Abstract
Background
PGC-1α can be activated by deacetylation reactions catalyzed by SIRT1. Resveratrol is currently known as a potent activator of SIRT1. However, it is unknown whether the renal-protective effect of resveratrol is further related to activation of the podocyte SIRT1/PGC-1α pathway.
Material/Methods
High glucose was used to stimulate mouse podocytes. Resveratrol and PGC-1α siRNA transfection were used to perform co-intervention treatments. The protein and mRNA expression levels of SIRT1, PGC-1α, NRF1, and TFAM were detect by immunofluorescence, Western blot analysis, and qRT-PCR in the podocytes, respectively. DCHF-DA and MitoSOX™ staining were used to monitor the total ROS and mitochondrial ROS levels, respectively. The specific activities of complexes I and III were measured using Complex I and III Assay Kits. Mitochondrial membrane potential and cell apoptosis were measured using JC-1 staining and Annexin V-FITC/PI double-staining, respectively.
Results
We found that high-glucose stimulation results in time-dependent decreases in the expression of SIRT1, PGC-1α, and its downstream genes NRF1 and mitochondrial transcription factor A (TFAM) for mouse podocytes, and increases ROS levels in cells and mitochondria. Moreover, the expression of nephrin was downregulated and the cell apoptotic rate was increased. Resveratrol treatment can improve abnormalities caused by high-glucose stimulation. In addition, it can also reduce the release of mitochondrial cytochrome C and DIABLO proteins to the cytoplasm and increase respiratory chain complex I and III activity and mitochondrial membrane potential.
Conclusions
Resveratrol can reduce the oxidative damage and apoptosis of podocytes induced by high-glucose stimulation via SIRT1/PGC-1α-mediated mitochondrial protection.
MeSH Keywords: Apoptosis; Genes, Mitochondrial; Oxidative Stress
Background
Diabetic nephropathy (DN) has high incidence, disability, mortality rates and has become an important focus of research. Studies have demonstrated that podocytes are required to maintain integrity of the glomerular filtration barrier and for prevention of proteinuria [1]. Podocytes are a highly differentiated terminal cell type with poor regeneration ability. In podocytes, the normal maintenance of multistage foot structure requires considerable energy consumption, and they are also relatively sensitive to oxidative stress. Enhancing oxidative stress can cause podocyte injury. A study has reported that podocytes are the initial target of cellular damage, especially in DN, apoptosis, and shedding, which leads to increased proteinuria and glomerular sclerosis in the early stage of disease progression [2].
Many studies have confirmed that oxidative stress plays an important role in the pathogenesis of DN [3]. In 2001, Brownlee proposed the unified mechanism theory that postulates the excess reactive oxygen species (ROS) produced by the high-glucose-induced mitochondrial respiratory chain is a triggering factor in the development of diabetic complications [4]. Mitochondria are considered a significant source of intracellular ROS and are involved in endogenous apoptotic pathways. Mitochondria are important players in the pathogenesis of DN and have been proved to be susceptible to peroxisomes [5].
Mitochondrial biosynthesis is a physiological activity required to maintain and repair mitochondrial structures and is an important process in regulation of mitochondrial gene and protein expression [6]. The peroxisome proliferator-activated receptor gamma co-activator-1α(PGC-1α) is a central regulator of mitochondrial biosynthesis, and mitochondrial DNA is activated and upregulated by the transcription factors nuclear respiratory factor (NRF)-1 and TFAM [7]. The downregulation of PGC-1α expression along with mitochondrial dysfunction were noted in a variety of diabetic complications, suggesting that PGC-1α-regulated mitochondrial biosynthesis can delay the development of diabetic complications. Further studies have shown that PGC-1α can be activated by deacetylation reactions catalyzed via the NAD+-dependent deacetylase silent mating-type information regulation 2 homolog 1 (SIRT1) [8,9]. Resveratrol is a potent activator of SIRT1, which exerts important biological effects on modulation of inflammation, oxidative stress, and cancer, as well as the regulation of glucose and lipid metabolism. Recently, it was found in a DN animal model that resveratrol exhibited renal protective effects by the reduction of proteinuria and extracellular matrix deposition [10,11]. In the present study, resveratrol was shown to regulate the SIRT1-downstream target genes PGC-1α and Forkhead box O (FOXO) by increasing mitochondrial function in myocardium and skeletal muscle tissues [12,13]. However, it remains unknown whether the renal-protective effect of resveratrol is further related to the activation of the podocyte SIRT1/PGC-1α pathway and improvement of mitochondrial function in DN.
In the present study, podocytes were cultured in vitro by high-glucose stimulation. In addition, experiments were carried out using resveratrol and PGC-1α siRNA transfection techniques to investigate the possible mechanisms of mitochondrial dysfunction in diabetic podocyte lesions and their possible protective targets.
Material and Methods
Reagents and antibodies
All culture media was purchased from Gibco-BRL (Grand Island, NY, USA). Resveratrol, D-glucose, and mannitol were obtained from Sigma (St. Louis, MO, USA). Recombinant murine IFN-γ was purchased from Peprotech Company (NJ, USA). Antibodies for TFAM and DIABLO were obtained from Proteintech (Chicago, IL). Antibodies for SIRT1, NRF1, and nephrin were purchased from Abcam (Cambridge, UK). PGC-1α antibody was purchased from Novus Biologicals (Littleton, CO, USA). Cytochrom C antibody was purchased from Signalway Antibody Company (College Park, MD, USA). Cleaved caspase-3 antibody was purchased from Cell Signaling Technology (Beverly, MA, USA). The β-actin antibody was purchased from Biosynthesis Biotechnology Co. (Beijing, China). The FITC Annexin V Apoptosis Detection Kit I was purchased from BD Pharmingen (San Diego, CA, USA). The polyvinylidene difluoride (PVDF) membrane was purchased from Millipore (Billerica, MA, USA). TRIzol, Lipofectamine 2000 reagent, and MitoSOX™ Red mitochondrial superoxide indicator were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
Podocyte culture and experimental groups
Conditionally immortalized mouse podocytes were purchased from the Basic Medical Cell Center in the Basic College of Peking Union Medical College. The undifferentiated podocytes were initially cultured in a 33°C incubator containing 5% CO2. Following culture and passage of the podocytes with PRMI1640 medium that contained 10U/ml mouse recombinant IFN-γ, 10% fetal bovine serum, 100U/ml penicillin, and 100 μg/ml streptomycin, the cells were transferred to a 37 incubator containing 5% CO2. The cells were diluted to 1: 4 and/or 1: 6 and were incubated for 10 to 14 days using RPMI1640 complete medium in the absence of IFN-γ. Following the maturation of cell differentiation, normal glucose (5.6 mmol/L glucose, NG), hyperosmotic control (24.5 mmol/L mannitol, MG), high glucose (30 mmol/L glucose, HG), and high glucose + resveratrol (30 mmol/L glucose + 10 μmol/L Rel, HG + Res) were used as intervention treatments for different time periods [14].
Transfection of podocytes using PGC-1α siRNA
Conditionally immortalized mouse podocytes were grown in 6-well plates for 24 h in the presence of RPMI 1640 medium with 10% FBS and were transfected with siRNA against PGC1α using Lipofectamine RNAi MAX according to the instructions provided by the manufacturer. siRNAs were synthesized by SBS Genetech Co. (Beijing, China). After 24 h transfection, the cells were treated with high glucose in the presence and/or absence of resveratrol.
Real-time fluorescence quantitative PCR
Total RNA and cDNA were prepared from cultured cells using TRIzol reagent and a TaKaRa RNA PCR kit (AMV) (TaKaRa Bio, Inc.), respectively. The cDNA was amplified using PCR with specific primers for SIRT1, PGC-1α, NRF1, TFAM, and β-Actin rRNA (Table 1), which were obtained from Sangon Biotech Co. (Shanghai, China). qRT-PCR was conducted using SYBR Premix Ex TaqTMII (TaKaRa Bio, Inc., Shiga, Japan) and the Agilent Mx3000P QPCR system (Agilent, CA, USA). The relative changes in gene expression were calculated using the 2−ΔΔCT method, and all experiments were repeated at least 3 times.
Table 1.
Primer sequences for real-time RT-PCR.
| Gene symbol | Forward primer | Reverse primer |
|---|---|---|
| SIRT1 | GCTGACGACTTCGACGACG | TCGGTCAACAGGAGGTTGTCT |
| PGC-1α | TATGGAGTGACATAGAGTGTGCT | GTCGCTACACCACTTCAATCC |
| NRF1 | CGGAAACGGCCTCATGTGT | CGCGTCGTGTACTCATCCAA |
| TFAM | ATTCCGAAGTGTTTTTCCAGCA | TCTGAAAGTTTTGCATCTGGGT |
| α-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
Extraction of mitochondrial cytoplasmic protein and the total cell protein
Cells treated with glucose were routinely digested and collected. Each sample contained 2×107 cells mixed with 1 ml mitochondrial separation reagent (Sangon Biotech Co., Shanghai) to gently suspend the cells. The samples were placed on ice for 10 to 15 min, and the cell suspension was homogenized approximately 30 times. The cell homogenate was collected and centrifuged at 1000 g at 4°C for 10 min, and the supernatant was transferred to a new centrifuge tube and then centrifuged at 1100 g at 4°C for 10 min. The supernatant was collected and centrifuged at 1200 g at 4°C for 10 min. Samples of cytoplasmic proteins without mitochondria were obtained. A total of 150~200 μl of mitochondrial solution was added and the mitochondrial pellet was redissolved for mitochondrial enzyme activity assay. The cytoplasmic protein concentration was measured by the BCA method and the protein was stored at −80°C after dispensing.
Western blot detection
A pre-cooled PBS solution was added to wash the cells 3 times, and the cells were lysed by addition of 300 μl of lysis solution on ice for 30 min. The samples were centrifuged, the supernatant was collected, and the BCA method was used to determine the protein concentration. Following denaturation of the protein, 10% polyacrylamide gel electrophoresis was carried out and the proteins were transferred from the gels to the polyvinylidene fluoride membrane. The membrane was incubated with 5% skimmed milk powder at 37°C for 2 h, then it was washed with TBST (Tris-buffered saline + Tween 20). The proteins were incubated with rabbit anti-SIRT1, anti-nephrin, and anti-cleaved caspase 3 monoclonal antibodies at a dilution of 1: 1000; rabbit anti-PGC-1α, NRF1, and TFAM polyclonal antibodies at a dilution of 1: 1000; and rabbit anti-Cyto C and DIABLO polyclonal antibodies at a dilution of 1: 500. Anti-β-actin polyclonal antibody was used at 1: 2000. The membrane was incubated with the primary antibodies overnight at 4°C. The next morning, the membranes were washed with TBST 3 times, and horseradish peroxidase-labeled goat anti-rabbit and/or anti-mouse secondary antibodies (1: 5,000) were added. The membranes were incubated with the antibodies at 3°C for 1 h. The detection was conducted using enhanced chemiluminescence (ECL) reaction reagents, and proteins were detected in 10% SDS-PAGE. The gray value of the strips was measured using the Gel-Pro image analysis system, and the ratio of the absorbance value of the target band to the β-actin bands represented the relative content of the target protein.
Immunofluorescence assay
The cells were fixed with 4°C acetone for 10 min and permeabilized with 0.2% Triton X-100 at room temperature for 10 min to expose the antigen. The histology slices containing the cells were incubated with 10% normal goat serum at 37°C for 30 min, and then mixed with mouse anti-SIRT1 monoclonal antibody (1: 200), rabbit anti-PGC-1α, NRF1, and TFAM polyclonal antibodies (1: 100) at 4°C overnight. After washing with PBS, FITC-labeled anti-mouse and rabbit secondary antibody (1: 100) were added and incubated for 1 h at 37°C. The histology slices were further washed with PBS, sealed with mounting agent, and observed under a fluorescence microscope.
Detection of intracellular total ROS
DCHF-DA (Sigma, MO, USA) was diluted to serum-free DMEM to a final concentration of 10 μmol/L. The original medium was then removed, and the cells were washed 2 times with PBS. The diluted DCHF-DA medium was added to the cells (incubated at 37°C for 30 min). After the cells were washed 2 times with PBS and routine digestion, collection was carried out. At last, the cells were re-suspended in PBS, and the fluorescence value was measured by flow cytometry. The parameter settings included an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Detection of the synthesis of mitochondrial ROS using MitoSOX staining
A total of 50 μg of MitoSOX™ powder was dissolved in 13 μl DMSO-prepared 5 mmol/L MitoSOX™ solution and diluted with HBSS/Ca/Mg buffer to prepare 5 μmol/L MitoSOX™ working solution. D-Hank’s solution was used to wash the cells twice, and 1 ml of 5 μmol/L MitoSOXTM working solution was added to the cells. The samples were incubated at 37°C for 10 min in the dark and washed gently with D-Hank’s solution thrice. The samples were observed and photographed under an inverted fluorescence microscope. Semi-quantitative analysis of the image was performed using Image-J software. The average fluorescence intensity of MitoSOX per unit cell area was calculated following correction of the background of the image. A total of 3 samples were used from the cultured cells under various conditions, and 5 high-power field views were randomly selected from each sample.
Detection of the activities of mitochondrial complexes I and III
The mitochondria were freshly isolated from podocytes as described previously. The specific activities of complexes I and III were measured using Complex I and III Assay Kits (GENMED Scientific, Shanghai, China) according to the manual provided by the manufacturer. The activity of complex I was detected at 340 nm based on the reduction of the ubiquinone analogue decylubiquinone, whereas the activity of complex III was detected by monitoring the reduction of cytochrome at 550 nm. The activity of the 2 complexes is expressed in milligrams of protein per milligram of sample, and the biological activity is characterized by nanomoles per milligram of protein per minute.
Detection of mitochondrial membrane potential using JC-1 staining
A total of 100 μl incubation buffer was added with 900 μl sterilized deionized water to prepare a dyed buffer solution, which was preheated to 37°C. A total of 10 μl JC-1 (SAB, MD, USA) was added to each 1 ml of dying buffer working solution to prepare the JC-1 working solution. Then, the cells were washed twice with PBS buffer solution, and the number of cells per dish was estimated to approximately 5×105. A total of 500 μl of JC-1 working solution was added and the samples were incubated at 37°C in the presence of 5% CO2 for 15 min. The cells were washed twice with the buffer that contained the dye working solution. Finally, 500 μl of the working solution was added to each petri dish, and the cells were observed and photographed under a confocal microscope.
Detection of cell apoptosis using Annexin V-FITC/PI double-staining
Each group of cells was collected and washed twice with pre-cooled PBS. A total of 500 μl of 1×binding buffer was added to suspend 1×106/ml of cells, whereas 5 μl of FITC-labeled Annexin V reagent mix was added along with 10 μl PI. The cells were incubated at room temperature in the dark for 15 min. The apoptotic rate was measured by flow cytometry for 1 h.
Statistical analysis
SPSS17.0 statistical software was used for statistical analysis. Continuous data are expressed as mean ± standard deviation (χ̄±S), and one-way ANOVA was used for comparison of 2 groups. Statistical significance was set at p<0.05.
Results
High-glucose treatment reduced the expression levels of SIRT1, PGC-1α, NRF1, and TFAM in podocytes
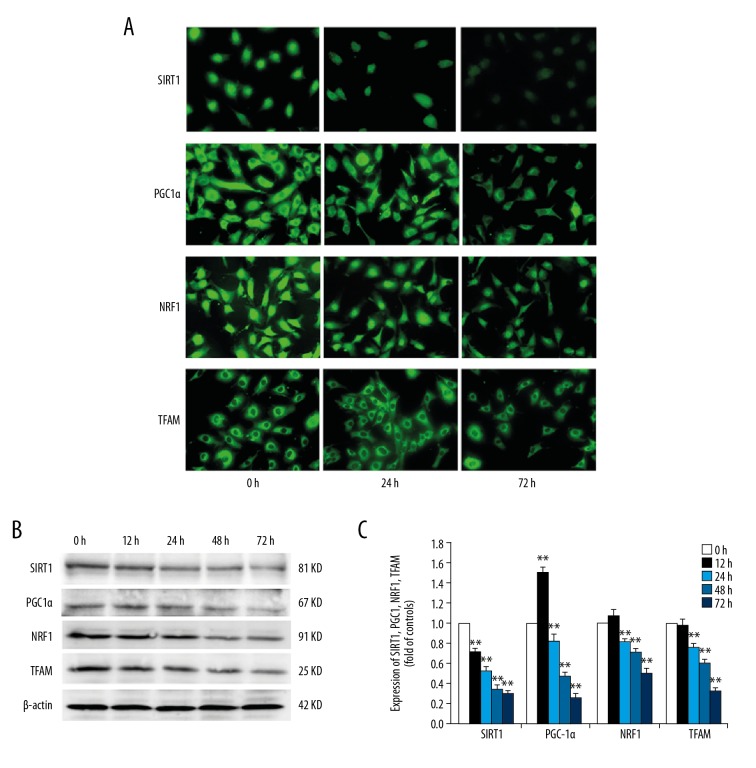
To investigate the effect of high glucose on the expression levels of protein and mRNA for SIRT1, PGC-1α, NRF1, and TFAM in the podocytes, immunofluorescence, Western blot, and real-time quantitative PCR assays, respectively, were used. The results indicated that SIRT1 and NRF1 were mainly expressed in the nucleus, while a lesser amount of protein was detected in the cytoplasm. Positive expression of PGC-1α was detected in the cytoplasmic and nuclear fractions. TFAM was mainly expressed in the cytoplasm. The protein expression levels of SIRT1, PGC-1α, NRF1, and TFAM in HG group was lower than those in the NG group (Figure 1A). Western blot analysis indicated that the expression of SIRT1 was decreased from 0 h to 72 h after high-glucose treatment. The expression of PGC-1α was upregulated at 12 h after hyperglycemia, whereas the expression of PGC-1α decreased after 24 h. The changes in the expression levels of NFR1 and TFAM were not statistically significant at 12 h of high-glucose treatment compared with those at 0 h, and the expression levels decreased after 24 h (Figure 1B). The results of qRT-PCR were similar to that of protein expression (Figure 1C).
Figure 1.
Effects of high glucose on the expression of SIRT1, PGC-1α, NRF1, and TFAM in podocytes. Podocytes were incubated with 5.6 mM glucose (NG group), 5.6 mM glucose plus 24.4 mM mannitol (M group), or 30 mM glucose (HG group) for 72 h. (A) SIRT1, PGC-1α, NRF1, and TFAM expression were detected using immunocytochemical staining at different time points (0, 12, 24, 48, and 72 h). (×200). (B) The expression levels of SIRT1, PGC-1α, NRF1, and TFAM were detected by Western blot analysis, and the relative intensities were normalized against β-actin. (C) The expression levels of SIRT1, PGC-1α, NRF1, and TFAM mRNA were analyzed by real-time PCR. The values are expressed as mean ±SD (n=6). * P<0.05, ** P<0.01 vs. control group.
High-glucose treatment increased the synthesis of total ROS in podocytes and mitochondria
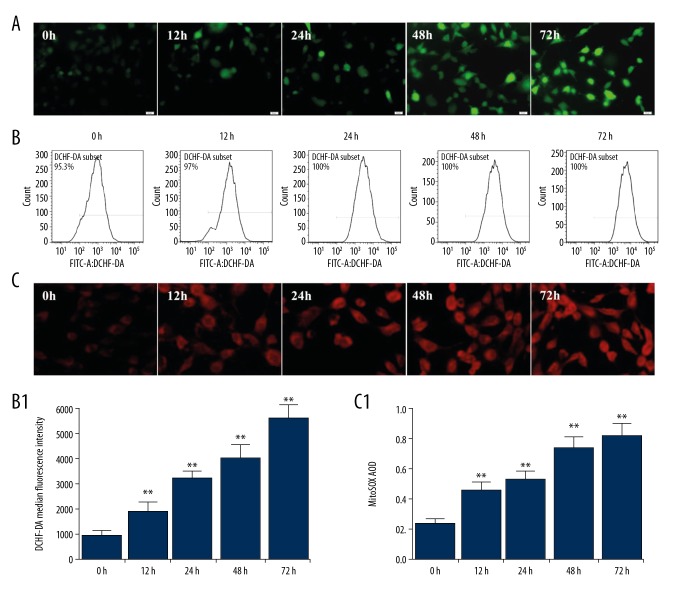
DCHF-DA and MitoSOX™ staining were used to monitor the total ROS and mitochondrial ROS levels, respectively. DCHF-DA was gradually increased following incubation with high glucose (Figure 2A). The results of the flow cytometry assays indicated that the total ROS was gradually increased following incubation with high glucose in a time-dependent manner (Figure 2B). The content of mitochondrial ROS was gradually increased in intensity following treatment with high glucose (Figure 2C). The results of fluorescence semi-quantitative analysis were consistent with the total ROS levels measured by flow cytometry.
Figure 2.
Effects of high glucose on the intracellular and mitochondrial ROS production in podocytes. Podocytes were incubated with 5.6 mM glucose (NG group), 5.6 mM glucose plus 24.4 mM mannitol (M group), and/or 30 mM glucose (HG group) for 72 h. (A) The intracellular ROS production in podocytes was detected by DCHF-DA staining (×400). (B, B1) The intracellular ROS production in podocytes was detected by flow cytometry. (C, C1) HG-induced mitochondrial ROS production in podocytes was evaluated by MitoSOX. The values are expressed as mean ±SD (n=6). ** P<0.01 vs. control group (0 h).
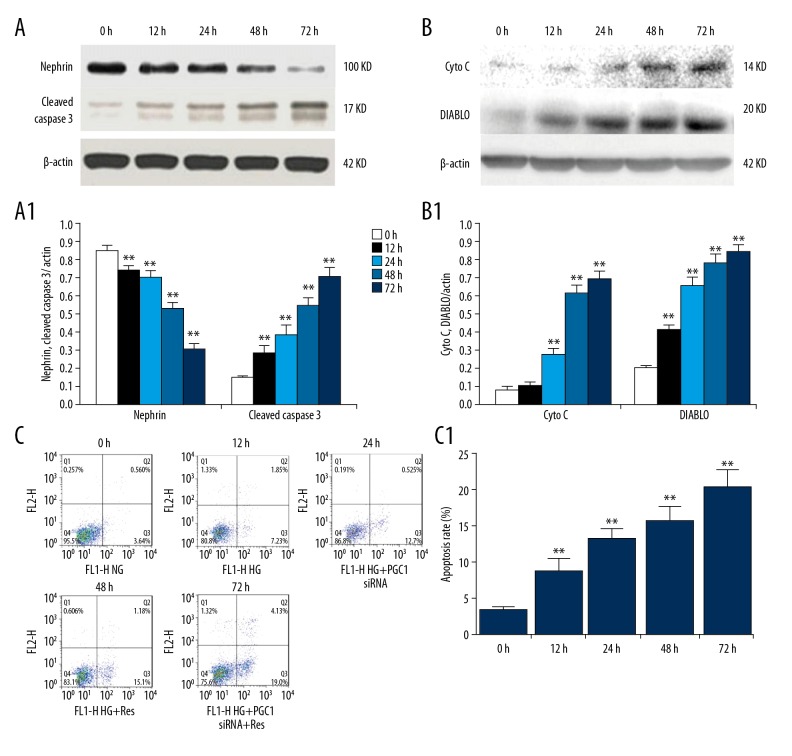
High-glucose treatment induced cytochrome C and DIABLO release, increased cell apoptosis, and induced nephrin decrease in podocyte
Cytochrome C and DIABLO proteins in the cytoplasm were upregulated in a time-dependent manner with high-glucose treatment, suggesting that the mitochondrial apoptotic pathway was activated (Figure 3A). High-glucose treatment reduced the expression of nephrin and increase cleaved caspase 3 protein expression in a time-dependent manner (Figure 3B). Flow cytometry analysis results indicated that the apoptotic rate of the cells was also increased in a time-dependent manner by high-glucose treatment at 72 h (Figure 3C).
Figure 3.
Effects of high glucose on nephrin expression and apoptosis induction in podocytes. (A, A1) The expression of cleaved caspase-3 and nephrin were analyzed by Western blotting. (B, B1) Western blot analysis of cytochrome C and DIABLO in cytosolic fractions of podocytes. The relative intensities were normalized against β-actin. (C, C1) Apoptosis was detected by flow cytometry. The values are expressed as mean ±SD. ** P<0.01 vs. control group.
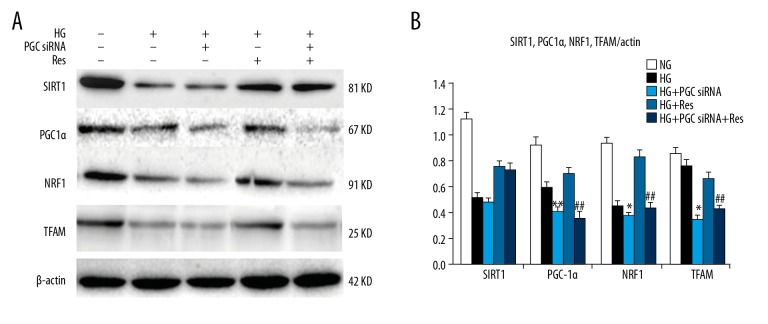
Effects of resveratrol and PGC1 siRNA on the expression levels of SIRT1, PGC-1α, NRF1, and TFAM in podocytes under high-glucose treatment conditions
To investigate the protective effect of resveratrol (SIRT1 agonist) on high-glucose-induced podocyte injury, we knocked down PGC1 by siRNA. Transfection was conducted in optimal condition of 6.25 μl siRNA and 5 μl transfection reagent, and the efficiency of PGC-1α knockdown was higher than 60%. Conversely, there were no significant effects on the expression of PGC-1α in control siRNA.
The protein expression levels of SIRT1, PGC-1α, NRF1, and TFAM in the HG group were significantly lower than those in the NG group after 48 h of high-glucose treatment. The expression levels of the 4 proteins in the HG+Res group were significantly higher than those in the HG group. The expression levels of the 4 proteins in the HG+PGC1 siRNA group were significantly lower than those in the HG group. The expression levels of the 4 protein in the HG+PGC1 siRNA+Res group were significantly lower than those in the HG group, but they were significantly higher than those in the HG+PGC1 siRNA group (Figure 4). These data suggest that resveratrol can increase the expression levels of SIRT1 and PGC-1α, and that PGC1 k/o by siRNA blocked the effects of SIRT1 on PGC-1α via NRF1 and TFAM. It may be concluded that SIRT1 acts upstream of PGC-1α.
Figure 4.
(A, B) Effects of resveratrol and PGC1α siRNA on HG-induced protein expression of SIRT1, PGC1α, NRF1, and TFAM in podocytes. Podocytes were incubated with normal glucose (5.6 mM, NG), high glucose (30 mM, HG), high glucose +PGC1 siRNA (HG+PGC1 siRNA), high glucose+resveratrol (10 μmol/L, HG+Res), and high glucose +PGC1 siRNA+resveratrol (HG+PGC1 siRNA+Res) for 48 h. The relative intensities were normalized against β-actin. The values are expressed as mean ±SD. * P<0.05, **P<0.01 vs. high glucose group; ## P<0.01 vs. high glucose + resveratrol group.
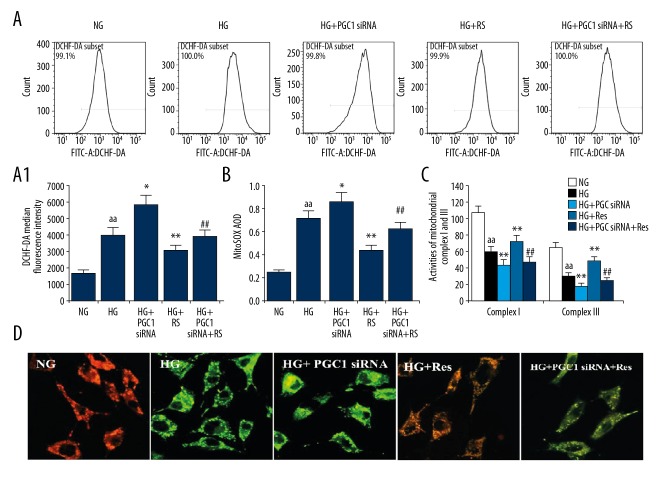
The effect of resveratrol and PGC1 siRNA on the total ROS and mitochondrial ROS synthesis in podocytes under high-glucose treatment conditions
The results of flow cytometry analysis indicated that the total content of ROS in the HG + Res group was significantly lower than in the HG group, and these levels were significantly higher than in the HG + PGC1 siRNA group (Figure 5A). The mitochondrial ROS change exhibited a similar trend in each group compared to that noted previously (Figure 5B), suggesting that the protective effect of resveratrol on the high-glucose-induced podocyte ROS synthesis was associated with the SIRT1/PGC-1α axis.
Figure 5.
Effects of resveratrol and PGC1α siRNA on HG-induced total ROS production and mitochondrial injury in podocytes. (A, A1) Intracellular ROS was detected by flow cytometry. (B) The mitochondrial ROS production in podocytes was detected by flow cytometry. (C) The changes of mitochondrial complex I and III activities in podocytes were detected by kit. (D) ΔΨYm was measured using a fluorescent probe JC-1. The ratio of red/green fluorescence represented ΔΨm in podocytes. The values are expressed as mean ±SD. aa P<0.01 vs. normal-glucose group; * P<0.05, ** P<0.01 vs. high-glucose group; ## P<0.01 vs. high-glucose + resveratrol group.
The effects of resveratrol and PGC1 k/o by siRNA on the activities of the mitochondrial respiratory chain complexes I and III and on the membrane potential in podocytes under high-glucose treatment conditions
To further clarify the relationship between SIRT1/PGC-1α axis and mitochondrial dysfunction, colorimetric method was used to detect the activity of mitochondrial respiratory chain complexes I and III among the different groups. The activity of the complexes I and III in the HG + Res group was higher than in the HG group, and it was higher than in the HG+PGC1 siRNA+Res group (Figure 5C). The mitochondrial membrane potential changes were monitored using the JC-1 probe, in which red fluorescence indicates high potential and green fluorescence indicates low potential (Figure 5D).
The mitochondrial membrane potential results were consistent with the activity of mitochondrial respiratory chain complexes I and III among the different groups. This indicates that mitochondrial dysfunction of the podocytes in the high-glucose environment was aggravated following inhibition of PGC-1α. However, this effect was reduced by PGC1 siRNA interference. Furthermore, resveratrol improved mitochondrial function via the SIRT1/PGC-1α axis.
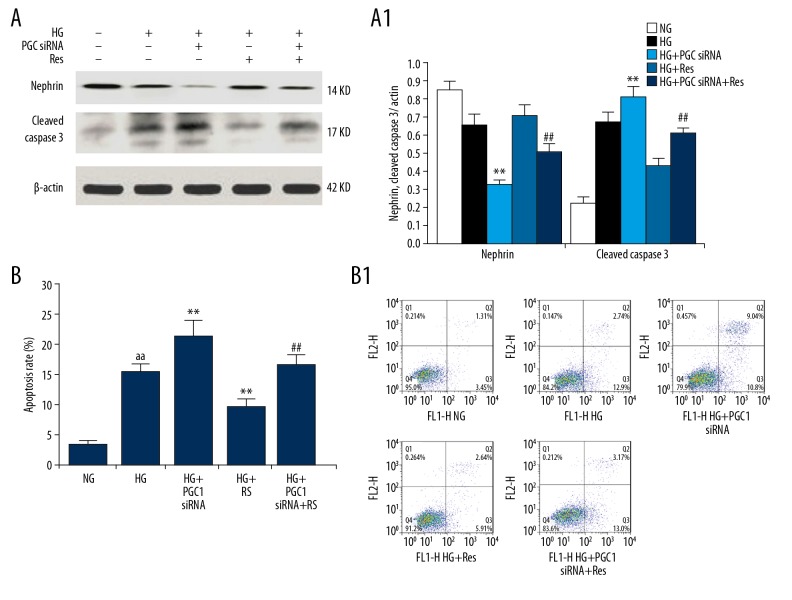
The effect of resveratrol and PGC1 siRNA on podocyte injury and apoptosis induction under high-glucose treatment conditions
Western blot analysis indicated that the expression of nephrin in the HG + PGC1 siRNA + Res group was significantly lower than in the HG + Res group, and was significantly higher than in the HG + PGC1 siRNA group. However, the expression of cleaved caspase 3 was the opposite of the expression of nephrin (Figure 6A). The podocyte apoptotic rate in the HG + PGC1 siRNA + Res group was significantly lower than in the HG + Res group, and was significantly higher than in the HG + PGC1 siRNA group (Figure 6B), suggesting that resveratrol reduces the high-glucose-induced podocyte injury and apoptosis induction via the SIRT1/PGC-1α axis.
Figure 6.
Effects of resveratrol and PGC1α siRNA on HG-induced apoptosis and nephrin expression of podocytes. (A, A1) The expression of cleaved caspase-3 and nephrin were analyzed by Western blotting. The relative intensities were normalized to the β-actin. (B, B1) Apoptosis was detected by flow cytometry. The values are expressed as mean ±SD. aa P<0.01 vs. normal glucose group; ** P<0.01 vs. high glucose group; ## P<0.01 vs. high-glucose + resveratrol group.
Discussion
Glomerular podocytes are the main component involved in maintenance of glomerular normal filtration barrier structure and function. The normal physiological function of podocytes, including the maintenance of cytoskeletal stability, and the normal secretion of cytokines and basement membrane related proteins all require a large amount of energy to support. Podocytes are highly differentiated terminal cells that are particularly sensitive and susceptible to oxidative stress [15]. Recent studies have shown that the onset of diabetic nephropathy is related to the mechanism of podocyte injury, and excessive ROS synthesis and mitochondrial dysfunction are the main causes of the development of diabetic nephropathy [16,17].
Studies have confirmed that the amount of ROS produced by mitochondria increases under high-glucose conditions and induces diabetic complications. Although mitochondria are the only subcellular organelles in the extranuclear genome, the repair system is not perfect due to the lack of mitochondrial DNA introns. With the protection of the lack of proteolysis, it can easily cause intracellular damage, resulting in mitochondrial dysfunction [18].
In addition, the ability of mitochondria to synthesize proteins is very limited because most mitochondrial structural proteins, including respiratory chain complex subunits and mitochondrial transcription factors, are encoded by nuclear genes. Mitochondrial biosynthesis plays a key role in the maintenance and repair of mitochondrial functions, as well as in information transfer between the nuclear and mitochondrial genes.
The most important regulatory factors mediating this process are the co-activator PGC-1α and its downstream transcription factors NRF1 and TFAM, of which PGC-1α plays an important role in the regulation of mitochondrial biogenesis and oxidation [6,7].
PGC-1α is a co-activator of the nuclear receptor PPARγ, and it is highly expressed when energy requirements are high. PGC-1α is not expressed in tissues such as myocardium, skeletal muscle, nervous system, liver, kidney, and adipose tissue, and mainly regulates the synthesis of mitochondrial proteins (including respiratory chain complex subunits) by activating the nuclear transcription factor NRF1 [19].
NRF1 regulates mitochondrial DNA replication and transcription by binding to the TFAM promoter [20]. Recent studies have identified PGC-1α as an important target for improving mitochondrial function. Decreased PGC-1α expression, which is associated with mitochondrial dysfunction and oxidative damage, was observed in diabetic neurodegenerative lesions, diabetic skeletal muscle cells, ob/ob mouse cardiomyocytes, and diabetic microenvironment adipose-derived stem cells [21–24]. Silent mating-type information regulation 2 homolog 2 1 (SIRT1) is a NAD+ (nicotinine adenine dinucleotide)-dependent histone/non-histone deacetylase, which has been found to activate coactivators, transcription factors, nuclear receptors, and DNA repair enzymes, specifically P300/CBP, PARP-1, Ku70, FOXOs [25], p53 [26], and NF-κB [27]. The transcription factor PPAR and its co-activator PGC1 are also substrates of SIRT1 [8]. SIRT1 activates a multitude of downstream factors involved in the regulation of cell proliferation, differentiation, aging, apoptosis, and metabolism, according to each tissue type. The majority of relevant studies have confirmed that SIRT1 gene exerts a potential protective effect on the kidneys. The abnormalities of SIRT1 expression and activity levels are noted in acute renal injury induced by lipopolysaccharide, in ischemia-reperfusion [28,29], and/or in chronic interstitial fibrosis and diabetic nephropathy [30,31]. The upregulation of SIRT1 expression by energy limitation, agonist-mediated induction, and transgene overexpression can ameliorate the pathological changes caused by oxidative stress. In addition, SIRT1 upregulation can improve the induction of apoptosis and fibrosis in nephropathy. The mechanism of action may be related to the de-acetylation of downstream genes and the activation of signaling pathways. The relationship between podocyte injury, SIRT1, PGC-1α and mitochondrial dysfunction under high glucose treatment conditions remains unclear.
PPAR and PGC1 are also substrates for SIRT1 [8]. According to different tissue types, SIRT1 activation is involved in the regulation of cell proliferation, differentiation, senescence, apoptosis, metabolism, and many other downstream factors. Most studies confirmed that the SIRT1 has a potential protective effect on the kidneys. It has been reported to show abnormal expression and activity levels of SIRT1In ischemia-reperfusion [28,29], chronic interstitial fibrosis and diabetic nephropathy [30], and acute renal injury induced by lipopolysaccharide [31]. Upregulation of SIRT1 expression can ameliorate pathological changes caused by oxidative stress via energy limitation, agonist-mediated induction, and transgene overexpression. Moreover, upregulation of SIRT1 can improve the induction of apoptosis and fibrosis in nephropathy, which may be related to the deacetylation of downstream genes and the activation of signaling pathways. However, the relationships between podocyte injury and SIRT1, PGC-1α, and mitochondrial dysfunction under high-glucose treatment conditions remain unclear.
In this study, mouse podocytes were treated with high glucose at different time points, and the expression levels of PGC-1α, NRF1, and TFAM were reduced. High glucose induced the downregulation of SIRT1 expression in podocytes, which was later than that of PGC-1α. In addition, high glucose can induce an increase in mitochondrial and intracellular ROS levels in a time-dependent manner, and these 2 changes show a consistent trend. After mitochondrial release of cytochrome C and DIABLO was detected, caspase-3 was induced the expression of apoptosis-related markers was also increased. Previous studies have shown that SIRT1 can be activated by deacetylation of PGC-1α, and it further regulates the expression of the kinase LKB, which acts upstream of AMPK [32]. Studies have shown that SIRT1 can increase the expression of PGC-1α at the transcriptional level [33,34]. Despite these findings, the role of SIRT1 in the downregulation of high-glucose-induced PGC-1α in podocytes remains unclear.
Previous studies have not shown whether mitochondrial biosynthesis can play a protective role in oxidative stress and podocyte apoptosis induction through high-glucose treatment. To explore these hypotheses, SIRT1 resveratrol was selected as an agonist in the podocyte in vitro model. The role of resveratrol in the metabolism of glycolipids has previously been studied and it has been demonstrated to have antioxidant, anti-apoptotic, and anti-aging effects. In diabetic nephropathy, resveratrol shows hypoglycemic and anti-inflammatory effects. Studies have shown that resveratrol inhibits both glomerular filtration and interstitial fibrosis in STZ-induced type 1 diabetic rats and db/db mice [11]. Most studies have shown that resveratrol is mediated through SIRT1/FOXO3a and/or through non-SIRT1-dependent regulatory mechanisms, and AMPK/NOX4/ROS plays an antioxidant role [35]. Xu et al. reported that the activation of SIRT1 by resveratrol improved the oxidative damage induced by high-glucose treatment in the mitochondria of mesangial cells [14]. In contrast to the study by Xu et al., our study indicated that resveratrol increases the expression of SIRT1, improves the synthesis of intracellular ROS in a high-glucose environment, and reduces the induction of apoptosis. The analysis of mitochondrial function showed that when mitochondrial ROS synthesis was reduced, the activity of respiratory chain complexes I and III and mitochondrial membrane potential increased accordingly. This result suggests that mitochondrial function is improved and the mitochondrial apoptotic pathway is inhibited. When resveratrol was added, it upregulated the expression of PGC-1α, NRF1, and TFAM, suggesting that reduction of oxidative stress and apoptosis in podocytes under high-glucose treatment conditions is related to regulation of mitochondrial biosynthesis by PGC-1α/NRF1/TFAM. Additionally, SIRT1 plays a regulatory role in the upstream of PGC-1α. To confirm this hypothesis, PGC-1α was knocked down by siRNA interference in the presence of resveratrol. The data indicated that oxidative damage and apoptosis were further aggravated in the HG + PGC-1α siRNA intervention group compared to the high-glucose group, whereas the protective effect of resveratrol on high-glucose-induced podocyte injury was further inhibited by knockdown of PGC-1α. In contrast to these observations, the upregulation of SIRT1 was not blocked, which further suggests that SIRT1 acts upstream of PGC-1α, and resveratrol improves mitochondrial dysfunction caused in high-glucose action by activating the SIRT1/PGC-1α axis, inhibiting oxidative stress and inducing apoptosis. These data provide an experimental basis for elucidating the mechanism underlying the renal-protective effects of resveratrol, and also show that SIRT1 has potential as a target in early prevention and treatment of diabetic nephropathy.
Conclusions
In this study, the downregulation of SIRT1 and PGC-1α was detected in mouse podocytes cultured under high-glucose conditions. The downregulation of SIRT1 and PGC-1α expression led to increased oxidative stress, mitochondrial apoptosis pathway activation, and impaired mitochondrial function. Resveratrol can upregulate the expression of mitochondrial biosynthesis-related proteins through the SIRT1/PGC-1α axis, improving mitochondrial function, reducing podocyte oxidative stress, and inhibiting the induction of apoptosis.
Acknowledgement
We thank the Department of Pathology of Hebei Medical University for providing assistance.
Abbreviations
- DCHF-DA
2,7-dichlorofluorescein diacetate
- DN
diabetic nephropathy
- MDA
malondialdehyde
- mtDNA
mitochondrial DNA
- NADH
nicotinamide adenine dinucleotide
- NRF
nuclear respiratory factor
- PGC-1α
peroxisome proliferator-activated receptor γ co-activator 1α
- ROS
reactive oxygen species
- SIRT1
Sir2-related enzymes, sirtuins1
- SOD
superoxide dismutase
- TFAM
mitochondrial transcription factor A
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weil EJ, Lemley KV, Mason CC, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82:1010–17. doi: 10.1038/ki.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sifuentesfranco S, Padillatejeda DE, Carrilloibarra S, et al. Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int J Endocrinol. 2018;2018 doi: 10.1155/2018/1875870. 1875870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 5.Lindblom R, Higgins G, Coughlan M, de Haan JB. Targeting mitochondria and reactive oxygen species-driven pathogenesis in diabetic nephropathy. Rev Diabet Stud. 2015;12(1–2):134–56. doi: 10.1900/RDS.2015.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li P, Yan L, Burns N, et al. SIRT1 is required for mitochondrial biogenesis reprogramming in hypoxic human pulmonary arteriolar smooth muscle cells. Int J Mol Med. 2017;39(5):1127–36. doi: 10.3892/ijmm.2017.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884–90. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khader A, Yang WL, Kuncewitch M, et al. Sirtuin 1 activation stimulates mitochondrial biogenesis and attenuates renal injury after ischemia-reperfusion. Transplantation. 2014;98(2):148–56. doi: 10.1097/TP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 9.Gurd BJ. Deacetylation of PGC-1α by SIRT1: Importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36(5):589–97. doi: 10.1139/h11-070. [DOI] [PubMed] [Google Scholar]
- 10.Kim MY, Lim JH, Youn HH, et al. Erratum to: Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db, mice. Diabetologia. 2013;56(3):681. doi: 10.1007/s00125-012-2747-2. [DOI] [PubMed] [Google Scholar]
- 11.He T, Xiong J, Nie L, et al. Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J Mol Med (Berl) 2016;94(12):1359–71. doi: 10.1007/s00109-016-1451-y. [DOI] [PubMed] [Google Scholar]
- 12.Mitra R, Nogee DP, Zechner JF, et al. The transcriptional coactivators, PGC-1alpha and beta, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J Mol Cell Cardiol. 2012;52(3):701–10. doi: 10.1016/j.yjmcc.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung HL, Cheng SY, Cheung YT, et al. A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol. 2018;14:7–19. doi: 10.1016/j.redox.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Nie L, Yin YG, et al. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259(3):395–401. doi: 10.1016/j.taap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–33. [PubMed] [Google Scholar]
- 16.Ranjbar A, Kheiripour N, Ghasemi H, et al. Antioxidative effects of tempol on mitochondrial dysfunction in diabetic nephropathy. Iran J Kidney Dis. 2018;12(2):84–90. [PubMed] [Google Scholar]
- 17.Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: The beginning and end to diabetic nephropathy? Br J Pharmacol. 2014;171(8):1917–42. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shokolenko I, Venediktova N, Bochkareva A, et al. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009;37(8):2539–48. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100(14):8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta. 2012;1819(9–10):921–29. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Mitra R, Nogee DP, Zechner JF, et al. The transcriptional coactivators, PGC-1alpha and beta, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J Mol Cell Cardiol. 2012;52(3):701–10. doi: 10.1016/j.yjmcc.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coll T, Jove M, Rodriguez-Calvo R, et al. Palmitate-mediated downregulation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha in skeletal muscle cells involves MEK1/2 and nuclear actor-kappaB activation. Diabetes. 2006;55(10):2779–87. doi: 10.2337/db05-1494. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury SKR, Dobrowsky RT, Fernyhough P. Nutrient excess and altered mitochondrial proteome and function contribute to neurodegeneration in diabetes. Mitochondrion. 2011;11(6):845–54. doi: 10.1016/j.mito.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang XY, Lu DB, Jiang YZ, et al. PGC-1alpha prevents apoptosis in adipose-derived stem cells by reducing reactive oxygen species production in a diabetic microenvironment. Diabetes Res Clin Pract. 2013;100:368–75. doi: 10.1016/j.diabres.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Hori YS, Kuno A, Hosoda R, Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One. 2013;8(9):e73875. doi: 10.1371/journal.pone.0073875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 27.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 2013;273(2):345–54. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung YJ, Lee AS, Nguyen-Thanh T, et al. SIRT2 regulates LPS-induced renal tubular CXCL2 and CCL2 expression. J Am Soc Nephrol. 2015;26(7):1549–60. doi: 10.1681/ASN.2014030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponnusamy M, Zhou X, Yan Y, et al. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J Pharmacol Exp Ther. 2014;350(2):243–56. doi: 10.1124/jpet.113.212076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Zhong Y, Li X, et al. Role of transcription factor acetylation in diabetic kidney disease. Diabetes. 2014;63(7):2440–53. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Um JH, Park SJ, Kang H, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–63. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–18. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Wang Z, Zhao J, et al. Resveratrol attenuates lipopolysaccharides (LPS)-induced inhibition of osteoblast differentiation in MC3T3-E1 cells. Med Sci Monit. 2018;24:2045–52. doi: 10.12659/MSM.905703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Meng L, Zhao L, et al. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res Clin Pract. 2017;126:172–81. doi: 10.1016/j.diabres.2016.12.005. [DOI] [PubMed] [Google Scholar]