Abstract
Background
Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) has comparable fusion rates and outcomes to the open approach, though many surgeons avoid the technique due to an initial learning curve. No current studies have examined the learning curve of MI-TLIF with respect to fluoroscopy time and exposure. Our objective with this retrospective review was to therefore use a repeatable mathematical model to evaluate the learning curve of MI-TLIF with a focus on fluoroscopy time and exposure.
Methods
We conducted a retrospective review of single level, primary fusions performed by a single surgeon during his initial experience with minimally invasive spine surgery. Chronologic case number was plotted against variables of interest, and learning was identified as the point at which the instantaneous rate of change of a curve fit to the data set equaled the average rate of change of the data set.
Results
One hundred nine cases were reviewed. Proficiency in operative time was achieved at 38 cases with the first 38 requiring a median of 137 minutes compared to 104 minutes for the latter 71 cases (P < .0001). Mastery of fluoroscopy use occurred at case 51. The median fluoroscopy time for the first 51 cases was 2.8 minutes, which dropped to 2.1 minutes for cases 52 to 109 (P < .0001). The complication rate plateaued after 43 cases, with 3 of 11 total complications occurring in the latter 76 cases.
Conclusions
Our results demonstrate the most gradual learning occurred with respect to fluoroscopy time and exposure, and operative time improved the quickest.
Level of Evidence
IV.
Clinical Relevance
These findings may guide spine surgeon education and training in minimally invasive techniques, and help determine safe case loads for radiation exposure during the initial learning phase of the technique. The model used to identify the learning curve can also be applied to several fields and surgical techniques.
Keywords: minimally invasive spine surgery, transforaminal lumbar interbody fusion, fluoroscopy, radiation exposure, learning curve
INTRODUCTION
Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) has gained popularity in recent years for its comparative effectiveness to open TLIF, with patient benefits including less blood loss, reduced postoperative narcotic requirements, faster return of functional status, and earlier return to work.1 Reduced length of hospital stay and shorter operative time also minimize the overall hospital costs associated with MI-TLIF when compared to the open procedure.2,3 Despite these potential benefits, the adoption of MI-TLIF has been impeded by concerns regarding the steep learning curve and significantly increased radiation exposure to both the surgeon and the patient.4–6
Fluoroscopic guidance is necessary during percutaneous pedicle screw instrumentation, appropriate dilator placement, and to verify placement of the interbody cage. In open TLIF, fluoroscopic imaging is typically only necessary to verify the operative level and confirm correct placement of the cage and pedicle screws. Consequently, the amount of fluoroscopy time required to safely perform an MI-TLIF is several fold greater compared to the traditional open technique. This additional radiation exposure presents a risk to the surgeon, the patient, and the entire operating room staff.7–12
Previous studies have demonstrated that operative time, estimated blood loss (EBL), complication rates, and patient outcomes all improve as the surgeon gains experience. However, examination of fluoroscopy time as a function of surgeon experience has not previously been described. Risks associated with occupational radiation exposure are a deterrent to the adoption of minimally invasive techniques, especially MI-TLIF. Additionally, there exists no standardized method for identifying a learning curve, making it difficult to interpret results of multiple different learning curve studies. We therefore sought to use a systematic mathematical model to accurately define the learning curve of MI-TLIF with a focus on fluoroscopy time.
METHODS
Patient and Operative Information
The institutional review board approved this retrospective study of patients who underwent a single-level, primary MI-TLIF with bilateral fixation performed by a single surgeon at an academic center between 2011 and 2015. The surgeon completed a residency in Orthopedic Surgery followed by a fellowship in Spine surgery; however, neither of these programs provided exposure to minimally invasive spine surgery. Therefore, this series catalogues his initial experience adopting minimally invasive techniques. No courses were attended to learn MI-TLIF techniques. Techniques were learned from the operative manuals accompanied with each instrument set. The main cases of the surgeon practice include degenerative spondylosis and spondylolisthesis that are refractory to conservative treatment. It should be noted that the first surgical assist varied between a second year resident, a fourth year resident and a spine fellow. There were 3 different surgical technicians and 3 different radiology technicians. All demographic and operative information was obtained via the electronic medical record. Fluoroscopy time and exposure were obtained from the PACS system. Postoperative complications were identified via inpatient progress notes, clinic follow-up notes, and subsequent operative reports. The indications for each procedure were symptomatic degenerative spondylosis or spondylolisthesis that did not respond to conservative management. Any cases of trauma or neoplasm were excluded from the analysis. All patients had at least 1 year of follow up.
Operative Procedure
The same screw and cage system was used for all cases. The minimally invasive TLIF procedure was performed using tubular retractor and percutaneous pedicle screw instrumentation with fluoroscopic assistance. The pedicles were instrumented percutaneously using uniplanar C-arm. Antero-posterior fluoroscopy shots were used to align a Jamshidi needle with the targeted pedicle, advance it onto the pedicle, and pass a guidewire. Guidewires were placed in the pedicles bilaterally. Following placement of all guidewires, the tubular retractor was docked on the facet joint on the side of primary symptoms and the initial dilator was inserted through either the superior or inferior incision to initiate the decompression procedure. Direct decompression was performed through a unilateral approach in all cases. Using microscopic visualization, the entire facet joint and pars were resected and exiting nerve root and disc space and nerve root were identified; this step may include removal of a portion of the lamina depending upon the patient's imaging and symptomatology. Once the disc space was visualized, a discectomy was performed. The central canal and opposite lateral recess were decompressed after the interbody cage was placed. The tubular retractor was angled and the Jackson table was rotated away from the surgeon in order to access the contralateral side. In cases, especially at L5S1, when the nerve root was draped over the disc space, a neurolysis was performed to allow for the nerve root to be mobilized. This was followed by serial distraction of the disc space using disc space distractors. If there was still limited ability to enter the disc space due to the nerve, the superior posterior edge of the S1 pedicle and vertebral body were drilled off to allow for a safer channel. Optimal interbody cage height was achieved when there was adequate tension during trial insertion and was verified with fluoroscopy. The anterior disc space was then filled with autologous and allograft bone under fluoroscopic guidance to ensure proper location of graft delivery. An extra-small bone morphogenetic protein (BMP) soaked collagen sponge was placed into the cage with autograft and allograft bone packed around the cage in the disc space. Insertion of an articulating TLIF cage was carried out under fluoroscopy to guide depth of insertion and appropriate rotation. Pedicle screws were then placed over the resting guide wires followed by percutaneous placement of a pre-cut rod.
Statistical Analysis
All statistical analyses were done using Prism Graphpad V6 (La Jolla, California). Identification of the learning curve began with testing correlation between each variable and chronologic case number. When a statistically significant correlation was found, a linear slope was fit to the overall data set, giving an average rate of change for all cases. This step provides a quantifiable average improvement in the variable of interest, such as operative time, per case. A nonlinear association curve was then fit to the data set and the derivative of the equation for this nonlinear curve was solved to find the case at which point the slope of the curve equaled the linear slope. This identified case thus equals the point at which the average rate of change on the linear curve equals the instantaneous rate of change on the nonlinear curve. Therefore, the rate of change after this case will always be less than the average rate of change, suggesting a plateau in learning had occurred. For continuous variables fit with a dissociation curve, the Runs test was computed to determine if the data were clustered on either side of the curve, rather than being randomly scattered. If the P-value for the Runs test is statistically significant, it suggests the data may not be well represented by the curve.
To solve the learning curve for a dichotomous outcome such as complication rates, cumulative complication number was plotted against time. All complications, whether related to the surgical technique, surgical indications, or other factors were included in the cumulative complication number because all these factors are positively impacted by surgeon experience, that is, learning curve. The average complication rate was the total number of complications for the entire case series, and therefore the derivative of the association curve fit to this data set was solved for that average complication rate. Proportion comparisons between groups were performed with a Fisher exact test, and numerical comparisons were conducted with a Mann-Whitney U test. Statistical significance was taken at P < .05.
RESULTS
Patient Demographics
One hundred nine consecutive single level, primary MI-TLIFs with bilateral fixation were performed between 2011 and 2015. There were 55 male patients and 54 female patients with an average age of 57 years ± 15. Of the 109 cases, only one was at the L3-L4 level, 72 at L4-L5, and 36 were at L5-S1. The average body mass index (BMI) was 28.16. There were 10 smokers, 19 patients with diabetes, and 46 with hypertension. There was no statistically significant correlation with chronologic case number and BMI, smoking status, diabetes, or hypertension.
Operative Time
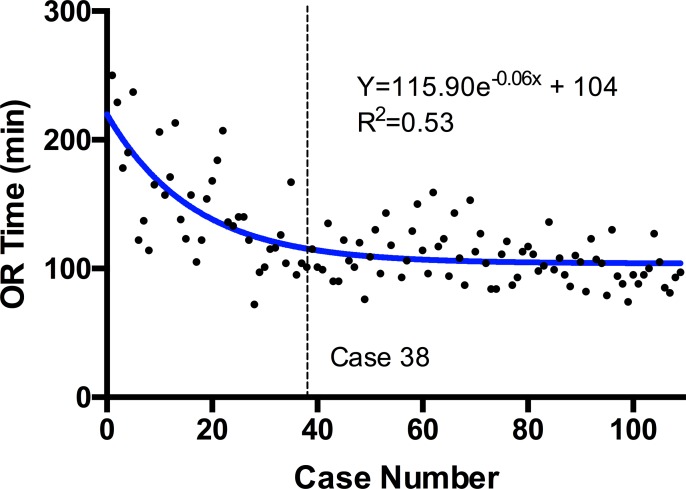
The average operative time for all 109 cases was 112 minutes with an interquartile range of 96 to 136 minutes. There was a statistically significant (P ≤ .0001, r = −0.61) negative correlation between case number and operative time when examining all cases. A linear slope fit to the overall data set was −0.69 (R2 = 0.38). A dissociation curve was fit to the data set (R2 = 0.53; Figure 1), and when solving the derivative of this equation for the case at which the slope of the curve = −0.69, the result was 38. The P-value for the Runs test for this curve was P = .35. When cases 1 to 38 and 39 to 109 were compared, the first cohort of cases had a median odds ratio (OR) time of 137 minutes, and the second cohort of cases had a median OR time of 104 minutes, demonstrating a 24% decrease in median OR time between the first 38 and all subsequent cases (P < .0001).
Figure 1.
Dissociation curve fit to scatterplot of operative (OR) time. Derivative of the equation solved for overall slope of the data set identifies the learning curve at case 38.
Fluoroscopy Time and Exposure
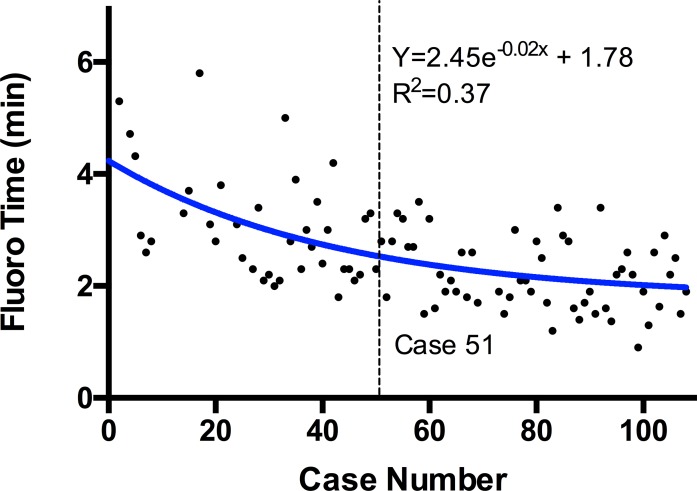
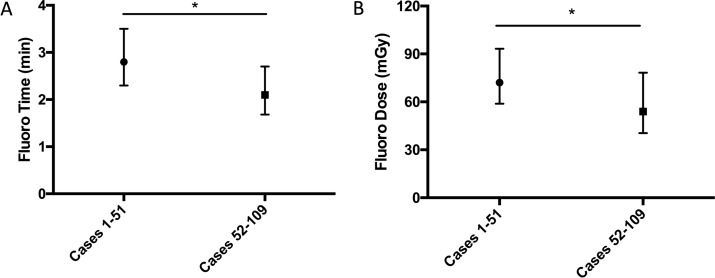
The median fluoroscopy time for all cases was 2.4 minutes with an interquartile range of 1.90 to 3.00 minutes. There was a statistically significant (P ≤ .0001, r = −0.57) negative correlation between fluoroscopy time and chronologic case number. A slope fit to the overall data set was −0.01748 (R2 = 0.33). A dissociation curve was fit to the data set (R2 = 0.37; Figure 2), and solving the derivative of this equation for the average rate of change of the model, −0.01748, resulted in case 51. The P-value for the Runs test for this curve was P = .33. Cases 1 to 51 had a median fluoroscopy time of 2.8 minutes with an interquartile range of 2.30 to 3.50 minutes, while cases 52 to 109 had a median fluoroscopy time of 2.1 minutes with an interquartile range of 1.68 to 2.70, a 25% reduction (Figure 3A; P ≤ .0001).
Figure 2.
Dissociation curve fit to scatterplot of fluoroscopy (fluoro) time. Derivative of the equation solved for overall slope of the data set identifies the learning curve at case 51.
Figure 3.
(A) Comparison of median and interquartile range of fluoroscopy (fluoro) time between cases 1 to 51 and cases 52 to 109. (B) Comparison of median and interquartile range of fluoroscopy dose between cases 1 to 51 and cases 52 to 109.
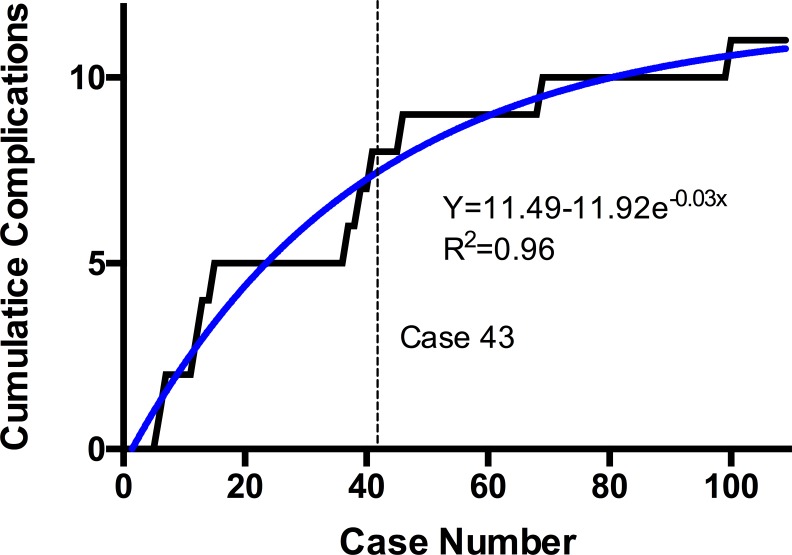
Figure 4.
Cumulative number of complications plotted against chronologic case number with association curve fit. Derivative of the equation solved for overall complication rate identifies the learning curve at case 43.
The median fluoroscopy dose for all 109 cases was 62.70 mGy. There was a difference between the median mGy dose for cases 1 to 51 (72.05) and cases 52 to 109 (53.95) that was statistically significant (Figure 3B; P ≤ .008).
Complication Rates
A total of 11 complications occurred from the 109 cases (10%). A graph of the cumulative number of complications over time displays a sharp plateau starting at case 43, which is where the derivative of the association curve equation equals the overall complication rate of 10%. A total of 8 complications occurred from cases 1 to 43 (18%) compared to only 3 from cases 44 to 109 (5%). Therefore, 73% of total complications occurred in the first 43 cases and this proportion was statistically significant (P = .0236). The 11 complications included 2 epidural hematomas requiring operative evacuation, 2 cases of failed hardware requiring revision, 2 cases of pseudarthrosis requiring revision, 2 cases recurrent stenosis requiring revision, and 3 cases of adjacent segment pathology requiring revision surgery.
EBL and Length of Stay
The mean EBL for the 109 cases was 64 milliliters ± 45. There was no statistically significant correlation between EBL and case number (P = .07). The average length of stay for all 109 cases was 1.8 days ± 1.4. There was not a significant correlation between length of stay and case number (P = .78)
DISCUSSION
In 2003, Foley et al13 described the MI-TLIF using tubular retractors via a muscle splitting approach to minimize soft tissue injury and atrophy of the erector musculature.14 The procedure has since seen a steady rise in popularity as surgeons transition from open to minimally invasive techniques. Studies have previously been conducted to characterize this learning curve; however, they largely focused on operative time and blood loss, neglecting to address the key issue of the increased reliance upon fluoroscopic imaging.4–6
In analyzing the experience of a single surgeon starting to use MI-TLIF, we have found that improvement in operative time plateaus after completing approximately 38 cases. This value is in agreement with Silva et al6 whom determined that 90% of the learning curve for operative time was achieved after 30 cases, as well as Lee et al4 whom also identified the learning curve at 30 cases.4,6 We can conclude that gaining the ability to progress through each step of the procedure in a consistent fashion does not require a large case volume. However, in terms of fluoroscopy time, a much larger learning curve was appreciated, with improvement continuing up until case 51. The same was true for complication rates, which were noted to plateau after 43 cases. Reduction in fluoroscopy time is achieved through refinement of technique, avoiding unnecessary steps, and confidence in the accuracy of each maneuver. Likewise, reduction in complication rate displays the evolution of a surgeons understanding of the procedure in terms of their ability to identify and avoid intraoperative risks and optimize each step of the operation to improve outcomes. These measures are therefore much more indicative of true mastery of the procedure.
Fluoroscopy of the lumbar spine requires high-energy beams to achieve adequate tissue penetration and image quality. These high-energy x-rays result in greater backscatter, which is the dominant source of radiation exposure to the surgeon, as well as the entire operating room staff. North American guidelines state that radiation exposure should not exceed a total body dose of 0.05 Gy (5 rads). When considering specific body parts, the dose should not exceed 15 roentgen equivalent man (REM) for the eyes, 30 REM for the forearms, and 50 REM for the hands.15 In our study, the reported radiation is the dosage emitted by the machine, which does not correlate with absorbed dose for the surgeon or patient. The radiation dose rates to the hands of an orthopedic surgeon have been identified using dosimeter rings on the hands and were found to be the greatest for intramedullary nailing of the femur or tibia where readings up to 8 milliREM (mREM) per minute of fluoroscopy time have been noted. Dose rates are much higher during percutaneous instrumentation of the spine reaching levels up to 58 mREM/min.16
Performance of an MI-TLIF likely carries a similar 58 mREM/min so considering the average fluoroscopy time of 189.6 seconds for our early cohort of patients; the radiation dose to the hands of our surgeon was approximately 0.18 REM per case. At this rate, the recommended hand exposure limit would be reached after 273 cases. For a surgeon proficient in the procedure only 138.8 seconds of fluoroscopy time would be required, meaning an average dose to the hands of 0.13 REM per case. This would allow for the performance of 373 cases until the recommended yearly limit has been reached. It is very unlikely for a surgeon to exceed this number of interbody fusions in a calendar year; however, all other procedures requiring fluoroscopic guidance must also be taken into account. Even in that case, a spine surgeon focused on minimally invasive techniques with a high case volume is unlikely to reach the recommended radiation dosage limit.
In spite of this, thorough knowledge of radiation safety measures is essential and should be employed in every case. Herscovici et al17 outlined 3 key variables that may be modified to limit radiation exposure including mechanical, span, and the use of barriers. The most effective form of mechanical control involves placing the beam source contralateral to the operating surgeon, however, in the setting of spine surgery where bilateral instrumentation is required, this variable sometimes cannot be maximized. For the same reasons, span is also difficult to limit in these cases, as the surgeon must often stand adjacent to the patient and fluoroscopy machine while holding an instrument in place. For percutaneous pedicle screw placement, localization shots to align each vertebral body prior to insertion of Jamshidi needles should be conducted with all staff standing greater than 6 feet from the C-arm. Once inserted, the Jamshidi should be held with a Kocher or another long instrument when taking images. Radiation dosage is inversely proportional to the distance squared; therefore, the additional 5 to 10 cm can reduce the hand dose by 25% to 45%.16 Regular use of barrier devices, including lead gowns and leaded glasses is standard practice.
The use of navigation is growing in popularity over recent years, with several products currently available on the market. These tools can reduce the amount of intraoperative fluoroscopy time; however, radiation dosage to the patient can still be significant as they may require a preoperative or intraoperative computed tomography scan (typically 7.5 mGy of radiation exposure). Kim et al18 described their experience with a navigation assisted fluoroscopy system that required twice as long to set up compared to regular fluoroscopy (9.67 vs 4.78 minutes) but resulted in significantly less fluoroscopy time (28.7 vs 41.9 seconds). Additionally, because the operating room staff was able to stand away from the navigation fluoroscope while it was actively shooting, the actual radiation exposure was undetectable on tracers placed on the surgeon. Alternative fluoroscopy protocols have also been used effectively to reduce radiation dose. Clark et al19 reported on the use pulsed low dose fluoroscopy or digital spot imaging protocols for single level MI-TLIF and saw a reduction in fluoroscopy time down to a mean of 18.72 seconds. However, the average operative time for this series was considerably longer at 177 minutes. This may be partly explained by the necessity for frequent adjustment of image brightness, contrast, and fluoroscopy settings.
Current literature examining the learning curve for MI-TLIF has utilized the arbitrary method of comparing the first half of cases to the last half, by using segmental linear regressions, or by fitting semilogarithmic curves and visually identifying plateaus without any mathematical technique.1,4,5 The disparity of methods for identifying the learning across other surgical specialties also makes it difficult to compare results between studies. Our technique for identifying the learning curve, and where learning is achieved, is a mathematical and systematic method based on the assumption that proficiency is achieved at the case number where the rate of improvement is smaller than the rate of improvement for the entire data set. In simpler terms, we identify the point at which the surgeon has achieved the majority of improvement to be expected and therefore has become proficient in that particular procedure.
The idea of the learning curve becomes an important part of presenting information to patients. While our study alone does not provide sufficient evidence to exactly define where learning has occurred with respect to our chosen variables, it does shed light on which variables, in our case radiation exposure, take longer to master. It is important for the surgeon to keep in mind the concept of the learning curve when discussing surgery with patients.
This study has several limitations largely stemming from its retrospective nature, which limited our ability to control sources of bias. Although all cases were performed by a single surgeon, the assistant surgeon included a rotating roster of either a second year resident, fourth year resident, or spine surgery fellow. Additionally, the operative team would include 1 of 3 surgical technicians, as well as 1 of 3 radiology technicians typically assigned to that room. This is a possible source of bias, however, when generalizing our findings to the broader group of surgeons, it is likely that many work under similar circumstances. Since our complications were identified retrospectively, they were also subject to bias. Longer follow-up time for the earlier cases meant that we were more likely to encounter complications, especially pseudarthrosis, recurrent stenosis, or revision surgery for these cases leading to skew in the complication rate over time. The single surgeon nature of the study allowed accurate mapping of the learning curve; however, it also makes generalization of the results more difficult as progression along the curve would vary with the frequency and difficulty of the case load and learning habits of each individual surgeon. Our radiation dose data reflected only the radiation emitted by the C-arm not the absorbed dose, as we did not have radiation detectors on the body or hands of the surgeon, which precluded us from making conclusions on the actual radiation exposure to the surgeon as they progress along the learning curve. Lastly, our model relies on the assumptions of fitting a linear slope to a data set and subsequently fitting a nonlinear curve to the data set. If applied to a very large data set, the linear slope would become very shallow meaning that the average rate of improvement would decline, thereby skewing the point where proficiency is achieved. Therefore, this model should not be applied to large data sets. While our method presented is not perfect, it is a standardized mathematical approach that can be applied to multiple variables for different techniques that would allow for comparison between studies and statistical validation. Future application to other surgical techniques and medical procedures would then reliably identify the relevant number of cases required to achieve proficiency and could be used to guide training and certification of surgeons and physicians. Lastly, we only had 1-year follow up as part of our inclusion criteria. Therefore, it is possible that the earlier cases may have had more complications simply because they had longer follow-up time.
CONCLUSION
In our retrospective review of the first 5 years of a single surgeon's experience with MI-TLIF, we identified that a surgeon may achieve reasonable comfort with the procedure in order to minimize operative time after 38 cases, whereas true mastery of the procedure with respect to reduction in the complication rate and minimizing radiation exposure to themselves and their operative team requires 43 and 51 cases, respectively. A surgeon choosing to learn MI-TLIF should therefore be aware that mastering fluoroscopy time and exposure is a much more gradual process than achieving a reduction in operative time or complication rates. Our results have important implications for guiding the education of surgeons adopting the MI-TLIF technique and for emphasizing proper fluoroscopy protocols and techniques to reduce radiation exposure when initially learning the technique. Additionally, our method of identifying the learning curve is reproducible and can be applied broadly to other procedures and fields.
REFERENCES
- 1.Lee K-H, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265–2270. doi: 10.1007/s00586-012-2281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh K, Nandyala SV, Marquez-Lara A, et al. A perioperative cost analysis comparing single-level minimally invasive and open transforaminal lumbar interbody fusion. Spine J. 2014;14(8):1694–1701. doi: 10.1016/j.spinee.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 3.Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1–2):230–238. doi: 10.1016/j.wneu.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Lee JC, Jang HD, Shin BJ. Learning curve and clinical outcomes of minimally invasive transforaminal lumbar interbody fusion: our experience in 86 consecutive cases. Spine (Phila Pa 1976) 2012;37(18):1548–1557. doi: 10.1097/BRS.0b013e318252d44b. [DOI] [PubMed] [Google Scholar]
- 5.Nandyala SV, Fineberg SJ, Pelton M, et al. Minimally invasive transforaminal lumbar interbody fusion: one surgeon's learning curve. Spine J. 2014;14(8):1460–1465. doi: 10.1016/j.spinee.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 6.Silva PS, Pereira P, Monteiro P, et al. Learning curve and complications of minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus. 2013;35(2):E7. doi: 10.3171/2013.5.FOCUS13157. [DOI] [PubMed] [Google Scholar]
- 7.Perisinakis K, Damilakis J, Theocharopoulos N, et al. Patient exposure and associated radiation risks from fluoroscopically guided vertebroplasty or kyphoplasty. Radiology. 2004;232(3):701–707. doi: 10.1148/radiol.2323031412. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan D, Than KD, Want AC, et al. Radiation safety and spine surgery: systematic review of exposure limits and methods to minimize radiation exposure. World Neurosurg. 2014;82(6):1337–1343. doi: 10.1016/j.wneu.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Bindal RK, Glaze S, Ognoskie M, et al. Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine. 2008;9(6):570–573. doi: 10.3171/SPI.2008.4.08182. [DOI] [PubMed] [Google Scholar]
- 10.Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16(5):1195–1199. doi: 10.1148/radiographics.16.5.8888398. [DOI] [PubMed] [Google Scholar]
- 11.Mettler FA, Jr, Koenig TR, Wagner LK, et al. Radiation injuries after fluoroscopic procedures. Semin Ultrasound CT MR. 2002;23(5):428–442. doi: 10.1016/s0887-2171(02)90014-4. [DOI] [PubMed] [Google Scholar]
- 12.Mastrangelo G, Fedeli U, Fadda E, et al. Increased cancer risk among surgeons in an orthopaedic hospital. Occup Med. 2005;55(6):498–500. doi: 10.1093/occmed/kqi048. [DOI] [PubMed] [Google Scholar]
- 13.Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 2003;28(15 suppl):S26–S35. doi: 10.1097/01.BRS.0000076895.52418.5E. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery: part 2: histologic and histochemical analyses in humans. Spine (Phila Pa 1976) 1994;19(22):2598–2602. doi: 10.1097/00007632-199411001-00018. [DOI] [PubMed] [Google Scholar]
- 15.United States Nuclear Regulatory Commission. NCR Rules and Regulations Standards for Protection Against Radiation. Code of Federal Regulations and Energy, Chapter 1, Title 10, Part 20. Bethesda, MD: United States Government Printing Office;; 1995. [Google Scholar]
- 16.Rampersaud YR, Foley KT, Shen AC, et al. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine (Phila Pa 1976) 2000;25(20):2637–2645. doi: 10.1097/00007632-200010150-00016. [DOI] [PubMed] [Google Scholar]
- 17.Herscovici D, Jr, Sanders RW. The effects, risks, and guidelines for radiation use in orthopaedic surgery. Clin Orthop Relat Res. 2000;(375):126–132. doi: 10.1097/00003086-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Kim CW, Lee YP, Taylor W, et al. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008;8(4):584–590. doi: 10.1016/j.spinee.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Clark JC, Jasmer G, Marciano FF, et al. Minimally invasive transforaminal lumbar interbody fusions and fluoroscopy: a low-dose protocol to minimize ionizing radiation. Neurosurg Focus. 2013;35(2):E8. doi: 10.3171/2013.5.FOCUS13144. [DOI] [PubMed] [Google Scholar]