Abstract
The goal of this review is to advance the understanding of the muscular and soft tissue palatal anatomy as it relates to palatal surgery for sleep apnea and the phenotypic variations that generate the shape and collapsibility of the retropalatal airway. Anatomically, the soft palate has both a proximal and distal segments separated by the palatal genu. The proximal palatal segment has a variable angle from the hard palate (ie, alpha angle) determined by the position and length of the levator veli palatini muscle. The palatopharyngeus muscle (PP) is a major defining element of the palate and lateral pharyngeal wall and forms the medial wall of the lateral palatal space. It is composed of two divisions: the longitudinal palatopharyngeus fasciculi which acts to elevate the pharynx and depress the soft palate and the transverse palatopharyngeus fascicle (Passavant's ridge) which function is a nasopharyngeal sphincter. The lateral palatal space incorporates the supra‐tonsilar fat, and is bounded by muscles that determine the structure of the palate and associated lateral pharyngeal walls. Understanding of palatal muscles and pharyngeal airway phenotypes provides insight into the steps and mechanisms of pharyngoplasty procedures.
Level of Evidence
N/A
Keywords: Palatopharyngeus, lateral palatal space, palate, anatomy, phenotypes, pharyngoplasty, obstructive sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) causes significant social and medical morbidity. Treatment success using nonsurgical methods such as positive airway pressure therapy (PAP) is limited due to patient noncompliance. However, many alternative surgical therapies in adults are sub‐optimal. To improve surgical success in adults, palatopharyngoplasty techniques have evolved from primarily excisional methods to those that reconstruct and reposition the palate. One such technique, expansion sphincter‐pharyngoplasty (ESP), was introduced by Pang and Woodson in 2007 to improve the airflow and decrease obstruction at the palatal and oropharyngeal level by realignment of the palatopharyngeus muscle and conceptually reduce lateral wall collapse.1 The goal of this review is to advance the understanding of palatal anatomy as it relates to palatal surgery for sleep apnea and the phenotypic variations that generate the shape and collapsibility of the retropalatal airway to better allow application of various pharyngoplasty procedures and evolving pharyngoplasty techniques. The importance of a novel description of known anatomic structures, the lateral palatal space, details of palatal muscles, and pharyngeal airway phenotypes provide insight into improving the application of palatal reconstructive techniques.
Reconstructive Pharyngoplasty
Expansion sphincter‐pharyngoplasty, lateral pharyngoplasty, relocation pharyngoplasty, Han UPPP, suspension palatoplasty, and others are surgical techniques that have the goals to alter the lateral pharyngeal wall and soft palate at the level of the velopharynx and oropharynx.1, 2, 3, 4, 5 A key element of many techniques is altering characteristics of the palatopharyngeus muscle and superior pharyngeal constrictor muscles (SC). The ESP which repositions the palatopharyngeus muscle laterally, anteriorly, and superiorly is an example of this class of technique, has demonstrated benefits in selected OSA patients (Fig. 1).1, 6, 7 It is suggested that the effect of ESP procedure may be mediated by three mechanisms: A) static opening of the lateral pharyngeal retropalatal recess, B) advancing the distal soft palate anteriorly; and C) reducing lateral wall compliance by mobilizing and repositioning the palatopharyngeus.1, 7
Figure 1.
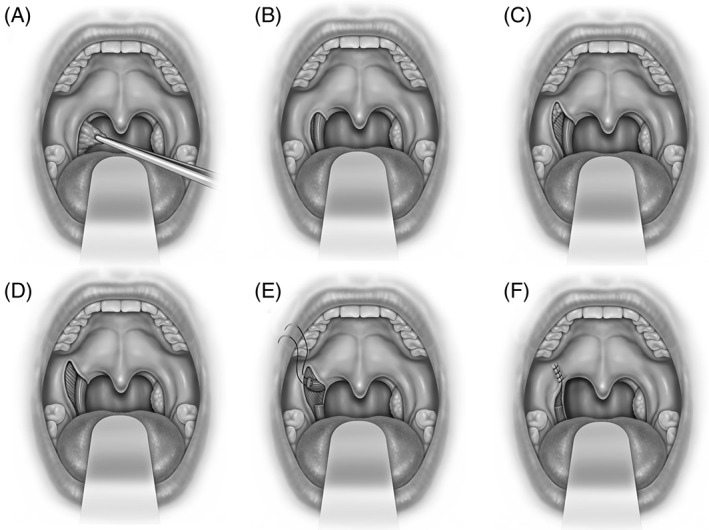
Expansion sphincterplasty is shown (A) tonsillectomy, (B) exposure of palatopharyngeus and superior constrictor muscles within the tonsillar fossa, (C) lateral palatal incision over lateral palatal space with exposure of supratonsillar fat, (D) removal of fat and fibers of palatoglossus provides exposure to superior constrictor and arching fibers of palatopharyngeus muscles, (E) palatopharyngeus muscle incised 1.5 cm. inferior to the fulcrum of rotation and the pedicle is sutured to pterygomandibular raphe or fibrous tissue lateral to the hamulus, and (F) mucosal closure.
There are other soft palate surgery techniques for OSA that also modify the lateral pharyngeal wall. The Australian modified UPPP creates a ventral palatal incision, removal of “supratonsillar fat,” followed by creating and rotating a posterior pillar mucosal flap into the newly created supra‐lateral velopharyngeal port.3 In 2015, a “barbed reposition pharyngoplasty” was introduced to combine tonsillectomy, a conservative ventral palatal mucosal excision, removal of associated submucosal tissues, and posterior pillar mucosal repositioning using a barbed bidirectional suture to the pterygomandibular raphe.8 Suspension palatoplasty proposes to enlarge the retropalatal space by pulling the palatopharyngeus to the pterygomandibular raphe.5
Surgical Anatomy and Phenotypes of the Palate
It is observed that the structure and obstructive pattern of the pharynx differs among individuals. However, finding a method to describe the complex shape, size, curvature, length, and collapsibility is difficult. Finite element analysis demonstrates that muscle position, angulation, and length determine the size, shape, and closure patterns of the retropalatal airway.9 Understanding and describing the muscular structures provide a method to comprehend more complex anatomy of the palate and palatal airway. The major muscles that create the form and shape of the palate include the tensor veli palatini and its aponeurosis (TVP), levator veli palatini (LVP), palatoglossus (PG), palatopharyngeus (PP), uvula (U), superior pharyngeal constrictor (SC), and salpingopharyngeus (SP). The soft palate and its related airway are complex but ultimately much of its form can be constructed using a model of muscle slings and buttresses (Fig. 2). Describing and perceiving the airway in relationship to these muscles provides a distinct understanding in contrast to Mueller's Maneuver and current techniques of drug induced sedated endoscopy (DISE) which give general levels and patterns of obstruction but do not describe more complex elements that are required to learn the airflow limitation and its implications or choice of surgical approach.
Figure 2.
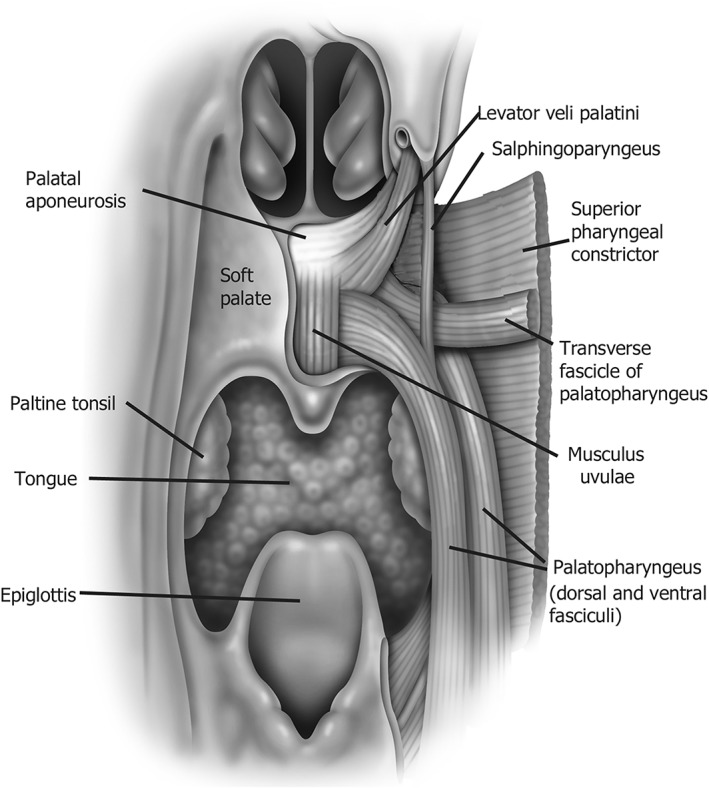
Depiction of the major muscular slings that make up the palate and associated airway.
Three palatal airway phenotypes based on cephalometric x‐rays, and three upper pharyngeal phenotypic patterns based on endoscopy (oblique, intermediate, vertical) have been described (Fig. 3).10, 11 These provide information on the shape and configuration of the airway and not just information on how the airway collapses at a single chokepoint. The endoscopic method is based on three midsagittal soft palate anatomic landmarks and their relationship to the posterior pharyngeal wall. The superior, middle, and inferior landmarks are the junction of the soft and hard palate, the palatal anatomic genu, and the free margin of the soft palate at the velopharynx. These landmarks are both a visual means to identify fixed and repeatable points along the airway but are also associated with the muscle slings that determine the shape and position of the palate, and with the aponeurotic, muscular, and velar anatomic divisions of the soft palate.11
Figure 3.
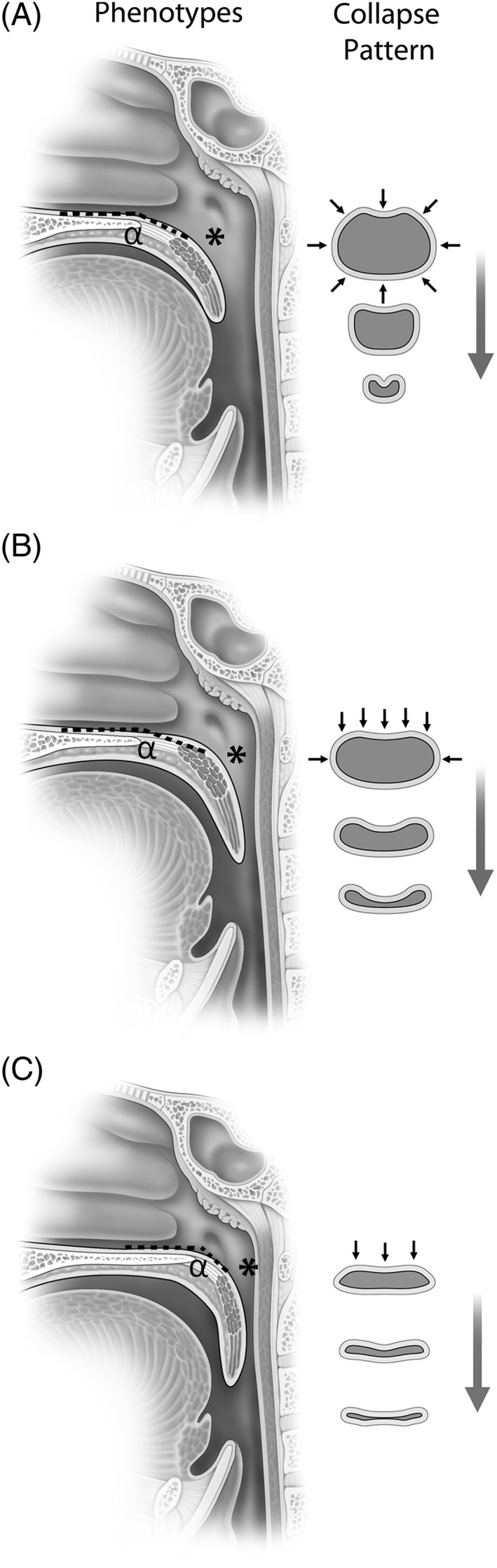
Palatal phenotypes and pattern of collapse are shown. (A) Oblique pattern demonstrates a less acute alpha (α) angle with airway size larger in an anterior posterior dimension at the hard palate, genu (*), and velum; shape is more circular and collapse pattern is circular. (B) Intermediate pattern demonstrates a less acute alpha angle (α) but narrowing at the genu (*) and velum. (C) Vertical pattern has a more acute alpha (α) angle with narrowing of the airway at the genu (*) and velum; airway shape is coronal and collapse patter is flat (anterior posterior).
Anatomically, the soft palate has both a proximal and distal segments separated by the palatal genu. The anatomic genu approximates the border between the muscular and aponeurotic segments of the palate (Fig. 4). The lengths of both the proximal and distal palate vary among individuals. Within the same individual, the soft palate configuration and the position of the genu are strongly correlated to levator veli palatini (LVP) length.10 The LVP compromises 40% of the soft palate length between the base of uvula and the hard palate. Inferior to this muscle, the uvula defines the midsagittal landmark of the velum.12, 13
Figure 4.
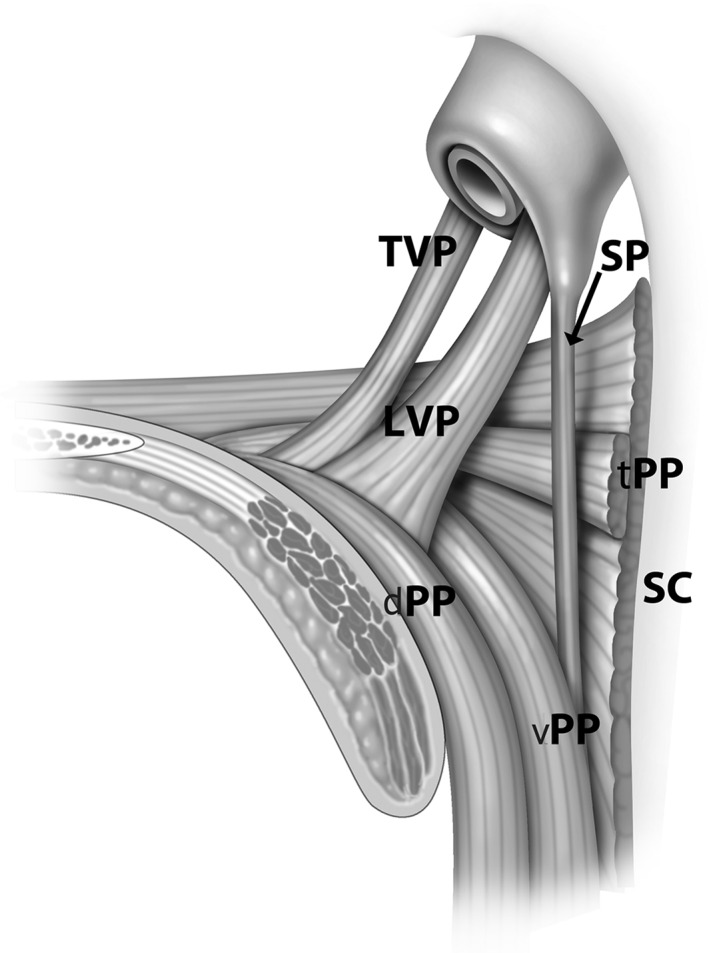
Muscles of the palate are shown tensor veli palatini (TVP), levator veli palatini (LVP), salphingopharyngeus (SP), superior pharyngeal constrictor (SC), and transverse fascicle (tPP), dorsal fascicle (dPP), and ventral fascicle (vPP) of the palatopharyngeus muscle (PP).
The proximal palatal segment has a variable angle from the hard palate (ie, alpha angle).14 Dynamic anatomic studies of speech and swallowing (and likely respiration) demonstrate that this angle is highly correlated to the length and position of the LVP. In individuals with oblique and intermediate palatal phenotypes, the angle is less acutely downward and more parallel to the hard palate. In vertical palatal phenotypes, it is more acutely downward and more parallel to the posterior pharyngeal wall. The distal palatal segment also has variable length and the length of its obstruction (>15 mm), has been associated with poorer palatopharyngoplasty outcomes.15
The structure of the skull base and maxilla demonstrates the correlation with the pharyngeal structure. CT measurements show that the “flat” or narrow anterior posterior velopharyngeal shape is associated with more posteriorly positioned hamuli and posterior maxilla.16 Velopharyngeal anatomic phenotypes are also associated with velopharyngeal closure patterns (Fig. 3). A “deep” pattern with increased anterior posterior distance from the posterior wall to the velum is associated with a circular pattern of closure. In contrast, a “flat” pattern with narrow anterior posterior distance between the posterior wall and velum is associated with an anterior posterior or coronal pattern of closure.16 Endoscopically, the flat pattern is associated with a vertical palatal phenotype while a deeper pattern is associated with an oblique phenotype and more prominent lateral pharyngeal walls.11
The muscular sling, that serve as the margin of the retropalatal lateral pharyngeal wall, is the salpingopharyngeus proximally and the palatopharyngeus distally. The position of the lateral wall margin is affected by multiple elements. Anatomically, obesity, the size of the supratonsillar fat pads, and size of the palatine tonsils are associated with lateral wall position.17 Lymphoid tissues (especially in children) contribute to lateral wall flow limitation in the pharyngeal isthmus above the free margin of the soft palate due to impingement of the superior pole of the palatine tonsil at the location of the narrowing of the nasopharynx.18 Physiologically, lateral wall position is also determined by dynamic strain and tension on the lateral wall muscles. This strain is mediated by tracheal traction, hyoid, and mandibular tension.19
Muscles of the Soft Palate
The LVP courses from the petrous part of the temporal bone and inserts into the midline of the velum to form a muscle sling along an oblique‐coronal plane (Fig. 4). The levator muscle is significantly longer in men than in women with an average difference of 7.56 mm. In contrast, minimal sex differences have been observed in the velar length and velopharyngeal depth measurements. The role of paired LVP is to retract and depending on the angle of insertion both elevates the velum and pulls the velum up to close against the posterior pharyngeal wall.12, 20 The angle of insertion contributes to the angle of the vertical segment of the soft palate. Position of the palatal genu is related to both the posterior pharyngeal wall and its distance from the hard palate. The muscle length of the paired LVP also creates a muscle sling that contributes to the maximal circumference of the retropalatal airway (as does the length of the transverse fascicle of the palatopharyngeus) at the level of the palatal genu.20
The conceptual anatomical antagonist of the LVP is the PG. The muscle courses within the palatoglossal fold after originating on the inferior edge of the palatal aponeurosis.21 The PG is small compared to many other palatal muscles and its respiratory function is unknown. The PG also separates the superior pole of the tonsil from the “supratonsillar fat” (Fig. 5).
Figure 5.
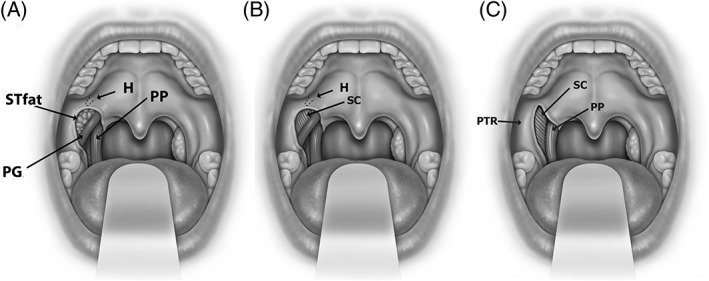
Anatomy of the lateral palatal space is shown. Exposure of palatoglossus muscle and supratonsillar fat (STfat) (in A), removal of supratonsillar fat in pterygoid humulus area (H) (in B) and palatoglossus muscle (PG) (in C) demonstrate the palatal anatomic relationships of the superior pharyngeal constrictor muscle (SC), palatopharyngeus muscle (PP), and pterygomandibular raphe (PTR).
The SC muscle serves as the lateral and posterior boundary of much of the pharynx and relates to the pharyngobasilar fascia. Fasciculi originate from the buccinators, maxilla, pterygomandibular raphe, and tongue to insert into the midline.21 The pharyngeal venous plexus is found at the inferior tonsillar pole and is lateral to the branches of external carotid artery (ECA). ECA runs approximately 1.8 cm from the lateral pharyngeal wall (LPW). The internal carotid artery (ICA) lies 2.1 cm from LPW at the level of C2‐3 interspace. It is essential to establish the distance of the ECA and ICA to the LPW and identify anatomic variations in the relationship of LPW with the carotid system which may occur in up to 5% of patients. In approximately 5% of individuals the ICA is aberrantly close or even posterior to the pharynx (Figure 6).22
Figure 6.
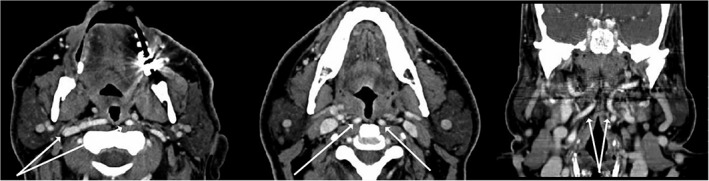
CT scan imaging showing aberrant internal carotid artery (white arrows).
The glossopharyngeal nerve (IXth cranial nerve) can be identified deep to the superior pharyngeal constrictor and traced infero‐medially toward the base of the tongue. The landmark for the main trunk of the glossopharyngeal nerve is found at the intersection of the posterior tonsillar pillar with the base of tongue. The IXth cranial nerve is divided into branches which innervate the lateral pharyngeal wall and the lateral part of tongue base.23
The palatopharyngeus (PP) is a significant longitudinal muscle of the pharynx (Figs. 2 and 4). It is composed of two major divisions: the longitudinal PP and the transverse PP. Dorsal and ventral bellies of PP muscle lie medial to the SC in the oropharynx before inserting on the thyroid cartilage and diffusing into fibers of the inferior constrictor muscle. The PP muscle diffusely originates as three fasciculi from the palatal aponeurosis. Two longitudinal fasciculi (the ventral (smaller) and dorsal (larger)) sandwiching the LVP before combining into a more concise muscle bundle within the palatopharyngeal arch and lateral wall of the pharynx. The function of the PP muscle constricts during swallow both medializing the lateral wall and shortening the pharynx.24 During respiration longitudinal traction of the pharynx stiffens this muscle and reduces the compliance of the palate and pharynx. The transverse fascicle passes dorsally from the soft palate to reach the pharyngeal raphe and this is associated with “Passavant's ridge” (Fig. 7). The transverse fascicle forms a ridge against which the soft palate is elevated in order to separate the nasopharynx from the oro‐pharynx. Therefore, the PP acts not only to elevate the pharynx or depress the soft palate but as a nasopharyngeal sphincter.25 It is speculated that the muscle length of the paired transverse fasciculi contributes to maximize retropalatal airspace size. The PP muscle's function in respiration is not well described. Anatomically, it may function as an airway constrictor or dilator depending on the resting position of the muscle. Further, during respiration, PP muscle contraction, combined with tracheal tug, stiffens the airway wall and displaces the palate inferiorly to open the velopharynx.25
Figure 7.
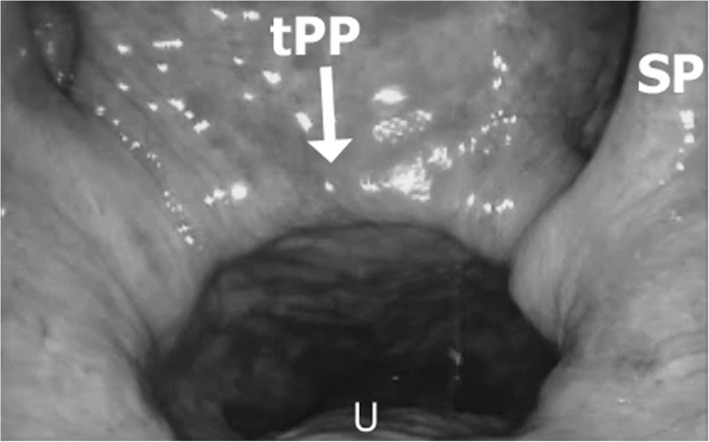
Transverse fascicle of the palatopharyngeus muscle (tPP) is shown on endoscopic view of the nasopharynx. Salphingopharygeus muscle (SP) courses medially and the tPP inserts into the palate in the soft palate lateral to the uvular muscle (U).
The current description contrasts to Finkelstein who attributed Passavant's ridge to the superior pharyngeal constrictor muscle.16 The transverse PP is a unique muscle interspaced between fibers of the longitudinal PP and the SC where the paired muscle fascicles insert in the midline of the posterior naso‐pharyngeal wall. Innervated by the glossopharyngeal nerve, it functions as a sphincter during swallow. Respiratory activity during sleep is unknown, however, during wake expiratory contraction can be observed.
The tensor veli palatini muscle (TVP) is not within the soft palate, however, its aponeurosis is the insertion of the uvular, PP and PG muscles, so it is included in this description. The course of the TVP postero‐lateral to the hamulus is similar to the course of LVP. The primary function of TVP is to open the Eustachian tube during swallowing and yawning. It originates at the base of the medial pterygoid plate of the sphenoid bone and lateral wall of the Eustachian tube. The TVP tendon firmly attaches and folds around the hamulus of the medial pterygoid plate.23 The tensor tendon (aponeurosis) is dorsal to a thick fibro‐adipose layer in the proximal palate. The TVP demonstrates phasic respiratory EMG activity. In the animal model, the stimulation of TVP increases the maximal airflow at the site of flow limitation by decreasing collapsibility of the pharyngeal airway at the soft palate level,26 however its respiratory function in humans to stabilize the palate is uncertain.
Lateral Palatal Space
The lateral palatal space (LPS) is an inadequately described, we believe it is critical and distinct anatomic space of the ventral soft palate (Fig. 5). Initially, the space was labeled as a “supratonsillar” fat and due to its limited tissue volumetric effect was not adequately appreciated, however, we consider this an important anatomic space with defined boundaries.
The LPS can be described as follows: Inferiorly—the tonsil and deep fibers of the PG; medially—the proximal intra‐velar segment of the PP; superiorly—the hamulus; and laterally—the SC which separates it from the pterygomandibular space and the parapharyngeal space. Ventrally, it is bounded by the superficial fibers of the PG and the ventral palatal mucosa. The geometric shape of LPS is pyramidal (wider at its base (close to the tonsil) and more narrow superiorly at the hamulus). As noted, fat contained in the lateral palatal space has been referred to as supra‐tonsillar fat.
Removal of the supra‐tonsillar fat exposes the anatomy of the palatal muscles that define the LPS. Upon removal, the ventral fascicle of the PP is medial to the space. The SC is lateral, the PP is inferior. Medially in the LPS, the PP originates as fan shaped muscle from the palatine aponeurosis. It then courses distally, inferiorly, and laterally. The ventral and dorsal fascicles combine into a more defined band of muscle. This contributes to the structure of the posterior tonsillar pillar and lateral pharyngeal wall. Repositioning and surgical relocation of these fascicles alter the shape, position, and compliance of the palate and lateral pharyngeal walls.
Lateral dissection of the LPS exposes the pterygomandibular raphe which is the anchoring point for several reconstructive palatal surgeries. Approached from a ventral dissection, the dorsal dissection between the PP and SC exposes both dorsal and ventral PP fasciculi and allow for surgical relocation. Cranial dissection in this plane provides access to the lateral wall anatomy if obstruction is at a level closer to the palatal genu. In contrast, caudal dissection in the tonsillar fossa with partial division and rotation is part of the original description of the expansion sphincter pharyngoplasty technique.
DISCUSSION
Reconstructive airway surgery may alter form and function through several mechanisms (volume removal, compliance changes, and realigning tissues). Historically UPPP was primarily a procedure based on tissue excision. Subsequent objective analysis of the sites of failures demonstrated residual obstruction occurred proximal to the oropharynx in the retropalatal region and from lateral wall collapse. To address this, reconstructive surgical approaches that reorient the pharyngeal and palatal structures have been applied. Maxillomandibular advancement which demonstrates high success rates moves the skeletal structure forward to reorient the pharynx to obtain dramatic changes in the retropalatal region.27
ESP relocates the PP muscle laterally, superiorly and anteriorly (conceptually a reverse Orticochea sphincter pharyngoplasty which augments the sphincteric function of the palate).28 This technique incises the body of PP within the tonsillar fossa, creating a muscle tether, and then suturing the muscle in positions to maximally expand the pharynx. This modification differes from other suture suspension of the anterior and posterior pillar of traditional UPPP by mobilizing the muscle from the superior pharyngeal constrictor and transecting the muscle to release tension to reduce dehiscence. The Australian modified UPPP, relocation pharyngoplasty, and lateral pharyngoplasty all relocate the PP.2, 3, 7, 8 Knowledge of the anatomy allows for better assessment of the similarities, differences, advantages, and disadvantages of the procedures.
As techniques evolve, relocation of the PP has not been limited to the tonsillar fossa and can be extended proximally into the lateral palatal space. Surgical removal of the supra‐tonsillar fat is a potential surgical pathway to manipulate the anatomy of the palate.
The PP is a major defining element of the palate and lateral pharyngeal wall. From superior (origin) to inferior (insertion), the longitudinal facsiculi course medial to lateral and ventral to dorsal. In sleep apnea surgery, the concept is to move the velopharyngeal segment of the muscle vector laterally, anteriorly and superiorly to increase muscle tension and airway size. Systematic reviews of pharyngeal expansion techniques that change the PP vector, such as ESP and lateral pharyngoplasty suggest better outcomes in improving sleep disordered breathing compared to traditional uvulopalatopharyngoplasty.6
CONCLUSION
A more detailed description of palatal anatomy benefits the understanding of the reconstructive palatal surgery. The orientation, size, length, and vectors of the palatal muscles are major determinates of the palatal airway. Reconstructive surgery changes the anatomy by altering the palatal and pharyngeal form and function (tension and vectors). Among the multiple muscles that contribute to the palatopharyngeal function, the palatopharyngeus muscle is the key to many current reconstructive pharyngoplasty techniques for OSA. Additionally, defining the anatomical “lateral palatal space” and the muscles that delineate provides an avenue for a better understanding of the techniques used for surgical treatments for OSA and observed improvements in the outcomes of sleep surgery.
Funding: Department of Otolaryngology Medical College Wisconsin.
Conflict of Interest: Dr Olszewska has no conflict of interest or financial disclosure. Dr Woodson has no conflict/ financial disclosure with the topic of this work but in the field of sleep medicine has the following: Inspire Medical Research/Consultant, Medtronic Consultant/royalty, Siesta medical Consultant/options, Lingualflex Consultant, Cryosa Consultant, Zelegent Consultant, Medrobotics Consultant.
BIBLIOGRAPHY
- 1. Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg 2007;137(1):110–114. [DOI] [PubMed] [Google Scholar]
- 2. Cahali MB. Lateral pharyngoplasty: a new treatment for OSAHS. Laryngoscope 2003;113:1961–1968. [DOI] [PubMed] [Google Scholar]
- 3. MacKay SG, Carney AS, Woods C, et al. Modified uvulopalatopharyngoplasty and coblation channeling of a tongue for obstructive sleep apnea: a multi‐center Australian trial. J Clin Sleep Med 2013;9(2):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han D, Ye J, Lin Z, Wang J, Wang J, Zhang Y. Revised uvulopalatopharyngoplasty with uvula preservation and its clinical study. ORL J Otorhinolaryngol Relat Spec 2005;67(4):213–219. [DOI] [PubMed] [Google Scholar]
- 5. Li HY, Lee LA, Kezirian EJ, Nakayama M. Suspension palatoplasty for obstructive sleep apnea—a preliminary study. Sci Rep 2018;8(1):4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pang KP, Pang EB, Win MT, Pang KA, Woodson BT. Expansion sphincter pharyngoplasty for the treatment of OSA: a systemic review and meta‐analysis. Eur Arch Otorhinolaryngol 2016;273(9):2329–2333. [DOI] [PubMed] [Google Scholar]
- 7. Vicini C, Montevecchi F, Pang KP, et al. Combined transoral tongue base surgery and palate surgery in obstructive sleep apnea‐hypopnea syndrome: expansion sphincter pharyngoplasty versus uvulopalatopharyngoplasty. Head Neck 2014: 36(1):77–83. [DOI] [PubMed] [Google Scholar]
- 8. Vicini C, Hendawy E, Campanini A, et al. Barbed reposition pharyngoplasty (BRP) for OSAHS: a feasibility, safety, efficacy and teachability pilot study. ‘We are on the giants's shoulders'. Eur Arch Otorhinolaryngol 2015;272(10):3065–3070. [DOI] [PubMed] [Google Scholar]
- 9. Srodon, PD , Miquel ME, Birch MJ. Finite element analysis animated simulation of velopharyngeal closure. Cleft Palate Craniofac J 2012;49:44–50. [DOI] [PubMed] [Google Scholar]
- 10. Finkelstein Y, Wolf L, Nachmani A, et al. Velopharyngeal anatomy in patients with obstructive sleep apnea versus normal subjects. J Oral Maxillofac Surg 2014;72:1350–1372. [DOI] [PubMed] [Google Scholar]
- 11. Woodson BT. A method to describe the pharyngeal airway. Laryngoscope 2015;125(5):1233–1238. [DOI] [PubMed] [Google Scholar]
- 12. Perry JL, Sutton BP, Kuehn DP, Gamage JK. Using MRI for assessing velopharyngeal structures and function. Cleft Palate Craniofac 2014;51(4):476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inouye JM, Perry JL, Lim KY, Blemker SS. A computational model quantifies the effect of anatomical variability on velopharyngeal function. J Speech Lang Hear Res 2015;58(4):1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipira AB, Grames LM, Molter D, Govier D, Kane AA, Woo AS. Videofluoroscopic and nasendoscopic correlates of speech in velopharyngeal dysfunction. Cleft Palate Craniofac J 2011;48:550–559. [DOI] [PubMed] [Google Scholar]
- 15. Zhang P, Ye J, Pan C, Sun N, Kang D. The role of obstruction length and height in predicting outcome of velopharyngeal surgery. Otolaryngol Head Neck Surg 2015;153(1):144–149. [DOI] [PubMed] [Google Scholar]
- 16. Finkelstein Y, Shapiro‐Feinberg M, Talmi YP, Nachmani A, DeRowe A, Ophir D. Axial configuration of the velopharyngeal valve and its valving mechanism. Cleft Palate Craniofac J 1995;32:299–305. [DOI] [PubMed] [Google Scholar]
- 17. Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003;168:522–530. [DOI] [PubMed] [Google Scholar]
- 18. Arens R, McDonough JM, Corbin AM, et al. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2003;167:65–70. [DOI] [PubMed] [Google Scholar]
- 19. Dempsey JA, Veasey SC, MorganN BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abe M, Murakami G, Naguchi M, Kitamura S, Shimada K, Kohama GI. Variations in the tensor veli palatyni muscle with special reference to its origin and insertion. Cleft Palate Craniofac J 2004;41(5):474–484. [DOI] [PubMed] [Google Scholar]
- 21. Sakamoto Y. Configuration of the extrinsic muscles of the tongue and their spatial interrelationships. Surg Radiol Anat 2017;39(5):497–506. [DOI] [PubMed] [Google Scholar]
- 22. Pfeiffer J, Ridder GJ. A clinical classification system for aberrant internal carotid arteries. Laryngoscope 2008;118(11):1931–1936. [DOI] [PubMed] [Google Scholar]
- 23. Lim CM, Mehta V, Chai R, et al. Transoral anatomy of the tonsillar fossa and lateral pharyngeal wall: anatomic dissection with radiographic and clinical correlation. Laryngoscope 2013;123(12):3021–3025. [DOI] [PubMed] [Google Scholar]
- 24. Sumida K., Yamashita K, Kitamura S. Gross anatomic study of the human palatopharyngeus muscle throughout its entire course from origin to insertion. Clin Anat 2012;25(3):314–323. [DOI] [PubMed] [Google Scholar]
- 25. Sumida K, Ando Y, Seki S, et al. Anatomical status of the human palatopharyngeal sphincter and its functional implications. Surg Radiol Anat 2017;39(11):1191–1201. [DOI] [PubMed] [Google Scholar]
- 26. McWhorter AJ, Rowley JA, Eisele DW, Smith PL, Schwartz AR. The effect of tensor veli palatini stimulation on upper airway patency. Arch Otolaryngol Head Neck Surg 1999; 125:937–940. [DOI] [PubMed] [Google Scholar]
- 27. Faria AC, da Silva‐Junior SN, Garcia LV, dos Santos AC, Fernandes MR, de Mello‐Filho FV. Volumetric analysis of the pharynx in patients with obstructive sleep apnea (OSA) treated with maxillomandibular advancement (MMA). Sleep Breath 2013;17(1):395‐401. [DOI] [PubMed] [Google Scholar]
- 28. Saint Raymond C, Bettega G, Deschaux C, et al. Sphincter pharyngoplasty as a treatment of velopharyngeal incompetence in young people: a prospective evaluation of effects on sleep structure and sleep respiratory disturbances. Chest 2004;125(3):864–871. [DOI] [PubMed] [Google Scholar]


