Abstract
Replacement of the exocrine parenchyma by fibrous tissue is a main characteristic of chronic pancreatitis. Understanding the mechanisms of pancreatic fibrogenesis is critical for the development of preventive and therapeutic interventions. Cyclooxygenase-2 (COX-2), a rate-limiting enzyme for prostaglandin synthesis, is expressed in patients with chronic pancreatitis. However, it is unknown whether COX-2 can cause chronic pancreatitis. To investigate the roles of pancreatic acinar COX-2 in fibrogenesis and the development of chronic pancreatitis, COX-2 was ectopically expressed specifically in pancreatic acinar cells in transgenic mice. Histopathological changes and expression levels of several profibrogenic factors related to chronic pancreatitis were evaluated. COX-2 was expressed in the pancreas of the transgenic mice, as detected by Western blot analysis. Immunohistochemical staining showed COX-2 was specifically expressed in pancreatic acinar cells. COX-2 expression led to progressive changes in the pancreas, including pancreas megaly, persistent inflammation, collagen deposition, and acinar-to-ductal metaplasia. Quantitative RT-PCR and immunostaining showed that profibrogenic factors were upregulated and pancreatic stellate cells were activated in the COX-2 transgenic mice. Expression of COX-2 in pancreatic acinar cells is sufficient to induce chronic pancreatitis. Targeting this pathway may be valuable in the prevention of chronic pancreatitis.
NEW & NOTEWORTHY COX-2 expression is observed in pancreatic tissues of human chronic pancreatitis. In this study, we showed that COX-2 expression caused the development of chronic pancreatitis in transgenic mice, supporting the idea that COX-2 inhibition may be an effective preventive and therapeutic strategy.
Keywords: chronic inflammation, fibrogenesis, pancreatic stellate cells, prostaglandin
INTRODUCTION
Chronic pancreatitis is a chronic inflammatory disease characterized by progressive destruction of pancreatic acinar cells, which are replaced by extracellular matrix tissue (27). Replacement of the exocrine parenchyma by fibrous tissue and inflammatory cell infiltration are the main histological characteristics of the disease (9, 21). Pancreatic stellate cells (PSCs) play a pivotal role in promoting pancreatic fibrosis. These cells are quiescent and store retinoids in normal pancreas, but become activated into highly proliferative myofibroblast-like cells, which express α-smooth muscle actin (α-SMA) and produce type I collagen and other extracellular matrix components (15, 19). Prolonged chronic inflammatory conditions of the pancreas predispose individuals to the development of pancreatic ductal adenocarcinoma (2).
Many proinflammatory cytokines, including interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), are known to be involved in promoting pancreatic pathogenesis that leads to proliferation of mesenchymal cells and promotes chronic pancreatitis (14, 30). Transforming growth factor-β (TGF-β) regulates PSCs activation, matrix synthesis, and cell proliferation in an autocrine manner through the SMAD-2, SMAD-3, and ERK pathways (11, 25). Phosphorylated signal transducers and activators (p-STAT3), as well as extracellular signal-regulated kinase (p-ERK) can induce the proliferation of PSCs through their downstream transcription factors (5, 10, 12, 16, 18, 20). Through an intricate network of several autocrine or paracrine molecular signaling pathways, these profibrogenic factors activate PSCs and cause fibrogenesis in pancreas during the development of chronic pancreatitis (14).
Cyclooxygenase (COX) catalyzes the rate-limiting step of arachidonic acid metabolism into prostaglandins, leukotrienes, and thromboxanes (22), which play critical roles in numerous biologic processes, including inflammation, reproductive biology, and immune function (32). Two isoforms of the COX enzyme exist; many tissues constitutively express the COX-1 isoform, including the kidney and gastrointestinal mucosa. In contrast, COX-2, the inducible isoform, usually is undetectable in most tissues, but may be induced by a wide range of extracellular and intracellular stimuli (31, 32). COX-2 expression has been observed in patients with chronic pancreatitis (9, 27). However, it is unclear whether COX-2 can cause chronic pancreatitis.
Pancreatic acinar cells play a key role in the initiation of pancreatitis. However, pancreatic fibrogenesis is not a default mechanism to replace dead pancreatic acinar cells (3). Instead, pancreatic acinar cells generate many proinflammatory factors, which may be critical to initiate pancreatic fibrogenesis. Since COXs catalyze the formation of prostaglandins, which play a critical role in inflammation, we sought to evaluate whether COX-2 expression in pancreatic acinar cells would lead to the development of chronic pancreatitis.
MATERIALS AND METHODS
Transgenic-specific expression of COX-2 in mouse pancreatic acinar cells.
A full-length elastase (Ela) promoter was used to drive tamoxifen-inducible CreERT expression in mouse pancreatic acinar cells (Ela-CreERT mice, referred to as Cre in this study), as previously described (6). COX-2 floxed mice, which will express COX-2 in cells only following Cre-recombinase-mediated activation, were a kind gift from Dr. Harvey R. Herschman (University of California-Los Angeles, Los Angeles, CA) (8). The COX-2 floxed mice were crossed with Ela-CreERT (Cre) mice to generate double transgenic mice (referred to as COX-2/Cre mice). Age- and sex-matched Cre mice were used as controls for all experiments. Both sexes were used. There were six mice in each group, unless otherwise indicated. This line of Cre mice has tamoxifen-independent recombination due to high-level expression of CreERT (6). Therefore, tamoxifen was not used in this study. All experiments were conducted with the approval of the Institutional Animal Care and Use Committee at the University of Texas MD Anderson Cancer Center.
Histology.
For routine histology, pancreatic tissues were fixed with 10% formaldehyde in phosphate-buffered saline (PBS), embedded in paraffin, sectioned, and stained with hematoxylin and eosin. A pathologist blinded to experimental procedures performed the histological analysis of pancreatic injury by light microscopy based on the severity of acinar cell atrophy, inflammatory cell infiltration, edema, and fibrosis. Pathological scores of pancreatic injury were evaluated, as previously described (23, 29).
Picro-Sirius red stain and Alcian blue staining.
Collagen deposits were detected using Picro-Sirius Red Stain Kit (Abcam, ab150681) in paraffin-embedded pancreatic tissue. Briefly, after being deparaffinized and hydrated in distilled water, the slides were incubated for 1 h with Picro-Sirius Red Solution and then quickly rinsed in two changes of 0.5% acetic acid solution. After dehydration in two changes of absolute alcohol, the slides were mounted.
Mucin detection was performed with Alcian Blue Stain Kit (Abcam, ab150662) and followed the manufacturer’s protocol.
Western blot analysis.
Pancreatic tissues were homogenized in lysis buffer (RIPA buffer with 1% SDS and proteinase inhibitor cocktails) and immunoblotted with the following antibodies: COX-1 (1:1,000, Santa Cruz), COX-2 (1:500, Cayman Chemicals), and GAPDH (1:1,000, Sigma). Proteins were separated by 10% SDS-PAGE gels, transferred to nitrocellulose membranes, and then blocked for 2 h with 5% nonfat milk in PBST (PBS + 0.05% Tween 20) buffer. The membrane was incubated with primary antibodies overnight at 4°C, and appropriate fluorescent dye-labeled secondary antibodies were used for detection with the Odyssey Infrared Imaging System (LI-COR Biosciences), as previously described (7).
RT-PCR.
Total RNA was isolated from mouse pancreas using TRIzol reagent (15596026, Invitrogen, Carlsbad, CA). RNA was further purified by DNase digestion (15 min) and recovered with RNeasy kit (Qiagen, 74106). Reverse transcription was performed with 1 μg of purified total RNA in a 25-μl volume of RT reaction mixture (A3500, Promega, Carlsbad, CA). Quantitative PCR was conducted using SYBR green I to monitor the PCR products on the I-Cycler thermal cycler and IQ real-time PCR detection system (BioRad). The following primers were used: IL-1β (NM_008361, forward: 5′-AGA GCA TCC AGC TTC AAA TCT C-3′; reverse: 5′-CAG TTG TCT AAT GGG AAC GTC A-3′); TNF-α (NM_001278601, forward: 5′-TTA GAA AGG GGA TTA TGG CTC A-3′; reverse: 5′-ACT CTC CCT TTG CAG AAC TCA G-3′); fibronectin (NM_001276408, forward: 5′-TGT GGT CTA CTC TGT GGG AAT G-3′; reverse: 5′-TTG AAT TGC CAC CAT AAG TCT G-3′); TGF-β (NM_011577, forward: 5′-GAA CCA AGG AGA CGG AAT ACA G-3′; reverse: 5′-AAC CCA GGT CCT TCC TAA AGT C-3′).
Immunohistochemistry.
Mice pancreas tissues were either frozen in optimal cutting temperature compound (6255001, Fisher Scientific, Chicago, IL) or fixed in 10% formalin (24–48 h) at room temperature and embedded in paraffin. Immunohistochemistry (IHC) staining was performed using formalin-fixed paraffin-embedded tissue sections. Briefly, after deparaffinization and antigen retrieval, endogenous peroxidase activity was blocked with H2O2, followed by washing and blocking. Primary antibodies were then applied: COX-2 (1: 300; Cayman Chemicals, Ann Arbor, MI), CD11b (1:2,000, Abcam, Cambridge, MA), amylase (1:500, Santa Cruz, Dallas, TX), p-STAT3 (1:200, CST, 9145), p-ERK (1:200, CST, 4370), α-SMA (1:200, Abcam, Cambridge, MA, ab5694), Ki-67 (1:200, Sigma-Aldrich, St. Louis, MO, AB9260). After overnight incubation (4°C), slides were washed with PBS and PBS-containing 0.05% Tween 20 and then incubated with appropriate secondary antibodies (Vectastatin Elite ABC Kit, Vector Laboratories, Burlingame, CA). Positive reactions were detected by exposing the sample to DAB + Substrate System (DAKO). The sections were counterstained with hematoxylin and mounted.
Statistical analysis.
The results are expressed as means ± SE. The Student’s t-test and Wilcoxon test were used for statistical analysis with the SPSS version 17.0 software. A P value of <0.05 was considered statistically significant.
RESULTS
COX-2 was expressed in the pancreas of transgenic mice.
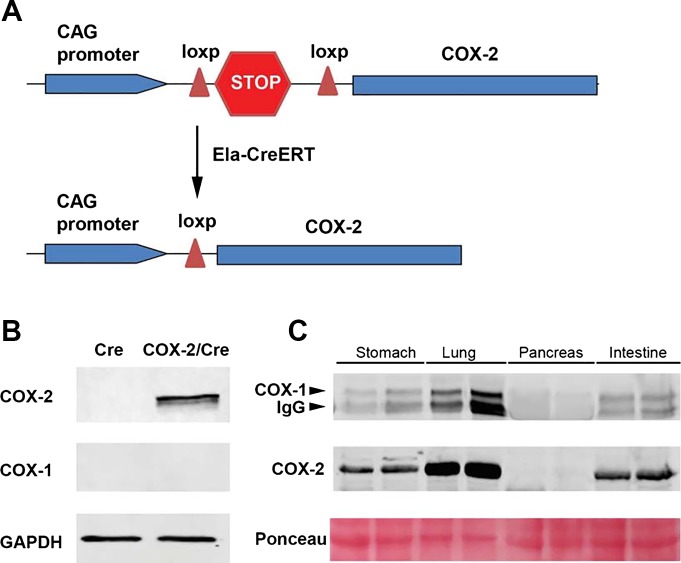
For pancreatic acinar-specific expression of COX-2, COX-2 floxed mice were crossed with Ela-CreERT mice to generate double transgenic (COX-2/Cre) mice (Fig. 1A). Western blot analysis showed that COX-2 was highly expressed in COX-2/Cre mice, but not Cre mice (Fig. 1B). Overexpression of COX-2 in the pancreas did not change the expression of COX-1 (Fig. 1B). We further examined the expression of COX isoforms in various mouse tissues. In contrast to other tissues, such as stomach, lung, and intestine, which exhibited a high basal level of COX-1 and COX-2 expression, neither COX-1 nor COX-2 was detectable by Western blot analysis in normal pancreas samples (Fig. 1C).
Fig. 1.
Pancreatic acinar cell-specific expression of cyclooxygenase (COX)-2. A: schema of transgenic expression of COX-2 in mouse pancreatic acinar cells. B: Western blot showed COX-2 was expressed in COX-2/Cre mice, but not Cre mice. COX-1 expression was not affected (20 wk). C: pancreas, stomach, lung, and intestine were harvested from wild-type mice, and Western blot analysis was performed using COX-1 and COX-2 antibodies. Note: anti-COX-1 is a mouse monoclonal antibody. Some immunoglobulin-rich tissues showed strong nonspecific bands from secondary anti-mouse antibody.
COX-2 expression induced pancreas megaly and progressive inflammatory changes.
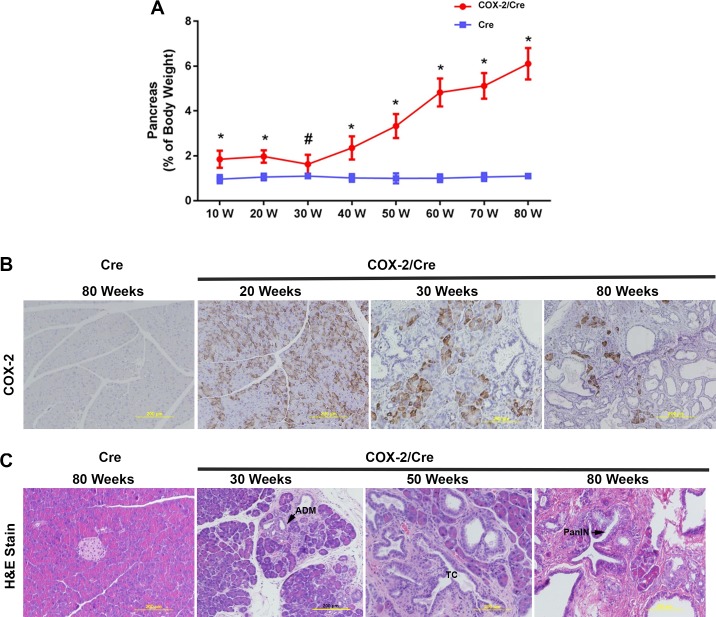
Normally the pancreas weight composes 1% of the total mouse body weight. However, in the COX-2/Cre transgenic mice, the ratio of pancreas to body weight was larger than in the Cre control mice, and the ratio increased dramatically with the age of mice (Fig. 2A). The average pancreas-to-body weight ratio in the COX-2/Cre transgenic mice reached 6% compared with 1% in control Cre mice at 80 wk of age. The body weight has no difference between these groups. The alterations seen in the pancreas-to-body weight ratio reflected increased pancreas weight in COX-2/Cre mice, at early ages. However, in older mice, numerous fluid-filled, macroscopic cystic lesions were observed. It was the accumulation of cystic fluid in the cystic ductal lesions that contributed significantly to the increased pancreatic weight. IHC with anti-COX-2 antibody showed that COX-2 was expressed in pancreatic acinar cells (Fig. 2B). The number of COX-2-positive acinar cells gradually decreased as mouse age increased, suggesting COX-2 expression may promote the acinar cells’ death and/or acinar-to-ductal metaplasia (ADM) during the disease progression (Fig. 2B). Histological examination of pancreas tissues showed no damage in Cre control mice, even at 80 wk. In COX-2/Cre mice, the pancreata did not reveal obvious pathological changes before 20 wk of age (data not shown). However, from ages of 30 wk, focal damages, including acinar atrophy, inflammatory cell infiltration, and fibrosis, developed in the COX-2/Cre double transgenic mice (Fig. 2C). At later ages, progressive inflammatory changes, including cystic ductal lesions, tubular complex, ADM, and low-grade pre-neoplastic pancreatic intraepithelial neoplasms (PanINs) were observed (Fig. 2C).
Fig. 2.
Pathological changes of pancreata in cyclooxygenase (COX)-2/Cre mice. A: pancreatic COX-2 expression led to an increased pancreas-to-body weight ratio (*P < 0.01, #P < 0.05). Values are means ± SE. Ela-CreERT (Cre) mice were used as control. B: expression level of COX-2 at different ages of mice. C: hematoxylin and eosin (H&E) staining shows the pathological changes caused by COX-2 expression in the pancreas. Cystic ductal lesions, tubular complex (TC), acinar-to-ductal metaplasia (ADM), and low-grade pre-neoplastic pancreatic intraepithelial neoplasms (PanINs) were observed.
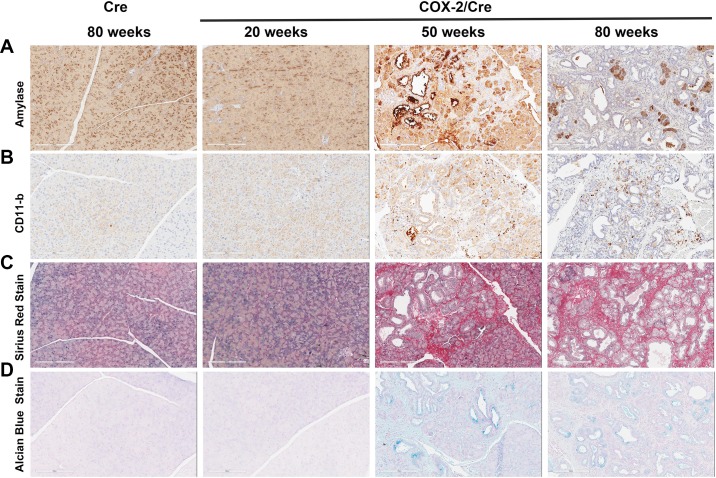
IHC staining with the acinar cell-specific marker amylase further revealed a gradual loss in the number of pancreatic acinar cells with age (Fig. 3A). The loss of pancreatic acinar cells was accompanied by increased CD11b-positive inflammatory cells (Fig. 3B). However, severe destruction of pancreas was seen in the COX-2-expressing mice and worsened with time (e.g., 50 vs. 80 wk). Sirus red staining of collagen indicated increased fibrogenesis, a feature of chronic pancreatitis (Fig. 3C). ADM and increased ductal structures were revealed by Alcian blue staining (Fig. 3D). Together, these changes reflected the severity of chronic inflammation of the pancreas, which is further summarized in Table 1.
Fig. 3.
Inflammation and fibrogenesis in cyclooxygenase (COX)-2/Cre mice. A: immunohistochemical stain for amylase showed a progressive loss of acinar cells in COX-2 expressing pancreata. B: CD11b staining showed dramatic inflammatory cell infiltration in COX-2 mice. C: Sirius red staining indicated progressive fibrogenesis in COX-2 expressing mice. D: Alcian blue staining revealed acinar-to-ductal metaplasia and pancreatic intraepithelial neoplasia in COX-2 expressing mice.
Table 1.
Pathological scoring of the pancreatic tissues in COX-2/Cre mice after different time periods
|
Weeks |
||||
|---|---|---|---|---|
| Pathology | 10/20 | 30 | 80 | P Values |
| Acinar atrophy | 0 | 0.80 ± 0.45 | 2.60 ± 0.55 | 0.0001 |
| Inflammatory cell infiltration | 0 | 1.00 ± 0.71 | 1.60 ± 0.55 | 0.172 |
| Edema | 0 | 0.40 ± 0.55 | 1.20 ± 0.84 | 0.111 |
| Fibrosis | 0 | 0.80 ± 0.45 | 3.00 ± 0.00 | <0.0001 |
| Tubular complex | 0 | 1.40 ± 0.55 | 2.80 ± 0.45 | 0.02 |
| Total score | 0 | 4.40 ± 1.95 | 11.2 ± 0.84 | <0.0001 |
Values are means ± SE; n = 5 mice. Histological scoring of acinar atrophy, inflammatory cell infiltration, edema, fibrosis, and tubular complex in cyclooxygenase (COX)-2/Cre mice. Lesion sizes in each microscopic field were evaluated as follows: 0 = absent; 1 = a lesion slightly shown in lobule or intralobular region (less than one-half); 2 = a lesion widely shown in lobule or intralobular region (more than one-half); 3 = a lesion shown across lobule and intralobular region or with destruction of lobular architecture. Significant differences (P < 0.05) between 80 wk and 30 wk indicated the progression of chronic pancreatitis.
Pancreatic acinar COX-2 expression activated profibrogenic and proliferative pathways.
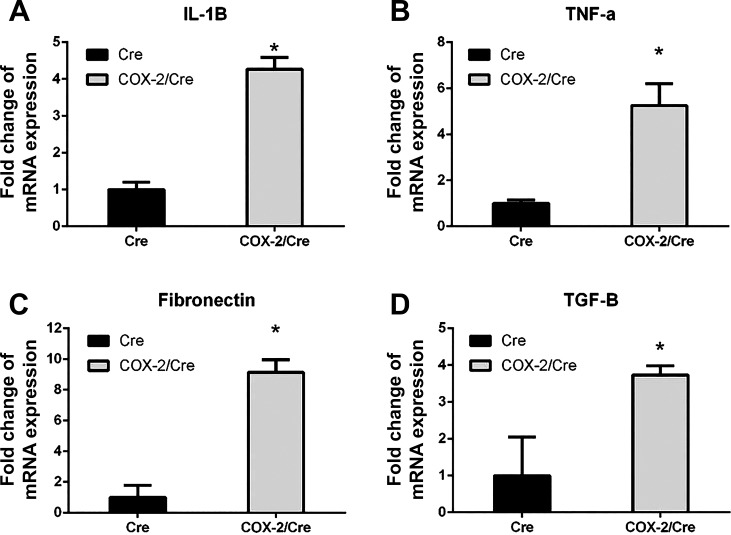
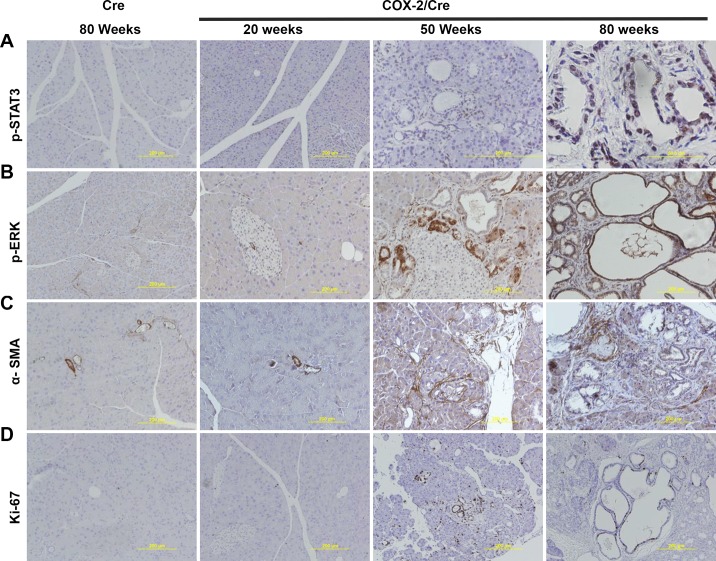
To investigate the potential mechanisms of COX-2 expression-induced pancreatic hyperplasia, inflammation, and fibrogenesis, we investigated the expression level of several key profibrogenic and proliferative signaling pathways by quantitative RT-PCR and immunohistochemical staining. Quantitative RT-PCR results indicated that, compared with Cre control mice, proinflammatory cytokines and profibrogenic factors, including IL-1β, TNF-α, fibronectin, and TGF-β, were significantly upregulated in the pancreata of COX-2/Cre mice at 30 wk (P < 0.05) (Fig. 4). IHC staining showed an evident positive signal for p-STAT3 (Fig. 5A) and p-ERK in the COX-2/Cre mice at both 50 and 80 wk (Fig. 5B). Notable increased PSC activation (α-SMA, Fig. 5C) as well as cell proliferation, as seen by Ki-67 staining (Fig. 5D) were also observed in COX-2/Cre mice.
Fig. 4.
Upregulation of key cytokines and profibrogenic factors in cyclooxygenase (COX)-2/Cre mice. Interleukin (IL)-1β (A), tumor necrosis factor (TNF)-α (B), fibronectin (C), and transforming growth factor (TGF)-β (D) were significantly increased in the pancreata of COX-2-expressing mice (30 wk) (*P < 0.05). Values are means ± SE.
Fig. 5.
Activation of phosphorylated signal transducers and activators (p-STAT3), phosphorylated extracellular signal-regulated kinase (p-ERK), α-smooth muscle actin (α-SMA), and Ki-67 signaling pathways in cyclooxygenase (COX)-2 mice. Immunohistochemical staining results showed the expression of p-STAT3 (A), p-ERK (B), α-SMA (C), and Ki-67 (D) in the pancreatic tissues of mice expressing COX-2 at different time points (20, 50, and 80 wk).
DISCUSSION
In this study, we expressed COX-2 specifically in the mouse pancreatic acinar cells via conditional transgenic mouse model. We observed that acinar cell COX-2 expression resulted in the upregulation of profibrogenic factors, PSC activation, and the development of chronic pancreatitis with pre-neoplastic lesions. These data suggest that, during the initiation of pancreatitis, pancreatic acinar cells can synthesize and secrete various proinflammatory factors, which can initiate the processes of inflammation and fibrosis. This study also highlights the interactions of pancreatic acinar cells and PSC in the development of inflammation and fibrogenesis,
During the initial stage of pancreatitis, acinar cells can synthesize as well as secrete a variety of proinflammatory factors, which contribute to the processes of inflammation and fibrosis. Our focus in this study was to use a genetically modified mouse model to address the roles of COX-2 in pancreatic inflammation and fibrogenesis. To accomplish this goal, we crossed a COX-2 floxed mouse with an elastase promoter driven CreERT (Cre) mouse to generate a transgenic mouse model that expressed COX-2, specifically in pancreatic acinar cells.
We confirmed that COX-2 was specifically expressed in the pancreatic acinar cells of COX-2/Cre mice by IHC staining. COX-2 expression was greatest in mouse tissue samples at 20 wk, but weakened as the pancreas began to degrade due to increases in inflammation and fibrosis from pancreatitis. Our histological results verified that COX-2 expression is associated with ADM and PanIN lesion formation in mice. COX-2 expression has been observed in patients with chronic pancreatitis (9, 27). The data from this study demonstrate that COX-2 expression can cause chronic pancreatitis in conditional transgenic mice.
We observed that pancreatic acinar cell COX-2 expression resulted in the development of chronic pancreatitis and advanced to precancerous PanIN lesion formation. Markers for pancreatic destruction, as well as the profibrogenic mediators, collagen and mucin, were upregulated. Histology from control and COX-2 mice showed amylase accumulation, immune cell infiltration, collagen deposition, and mucin production increased in the pancreata from COX-2/Cre mice. Taken together, the results demonstrate that there is a shift in the pancreatic tissue, during prolonged chronic pancreatitis, from an acinar to more ductal phenotype.
Expression of COX-2 led to continuous activation of inflammatory mediators and profibrogenic factors since the age of 30 wk. IL-1β, TNF-α, TGF-β, p-STAT3, and p-ERK levels were significantly increased in COX-2/Cre mice. It has previously been reported that, during pancreatic injury, atrophic acinar cells can induce macrophages and granulocytes to release IL-1β and TNF-α, resulting in activation of PSCs to promote chronic pancreatitis (4, 14, 24). Various molecular mechanistic pathways, such as TGF-β/SMAD (25, 28), JAK/STAT (10, 17), IL-1β/ERK (16, 18), and their cross talks have been previously shown to play a critical role in the activation of PSCs and trigger the phenomenon of pancreatic fibrogenesis. These profibrogenic signaling pathways were increased in COX-2-expressing mice and might participate in the activation of PSCs, resulting in chronic damage, including acinar cell atrophy and fibrosis. Increased expression of α-SMA indicated that PSCs were activated. Overall, our study demonstrated that expression of COX-2 in pancreatic acinar cells can activate PSCs through the involvement of various profibrogenic mediators.
The highest COX-2 expression was observed at 20 wk with decreasing in older animals, but the majority of destruction to pancreatic acinar cells occurred after 30 wk in mice. The corresponding profibrogenic factors as well as PSC activation showed dramatic increases, accompanied by aggravating chronic inflammation after 30 wk. Even the development of PanIN lesions was observed at later times, associated with an increase in the proliferation rate, as assessed by Ki-67 staining. COX-2 expression was previously reported to increase progression of the PanIN lesions in pancreatic specimens (1, 26). Our results further verified that COX-2 expression is associated with ADM and PanIN lesions in mice. Based on our prolonged period of observation, it appears that overexpression of COX-2 activates proliferative signaling at earlier times and causes inflammatory and profibrogenic mediator expression at later times. Together, these signaling pathways contribute to pancreas megaly and the pathogenesis of pancreatic fibrosis, and ultimately lead to the development of chronic pancreatitis and pre-neoplastic lesions.
Although the mechanisms responsible for the COX-2-induced proliferation and inflammation require further study, it is expected that these effects are mediated by the products of COX-2 prostaglandins. The COX enzymes catalyze a key reaction in the conversion of arachidonic acid to prostaglandins, such as prostaglandin E2 (PGE2). PGE2 can increase cell growth through the E-prostanoid receptor signaling pathways (33). COX-2-PGE2-EP receptor pathway is also a potent inflammatory mediator (13).
In summary, expression of COX-2 in pancreatic acinar cells is sufficient to induce chronic pancreatitis and lead to pre-neoplastic lesions in mice through persistently activating profibrogenic factors and PSCs. Targeting this pathway may be valuable in the treatment and prevention of chronic pancreatitis. Although the precise cellular mechanisms remain to be fully elucidated, our data suggest that this mouse model may be valuable for the investigation of the mechanisms involved in the pathogenesis of chronic pancreatitis and pancreatic cancer initiation.
GRANTS
This work was supported in part by National Institutes of Health (NIH) Grants DK-05206 and AA-020822 and The Lockton Endowment (to C. D. Logsdon); U.S. Department of Defense Grant W81XWH-15-1-0257 and NIH Grants 5K12 CA-090628-18 and P50 CA-102701 (to B. Ji); and National Natural Science Foundation of China Grants 81300353 and 81770642 (to H. Huang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.H., J.C., L.P., Y.Y., and W.L. performed experiments; H.H., J.C., Y.Y., D.D., Y.L., and Y.B. interpreted results of experiments; H.H., J.C., L.P., D.D., and Y.Z. prepared figures; J.C., Y.Y., and H.W. analyzed data; J.C., L.P., and C.D.L. drafted manuscript; J.C., Z.L., Y.B., A.N.H., X.Z., C.D.L., and B.J. edited and revised manuscript; Y.B., X.Z., W.L., and C.D.L. conceived and designed research; C.D.L. and B.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciated Brandy H. Edenfield for histology assistance.
REFERENCES
- 1.Albazaz R, Verbeke CS, Rahman SH, McMahon MJ. Cyclooxygenase-2 expression associated with severity of PanIN lesions: a possible link between chronic pancreatitis and pancreatic cancer. Pancreatology 5: 361–369, 2005. doi: 10.1159/000086536. [DOI] [PubMed] [Google Scholar]
- 2.Colby JKL, Klein RD, McArthur MJ, Conti CJ, Kiguchi K, Kawamoto T, Riggs PK, Pavone AI, Sawicki J, Fischer SM. Progressive metaplastic and dysplastic changes in mouse pancreas induced by cyclooxygenase-2 overexpression. Neoplasia 10: 782–796, 2008. doi: 10.1593/neo.08330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaiser S, Daniluk J, Liu Y, Tsou L, Chu J, Lee W, Longnecker DS, Logsdon CD, Ji B. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut 60: 1379–1388, 2011. doi: 10.1136/gut.2010.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasiorowska A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Małecka-Panas E. Subclinical inflammation and endothelial dysfunction in patients with chronic pancreatitis and newly diagnosed pancreatic cancer. Dig Dis Sci 61: 1121–1129, 2016. doi: 10.1007/s10620-015-3972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaster R, Sparmann G, Emmrich J, Liebe S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut 51: 579–584, 2002. doi: 10.1136/gut.51.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji B, Song J, Tsou L, Bi Y, Gaiser S, Mortensen R, Logsdon C. Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis 46: 390–395, 2008. doi: 10.1002/dvg.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji B, Tsou L, Wang H, Gaiser S, Chang DZ, Daniluk J, Bi Y, Grote T, Longnecker DS, Logsdon CD. Ras activity levels control the development of pancreatic diseases. Gastroenterology 137: 1072–1082.e6, 2009. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamei K, Ishikawa TO, Herschman HR. Transgenic mouse for conditional, tissue-specific Cox-2 overexpression. Genesis 44: 177–182, 2006. doi: 10.1002/dvg.20199. [DOI] [PubMed] [Google Scholar]
- 9.Koliopanos A, Friess H, Kleeff J, Roggo A, Zimmermann A, Büchler MW. Cyclooxygenase 2 expression in chronic pancreatitis: correlation with stage of the disease and diabetes mellitus. Digestion 64: 240–247, 2001. doi: 10.1159/000048868. [DOI] [PubMed] [Google Scholar]
- 10.Komar HM, Serpa G, Kerscher C, Schwoegl E, Mace TA, Jin M, Yang MC, Chen CS, Bloomston M, Ostrowski MC, Hart PA, Conwell DL, Lesinski GB. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci Rep 7: 1787, 2017. doi: 10.1038/s41598-017-01973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korc M, Friess H, Yamanaka Y, Kobrin MS, Buchler M, Beger HG. Chronic pancreatitis is associated with increased concentrations of epidermal growth factor receptor, transforming growth factor alpha, and phospholipase C gamma. Gut 35: 1468–1473, 1994. doi: 10.1136/gut.35.10.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grünert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest 80: 47–55, 2000. doi: 10.1038/labinvest.3780007. [DOI] [PubMed] [Google Scholar]
- 13.Mann JR, Backlund MG, DuBois RN. Mechanisms of disease: Inflammatory mediators and cancer prevention. Nat Clin Pract Oncol 2: 202–210, 2005. doi: 10.1038/ncponc0140. [DOI] [PubMed] [Google Scholar]
- 14.Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther 8: 10–25, 2017. doi: 10.4292/wjgpt.v8.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masamune A, Kikuta K, Satoh M, Satoh K, Shimosegawa T. Rho kinase inhibitors block activation of pancreatic stellate cells. Br J Pharmacol 140: 1292–1302, 2003. doi: 10.1038/sj.bjp.0705551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masamune A, Satoh A, Watanabe T, Kikuta K, Satoh M, Suzuki N, Satoh K, Shimosegawa T. Effects of ethanol and its metabolites on human pancreatic stellate cells. Dig Dis Sci 55: 204–211, 2010. doi: 10.1007/s10620-008-0695-y. [DOI] [PubMed] [Google Scholar]
- 17.Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Activation of JAK-STAT pathway is required for platelet-derived growth factor-induced proliferation of pancreatic stellate cells. World J Gastroenterol 11: 3385–3391, 2005. doi: 10.3748/wjg.v11.i22.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masamune A, Watanabe T, Kikuta K, Satoh K, Kanno A, Shimosegawa T. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol 299: G821–G832, 2010. doi: 10.1152/ajpgi.00178.2010. [DOI] [PubMed] [Google Scholar]
- 19.Masamune A, Watanabe T, Kikuta K, Shimosegawa T. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol 7, Suppl: S48–S54, 2009. doi: 10.1016/j.cgh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 20.Masamune A, Kikuta K, Satoh M, Suzuki N, Shimosegawa T. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther 312: 651–658, 2005. doi: 10.1124/jpet.104.076232. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura N, Ochi K, Ichimura M, Mizushima T, Harada H, Harada M. Study on free radicals and pancreatic fibrosis—pancreatic fibrosis induced by repeated injections of superoxide dismutase inhibitor. Pancreas 22: 53–57, 2001. doi: 10.1097/00006676-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Needleman P, Truk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem 55: 69–102, 1986. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 23.Niina Y, Ito T, Oono T, Nakamura T, Fujimori N, Igarashi H, Sakai Y, Takayanagi R. A sustained prostacyclin analog, ONO-1301, attenuates pancreatic fibrosis in experimental chronic pancreatitis induced by dibutyltin dichloride in rats. Pancreatology 14: 201–210, 2014. doi: 10.1016/j.pan.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Norman JG, Fink GW, Sexton C, Carter G. Transgenic animals demonstrate a role for the IL-1 receptor in regulating IL-1beta gene expression at steady-state and during the systemic stress induced by acute pancreatitis. J Surg Res 63: 231–236, 1996. doi: 10.1006/jsre.1996.0253. [DOI] [PubMed] [Google Scholar]
- 25.Ohnishi H, Miyata T, Yasuda H, Satoh Y, Hanatsuka K, Kita H, Ohashi A, Tamada K, Makita N, Iiri T, Ueda N, Mashima H, Sugano K. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J Biol Chem 279: 8873–8878, 2004. doi: 10.1074/jbc.M309698200. [DOI] [PubMed] [Google Scholar]
- 26.Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, Gomez SB, Ji B, Huang H, Wang H, Fleming JB, Logsdon CD, Cruz-Monserrate Z. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology 145: 1449–1458, 2013. doi: 10.1053/j.gastro.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlosser W, Schlosser S, Ramadani M, Gansauge F, Gansauge S, Beger HG. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas 25: 26–30, 2002. doi: 10.1097/00006676-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR, Iredale JP. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 160: 1787–1798, 2002. doi: 10.1016/S0002-9440(10)61125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takano S, Kimura T, Yamaguchi H, Kinjo M, Nawata H. Effects of stress on the development of chronic pancreatitis. Pancreas 7: 548–555, 1992. doi: 10.1097/00006676-199209000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Van Laethem JL, Devière J. Pancreatitis and cytokines. Acta Gastroenterol Belg 59: 186–187, 1996. [PubMed] [Google Scholar]
- 31.Williams CS, DuBois RN. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol Gastrointest Liver Physiol 270: G393–G400, 1996. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- 32.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18: 7908–7916, 1999. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 33.Zweifel BS, Davis TW, Ornberg RL, Masferrer JL. Direct evidence for a role of cyclooxygenase 2-derived prostaglandin E2 in human head and neck xenograft tumors. Cancer Res 62: 6706–6711, 2002. [PubMed] [Google Scholar]