Abstract
Precision-cut liver tissue slice (PCLS) contains all major cell types of the liver parenchyma and preserves the original cell-cell and cell-matrix contacts. It represents a promising ex vivo model to study liver fibrosis and test the antifibrotic effect of experimental compounds in a physiological environment. In this study using RNA sequencing, we demonstrated that various pathways functionally related to fibrotic mechanisms were dysregulated in PCLSs derived from rats subjected to bile duct ligation. The activin receptor-like kinase-5 (Alk5) inhibitor SB525334, nintedanib, and sorafenib each reversed a subset of genes dysregulated in fibrotic PCLSs, and of those genes we identified 608 genes whose expression was reversed by all three compounds. These genes define a molecular signature characterizing many aspects of liver fibrosis pathology and its attenuation in the model. A panel of 12 genes and 4 secreted biomarkers including procollagen I, hyaluronic acid (HA), insulin-like growth factor binding protein 5 (IGFBP5), and WNT1-inducible signaling pathway protein 1 (WISP1) were further validated as efficacy end points for the evaluation of antifibrotic activity of experimental compounds. Finally, we showed that blockade of αV-integrins with a small molecule inhibitor attenuated the fibrotic phenotype in the model. Overall, our results suggest that the rat fibrotic PCLS model may represent a valuable system for target validation and determining the efficacy of experimental compounds.
NEW & NOTEWORTHY We investigated the antifibrotic activity of three compounds, the activin receptor-like kinase-5 (Alk5) inhibitor SB525334, nintedanib, and sorafenib, in a rat fibrotic precision-cut liver tissue slice model using RNA sequencing analysis. A panel of 12 genes and 4 secreted biomarkers including procollagen I, hyaluronic acid (HA), insulin-like growth factor binding protein 5 (IGFBP5), and WNT1-inducible signaling pathway protein 1 (WISP1) were then established as efficacy end points to validate the antifibrotic activity of the αV-integrin inhibitor CWHM12. This study demonstrated the value of the rat fibrotic PCLS model for the evaluation of antifibrotic drugs.
Keywords: bile duct ligation, fibrosis, precision-cut liver tissue slice
INTRODUCTION
Chronic liver injury due to various etiologies, such as chronic hepatitis virus infections, excessive alcohol consumption, and nonalcoholic steatohepatitis, leads to the development of liver fibrosis (10). Progressive liver fibrosis may ultimately lead to cirrhosis characterized by disrupted hepatic architecture, vascular structure, and aberrant regeneration (39). Liver fibrosis and in particular cirrhosis are among the leading causes of morbidity and mortality worldwide (32). Although tremendous progress has been made in our understanding of the pathogenic mechanisms of liver fibrosis, effective antifibrotic therapies for liver fibrosis are still lacking. One challenge in the development of effective therapies is the lack of in vitro models that may better predict the translation of preclinical findings to human disease. In recent years, several research groups reported the use of precision-cut liver tissue slice (PCLS) as an ex vivo model to study liver fibrosis. For example, healthy PCLSs were exposed to various fibrogenic chemicals including ethanol (38), bile acids (4), CCL4 (46, 47), and thioacetamide (12) to induce fibrotic responses. Sadasivan et al. (37) developed a PCLS fibrosis model using a cocktail of transforming growth factor-β (TGF-β), platelet-derived growth factor, lysophosphatidic acid, sphingosine 1 phosphate, lipopolysaccharide, and palmitate. More importantly, Veidal et al. (48) and Westra et al. (49) investigated antifibrotic activity of various compounds on the fibrotic PCLSs derived from rats that were administered with CCL4 or subjected to bile duct ligation (BDL). PCLS fibrosis models allow the study of liver fibrogenesis in a multicellular system with complex tissue-like structures in which cell-cell and cell-matrix interactions are maintained. It represents a promising ex vivo model to study liver fibrosis and to evaluate the efficacy of experimental compounds in a physiological environment.
While PCLS fibrosis models are being increasingly used, to our knowledge, global gene expression analysis of the fibrotic PCLS in response to antifibrotic compounds has not been reported. In this study, we used RNA Sequencing (RNA-seq) to investigate the antifibrotic activity of three compounds, SB525334, nintedanib, and sorafenib, in the fibrotic PCLSs derived from rats subjected to BDL. BDL causes cholestatic injury and periportal biliary fibrosis. The BDL model is one of the most commonly used in vivo models of experimental fibrosis to test concepts and exploratory treatments due to its robust fibrotic phenotype (19). SB525334, nintedanib, and sorafenib have been demonstrated to attenuate fibrosis in multiple preclinical fibrosis models including models of liver fibrosis (7, 14, 15, 31, 45). SB525334 is an activin receptor-like kinase-5 (Alk5) inhibitor, and both nintedanib and sorafenib are multikinase inhibitors that are also being used in the clinical practice for the treatment lung fibrosis and of various cancers including liver cancer. A gene expression signature including 608 genes that were dysregulated in fibrotic PCLSs and inversely modulated by all three antifibrotic compounds was identified. A panel of 12 genes and 4 secreted factors including procollagen I, hyaluronic acid (HA), insulin-like growth factor binding protein 5 (IGFBP5), and WNT1 inducible signaling pathway protein 1 (WISP1) were selected as efficacy end points to validate the antifibrotic activity of a small molecule inhibitor of αV-integrins. Overall, our study provides a detailed transcriptomic characterization of the BDL-induced rat fibrotic PCLS model and demonstrates its value for the testing of antifibrotic drugs.
METHODS
PCLS preparation and culture.
All animal procedures were performed in adherence with the National Institute of Health guidelines on the use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Bristol Myers Squibb. Male Sprague-Dawley rats weighing between 250 and 300 g were purchased from Charles River Laboratories. They were housed in a humidity- and temperature-controlled room on a 12-h:12-h light-dark cycle with free access to chow and water. To perform BDL, rats were anesthetized with isoflurane and a midline laparotomy was performed. The common bile duct was then ligated twice with 4.0 silk. Sham-operated rats were subjected to laparotomy without BDL. Four weeks after surgery, the livers were excised. The livers were either used to make PCLS or snap frozen in liquid nitrogen for RNA extractions. To make PCLS, liver tissue cores with a diameter of 8 mm were prepared from liver tissues using tissue corning press, which were then loaded into Alabama R&D tissue slicer (Alabama Research & Development, Munford, AL) to make PCLSs at a thickness of 220 μm. The PCLSs were then placed on stainless-steel roller inserts and loaded into glass vials containing 2.5 ml of Williams’ Media E with glutaMAX (Life Technologies, Carlsbad, CA) supplemented with 50 U penicillin-streptomycin. The vials containing the PCLS were incubated in the dynamic organ culture incubator (Vitron, Tucson, AZ) gassed with carbogen (95% O2-5% CO2) with gentle rotation at 37°C for 48 h. For each ex vivo study, three sham or BDL-operated rats were used to make PCLSs. PCLSs from the same surgical procedure were pooled and randomized to treatment groups. To determine the antifibrotic effect of compounds, PCLSs were treated with vehicle DMSO, the Alk5 inhibitor SB525334 (1.0 μM), nintedanib (1.0 μM), sorafenib (1.0 μM) (Selleckchem, Houston, TX), or CWHM12 (1.0 and 3.0 μM) (synthesized at Bristol Myers Squibb). All compounds were dissolved in DMSO. After a 48-h incubation, PCLSs were snap frozen and the conditioned medium was collected for future use.
Real-time quantitative PCR.
Total RNA was prepared from PCLSs or liver using the RNeasy 96 kit (Qiagen) according to the manufacturer's instructions. cDNA synthesis was performed with high-capacity RNA to cDNA kit (Life Technologies, Carlsbad, CA). Real-time PCR was performed using the ViiA 7 (Life Technologies).
Gene expression profiling and bioinformatics analysis.
RNA-seq was used to determine gene expression. Sequencing libraries were generated using the Illumina TruSeq Stranded Total RNA Library Prep Kit with Ribo-Zero (Illumina, San Diego, CA) following the manufacturer’s instruction. RNA-Seq libraries were run on an Illumina HiSeq 2500 platform producing 75-bp paired-end reads. We generated on average 40 million PE read for each sample. Reads were aligned to the Rattus norvegicus Rnor_6.0 reference genome (National Center for Biotechnology Information Assembly Accession No. GCF_000001895.5) (11) with the Omicsoft Sequence Aligner (16). Gene and transcript abundance was determined with Ensembl R89 rat gene models (50) using RSEM (24). All RNA-Seq data were processed in R with Bioconductor packages (18). RNA-Seq samples were TMM normalized (36) with the edgeR package (26, 35). Outlier detection was performed using t-distributed Stochastic Neighbor Embedding (t-SNE). All the gene expression data were deposited in National Center for Biotechnology Information Gene Expression Omnibus Accession No. GSE120804. Differential gene expression contrasts between treatment groups were performed using the limma package (34). Pathway enrichment was computed using MetaBase (Clarivate Analytics) version 6.34.69200.
ELISA.
Secreted biomarkers including procollagen I, HA, IGFBP5, and WISP1 in the conditioned medium were measured using procollagen I (Ab210579; Abcam, Cambridge, UK), hyaluronan quantikine (DHYAL0; R&D Systems, Minneapolis, MN), IGFBP5 (Ab208345; Abcam), and WISP1 quantikine (MWSP10; R&D Systems) ELISA kits following manufacturer's instructions.
Statistical analysis.
The results (means ± SE) were subjected to statistical analysis by ANOVA using Graphpad Prism software. P < 0.05 determined statistical significance.
RESULTS
Antifibrotic compounds suppressed the fibrotic phenotype in the rat PCLSs.
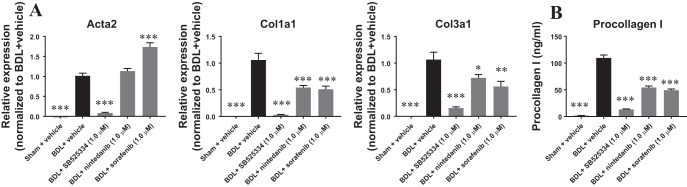
PCLSs from sham or BDL rats were prepared and treated with vehicle or 1.0 μM of various compounds for 48 h. Compound treatments did not affect viability of PCLSs as shown by comparable ATP contents in the PCLSs with or without compound treatments (data not shown). Compared with PCLSs derived from sham rats, the expression of the fibrotic markers Col1a1 and Col3a1 was significantly elevated in the vehicle-treated fibrotic PCLSs. Similarly, expression of Acta2, a key marker of fibroblast to myofibroblast transdifferentiation, was also upregulated (Fig. 1A). All three compounds, SB525334, nintedanib, and sorafenib, demonstrated antifibrotic effects as shown by the suppression of Col1a1 and Col3a1 gene expression (Fig. 1A). However, only SB525334 was able to abrogate upregulation of Acta2. We next investigated the effect of these compounds on procollagen I protein levels in the conditioned medium. Consistent with the gene expression results, procollagen I protein levels in the conditioned medium were significantly increased in fibrotic PCLSs and treatment with all three compounds decreased its levels (Fig. 1B). Together, these results demonstrated that SB525334, nintedanib, and sorafenib attenuated the fibrotic phenotype in the rat fibrotic PCLS model.
Fig. 1.
Antifibrotic compounds attenuated the fibrotic phenotype in rat fibrotic precision-cut liver tissue slices (PCLSs) after 48 h of incubation. SB525334, nintedanib, and sorafenib suppressed fibrotic gene expression in rat fibrotic PCLSs (A) and procollagen I secretion in the conditioned medium (B). Data are means ± SE; n = 3 rats for both sham and bile duct ligation (BDL); n = 8–10 liver slices for each condition. *P < 0.05, **P < 0.01, ***P < 0.001.
Transcriptome analysis.
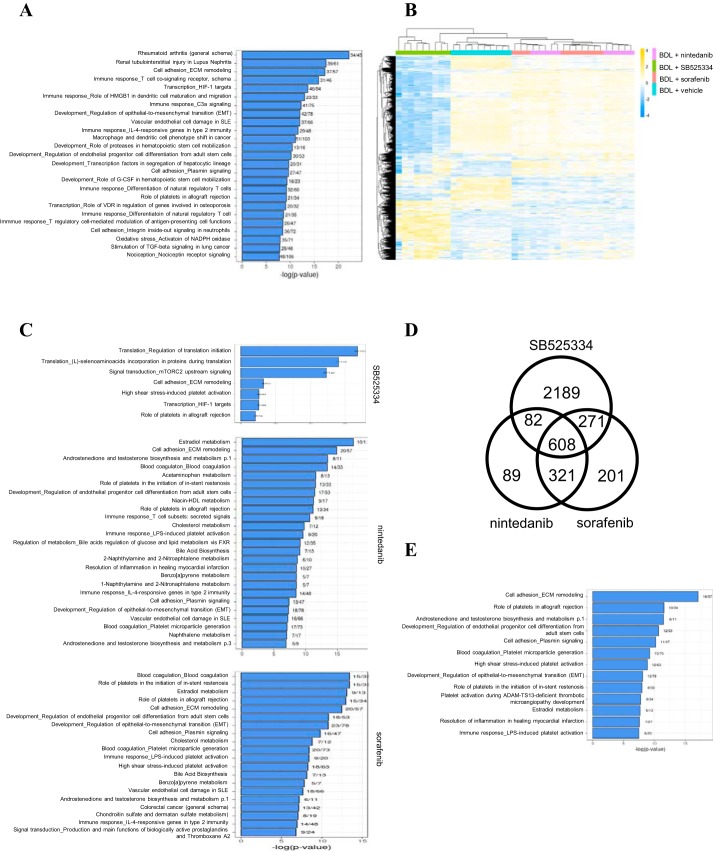
Having shown the antifibrotic effect of these compounds, we then performed RNA-seq analysis to evaluate the global gene expression changes in fibrotic PCLSs with or without compound treatments. Compared with sham PCLSs, 3,788 genes were differentially expressed in fibrotic PCLSs derived from BDL rats (absolute fold change > 3.0, false discovery rate < 0.05). Pathway analysis indicated that genes involved in profibrotic mechanisms including cell adhesion, extracellular matrix (ECM) remodeling, various immune responses, epithelial-to-mesenchymal (EMT) transition, oxidative stress, and stimulation of TGF-β signaling were extensively dysregulated in fibrotic PCLSs (Fig. 2A). Over 400 genes encoding matrisome proteins including 130 core matrisome and 280 matrisome-associated proteins according to the definition by MatrisomeDB 2.0 (28) were dysregulated in fibrotic PCLSs, 379 of which were upregulated and 31 were downregulated (Supplemental Table S1; Supplemental Material for this article is available online at the Journal website). One-hundred twenty-six of 130 core matrisome genes were upregulated in fibrotic PCLSs, which reflected increased synthesis of structural ECM proteins under the fibrotic condition. Both innate and adaptive immune mechanisms contribute to fibrogenesis (25). Accordingly, genes encoding various cytokines and chemokines that are known to promote fibrogenesis including Il-1β, Il-6, Il-10, Il-13, Il-17, Ccl2, Ccl17, and Tnf were found upregulated in fibrotic PCLSs. EMT, a process during which epithelial cells gradually transform into mesenchymal-like cells and lose their epithelial functionality and characteristics, is thought to play an important role in the development of fibrosis (22). In agreement with the results of the pathway analysis, the expression of many EMT-associated genes including Fn1, Snail1, Snail2, Twist1, Vim, Zeb2, as well as many members of Wnt family were upregulated in fibrotic PCLSs. Oxidative stress is implicated as an important molecular mechanism underlying fibrogenesis (27). Consistently, genes encoding NADPH oxidases including Cybb, Duox1, Duox2, and Nox4 and their regulatory subunits including Cyba, Ncf1, Ncf4, Noxa1, Noxo1, and Rac2 were significantly elevated in fibrotic PCLSs, which strongly suggested increased production of reactive oxygen species under the fibrotic condition. TGF-β is a central mediator that drives fibrosis by regulating important biological processes such as cellular proliferation, ECM production, and EMT (3). Genes encoding three isoforms of the TGF-β family including TGFβ1, 2, and 3, and their receptor TGFBR1 as well as downstream targets of TGF-β signaling including Fn1, Serpine1, Snail, and Vim were significantly elevated in fibrotic PCLSs. This was consistent with the induction of TGF-β signaling in fibrotic PCLSs.
Fig. 2.
Transcriptomic analysis of rat fibrotic precision-cut liver tissue slices (PCLSs) after 48 h of incubation with antifibrotic compounds. A: pathway analysis of differentially expressed genes between sham and fibrotic PCLSs. B: heat map analysis shows that each compound reversed a subset of genes dysregulated in fibrotic PCLSs. A total of 3,761 genes were reversed by at least 1 compound, and they were hierarchically clustered both by genes and by samples. Yellow indicates upregulated, and blue indicates downregulated genes. C: pathway analysis of genes that were dysregulated in fibrotic PCLSs and inversely modulated by SB525334, nintedanib, and sorafenib, respectively. D: Venn diagram showing numbers of genes that were dysregulated in fibrotic PCLSs and reversely regulated by each compound. E: pathway analysis of the 608 genes that were reversely regulated by all 3 compounds.
To understand the molecular mechanism of how these compounds attenuated the fibrotic phenotype, we focused on genes whose expression was dysregulated in fibrotic PCLSs and inversely modulated by SB525334, nintedanib, or sorafenib (fold-change > 1.5 or < −1.5, false discovery rate < 0.05). With the use of these criteria, over 8,000 genes were found to be dysregulated in fibrotic PCLSs and 3,150, 1,100, and 1,401 of those were inversely modulated by SB525334, nintedanib, or sorafenib, respectively. Overall, a total of 3,761 genes were inversely modulated by at least one of these compounds. Hierarchical clustering of these genes clearly illustrated the difference in the subset of genes reversed by each compound (Fig. 2B). Nintedanib- and sorafenib-treated groups were clustered together indicating their more similar mechanism of action when compared with SB525334. Gene pathway analysis revealed that these inversely modulated genes were highly enriched in pathways related to cell adhesion, ECM remodeling, immune responses, and EMT, which highlighted the antifibrotic effect of these compounds (Fig. 2C). The Venn diagram analysis further depicts numbers of common and differential reversely modulated genes in response to drug treatments (Fig. 2D). Of the 3,761 genes, 2,189, 89, and 201 genes were uniquely reversely-modulated by SB525334, nintedanib, and sorafenib. A total of 608 genes were found to be reversely modulated by all three drugs. Of these 608 genes, 433 were upregulated in fibrotic PCLSs and downregulated with compound treatments, while 175 were downregulated in fibrotic PCLSs and upregulated with compound treatments. The list of genes reversed by all three compounds is shown in Supplemental Table S2. Many of these 608 genes were functionally associated with ECM homeostasis and/or fibrogenesis. Indeed, 106 of these 608 genes encode matrisome proteins and the majority of them (94 out of 106) were upregulated in fibrotic PCLSs and suppressed by the test compounds. The matrisome genes identified include 38 core matrisome genes and 68 matrisome-associated genes. Further pathway analysis of these 608 genes highlighted pathways related to cell adhesion, ECM remodeling, platelet regulations, EMT, and resolution of inflammation and were commonly regulated by these compounds (Fig. 2E). Overall, our transcriptomic analysis showed that genes functionally related to fibrotic pathways were dysregulated in fibrotic PCLSs and they could at least be partially reversed by antifibrotic compounds. In addition, a set of 608 genes that were dysregulated in fibrotic PCLSs and inversely modulated by all 3 antifibrotic compounds were identified. They represent a gene signature that is associated with the pathology of liver fibrosis and whose expression can be reversed upon liver fibrosis resolution.
Establishment of end points for evaluation of antifibrotic compounds.
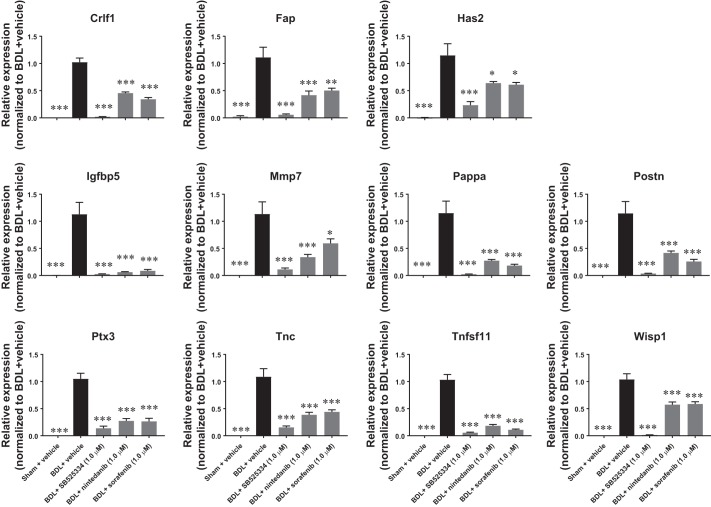
To further use the BDL-induced rat fibrotic PCLS model as a preclinical model for the evaluation of antifibrotic experimental compounds, it is important to establish robust and sensitive end points with translational relevance. We therefore focused on the top 60 genes that were most strongly upregulated in the fibrotic PCLSs and were also downregulated by all three compounds tested. Of the top 60 genes, 15 had been reported to be dysregulated and/or functionally involved in liver fibrosis. Eleven of them including cytokine receptor like factor 1 (Crlf1), fibroblast activation protein alpha (Fap), hyaluronan synthase 2 (Has2), Igfbp5, matrix metallopeptidase 7 (Mmp7), Pappalysin 1 (Pappa), periostin (Postn), pentraxin 3 (Ptx3), TNF superfamily member 11 (Tnfsf11), tenascin C (Tnc), and Wisp1 were selected for further validation. Consistent with the RNA-seq results, real-time PCR analysis shows that all of the 11 genes were strongly upregulated in fibrotic PCLSs and suppressed by the compounds in the same set of samples that had been previously used for RNA-seq study (Fig. 3).
Fig. 3.
Validation of top reversely expressed genes. SB525334, nintedanib, and sorafenib suppressed fibrotic gene expression elevated in rat fibrotic precision-cut liver tissue slices (PCLSs) after 48 h of incubation. Data are means ± SE; n = 3 rats for both sham and bile duct ligation (BDL); n = 8–10 liver slices for each condition. *P < 0.05, **P < 0.01, ***P < 0.001.
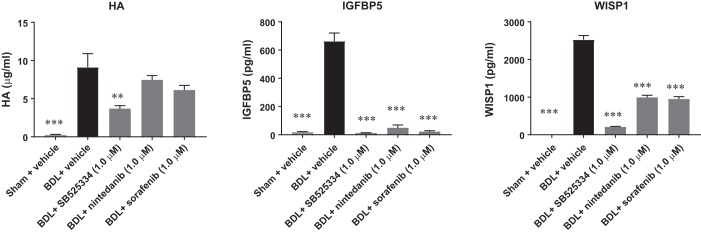
We next selected three of the secreted factors including HA, IGFBP5, and WISP1 and assessed the effect of the three test compounds on their secretion in the conditioned medium. Consistent with the gene expression analysis results, levels of HA, IGFBP5, and WISP1 in the conditioned medium were significantly increased in fibrotic PCLSs and decreased upon compound treatments (Fig. 4). These 11 genes together with Col1a1 as well as 4 secreted biomarkers, procollagen I, HA, TGF-β1, and WISP1 were then used as efficacy end points for the assessment of the antifibrotic activity of experimental compounds.
Fig. 4.
ELISA analysis of secreted biomarkers. SB525334, nintedanib, and sorafenib suppressed secretion of hyaluronic acid (HA), insulin-like growth factor binding protein 5 (IGFBP5), and WNT1-inducible signaling pathway protein 1 (WISP1) in the conditioned medium after 48 h of incubation. Data are means ± SE; n = 3 rats for both sham and bile duct ligation (BDL); n = 8–10 liver slices for each condition. **P < 0.01 and ***P < 0.001.
Blockade of αV integrins suppressed TGF-β1-induced fibrotic phenotype in the rat PCLS model.
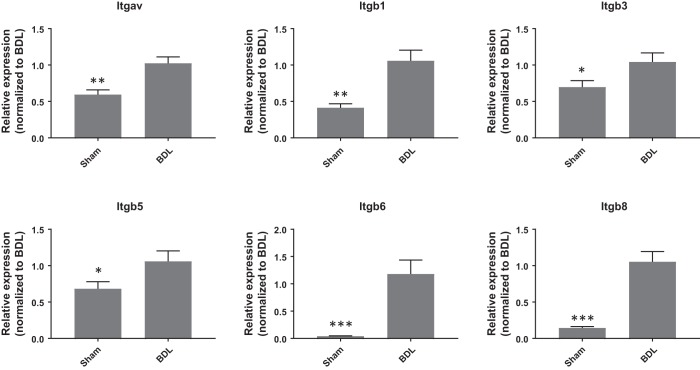
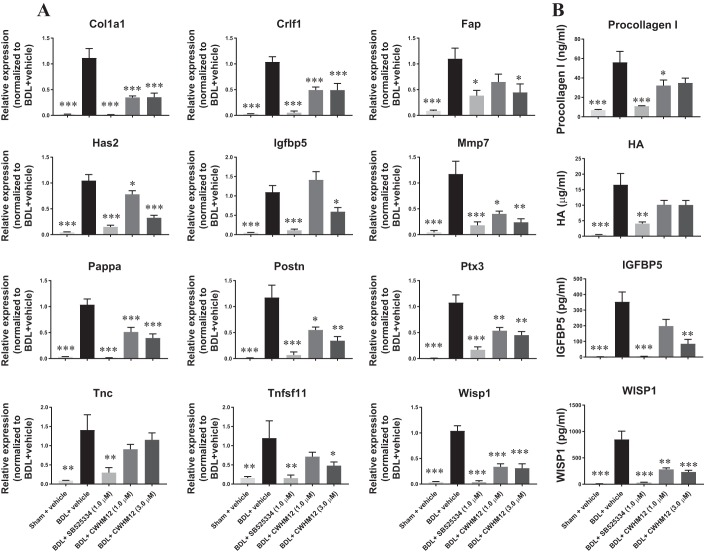
Integrins are a large family of transmembrane heterodimeric receptors that mediate bidirectional signaling through the cell membrane. The αV-subunit forms heterodimers with the β1-, β3-, β5-, β6-, or β8-subunit. αV-Subunit-containing integrins appear to be central mediators of fibrosis, and the modulation of various αV-subunit-containing integrins was found to have profound effects on fibrosis in multiple organs in preclinical models (5, 40). We first quantified αV-, β1-, β3-, β5-, β6-, and β8-subunit transcripts in BDL-induced liver fibrosis model. Compared with sham control, expression of Itgav, Itgb1, Itgb3, Itgb5, Itgb6, and Itgb8 was significantly elevated in the fibrotic livers 4 wk after BDL surgery (Fig. 5). We therefore sought to investigate the effects of targeting αV-subunit-containing integrins using the rat fibrotic PCLS fibrosis model with a small molecule αV-integrin inhibitor CWHM12 (13) using the end points established earlier. As shown in Fig. 6A, CWHM12 significantly suppressed 11 of the 12 genes upregulated in fibrotic PCLSs, namely, Col1a1, Crlf1, Fap, Has2, Igfbp5, Mmp7, Pappa, Postn, Ptx3, Tnfsf11, and Wisp1. In addition, CWHM12 significantly decreased levels of procollagen I, IGFBP5, and WISP1 while modestly decreased levels of HA in the conditioned medium when compared with the vehicle-treated group (Fig. 6B). These results indicate that inhibition of αV-integrins attenuated the fibrotic phenotype in the BDL-induced rat fibrotic PCLS model and provide further evidence that αV-integrins may represent a promising target for the treatment of liver fibrosis.
Fig. 5.
Upregulation of integrin genes expression in the fibrotic livers of rats subjected to bile duct ligation (BDL) for 4 wk. Data are means ± SE; n = 7 for both sham and BDL; *P < 0.05, **P < 0.01 ***P < 0.001.
Fig. 6.
The small molecule αV integrins inhibitor CWHM12 attenuated fibrotic phenotype in fibrotic precision-cut liver tissue slices (PCLSs) after 48 h of incubation. A: CWHM12 suppressed expression of a panel of fibrotic genes. B: CWHM12 decreased secretion of procollagen I, hyaluronic acid (HA), insulin-like growth factor binding protein 5 (IGFBP5), and WNT1-inducible signaling pathway protein 1 (WISP1) in the conditioned medium. Data are means ± SE; n = 3 rats for both sham and bile duct ligation (BDL); n = 6–8 liver slices for each condition. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
The translation of novel antiliver fibrosis therapies from preclinical models into clinical use remains a major challenge. Rodent liver fibrosis models have been invaluable to study the mechanism of liver fibrogenesis and remain the gold standard for the evaluation of preclinical drug candidates. Recently, the PCLS model has emerged as a promising tool to study liver fibrosis and test the efficacy of putative antifibrotic compounds. Compared with the in vivo rodent models, the PCLS model requires much fewer animals and it has the capacity to test a larger number of experimental compounds in a shorter period of time. On the other hand, compared with the typically used in vitro models of monoculture or occasionally coculture systems of hepatic stellate cell, the PCLS model represents a more physiologically relevant system. Particuarly, PCLSs generated from fibrotic livers derived from rodent models or patients allow the testing of the effects of compounds in a disease-like pathological environment, which increases the translational relevance of the PCLS fibrosis model.
In this study, we used fibrotic PCLSs derived from rats that had been subjected to BDL to determine antifibrotic activity of SB525334, nintedanib, or sorafenib. SB525334 is a small molecule inhibitor of TGF-β type I receptor ALK5. TGF-β is a master profibrotic cytokine and plays extensive roles in fibrotic diseases including liver fibrosis. This signaling pathway involves TGF-β binding to the TGF-β type II receptor, which recruits the TGF-β type I receptor ALK5, resulting in the activation of downstream signaling via both canonical and noncanonical signaling pathways (1). Therefore, inhibition of the TGF-β signaling pathway could be a very effective strategy for controlling liver fibrosis. Indeed, the ALK5 inhibitor GW6604 was shown to protect rats from dimethylnitrosamine-induced liver fibrosis (6). Nintedanib is approved for the treatment of idiopathic pulmonary fibrosis, and sorafenib is an approved drug for the treatment of multiple cancers. Nintedanib and sorafenib are both tyrosine kinase inhibitors with overlapping but different kinase selectivity profiles, and they both demonstrated antifibrotic activity in multiple preclinical liver fibrosis models (7, 15, 31, 45). A global gene expression analysis enabled our study to reveal that each compound inversely modulated a unique subset of genes perturbed under fibrotic condition. Many of those are functionally associated with cell adhesion, ECM remodeling, immune responses, and EMT. Interestingly, 2,189, 89, and 201 genes were uniquely reversely modulated by SB525334, nintedanib, and sorafenib, respectively. These results not only illustrated the antifibrotic function of these compounds but also reflected on their different mechanism of action. However, it needs to be emphasized that since only a single concentration of drug was studied in the current study, it is highly likely that some of the differences observed may be due to differences in the efficacy at the concentrations tested. Importantly, a panel of 608 genes that were modulated by all 3 compounds in a similar manner were also identified. They may define a gene signature that can be used to characterize the pathogenesis of liver fibrosis and whose expression is expected to be modulated by efficacious antifibrotic compounds in this model.
To further use the BDL-induced fibrotic PCLS model as an efficacy model, it is necessary to establish robust and sensitive end points that are able to effectively predict the antifibrotic potential of experimental compounds and that also have translational relevance. We validated a panel of 12 genes that were dysregulated in fibrotic PCLSs and were inversely modulated by all three antifibrotic compounds. This panel of genes includes genes encoding matrisome proteins (Col1a1, Crlf1, Igfbp5, Mmp7, Pappa, Postn, Ptx3, Tnc, Tnfsf11, and Wisp1), markers of myofibroblast (Fap and Pappa), and HA synthase (Has2). Type 1 collagen is the major fibrillar collagen deposited in the ECM and increased production of interstitial collagen I is a hallmark of all fibrotic diseases (33). Crlf1 encodes a member of the cytokine type I receptor family. Expression of CRLF1 was undetectable in quiescent hepatic stellate cells (HSCs) and was highly upregulated in activated HSCs (44). FAP is a cell surface-associated serine protease, and it is a selective marker for HSC in cirrhotic patient’s liver tissues undergoing remodeling (23). Has2 encodes one of the HA synthases that synthesize HA, a major component of the ECM that plays an important role in the pathobiology of liver fibrosis (2). IGFBP5 is strongly induced upon activation of HSCs and their transdifferentiation into myofibroblasts. IGFBP5 was shown to enhance survival of LX2 human HSCs (43). MMP7 is a member of matrix metalloproteinases protein family, and it was identified as a novel serum biomarker of advanced liver fibrosis (20). Pappa encodes a secreted metalloproteinase, which cleaves IGFBPs and it was identified as one of the biomarkers for myofibroblasts associated with human hepatocellular carcinoma (42). Postn encodes a secreted extracellular matrix protein, and its expression was significantly upregulated in preclinical rodent liver fibrosis models. Postn deficiency significantly attenuated liver fibrosis in mice (17). Plasma PTX3, a member of the pentraxin protein family, may be used as a novel marker for patients with liver cirrosis (29). TNFSF11 encodes a member of the TNF cytokine family and serum TNFSF11 levels were significantly elevated in patient with cirrhosis (9). TNC is an extracellular matrix protein. Deficiency of TNC attenuated liver fibrosis in immune-mediated chronic hepatitis in mice (8). Wisp1, a downstream signaling target of WNT1, belongs to the CCN family of matricellular proteins and functions as an extracellular signaling modulator and may play a critical role in liver fibrosis (21). Importantly, as all of these genes and/or gene products had been reported to be dysregulated in preclinical fibrotic models and/or in fibrotic tissues/serum derived from patients, they represent relevant end points directly related to liver fibrosis in vivo. In addition to the gene panel, the four secreted biomarkers including procollagen 1, HA, IGFBP5, and WISP1 were also included as end points. Fragments of collagen I are used as serum biomarkers for fibrotic diseases both preclinically and in patients (33). HA is a high molecular weight polysaccharide and plays an important structural role in the formation of ECM. Furthermore, HA contributes to fibrosis by effecting fibroblast motility, fibroblast proliferation, and fibroblast-to-myofibroblast differentiation (2). Serum HA is extensively used as a noninvasive biomarker to assess the presence of fibrosis especially in the liver (30). Although there has been no reports of using either IGFBP5 or WISP1 as liver fibrosis biomarkers, both have been used as biomarkers for other diseases including renal fibrosis (41, 51).
The potential of this panel of genes in combination with the four soluble biomarkers as efficacy end points to predict antifibrotic activity of experimental compounds was assessed by testing the small molecule αV-integrin inhibitor CWHM12. CWHM12 demonstrated high potency against all of the five αV-subunit-containing integrins and it attenuated the progression of fibrosis in mouse models of liver and lung fibrosis (13). Consistent with these data, our results showed that CWHM12 suppressed the expression of 11 of the 12 genes included in the panel. In addition, CWHM12 decreased secretion of three of the four soluble biomarkers (procollagen I, IGFBP5, and WISP1) in the conditioned medium. These results clearly demonstrate the antifibrotic activity of CWHM12 in the BDL-induced fibrotic PCLS model and suggest that these end points may be predictive of the efficacy of experimental compounds. Undoubtedly, to further validate these end points as efficacy readouts, it will be necessary to test a variety of additional compounds with a wide range of mechanisms of action. It will also be interesting to test whether these end points are valid in the in vivo rat BDL model and in human, e.g., using PCLSs derived from patients with fibrotic liver diseases.
In summary, we developed a BDL-induced rat fibrotic PCLS model and demonstrated the antifibrotic activity of three test compounds in this system. We performed a detailed RNA-seq analysis of the fibrotic phenotype and its reversion by three antifibrotic compounds. A panel of 12 genes and 4 secreted biomarkers was established and used as the efficacy end point to validate the antifibrotic effect of the small molecule αV-integrin inhibitor CWHM12. Altogether, this study suggests that the BDL-induced rat fibrotic PCLS model may provide a valuable tool to evaluate potential novel antifibrotic drugs for the treatment of liver fibrosis.
GRANTS
This work was funded by Bristol-Myers Squibb.
DISCLOSURES
All authors are employees of Bristol-Myers Squibb.
AUTHOR CONTRIBUTIONS
X.H. and G.J. conceived and designed research; X.H., H.C., Y.Z., and Y.W. performed experiments; X.H., H.C., R.A., K.R., and J.T. analyzed data; X.H. and G.J. interpreted results of experiments; X.H. prepared figures; X.H. drafted manuscript; X.H. and G.J. edited and revised manuscript; X.H. and G.J. approved final version of manuscript.
Supplemental Data
REFEERENCES
- 1.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 11: 790–811, 2012. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albeiroti S, Soroosh A, de la Motte CA. Hyaluronan’s role in fibrosis: a pathogenic factor or a passive player? BioMed Res Int 2015: 790203, 2015. doi: 10.1155/2015/790203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors 29: 196–202, 2011. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clouzeau-Girard H, Guyot C, Combe C, Moronvalle-Halley V, Housset C, Lamireau T, Rosenbaum J, Desmoulière A. Effects of bile acids on biliary epithelial cell proliferation and portal fibroblast activation using rat liver slices. Lab Invest 86: 275–285, 2006. doi: 10.1038/labinvest.3700386. [DOI] [PubMed] [Google Scholar]
- 5.Conroy KP, Kitto LJ, Henderson NC. αv integrins: key regulators of tissue fibrosis. Cell Tissue Res 365: 511–519, 2016. doi: 10.1007/s00441-016-2407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Gouville AC, Boullay V, Krysa G, Pilot J, Brusq JM, Loriolle F, Gauthier JM, Papworth SA, Laroze A, Gellibert F, Huet S. Inhibition of TGF-beta signaling by an ALK5 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br J Pharmacol 145: 166–177, 2005. doi: 10.1038/sj.bjp.0706172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng YR, Ma HD, Tsuneyama K, Yang W, Wang YH, Lu FT, Liu CH, Liu P, He XS, Diehl AM, Gershwin ME, Lian ZX. STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib. J Autoimmun 46: 25–34, 2013. doi: 10.1016/j.jaut.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 8.El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol 211: 86–94, 2007. doi: 10.1002/path.2099. [DOI] [PubMed] [Google Scholar]
- 9.Fábrega E, Orive A, García-Suarez C, García-Unzueta M, Antonio Amado J, Pons-Romero F. Osteoprotegerin and RANKL in alcoholic liver cirrhosis. Liver Int 25: 305–310, 2005. doi: 10.1111/j.1478-3231.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 10.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134: 1655–1669, 2008. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, Okwuonu G, Hines S, Lewis L, DeRamo C, Delgado O, Dugan-Rocha S, Miner G, Morgan M, Hawes A, Gill R, Celera, Holt RA, Adams MD, Amanatides PG, Baden-Tillson H, Barnstead M, Chin S, Evans CA, Ferriera S, Fosler C, Glodek A, Gu Z, Jennings D, Kraft CL, Nguyen T, Pfannkoch CM, Sitter C, Sutton GG, Venter JC, Woodage T, Smith D, Lee HM, Gustafson E, Cahill P, Kana A, Doucette-Stamm L, Weinstock K, Fechtel K, Weiss RB, Dunn DM, Green ED, Blakesley RW, Bouffard GG, De Jong PJ, Osoegawa K, Zhu B, Marra M, Schein J, Bosdet I, Fjell C, Jones S, Krzywinski M, Mathewson C, Siddiqui A, Wye N, McPherson J, Zhao S, Fraser CM, Shetty J, Shatsman S, Geer K, Chen Y, Abramzon S, Nierman WC, Havlak PH, Chen R, Durbin KJ, Egan A, Ren Y, Song XZ, Li B, Liu Y, Qin X, Cawley S, Worley KC, Cooney AJ, D’Souza LM, Martin K, Wu JQ, Gonzalez-Garay ML, Jackson AR, Kalafus KJ, McLeod MP, Milosavljevic A, Virk D, Volkov A, Wheeler DA, Zhang Z, Bailey JA, Eichler EE, Tuzun E, Birney E, Mongin E, Ureta-Vidal A, Woodwark C, Zdobnov E, Bork P, Suyama M, Torrents D, Alexandersson M, Trask BJ, Young JM, Huang H, Wang H, Xing H, Daniels S, Gietzen D, Schmidt J, Stevens K, Vitt U, Wingrove J, Camara F, Mar Albà M, Abril JF, Guigo R, Smit A, Dubchak I, Rubin EM, Couronne O, Poliakov A, Hübner N, Ganten D, Goesele C, Hummel O, Kreitler T, Lee YA, Monti J, Schulz H, Zimdahl H, Himmelbauer H, Lehrach H, Jacob HJ, Bromberg S, Gullings-Handley J, Jensen-Seaman MI, Kwitek AE, Lazar J, Pasko D, Tonellato PJ, Twigger S, Ponting CP, Duarte JM, Rice S, Goodstadt L, Beatson SA, Emes RD, Winter EE, Webber C, Brandt P, Nyakatura G, Adetobi M, Chiaromonte F, Elnitski L, Eswara P, Hardison RC, Hou M, Kolbe D, Makova K, Miller W, Nekrutenko A, Riemer C, Schwartz S, Taylor J, Yang S, Zhang Y, Lindpaintner K, Andrews TD, Caccamo M, Clamp M, Clarke L, Curwen V, Durbin R, Eyras E, Searle SM, Cooper GM, Batzoglou S, Brudno M, Sidow A, Stone EA, Venter JC, Payseur BA, Bourque G, López-Otín C, Puente XS, Chakrabarti K, Chatterji S, Dewey C, Pachter L, Bray N, Yap VB, Caspi A, Tesler G, Pevzner PA, Haussler D, Roskin KM, Baertsch R, Clawson H, Furey TS, Hinrichs AS, Karolchik D, Kent WJ, Rosenbloom KR, Trumbower H, Weirauch M, Cooper DN, Stenson PD, Ma B, Brent M, Arumugam M, Shteynberg D, Copley RR, Taylor MS, Riethman H, Mudunuri U, Peterson J, Guyer M, Felsenfeld A, Old S, Mockrin S, Collins F; Rat Genome Sequencing Project Consortium . Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428: 493–521, 2004. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 12.Guyot C, Combe C, Clouzeau-Girard H, Moronvalle-Halley V, Desmoulière A. Specific activation of the different fibrogenic cells in rat cultured liver slices mimicking in vivo situations. Virchows Arch 450: 503–512, 2007. doi: 10.1007/s00428-007-0390-y. [DOI] [PubMed] [Google Scholar]
- 13.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19: 1617–1624, 2013. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higashiyama H, Yoshimoto D, Kaise T, Matsubara S, Fujiwara M, Kikkawa H, Asano S, Kinoshita M. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Exp Mol Pathol 83: 39–46, 2007. doi: 10.1016/j.yexmp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Hong F, Chou H, Fiel MI, Friedman SL. Antifibrotic activity of sorafenib in experimental hepatic fibrosis: refinement of inhibitory targets, dosing, and window of efficacy in vivo. Dig Dis Sci 58: 257–264, 2013. doi: 10.1007/s10620-012-2325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Ge H, Newman M, Liu K. OSA: a fast and accurate alignment tool for RNA-Seq. Bioinformatics 28: 1933–1934, 2012. doi: 10.1093/bioinformatics/bts294. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Liu W, Xiao H, Maitikabili A, Lin Q, Wu T, Huang Z, Liu F, Luo Q, Ouyang G. Matricellular protein periostin contributes to hepatic inflammation and fibrosis. Am J Pathol 185: 786–797, 2015. doi: 10.1016/j.ajpath.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, Gottardo R, Hahne F, Hansen KD, Irizarry RA, Lawrence M, Love MI, MacDonald J, Obenchain V, Oleś AK, Pagès H, Reyes A, Shannon P, Smyth GK, Tenenbaum D, Waldron L, Morgan M. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods 12: 115–121, 2015. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 117: 539–548, 2007. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irvine KM, Wockner LF, Hoffmann I, Horsfall LU, Fagan KJ, Bijin V, Lee B, Clouston AD, Lampe G, Connolly JE, Powell EE. Multiplex serum protein analysis identifies novel biomarkers of advanced fibrosis in patients with chronic liver disease with the potential to improve diagnostic accuracy of established biomarkers. PLoS One 11: e0167001, 2016. doi: 10.1371/journal.pone.0167001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jian YC, Wang JJ, Dong S, Hu JW, Hu LJ, Yang GM, Zheng YX, Xiong WJ. Wnt-induced secreted protein 1/CCN4 in liver fibrosis both in vitro and in vivo. Clin Lab 60: 29–35, 2014. doi: 10.7754/Clin.Lab.2013.121035. [DOI] [PubMed] [Google Scholar]
- 22.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003. doi: 10.1172/JCI200320530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Müller E, Rettig WJ, Gorrell MD. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology 29: 1768–1778, 1999. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Ruotti V, Stewart RM, Thomson JA, Dewey CN. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 26: 493–500, 2010. doi: 10.1093/bioinformatics/btp692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mack M. Inflammation and fibrosis. Matrix Biol 68-69: 106–121, 2018. doi: 10.1016/j.matbio.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297, 2012. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morry J, Ngamcherdtrakul W, Yantasee W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol 11: 240–253, 2017. doi: 10.1016/j.redox.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol 49: 10–24, 2016. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narciso-Schiavon JL, Pereira JG, Silva TE, Bansho ET, Morato EF, Pinheiro JT, Muraro-Wildner L, Bazzo ML, Dantas-Corrêa EB, Schiavon LL. Circulating levels of pentraxin-3 (PTX3) in patients with liver cirrhosis. Ann Hepatol 16: 780–787, 2017. doi: 10.5604/01.3001.0010.2789. [DOI] [PubMed] [Google Scholar]
- 30.Neuman MG, Cohen LB, Nanau RM. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem 49: 302–315, 2016. doi: 10.1016/j.clinbiochem.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Öztürk Akcora B, Storm G, Prakash J, Bansal R. Tyrosine kinase inhibitor BIBF1120 ameliorates inflammation, angiogenesis and fibrosis in CCl4-induced liver fibrogenesis mouse model. Sci Rep 7: 44545, 2017. doi: 10.1038/srep44545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poynard T, Lebray P, Ingiliz P, Varaut A, Varsat B, Ngo Y, Norha P, Munteanu M, Drane F, Messous D, Bismut FI, Carrau JP, Massard J, Ratziu V, Giordanella JP. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol 10: 40, 2010. doi: 10.1186/1471-230X-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricard-Blum S, Baffet G, Théret N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol 68-69: 122–149, 2018. doi: 10.1016/j.matbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11: R25, 2010. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadasivan SK, Siddaraju N, Khan KM, Vasamsetti B, Kumar NR, Haridas V, Reddy MB, Baggavalli S, Oommen AM, Pralhada Rao R. Developing an in vitro screening assay platform for evaluation of antifibrotic drugs using precision-cut liver slices. Fibrogenesis Tissue Repair 8: 1, 2014. doi: 10.1186/s13069-014-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaffert CS, Duryee MJ, Bennett RG, DeVeney AL, Tuma DJ, Olinga P, Easterling KC, Thiele GM, Klassen LW. Exposure of precision-cut rat liver slices to ethanol accelerates fibrogenesis. Am J Physiol Gastrointest Liver Physiol 299: G661–G668, 2010. doi: 10.1152/ajpgi.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol 68-69: 435–451, 2018. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Sheppard D. Roles of alphav integrins in vascular biology and pulmonary pathology. Curr Opin Cell Biol 16: 552–557, 2004. doi: 10.1016/j.ceb.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Shersher DD, Vercillo MS, Fhied C, Basu S, Rouhi O, Mahon B, Coon JS, Warren WH, Faber LP, Hong E, Bonomi P, Liptay MJ, Borgia JA. Biomarkers of the insulin-like growth factor pathway predict progression and outcome in lung cancer. Ann Thorac Surg 92: 1805–1811, 2011. doi: 10.1016/j.athoracsur.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 42.Slany A, Haudek-Prinz V, Zwickl H, Stättner S, Grasl-Kraupp B, Gerner C. Myofibroblasts are important contributors to human hepatocellular carcinoma: evidence for tumor promotion by proteome profiling. Electrophoresis 34: 3315–3325, 2013. doi: 10.1002/elps.201300326. [DOI] [PubMed] [Google Scholar]
- 43.Sokolović A, Sokolović M, Boers W, Elferink RP, Bosma PJ. Insulin-like growth factor binding protein 5 enhances survival of LX2 human hepatic stellate cells. Fibrogenesis Tissue Repair 3: 3, 2010. doi: 10.1186/1755-1536-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefanovic L, Stefanovic B. Role of cytokine receptor-like factor 1 in hepatic stellate cells and fibrosis. World J Hepatol 4: 356–364, 2012. doi: 10.4254/wjh.v4.i12.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su TH, Shiau CW, Jao P, Liu CH, Liu CJ, Tai WT, Jeng YM, Yang HC, Tseng TC, Huang HP, Cheng HR, Chen PJ, Chen KF, Kao JH, Chen DS. Sorafenib and its derivative SC-1 exhibit antifibrotic effects through signal transducer and activator of transcription 3 inhibition. Proc Natl Acad Sci USA 112: 7243–7248, 2015. doi: 10.1073/pnas.1507499112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Bovenkamp M, Groothuis GM, Draaisma AL, Merema MT, Bezuijen JI, van Gils MJ, Meijer DK, Friedman SL, Olinga P. Precision-cut liver slices as a new model to study toxicity-induced hepatic stellate cell activation in a physiologic milieu. Toxicol Sci 85: 632–638, 2005. doi: 10.1093/toxsci/kfi127. [DOI] [PubMed] [Google Scholar]
- 47.van de Bovenkamp M, Groothuis GM, Meijer DK, Slooff MJ, Olinga P. Human liver slices as an in vitro model to study toxicity-induced hepatic stellate cell activation in a multicellular milieu. Chem Biol Interact 162: 62–69, 2006. doi: 10.1016/j.cbi.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Veidal SS, Nielsen MJ, Leeming DJ, Karsdal MA. Phosphodiesterase inhibition mediates matrix metalloproteinase activity and the level of collagen degradation fragments in a liver fibrosis ex vivo rat model. BMC Res Notes 5: 686, 2012. doi: 10.1186/1756-0500-5-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westra IM, Oosterhuis D, Groothuis GM, Olinga P. The effect of antifibrotic drugs in rat precision-cut fibrotic liver slices. PLoS One 9: e95462, 2014. doi: 10.1371/journal.pone.0095462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P. Ensembl 2018. Nucleic Acids Res 46: D754–D761, 2018. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong X, Tu YJ, Li Y, Zhang P, Wang W, Chen SS, Li L, Chung AC, Lan HY, Chen HY, Li GS, Wang L. Serum levels of WNT1-inducible signaling pathway protein-1 (WISP-1): a noninvasive biomarker of renal fibrosis in subjects with chronic kidney disease. Am J Transl Res 9: 2920–2932, 2017. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.