Abstract
The liver is an organ that, when dysfunctional in a septic patient, is strongly associated with morbidity and mortality. Understanding the pathophysiology of liver failure during sepsis may lead to improved diagnostics and potential therapeutic targets. Historically, programmed cell death receptor (PD) ligand 1 (PD-L1) has been considered the primary ligand for its checkpoint molecule counterpart, PD-1, with PD-L2 rarely in the immunopathological spotlight. PD-1 and PD-L1 contribute to liver dysfunction in a murine cecal ligation and puncture (CLP) model of sepsis, but virtually nothing is known about PD-L2’s role in sepsis. Therefore, our central hypothesis was that sepsis-induced changes in hepatic PD-L2 expression contributed to worsened liver function and, subsequently, more pronounced morbidity and mortality. We found that although PD-L1 gene deficiency attenuated the hepatic dysfunction seen in wild-type mice after CLP, the loss of PD-L2 appeared to actually worsen indices of liver function along with a trend toward higher liver tissue vascular permeability. Conversely, some protective effects of PD-L2 gene deletion were noted, such as reduced liver/peritoneal bacterial load and reduced IL-6, IL-10, and macrophage inflammatory protein 2 levels following CLP. These diverse actions, as well as the unique expression pattern of PD-L2, may explain why no overt survival advantage could be witnessed in the septic PD-L2−/− mice. Taken together, these data suggest that although PD-L2 has some selective effects on the hepatic response seen in the septic mouse, these factors are not sufficient to alter septic mortality in this adult murine model.
NEW & NOTEWORTHY Our study shows not only that ligands of the checkpoint protein PD-1 respond inversely to a stressor such as septic challenge (PD-L2 declines, whereas PD-L1 rises) but also that aspects of liver dysfunction increase in septic mice lacking the PD-L2 gene. Furthermore, these differences in PD-L2 gene-deficient animals culminated in the abrogation of the survival advantage seen in the septic PD-L1-knockout mice, suggesting that PD-L2 may have roles beyond a simple immune tolerogen.
Keywords: inflammation, posttranslational modification, programmed cell death receptor-1, programmed cell death receptor ligand 1, sepsis
INTRODUCTION
Sepsis is a syndrome resulting from a dysfunctional response to infection and is a leading cause of critical illness and mortality worldwide. From an epidemiological perspective, sepsis is a serious problem that contributes to both sustained patient morbidity and burdensome healthcare costs (6, 7).
Programmed cell death receptor 1 (PD-1) is a cell-surface coinhibitory protein on immune cells that, when bound to its ligands, has been shown to downregulate immune cell function (12, 26). PD-1-knockout mice appear to be protected from experimental septic challenge [cecal ligation and puncture (CLP)]-induced lethality, and their macrophages are resistant to sepsis-induced cellular dysfunction (12). However, it is unclear whether this effect is due to the knockdown of PD-1 itself or due to the resultant attenuation of PD-1’s interactions with its ligands, namely PD-L1 and PD-L2.
Regarding PD-L1, it has been previously established that this ligand’s expression is significantly upregulated during sepsis (11, 22). Gene deficiency or blockade of PD-L1 has also been shown to have a protective effect: it improves survival in septic mice via the inhibition of lymphocyte apoptosis and reversal of monocyte dysfunction (11, 29). Furthermore, the protective role of PD-L1 blockade resulted not only in the suppression of proinflammatory cytokines (IL-6 and TNF-α) but also anti-inflammatory cytokine IL-10 (11, 29). This is further supported by a study indicating the suppression of lymphocyte proliferation in the case of PD-L1 upregulation via IFN-γ (5). In this regard, we recently reported that PD-L1 expression is increased in the liver after CLP and that there is a link between PD-L1 blockade and alleviation of vascular permeability defects mediated by liver sinusoidal endothelial cells (LSECs), as well as an attenuation of LSEC apoptosis and cell loss during sepsis. PD-1:PD-L1 blockade also appeared to restore LSEC angiogenesis and proliferation (13, 14).
By comparison, however, very little is known about the role of PD-L2 expression in the response to septic challenge. PD-L2 is a cell-surface glycoprotein that was first identified in 2001 during the search for PD-L1 homologs on GENBANK (16). Their studies revealed that interaction between PD-1 and PD-L2 led to a downregulation of T cell responses, thus inhibiting T cell activation and proliferation. Since little is known about PD-L2 expression in relation to a variety of pathological conditions, including sepsis, there is a significant appeal in studying this novel protein to deduce what its roles could be in relation to its sister molecule, PD-L1.
Finally, although all organs are eventually affected by sepsis, our group has had a particular interest in the liver because it plays a key role in metabolic and homeostatic activities, as well as host defense (2). Sepsis-induced liver dysfunction is strongly associated with mortality, as it is a frequent event in the multiple organ dysfunction syndrome seen in critically ill septic patients (8). In fact, it accounts for the highest percentage of mortality when compared with other organ dysfunction (i.e., lung and kidney). Since we previously demonstrated a correlation between PD-L1 expression and declining liver function in murine sepsis (13, 14), we are interested in discovering what effects PD-L2 has on murine liver function after experimental sepsis. A better understanding of hepatic PD-L2 pathophysiology could have eventual progressive impacts on developing the tools to diagnose liver dysfunction and manage the sequelae. Our central hypothesis is that sepsis-induced changes in PD-L2 expression in the liver contribute to worsened liver function and, subsequently, more severe morbidity and mortality.
MATERIALS AND METHODS
Animals.
Wild-type (WT) C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and acclimated to a facility for no less than a week before using these animals (20). Alternatively, C57BL/6 mice deficient in the PD-L1 gene (PD-L1−/−) (provided by Tasuku Honjo, Kyoto University, Kyoto, Japan, through Megan Sykes at Massachusetts General Hospital, Boston, MA) as well as mice deficient in the PD-L2 gene (PD-L2−/−) (Jackson) were bred at the Rhode Island Hospital rodent facility (12-h:12-h light-dark cycle, 15.5–22°C, 30–70% humidity) and received standard care and diet ad libitum. Adult male mice ~8–12 wk of age were used for all experiments. All protocols were carried out in the morning (8:00 am–11:00 am), were approved by the Institutional Animal Care and Use Committee of Rhode Island Hospital (AWC nos. 0228-13 and 0040-16) and were in accordance with the Animal Welfare Act and National Institutes of Health Guide for Animal Use and Care.
CLP.
The CLP method used to induce polymicrobial sepsis in animals was carried out as previously described by our group (1). Briefly, this model entails a midline laparotomy and ligation of the cecum followed by extrusion of cecal contents through perforations made from a 21-gauge needle. For sham procedure, laparotomy was performed with identification of the cecum but without ligation or perforation. All laparotomies were sutured closed in two layers; mice received crystalloid resuscitation postoperatively and were monitored in the postoperative period.
Survival analysis.
Following septic challenge on WT, PD-L1−/−, and PD-L2−/− mice, survival was monitored daily for a period of 14 consecutive days.
Liver perfusion and tissue/cell harvest.
Twenty-four hours following CLP, mice were euthanized, the peritoneal cavity was exposed, and the portal vein was clamped. A syringe filled with collagenase buffer was inserted cannula side up and slowly perfused until the liver was cleared of blood (i.e., the organ blanched). The liver was then harvested for several purposes: to obtain single cell preparations, to lyse the tissue to obtain protein, or to isolate mRNA as we have previously described (13).
Western blot analysis.
Western blots of PD-L2 and GAPDH protein levels from whole tissue lysates harvested 24 h following CLP or sham were performed as previously described (4). PD-L2 (clone TY25; ab21107) densitometric expression (fold expression) was compared against GAPDH (rabbit polyclonal; ab9484) (both from Abcam, Cambridge, MA) as a loading control.
Tissue vascular permeability assay.
The Evans blue dye (EBD) leakage method was used to deduce vascular permeability in the veins of various organs, and the level of vascular permeability was evaluated by quantitative measurement of a set amount of dye incorporated per milligram of tissue over a set period of time in the tissue samples as described previously (21, 28). Twenty-four hours after sham and/or CLP surgery, animals were intravenously injected with 200 μl of 0.5% (wt/vol) EBD (Sigma-Aldrich; St. Louis, MO). After the dye was allowed to percolate to the subendothelial spaces for 20 min, mice were euthanized, blood samples were collected, and the livers were perfused with 1X PBS, weighed, and then dissociated with formamide (Sigma-Aldrich) for 48 h at 37°C. Supernatants were spun down and read at 610 nm. The amount of EBD extracted (vascular leakage) from each liver sample was calculated and normalized to the amount of EBD (mg/ml) collected in the plasma (13).
Alanine aminotransferase, aspartate aminotransferase, and bilirubin levels of mouse plasma.
Whole blood samples were collected by cardiac puncture, using heparinized needles from animals 24 h after surgery, and spun down at 9,300 g for 10 min. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in plasma were detected by a Biotron Diagnostics kit (Hemet, CA) and read at 540 nm on a microplate spectrophotometer. Normal ranges for ALT and AST in mice are 10–35 U/l, with the linear limit at 800 U/l, and 10–45 U/l, with the linear limit at 600 U/l, respectively. The plasma bilirubin levels were determined according to the manufacturer’s directions with a commercial assay kit obtained from Sigma Aldrich and read at 540 nm. Normal ranges for bilirubin in mice are 0–1 mg/dl, with the linear limit at 1.6 mg/dl.
Nonparenchymal liver cell purification.
Because PD-L2 is primarily expressed on LSECs, which compose a subpopulation of nonparenchymal liver cells (NPCs), isolation of NPCs from hepatocytes provides a narrower scope for identifying the target cell population within tissue samples. Liver tissue was perfused and digested with a collagenase IV (Sigma-Aldrich) buffer (1% collagenase IV from clostridium histolyticum and 0.02% DNAse I in 10 mM HEPES-NaOH (pH 7.4), 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2 in 1X PBS) for 30 min at 37°C; cell suspensions were then collected in 40-mm strainers, and hepatocyte pellets were removed by slow centrifugation at 30 g for 10 min. The supernatant cells were enriched with 30% Histodenz nonionic density gradient medium and spun down at 1,650 g for 30 min. NPCs in the interface layer were collected and washed (14).
ELISA.
Blood was extracted from the heart via cardiac puncture 24 h after surgery, and plasma was separated by centrifugation. Local tissue pro-/anti-inflammatory cytokine/chemokine load and select acute phase protein expression changes were measured. These included the use of commercial ELISA kits for proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) (BD Biosciences, San Diego, CA), anti-inflammatory interleukin 10 (IL-10), the chemokines monocyte chemoattractant protein 1 (MCP-1) (BioLegend, San Diego, CA), and chemokine macrophage inflammatory protein 2 (MIP-2) (R&D Systems, Minneapolis, MN).
Quantitative real-time PCR.
mRNA levels of PD-L2 were measured using quantitative real-time PCR. In brief, total RNA was extracted from the liver following harvest and then purified as previously described (3). cDNA was synthesized via reverse transcription of RNA, and primer pairs (Thermo Fisher Scientific, Waltham, MA) for the PD-L2 gene were: sense, 5′-GTA CCG TTG CCT GGT CAT CT-3′; and antisense, 5′-GCC AGG ACA CTT CTG CTA GG-3′; for GAPDH: sense, 5′-CAT GGC CTC CAA GGA GTA AG-3′; antisense, 5′-CCT AGG CCC CTC CTG TTA TT-3′, designed by Menke et al. (19).
Flow cytometry.
Fluorescent labeling of various cell populations and the flow cytometric analysis was carried out as previously described (14). Markers used included monoclonal antibodies to CD31 (LSECs), F4/80 (Kupffer cells), and CD273 (PD-L2); all were directly conjugated to various fluorochromes (BioLegend). The scattering of light seen during flow cytometry allows for the determination of volume and morphological complexity of cells and other particles. Furthermore, cell apoptosis was identified and gated to ensure that only live cells were considered (Propidium Iodide Flow Cytometry Kit, Abcam, Cambridge, MA).
Bacterial burden.
Whole blood (diluted to 1:10 in sterile PBS) and peritoneal fluids (diluted to 1:100 in sterile PBS) were collected from mice. These samples were plated on sheep’s blood agar and incubated for 24 h at 37°C. The plates were then visually inspected, and total numbers of bacterial colony-forming units (CFU) were determined per milliliter or milligram of tissue samples.
Statistical analysis.
The data were expressed as means ± SE and were prepared using SigmaPlot version 11.0. We used the nonparametric Mann-Whitney U-test to evaluate P values when testing two subgroups. For multiple groups analysis, intergroup comparisons were performed by the Holm-Sidak test. Survival study of subgroups was done with a Kaplan-Meier analysis, and comparisons were performed by the log-rank test. Significant differences were confirmed between groups when the P value was <0.05.
RESULTS
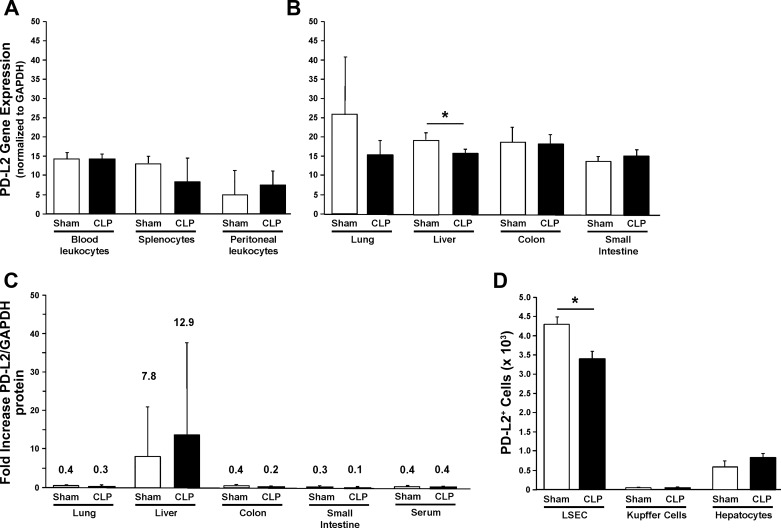
The first goal of this investigation was to determine the location of PD-L2 expression throughout adult mice in response to sham or septic challenges. Here, we found that PD-L2 gene expression was evident in almost every whole undifferentiated immune tissue (Fig. 1A) or whole organ tissue extract (Fig. 1B) examined in sham and CLP mice. However, for the most part, we either detected no marked change in PD-L2 expression or a decline in PD-L2 message expression in CLP mice (Fig. 1, A and B). In this respect, the decline in expression, although a nonsignificant trend in the lung, was consistently markedly (P < 0.05) decreased only in the liver (Fig. 1B). Of note, using Western immunoblot analysis on these whole tissue lysates, although we obtained the strongest signal for PD-L2 protein levels from the liver and modest background signals from the other tissues, we were not able to clearly identify a marked difference between CLP and sham animals’ PD-L2 levels by this method (Fig. 1C). In an attempt to clarify the PD-L2 expression situation relative to the gene and protein levels in the liver, we chose to further examine the hepatic cellular subpopulations’ PD-L2 expression using flow cytometric analysis. Here, we found that the number of PD-L2+ LSECs decreased significantly after CLP compared with sham mice (P < 0.05) (Fig. 1D). Conversely, the number of PD-L2+ cells did not change significantly in Kupffer cells and hepatocytes, and the overall number of PD-L2+ cells in both these groups was substantially smaller when compared with LSECs (Fig. 1D). Because of the apparent links between PD-L2 and the liver immune function, we wanted to test the hypothesis that sepsis-induced changes in PD-L2 expression in the liver contribute to worsened liver function and, subsequently, more severe morbidity and mortality. To do this, we chose to measure the effects of PD-L2 gene deficiency on liver function in experimental sepsis/CLP and, where possible, how it compared with PD-L1 gene deficiency. This was considered because we previously have shown that PD-L1 gene deficiency mitigates aspects of liver dysfunction (in part through its actions on LSECs) (13) and general animal mortality (11).
Fig. 1.
Programmed cell death receptor ligand 2 (PD-L2) is expressed on a variety of organs and leukocytes present in the blood, peritoneum, or spleen, and with the exception of a consistent decline in the gene expression of the liver, is not altered in mice 24 h post-cecal ligation and puncture (CLP). PD-L2 gene expression (normalized to housekeeping gene GAPDH) in blood leukocytes, splenocytes, and peritoneal exudate cells (A) (n = 4/group) or lung, liver, large intestine, and small intestinal tissue lysates (B) (n = 7–8/group) obtained from mice 24 h after CLP was extracted for mRNA and analyzed using quantitative real-time PCR. PD-L2 protein expression in various whole tissue lysates or serum was determined by Western immunoblot analyses, in which the bands detected were quantitated by densitometry. Summary of the fold expression change (ratio of densitometry) for PD-L2/GAPDH (C) (n = 4/group). The number of PD-L2+ liver cells by hepatic subpopulations [liver sinusoidal endothelial cells (LSEC), Kupffer cells, or hepatocytes] at 24 h after CLP was determined by flow cytometry of the various isolated/enriched cell subpopulations (D) (n = 6/group). Data are expressed as mean ± SE; *P < 0.05 by nonparametric Mann-Whitney U-test to evaluate P values when testing two subgroups.
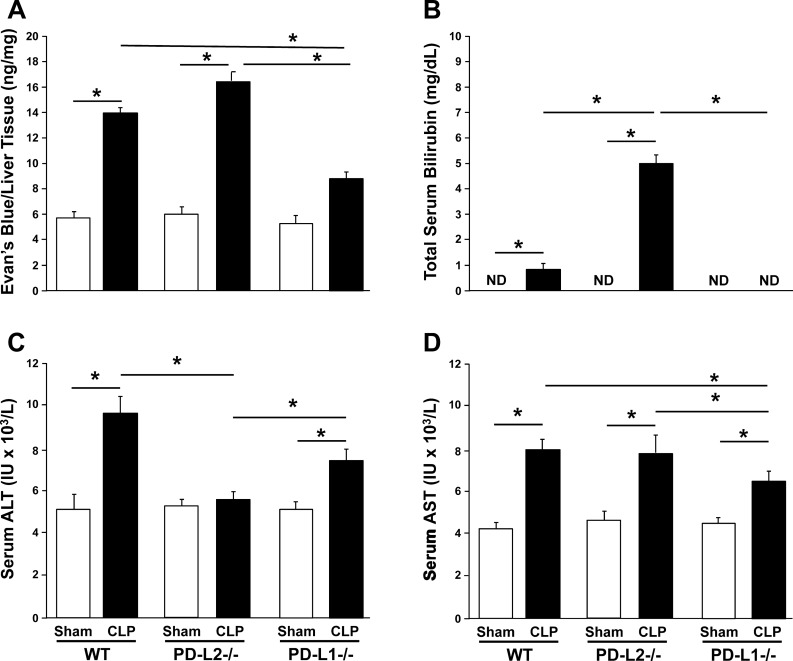
We first examined endothelial barrier function by using an EBD leakage assay. Compared with shams, liver vascular permeability increased significantly in WT and PD-L2−/− mice after CLP (P < 0.05); however, this increase was attenuated in PD-L1−/− CLP mice (Fig. 2A).
Fig. 2.
Indices of dysfunction and damage as a measure of septic mouse liver injury. Vascular permeability measured in nanograms of Evans blue dye over milligrams of liver tissue in wild-type (WT), programmed cell death receptor ligand 2 (PD-L2)−/−, and PD-L1−/− mice 24 h after cecal ligation and puncture (CLP) (n = 4/group) (A). Total blood bilirubin measured in mg/dL in WT, PD-L2−/−, and PD-L1−/− mice 24 h after CLP (n = 5/group) (B). Blood alanine aminotransferase (ALT) and aspartate aminotransferase (AST) measured in IU/L in WT, PD-L2−/−, and PD-L1−/− mice 24 h after CLP (n = 5/group) (C and D, respectively). Data are expressed as mean ± SE; *P < 0.05 by nonparametric Mann-Whitney U-test to evaluate P values when testing two subgroups. For multiple groups analysis, intergroup comparisons were performed by the Holm-Sidak test. ND, not detected.
Turning now to markers of hepatic dysfunction, we determined that, compared with sham mice, total blood bilirubin levels increased in WT CLP mice and increased further in PD-L2−/− CLP mice. Bilirubin was not detected in PD-L1−/− sham or CLP mice (Fig. 2B). As expected, ALT levels in the blood were markedly increased after CLP among WT mice. This effect was similar in septic PD-L1−/− mice, but to a lesser extent, versus their respective sham controls. However, there was no significant change in ALT levels between PD-L2−/− sham and CLP mice (Fig. 2C). Interestingly, AST levels echoed a similar pattern across all three groups, with CLP inducing a rise in AST, indicating, to some extent, liver damage. This rise was significantly attenuated in PD-L1−/− CLP mice compared with WT and PD-L2−/− CLP mice (Fig. 2D).
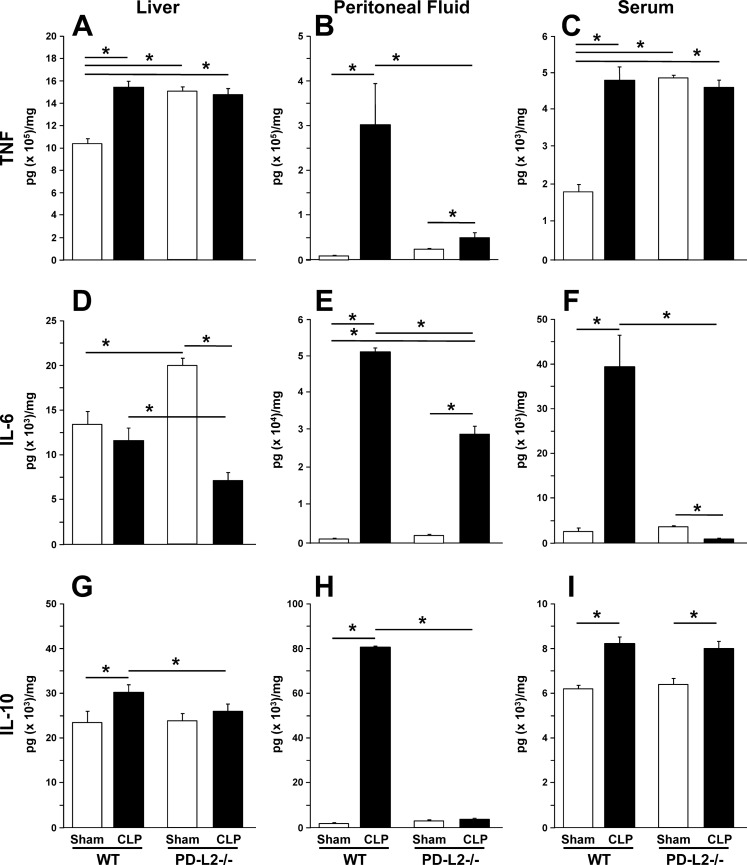
Given the heterogeneity of the blood markers, we investigated tissue inflammation as a possible process underlying the hepatic dysfunction seen following septic insult in the presence or absence of PD-1’s ligand, PD-L2. In the liver (Fig. 3A), TNF-α levels increased in the WT but not in PD-L2−/− mice after CLP. However, sham levels of TNF-α appeared to be higher in PD-L2−/− mice when compared with sham levels in WT mice. In the peritoneal fluid (Fig. 3B), TNF-α increased significantly after CLP in the WT mice, and its increase was attenuated in PD-L2−/− CLP mice. In the plasma (Fig. 3C), a similar pattern to the results in the liver was seen. Although TNF-α increased in the WT mice after CLP and not in the PD-L2−/− mice, the sham levels were already higher in the latter group. In the liver (Fig. 3D), IL-6 did not increase in the WT mice but decreased in the PD-L2−/− mice after CLP. However, IL-6 levels were significantly higher in PD-L2−/− sham mice when compared with WT sham mice. In peritoneal fluid (Fig. 3E), IL-6 increased significantly in WT and PD-L2−/− mice after CLP. However, this increase was attenuated in PD-L2−/− mice. In the plasma (Fig. 3F), IL-6 increased significantly in WT mice and decreased in PD-L2−/− mice compared with their correspondent shams. In the liver (Fig. 3G), levels of the anti-inflammatory mediator IL-10 increased significantly in the WT mice after CLP, and its increase was attenuated in PD-L2−/− mice. In the peritoneal fluid (Fig. 3H), IL-10 increased significantly in the WT mice after CLP, and its increase was greatly attenuated in the PD-L2−/− group. In the plasma (Fig. 3I), IL-10 increased after CLP in both the WT and PD-L2−/− groups with a similar amount.
Fig. 3.
TNF-α, IL-6, and IL-10 as markers of pro-/anti-inflammation in the liver, locally in the peritoneum (relative to the septic insult), and systemically in blood. TNF-α (A–C), IL-6 (D–F), and IL-10 (G–I) levels 24 h after CLP were determined for the liver tissue lysates (A, D, and G), clarified peritoneal fluid (B, E, and H), and plasma (C, F, and I) by commercial ELISA and reported in pg/mg protein (n = 6/group). Data are expressed as mean ± SE; *P < 0.05 by nonparametric Mann-Whitney U-test to evaluate P values when testing two subgroups. For multiple groups analysis, intergroup comparisons were performed by the Holm-Sidak test. CLP, cecal ligation and puncture; PD-L2, programmed cell death receptor ligand 2; WT, wild-type.
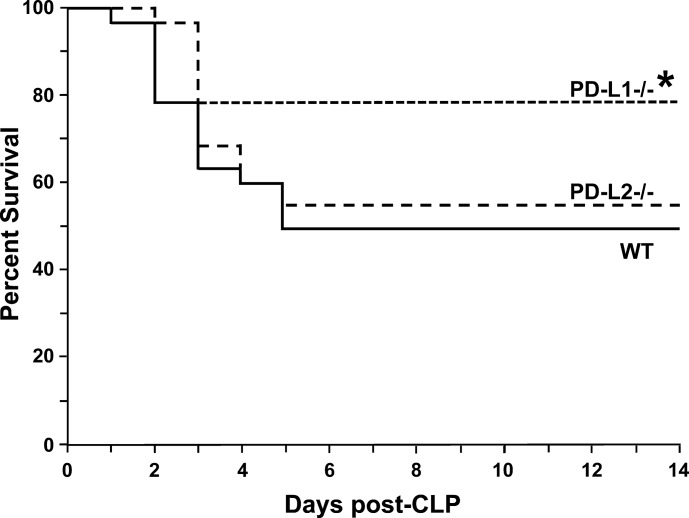
Overall, hepatic tissue derived from PD-L2−/− mice did not demonstrate worsened inflammation when compared with WT mice. Consequently, we conducted a survival analysis to determine whether any difference in mortality between the groups existed. Survival after sepsis was significantly enhanced in the PD-L1−/− mice but not in the PD-L2−/− mice when compared with the WT group (Fig. 4).
Fig. 4.
Survival is enhanced in programmed cell death receptor ligand 1 (PD-L1)−/− mice compared with wild-type (WT) post-cecal ligation and puncture (CLP) but not in PD-L2−/− mice. Survival of PD-L1−/−, PD-L2−/−, and WT mice after CLP measured at 12 h time points for the first 3 days and then at 24 h time points for a total of 360 h (14 days) (n = 15/group). *P < 0.05 by a Kaplan-Meier analysis, and comparisons were performed by the log-rank test.
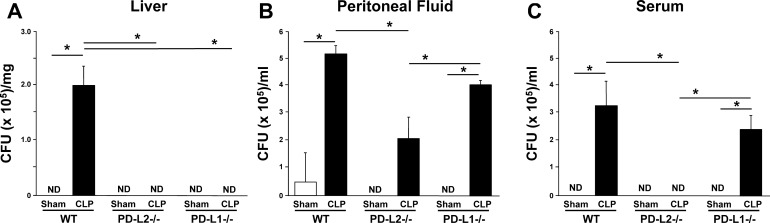
In the context of our studies above, with evidence of liver dysfunction/damage potentiation (excluding the blood ALT results), but no survival benefit in the PD-L2 gene-deficient mice, we attempted to ascertain whether these animals had comparable capacity to clear their septic bacterial burdens (bacterial load or CFU/ml at 24 h post-CLP). What we found was, not unexpectedly, that the bacterial load was increased significantly in WT and PD-L1−/− CLP mice compared with sham mice in the blood (P < 0.05) (Fig. 5C). As expected, the WT CLP mouse blood CFU increase was mildly attenuated in PD-L1−/− mice but was surprisingly completely blunted in PD-L2−/− CLP mice (Fig. 5C). This trend held true for bacterial load in peritoneal fluid, though to a lesser degree (Fig. 5B). In the liver, the bacterial load increased significantly after CLP only in WT mice, whereas both PD-L2−/− and PD-L1−/− CLP and all sham samples showed no evidence of bacterial presence (Fig. 5A). These results brought us to our final set of experiments, namely assessing neutrophil and monocyte/macrophage infiltration (in the liver, locally, and systemically), which could offer insight into worsened or enhanced immunity in the PD-L2−/− mice.
Fig. 5.
Bacterial burden in blood, peritoneal fluid, and liver post-cecal ligation and puncture (CLP). Bacterial load in liver tissue (A), peritoneal fluid (B), or the blood (C) of mice harvested 24 h after CLP or sham protocol, as measured in colony-forming unit (CFU)/ml or CFU/mg tissue (n = 6). Data are expressed as mean ± SE; *P < 0.05 by nonparametric Mann-Whitney U-test to evaluate P values when testing two subgroups. For multiple groups analysis, intergroup comparisons were performed by the Holm-Sidak test. PD-L, programmed cell death receptor ligand; WT, wild-type; ND, not detected.
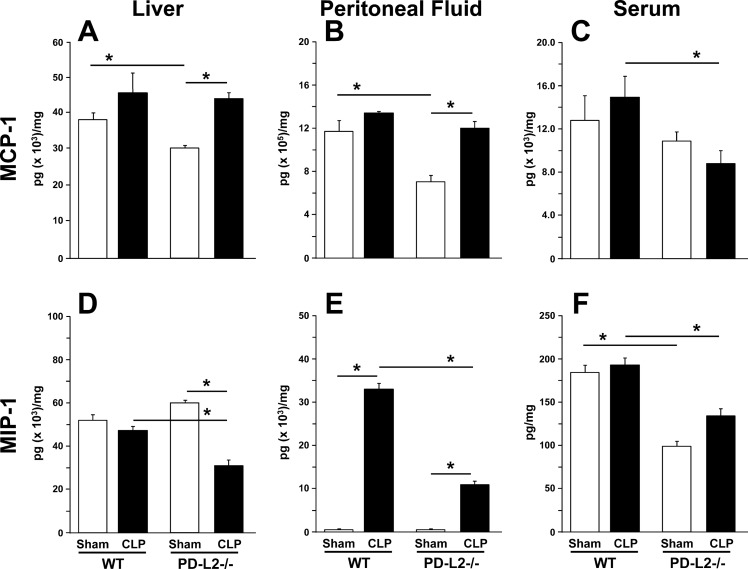
In the liver (Fig. 6A), MCP-1 increased in both the WT and PD-L2−/− mice after CLP, but there were no differences in the increase across the two groups. In the peritoneal fluid (Fig. 6B), MCP-1 levels were not changed between sham and CLP WT mice; however, MCP-1 levels increased significantly in the PD-L2−/− mice after CLP when compared with its sham group. In the plasma (Fig. 6C), MCP-1 levels did not change after CLP compared with their correspondent shams in either group, but the MCP-1 levels were significantly lower in the PD-L2−/− CLP group compared with WT CLP mice. In the liver (Fig. 6D), MIP-2 levels did not change between WT sham and CLP groups. Although significantly decreased after CLP compared with shams in PD-L2−/− mice, these changes were also significant when compared with WT CLP mice. In the peritoneal fluid (Fig. 6E), MIP-2 increased significantly in both WT and PD-L2−/− mice after CLP, and its increase was markedly attenuated in the PD-L2−/− CLP mice. In the plasma (Fig. 6F), there were no significant changes in MIP-2 levels after CLP when compared with their correspondent shams in both the WT and PD-L2−/− groups. However, the levels of MIP-2 were markedly lower in the PD-L2 groups when compared with WT mouse levels.
Fig. 6.
Monocyte chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 2 (MIP-2) as markers of leukocyte chemotactic environment in the liver, locally in the peritoneum (relative to the septic insult), and systemically in blood. MCP-1 (A–C) and MIP-2 (D–F) levels 24 h after cecal ligation and puncture (CLP) were determined for the liver tissue lysates (A, and D), clarified peritoneal fluid (B and E), and plasma (C and F) by commercial ELISA and reported in pg/mg protein (n = 6/group). Data are expressed as mean ± SE; *P < 0.05 by nonparametric Mann-Whitney U-test to evaluate P values when testing two subgroups. For multiple groups analysis, intergroup comparisons were performed by the Holm-Sidak test. PD-L2, programmed cell death receptor ligand 2; WT, wild-type.
DISCUSSION
Our findings suggest that although PD-L2 is expressed across several organs and leukocytes in peritoneal fluid/blood in naïve/sham mice, its expression changes after CLP only consistently within the liver. We further found that within the sham mouse liver, similar to PD-L1, PD-L2 expression is primarily restricted to LSECs. However, unlike the increase in PD-L1’s expression seen post-CLP, PD-L2 expression is actually decreased. These initial findings led us to hypothesize that the decline in PD-L2 in the liver may have an opposite role when compared with PD-L1 during sepsis. Interpreted another way, although PD-L1 knockout seemed to provide protective effects against septic morbidity and mortality, we speculated that loss of PD-L2 expression would worsen organ damage and survival outcomes following sepsis.
To examine this concept further, we queried whether liver vascular endothelial permeability had been compromised by using the EBD exclusion assay. We found that, in the case of PD-L1-knockout mice, there was less “leakage” of EBD into the liver, consistent with our previous findings that PD-L1 knockout alleviated the increased permeability after CLP seen in WT mice (14). We expected the PD-L2-knockout group to show increased permeability when compared with the WT mice. However, the slight trend upwards was not statistically significant.
We next turned our attention to differentiating the extent to which PD-L2 as opposed to PD-L1 gene deficiency altered aspects of liver dysfunction or damage. In general clinical diagnostics, bilirubin is used as a measure of liver dysfunction, whereas ALT and AST are used as a measure of liver damage (15). The latter two measurements vary in their elevation depending on the cause of hepatocellular injury. Here, we noted that although PD-L1 gene deficiency in mice appeared to bring down total blood bilirubin post-CLP when compared with the WT mice, PD-L2-knockout septic mice exhibited significantly higher levels. This supports our hypothesis that PD-L2 may contribute to reducing liver dysfunction; it also supports the narrative that PD-L1 knockout has protective effects against CLP-induced liver dysfunction (14).
To the extent that changes in liver function were a reflection of altered local liver/infected peritoneal site and systemic septic mouse inflammatory cytokine response, we noted that PD-L2 gene deficiency in mice strongly attenuated the rise of pro-/anti-inflammatory cytokines such as TNF-α, IL-10, and IL-6. This is in line with what has been reported previously for PD-L1-knockout mice (11). PD-L2-knockout mice also showed lower basal levels of chemokines such as MCP-1 and MIP-2; however, the increase of MIP-2 post-CLP was significantly lower when compared with the WT CLP group. Nevertheless, changes in the pro-/anti-inflammatory systemic and/or local hepatic milieu do not seem sufficient to explain the differential hepatic function and overall change in morbidity/mortality seen here.
Bacterial clearance in the blood (systemic), peritoneal fluid (local), and the liver was significantly improved in PD-L2-knockout mice compared with WT mice after CLP (Fig. 5). Although this increased bacterial clearance was also observed in PD-L1-knockout mice, there was only mild (not statistically significant) improvement in both systemic blood and local peritoneal bacterial clearance compared with WT mice after CLP. This latter aspect is consistent with our previous studies that showed no marked change in systemic and local bacterial burden (11). This shows that PD-L2 may have a role in the regulation of bacterial clearance during sepsis more like the septic PD-1 gene-deficient mice (12). This is consistent with the sinusoidal location of PD-L2 in the liver, suggesting that PD-L2 may influence the inflammatory and filtration functions of this complex organ (23).
Given the above, it is surprising to have found that, unlike PD-L1 gene-knockout animals, PD-L2 gene deficiency appears to produce no effect on adult murine mortality in our model of intraabdominal sepsis. The underlying reasons for this effect remain unclear, although the septic PD-L2-knockout mice appeared to also have a greatly reduced bacteremia and reduced peritoneal and systemic inflammatory mediator levels, as was the case with PD-1 gene-deficient mice (12). We can only speculate that the inability to mitigate the development of organ dysfunction here, as documented by the sustained rise in liver vascular permeability, AST, and the potentiated bilirubin levels, had a nullifying effect on the capacity of these PD-L2−/− mice to clear bacteria and/or reduce inflammatory cytokine levels. Stated another way, although reducing bacterial burden and suppressing inflammation are important aspects of protecting against the negative sequelae of polymicrobial sepsis, they may be insufficient if hepatic function and/or other organ functions are not preserved.
At the cellular level, one might conclude that the loss of some facets of LSEC function (related to either liver tissue edema and/or intracellular solute trafficking, which is directly affected by PD-L2 expression) is more critical than PD-L’s role as a simple immune tolerogen. In this respect, we have recently published that PD-L1 gene deficiency appears to have tolerogen-independent activities in epithelial cells in the intestine that appear to have an impact on CLP mouse gut permeability (25). Studies restricted to myeloid cells have suggested that unlike PD-L1, PD-L2 expression appears to also have a role in driving “alternative activation” in macrophage/dendritic cells (17, 18); whether such a process occurs in LSECs in our study is unclear. Inasmuch, although it is tempting to speculate that PD-L2 could have such distinct roles, this remains to be determined. That said, since we only looked at the impact of sepsis on the expression of PD-L2 and not its primary receptor PD-1, which we have previously reported to be altered on Kupffer cells and liver invariant natural killer T cells (9, 24, 27), we cannot rule out that reduced capacity to ligate PD-1 on these or other hepatic immune cells may not indirectly account for these changes. This also does not preclude the roles that altered PD-L2 expression/gene deficiency (and indirectly the inability to ligate PD-1 expressed there) may have played in the septic changes in other tissue bed/organ inflammatory/immune/homeostatic functions that could have contributed to the responses seen here. However, as CLP did not induce overt changes in splenic, peritoneal, intestinal, or pulmonary expression of PD-L2, we did not take these under consideration as significant cellular/tissue sights to further focus on here in trying to understand PD-L2’s role in septic morbidity/mortality.
One limitation with the systemic PD-L1 or PD-L2-knockout mouse approach we used here is, like all unconditional-knockout mice, such animals represent a predispositional state not typically encountered clinically relative to the contribution of a gene or its associated signaling pathways to the development of sepsis. Furthermore, such mice may have a variety of unanticipated compensatory conditions/responses not seen in the background control animals that should be kept in mind when considering the data (10). Although these issues might, in part, be addressed by using neutralizing antibodies and/or siRNA approaches in a temporal/posttreatment fashion, this approach by the nature of the experimental design will still not be able to tell us at what cellular level lack of PD-L1 or PD-L2 are active. To address this, we need to apply either a targeted delivery system (which is unknown to us at present) and/or use/build an inducible cell lineage-specific PD-L1 or PD-L2 gene “deleter mouse,” the components of which do not presently exist and are well outside the breath of this study.
To the extent that the basal gut microbiome of the animals we used significantly affected the outcomes reported here, we would make two points. First, relative to the comparison of WT background control animals to the two knockout strains, we bred in-house at our animal facility. It is worth noting that animals obtained from our outside vendor were acclimated no less than 7 days, and often longer (maximum ~5 wk), before using these animals in studies done here. In female mice, the time frame of 7 days has been reported to be sufficient for stabilization (though at the minimal end) of the mouse microbiota following transportation to another institution (20). Second, since both PD-L2−/− and PD-L1−/− strains were bred in-house in our animal facility/room and as we did not see any overt differences from a health/husbandry perspective, it is unlikely that basal differences in intestinal microbiota accounted for the differences we reported here in survival or otherwise. That said, we do not rule out (we would anticipate) differential/unique changes in the gastrointestinal microbiota in response to CLP, but the nature of these changes remains to be established in future studies. Nevertheless, there is much to be learned about PD-L2 (which is clearly not just a redundant homologue of PD-L1) and how it fits into the grand scheme of sepsis and innate immunity.
GRANTS
This work was funded in part by National Institute of General Medical Sciences Grants T32-GM-077995 (to M. Le) and R35-GM-118097 (to A. Ayala), an “Armand D. Versaci” Research Scholar in Surgical Sciences Fellowship (to E. A. Fallon and T. T. Chun), and a Surgical Infection Society Foundation Basic and Translational Research Fellowship Award (to E. A. Fallon).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.-L.R., M.L., and A.A. conceived and designed research; A.-L.R., M.L., Y.C., A.M., S.X., T.T.C., and C.P.E. performed experiments; A.-L.R., M.L., and C.P.E. analyzed data; A.-L.R., M.L., and C.-S.C. interpreted results of experiments; A.-L.R., M.L., and A.A. prepared figures; A.-L.R., M.L., and A.A. drafted manuscript; M.L., C.-S.C., E.A.F., and A.A. edited and revised manuscript; A.-L.R., M.L., C.-S.C., Y.C., E.A.F., A.M., S.X., T.T.C., C.P.E., and A.A. approved final version of manuscript.
REFERENCES
- 1.Ayala A, Perrin MM, Kisala JM, Ertel W, Chaudry IH. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (interleukins-1 and -6 and tumor necrosis factor). Circ Shock 36: 191–199, 1992. [PubMed] [Google Scholar]
- 2.Canabal JM, Kramer DJ. Management of sepsis in patients with liver failure. Curr Opin Crit Care 14: 189–197, 2008. doi: 10.1097/MCC.0b013e3282f6a435. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 4.Chung CS, Chen Y, Grutkoski PS, Doughty L, Ayala A. SOCS-1 is a central mediator of steroid-increased thymocyte apoptosis and decreased survival following sepsis. Apoptosis 12: 1143–1153, 2007. doi: 10.1007/s10495-007-0059-7. [DOI] [PubMed] [Google Scholar]
- 5.de Kleijn S, Langereis JD, Leentjens J, Kox M, Netea MG, Koenderman L, Ferwerda G, Pickkers P, Hermans PW. IFN-γ-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS One 8: e72249, 2013. doi: 10.1371/journal.pone.0072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K; International Forum of Acute Care Trialists . Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 193: 259–272, 2016. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 7.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 41: 1167–1174, 2013. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 8.Hebert PC, Drummond AJ, Singer J, Bernard GR, Russell JA. A simple multiple system organ failure scoring system predicts mortality of patients who have sepsis syndrome. Chest 104: 230–235, 1993. doi: 10.1378/chest.104.1.230. [DOI] [PubMed] [Google Scholar]
- 9.Heffernan DS, Monaghan SF, Thakkar RK, Tran ML, Chung CS, Gregory SH, Cioffi WG, Ayala A. Inflammatory mechanisms in sepsis: elevated invariant natural killer T-cell numbers in mouse and their modulatory effect on macrophage function. Shock 40: 122–128, 2013. doi: 10.1097/SHK.0b013e31829ca519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holschneider DP, Shih JC. Genotype to phenotype: challenges and opportunities. Int J Dev Neurosci 18: 615–618, 2000. doi: 10.1016/S0736-5748(00)00026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Chen Y, Chung CS, Yuan Z, Monaghan SF, Wang F, Ayala A. Identification of B7-H1 as a novel mediator of the innate immune/proinflammatory response as well as a possible myeloid cell prognostic biomarker in sepsis. J Immunol 192: 1091–1099, 2014. doi: 10.4049/jimmunol.1302252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA 106: 6303–6308, 2009. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchins NA, Chung CS, Borgerding JN, Ayala CA, Ayala A. Kupffer cells protect liver sinusoidal endothelial cells from Fas-dependent apoptosis in sepsis by down-regulating gp130. Am J Pathol 182: 742–754, 2013. doi: 10.1016/j.ajpath.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchins NA, Wang F, Wang Y, Chung CS, Ayala A. Kupffer cells potentiate liver sinusoidal endothelial cell injury in sepsis by ligating programmed cell death ligand-1. J Leukoc Biol 94: 963–970, 2013. doi: 10.1189/jlb.0113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician 59: 2223–2230, 1999. [PubMed] [Google Scholar]
- 16.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2: 261–268, 2001. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 17.Mayuzumi N, Matsushima H, Takashima A. IL-33 promotes DC development in BM culture by triggering GM-CSF production. Eur J Immunol 39: 3331–3342, 2009. doi: 10.1002/eji.200939472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina-Colorado AA, Osorio EY, Saldarriaga OA, Travi BL, Kong F, Spratt H, Soong L, Melby PC. Splenic CD4+ T cells in progressive visceral leishmaniasis show a mixed effector-regulatory phenotype and impair macrophage effector function through inhibitory receptor expression. PLoS One 12: e0169496, 2017. doi: 10.1371/journal.pone.0169496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N, Mayadas TN, Lan HY, Sharpe AH, Kelley VR. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J Immunol 179: 7466–7477, 2007. doi: 10.4049/jimmunol.179.11.7466. [DOI] [PubMed] [Google Scholar]
- 20.Montonye DR, Ericsson AC, Busi SB, Lutz C, Wardwell K, Franklin CL. Acclimation and institutionalization of the mouse microbiota following transportation. Front Microbiol 9: 1085, 2018. doi: 10.3389/fmicb.2018.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp 73: e50062, 2013. doi: 10.3791/50062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8: 239–245, 2007. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 23.Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol 14: 55–66, 2017. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Huang X, Chung CS, Chen Y, Hutchins NA, Ayala A. Contribution of programmed cell death receptor (PD)-1 to Kupffer cell dysfunction in murine polymicrobial sepsis. Am J Physiol Gastrointest Liver Physiol 311: G237–G245, 2016. doi: 10.1152/ajpgi.00371.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Chung CS, Chen Y, Monaghan SF, Patel S, Huang X, Heffernan DS, Ayala A. A novel role for programmed cell death receptor ligand-1 (PD-L1) in sepsis-induced intestinal dysfunction. Mol Med 22: 830–840, 2017. doi: 10.2119/molmed.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Chen PW, Li H, Alizadeh H, Niederkorn JY. PD-L1: PD-1 interaction contributes to the functional suppression of T-cell responses to human uveal melanoma cells in vitro. Invest Ophthalmol Vis Sci 49: 2518–2525, 2008. doi: 10.1167/iovs.07-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young JS, Heffernan DS, Chung CS, Kettenmann ML, Young WA, Guillen VS, Cioffi WG, Ayala A. Effect of PD-1: PD-L1 in invariant natural killer T cell emigration and chemotaxis following sepsis. Shock 45: 534–539, 2016. doi: 10.1097/SHK.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 7: 128–139, 2010. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care 14: R220, 2010. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]