Abstract
This study aimed to establish mechanistic links between the prolonged intake of desloratadine, a common H1 receptor blocker (i.e., antihistamine), and development of obesity and metabolic syndrome. Male Sprague-Dawley rats were treated for 16 wk with desloratadine. We analyzed the dynamics of body weight gain, tissue fat accumulation/density, contractility of isolated mesenteric lymphatic vessels, and levels of blood lipids, glucose, and insulin, together with parameters of liver function. Prolonged intake of desloratadine induced development of an obesity-like phenotype and signs of metabolic syndrome. These alterations in the body included excessive weight gain, increased density of abdominal subcutaneous fat and intracapsular brown fat, high blood triglycerides with an indication of their rerouting toward portal blood, high HDL, high fasting blood glucose with normal fasting and nonfasting insulin levels (insulin resistance), high liver/body weight ratio, and liver steatosis (fatty liver). These changes were associated with dysfunction of mesenteric lymphatic vessels, specifically high lymphatic tone and resistance to flow together with diminished tonic and abolished phasic responses to increases in flow, (i.e., greatly diminished adaptive reserves to respond to postprandial increases in lymph flow). The role of nitric oxide in this flow-dependent adaptation was abolished, with remnants of these responses controlled by lymphatic vessel-derived histamine. Our current data, considered together with reports in the literature, support the notion that millions of the United States population are highly likely affected by underevaluated, lymphatic-related side effects of antihistamines and may develop obesity and metabolic syndrome due to the prolonged intake of this medication.
NEW & NOTEWORTHY Prolonged intake of desloratadine induced development of obesity and metabolic syndrome associated with dysfunction of mesenteric lymphatic vessels, high lymphatic tone, and resistance to flow together with greatly diminished adaptive reserves to respond to postprandial increases in lymph flow. Data support the notion that millions of the USA population are highly likely affected by underevaluated, lymphatic-related side effects of antihistamines and may develop obesity and metabolic syndrome due to the prolonged intake of this medication.
Keywords: desloratadine, lymphatic vessel, metabolic syndrome, obesity
INTRODUCTION
Chylomicrons, containing dietary lipids absorbed from the intestine, reach the blood through the mesenteric lymphatic network, where mesenteric lymphatic vessels (MLVs) play a key functional role. Spontaneous phasic contractions of MLVs create forces that drive the lipid-enriched lymph through the mesenteric lymphatic network (3, 26, 36, 49). The tone of MLVs plays an important role in the resistance of lymphatic vessels to lymph flow. Postprandial increases in gastrointestinal lymph formation and flow (20, 24, 44) inhibit phasic contractions and reduce tone in the MLVs in a shear-dependent manner (16). During these periods, when “passive” lymph flow (13) is elevated, such mechanisms maintain MLV function in an energy-saving mode (16) by diminishing phasic pumping contractions and tone, thereby converting MLV functional behavior from acting primarily like a pump to acting more like a conduit (39). Flow-induced regulation of MLV function depends on postprandial increases in wall shear stress experienced by lymphatic endothelium associated with periods of higher lymph flow rates and higher viscosity of mesenteric lymph following a meal. Nitric oxide (NO) plays an important role as an endothelium-derived relaxing factor (EDRF) in the fast components of this flow-dependent adaptation of phasic and tonic contractions in MLVs (1, 7, 16). Recently, we (31) confirmed the functional role of another EDRF in MLVs, histamine, that is responsible for the more prolonged components of flow-dependent postprandial adaptation of MLV contractility and tone. This role of histamine is increased with aging (32).
Any dysfunction of these lymph flow-dependent regulatory mechanisms of lymphatic contractility and tone would alter the flow of lymph and, as a consequence, would worsen postprandial absorption of lipids from the gut via lymph. As a particular example that illustrates this conclusion, a hypothetical chronic 10% increase in tone of a MLV with typical diameter ~120 μm will lead to a 58% increase in resistance of this MLV to lymph flow, with subsequent negative influence on postprandial absorption of lipids and transport of chylomicrons by lymph.
Recently, Ratliff et al. (40) presented important data to the research community obtained from the 2005–2006 National Health and Nutrition Examination Survey. In this study, 268 adults with reported use of an H1 antihistamine (predominantly cetirizine and fexofenadine in this study) and 599 age- and sex-matched controls were studied. Their results demonstrated that H1 antihistamine users had significantly higher body weight, body mass index, waist circumference, and insulin levels than the matched controls. The authors concluded that it is imperative that the relationship among increased antihistamine use, obesity, and underlying risk factors be explored (40). With consideration of these clinical findings and on the basis of our novel data on the role of histamine as an EDRF in MLVs (31, 32), we hypothesized that prolonged intake of antihistamines might alter the histamine-mediated components of flow-dependent postprandial adaptation of MLV contractility and tone. We proposed that such alterations would likely lead to a chronic increase in MLV tone and, subsequently, to chronically increased MLV resistance to lymph flow. Therefore, chronic use of antihistamines can induce abnormalities in lipid absorption with potential excessive accumulation of lipids in the mesentery as a starting point for development of obesity and, subsequently, the development of metabolic syndrome.
To test our central hypothesis, we designed a study in which we implemented daily treatment of rats with desloratadine (trade name Clarinex in the USA and Aerius in Europe), a commonly used blocker of H1 histamine receptors, for 16 wk (group D). A control group (group C) of animals was used for comparisons of data. In both study groups, we evaluated the dynamics of weight gain and, upon completion of the treatment period, regional fat accumulation/density, mesenteric lymphatic phasic contractility and tone, and levels of various substances in central and portal blood, as well as characteristics of liver function.
MATERIALS AND METHODS
Animal procedures.
For the present studies, we used male Sprague-Dawley rats with an average entry body weight for all experimental groups of 171 ± 13 g (6 wk old). All animal procedures were reviewed and approved by the Texas A&M University Institutional Animal Care and Use Committee and were in accordance with federal and local regulations.
Animals were randomly separated into two experimental groups (n = 6 for each group): a control group (group C) and a desloratadine-treated group (group D). Animals were fed a purified rodent diet with 10% energy from fat (catalog no. 58Y2; TestDiet, St. Louis, MO). We measured daily food consumption, and it was not different in group C and group D.
To mimic prolonged intake of antihistamines in humans, animals from experimental group D underwent daily intake of desloratadine at a dosage of 50 μg/kg body wt, which is estimated to be equivalent to one 5-mg tablet of Clarinex taken once a day by a patient. Desloratadine (Sigma-Aldrich, St. Louis, MO; catalog no. D1069) was administered to animals in group D daily for 16 wk via gavage, with the volume of the gavage solution not exceeding 0.5 ml. Desloratadine powder was initially diluted in dimethyl sulfoxide (DMSO; Sigma-Aldrich, catalog no. D8418) to prepare a stock solution based on the stated solubility of desloratadine (10 mg/ml DMSO). Daily gavage solutions were prepared with subsequent dilution of the stock in sterile-filtered water (Sigma-Aldrich, catalog no. W3500), according to the current body weight of the animal. Body weight corrections were implemented at the end of each week to take into account weekly weight gain. Animals from group C received daily gavage of the vehicle (DMSO diluted in sterile filtered water to the same final concentration as for group D), based on body weights with similar weekly weight gain adjustments. At the end of the 16-wk treatment period, the DMSO concentration in the gavage water varied between 0.6 and 0.8%, which corresponds to an intake of 3–4 μg DMSO/kg body wt. These dosages of DMSO in the daily gavage solution were between 500- and 1,000-fold lower than the toxic dosage of DMSO in gavage solutions for rats reported by various sources (2, 11, 33).
The following procedures and methodologies were applied to animals from both experimental groups (C and D). Weights of animals were measured at the end of each week for the entire 16 wk of study. During week 15, blood was collected from the cephalic vein of each fasted animal to determine blood lipids, glucose, and insulin. On the experimental day, all nonfasted rats were anesthetized with a solution containing a combination of Fentanyl-Droperidol (0.3 ml/kg im) and diazepam (2.5 mg/kg im). Subsequently, the chest was opened (which is a terminal procedure), and death was confirmed. Immediately post mortem, a midline abdominal incision was made, and blood was collected from the portal vein for determination of lipids in portal blood. Directly after portal blood collection, blood was collected from the heart for determination of insulin in systemic blood and blood lipids to compare with portal blood lipids in samples taken the same day.
The whole gut next was removed by cutting at the root of the mesentery after clamping it to avoid excessive bleeding. Mesentery of the small intestine (without gut), containing MLVs, was used for isolation of lymphatic vessels. Last, samples of adipose tissue were collected from various regions of the body: white adipose tissue (WAT) samples, from subcutaneous abdominal fat deposits in the upper part of the abdomen, inguinal adipose tissue samples, from fat deposits located between the abdomen and the hindlimb, and perivascular femoral fat tissue samples, from fat deposits located near the femoral artery and vein. Samples of brown adipose tissue were obtained from the intracapsular brown adipose tissue (IBAT) deposit. The animal body was placed with the abdomen down, and the skin, approximately one inch below the shoulder blades, was grasped with tweezers, lifted, and incised. The skin was widely cut laterally and forward to open the shoulder region. The revealed light-colored fat pad contained IBAT covered with a thin layer of white adipose. The WAT was carefully lifted and excised, revealing butterfly-shaped darker-colored BAT. The brown fat pad was carefully dissected to ensure no white adipose or muscle remained attached to the sample. All WAT and IBAT samples were placed in sealed containers on wet ice and transported for subsequent analysis. Samples of WAT and BAT were analyzed using Raman and Brillouin spectroscopies to determine Brillouin shift, as an indicator of stiffness of fat tissues, i.e., measure of fat density. The methodology was similar to that described in a study by Troyanova-Wood et al. (43).
Analysis of lymphatic contractility.
Once exteriorized, the sections of the mesentery containing MLVs were positioned in a dissection chamber within the field of view of a dissecting microscope and continuously suffused with standard Dulbecco’s phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA, catalog no. 14040-133). Suitable MLVs were identified and cleared of all surrounding tissue. At least three sections of MLVs 1.4–1.5 cm in length (equal for all preparations) were carefully dissected from each animal and used for experiments. When pressurized to a transmural pressure of 5 cmH2O in isolated vessel chambers for analysis of lymphatic contractility, the outer diastolic diameters of the MLV segments averaged 181 ± 14 μm (group C) and 173 ± 6 μm (group D) and were not statistically different.
Isolated MLVs were transferred to standard 35-mm petri dishes (one MLV specimen/dish) completely filled with ~38°C Dulbecco’s modified Eagle’s medium-F12 (DMEM-F12) solution (Invitrogen, catalog no. 11039) supplemented with an antibiotic mixture (Invitrogen, catalog no. 15140) to achieve a concentration of 100 IU/ml penicillin and 100 µg/ml streptomycin per milliliter of DMEM-F12. We (15, 31, 32) have demonstrated that lymphatic contractility is not affected by overnight incubation in this solution. Care was taken to ensure that the mesenteric segment was completely submerged in the medium in the dish. For purposes of this study, the MLVs from each animal were randomly divided into three groups: fresh control, sham overnight control, and overnight α-methyl-dl-histidine dihydrochloride (α-MHD)-treated groups. On each experimental day, we used MLVs from a fresh control set of vessels taken from each experimental group to record parameters of their contractility under various transmural pressures and high imposed flow, as described below. We also treated all of these fresh control MLVs with Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) as described below (but without preceding treatment by α-MHD).
The next day, we used both sham overnight control and α-MHD-treated MLVs obtained from the same animal. The α-MHD (Sigma-Aldrich, catalog no. M8628) was used at a concentration of 10 μM (overnight, 18–20 h) to effectively block histidine decarboxylase (HDC), the histamine-producing enzyme in MLVs, and effectively deplete MLVs of the internally stored histamine produced earlier (31). Isolated MLVs, both sham overnight control and α-MHD treated, were transferred to an isolated vessel chamber (modified Living Systems Instrumentation single vessel chamber, model CH/1; St. Albans City, VT) filled with prewarmed 38°C DMEM-F12 (pH 7.36). Cannulation and vessel setup were performed, the contractions of MLVs were recorded, and their diameters were tracked continuously in all experiments, as described in our previous studies (16, 17).
In every experiment, we evaluated the contractile responses of MLV segments at transmural pressures of 1, 3, and 5 cmH2O for 5 min at each pressure and then in response to an axial intraluminal pressure gradient (imposed flow gradient) of 5 cmH2O for another 5 min at transmural pressure of 5 cmH2O. The methodology for creating the various transmural pressures and imposed flow in MLVs and the selection criteria of the mentioned values are well established and described in detail in previous studies (16, 31, 32). The measurements of lymphatic contractile activity were performed while MLVs were being superfused with DMEM-F-12 for sham overnight control vessels, and with DMEM-F12 supplemented with 10 μM α-MHD for the α-MHD-treated MLV segments.
After completion of measurements of contractile activity of MLVs in the subsequent parts of the experiments with the transmural pressure range and imposed flow gradient in DMEM-F-12 containing α-MHD (α-MHD-treated group), the solution in the chamber was additionally supplemented for 15 min with the nitric oxide synthase (NOS) inhibitor l-NAME (Sigma-Aldrich, catalog no. N5751) at 100 μM. The effectiveness of NOS blockade in rat lymphatic vessels induced by application of l-NAME at this concentration has been demonstrated by us in numerous previous reports (7, 17, 31, 32). After the initial 15 min of incubation in the l-NAME-containing solution, measurements of contractile activity of MLVs in response to the transmural pressure range and imposed flow gradient were repeated in the presence of l-NAME in vessels from the α-MHD-treated group.
At the end of each experiment, the passive (relaxed) diameters of all used MLVs were measured at each pressure (1, 3, and 5 cmH2O for 3 min at each pressure) after the MLVs were exposed to a nominally calcium-free, ethylenediaminetetraacetic acid (EDTA)-supplemented physiological saline solution (PSS; in mM: 145.0 NaCl, 4.7 KCl, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 3.0 EDTA, 3.0 3-(N-morpholino)propanesulfonic acid (MOPS) for 15 min.
Data analysis for isolated lymphatic vessel experiments.
Lymphatic diameters were tracked continuously during experiments using “Vessel Track” software, developed previously (8). We used cardiac pump analogies to define systole and diastole in reference to the lymphatic contractile cycle (4, 16), and the end-diastolic and end-systolic points in the diameter tracings were recorded for each 5-min interval for a transmural pressures of 1, 3, and 5 cmH2O and for imposed flow gradients of 1, 3, and 5 cmH2O. From the lymphatic end-diastolic and end-systolic diameters (EDD and ESD), the following lymph pump parameters were calculated: lymphatic tone index (LTI, the difference between the passive lymphatic diameter in Ca2+-free PSS and EDD, expressed as a percentage of the passive lymphatic diameter in Ca2+-free PSS), relative diastolic resistance to flow (RDRF), calculated for each level of transmural pressure using formula RDRF = (Ca2+-free outer lymphatic diameter/2)4/(EDD/2)4, contraction amplitude (the difference between the diastolic and systolic diameters), contraction frequency, ejection fraction (EF, the fraction of end-diastolic volume ejected during the single lymphatic contraction, calculated using the formula EF = (EDD2 − ESD2)/EDD2) (4), and fractional pump flow (FPF, an index of lymph pump flow, calculated as ejection fraction × contraction frequency). Because of the anatomic variations between lymphatic vessels, to compare the changes in diameter during the lymphatic contractile cycle, the diastolic and systolic diameters were normalized to the passive lymphatic diameters in Ca2+-free PSS at the corresponding transmural pressure. From each animal, one MLV segment was used for the fresh control group, one for the overnight sham control group, and one for the α-MHD-treated group. We used data from six animals for each study group for functional analysis of MLV contractility.
Measurements of parameters in systemic and portal blood.
Blood samples, collected as described above and used to measure lipids, glucose, and insulin, were transferred to the clinical laboratory at Baylor Scott & White Hospital, Temple, TX. Analyses were performed using standard methodology.
Liver-to-body weight ratio, estimated bile duct mass, and assessment of liver steatosis.
Liver weight, body weight, and liver-to-body weight ratios were calculated for all groups of rats. Intrahepatic bile duct mass (%total liver weight) was evaluated by semiquantitative immunohistochemistry and immunoblotting for CK-19 (a cholangiocyte-specific marker), as described in previous studies (21, 22). To assess the degree of hepatic steatosis, Oil Red O staining (21, 27) was performed in OCT-embedded liver sections (10 mm thick; 10 different fields were analyzed from each sample from animals from all experimental groups) using the Oil Red O (Lipid Stain) kit purchased from Abcam (Abcam, Cambridge, MA; catalog no. ab150678) and performed as outlined by the manufacturer’s protocol. Normalized pixel intensity per image and average liver fat droplet size were used as quantitative measures.
Statistical differences.
Statistical differences were determined by analysis of variance, regression analysis, and Student’s t-test (JMP software v. 9.0.2 for Windows; SAS Institute, Cary, NC) and considered significant at P < 0.05. We used data from all six animals from each of experimental group.
RESULTS
Weight gain and tissue lipids.
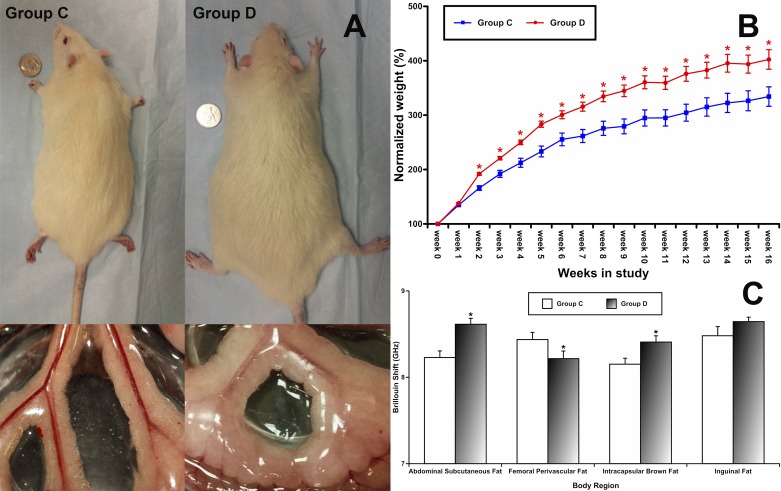
In all study groups we evaluated weight gain weekly. We found that animals in in group D (beginning in week 2) gained more weight than animals in control group C. With an average entry body weight for all experimental groups of 171 ± 13 g, at the end of week 16, the animals in group D demonstrated a 402.4 ± 17.9% gain, which was greater (P < 0.05) than the body weight gain of animals in group C (334.1 ± 17.8%). A series of photographs in Fig. 1A demonstrate representative pictures of animals from each group and fat accumulation patterns in their mesenteric tissues (with large mesenteric fat deposits in group D compared with group C) at the end of the 16-wk treatment period. Weekly body weights increased throughout the entire study, as shown in Fig. 1B. Using a recently developed approach to determine Brillouin shift, as an indicator of stiffness of fat tissues, i.e., measure of fat density (43), we assessed lipid accumulation in various tissues (Fig. 1C). These data demonstrate variability in the patterns of changes in group D. Abdominal subcutaneous fat and intracapsular brown fat densities were increased (P < 0.05) in group D, while femoral perivascular fat density was decreased (P < 0.05) in group D compared with control.
Fig. 1.
Effects of prolonged intake of desloratadine on weight gain and tissue lipids. group C, control group; group D, desloratadine-treated group. A: representative pictures of animals from both groups at week 16 and fat accumulation patterns in their mesenteric tissues. B: weekly body weights. C: fat density in various tissues. *Significant differences (P < 0.05) between data from group C and group D; n = 6 for each group.
Functional characteristics of mesenteric lymphatic vessels.
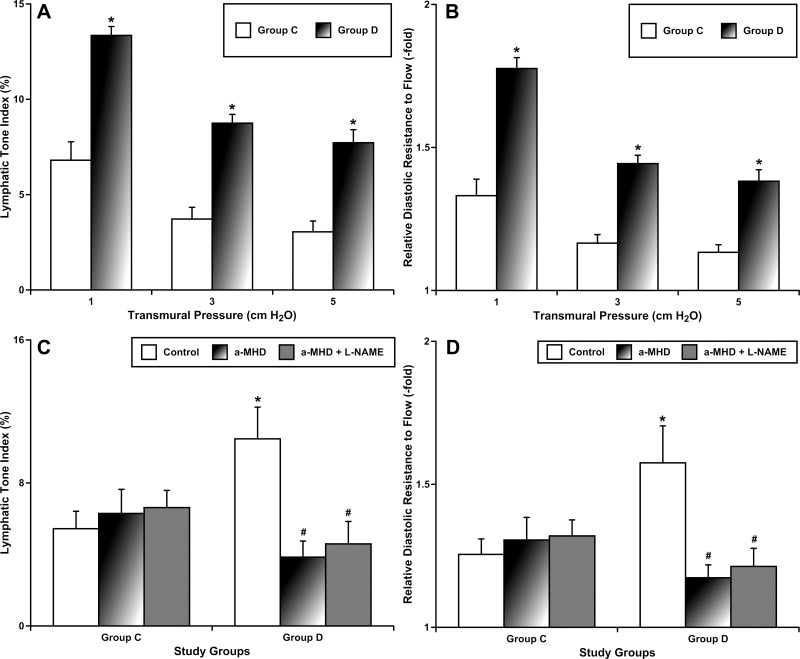
After completion of 16 wk of treatment, we evaluated the phasic and tonic contractions of MLVs isolated from animals of all groups. In the first set of experiments, we evaluated the sensitivity of MLVs to stretch by application of a standard range (14, 30) of transmural pressures from 1 to 5 cmH2O. We did not find any significant differences in lymphatic phasic contraction amplitude, phasic contraction frequency, or fractional pump flow (index of active pumping per minute) between MLVs from the control group compared with group D. The parameters of MLV contractility in fresh controls and sham overnight controls were also not statistically different. In contrast to phasic contractility, the tone of MLVs was significantly changed in group D. Figure 2A demonstrates that after prolonged intake of desloratadine we observed increases in lymphatic tone of 96, 135, and 153% at transmural pressures of 1, 3, and 5 cmH2O, respectively. The resulting changes in the LTI (see materials and methods) in group D induced significant increases in MLV relative diastolic resistance to flow (RDRF) compared with control (Fig. 2B): 33, 28, and 22% of the control value at the same levels of transmural pressure.
Fig. 2.
Effects of prolonged intake of desloratadine on lymphatic tone and lymphatic diastolic resistance of mesenteric lymphatic vessels before (A and B, at range of transmural pressures 1, 3, and 5 cmH2O) and after (C and D, at transmural pressure 5 cmH2O) elimination of the effects of histamine and nitric oxide. group C, control group; group D, desloratadine-treated group. α-HMD, α-methyl-dl-histidine dihydrochloride; l-NAME, Nω-nitro-l-arginine methyl ester hydrochloride. *Significant differences (P < 0.05) between group C and group D under similar conditions; #significant differences (P < 0.05) between control conditions and any treatment condition within each experimental group; n = 6 for each group.
To determine potential changes in the roles of NO and histamine as known lymphatic EDRFs (16, 17, 31, 32), we evaluated lymphatic contractile parameters under conditions of pharmacological blockade of histamine production with α-MHD (10 μM) alone and together with blockade of NO with l-NAME (100 μM), as commonly performed in the past (17, 31, 32). It is important to mention that all transmural pressure- and imposed flow-dependent reactions of fresh MLVs from both experimental groups to l-NAME without preceding α-MHD treatment were not statistically different from the reactions of MLVs from both experimental groups to l-NAME after overnight α-MHD treatment (described below). In the absence of imposed flow, as described above (transmural pressure range from 1 to 5 cmH2O), we found that elimination of histamine was able to reduce LTI significantly in group D, whereas it did not change LTI significantly in group C. Similarly, RDRF was also diminished in group D after blockade of histamine production. NO did not appear to play a significant role under these conditions in either experimental group, since the addition of NO blockade did not induce further significant changes in the studied parameters. Figure 2, C and D, demonstrate these findings at a transmural pressure of 5 cmH2O. Elimination of histamine reduced the LTI in groups D to 73%, and RDRF to 25%, compared with their respective control values.
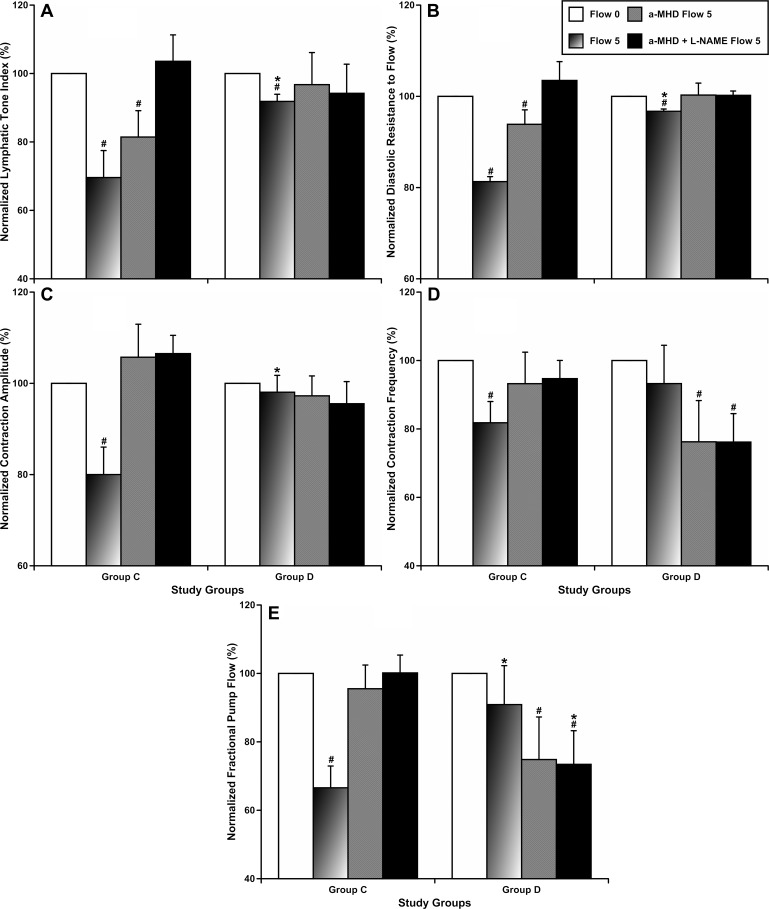
In a second set of experiments, we evaluated sensitivity of MLVs to wall shear stress by application of a high (16, 30) imposed flow gradient (5 cmH2O) after zero-imposed flow conditions, all at a constant transmural pressure of 5 cmH2O. MLVs from the control group demonstrated the typical NO-/histamine-dependent partial relaxation and pump inhibition by increased imposed flow, as described previously (16, 30). In MLVs from group D, the LTI decreased only 8.2% compared with 30.4% in group C (Fig. 3A) and RDRF only 3.3% compared with 18.7% in group C (Fig. 3B). Furthermore, we found that in group D an imposed flow did not induce negative inotropy (decrease of contraction amplitude) in MLVs compared with the control group (Fig. 3C). In addition, in group D we found that imposed flow-induced inhibition of contraction frequency was abolished (Fig. 3D). As a result, fractional pump flow (FPF, an index of minute pumping of MLVs) was not changed in group D due to increased imposed flow in MLVs (Fig. 3E). Cumulatively, experiments with high imposed flow demonstrated a diminished tonic response to increased imposed flow and an abolished phasic component of this response in group D.
Fig. 3.
Effects of prolonged intake of desloratadine on parameters of tonic and phasic contractility of mesenteric lymphatic vessels under conditions of increased imposed flow (from 0 to 5 cmH2O at transmural pressure of 5 cmH2O) before and after elimination of the effects of histamine and nitric oxide. group C, control group; group D, desloratadine-treated group. *Significant differences (P < 0.05) between the control group and group D under similar conditions; #significant differences (P < 0.05) between control conditions and any imposed flow/treatment condition within each experimental group; n = 6 for each group.
Elimination of NO and histamine as EDRF molecules in isolated MLVs demonstrated the lack of importance of NO in any imposed flow-induced effects in group D (including freshly isolated MLVs without preceding HDC blockade), whereas in group C we observed the usual reaction (31, 32) of MLVs to this combination of treatments (α-MHD and l-NAME). These findings underscore the predominant role of lymphatic-derived histamine for flow-induced changes in desloratadine-treated animals. The flow-induced changes in LTI and RDRF disappeared after elimination of histamine in group D (Fig. 3, A and B), whereas phasic contraction amplitude was unaffected by flow at all in this group (Fig. 3C). In addition, in group D, elimination of histamine led to development of flow-induced negative chronotropy (decrease in contraction frequency) (Fig. 3D) and corresponding changes in FPF (Fig. 3E). Cumulatively, with the absence of imposed flow-dependent regulation of MLVs by NO in group D, the lymphatic-derived histamine was the predominant molecule involved in control of lymphatic tone in MLVs in desloratadine-treated animals. In addition, flow-induced effects of histamine on contraction frequency and FPF were abnormal in this experimental group (excessive inhibitory effect compared with control).
Blood: lipids, glucose, and insulin.
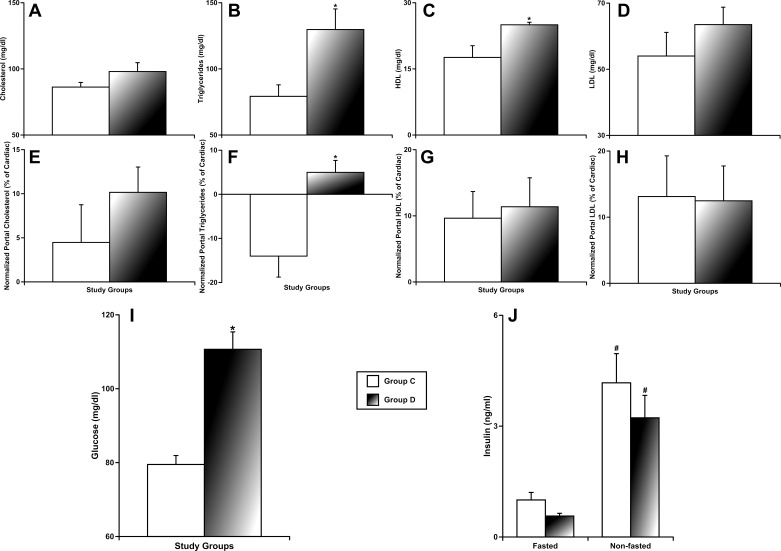
We evaluated potential changes in blood lipids, glucose, and insulin in systemic blood and changes in levels of the portal blood lipids, in both experimental groups. While cholesterol was unchanged in group D (Fig. 4A), triglycerides were significantly higher in group D (129.8 ± 15.6 mg/dl) than in control (79.3 ± 8.7 mg/dl) (Fig. 4B). High-density lipoprotein (HDL) was also significantly higher in desloratadine-treated animals (25.0 ± 0.6 mg/dl) compared with group C (17.6 ± 2.7 mg/dl) (Fig. 4C). Low-density lipoprotein (LDL) was unchanged (Fig. 4D). Comparison of systemic and portal blood revealed higher portal triglycerides in group D (Fig. 4F), whereas all other measured parameters were similar to observed systemic values (Fig. 4, E, G, and H). Additionally, we found elevated fasting glucose levels in group D (110.7 ± 4.7 mg/dl) compared with control (79.5 ± 2.4 mg/dl) (Fig. 4I). The nonfasting insulin was significantly higher than fasting insulin levels in both groups C and D (Fig. 4J). Cumulatively, these data demonstrate that prolonged intake of desloratadine was associated with increased blood triglycerides (with signs of their rerouting toward portal blood) and high HDL. Higher levels of fasting glucose in group D coexisted with normal levels of fasting and nonfasting insulin, which may be indicative of development of insulin resistance in desloratadine-treated animals.
Fig. 4.
Effects of prolonged intake of desloratadine on blood lipids (fasted), glucose (fasted), and insulin (fasted and nonfasted). group C, control group; group D, desloratadine-treated group; HDL/LDL, high/low-density lipoprotein. A–D and I–J: measurements in systemic blood. panels F–H: measurements in portal blood normalized to corresponding levels in systemic blood under the same conditions (nonfasted). *Significant differences (P < 0.05) between the control group and group D; #significant differences (P < 0.05) between nonfasted and fasted insulin within each experimental group; n = 6 for each group.
Characteristics of liver functionality.
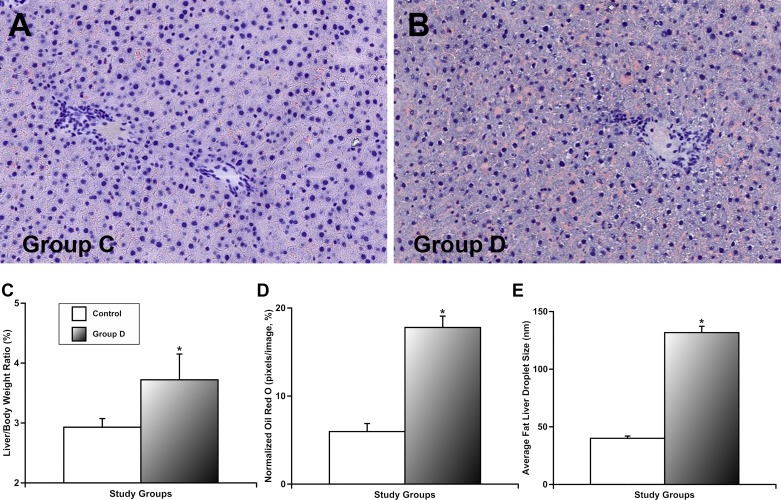
In the next set of experiments, we evaluated parameters related to liver function. Figure 5 demonstrates the results of these measurements. We found that the liver/body weight ratio was higher in group D than in control (Fig. 5C). Using the Oil Red O staining of liver sections, we evaluated and quantified the effects of prolonged intake of desloratadine on fat accumulation in the liver. Figure 5, A and B shows representative images of liver sections from groups C and D. Quantitative analyses (Fig. 5, D and E) confirmed excessive fat accumulation in the liver in desloratadine-treated animals compared with control. Cumulatively, these data demonstrate development of liver steatosis (“fatty liver”) in animals that underwent prolonged intake of desloratadine, which coexisted with an increased total liver mass.
Fig. 5.
Effects of prolonged intake of desloratadine on fat accumulation in the liver (based on Oil Red O staining) and its weight. group C, control group; group D, desloratadine-treated group. A and B: representative images of liver sections stained by Oil Red O in corresponding study groups. C: average liver/body weight ratio. Normalized intensity of staining Oil Red O (D) and average fat liver droplet size (E), results of quantitative analyses of fat accumulation in the liver. *Significant differences (P < 0.05) between the control group and group D; n = 6 for each group.
DISCUSSION
Our current data establish clear links between prolonged intake of desloratadine (an H1 receptor blocker, i.e., antihistamine), development of an obese phenotype, and signs of metabolic syndrome in experimental animals. The alterations include excessive weight gain, increased density of abdominal subcutaneous fat and intracapsular brown fat, high blood triglycerides with indication of their rerouting toward portal blood, high HDL, high fasting blood glucose levels coexisting with normal levels of fasting and nonfasting insulin, high liver/body weight ratio, and liver steatosis (“fatty liver”). These changes are clearly associated with dysfunction of mesenteric lymphatic vessels, i.e., their high tone and resistance to flow together with diminished tonic and abolished phasic responses to increases in flow (i.e., a greatly diminished reserve to adapt to postprandial increases in lymph flow). The role of NO in this flow-dependent adaptation is abolished, although remnants of these responses were controlled by lymphatic-derived histamine. Therefore, the central hypothesis of this study that prolonged intake of H1 receptor blocker will alter mesenteric lymphatic function linked to the subsequent development of obesity and metabolic syndrome is confirmed. These our data lead to important health considerations and raise essential questions.
Antihistamines and obesity.
Over the past decades, the development of obesity with excessive accumulation of fat in the abdominal compartment of the body, and its potential consequences (including metabolic syndrome), has become a serious health problem in developed countries. In particular, in the United States it is estimated that one-third of the adult population and nearly 17% of youth suffer from excessive fat accumulation and its consequences (34, 35). With the increase occurring since the mid-1980s, obesity among the U.S. population has gained epidemic characteristics (10) and demonstrates a tendency for further growth. Various sources monitoring the geographical distribution of obesity in the U.S. (e.g., https://stateofobesity.org/adult-obesity/) point to a higher rate of obesity in the south central U.S. While numerous studies have been performed and tremendous efforts have been implemented to try to solve the obesity problem, until now there has been no commonly accepted universal explanation of this situation; nor has there been a commonly accepted theory of the pathogenesis of obesity and metabolic syndrome. This likely relates to the multifactorial nature of obesity, where variations in diets overlap with differences in sex, age, race, profession, levels of daily exercise, unrelated pathologies, income, etc. Therefore, we will not provide here a broad overview of the literature on this problem; instead, we will focus on our hypothesis that may provide a novel explanation of a potential cause of obesity in a significant part of the affected population in the U.S.
We believe that it is important to note that the regions with the highest distribution of obesity in the U.S. (south central U.S.) overlap on the maps with regions exhibiting the highest rates of several seasonal allergies, e.g., asthma and sinusitis (5). Noteworthy, historically a major advance in antihistamine development occurred in the 1980s with the introduction of second-generation H1-antihistamines (18). The timing of this mass introduction of new and improved antihistamines into the U.S. market overlaps in time with the beginning of the increase in obesity rates in a significant portion of the U.S. population (10). Therefore, we believe that our current data support the notion that millions within the U.S. population are highly likely to have been affected by underevaluated lymphatic-related side effects of antihistamines taken to cure various allergic disorders, and many of them may have developed obesity and metabolic syndrome due to the prolonged intake of this medication.
Importantly, our study with 16 wk of antihistamine intake by rats mimics ~8 yr of daily intake of one tablet of antihistamine by humans [2 wk of laboratory rat life corresponds to ~1 yr of human life (42)]. Notably, rats from the desloratadine-treated group exhibited an increase in weight gain over their control counterparts after only 2 wk of regular daily desloratadine intake, mimicking only 1 yr of daily consumption of antihistamine by humans. At the same time, in humans, socioeconomic factors may affect individuals who need to take this type of anti-allergy medicine. With easy availability of cheap over-the-counter antihistamines in the U.S., income must be considered as a factor when a choice must be made between an expensive visit to the allergy doctor or the purchase of a cheap nonprescription treatment. Minority and low-socioeconomic-status groups in the U.S. are disproportionately affected by obesity at all ages (45), which, in part, may be due to cheap antihistamine use. Regularity and frequency of uncontrolled antihistamines intake should also be considered. It is clear that obesity and metabolic syndrome have a multifactorial nature, where variations in diets (including prevailing source of nutrients, amounts and frequency of meals) overlap with differences in sex, age, race, profession, levels of daily exercise, unrelated pathologies, income, etc. Any of these factors, including prolonged intake of antihistamines, may separately or cumulatively lead to obesity and potentially to development of metabolic syndrome.
Lymphatic transport function and obesity.
There is currently no commonly accepted understanding of the mechanisms of alterations in lymphatic vessel transport function during development of obesity and metabolic syndrome. The scientific literature provides evidence that obesity and metabolic syndrome diminish lymphatic clearance of peripheral tissues (6, 9, 12, 29, 41). However, the conclusions that “obesity but not high-fat diet impairs lymphatic function” (12) appears to be somewhat one-sided. While obesity definitely reduced lymphatic clearance in certain peripheral lymphatic beds (6, 9, 12, 29, 41), these regional lymphatic vessels (popliteal, ear, etc.) are not involved in dietary uptake of lipids. Potential alterations in mesenteric lymphatic transport function linked to dynamics of excessive weight gain and the development of overweight and obesity have not been carefully evaluated in these studies, and it is quite possible that initial alterations in the transport function of MLVs were overlooked. At the same time, it is well known that lymphatic contractile elements and transport function are heterogeneous in various regional lymphatic beds (14, 28). This functional heterogeneity between peripheral and visceral lymphatics in mice is even more profound (46); so wide-ranging conclusions about transport function of all lymphatic beds during development of obesity are not yet well supported.
In the case of prolonged intake of antihistamine, its inhibitory action on the H1 receptors [responsible for the histamine-induced relaxation of MLVs (23)] induces both chronically increased lymphatic tone and lymphatic resistance to flow, which may likely be an initial cause of alterations of lipid adsorption in the gut and subsequently alterations in lipid metabolism. However, it is important to consider postprandial activation of intestinal mucosal and mesenteric perilymphatic mast cells (19, 38). Additionally, the concentration-dependent effects of histamine on lymphatic contractility (37) are by themselves able to shift the balance between EDRF-related influences of low concentrations of the lymphatic-derived histamine and the potentially contrary effects of excessive mast cell-released histamine, acting together with other mast cell-derived mediators. Such possibilities should be properly evaluated in future studies.
Recent studies demonstrated that a high-fructose diet induces development of obesity and metabolic syndrome, which correlates with alterations of lymphatic contractility in MLVs and thoracic duct (47, 48). Those authors considered such changes in MLVs to be secondary to the development of metabolic syndrome and further established that hyperglycemia/hyperinsulinemia-induced insulin resistance causes alterations in cellular bioenergetics and major regulatory pathways responsible for proper contractile response of lymphatic muscle (25). Our current study revealed some similarities to the effects of the high-fructose diet. In particular, the regulatory role of NO for the contractile responses of MLVs was ablated in all of our treated experimental groups. However, in group D we observed major alterations in lymphatic tone, but we did not find the reduced lymphatic contraction frequency typical for high-fructose-induced obesity and metabolic syndrome (48). Such differences are clearly related to the influences of desloratadine and require further in-depth investigations. However, in our desloratadine-treated experimental group, the predicted influences of H1 receptor blockade on lymphatic tone and resistance of lymphatic vessels to flow (i.e., increases in these parameters) are confirmed and are clearly linked to the development obesity and metabolic syndrome.
In conclusion, our studies provide experimental evidence of harmful effects of prolonged intake of desloratadine and serve as a warning sign of likely serious health problems for a significant portion of the U.S. population. Larger research efforts, including patient-based studies, are necessary to evaluate the described effects in humans by use of various available antihistamines and to elucidate the pathophysiology of changes in order to develop countermeasures to prevent the antihistamine-induced mesenteric lymphatic dysfunction and the development of obesity and metabolic syndrome.
GRANTS
This work was supported in its major parts by the National Institutes of Health (NIH) Grants DK-099161 (A. A. Gashev) and HL-123420 (D. C. Zawieja). Additional support was provided by NIH Grants AG-030578 (A. A. Gashev) and DK-108959 (H. Francis); Texas A&M University Health Science Center College of Medicine and Department of Medical Physiology (A. A. Gashev); Veterans Affairs Merit Award (1I01BX003031) from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service (H. Francis); National Science Foundation (DBI-1455671) (V. V. Yakovlev); U.S. Department of Defense (FA9550-15-1-0517; FA9550-18-1-0141) (V. V. Yakovlev); and by Cancer Prevention and Research Institute of Texas (RP160834) (V. V. Yakovlev).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.Y.G., I.T.N., L.H., C.G., M.T.-W., S.F.A., S.P., C.D., A.R.H., V.V.Y., M.K.N.-R., D.C.Z., C.J.M., G.D.A., H.F., and A.A.G. conception and design of research; O.Y.G., I.T.N., L.H., C.G., M.T.-W., S.F.A., S.P., C.D., A.R.H., V.V.Y., M.K.N.-R., D.C.Z., C.J.M., G.D.A., H.F., and A.A.G. performed experiments; O.Y.G., I.T.N., L.H., C.G., M.T.-W., S.F.A., S.P., C.D., A.R.H., V.V.Y., M.K.N.-R., D.C.Z., C.J.M., G.D.A., H.F., and A.A.G. analyzed data; O.Y.G., I.T.N., L.H., M.T.-W., S.F.A., S.P., C.D., A.R.H., V.V.Y., M.K.N.-R., D.C.Z., C.J.M., G.D.A., H.F., and A.A.G. interpreted results of experiments; O.Y.G., L.H., C.G., M.T.-W., S.F.A., S.P., C.D., A.R.H., V.V.Y., M.K.N.-R., D.C.Z., C.J.M., G.D.A., H.F., and A.A.G. prepared figures; O.Y.G., I.T.N., S.P., C.D., A.R.H., V.V.Y., M.K.N.-R., D.C.Z., C.J.M., G.D.A., H.F., and A.A.G. drafted manuscript; O.Y.G., I.T.N., S.P., C.D., A.R.H., V.V.Y., M.K.N.-R., D.C.Z., C.J.M., G.D.A., H.F., and A.A.G. edited and revised manuscript; O.Y.G., I.T.N., L.H., C.G., M.T.-W., S.F.A., S.P., C.D., A.R.H., V.V.Y., M.K.N.-R., D.C.Z., C.J.M., G.D.A., H.F., and A.A.G. approved final version of manuscript.
REFERENCES
- 1.Akl TJ, Nagai T, Coté GL, Gashev AA. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol 301: H1828–H1840, 2011. doi: 10.1152/ajpheart.00538.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsch W, Sponer G, Dietmann K, Fuchs G. Acute toxicity of various solvents in the mouse and rat. LD50 of ethanol, diethylacetamide, dimethylformamide, dimethylsulfoxide, glycerine, N-methylpyrrolidone, polyethylene glycol 400, 1,2-propanediol and Tween 20. Arzneimittelforschung 26: 1581–1583, 1976. [PubMed] [Google Scholar]
- 3.Benoit JN. Relationships between lymphatic pump flow and total lymph flow in the small intestine. Am J Physiol 261: H1970–H1978, 1991. doi: 10.1152/ajpheart.1991.261.6.H1970. [DOI] [PubMed] [Google Scholar]
- 4.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol 257: H2059–H2069, 1989. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell DL, Villarroel MA. Tables of Summary Health Statistics for U.S. Adults: 2016 National Health Interview Survey. National Health Interview Survey. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Health Statistics; http://www.cdc.gov/nchs/nhis/SHS/tables.htm [3/18/2017]. [Google Scholar]
- 6.Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, Detmar M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One 9: e94713, 2014. doi: 10.1371/journal.pone.0094713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 297: H1319–H1328, 2009. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol 4: 3–10, 2006. doi: 10.1089/lrb.2006.4.3. [DOI] [PubMed] [Google Scholar]
- 9.Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, Oliver G. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 1: e85096, 2016. doi: 10.1172/jci.insight.85096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav 86: 599–602, 2005. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Gad SC, Cassidy CD, Aubert N, Spainhour B, Robbe H. Nonclinical vehicle use in studies by multiple routes in multiple species. Int J Toxicol 25: 499–521, 2006. doi: 10.1080/10915810600961531. [DOI] [PubMed] [Google Scholar]
- 12.García Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, Gardenier JC, Savetsky IL, Aschen SZ, Nitti MD, Mehrara BJ. Obesity but not high-fat diet impairs lymphatic function. Int J Obes 40: 1582–1590, 2016. doi: 10.1038/ijo.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gashev AA. Lymphatic vessels: pressure- and flow-dependent regulatory reactions. Ann N Y Acad Sci 1131: 100–109, 2008. doi: 10.1196/annals.1413.009. [DOI] [PubMed] [Google Scholar]
- 14.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11: 477–492, 2004. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 15.Gashev AA, Davis MJ, Gasheva OY, Nepiushchikh ZV, Wang W, Dougherty P, Kelly KA, Cai S, Von Der Weid PY, Muthuchamy M, Meininger CJ, Zawieja DC. Methods for lymphatic vessel culture and gene transfection. Microcirculation 16: 615–628, 2009. doi: 10.1080/10739680903120778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holgate ST, Canonica GW, Simons FE, Taglialatela M, Tharp M, Timmerman H, Yanai K; Consensus Group on New-Generation Antihistamines . Consensus Group on New-Generation Antihistamines (CONGA): present status and recommendations. Clin Exp Allergy 33: 1305–1324, 2003. doi: 10.1046/j.1365-2222.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- 19.Ji Y, Sakata Y, Yang Q, Li X, Xu M, Yoder S, Langhans W, Tso P. Activation of rat intestinal mucosal mast cells by fat absorption. Am J Physiol Gastrointest Liver Physiol 302: G1292–G1300, 2012. doi: 10.1152/ajpgi.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassis T, Yarlagadda SC, Kohan AB, Tso P, Breedveld V, Dixon JB. Postprandial lymphatic pump function after a high-fat meal: a characterization of contractility, flow, and viscosity. Am J Physiol Gastrointest Liver Physiol 310: G776–G789, 2016. doi: 10.1152/ajpgi.00318.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy L, Hargrove L, Demieville J, Bailey JM, Dar W, Polireddy K, Chen Q, Nevah Rubin MI, Sybenga A, DeMorrow S, Meng F, Stockton L, Alpini G, Francis H. Knockout of l-histidine decarboxylase prevents cholangiocyte damage and hepatic fibrosis in mice subjected to high-fat diet feeding via disrupted histamine/leptin signaling. Am J Pathol 188: 600–615, 2018. doi: 10.1016/j.ajpath.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy LL, Hargrove LA, Graf AB, Francis TC, Hodges KM, Nguyen QP, Ueno Y, Greene JF, Meng F, Huynh VD, Francis HL. Inhibition of mast cell-derived histamine secretion by cromolyn sodium treatment decreases biliary hyperplasia in cholestatic rodents. Lab Invest 94: 1406–1418, 2014. doi: 10.1038/labinvest.2014.129. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz KH, Moor AN, Souza-Smith FM, Breslin JW. Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation 21: 593–605, 2014. doi: 10.1111/micc.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JS. Motility, lymphatic contractility, and distention pressure in intestinal absorption. Am J Physiol 208: 621–627, 1965. doi: 10.1152/ajplegacy.1965.208.4.621. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y, Fluckey JD, Chakraborty S, Muthuchamy M. Hyperglycemia- and hyperinsulinemia-induced insulin resistance causes alterations in cellular bioenergetics and activation of inflammatory signaling in lymphatic muscle. FASEB J 31: 2744–2759, 2017. doi: 10.1096/fj.201600887R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261: 255–269, 1976. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mells JE, Fu PP, Kumar P, Smith T, Karpen SJ, Anania FA. Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J Nutr Biochem 26: 285–292, 2015. doi: 10.1016/j.jnutbio.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17: 920–922, 2003. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- 29.Nitti MD, Hespe GE, Kataru RP, García Nores GD, Savetsky IL, Torrisi JS, Gardenier JC, Dannenberg AJ, Mehrara BJ. Obesity-induced lymphatic dysfunction is reversible with weight loss. J Physiol 594: 7073–7087, 2016. doi: 10.1113/JP273061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nizamutdinova IT, Dusio GF, Gasheva OY, Skoog H, Tobin R, Peddaboina C, Meininger CJ, Zawieja DC, Newell-Rogers MK, Gashev AA. Mast cells and histamine are triggering the NF-κB-mediated reactions of adult and aged perilymphatic mesenteric tissues to acute inflammation. Aging (Albany NY) 8: 3065–3090, 2016. doi: 10.18632/aging.101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, Chatterjee V, Meininger CJ, Gashev AA. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 21: 640–648, 2014. doi: 10.1111/micc.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nizamutdinova IT, Maejima D, Nagai T, Meininger CJ, Gashev AA. Histamine as an endothelium-derived relaxing factor in aged mesenteric lymphatic vessels. Lymphat Res Biol 15: 136–145, 2017. doi: 10.1089/lrb.2016.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noel PR, Barnett KC, Davies RE, Jolly DW, Leahy JS, Mawdesley-Thomas LE, Shillam KW, Squires PF, Street AE, Tucker WC, Worden AN. The toxicity of dimethyl sulphoxide (DMSO) for the dog, pig, rat and rabbit. Toxicology 3: 143–169, 1975. doi: 10.1016/0300-483X(75)90081-5. [DOI] [PubMed] [Google Scholar]
- 34.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311: 806–814, 2014. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988-1994 Through 2013–2014. JAMA 315: 2292–2299, 2016. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol 239: H88–H95, 1980. doi: 10.1152/ajpheart.1980.239.1.H88. [DOI] [PubMed] [Google Scholar]
- 37.Petunov SG, Egorova AA, Orlov RS, Nikitina ER. Effect of histamine on spontaneous contractions of mesenteric lymphatic vessels and lymph nodes of white rats: endothelium-dependent responses. Dokl Biol Sci 432: 176–180, 2010. doi: 10.1134/S0012496610030038. [DOI] [PubMed] [Google Scholar]
- 38.Plaku KJ, von der Weid PY. Mast cell degranulation alters lymphatic contractile activity through action of histamine. Microcirculation 13: 219–227, 2006. doi: 10.1080/10739680600556902. [DOI] [PubMed] [Google Scholar]
- 39.Quick CM, Venugopal AM, Gashev AA, Zawieja DC, Stewart RH. Intrinsic pump-conduit behavior of lymphangions. Am J Physiol Regul Integr Comp Physiol 292: R1510–R1518, 2007. doi: 10.1152/ajpregu.00258.2006. [DOI] [PubMed] [Google Scholar]
- 40.Ratliff JC, Barber JA, Palmese LB, Reutenauer EL, Tek C. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring) 18: 2398–2400, 2010. doi: 10.1038/oby.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, Joseph WJ, Mehrara BJ. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol 307: H165–H172, 2014. doi: 10.1152/ajpheart.00244.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med 4: 624–630, 2013. [PMC free article] [PubMed] [Google Scholar]
- 43.Troyanova-Wood M, Gobbell C, Meng Z, Gashev AA, Yakovlev VV. Optical assessment of changes in mechanical and chemical properties of adipose tissue in diet-induced obese rats. J Biophotonics 10: 1694–1702, 2017. doi: 10.1002/jbio.201600281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tso P, Pitts V, Granger DN. Role of lymph flow in intestinal chylomicron transport. Am J Physiol 249: G21–G28, 1985. doi: 10.1152/ajpgi.1985.249.1.G21. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 29: 6–28, 2007. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 46.Zawieja SD, Castorena-Gonzalez JA, Scallan JP, Davis MJ. Differences in L-type Ca2+ channel activity partially underlie the regional dichotomy in pumping behavior by murine peripheral and visceral lymphatic vessels. Am J Physiol Heart Circ Physiol 314: H991–H1010, 2018. doi: 10.1152/ajpheart.00499.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zawieja SD, Gasheva O, Zawieja DC, Muthuchamy M. Blunted flow-mediated responses and diminished nitric oxide synthase expression in lymphatic thoracic ducts of a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 310: H385–H393, 2016. doi: 10.1152/ajpheart.00664.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 302: H643–H653, 2012. doi: 10.1152/ajpheart.00606.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zweifach BW, Prather JW. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am J Physiol 228: 1326–1335, 1975. doi: 10.1152/ajplegacy.1975.228.5.1326. [DOI] [PubMed] [Google Scholar]