Abstract
Background
Simvastatin, an HMG-CoA reductase inhibitor, has been reported to exert multiple protective effects on the cardiovascular system. However, the molecular mechanism remains to be examined. The present study was designed to study the effects of simvastatin on cardiac hypertrophy in diabetic rats and to explore its potential mechanism.
Material/Methods
Sprague-Dawley rats were assigned into a control (Con) group, a streptozotocin (STZ) group, and a STZ+simvastatin (STZ+SIM) group. The level of reactive oxygen species (ROS) was measured by using dihydroethidium (DHE) staining. The protein expressions of p65, IκBα, vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), calpain-1, and endothelial nitric oxide synthase (eNOS) were examined by Western blot analysis. qPCR was used to detect the levels of brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP).
Results
Simvastatin improved the cardiac hypertrophy of diabetic rats, as demonstrated by decreases in the ratios of left ventricular weight/body weight (LVW/BW) and heart weight/body weight (HW/BW) and by the downregulation of mRNA expression of BNP and ANP in the heart tissue. Simvastatin decreased the protein expressions of VCAM-1, ICAM-1, IL-6, and TNF-α, increased eNOS protein expression, and limited an increase in ROS levels in the heart tissue. Simvastatin increased IκBα protein expression in cytoplasm and inhibited the translocation of p65, the subunit of nuclear factor-κB (NF-κB) to the nucleus from the cytoplasm of the heart tissue. Furthermore, simvastatin attenuated the activity of calpain and calpain-1 protein expression in heart tissue.
Conclusions
Simvastatin attenuates cardiac hypertrophy in diabetic rats, which might be due to the attenuation of oxidative stress and inflammation induced by calpain-1-mediated activation of NF-κB.
MeSH Keywords: Calpain, Diabetic Cardiomyopathies, Inflammation, Oxidative Stress
Background
Diabetic cardiomyopathy (DCM) is an acknowledged specific cardiomyopathy that develops in diabetic patients in the absence of coronary atherosclerosis and hypertension. The total number of adults worldwide experiencing diabetes mellitus was 415 million in 2015, and is estimated to increase to 642 million by 2040 [1]. Cardiac hypertrophy often precedes the pathological phenotype of DCM, as evidenced by the increase in heart size and mass, which ultimately results in stiffer ventricles, irreversible cardiac remodeling, and subsequent heart failure.
The multifaceted mechanisms of diabetic cardiac hypertrophy involve elevated oxidative stress [2], cardiac inflammatory responses, and myocardial remodeling [3]. Hyperglycemia induces glucose auto-oxidation and surplus generation of reactive oxygen species (ROS). Excess ROS activates protein kinase C and subsequently nuclear factor-κB (NF-κB), leading to myocardial injury [4]. A previous study demonstrated that vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) directly participate in development of diabetic complications and heart disease [5]. Oxidative stress and inflammatory responses have been shown to cross-talk and interact with each other [6]. The NF-κB family plays an important part in inflammatory responses by promoting the expression of inflammatory mediators.
Calpain-1 belongs to the family of cysteine proteases. Calpain-1 activation has been demonstrated to induce IκBα degradation and NF-κB activation [7]. In a previous study, we found that calpain-1 plays an critical role in atherogenesis through endothelial disorder and inflammation [8]. However, whether calpain-1-mediated NF-κB signaling is involved in the diabetic heart remains to be investigated. In this study, we hypothesized that calpain-1 mediates IκBα degradation and NF-κB activation, which induce oxidative stress and inflammatory responses in cardiac hypertrophy of diabetic rats.
Simvastatin is a 3-hydroxy-3-methyl-glutarylcoenzyme-CoA (HMG-CoA) reductase inhibitor. Treatment with simvastatin has been reported to exert multiple protective effects on the cardiovascular system, independent of their classical functions on lipoproteins. One study has shown simvastatin exerted protective effect against cardiac hypertrophy in a rat model of abdominal aortic constriction [9]. However, the effects and underlying mechanisms of simvastatin on cardiac hypertrophy in diabetic rats remains to be investigated. The present study was designed to provide insight into the effects of simvastatin on cardiac hypertrophy in streptozotocin (STZ)-induced diabetic rats with focus on oxidative stress and inflammation induced by calpain-1-mediated activation of NF-κB. The results showed that simvastatin is effective in protecting against cardiac hypertrophy in diabetic rats by attenuating oxidative stress and inflammation, which might be largely due to the activation of NF-κB mediated by calpain-1.
Material and Methods
Agents and diabetic animal model
Simvastatin tablets were purchased from Shandong Lukang Pharmacy (Jining, China). Antibodies against IκBα, p65, ICAM-1, VCAM-1, IL-6, TNF-α, endothelial nitric oxide synthase (eNOS), and glyceraldehyde-3-phosphatedehydrogenase (GAPDH) were provided by Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against calpain-1 were purchased from Abcam (Cambridge, MA, USA). The primers of ANP and BNP were obtained from TaKaRa Biotechnology Co. (Dalian, China). Nuclear and cytoplasmic protein extraction kits were from Beyotime Biotechnology (Shanghai, China). HE staining kits were purchased from Nan Jing Jian Cheng Bioengineering Institute (Nanjing, China). PCR kits were purchased from Nanjing Vazyme Biotech Co. (Nanjing, China). Enzyme-linked immunosorbent assay (ELISA) kits for IL-6 and TNF-α were purchased from R&D Systems (Minneapolis, MN, USA). The investigation was not financed by any pharmaceutical company.
Male Sprague-Dawley rats age of 4–6 weeks (200±20 g) were provided by the Experimental Animal Center of Jinzhou Medical University (certificate No. SCXK 2016-0004). The Committee on the Ethics of Animal Experiments of Jinzhou Medical University approved this study (permit No. LNMU-2016-118), which was performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All rats were maintained at an ambient room temperature (22±2°C) under a 12/12-h light-dark cycle and had free access to water and food. Rats were considered diabetic and were used for the study only if they had hyperglycemia (≥15 mmol/L) 72 h after STZ (50mg/kg) injection. The diabetic rats were divided into 2 groups: an STZ group (n=10) and an STZ+ SIM group (n=10), which were orally administered the vehicle (i.g.) and simvastatin (20 mg/kg/d) for 12 weeks. Another 10 healthy non-diabetic rats were used as a control group (n=10) and were given vehicle only.
Heart weight index measurement
The heart weight indexes represented by ratios of heart weight/body weight (HW/BW) and left ventricle weight (LVW/BW) were measured as previously reported by our laboratory [10].
Biochemical analysis
The serum levels of total cholesterol (TC) and triglyceride (TG) were measured with commercial kits (Nanjing Jian Cheng Bioengineering Institute, Nanjing, China). The levels of glucose (GLU) in serum were determined using commercial kits provided by Bio Med Diagnostics (White City, OR, USA).
ELISA assay
The serum concentrations of IL-6 and TNF-α were estimated using ELISA kits in accordance with the manufacturer’s instructions.
The ROS production measurement
The ROS production in diabetic heart tissues was determined by dihydroethidium (DHE) staining as reported previously by our laboratory [11].
Histomorphology and immunohistochemistry examination
The myocardial tissues were fixed in 10% phosphate-buffered formalin, dehydrated in an ascending series of ethyl alcohol, cleared in xylene, and embedded in paraffin wax. Sections of 5-μm thickness were prepared and stained with hematoxylin and eosin (HE). The sections were examined under a light microscope and photographed. For immunohistochemical staining, myocardial tissues were embedded in paraffin using standard histological procedures, subjected to antigen retrieval in 0.01 M citrate buffer (pH 6.0) by microwaving, and then placed in 3% hydrogen peroxide in methanol for 30 min at room temperature. Slides were blocked with 5% BSA in PBS for 20 min and then incubated with primary antibody at 4°C overnight (P65, 1: 200). After washing 3 times with phosphate-buffered saline (PBS), sections were incubated with anti-rabbit IgG (1: 200) secondary antibody labeled with horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Diaminobenzidine (DAB) substrate kits were used to reveal the immunohistochemical reaction.
Western blot analysis
To extract the total protein, heart samples were homogenized in radio-immunoprecipitation assay (RIPA) lysis buffer containing proteinase inhibitors. Cytoplasmic and nuclear protein extracts were prepared from heart tissue using nuclear and cytoplasmic protein extraction kits and then placed on ice. The bicinchoninic acid protein assay (BCA) was used to measure the protein concentration, followed by the separation of protein samples with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which was then transferred to hydrophobic polyvinylidene fluoride (PVDF) membranes and soaked in 1% BSA for 1.5 h at room temperature. The primary antibodies against eNOS, IL-6, TNF-α, ICAM-1, VCAM-1, IκBα, P65, calpain-1, Lamin B, and GAPDH were added onto the membrane and incubated overnight at 4°C, followed by washing with TBST. HRP-conjugated secondary antibodies were added onto the membrane and incubated for 1 h at room temperature. The chemiluminescence reagents were used to detect the blotting, which was analyzed using Quantity One software (Bio-Rad Laboratories).
Real-time RT-PCR analysis
RNA was isolated from myocardial tissue using Trizol reagent (Invitrogen, Carlsbad, CA). Quantitative real-time polymerase chain reaction (qPCR) of cDNA with the SYBR II Green QPCR system was performed with GAPDH as the internal control. Briefly, real-time RT-PCR was performed with One Step qRT-PCR SYBR® Green Kit (Vazyme) based on the following reaction conditions: pre-denaturation at 95°C for 30 s, 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s, and extension at 95°C for 15 s, followed by a final extension at 60°C for 1 min. The relative mRNA expression levels were calculated by the 2−ΔΔCt method. The primers used in the study were: ANP (forward, reverse): CAG CAC AAT AGA GCC GCT GA, GGG CAG GAG CTT GAA CAC G; BNP (forward, reverse): GCA GAA GCT GCT GGA GCT GA, ATC CGG AAG GCG CTG TCT TG; GAPDH (forward, reverse): GAG ACA GCC GCA TCT TCT TG, ATA CGG CCA AAT CCG TTC AC.
Calpain activity in diabetic myocardium
The activity of calpain was measured using an assay kit (Amyjet Scientific, Inc., China) according to the manufacturer’s protocol.
Statistical analysis
All data were analyzed using SPSS 17.0 statistical software. Measurement data are expressed as mean ± standard deviation (SD). All statistical comparisons were compared using one-way analysis of variance (ANOVA) or t test. Values of p<0.05 were regarded as statistically significant.
Results
Simvastatin improves the indexes related to the cardiac hypertrophy in diabetic rats
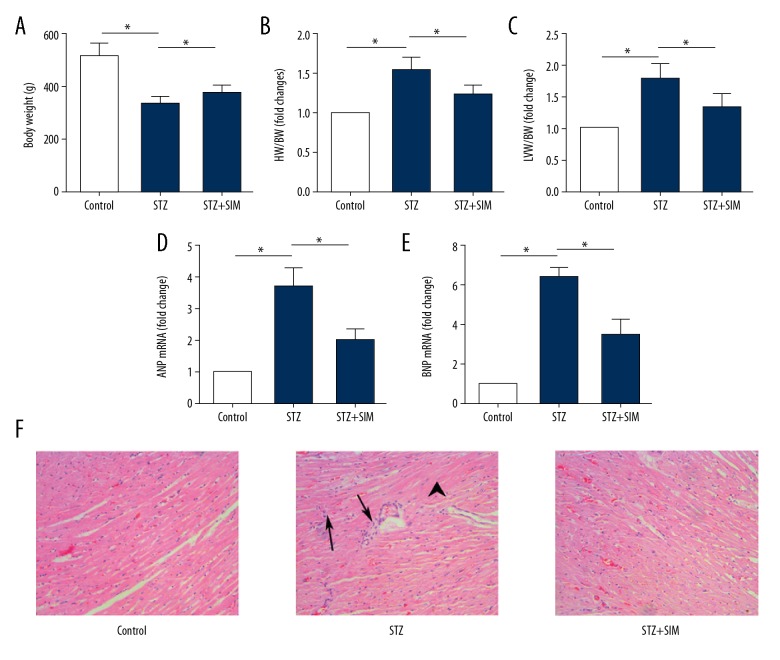
Injection of STZ decreased the body weight of rats (Figure 1A), resulting in increased ratios of HW/BW (Figure 1B) and LVW/BW (Figure 1C), as well as the mRNA expressions of ANP (Figure 1D) and BNP (Figure 1E), the specific marker of hypertrophy, in heart tissue (all p<0.05). However, simvastatin increased body weight, decreased the ratios of LVW/BW and HW/BW, and decreased the mRNA expressions of BNP and ANP. According to HE staining (Figure 1F), compared with that in the control group (all p<0.05), the myocardial cells in the STZ group were disordered, most of the myocardial cells were hypertrophic, and there was inflammatory cell infiltration. These pathological changes were improved by simvastatin.
Figure 1.
Simvastatin improves the indexes related to the cardiac hypertrophy in STZ-induced diabetic rats. (A) Body weight. (B) HW/BW. (C) LVW/BW. (D) mRNA expression of ANP. (E) mRNA expression of BNP. (F) Histological changes of heart tissue (×200, Arrows, inflammatory cells aggregation; Arrow head, hypertrophic cardiomyocytes). Data are expressed as means ±SD. n=10 for A–C; n=4 for D–F. * p<0.05 was considered statistically significant.
Effect of simvastatin on biochemical indexes
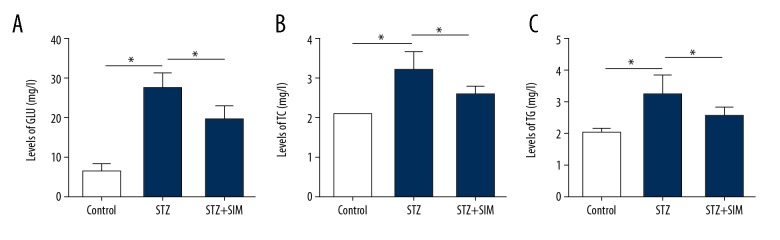
Compared with that in the control group, the contents of serum glucose (GLU) (Figure 2A), TC (Figure 2B), and TG (Figure 2C) in the STZ group were increased significantly (all p<0.05), and these changes were attenuated by simvastatin treatment (all p<0.05).
Figure 2.
Effect of simvastatin on biochemical indexes. (A) GLU. (B) TC. (C) TG. Data are expressed as means ±SD. n=10. * p<0.05 was considered statistically significant.
Simvastatin reduced the production of superoxide anion in myocardial tissue of diabetic rats
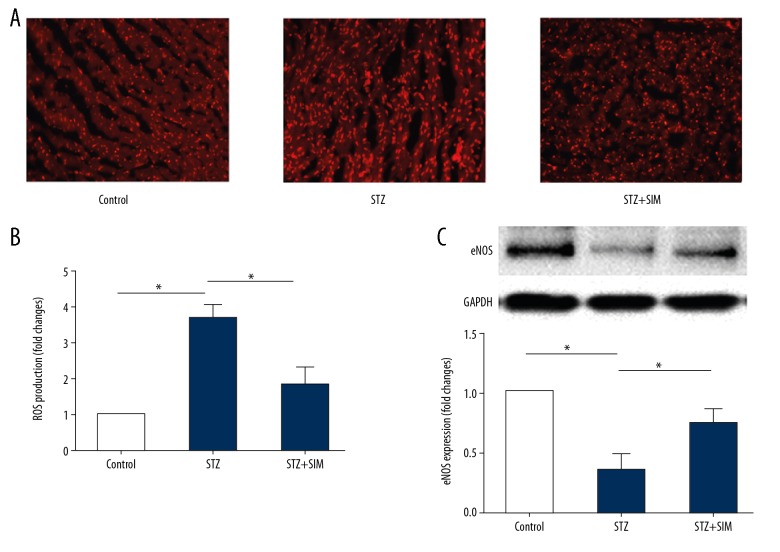
The results showed that the heart tissue of STZ-treated rats had increased superoxide anion production as compared with that of control rats (Figure 3) (p<0.05). The increase in the superoxide anion production was significantly reduced by simvastatin (Figure 3A, 3B) (p<0.05). In addition, compared with the control rats, injection of STZ decreased the protein expression of eNOS (Figure 3C) (p<0.05), which was reversed by simvastatin (p<0.05).
Figure 3.
Effects of simvastatin on the ROS production in heart tissue of diabetic rats. (A) Representative ethidium fluorescence images (×400). (B) Statistical results of simvastatin on the ROS production represented as relative fluorescence intensity. (C) Representative Western blot photograph of eNOS in different treatment rats. Data are presented as means ±SD. n=5 for A, B; n=4 for C. * p<0.05 was considered statistically significant.
Simvastatin downregulates the protein expression of inflammatory cytokines in diabetic heart tissue
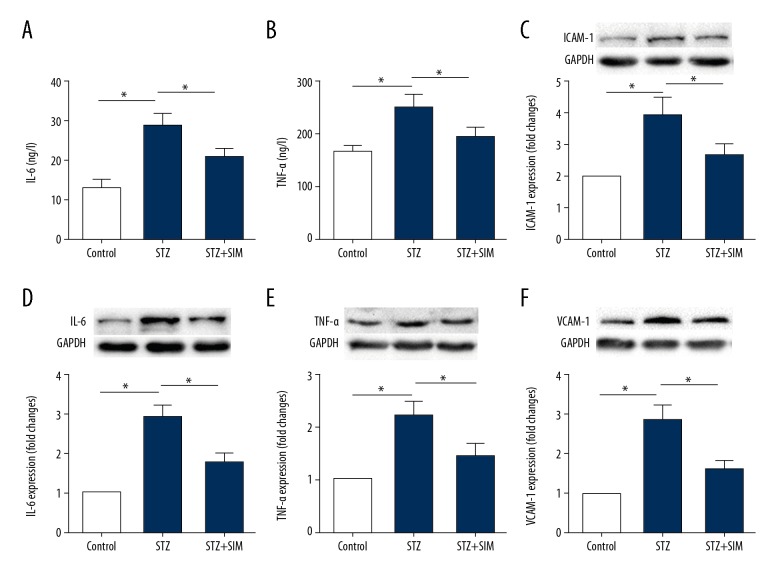
Inflammation plays an important role in the pathogenesis of DCM. The present study showed that simvastatin lowered the contents of proinflammatory cytokines TNF-α in diabetic heart tissue (Figure 4A, 4B), and downregulated the protein expression of TNF-α, IL-6, ICAM-1, and VCAM-1 in diabetic heart tissue (Figure 4C–4F) (all p<0.05).
Figure 4.
Simvastatin decreases the content and protein expression of inflammatory cytokines in diabetic heart tissue. Simvastatin decreases the contents of IL-6 and TNF-α in serum of diabetic rats (A, B) and downregulates the protein expression of ICAM-1, IL-6, TNF-α and VCAM-1 in heart tissue (C–F). Data are expressed as the mean ±SD. n=10 for A and B, n=4 for C–F. * p<0.05 was considered statistically significant.
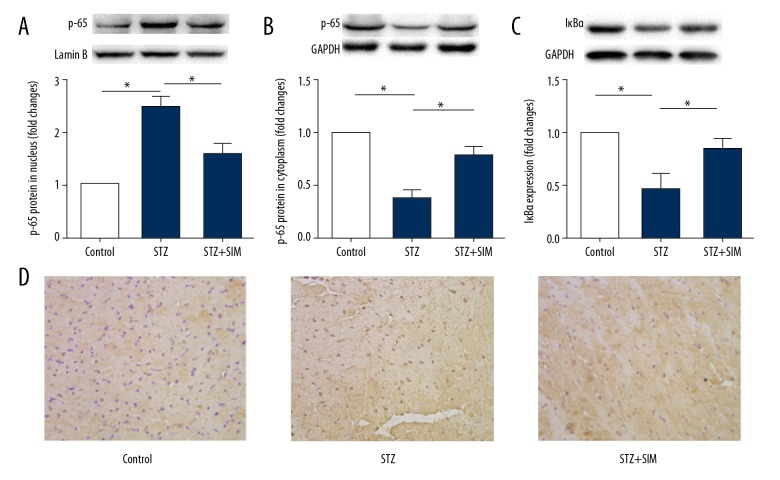
Simvastatin inhibits NF-κB nuclear translocation in heart tissue of diabetic rats
NF-κB activation followed by nuclear translocation suggests in the pathogenesis of diabetic cardiac hypertrophy. Therefore, we examined whether inhibition of NF-κB nuclear translocation participates in the attenuation of diabetic cardiac hypertrophy by simvastatin. As shown in Figure 5, compared with the control group, the protein expression of p65 was decreased in the cytoplasm (Figure 5A, 5B 5D) but was increased in the nucleus in the STZ group (all p<0.05). The expression of IκBα (Figure 5C) in the STZ group was also decreased (p<0.05). However, the increased translocation of p65 was partly abolished by simvastatin (p<0.05). These results suggest that the mechanism underlying the attenuation of cardiac hypertrophy by simvastatin is related to inhibition of NF-κB nuclear translocation by simvastatin.
Figure 5.
Simvastatin inhibits the nuclear translocation of NF-κB in heart tissue of diabetic rats. Representative photographs of Western blot of protein expression in nucleus (upper panel) and the quantification (lower panel). (A) p65 in nucleus. (B) p65 in cytoplasm. (C) IκBα in cytoplasm. (D) Representative images for the histochemical staining for p65 accumulation in the formalin-fixed heart tissues (400× magnification). Data are expressed as the mean ±SD. n=4. * p<0.05 was considered statistically significant.
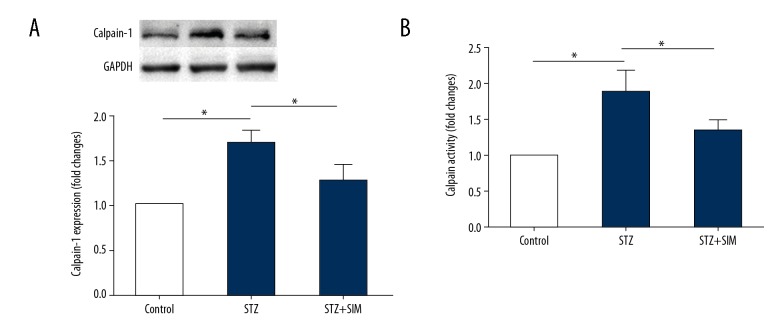
Simvastatin downregulates calpain-1 protein expression and reduces the activity of calpain in heart tissue
Calpain activation has an essential role in inflammation. To investigate the mechanism by which simvastatin inhibits inflammation, we assessed the protein expression of calpain-1 (Figure 6A) and the activity of calpain (Figure 6B) in the heart tissue of STZ-induced diabetic rats. As shown in Figure 6, combination with simvastatin downregulated calpain-1 protein expression and reduced calpain activity compared to that in the STZ group (all p<0.05).
Figure 6.
Simvastatin downregulates the protein expression of calpain-1 and reduces the calpain activity in heart tissue. (A) Representative photograph of Western blot of calpain-1 protein expression (upper panel) and the quantitation (lower panel). (B) Calpain activity. Data are expressed as the mean ±SD. n=4 for A and n=10 for B. * p<0.05 was considered statistically significant.
Discussion
We found that simvastatin: (1) reduced the ratios of LVW/BW and HW/BW, decreased BNP and ANP at the level of mRNA expression in the heart tissue, and improved the pathological disorders; (2) reduced the levels of blood glucose and blood lipids; (3) decreased the superoxide anion production and protein expression of eNOS in myocardial tissue of diabetic rats; (4) reduced the content of IL-6 and TNF-α and downregulated the protein expression of TNF-α, IL-6, ICAM-1, and VCAM-1 in heart tissue; (5) inhibited NF-κB nuclear translocation in the heart tissue; and (6) downregulated calpain-1 protein expression and reduced the calpain activity in heart tissue. These results suggest that simvastatin improves diabetic cardiac hypertrophy through attenuation of oxidative stress and inflammation induced by calpain-1-mediated activation of NF-κB. The present study confirmed and extended the findings of previous studies [12,13] by adding a new mechanism underlying the protection against cardiac hypertrophy in diabetic rats by simvastatin.
Cardiac hypertrophy, oxidative stress, inflammation, apoptosis, and myocardial interstitial fibrosis are the major features of DCM [14]. Cardiac hypertrophy often precedes the pathological phenotype of DCM, as evidenced by the increase in heart size and mass, which ultimately leads to stiffer ventricles, irreversible cardiac remodeling, and subsequent heart failure. Consistent with the above-described characteristics of cardiac hypertrophy, the present study demonstrated that diabetic rats undergo cardiac hypertrophy, as shown by the increased ratios of LVW/BW and HW/BW and by the upregulation of mRNA expression of BNP and ANP, the specific marker of hypertrophy, compared with the control group. Simvastatin significantly reversed these alterations. These results showing the cardioprotective effect of simvastatin are consistent with results of previous studies [12,15].
Oxidative stress is an imbalance between oxidants (e.g., ROS) and antioxidants, and probably contributes to the development, progression, and complications of diabetes [16]. In diabetic myocardium, hyperglycemia and the fatty acid oxidation pathway are major inducers of ROS. Endothelial NOS (eNOS) is the primary constitutive NOS in the myocardium, expressed in both endothelial cells and the cardiac myocyte itself. eNOS mediates endogenous NO output. NO is able to inhibit vascular smooth muscle cell proliferation, platelet adhesion and accumulation, and induce resistance to oxidative damage. Our previous study has demonstrated that oxidative stress is the critical factor contributing to inflammation of hypertrophic cardiomyopathy [17]. In the present study, we prove that simvastatin also has a protective effect on myocardial tissue via antioxidation, which can effectively ameliorate diabetic cardiac hypertrophy.
Inflammation plays an important role in the pathogenesis of diabetic cardiac hypertrophy. Proinflammatory cytokines, including IL-6 and TNF-α [18], participate directly in the inflammatory response responsible for diabetic cardiac hypertrophy. Endothelial cells release multiple inflammatory mediators and express various adhesion molecules such as ICAM-1 and VCAM-1, which induce firm adhesion of inflammatory cells at the vascular surface, increasing the incidence of vascular inflammation in the early stage of heart disease [19]. The present study shows that simvastatin reduced the contents of IL-6 and TNF-α in the diabetic rats injected with STZ and downregulated the protein expression of IL-6, TNF-α, ICAM-1, and VCAM-1 in the diabetic heart tissue. These results suggest that the inhibition of diabetic cardiac hypertrophy by simvastatin is also due to its anti-inflammatory action. Consistent with the present results, a previous study reported that simvastatin can reduce inflammatory response in cardiovascular diseases [20].
NF-κB activation and nuclear translocation play important roles in the pathogenesis of diabetic cardiomyopathy and cardiac hypertrophy [13]. In normal conditions, NF-κB is bound to the inhibitory protein IκBα in the cytoplasm. In the condition of diabetic cardiomyopathy, NF-κB is released and translocated into the nucleus as a result of degradation of IκBα proteins. Inactive NF-κB is primarily located in the cytoplasm and is associated with the inhibitor IκBα. Therefore, we examined whether inhibition of NF-κB nuclear translocation results in attenuation of diabetic cardiac hypertrophy by simvastatin. We found that the STZ group had decreased p65 and IκBα protein expression levels in the cytoplasm and increased p65 expression in the nucleus of heart tissue compared with the control group. These results indicate that NF-κB activation and nuclear translocation play critical roles in diabetic cardiac hypertrophy. However, simvastatin increased p65 and IκBα expression in cytoplasm and decreased the expression of p65 in the nucleus compared with that in the STZ group. These results suggest that inhibition of NF-κB nuclear translocation contributes to the attenuation of diabetic cardiac hypertrophy by simvastatin.
Calpain-1 is a calcium-activated neutral protease. In cardiovascular disease, calpain is over-activated and participates in the occurrence and development of various cardiovascular diseases such as myocardial ischemia-reperfusion injury, diabetic cardiomyopathy, hypertension, and atherosclerosis. Our previous study demonstrated that the protein expression of calpain-1 and the activity of calpain were increased in ISO-induced hypertrophic heart tissues and cells [10]. We also found that simvastatin decreased calpain-1 protein expression in macrophages treated with oxLDL [21]. A recent study shows that calpain activity in the diabetic myocardium is significantly increased and is an important cause of the myocardial damage in diabetes [22]. As expected, in this study, simvastatin reduced the protein expression of calpain-1 and the activity of calpain in myocardial tissue of diabetic rats. Consistently, a study showed that calpastatin overexpression can inhibit the activity of calpain to reduce diabetic myocardial ischemia/reperfusion (I/R) injury [23]. Targeted inhibition of calpain is protective against hypertrophy and fibrosis, 2 general hallmarks of diabetic cardiomyopathy [22]. Clinically, calpain-1 expression is positively correlated with the contents of TNF-α and IL-6, but calpain-2 protein expression is not [24]. Previous studies showed that the elevated calpain activity is associated with nuclear translocation of NF-κB [25] and calpain regulates ROS generation in the development of DCM [26]. These results suggest that downregulation of calpain-1 and calpain inactivation by simvastatin contribute to its anti-inflammation effect on diabetic cardiac hypertrophy.
Simvastatin is an HMG-CoA reductase inhibitor with lipid-lowering effect. In recent studies, simvastatin alleviated the progression of experimental DCM, possibly by a protective effect independent of its low-density lipoprotein (LDL)-cholesterol-lowering properties. It is well-established that the expressions of inflammatory cytokines can be increased by NF-κB activation. Following stimulation, activated NF-κB induces the upregulation of TNF-α, IL-6, ICAM-1, VCAM-1, and other molecules contributing to cardiovascular damage [27]. ROS and NF-κB are closely related to cardiac hypertrophy in hyperglycemia [28]. NF-κB has been shown to regulate ROS formation [29]. A previous study has shown that calpain induces the NF-κB signaling, inflammatory response, and apoptosis following myocardial infarction (MI) [30]. The results of the present study and previous research demonstrate that simvastatin improves oxidative stress and inflammation in diabetic cardiac hypertrophy, at least partly mediated by calpain-1 activation of NF-κB. The present study reveals that inhibition of calpain-mediated activation of NF-κB plays a key role in the improvement of diabetic cardiac hypertrophy by simvastatin treatment.
Conclusions
In summary, simvastatin attenuates cardiac hypertrophy in diabetic rats, at least partly due to attenuation of oxidative stress and inflammation induced by calpain-1-mediated activation of NF-κB.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the National Natural Science Foundation of China (81870329), the Natural Science Foundation of Liaoning Province (20180530069), and the Talent Fund of Jinzhou Medical University (No. 2014-18)
References
- 1.Zhang B, Shen Q, Chen Y, et al. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci Rep. 2017;7:44239. doi: 10.1038/srep44239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Blasio MJ, Huynh K, Qin C, et al. Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110alpha) signaling. Free Radical Biol Med. 2015;87:137–47. doi: 10.1016/j.freeradbiomed.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Frati G, Schirone L, Chimenti I, et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res. 2017;113:378–88. doi: 10.1093/cvr/cvx011. [DOI] [PubMed] [Google Scholar]
- 4.Khanra R, Dewanjee S, K Dua T, et al. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med. 2015;13:6. doi: 10.1186/s12967-014-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen HL, Liang ZS, Zhang R, Yang K. Anti-inflammatory effects of triptolide improve left ventricular function in a rat model of diabetic cardiomyopathy. Cardiovasc Diabetol. 2013;12:50. doi: 10.1186/1475-2840-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao MX, Zhou B, Ling L, et al. Salusin-beta contributes to oxidative stress and inflammation in diabetic cardiomyopathy. Cell Death Dis. 2017;8:e2690. doi: 10.1038/cddis.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kartkaya K, Kanbak G, Oglakci A, et al. Protective effect of calpain inhibitor N-acetyl-L-leucyl-L-leucyl-L-norleucinal on acute alcohol consumption related cardiomyopathy. Mol Biol Rep. 2014;41:6743–53. doi: 10.1007/s11033-014-3560-4. [DOI] [PubMed] [Google Scholar]
- 8.Yu L, Yin M, Yang X, et al. Calpain inhibitor I attenuates atherosclerosis and inflammation in atherosclerotic rats through eNOS/NO/NF-kappaB pathway. Can J Physiol Pharmacol. 2018;96:60–67. doi: 10.1139/cjpp-2016-0652. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Liu J. [Simvastatin attenuated cardiac hypertrophy via inhibiting JAK-STAT pathways]. Zhonghua Xin Xue Guan Bing Za Zhi. 2008;36:738–43. [in Chinese] [PubMed] [Google Scholar]
- 10.Mei M, Tang FT, Lu ML, et al. Astragaloside IV attenuates apoptosis of hypertrophic cardiomyocyte through inhibiting oxidative stress and calpain-1 activation. Environ Toxicol Phar. 2015;40:764–73. doi: 10.1016/j.etap.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Han R, Tang F, Lu M, et al. Protective effects of Astragalus polysaccharides against endothelial dysfunction in hypertrophic rats induced by isoproterenol. Int Immunopharmac. 2016;38:306–12. doi: 10.1016/j.intimp.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Al-Rasheed NM, Al-Rasheed NM, Hasan IH, et al. Simvastatin ameliorates diabetic cardiomyopathy by attenuating oxidative stress and inflammation in rats. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/1092015. 1092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Zhuang X, Huang Z, et al. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-kappaB-mediated inflammation both in vitro and in vivo. Biochim Biophys Acta. 2018;1864:238–51. doi: 10.1016/j.bbadis.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Othman AI, El-Sawi MR, El-Missiry MA, Abukhalil MH. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed Pharmacother. 2017;94:362–73. doi: 10.1016/j.biopha.2017.07.129. [DOI] [PubMed] [Google Scholar]
- 15.Osorio JC, Cheema FH, Martens TP, et al. Simvastatin reverses cardiac hypertrophy caused by disruption of the bradykinin 2 receptor. Can J Physiol Pharmacol. 2008;86:633–42. doi: 10.1139/y08-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen P, Nawroth PP, King G, et al. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 17.Xu CH, Tang FT, Lu ML, et al. Pretreatment with Astragaloside IV protects human umbilical vein endothelial cells from hydrogen peroxide induced oxidative stress and cell dysfunction via inhibiting eNOS uncoupling and NADPH oxidase – ROS – NF-kappa B pathway. Can J Physiol Pharmacol. 2016;94:1132–40. doi: 10.1139/cjpp-2015-0572. [DOI] [PubMed] [Google Scholar]
- 18.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur Heart J. 2013;34:2436–43. doi: 10.1093/eurheartj/eht142. [DOI] [PubMed] [Google Scholar]
- 19.Qin WD, Liu GL, Wang J, et al. Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy. Oncotarget. 2016;7:35618–31. doi: 10.18632/oncotarget.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CP, Huang PH, Lai CF, et al. Simvastatin attenuates oxidative stress, NF-kappaB activation, and artery calcification in LDLR−/− mice fed with high fat diet via down-regulation of tumor necrosis factor-alpha and TNF receptor 1. PLoS One. 2015;10:e0143686. doi: 10.1371/journal.pone.0143686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Yin M, Yu L, et al. Simvastatin inhibited oxLDL-induced proatherogenic effects through calpain-1-PPARgamma-CD36 pathway. Can J Physiol Pharmacol. 2016;94:1336–43. doi: 10.1139/cjpp-2016-0295. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Ma J, Zhu H, et al. Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes. 2011;60:2985–94. doi: 10.2337/db10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan L, Li J, Wei M, et al. Disruption of Rac1 signaling reduces ischemia-reperfusion injury in the diabetic heart by inhibiting calpain. Free Radical Biol Med. 2010;49:1804–14. doi: 10.1016/j.freeradbiomed.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Choi H, Myung K. Calcitriol enhances fat synthesis factors and calpain activity in co-cultured cells. Cell Biol Int. 2014;38:910–17. doi: 10.1002/cbin.10281. [DOI] [PubMed] [Google Scholar]
- 25.Kamal F, Yanakieva-Georgieva N, Piao H, et al. Local delivery of Angiotensin II receptor blockers into the kidney passively attenuates inflammatory reactions during the early phases of streptozotocin-induced diabetic nephropathy through inhibition of calpain activity. Nephron Exp Nephrol. 2010;115:E69–79. doi: 10.1159/000313832. [DOI] [PubMed] [Google Scholar]
- 26.Ni R, Zheng D, Xiong S, et al. Mitochondrial Calpain-1 disrupts ATP synthase and induces superoxide generation in type 1 diabetic hearts: A novel mechanism contributing to diabetic cardiomyopathy. Diabetes. 2016;65:255–68. doi: 10.2337/db15-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–29. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong P, Wu L, Qian Y, et al. Blockage of ROS and NF-kappaB-mediated inflammation by a new chalcone L6H9 protects cardiomyocytes from hyperglycemia-induced injuries. Biochim Biophys Acta. 2015;1852:1230–41. doi: 10.1016/j.bbadis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Wang J, Xu W, et al. Magnolol inhibits Streptococcus suis-induced inflammation and ROS formation via TLR2/MAPK/NF-kappaB signaling in RAW264.7 cells. Pol J Vet Sci. 2018;21:111–18. doi: 10.24425/119028. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Wei M, Wang Q, et al. Deficiency of Capn4 gene inhibits nuclear factor-kappaB (NF-kappaB) protein signaling/inflammation and reduces remodeling after myocardial infarction. J Biol Chem. 2012;287:27480–89. doi: 10.1074/jbc.M112.358929. [DOI] [PMC free article] [PubMed] [Google Scholar]