criswell HE & Breese GR The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. J Neurophysiol 100, 3417–3428 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bajo M, Cruz MT, Siggins GR, Messing R & Roberto M Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A 105, 8410–8415 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hirono M, Yamada M & Obata K Ethanol enhances both action potential-dependent and action potential-independent GABAergic transmission onto cerebellar Purkinje cells. Neuropharmacology 57, 109–120 (2009). [DOI] [PubMed] [Google Scholar]
- 111.Cruz MT, et al. Type 7 Adenylyl Cyclase is Involved in the Ethanol and CRF Sensitivity of GABAergic Synapses in Mouse Central Amygdala. Front Neurosci 4, 207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Q, et al. Control of synaptic strength, a novel function of Akt. Neuron 38, 915–928 (2003). [DOI] [PubMed] [Google Scholar]
- 113.Solovyova N, Moult PR, Milojkovic B, Lambert JJ & Harvey J Bi-directional modulation of fast inhibitory synaptic transmission by leptin. J Neurochem 108, 190–201 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang H, Nei H & Dougherty PM A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factoralpha. J Neurosci 30, 12844–12855 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pribiag H & Stellwagen D TNF-alpha downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J Neurosci 33, 15879–15893 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]