Abstract
Background
The POU domain class 5 transcription factor 1B (POU5F1B), is a pseudogene that is homologous to octamer-binding transcription factor 4 (OCT4), and is located adjacent to the MYC gene on human chromosome 8q24. POU5F1B has been reported to be transcribed in several types of cancer, but its role in cervical cancer remains unclear. This study aimed to investigate the expression and function of POU5F1B in tissue samples of human cervical cancer and in cervical cancer cell lines in vitro.
Material/Methods
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to quantify POU5F1B expression in cervical cancer tissues and in SiHa, HeLa, CaSki, and C33A human cervical cancer cell lines. Functional in vitro studies included analysis of the effects of POU5F1B expression on cervical cancer cell proliferation, migration, and apoptosis using a Cell Counting Kit-8 (CCK-8) assay, cell migration assays, and flow cytometry. Luciferase activity assays, qRT-PCR, and Western blot were performed to confirm the expression of POU5F1B.
Results
POU5F1B was significantly upregulated in cervical cancer tissues and cell lines. Interference with the expression of POU5F1B significantly inhibited cell proliferation, apoptosis, migration and invasion, and induced apoptosis in vitro. Western blot demonstrated that POU5F1B could modulate the expression of the OCT4 protein.
Conclusions
POU5F1B was upregulated in cervical cancer and down-regulation inhibited cell proliferation and migration and induced apoptosis in cervical cancer cell lines by modulating OCT4. Further studies are required to determine whether POU5F1B might be a diagnostic or prognostic biomarker or therapeutic target in cervical cancer.
MeSH Keywords: Octamer Transcription Factor-3, Pseudogenes, Uterine Cervical Neoplasms
Background
Worldwide, cancer of the uterine cervix is the fourth most common malignancy among women [1]. More than 90% of cases of cervical cancer result from infection with the human papillomavirus (HPV) [2]. Globally in 2015, an estimated 530,000 new cases of cervical cancer were annually, and approximately 270,000 women died from cervical cancer [3,4]. Almost 85% of cervical cancer deaths occur in undeveloped or developing countries [5]. Despite the recent progress in cervical cancer diagnosis and treatment, the 5-year survival rate of patients with advanced-stage cervical cancer has remained below 40% [6]. Therefore, it is important to understand the molecular pathology of cervical cancer and develop novel strategies for the management of this malignancy.
A pseudogene is a DNA fragment with high sequence similarity to the corresponding functional gene. Because of accumulation of multiple mutations, some pseudogenes have lost their original function [7]. Discovered in 1977, pseudogenes used to be considered as relics of genomes, and until recently, they received little attention from research molecular biologists [8]. Microarray technology has allowed detection of pseudogene transcription at the whole genome level in human malignancy and has shown that several pseudogenes are transcribed. Also, increasing numbers of studies have confirmed that several pseudogenes are involved in a variety of biological processes, and their deregulation may participate in human diseases, including human cancer [9–11].
The POU domain class 5 transcription factor 1B (POU5F1B), is a pseudogene that is homologous to OCT4, and is located adjacent to the MYC gene on human chromosome 8q24 [12–14]. POU5F1B has been shown to be an oncogenic in several types of cancer, but its role in cervical cancer remains unclear [14,15]. An increasing body of evidences has shown that the aberrant expression of POU5F1B plays an important role in carcinogenesis [14]. However, to the best of our knowledge, its function and molecular mechanisms in cervical cancer remains unknown.
Therefore, the aims of this study were to investigate the expression and function of POU5F1B in tissue samples of human cervical cancer and in cervical cancer cell lines in vitro.
Material and Methods
Patients, ethical approval, and tissue samples
This study was approved by the Ethical Review Board of Taixing Peoples’ Hospital and the study protocol complied with the Declaration of the Helsinki. All patients who participated in the study by providing tissue samples provided written informed consent prior to surgery. The animal study was approved by the Ethics Animal Care and Use Committee of Taixing Peoples’ Hospital.
In this study, paired human cervical cancer tissues and corresponding normal adjacent tissues from 50 patients were collected from the Department of Obstetrics and Gynecology, Taixing Peoples’ Hospital, between January 2015 and January 2018. Following surgery, the cervical tissue specimens were placed immediately in liquid nitrogen and stored at −80°C for further study. Histopathologic diagnoses of cervical cancer were evaluated and confirmed by at least two experienced pathologists. No patients had received chemotherapy or radiotherapy prior to surgery.
Cell lines and culture conditions
Four human cervical cancer cell lines, SiHa, HeLa, CaSki, and C33A were obtained from the Chinese Academy of Science cell bank in Shanghai, China. A normal cervical epithelial cell line, End1/E6E7 was obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). RPMI-1640 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 ug/ml streptomycin (Beyotime, Haimen, China) was used for cell culture. Cell culture plates were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Total RNA isolation and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA from clinical specimens and cells was extracted using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The A260/A280 ratio used to assess the purity of the RNA (>1.8 indicated high purity), and the RNA concentration was determined using an Eppendorf BioPhotometer spectrophotometer (Eppendorf, Hamburg, Germany). Reverse transcription was performed using a PrimeScript RT Reagent Kit (Takara Bio, Kusatsu, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in triplicate using a SYBR Premix Ex Taq II kit (Takara Bio, Kusatsu, Japan) with a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
Primer sequences used included:
POU5F1B (forward): GCGATCAAGCAGCGACTA;
POU5F1B (reverse): AGGGAAAGGGACTGAGGAG;
OCT4 (forward): TGAAGCTGGAGAAGGAGAAGCTG;
OCT4 (reverse): TCTTTCTGCAGAGCTTTGATGTCCT.
GAPDH was used as normalization gene. To normalize the RNA expression level, GAPDH (sense: CTC GCT TCG GCA GCA CAT ATA CT, antisense: ACG CTT CAC GAA TTT GCG TGT C) was used.
The PCR reaction conditions were as follows: initial denaturation at 95°C for 5 min followed by 40 cycles of at 95°C for 5 s, annealing at 58°C for 30 s, extension at 72°C for 30 s. Relative expression of the target gene was calculated using the 2−ΔΔCT method. ΔΔCT=Ct target gene–Ct reference gene (experimental group), Ct target gene–Ct reference gene (control group). CT was the number of cycles at which the fluorescence exceeded the threshold. Each experiment was performed in triplicate.
Knockdown vector construction
To knock down POU5F1B expression, the short hairpin RNA (shRNA) for knockdown of POU5F1B, small interfering (si-POU5F1B) and its negative control (si-NC) were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The shRNA for POU5F1B was cloned into the pGPU6/RFP vector. The vector sequence was as follows: 5′GAAGAGTTCCTAACACATTCA3′. Scramble vector was used as the negative control. The vector was transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) following the manufacturer’s protocol. The stable cell line was screened using puromycin (Sigma-Aldrich, St Louis, MO, USA).
Cell proliferation Cell Counting Kit-8 (CCK-8) assay
Cell proliferation assay was performed using a Cell Counting Kit-8 (CCK-8) assay (Beyotime, Haimen, China) in accordance with the manufacturer’s protocol. Cells that were transfected with the si-POU5F1B plasmid or empty vector were cultured at 37°C. After 12, 24, 36, 48, and 72 h of cell culture, 10 μl of CCK-8 solution was added to each well and the plates were incubated at 37°C for 30 minutes. Measurement of absorbance at 450 nm was performed using a Multiskan Microplate Photometer (Thermo Fisher Scientific, Waltham, MA, USA) with a reference wavelength of 650 nm. All experiments were performed in triplicate.
Colony formation assay and the EDU proliferation assay
For the colony formation assay, cells transfected with shRNA (200 cells per well) were seeded in triplicate in DMEM with 10% FBS in six-well plates and cultured at 37°C in a humidified incubator with 5% CO2 for 2 weeks. Cells were fixed in methanol and stained by incubation with 0.1% crystal violet. The visible colonies were photographed using an inverted microscope.
A colorimetric immunoassay was performed using a Cell-Light EdU Apollo 567 in vitro Imaging Kit (Ribobio Co Ltd., Guangzhou, China) was used to measure cell proliferation (EDU proliferation assay), according to the manufacturer’s instructions. Cells were cultured in 24-well plates at a density of 1×105 cells per plate for 24 h. Cells were counted and viewed using an inverted microscope.
Cell migration assay
Cells were inoculated onto 6-well plates and cultured until cells reached 100% confluence. A wound was formed with a pipette tip and then washed to remove the medium. Cells were then cultured in DMEM with serum-free medium at 37°C in a humidified atmosphere of with 5% CO2 for 48 h. Images were taken using the microscope and the distance between wound boundaries was measure within 48 h.
Transwell cell migration assay
A transwell cell migration assay was performed to examine cell invasion using a 24-well transwell chamber with a layer of Matrigel (Becton Dickinson, San Jose, CA, USA). The cells were starved in serum-free RPMI-1640 for 24 h. Then, 5×105 cells in 200 ul serum-free medium were added to the upper chamber and DMEM containing 10% fetal bovine serum was added to the lower chamber. After 24 h incubation, the chambers were removed and non-migrated cells were removed using a cotton-tipped swab. Then, 95% ethanol was used to fixed migrated cells on the bottom surface of the membrane and stained with gentian violet for 10 min at room temperature. Images were taken of each group with an inverted microscope.
Cell apoptosis assay
Cells were transfected with si-POU5F1B and si-NC. The cells were seeded in six-well plates. Cells were washed twice with cold PBS and then resuspended in Annexin V 1X Binding Buffer at a concentration of 1×106 cells/ml and 100 μl of the solution was transferred to a culture tube. Then 5 μl of annexin V conjugated to fluorescein isothiocyanate (FITC) and 5 μl propidium iodide (PI) were added to each culture tube. The cells were gently vortexed and incubated for 15 min at room temperature (25°C) in the dark. Finally, 400 μl of Annexin V 1X Binding Buffer was added to each tube followed by analysis by flow cytometry within one hour.
Western blot
The cells were lysed with RIPA lysis buffer (Beyotime, Haimen, China) supplemented with protease inhibitors (Roche, Basel, Switzerland), according to the manufacturer’s protocol. Protein concentration was determined using the BCA protein assay kit, following the manufacturer’s instructions. For each well, protein lysate (50 mg) was separated on 6–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride (PVDF) membranes (Beyotime, Haimen, China). The membranes were blocked with 5% dried skimmed milk powder for an hour and incubated in primary antibodies to OCT4 (Boster, Wuhan, China) at 4°C overnight and GAPDH (Beyotime, Haimen, China) at 4°C overnight. Subsequently, the membranes were removed and washed with TBST three times for 5 min, followed by incubation with secondary antibody conjugated to horseradish peroxidase (HRP) (Beyotime, Haimen, China) at 1: 11000 dilution at room temperature for 2 h. GAPDH was used as an internal control. Protein bands were was visualized using an enhanced chemiluminescence (ECL) kit (Millipore, Burlington, MA, USA) with a FluorChem™ FC3 system molecular imager (ProteinSimple, San Jose, California, USA).
Xenograft assays in nude mice
Twelve female nude mice (BALB/c-nu), 4–5 weeks old, were obtained from Deep Biological Tech (Nanjing, China). To confirm the function of POU5F1B in vivo, the mice were randomly divided into two groups, a si-POU5F1B group (n=6) and a control group (n=6). Transfected cells were collected and cultured in the logarithmic growth phase (1×107 ml/mouse). Cells were suspended in 0.2 ml PBS and injected subcutaneously into the right flank of female BALB/c nude mice. The xenografts were measured with calipers twice weekly, and relative data were recorded, using the formula:
where L represented the longest diameter and W represented the shortest diameter of the tumor. The mice were sacrificed 28 days post-injection and the xenograft tumors were removed for weighing, histology, and immunohistochemistry staining.
Statistical analysis
The data were presented as the mean ± standard deviation (SD) and analyzed using SPSS version 20.0 software (IBM Corp, Armonk, NY, USA). The differences between independent groups were analyzed using a Student’s t-test and one-way analysis of variance (ANOVA). P-values <0.05 were considered to indicate a statistically significant difference.
Results
The expression of POU5F1B was upregulated in cervical cancer tissue and cell lines
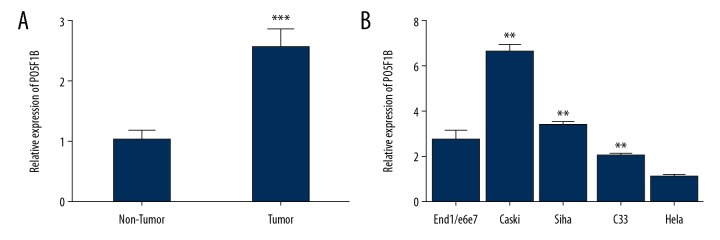
To determine whether POU5F1B exhibited aberrant expression in cervical cancer tissue and cell lines, POU5F1B expression was evaluated in 50 paired cervical cancer and normal cervical tissues. Expression of POU5F1B was significantly increased in tumor samples compared with adjacent non-tumor tissues (P<0.0001) (Figure 1A). The relative expression levels of POU5F1B were measured in the cervical cancer cell lines by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Expression levels of POU5F1B in the human cervical cancer cell lines, SiHa, HeLa, CaSki, and C33A were significantly increased when compared with the normal cervical epithelial cells, End1/E6E7 (P<0.01) (Figure 1B). CaSki cells were selected for the following experimental study, as they showed the highest expression levels of POU5F1B.
Figure 1.
POU5F1B was upregulated in cervical cancer cell lines and tissues, (A) In all tissue pairs, POU5F1B was significantly upregulated in cervical cancer tissues compared to normal cervical tissues (P <0.0001, tumor vs. non-tumor). Data are presented as ΔΔCT. (B) The expression level of POU5F1B is higher in cervical cancer cell lines, SiHa, CaSki, and C33A compared with the normal cervical epithelial cells. Each cell line was analyzed in triplicate. ** P<0.005 vs. si-NC.
Suppression of POU5F1B inhibited cervical cancer cell proliferation in vitro
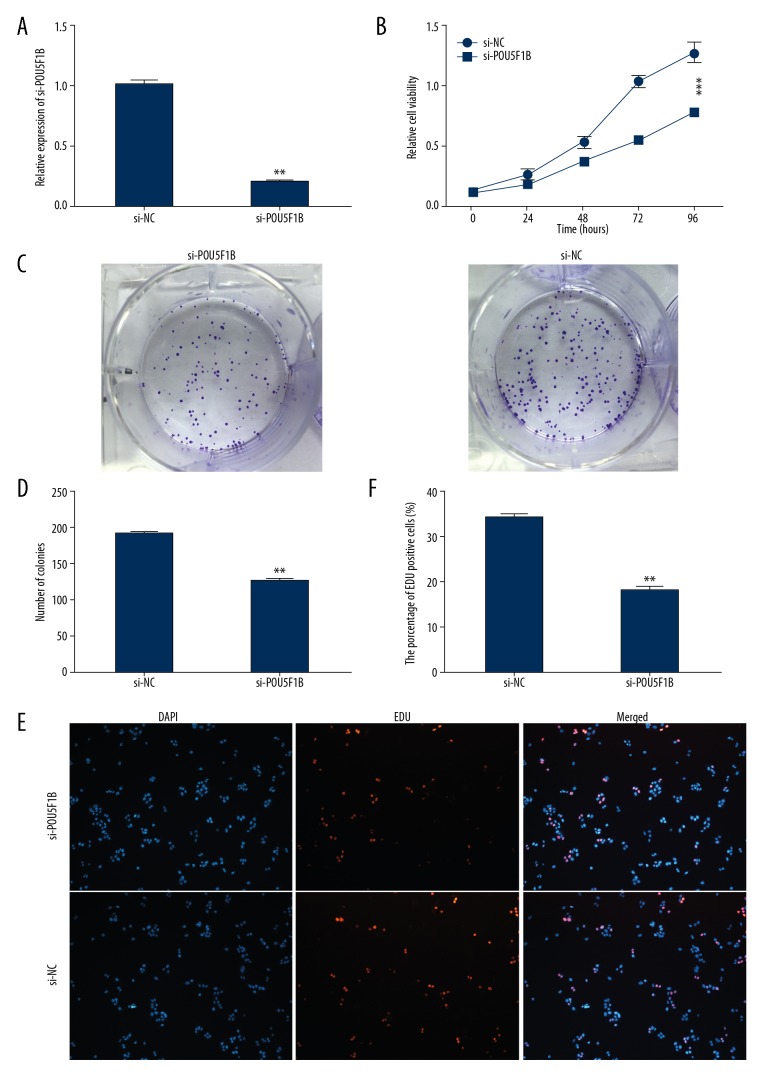
To determine the role of POU5F1B in the progression of cervical cancer, CaSki cervical cancer cells were transfected with si-NC or si-POU5F1B which resulted in the inhibition of the expression of POU5F1B (Figure 2A). To determine whether POU5F1B modulated proliferation and colony formation of cervical cancer, the cells underwent a Cell Counting Kit-8 (CCK-8) assay for assessment of cell viability. As shown in Figure 2B, the inhibition of POU5F1B in cervical CaSKi cancer cells significantly inhibited their proliferation in a time-dependent manner (P<0.01). The colony formation assay showed that the colonies were smaller and fewer after knockdown of POU5F1B and the number of colonies was reduced (Figure 2C, 2D). Also, the EDU proliferation assays show that the percentages of proliferating cells significantly decreased after knockdown of POU5F1B when compared with the control group (Figure 2E, 2F).
Figure 2.
Knockdown of POU5F1B inhibited proliferation and viability in cervical cancer cells. (A) POU5F1B expression was examined by quantitative reverse transcription polymerase chain reaction (qRT-PCR) in cervical cancer cells transfected with si-POU5F1B or si-NC. (B) The effects of POU5F1B knockdown on cell proliferation were analyzed by the Cell Counting Kit-8 (CCK-8) assay. Colonies were counted and the images were captured. (C, D) The effects of POU5F1B knockdown on cell proliferation, analyzed by colony formation assays. (E, F) The effects of POU5F1B knockdown on cell proliferation was analyzed by EDU proliferation assays, The he Click-it reaction showed EdU staining (red). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). The experiments were performed in triplicate ±SD. The values are considered significant at * P<0.01, ** P<0.01.
Suppression of POU5F1B inhibited cervical cancer cell migration in vitro
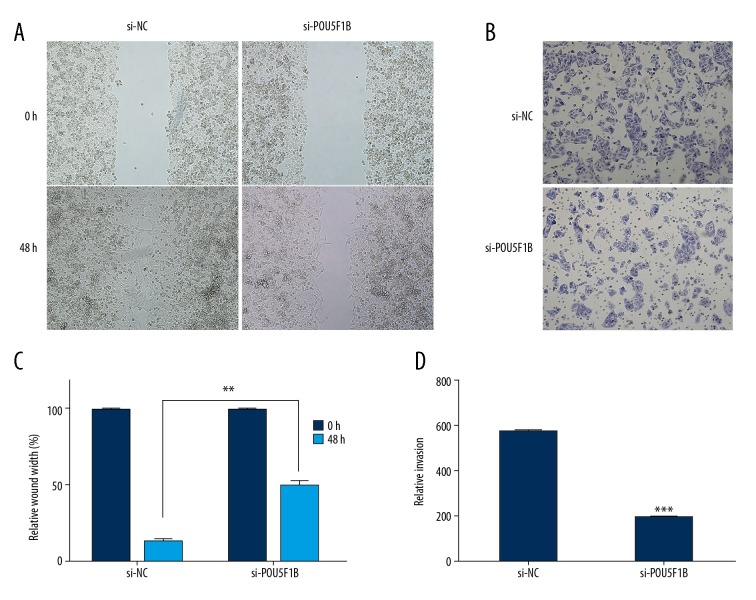
The effect of the inhibition of POU5F1B on the invasion and migration of cervical cancer cells were assessed by transwell assays and wound-healing assays. As shown in Figure 3A, and 3B, the results of the wound-healing assays showed that suppression of POU5F1B in CaSki cervical cancer cell lines caused significant (P<0.01) reduction in cell migration properties. Similar effects were observed for cell invasion as inhibition of POU5F1B expression was associated with a reduction in the cell invasion properties (Figure 3C, 3D). These results indicated that POU5F1B may have a role in the proliferation, migration, and invasion of the cervical cancer cells.
Figure 3.
Knockdown of POU5F1B decreased the migratory and invasive capabilities of cervical cancer cells. (A, B) Cell migration in Si-NC and Si-POU5F1B transfected CaSki breast cancer cells as determined by the wound healing assay. (C, D) Cell invasion of Si-NC and Si-POU5F1B transfected CaSki breast cancer cells as determined by transwell assays. The experiments were performed in triplicate ±SD. The values are considered significant at * P<0.05, ** P<0.01, *** P<0.001.
Suppression of POU5F1B promoted G1 arrest and resulted in apoptosis of cervical cancer cells in vitro
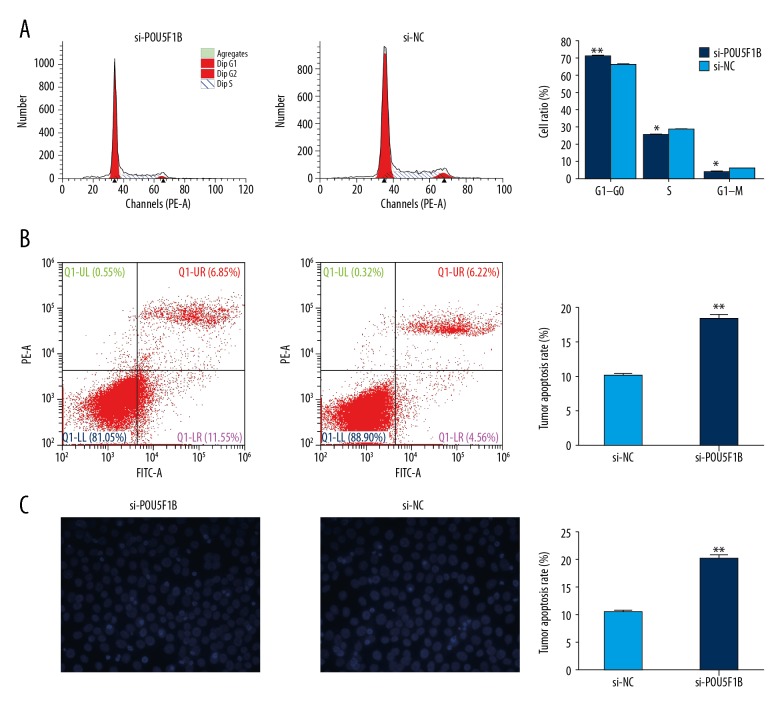
To investigate the function of POU5F1B on cell apoptosis, flow cytometry was performed using propidium iodide (PI) staining was used to access whether POU5F1B modulated cell cycle distribution. As shown in Figure 4A and 4B, suppression of POU5F1B caused a significant number of cells to undergo G0/G1-phase arrest, and the number of S-phase cells was significantly decreased (P <0.05). These results suggested that POU5F1B might be involved in cell-cycle regulation. Also, cell apoptosis analysis by flow cytometry showed that the number of apoptotic cells was significantly increased after knockdown of POU5F1B when compared with the control group. Also, a Hoechst staining assay showed that the number of cells with condensed and fragmented nuclei consistent with apoptosis was increased after knockdown of POU5F1B when compared with the control group (Figure 4C). These results indicated that POU5F1B might affect cervical cancer cell proliferation by regulating the cell cycle and apoptosis.
Figure 4.
Suppression of POU5F1B promoted G1 cell cycle arrest and increased apoptosis of cervical cancer cells in vitro. (A) Flow cytometry was used to detect the cell cycle regulation. The bar chart represents the percentage of cancer cells in G0/G1, S, or G2/M phase of the cell cycle. (B) The apoptosis rate of cells determined by flow cytometry analysis. (C) The apoptosis of cells was detected by Hoechst 33258 staining. * P<0.05, ** P<0.01, *** P<0.001. Results represent the mean ±SD of triplicate wells in three independent experiments.
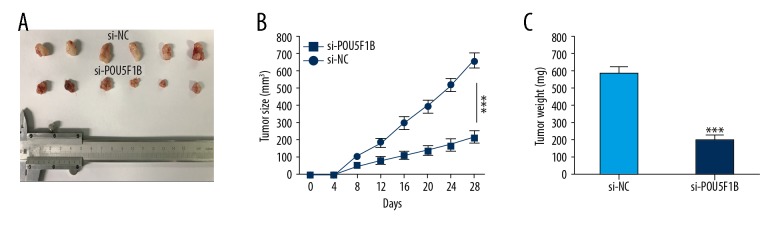
POU5F1B knockdown suppressed tumorigenicity in vivo
To explore whether POU5F1B expression affected tumor formation and growth in vivo, a xenograft model in nude mice was used to determine whether POU5F1B might affect tumorigenicity or tumor growth in vivo. As shown in Figure 5, there was significant inhibition of tumor growth in the POU5F1B-depleted group compared with the control group (Figure 5A). There was significantly less tumor mass in the POU5F1B-depleted group relative to the control group (Figure 5B, 5C). These results indicated that suppression of POU5F1B limited tumor progression in vivo.
Figure 5.
Knockdown of POU5F1B suppressed tumorigenicity in vivo. (A) Representative images show the mouse tumors in the si-POU5F1B group and the NC group. (B, C) Tumor sizes and weights were measured in the tumorigenesis assay. Data are presented as the mean ±SD. * P<0.05, ** P<0.01, *** P<0.001.
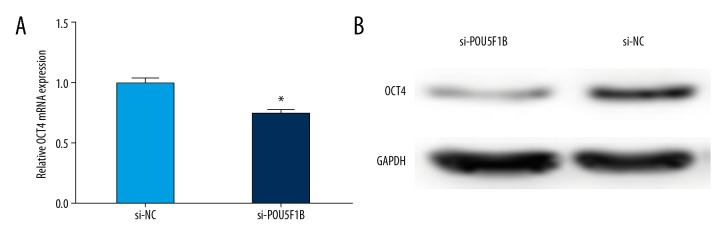
POU5F1B controlled the expression of OCT4
POU5F1B is an OCT4 pseudogene and has high homology with OCT4. The biological role of POU5F1B was studied in cervical cancer cells by applying the loss-of-function approach. POU5F1B might serve as a miRNA decoy and to exerts an oncogenic role by protecting OCT4 from miRNA-mediated downregulation. As shown in Figure 6A, the expression of OCT4 was significantly decreased in the POU5F1B-depleted group compared with the control group. The results of Western blot showed that the level of OCT4 protein in the POU5F1B-depleted group was lower than that of the control group (Figure 6B). These data indicated that POU5F1B might exert an oncogenic role by activation of OCT4 expression both at the mRNA and protein levels in cervical cancer.
Figure 6.
POU5F1B controls the expression of OCT4. (A) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to determine the mRNA levels of OCT4 after transfected with si-POU5F1B or si-NC. The data are represented as the mean ±SD. * P<0.05. (B) Western blot analysis was performed to determine the OCT4 protein expression after transfection with si-POU5F1B or si-NC.
Discussion
Pseudogenes are a subtype of long noncoding RNAs (lncRNAs) that arose from protein-coding genes that have lost the ability to translate into functional proteins [16]. Recently, mounting evidence suggests that in normal or cancer cells [17], pseudogene can alter carcinogenesis by regulating a variety of biological processes, including cell proliferation, differentiation, and apoptosis in several cancer types [18–20]. In general, pseudogenes are capable of regulating the expression of tumor suppressor genes and oncogenes by serving as microRNA decoys. For example, Wang et al. [21] showed that OCT4-pg4, an OCT4 pseudogene that expressed noncoding RNA, could act as suppressor genes in hepatocellular carcinoma (HCC), and indirectly affect the activity of OCT4 by competing for binding to miR-145.
The role of the OCT4 pseudogene, POU5F1B, has been extensively investigated in several critical biological processes in previously published studies, including in colorectal cancer, urinary bladder cancer, prostate cancer, chronic lymphocytic leukemia (CLL), gastric cancer, and hepatocellular carcinoma (HCC) [12–15,22–24]. Previous studies have shown that POU5F1B acts as a tumor oncogenic pseudogene in cancer progression [14,15]. A recently published study showed that the OCT4 pseudogene, POU5F1B, was amplified and promoted a more aggressive phenotype in gastric cancer (GC) [14]. Pan et al. [15] showed that POU5F1B was significantly upregulated in human HCC cells and tissues and promoted tumor cell proliferation by activating AKT [15]. Therefore, it might be assumed that POU5F1B competes with miRNAs as a form of miRNA ‘sponge’ in the regulation of OCT4A expression. Although POU5F1B has been reported to play an important role in the carcinogenesis of various cancers, the role of POU5F1B in cervical cancer remains unclear and the molecular mechanisms require further investigation.
In the present study, the levels of POU5F1B were shown to be significantly overexpressed in human cervical cancer tissues or cervical cancer cells when compared with paired adjacent normal breast tissues and normal cervical epithelial cells grown in vitro. Further reduced expression of POU5F1B inhibited cell proliferation, and cell migration and promoted cell apoptosis in vitro and in vivo. These data showed that POU5F1B functions as a novel tumor oncogene in cervical cancer, which may serve as an effective diagnostic biomarker and a potential therapeutic target in the treatment of cervical cancer. Although the upregulated expression and critical biological roles of POU5F1B have been shown in cervical cancer, the underlying mechanism of POU5F1B in regulating gene expression remains unknown. OCT4, as an important tumor oncogene, is capable of modulating several signaling pathways, resulting in the promotion of cell proliferation, cell migration, and invasion, as well as inhibition of apoptosis [25–27]. By Western blot, this study also showed that POU5F1B knockdown inhibited the expression of the OCT4 protein in cervical cancer cell lines. Some OCT4-targeting and POU5F1B-targeting miRNAs, such as miR-335 [28,29] and miR- 299-3p [30] have been reported to directly target OCT4 and regulate its expression. Therefore, these miRNAs might be competitively bound by OCT4, and consequently protect OCT4 from miRNA-mediated downregulation.
Conclusions
OCT4, a POU-domain transcription factor, plays a key role in maintaining cell pluripotency and cell renewal [31]. Targeting OCT4 might be a new strategy for cancer treatment, but this approach might be problematic given its key role in cell regulation and proliferation, and changes in OCT4 expression can give rise to profound biological effects. Therapeutic approaches to reduce OCT4 levels have anti-cancer properties. The challenge remains to identify the pathways of intrinsic and acquired resistance and identify potential candidate cancer-related intermediaries, such as the OCT4 pseudogene, as an effective diagnostic biomarker and a potential therapeutic target. Investigation of OCT4 and POU5F1B dysregulation in different types of human cancer may help clarify the complex regulatory role for the OCT4 pseudogene in tumorigenesis and to determine whether miRNA-based treatments or other approaches, will be effective cancer treatment strategies [14,32]. the present study showed that pseudogene POU5F1B is frequently upregulated in cervical cancer tissues and cell lines. Silencing of POU5F1B modulated OCT4 expression and inhibited cervical cancer cell proliferation, and migration in vitro while promoting apoptosis of cervical cancer cells and inhibiting tumor growth in xenograft mice. POU5F1B may act as an oncogene in cervical cancer and might be considered as an effective diagnostic biomarker and a potential therapeutic target in cervical cancer treatment. Further studies should be conducted to verify the competing endogenous RNA (ceRNA) network of POU5F1B, which may play a crucial role in the pathogenesis of cervical cancer. Further understanding of functions and molecular mechanisms of POU5F1B in the evolution and progression of cervical cancer may lead to new diagnostic and therapeutic approaches.
Footnotes
Source of support: This study was supported by research project funding from Taixing Peoples’ Hospital Fund Research Project (No: try1727) and the Bengbu Medical School Graduate Student Scientific Research Innovation Plan Project (No: Byycx1747)
Conflict of interest
None.
References
- 1.Small W, Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer. 2017;123(13):2404–12. doi: 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- 2.Moody CA, Laimins LA. Human papillomavirus oncoproteins: Pathways to transformation. Nat Rev Cancer. 2010;10(8):550–60. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Roy L, Bobbs A, Sattler R, et al. CD133 promotes adhesion to the ovarian cancer metastatic niche. Cancer Growth Metastasis. 2018;11 doi: 10.1177/1179064418767882. 1179064418767882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: A population-based study. Lancet Oncol. 2010;11(2):165–73. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 7.Proudfoot N. Pseudogenes. Nature. 1980;286(5776):840–41. doi: 10.1038/286840a0. [DOI] [PubMed] [Google Scholar]
- 8.Jacq C, Miller JR, Brownlee GG. A pseudogene structure in 5S DNA of Xenopus laevis. Cell. 1977;12(1):109–20. doi: 10.1016/0092-8674(77)90189-1. [DOI] [PubMed] [Google Scholar]
- 9.Tutar Y, Ozgur A, Tutar E, et al. Regulation of oncogenic genes by microRNAs and pseudogenes in human lung cancer. Biomed Pharmacother. 2016;83:1182–90. doi: 10.1016/j.biopha.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 10.Pink RC, Carter DR. Pseudogenes as regulators of biological function. Essays Biochem. 2013;54:103–12. doi: 10.1042/bse0540103. [DOI] [PubMed] [Google Scholar]
- 11.Groen JN, Capraro D, Morris KV. The emerging role of pseudogene expressed non-coding RNAs in cellular functions. Int J Biochem Cell Biol. 2014;54:350–55. doi: 10.1016/j.biocel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanke BW, Greenwood CM, Rangrej J, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat Genet. 2007;39(8):989–94. doi: 10.1038/ng2089. [DOI] [PubMed] [Google Scholar]
- 13.Speedy HE, Di Bernardo MC, Sava GP, et al. A genome-wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2014;46(1):56–60. doi: 10.1038/ng.2843. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H, Arao T, Togashi Y, et al. The OCT4 pseudogene POU5F1B is amplified and promotes an aggressive phenotype in gastric cancer. Oncogene. 2015;34(2):199–208. doi: 10.1038/onc.2013.547. [DOI] [PubMed] [Google Scholar]
- 15.Pedraszewski P, Wlazlak E, Panek W, et al. Cesarean scar pregnancy – a new challenge for obstetricians. J Ultrason. 2018;18(72):56–62. doi: 10.15557/JoU.2018.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pink RC, Wicks K, Caley DP, et al. Pseudogenes: Pseudo-functional or key regulators in health and disease? RNA. 2011;17(5):792–98. doi: 10.1261/rna.2658311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5) doi: 10.3390/ijms19051310. pii: E1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Martino M, Palma G, Azzariti A, et al. The HMGA1 pseudogene 7 induces miR-483 and miR-675 upregulation by activating Egr1 through a ceRNA mechanism. Genes (Basel) 2017;8(11) doi: 10.3390/genes8110330. pii: E330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong Y, Zhang L, Huang Y, et al. Pseudogene PDIA3P1 promotes cell proliferation, migration and invasion, and suppresses apoptosis in hepatocellular carcinoma by regulating the p53 pathway. Cancer Lett. 2017;407:76–83. doi: 10.1016/j.canlet.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Gong T, Zheng S, Huang S, et al. PTENP1 inhibits the growth of esophageal squamous cell carcinoma by regulating SOCS6 expression and correlates with disease prognosis. Mol Carcinog. 2017;56(12):2610–19. doi: 10.1002/mc.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Guo ZY, Zhang R, et al. Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis. 2013;34(8):1773–81. doi: 10.1093/carcin/bgt139. [DOI] [PubMed] [Google Scholar]
- 22.Rafnar T, Sulem P, Thorleifsson G, et al. Genome-wide association study yields variants at 20p12.2 that associate with urinary bladder cancer. Hum Mol Genet. 2014;23(20):5545–57. doi: 10.1093/hmg/ddu264. [DOI] [PubMed] [Google Scholar]
- 23.Hu Z, Zhu D, Wang W, et al. Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet. 2015;47(2):158–63. doi: 10.1038/ng.3178. [DOI] [PubMed] [Google Scholar]
- 24.Kastler S, Honold L, Luedeke M, et al. POU5F1P1, a putative cancer susceptibility gene, is overexpressed in prostatic carcinoma. Prostate. 2010;70(6):666–74. doi: 10.1002/pros.21100. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24(4):372–76. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 27.Fogarty NME, McCarthy A, Snijders KE, et al. Genome editing reveals a role for OCT4 in human embryogenesis. Nature. 2017;550(7674):67–73. doi: 10.1038/nature24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L, Yang Y, Xu H, et al. MiR-335 functions as a tumor suppressor in pancreatic cancer by targeting OCT4. Tumour Biol. 2014;35(8):8309–18. doi: 10.1007/s13277-014-2092-9. [DOI] [PubMed] [Google Scholar]
- 29.Schoeftner S, Scarola M, Comisso E, et al. An Oct4-pRb axis, controlled by MiR-335, integrates stem cell self-renewal and cell cycle control. Stem Cells. 2013;31(4):717–28. doi: 10.1002/stem.1315. [DOI] [PubMed] [Google Scholar]
- 30.Zhao R, Liu Q, Lou C. MicroRNA-299-3p regulates proliferation, migration and invasion of human ovarian cancer cells by modulating the expression of OCT4. Arch Biochem Biophys. 2018;651:21–27. doi: 10.1016/j.abb.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Roy L, Cowden Dahl KD. Can stemness and chemoresistance be therapeutically targeted via signaling pathways in ovarian cancer? Cancers (Basel) 2018;10(8) doi: 10.3390/cancers10080241. pii: E241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan Y, Zhan L, Chen L, et al. POU5F1B promotes hepatocellular carcinoma proliferation by activating AKT. Biomed Pharmacother. 2018;100:374–80. doi: 10.1016/j.biopha.2018.02.023. [DOI] [PubMed] [Google Scholar]