Abstract
The irritable bowel syndrome (IBS) is a functional gastrointestinal motor and visceral sensation disorder that is more common in women than men. Female serotonin transporter (SERT)-gene knockout (KO) rats exhibit hypersensitivity to colorectal balloon distention (CRD) that mimics colonic hypersensitivity occurring in female IBS patients. Alosetron (5-HT3 receptor antagonist) is used to treat diarrhea-predominant IBS in female patients. Other 5-HT3 receptor antagonists are ineffective at treating IBS symptoms. The visceromotor response (VMR) to CRD in SERT-KO and wild-type (WT) rats was measured following subcutaneous (sc), intracerobroventricular (icv), or intrathecal (it) treatment with 5-HT3 receptor antagonists and an agonist. Alosetron (sc) and granisetron (antagonists) caused a paradoxical increase in the VMR to CRD in SERT-KO female rats. Alosetron (sc) increased the VMR to CRD in WT male rats. Alosetron (it) increased the VMR to CRD in SERT-KO female rats only, and the 5-HT3 receptor agonist SR-52772 increased the VMR to CRD in SERT-KO male rats. Depletion of spinal 5-HT using 5,7-dihydroxytryptamine prevented the increase in VMR to CRD in SERT-KO female and male rats treated it with alosetron and SR-52772, respectively. Alosetron (icv) did not affect the VMR to CRD in WT or KO female rats, but it increased the VMR in male SERT-KO but not WT male rats. These data suggest that 5-HT3 receptor signaling at the dorsal spinal cord mediates visceral hypersensitivity in female SERT-KO rats. Such differences could facilitate development of sex-specific drug treatments for visceral pain.
NEW & NOTEWORTHY We studied a model of female sex-specific visceral hypersensitivity using rats that had a loss of function of the serotonin transporter (SERT) caused by gene truncation. Female SERT-KO rats exhibited visceral hypersensitivity in response to colorectal balloon distention. We found that increased 5-HT signaling at dorsal spine 5-HT3 receptors was responsible for visceral hypersensitivity in female but not male SERT-KO rats.
Keywords: 5-hydroxytryptamine receptors, irritable bowel syndrome, serotonin transporter, sex differences, visceral pain
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by altered bowel habits and abdominal pain, especially in women (15). 5-Hydroxytryptamine (5-HT) signaling at 5-HT3 receptors may contribute to visceral pain observed in IBS patients. 5-HT3 receptors are expressed by central and peripheral neurons (32, 39), including enteric neurons and the peripheral and central terminals of spinal and vagal primary afferent neurons that innervate the gut (23). Thus, 5-HT3 receptors modulate gut motility and visceral pain signaling (9, 43).
5-HT3 receptors are heteropentameric ligand-gated cation channels that belong to the Cys-loop receptor family, which includes GABAA receptors and nicotinic acetylcholine receptors (32). At the time of their discovery, it was concluded that pentameric 5-HT3 receptors were comprised only of 5-HT3A subunits. After the 5-HT3A receptor subunit was cloned, other subunits and splice variants were discovered, confirming the existence of multiple 5-HT3 receptor subtypes. In humans, five subunits (A–E) have been identified, some of which have splice variants. Subunits A–C are expressed centrally and peripherally, whereas the D and E subunits are localized only to the gastrointestinal tract (5). Functional 5-HT3 receptors require the A subunit. Polymorphisms in the A and E subunits are associated with increased IBS/diarrhea risk (14, 29), and polymorphisms in the 5-HT3A and 5-HT3E genes increase 5-HT3 receptor density in the membrane of transfected human embryonic kidney-293 cells, suggesting that receptor upregulation could occur in IBS patients with these polymorphisms (33).
In the United States, alosetron is approved for the treatment of severe IBS/diarrhea in female patients only (47). Furthermore, the mechanism by which alosetron exerts its visceral anti-nociceptive effect in females is unknown. c-Fos expression is increased in the dorsal spinal cord of rats undergoing colorectal balloon distention (CRD), and this response was inhibited by alosetron administered intravenously. This result suggests that alosetron can act the spinal level to block visceral afferent nociceptive signaling (37). Positron emission tomography (PET) scans measure increases in blood flow as an indicator of activation of discreet brain regions. PET scans in IBS patients showed that alosetron reduces cerebral blood flow in the left anterior insula, which is a viscerosensory region of the brain involved in emotional responses during anticipation of rectal distension. In addition, alosetron caused an inhibitory effect in the ventromedial frontal cortex at baseline and during anticipation but not during balloon inflation, suggesting an inhibitory effect on autonomic and emotional processing networks (41). These data suggest that peripheral 5-HT3 receptors are targets for alosetron actions in reducing visceral pain (37), whereas central sites of action mediate the emotional responses to visceral pain (7, 42). The contribution of 5-HT3 receptors in visceral pain has been studied in animals. Intraplantar formalin injection or intrathecal injection of 5-HT produces pain-related behaviors (paw licking and scratching) in wild-type mice, whereas these somatic pain behaviors are significantly reduced in 5-HT3A subunit-knockout mice. These data support a pro-nociceptive role for spinal 5-HT3 receptors (57). Also, mice treated with dextran sulfate sodium solution to induce colonic inflammation exhibit increased visceral pain behavior and increased 5-HT3 receptor expression in dorsal root ganglia (41). Similarly, rats treated with intracolonic acetic acid develop colitis and exhibit 5-HT3-mediated increases in visceral sensitivity (17, 26). Kozlowski et al. (37) showed that rats injected intramuscularly (im) with an acidic saline (pH = 4) develop somatic and visceral hypersensitivity. Intravenous (iv) and intrathecal (it) treatment with alosetron increased von Frey hair paw withdrawal thresholds in rats following intraplantar acetic acid injections. In addition, alosetron (iv and it) reduced the visceromotor response (VMR) to CRD in rats after im acidic saline injections. These data indicate that alosetron acts centrally to reverse somatic and visceral hyperalgesia. In another study, infusion of 5-hydroxytryptophan caused bladder hyperalgesia in female rats, and this response was attenuated by it but not subcutaneous (sc) ondansetron, suggesting a pro-nociceptive role of spinal 5-HT3 receptors (31). Bradesi et al. (8) showed that spinal 5-HT3 receptors mediate sensitization of spinal afferents in response to water avoidance stress, and upregulation of a spino-bulbo-spinal loop enhances visceral pain perception via 5-HT3 receptor signaling in male rats.
Saria et al. (48) showed that spinal 5-HT3 receptor activation releases substance P, neurokinin A, and calcitonin gene-related peptide from primary afferent nerves. These peptides increase pain transmission to corroborate the spino-bulbo-spinal loop theory. Other 5-HT receptors expressed in the spinal cord can modulate pain signaling. For instance, 5-HT1A receptor agonists reduce somatic pain signaling, whereas 5-HT3 receptor signaling is pro-nociceptive (46). Depletion of spinal 5-HT by the neurotoxin 5,7-dihydroxytryptamine (5,7-DHT) reversed the anti-nociceptive effects of 5-HT3 receptor antagonists, suggesting that endogenous 5-HT activates 5-HT3 receptors to enhance visceral and somatic pain transmission at the spinal level. 5,7-DHT rats treated with a high dose of 5-HT administered it developed somatic hyperalgesia (biting and licking following intraplantar formalin injection) via 5-HT3 receptor activation, whereas low-dose 5-HT was anti-nociceptive by acting at 5-HT1A receptors (46, 49). Data from other studies suggest that 5-HT signaling at spinal 5-HT3 receptors can be anti-nociceptive (3). Mice treated it with 5-HT or 2-methyl-5-HT (a 5-HT3 receptor agonist) had increased tail flick latencies (a somatic pain measure) and decreased pain behaviors (biting and scratching) in response to it administration of N-methyl-d-aspartate or substance P. This increase in tail flick latency and decreased pain behaviors were reversed following treatment with 5-HT3 receptor antagonists (3). In rats, hot plate latency (a measure of somatic pain) was increased following it treatment with 5-HT or 2-methy-5-HT and was reversed with 5-HT3 receptor blockade (24). Both studies concluded that the anti-nociceptive effect of 5-HT3 receptor agonists was caused by GABA released from inhibitory interneurons as a GABAA receptor antagonist blocked the analgesic effects of 5-HT3 receptor agonist (3, 24).
Clinical and basic research studies have shown that the role of 5-HT3 receptors in pain modulation is unclear, as results using 5-HT3 receptor ligands are dependent on the animal model, pain assay, and route of drug administration. In addition, IBS is more common in women compared with men, but most studies of IBS models using rats or mice are done using male animals only. Serotonin transporter-knockout (SERT-KO) rats circumvent these issues, as inflammation or stress is not required to induce visceral hypersensitivity specific for female rats (21). We used male and female SERT-KO rats to test the hypothesis that 5-HT3 receptor signaling contributes to visceral hypersensitivity and that this effect is female sex specific.
MATERIALS AND METHODS
Wild-type and SERT-KO rats.
All animal use protocols were approved by the Institutional Animal Use and Care Committee at Michigan State University. Serotonin transporter (SERT)-knockout (KO) and wild-type (WT) control rats were purchased under license from Genoway (http://genoway.com/) and were bred to maintain a colony at Michigan State University. Age-matched 3- to 4-mo-old rats were used for all experiments. Male and female rats in this age group weighed ∼350 and 220 g, respectively.
EMG electrode implantation.
Rats were anesthetized using 4% isoflurane gas and placed on a heating pad (∼30°C). A midscapular incision was made, followed by a ventral midline incision. A hemostat was used to gently separate the skin from the muscles. Teflon-coated silver wires (A-M systems, Sequim, WA) were stitched into the external oblique muscles above the inguinal ligaments, and the incision was closed with wound clips. The electrodes were tunneled sc, secured to the back musculature, and externalized in the midscapular region for future access. Sutures were used to close the midscapular incision. Carprofen (5 mg/kg sc) and piperacillin-tazobactam (120 mg/kg im) were administered postsurgery to minimize pain and infection risk, respectively. Rats were housed individually and allowed to recover for 3 days before visceromotor response to colorectal distension was assessed.
Visceromotor response to colorectal distension.
Under light isoflurane anesthesia, a flexible and lubricated latex balloon (4.5 cm, females; 7 cm, males) was inserted into the distal colon 1 cm past the anal sphincter and secured to the base of the tail using surgical tape. Balloon size differed for male and female rats, as male rats were larger than the female rats (see body weight above). After recovery from anesthesia, rats were placed in the plexiglass restrainer and allowed to acclimate for 30 min before experiments were stared. The balloon was connected via a flexible tube to an animal barostat (G & J Electronics, Toronto, ON, Canada), and the abdominal muscle electrode leads were connected to an amplifier (7P1, Grass-Instruments-Astro-Med, West Warwick, RI). The barostat and amplifier were connected to an A/D converter amplifier (Digidata 1223A; Molecular Devices, Sunnyvale, CA), and signals were digitized at 1 kHz and recorded using Axoscope 10 software (Molecular Devices). The visceromotor response (VMR) to colorectal distension (CRD) was assessed before and after drug treatments. The CRD protocol consisted of a series of five phasic distensions at pressures of 10, 20, 40, 60, and 80 mmHg (10-s duration; 5-min interval between distension). The VMR to CRD was measured by recording electromyographic (EMG) activity of the external oblique muscles 10 s before, 10 s during, and 10 s after each distension episode. The EMG signal was rectified, and the area under the curve was calculated using Clampfit 10 (Molecular Devices) and normalized to the 10-s baseline.
Intrathecal drug injection.
Rats were lightly anesthetized with 4% isoflurane, and the hair along the dorsal aspect of the lower spine was clipped. The L5–L6 intervertebral space was exaggerated by placing a 50-ml centrifuge tube under the lower limbs. A 27-G half-inch needle connected to a 50-μl glass syringe was gently inserted into the vertebral canal. Tail flick confirmed entry of the needle into the vertebral canal. Drugs were dissolved in 5–10 μl of solvent and administered using a 50-μl glass syringe to minimize back flow of CSF upon removal of the needle following injection.
Intracebroventicular drug injections.
Cannulas were implanted intracebroventicularly (icv) in rats under 4% isoflurane anesthesia and using a stereotaxis apparatus. Surgery was conducted on a heating pad (∼30°C), an incision was made to expose the skull, and a hole was drilled to place the cannula in the lateral ventricle. Jeweler’s screws and dental cement were used to anchor the cannula in place. Carprofen (5 mg/kg sc) and piperacillin/tazobactam (120 mg/kg im) were used to reduce pain and infection risk, respectively.
Cerebrospinal fluid collection.
Rats were deeply anesthetized with 4% isoflurane and positioned in a stereotaxic apparatus to exaggerate the atlanto-occiptal (AO) joint. A midline incision was made at the base of the skull, and retractor was used to expose the AO joint. A pulled capillary was used to puncture the ligaments and access the cerebrospinal fluid (CSF) in the cisterna magna. CSF samples were collected in Eppendorf tubes and stored at −80°C.
5-HT and norepinephrine extraction from spinal cord.
Rats were euthanized, and lumbosacral spinal cord was isolated and weighed on ice. Spinal cord samples were then treated with 30% tricholoractetic acid solution and homogenized using bead ruptor (Omni inc) at 4°C. The samples were centrifuged for 10 min and eluent collected and recentrifuged using optima max ultracentrifuge (Beckman-Coulter) for 1 h at 4°C. The eluent was stored at −80°C for HPLC analysis.
Drug treatments.
Rats were acclimated to the plexiglass restrainer in 1-h training sessions on days 3 and 4 post-abdominal electrode implantation. Alosetron (0.1 mg/kg) (52), granisetron (0.1 mg/kg) (38), and sterile saline vehicle were administered sc in random order. On days 5, 8, and 11, VMR to CRD was assessed before and after treatment with each drug or saline. Rats were euthanized on day 11 following the final experiment.
Ramosetron (0.01, 0.1 and 1 mg/kg) and sterile saline were administered sc to each rat in random order. We chose these doses based on the limited blood-brain barrier permeability of this drug (54) and our measurements of CSF levels of ramosetron in rats treated with each dose. Rats were equipped with electrodes on day 1. On days 3 and 4, rats were acclimated to the restrainer for 1 h each day. On days 5, 8, 11, and 14, VMR to CRD was assessed before and after treatment with the drug or vehicle. Rats were euthanized on day 14 following the final experiment. A similar procedure was performed on another group of rats to determine the alosetron dose-response curve. Alosetron (0.01, 0.1, and 1 mg/kg sc) or sterile saline vehicle was given to each rat. For it studies, alosetron, granisetron, and ondansetron were administered in random order at a dose of 25 nmol in 10 μl of sterile saline.
In another group of rats, EMG recording electrodes and an icv cannula were implanted 1.5 mm lateral and 1.5 mm sagittal to bregma and 3 mm from the skull surface in the right lateral cerebral ventricle on day 1 (51). Rats recovered for 6 days postsurgery. On days 7 and 8, rats were acclimated to the restrainer for 1 h each day. On days 9, 12, and 15, rats were treated icv with alosetron (25 nmol/10 μl), alosetron (2.5 nmol/10 μl), or 10 μl of sterile saline vehicle in random order.
In the last group of rats, EMG electrodes were implanted on day 1. On day 3, rats were treated with desipramine (25 mg/kg) via an ip injection (16). Desipramine is a selective norepinephrine reuptake inhibitor used to limit the uptake of 5,7-dihdroxytryptamine (5,7-DHT) to serotonergic neurons. Forty-five minutes after desipramine treatment, rats were treated it with 100 μg of 5,7-DHT dissolved in 10 μl of sterile saline or sterile saline only (10 μl) (16). Rats recovered for 7 days following 5,7-DHT treatment. On days 8 and 9 rats were acclimated to the restrainer, and on days 10, 13, and 16 rats were treated with alosetron (25 nmol it) (SR-57227; (100 pmol it) (16) or sterile saline in random order. VMR to CRD was recorded before and after treatment.
High-performance liquid chromatography.
Ramosetron levels were measured using a Phenomenex Luna 5u C-18 250 × 4.6 mm column. A photo diode array detector was used, and ramosetron was detected using a 213-nm wavelength. The mobile phase was 70% 0.05M sodium phosphate (pH 5.2) and 30% acetonitrile with flow rate at 1.0 ml/min. 5-HT detection was performed using a Thermo Scientific ODS Hypersil 3 μm, 150 × 3 mm column with detection at 200 mV. The mobile phase used was 90 mM dihydrogen sodium phosphate, 50 mM citrate, 50 μM EDTA, and 1.7 mM sodium octyl sulfate with 10% acetonitrile at a flow rate at 0.6 ml/min. Norepinephrine was detected using a Thermo Scientific HR-80 3 μm 80 × 4.6 mm column with Cat-A-Phase II mobile phase with flow rate at 1.1 ml/min and detection at −300 mV.
5-HT was measured using Thermo Scientific ODS Hypersil 3 μm 150 × 3 mm column with detection at 200 mV. The mobile phase was 90 mM dihydrogen sodium phosphate, 50 mM citrate, 50 μM EDTA, and 1.7 mM sodium octyl-sulfate with 10% acetonitrile at flow rate of 0.6 ml/min.
Statistics.
EMG recordings of the VMR to CRD were analyzed using two-way repeated-measures ANOVA and Bonferonni’s post hoc test. We used six to eight animals per group to achieve a power of 0.8, unless specified otherwise. Data are means ± SE. High-performance liquid chromatography (HPLC) data were analyzed using Student’s t-test. For all studies, P < 0.05 was considered statistically significant.
RESULTS
5-HT3 receptor antagonists administered sc increase the VMR to CRD in female SERT-KO rats.
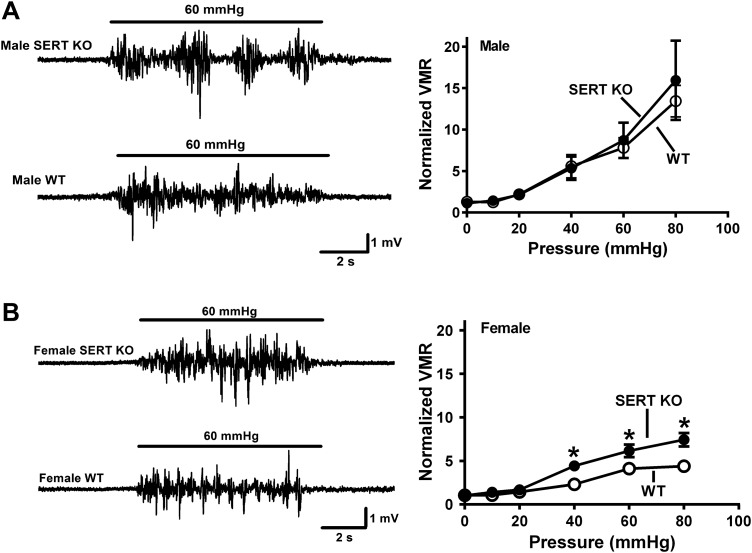
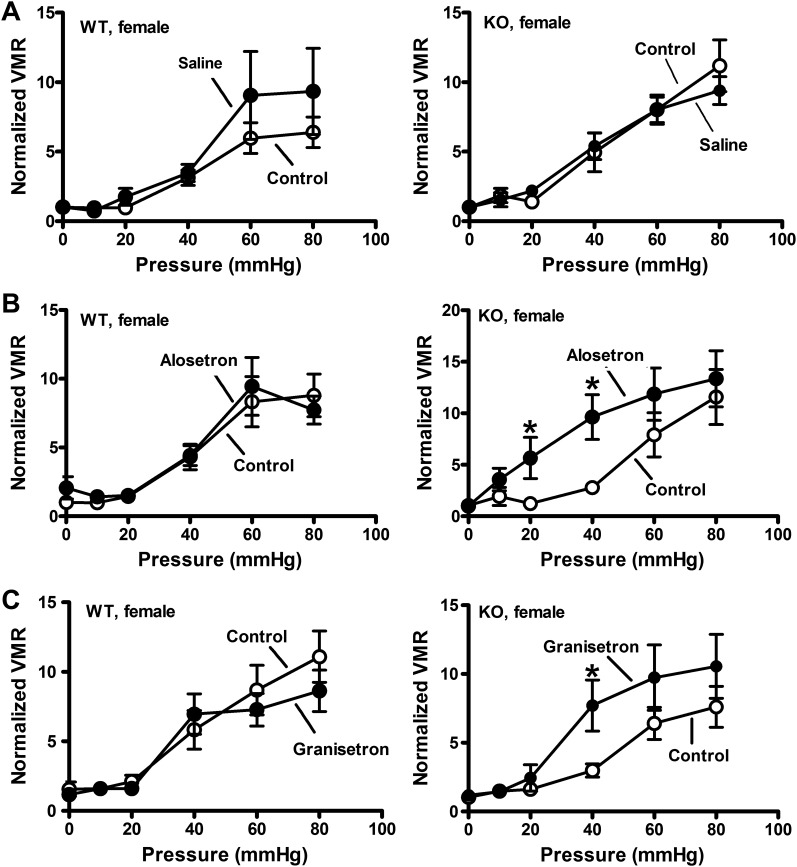
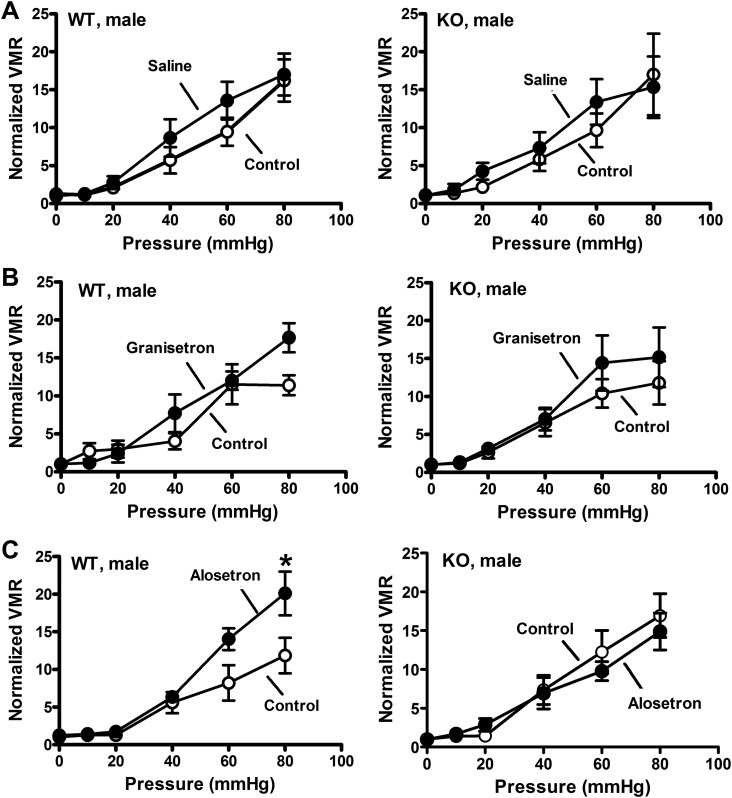
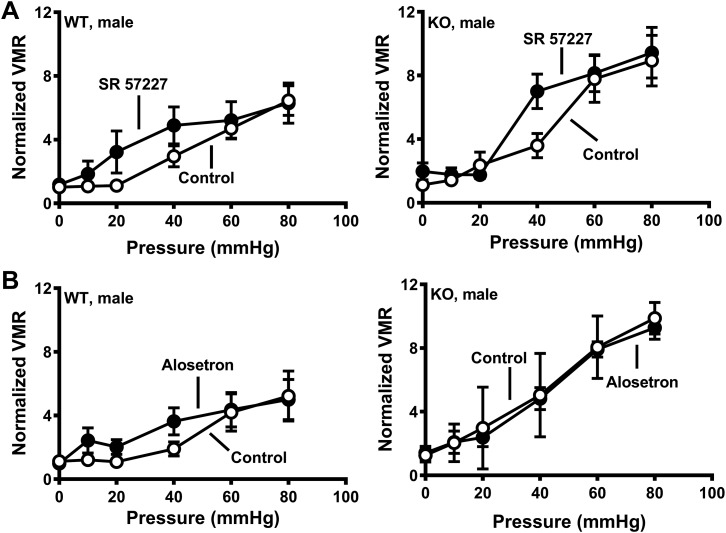
Initial studies verified sex-specific visceral hypersensitivity to CRD in SERT-KO female rats. These studies showed that the VMR in WT and SERT-KO male rats was similar (Fig. 1A), whereas female SERT-KO rats exhibited a greater VMR response to CRD compared with WT female rats (Fig. 1B). We next used 5-HT3 receptor antagonists administered sc to test the hypothesis that blocking 5-HT3 receptors would decrease the VMR to CRD. Saline injections did not alter the VMR in female WT or SERT-KO rats (Fig. 2A), whereas alosetron and granisetron (each at 0.1 mg/kg sc) increased the VMR to CRD in SERT-KO but not WT female rats (Fig. 2, B and C). Ondansetron (0.1 mg/kg sc) did not change the VMR to CRD in SERT-KO female rats (data not shown). Saline and granisetron (0.1 mg/kg sc) did not change the VMR to CRD in WT or SERT-KO male rats (Fig. 3, A and B), whereas alosetron (0.1 mg/kg sc) increased the VMR to CRD in WT but not SERT-KO male rats (Fig. 3C).
Fig. 1.
Enhanced visceromotor response to colorectal balloon distention in female but not male serotonin transporter-knockout (SERT-KO) rats. A: representative recordings of the visceromotor response (VMR) to colorectal balloon distention (CRD) obtained from a SERT-KO male rat (top trace) and a wild-type (WT) male rat (bottom trace). Bar indicates period of CRD (60 mmHg). Pressure response curves obtained from male SERT-KO (n = 8) and WT (n = 7) rats are shown on the right. There were no differences between these curves. B: leftward shift in the pressure/VMR curve from female SERT-KO rats (n = 6) compared with female WT rats (n = 6). *Significantly different from WT responses (P < 0.05). Data are means ± SE.
Fig. 2.
Alosetron and granisetron increase the visceromotor response (VMR) to colorectal balloon distention (CRD) in serotonin transporter-knockout (SERT-KO) but not wild-type (WT) female rats. A: VMR recorded at the indicated pressure before (control; n = 5) and after saline treatment in WT (n = 5; left) and SERT-KO (n = 5) rats (right). B: alosetron (0.1 mg/kg sc) increased the VMR in female SERT-KO (n = 6; right) but not WT (n = 6; left) rats. C: granisetron (0.1 mg/kg sc) also increased the VMR in SERT-KO (n = 6) but not WT (n = 6) female rats. *Significantly different from control (P < 0.05). Data are means ± SE.
Fig. 3.
Subcutaneous administration of the 5-HT3 receptor antagonist alosetron increased visceromotor response (VMR) to colorectal balloon distention (CRD) in wild-type (WT) but not serotonin transporter-knockout (SERT-KO) male rats. A: VMR recorded at the indicated pressure before (control) and after saline treatment. B: granisetron (0.1 mg/kg) did not affect VMR to CRD in WT (n = 5; left) or SERT-KO (n = 5; right) male rats. C: alosetron (0.1 mg/kg) increased VMR to CRD in WT (n = 6; left) but not SERT-KO male (n = 6) rats (right). *P < 0.05 compared with control. Data are means ± SE.
5-HT3 receptor antagonists act in the central nervous system to increase the VMR to CRD.
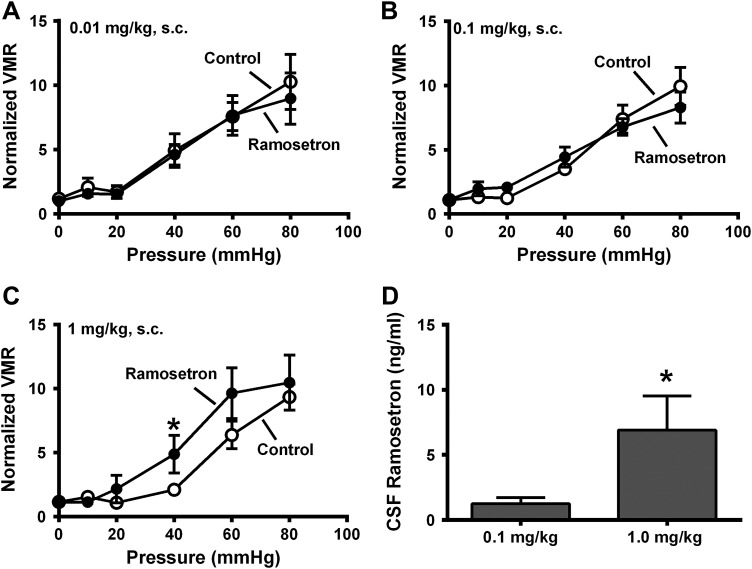
Alosetron and granisetron readily cross the blood-brain barrier (BBB) so that the increased VMR caused by these drugs might be mediated by blocking central nervous system (CNS) 5-HT3 receptors. We hypothesized that low doses (0.01 and 0.1 mg/kg) of ramosetron (which would not cross the BBB) would not affect the VMR to CRD, whereas at 1 mg/kg some ramosetron would cross the BBB and increase the VMR to CRD. We tested this hypothesis in female rats only as they showed the genotype-specific visceral hypersensitivity. We also measured ramosetron levels in the cerebrospinal fluid (CSF) using HPLC. Ramosetron at 0.01 and 0.1 mg/kg (Fig. 4, A and B) did not affect the VMR to CRD, whereas at 1 mg/kg sc, ramosetron increased this response (Fig. 4C). CSF measurements revealed low levels of ramosetron after the 0.1 mg/kg dose, whereas CSF levels of ramosetron were significantly increased following the 1 mg/kg dose (Fig. 4D).
Fig. 4.
Dose-response curves for effects of ramosetron (sc) on the visceromotor response (VMR) in serotonin transporter-knockout (SERT-KO) female rats. A–C: VMR recorded at the indicated pressure before (control) and after treatment with ramosetron at 0.01 (n = 6; A), 0.1 (n = 6; B), and 1.0 mg/kg (n = 6; C). Ramosetron increased the VMR to colorectal balloon distention (CRD) only at the 1 mg/kg dose. D: high-performance liquid chromatography analysis of cerebrospinal fluid (CSF) indicates that ramosetron was detected after the 0.1 and 1.0 mg/kg doses. *Significantly different from control for VMR or significantly different from 0.1 mg/kg. (P < 0.05). Data are means ± SE.
5-HT3 receptor antagonists at the spinal level to increase the VMR to CRD.
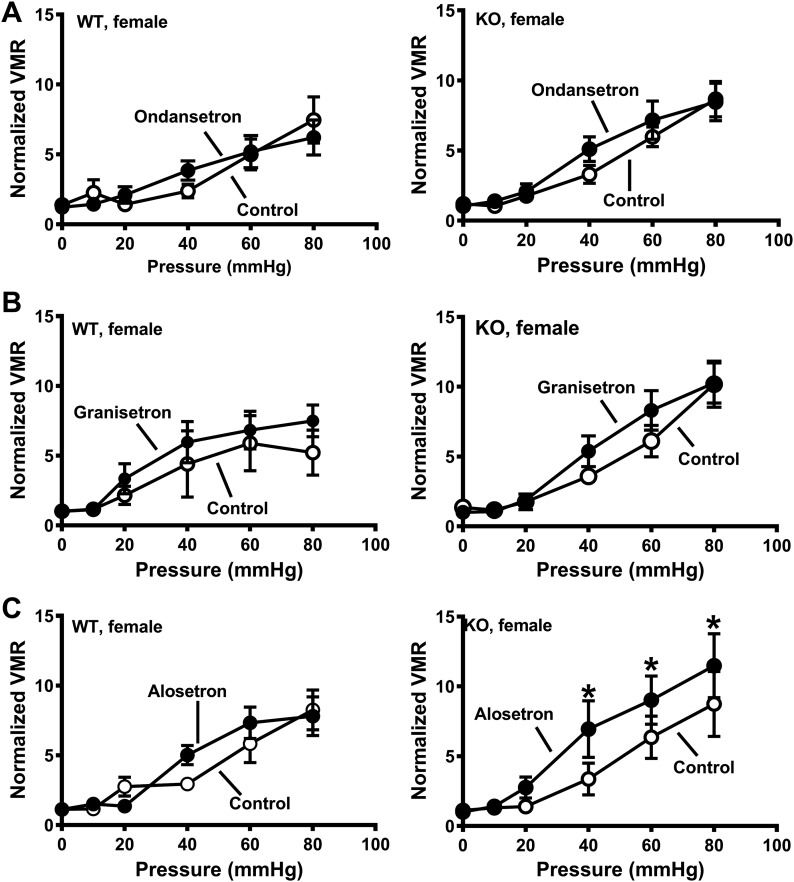
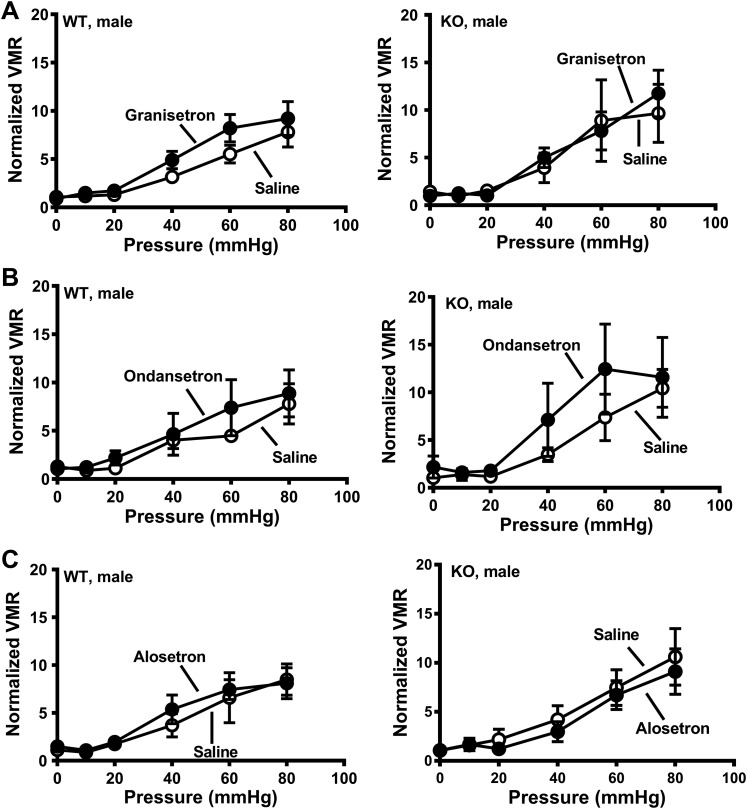
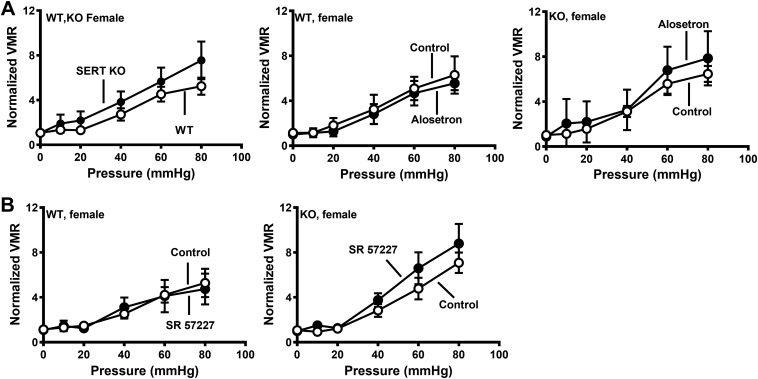
A more direct way of testing the effects of central inhibition of 5-HT3 receptors is to directly administer the drugs into the CNS. This was accomplished using intrathecal (it) drug injections. Alosetron, granisetron, ondansetron (each at 25 nmol), or saline was administered it to SERT-KO and WT rats. Neither ondansetron or granisetron altered visceral sensitivity in female WT or KO rats (Fig. 5A), whereas alosetron increased the VMR to CRD in SERT-KO female rats only (Fig. 5C). Intrathecal administration of alosetron, granisetron, and ondansetron (each at 25 nmol) did not affect VMR to CRD in male rats regardless of genotype (Fig. 6).
Fig. 5.
Intrathecal administration of alosetron, but not granisetron or ondansetron, increased visceromotor response (VMR) to colorectal balloon distention (CRD) in serotonin transporter-knockout (SERT-KO) but not wild-type (WT) female rats. A: ondansetron (25 nmol) did not change the VMR recorded from WT (n = 6; left) and SERT-KO (right; n = 8) rats. B: granisetron (25 nmol) did not affect VMR in WT (n = 5; left) or SERT-KO (n = 5; right) female rats. C: alosetron did not affect VMR to CRD in WT female rats (left; n = 5) but increased VMR to CRD in SERT-KO female rats (n = 8). *Significantly different than control (P < 0.05). Data are means ± SE.
Fig. 6.
Intrathecal administration of 5-HT3 receptor antagonists did not affect visceromotor response (VMR) to colorectal balloon distention (CRD) in wild-type (WT) or serotonin transporter-knockout (SERT-KO) male rats. VMR recorded at the indicated pressure before (control) and after treatment with the 5-HT3 receptor antagonists granisetron (25 nmol; n = 7 WT and n = 5 KO; A), ondansetron (25 nmol; n = 4 WT and n = 5, KO; B), and alosetron (25 nmol; n = 6 WT and n = 6 KO; C). Data are means ± SE.
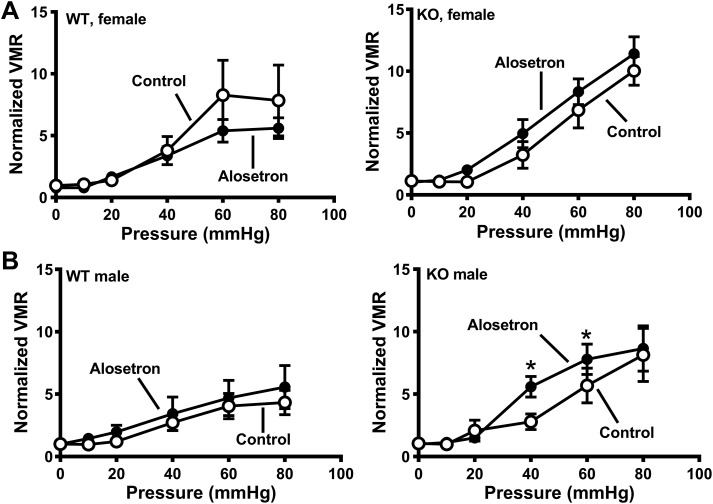
Drugs administered it circulate throughout the central nervous system via CSF. Therefore, the increase in VMR to CRD observed with it alosetron could be mediated in the brain or brainstem. To test this possibility, we administered alosetron icv to SERT-KO and WT rats. Confirmation of proper placement of the icv cannula was determined at the end of the last experiments by injecting methylene blue and inspection of the ventricles. Alosetron administered icv did not affect the VMR to CRD in WT or KO female rats or WT male rats (Fig. 7). However, alosetron increased the VMR to CRD in SERT-KO male rats. (Fig. 7B).
Fig. 7.
Intracerebroventricular administration of alosetron increased the visceromotor response (VMR) to colorectal balloon distention (CRD) in serotonin transporter-knockout (SERT-KO) male rats but not female SERT-KO or wild-type (WT) rats. VMR recorded at the indicated pressure before (control) and after treatment with alosetron (25 nmol) in WT (n = 5) and KO (n = 7) female rats (A) or in WT (n = 5) and KO (n = 6) male rats (B). *P < 0.05 vs. control. Data are means ± SE.
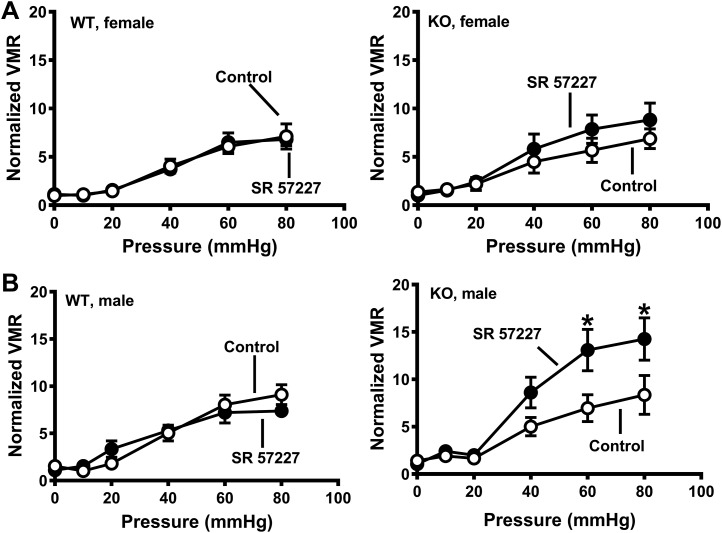
Beause 5-HT3 receptor antagonists increased the VMR to CRD in SERT-KO female rats, we wanted to test whether a 5-HT3 receptor agonist would decrease visceral hypersensitivity. The 5-HT3 receptor agonist SR-57227 (100 pmol) administered it caused an increase in VMR to CRD in SERT-KO males but not SERT-KO female rats or WT rats (Fig. 8).
Fig. 8.
Intrathecal administration of the 5-HT3 receptor agonist SR-57227 increased visceromotor response (VMR) to colorectal balloon distention (CRD) in male serotonin transporter-knockout (SERT-KO) rats. A: SR-57227 (100 pM) did not affect the VMR recorded from wild-type (WT) (n = 4; left) or SERT-KO (n = 5; right) female rats. B: SR-57227 increased the VMR in SERT-KO male (n = 5; right) but not WT male rats (n = 4; left). *Significantly different from control (P < 0.05). Data are means ± SE.
Spinal 5-HT depletion prevents visceral hypersensitivity.
Blocking the action of 5-HT released in the dorsal spinal cord from the descending projection of caudal raphe serotonergic neurons synapsing with inhibitory interneurons might be the mechanism by which alosetron increases VMR to CRD in SERT-KO female rats. To test this hypothesis, the effects of it alosetron on VMR to CRD were assessed after spinal 5-HT nerve terminals were reduced using the neurotoxin 5,7-DHT administered it (16). We pretreated the rats with desipramine, a norepinephrine transporter inhibitor used to restrict the actions of 5,7-DHT to 5-HT nerve terminals (16). 5,7-DHT significantly reduced5-HT levels in the lumbosacral spinal cord (Table 1), and 5-HT depletion was greater in the spinal cord of WT rats. HPLC analysis of lumbosacral spinal cord homogenates showed that 5-HT was reduced by 60% in WT female rats, 40% in WT male rats, 32% in SERT-KO male rats, and 25% in SERT-KO female rats. Norepinephrine levels were not affected by 5,7-DHT (Table 1).
Table 1.
5-HT and norepinephrine levels in the spinal cord of control and 5,7-DHT WT and SERT-KO rats
| 5-HT Levels (n) |
Norepinephrine Levels (n) |
|||
|---|---|---|---|---|
| Control | 5,7-DHT treated | Control | 5,7-DHT treated | |
| WT male | 279 ± 33 (5) | 140 ± 40 (4)* | 133 ± 41 (2) | 132 ± 11 (3) |
| SERT-KO male | 161 ± 34 (5) | 87 ± 7 (5) | 158 + 39 (2) | 101 ± 9 (3) |
| WT female | 224 ± 38 (5) | 72 ± 18 (5)* | 121 ± 24 (2) | 144 + 30 (5) |
| SERT-KO female | 178 ± 10 (7) | 122 ± 9 (6)* | 190 ± 13 (3) | 182 ± 25 (5) |
Values are means ± SE. 5,7-DHT, 5,7-dihydroxytryptamine; 5-HT, 5-hydroxytryptamine; SERT-KO, serotonin transporter-knockout; WT, wild type.
Significantly different from control rats.
Functional studies showed that the 25% reduction in 5-HT was sufficient to prevent the increase in VMR to CRD in SERT-KO female rats (Fig. 9A, left). Spinal 5-HT depletion did not change WT female rat sensitivity to alosetron (Fig. 9A, middle), but spinal 5-HT depletion did prevent the increased VMR to CRD caused by alosetron in female SERT-KO rats (Fig. 9A, right). The 5-HT3 receptor agonist, SR 57227 (100 pmol it) did not alter the VMR to CRD in female WT (Fig. 9B, left) or SERT-KO (Fig. 9B, right) that had been treated it with 5,7-DHT. SR-5727 (100 pmol it) did not change the VMR to CRD in WT or SERT-KO male rats treated it with 5,7-DHT to deplete spinal 5-HT (Fig. 10A). Similarly, alosetron administered it did not change the VMR to CRD in WT or KO male rats treated with 5,7-DHT (Fig. 10B, left and right).
Fig. 9.
A, left: intrathecal 5,7-dihydroxytryptamine (5,7-DHT) treatment blocked the increased visceral sensitivity in serotonin transporter-knockout (SERT-KO) female rats. A, middle: 5-HT depletion following 5,7-DHT treatment blocked the increase in visceromotor response (VMR) to colorectal balloon distention (CRD) in female SERT-KO (n = 7) compared with female wild-type (WT) rats (n = 7). A, right: 5,7-DHT treatment (it) did not change in VMR to CRD following treatment with alosetron (25 nmol it) in WT female rats (n = 6) or SERT-KO female rats (n = 5). B: the 5-HT3 receptor agonist SR-57227 SR (25 nmol it) did not alter the VMR to CRD in WT female (n = 6; left) or SERT-KO female rats treated with 5,7-DHT (n = 6; right). Data are means ± SE.
Fig. 10.
Intrathecal 5,7-dihydroxytryptamine (5,7-DHT) treatment blocked the increased visceromotor response (VMR) to colorectal balloon distention (CRD) in serotonin transporter-knockout (SERT-KO) male rats treated it with SR-57227. A: the 5-HT3 receptor agonist SR-57227 (100 pM it) did not affect the VMR to CRD in wild-type (WT) male rats (n = 5; left) or in SERT-KO male rats (n = 5; right) compared with control. B: 5-HT depletion with 5,7-DHT did not affect the VMR to CRD in male WT (n = 6; left) or male SERT-KO rats treated with alosetron (n = 6; right). Data are means ± SE.
See Table 2 for a summary of all our studies of sex differences and the effects of drug treatments on the VMR to CRD in WT and SERT-KO rats.
Table 2.
Summary of visceromotor responses to colorectal balloon distention in male and female WT and SERT-KO rats
| WT Female | SERT-KO Female | WT Male | SERT-KO Male | |
|---|---|---|---|---|
| No drug | Increased vs. WT | No change vs. WT | ||
| Alosetron, sc | No change vs. control | Increased vs. control | Increased vs. control | No change vs. control |
| Granisetron, sc | No change vs. control | Increased vs. control | No change vs. control | No change vs. control |
| Ondansetron, sc | No change vs. control | No change vs. control | Not tested | Not tested |
| Ramosetron, 0.01 mg/kg sc | Not tested | No change vs. control | Not tested | Not tested |
| Ramosetron, 0.1 mg/kg sc | Not tested | No change vs. control | Not tested | Not tested |
| Ramosetron, 1.0·mg/kg sc | Not tested | Increased vs. control | Not tested | Not tested |
| Alosetron, icv | No change vs. control | No change vs. control | No change vs. control | Increased vs. control |
| Alosetron, it | No change vs. control | Increased vs. control | No change vs. control | No change vs. control |
| Granisetron, it | No change vs. control | No change vs. control | No change vs. control | No change vs. control |
| Ondansetron, it | No change vs. control | No change vs. control | No change vs. control | No change vs. control |
| 5,7-DHT, it | No difference vs. WT | Not tested | Not tested | |
| 5,7-DHT, it + SR-57227, it | No change vs. control | No change vs. control | No change vs. control | No change vs. control |
| 5,7-DHT, it + alosetron it | No change vs. control | No change vs. control | No change vs. control | No change vs. control |
5,7-DHT, 5,7-dihydroxytryptamine; icv, intracerebroventricular; it, intrathecal; sc, subcutaneous; SERT-KO, serotonin transporter knockout; WT, wild type.
DISCUSSION
Drugs that target 5-HT receptors effectively treat some gastrointestinal motility and sensation disorders, including IBS. 5-HT4 receptor agonists like tegaserod, velusetrag, and prucalopride alleviate constipation (45), whereas the 5-HT3 receptor antagonists alosetron, ondansetron, and ramosetron reduce abdominal pain and diarrhea in some IBS patients (10). There is also a strong association between an insertion/deletion polymorphism (s/s, s/l, and reduced SERT expression vs. l/l and higher SERT expression) in the promoter region of the SERT gene and constipation-predominant IBS, and this polymorphism produces reduced SERT expression (58). These clinical data highlight a role for 5-HT signaling in the gut and in visceral pain pathways. In the present study, we used SERT-KO rats to study the sites of 5-HT signaling that contribute to visceral hypersensitivity in this rat. SERT-KO rats exhibit female-specific visceral hypersensitivity, which recapitulates the higher incidence of visceral pain in women with IBS compared with men (11).
Visceral hypersensitivity in male rats.
Alosetron administered icv increased the VMR to CRD in SERT-KO but not WT male rats. Others have also shown that alosetron increases the VMR to CRD in a model of visceral hypersensitivity using male rats (8). These investigators also showed that capsaicin-induced vagal ablation prevented the alosetron-induced increase in VMR to CRD. It was concluded that blocking 5-HT3 receptors on the peripheral terminals of vagal nerve fibers decreased descending inhibition at the level of the spinal cord, which increased visceral sensation. Bradesi et al. (8) ruled out the possibility that alosetron acted at the spinal level by showing that it administered alosetron did not affect the VMR to CRD. This result agrees with our data showing that in WT male rats, blockade of spinal 5-HT3 receptors does not affect the VMR to CRD. Similarly, 5-HT3 receptor antagonists administered it did not affect the VMR in WT female rats or SERT-KO male rats. Furthermore, alosetron but not granisetron increased the VMR to CRD in male but not female rats. However, it administration of the 5-HT3 receptor agonist SR-57227 caused an increase in VMR to CRD in SERT-KO male rats that may be due to expression of 5-HT3 receptors on terminals of primary afferent neurons in the dorsal horn of the spinal cord of male SERT-KO rats. Depletion of 5-HT from descending serotonergic neurons using 5,7-DHT prevented the increase in visceral sensitivity in SERT-KO male rats.
Visceral hypersensitivity in female rats.
Alosetron, a 5-HT3 receptor antagonist, reduces visceral pain in female IBS patients (17). However, we found that peripheral administration of alosetron and another 5-HT3 receptor antagonist, granisetron, increased the VMR to CRD in SERT-KO female rats. Volume-pressure loop analysis revealed that the increase in VMR to CRD in response to alosetron was not due to reduced compliance of the colon and rectum. Compliance is a measure of wall tension in response to change in pressure (27). A reduction in compliance would suggest that inhibition of 5-HT3 receptors in the gut prevents distention-evoked relaxations of the colon. In SERT-KO female rats, it alosetron, but not granisetron or ondansetron, increased the VMR to CRD. It is possible that alosetron, but not granisetron or ondansetron, administered it reaches the brain via the CSF to increase the VMR to CRD. However, alosetron administered icv did not affect VMR to CRD in any rats, ruling out a brain site of action. Using female rats, we also tested the effects of ramosetron, a 5-HT3 receptor antagonist with limited BBB permeability (55). Peripheral administration of a low-dose ramosetron did not affect VMR to CRD and did not produce detectable levels of ramosetron in the CSF. A higher dose of ramosetron produced detectable levels in the CSF and increased the VMR to CRD, confirming that CNS 5-HT3 receptors are targeted by 5-HT3 receptor antagonists to mediate visceral hyperalgesia in female SERT-KO rats.
Sites of action for 5-HT-mediated visceral hypersensitivity.
The data discussed above indicate that alosetron increased visceral sensitivity in female SERT-KO rats most likely by blocking lumbosacral spinal 5-HT3 receptors. However, although peripherally administered granisetron increased visceral sensitivity, it did not increase the VMR to CRD after it or icv administration. These observations are in line with previous studies showing variations in responses caused by different 5-HT3 receptor antagonists. For example, rats treated with acetic acid to increase colonic sensitivity showed a reduction in visceral sensitivity following sc treatment with granisetron but not ondansetron even when administered at a dose 10-fold higher than granisetron (38). In another study where visceral sensitivity was induced via systemic injection of 5-hydroxytryptophan, granisetron, but not ondansetron, reduced visceral sensation (4). In both studies, it was speculated that the variable response to 5-HT3 receptor antagonists is likely due to the different potencies for different 5-HT3 receptor subtypes. For example, granisetron may have higher affinity for 5-HT3 receptors composed of two A and three B subunits, whereas ondansetron has higher affinity for homomeric 5-HT3A receptors. However, studies of antagonist binding to 5-HT3 receptors have all used homomeric 5-HT3A receptors as the antagonist target (1, 20). Variable responses to 5-HT3 receptor antagonists have also been noted in clinical studies of these drugs. In one study, it was found that ondansetron increased pain thresholds to rectal electrical stimulation but decreased maximum tolerated volume in rectal distension studies (25). A more recent study assessing clinical end points in 120 IBS patients showed that ondansetron decreased stool frequency, urgency, and bloating but failed to improve pain scores (22). Others found that granisetron improved pain scores in IBS patients undergoing rectal balloon distension (11). Aside from the fact that this study had only 12 participants, pain as a clinical end point was not measured, weakening the conclusion that granisetron reduces visceral pain in IBS patients (13). Alosetron is used to treat IBS and is the only FDA-approved 5-HT3 receptor antagonist shown to reduce abdominal pain in female IBS-diarrhea patients (19, 47). This is supported by a study of >600 IBS-D male patients that showed that alosetron improved stool consistency but did not reduce abdominal pain (12, 28). Our studies also showed that icv administration of alosetron increased VMR to CRD in SERT-KO male but not SERT-KO female rats. The difference in alosetron efficacy between males and females could be due to sex differences in drug metabolism by CYP2C19, SERT gene polymorphisms, or limbic system activation (women exhibit higher limbic activity during rectal distension) (6, 35, 44). Furthermore, alosetron is more potent in female IBS-D patients homozygous for the l allele (higher SERT expression) of the gene encoding SERT (2). This may be due to increased 5-HT clearance in l/l carriers and suggests that patients with the s allele (reduced SERT expression) do not respond to alosetron as well as patients with the l/l genotype. Our rats lack SERT and exhibit increased visceral sensitivity due to the lack of the 5-HT clearance mechanism. In SERT-KO female rats, alosetron exhibited a bell-shaped dose-response curve, as shown previously (18, 38). This could be due to high-dose steric hindrance at the receptor or to actions at non-5-HT3 receptors (54), such as histamine H3 receptors and 5-HT1C receptors (18).
Data from our studies using it drug administration indicate that the mechanism by which alosetron increases visceral sensitivity in SERT-KO female and male WT rats differs. Descending serotonergic input to the spinal dorsal horn modulates pain signaling caused by inflammation, nerve injury, or serotonergic dysfunction (56). SERT-KO mice exhibit mechanical allodynia but not thermal hyperalgesia following sciatic nerve injury. This is thought to be due to specific changes in the function of Aδ sensory nerve fibers (53). When assessing visceral pain produced by intraperitoneal (ip) injection of acetic acid, SERT-KO and WT mice showed similar responses (30). The sex of the mice used in this study was not reported. Alosetron may increase visceral sensitivity by blocking the effects of 5-HT released from the nerve terminals of descending raphe neurons onto 5-HT3 receptors expressed by dorsal horn inhibitory interneurons. To test this hypothesis, we measured the effect of alosetron on visceral sensitivity in rats treated with the neurotoxin 5,7-DHT, which reduced spinal 5-HT levels, suggesting decreased descending 5-HT input to the lumbosacral spinal cord. Reduction in spinal 5-HT levels prevented the increase in visceral sensitivity observed following it alosetron treatment, suggesting that 5-HT signaling at 5-HT3 receptors in SERT-KO female rats is anti-nociceptive. A similar observation was reported in a somatic pain model, where formalin was injected into the hind paw (41). These investigators showed that reduction of spinal 5-HT reduced 5-HT3 receptor-mediated analgesia. In this study, 5,7-DHT treatment caused a 15% reduction in spinal 5-HT, which is comparable with the 5-HT reduction we saw in SERT-KO female rats. In another study, HPLC analysis of microdialysis samples from rat spinal cord treated it with a 5-HT3 receptor agonist showed increased GABA release compared with control rats (34). Therefore, a potential mechanism by which 5-HT3 receptors mediate nociception is via activation of inhibitory GABAergic interneurons in the dorsal spinal cord (3, 50).
Because inhibition of 5-HT3 receptors increased the VMR in SERT-KO female rats, we tested whether activation of 5-HT3 receptors produced analgesia. We treated rats it with the 5-HT3 receptor agonist SR-57227 and found that agonist-induced 5-HT3 receptor activation did not affect the VMR in SERT-KO or WT female rats.
Summary and conclusions.
Our data suggest that there are sex differences in 5-HT3 receptor-mediated visceral pain transmission in SERT-KO rats. In SERT-KO female rats, inhibition of spinal 5-HT3 receptors caused an increase in visceral sensitivity. On the other hand, spinal 5-HT3 receptor inhibition with alosetron did not affect visceral sensation in SERT-KO male rats, whereas alosetron administered icv did increase visceral sensation in these animals. It is well established that there are substantial sex differences in pain pathways in the central nervous system (CNS) (36, 40). We conclude that there are sex differences in the contributions of 5-HT signaling in pain pathways in the CNS. 5-HT signaling in the dorsal spinal cord is an important site of action in female rats, whereas brain sites of action may be more important in male rats.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-103759.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.E.-A. and J.J.G. conception and design of research; N.E.-A. performed experiments; N.E.-A. and J.J.G. analyzed data; N.E.-A. and J.J.G. interpreted results of experiments; N.E.-A. and J.J.G. prepared figures; N.E.-A. drafted manuscript; N.E.-A. and J.J.G. edited and revised manuscript; N.E.-A. and J.J.G. approved final version of manuscript.
REFERENCES
- 1.Abdel-Aal RA, Assi AA, Kostandy BB. Rivastigmine reverses aluminum-induced behavioral changes in rats. Eur J Pharmacol 659: 169–176, 2011. doi: 10.1016/j.ejphar.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Acosta A, Camilleri M. Pharmacogenetics in irritable bowel syndrome. Expert Opin Drug Metab Toxicol 11: 1187–1191, 2015. doi: 10.1517/17425255.2015.1048223. [DOI] [PubMed] [Google Scholar]
- 3.Alhaider AA, Lei SZ, Wilcox GL. Spinal 5-HT3 receptor-mediated antinociception: possible release of GABA. J Neurosci 11: 1881–1888, 1991. doi: 10.1523/JNEUROSCI.11-07-01881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banner SE, Sanger GJ. Differences between 5-HT3 receptor antagonists in modulation of visceral hypersensitivity. Br J Pharmacol 114: 558–562, 1995. doi: 10.1111/j.1476-5381.1995.tb13263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor–the relationship between structure and function. Neuropharmacology 56: 273–284, 2009. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain 4: 157–172, 2000. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 7.Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, Fitzgerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD, Mayer EA. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist Alosetron. Gastroenterology 123: 969–977, 2002. doi: 10.1053/gast.2002.35990. [DOI] [PubMed] [Google Scholar]
- 8.Bradesi S, Lao L, McLean PG, Winchester WJ, Lee K, Hicks GA, Mayer EA. Dual role of 5-HT3 receptors in a rat model of delayed stress-induced visceral hyperalgesia. Pain 130: 56–65, 2007. doi: 10.1016/j.pain.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Browning KN. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front Neurosci 9: 413, 2015. doi: 10.3389/fnins.2015.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M, Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 66: 966–974, 2017. doi: 10.1136/gutjnl-2016-313425. [DOI] [PubMed] [Google Scholar]
- 11.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 6: 71–80, 2014. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Ameen VZ, Dukes GE, McSorley DJ, Carter EG, Mayer EA. A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. Am J Gastroenterol 100: 115–123, 2005. doi: 10.1111/j.1572-0241.2005.40365.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Ilham SJ, Feng B. Pharmacological Approach for Managing Pain in Irritable Bowel Syndrome: A Review Article. Anesth Pain Med 7: e42747, 2017. doi: 10.5812/aapm.42747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung CK, Wu JC. Genetic polymorphism in pathogenesis of irritable bowel syndrome. World J Gastroenterol 20: 17693–17698, 2014. doi: 10.3748/wjg.v20.i47.17693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 313: 949–958, 2015. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 16.Choi S, Jonak E, Fernstrom JD. Serotonin reuptake inhibitors do not prevent 5,7-dihydroxytryptamine-induced depletion of serotonin in rat brain. Brain Res 1007: 19–28, 2004. doi: 10.1016/j.brainres.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 17.Choi YD, Sung TS, Kim HJ, La JH, Kim TW, Yang IS. Increased 5-hydroxytryptamine mediates post-inflammatory visceral hypersensitivity via the 5-hydroxytryptamine 3 receptor in rats. Dig Dis Sci 53: 2909–2916, 2008. doi: 10.1007/s10620-008-0244-8. [DOI] [PubMed] [Google Scholar]
- 18.Clayton NM, Sargent R, Butler A, Gale J, Maxwell MP, Hunt AA, Barrett VJ, Cambridge D, Bountra C, Humphrey PP. The pharmacological properties of the novel selective 5-HT3 receptor antagonist, alosetron, and its effects on normal and perturbed small intestinal transit in the fasted rat. Neurogastroenterol Motil 11: 207–217, 1999. doi: 10.1046/j.1365-2982.1999.00148.x. [DOI] [PubMed] [Google Scholar]
- 19.Cremonini F, Nicandro JP, Atkinson V, Shringarpure R, Chuang E, Lembo A. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther 36: 437–448, 2012. doi: 10.1111/j.1365-2036.2012.05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy NH, Lester HA, Dougherty DA. Ondansetron and granisetron binding orientation in the 5-HT(3) receptor determined by unnatural amino acid mutagenesis. ACS Chem Biol 7: 1738–1745, 2012. doi: 10.1021/cb300246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galligan JJ, Patel BA, Schneider SP, Wang H, Zhao H, Novotny M, Bian X, Kabeer R, Fried D, Swain GM. Visceral hypersensitivity in female but not in male serotonin transporter knockout rats. Neurogastroenterol Motil 25: e373–e381, 2013. doi: 10.1111/nmo.12133. [DOI] [PubMed] [Google Scholar]
- 22.Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, Henry A, Hall I, Whorwell P, Spiller R. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 63: 1617–1625, 2014. doi: 10.1136/gutjnl-2013-305989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR Jr, Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 123: 217–226, 2002. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- 24.Glaum SR, Proudfit HK, Anderson EG. 5-HT3 receptors modulate spinal nociceptive reflexes. Brain Res 510: 12–16, 1990. doi: 10.1016/0006-8993(90)90721-M. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg PA, Kamm MA, Setti-Carraro P, van der Sijp JR, Roth C. Modification of visceral sensitivity and pain in irritable bowel syndrome by 5-HT3 antagonism (ondansetron). Digestion 57: 478–483, 1996. doi: 10.1159/000201377. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood-Van Meerveld B, Mohammadi E, Tyler K, Pietra C, Bee LA, Dickenson A. Synergistic effect of 5-hydroxytryptamine 3 and neurokinin 1 receptor antagonism in rodent models of somatic and visceral pain. J Pharmacol Exp Ther 351: 146–152, 2014. doi: 10.1124/jpet.114.216028. [DOI] [PubMed] [Google Scholar]
- 27.Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterol Motil 8: 277–297, 1996. doi: 10.1111/j.1365-2982.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 28.Grover M, Camilleri M. Ramosetron in irritable bowel syndrome with diarrhea: new hope or the same old story? Clin Gastroenterol Hepatol 12: 960–962, 2014. doi: 10.1016/j.cgh.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu QY, Zhang J, Feng YC, Dai GR, Du WP. Association of genetic polymorphisms in HTR3A and HTR3E with diarrhea predominant irritable bowel syndrome. Int J Clin Exp Med 8: 4581–4585, 2015. [PMC free article] [PubMed] [Google Scholar]
- 30.Hall FS, Schwarzbaum JM, Perona MT, Templin JS, Caron MG, Lesch KP, Murphy DL, Uhl GR. A greater role for the norepinephrine transporter than the serotonin transporter in murine nociception. Neuroscience 175: 315–327, 2011. doi: 10.1016/j.neuroscience.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall JD, DeWitte C, Ness TJ, Robbins MT. Serotonin enhances urinary bladder nociceptive processing via a 5-HT3 receptor mechanism. Neurosci Lett 604: 97–102, 2015. doi: 10.1016/j.neulet.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen AA, Davies PA, Bräuner-Osborne H, Krzywkowski K. 3B but which 3B and that’s just one of the questions: the heterogeneity of human 5-HT3 receptors. Trends Pharmacol Sci 29: 437–444, 2008. doi: 10.1016/j.tips.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Büchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet 17: 2967–2977, 2008. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 34.Kawamata T, Omote K, Toriyabe M, Yamamoto H, Namiki A. The activation of 5-HT(3) receptors evokes GABA release in the spinal cord. Brain Res 978: 250–255, 2003. doi: 10.1016/S0006-8993(03)02952-4. [DOI] [PubMed] [Google Scholar]
- 35.Koch KM, Corrigan BW, Manzo J, James CD, Scott RJ, Stead AG, Kersey KE. Alosetron repeat dose pharmacokinetics, effects on enzyme activities, and influence of demographic factors. Aliment Pharmacol Ther 20: 223–230, 2004. doi: 10.1111/j.1365-2036.2004.02031.x. [DOI] [PubMed] [Google Scholar]
- 36.Kogler L, Müller VI, Seidel EM, Boubela R, Kalcher K, Moser E, Habel U, Gur RC, Eickhoff SB, Derntl B. Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage 134: 410–423, 2016. doi: 10.1016/j.neuroimage.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut 46: 474–480, 2000. doi: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langlois A, Pascaud X, Junien JL, Dahl SG, Rivière PJ. Response heterogeneity of 5-HT3 receptor antagonists in a rat visceral hypersensitivity model. Eur J Pharmacol 318: 141–144, 1996. doi: 10.1016/S0014-2999(96)00857-6. [DOI] [PubMed] [Google Scholar]
- 39.Lummis SC. 5-HT(3) receptors. J Biol Chem 287: 40239–40245, 2012. doi: 10.1074/jbc.R112.406496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mapplebeck JC, Beggs S, Salter MW. Molecules in pain and sex: a developing story. Mol Brain 10: 9, 2017. doi: 10.1186/s13041-017-0289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto K, Lo MW, Hosoya T, Tashima K, Takayama H, Murayama T, Horie S. Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice. Lab Invest 92: 769–782, 2012. doi: 10.1038/labinvest.2012.14. [DOI] [PubMed] [Google Scholar]
- 42.Mayer EA, Berman S, Derbyshire SW, Suyenobu B, Chang L, Fitzgerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther 16: 1357–1366, 2002. doi: 10.1046/j.1365-2036.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- 43.Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol 402: 385–401, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 44.Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology 124: 1738–1747, 2003. doi: 10.1016/S0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 45.Nelson AD, Camilleri M, Chirapongsathorn S, Vijayvargiya P, Valentin N, Shin A, Erwin PJ, Wang Z, Murad MH. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis. Gut 66: 1611–1622, 2017. doi: 10.1136/gutjnl-2016-311835. [DOI] [PubMed] [Google Scholar]
- 46.Oyama T, Ueda M, Kuraishi Y, Akaike A, Satoh M. Dual effect of serotonin on formalin-induced nociception in the rat spinal cord. Neurosci Res 25: 129–135, 1996. doi: 10.1016/0168-0102(96)01034-6. [DOI] [PubMed] [Google Scholar]
- 47.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther 30: 884–901, 2008. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Saria A, Javorsky F, Humpel C, Gamset R. 5-HT3 receptor antagonists inhibit sensory neuropeptide release from the rat spinal cord. Neuroreport 1: 104–106, 1990. doi: 10.1097/00001756-199010000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Sawynok J, Reid A. Interactions of descending serotonergic systems with other neurotransmitters in the modulation of nociception. Behav Brain Res 73: 63–68, 1995. doi: 10.1016/0166-4328(96)00072-1. [DOI] [PubMed] [Google Scholar]
- 50.Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 152: 1666–1673, 2011. doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Stewart JJ, Weisbrodt NW, Burks TF. Central and peripheral actions of morphine on intestinal transit. J Pharmacol Exp Ther 205: 547–555, 1978. [PubMed] [Google Scholar]
- 52.Taguchi R, Shikata K, Furuya Y, Hirakawa T, Ino M, Shin K, Shibata H. Selective corticotropin-releasing factor 1 receptor antagonist E2508 reduces restraint stress-induced defecation and visceral pain in rat models. Psychoneuroendocrinology 75: 110–115, 2017. doi: 10.1016/j.psyneuen.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 53.Vogel C, Mössner R, Gerlach M, Heinemann T, Murphy DL, Riederer P, Lesch KP, Sommer C. Absence of thermal hyperalgesia in serotonin transporter-deficient mice. J Neurosci 23: 708–715, 2003. doi: 10.1523/JNEUROSCI.23-02-00708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolf H. Preclinical and clinical pharmacology of the 5-HT3 receptor antagonists. Scand J Rheumatol Suppl 29: 37–45, 2000. doi: 10.1080/030097400446625. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto C, Murakami H, Koyabu N, Takanaga H, Matsuo H, Uchiumi T, Kuwano M, Naito M, Tsuruo T, Ohtani H, Sawada Y. Contribution of P-glycoprotein to efflux of ramosetron, a 5-HT3 receptor antagonist, across the blood-brain barrier. J Pharm Pharmacol 54: 1055–1063, 2002. doi: 10.1211/002235702320266208. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura M. Plastic changes of the descending serotonergic system in the spinal dorsal horn. Curr Anaesth Crit Care 17: 3–8, 2006. doi: 10.1016/j.cacc.2006.05.003. [DOI] [Google Scholar]
- 57.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 22: 1010–1019, 2002. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Y, Zheng G, Hu Z. Association between SERT insertion/deletion polymorphism and the risk of irritable bowel syndrome: a meta-analysis based on 7039 subjects. Gene 679: 133–137, 2018. doi: 10.1016/j.gene.2018.08.059. [DOI] [PubMed] [Google Scholar]