Abstract
Background and Aims:
Recently, low-dose intravenous (IV) dexmedetomidine has been evaluated for obtunding the pneumoperitoneum-induced haemodynamic changes and its analgesic efficacy in laparoscopic cholecystectomy. The aim was to determine the postoperative analgesic efficacy of low-dose bolus of 0.5 μg/kg dexmedetomidine via IV and intraperitoneal (IP) route in laparoscopic cholecystectomy.
Methods:
Seventy-five patients, aged 18–60 years of ASA physical status I and II, undergoing laparoscopic cholecystectomy under general anaesthesia were included. Patients in Group C received IP bupivacaine. Patients in Group IV received 0.5 μg/kg dexmedetomidine infusion IV after removal of gall bladder along with IP bupivacaine and Group IP received 0.5 μg/kg dexmedetomidine in 40 mL of 0.25% bupivacaine IP. The primary outcome was 'time to first request of analgesia' and the secondary outcomes were 'total consumption of tramadol in 24 hours,' visual analogue scale (VAS) pain score.
Results:
In total, 75 patients with 25 in each group were included. Time to first request of analgesia was found to be significantly lower in IV (59.68 ± 71.05 min, P = 0.00) and IP group (90.80 ± 80.46 min, P = 0.001) compared tp Group C (59.68 ± 71.05 min). Mean tramadol consumption in 24 hours (152.40 ± 60.958 vs 137.64 ± 52.40 mg) and mean VAS pain score were comparable in both IV and IP groups in the initial 12 h.
Conclusion:
Low bolus dose of IP dexmedetomidine is as efficacious as IV dexmedetomidine (0.5 μg/kg) along with IP bupivacaine in laparoscopic cholecystectomy.
Key words: Analgesia, bupivacaine, cholecystectomy, dexmedetomidine, intraperitoneal, intravenous
INTRODUCTION
Dexmedetomidine is a highly selective α2 receptor agonist with sympatholytic, sedative, analgesic, amnestic and opioid-sparing properties.[1,2] Majority of the studies evaluating the role of intravenous (IV) dexmedetomidine in laparoscopic cholecystectomy have used a dose of 1 μg/kg bolus followed by infusion in a dose of 0.2–0.7 μg/kg/h. This dose is known to produce a biphasic response, i.e., hypertension followed by hypotension and reflex bradycardia.[3] This can be prevented by omitting the bolus dose.[3] Recently, low-dose dexmedetomidine in an infusion dose of 0.2 and 0.4 μg/kg/h[4,5,6] have also been studied in laparoscopic cholecystectomy mainly for obtundation of haemodynamic response; however, sparingly studied for its efficacy in providing postoperative analgesia.[7]
Nowadays, the practice of intraperitoneal (IP) local anaesthetic administration has been a routine. Recently, some authors have evaluated the role of IP administration of dexmedetomidine (1 μg/kg) combined with bupivacaine for postoperative analgesia in patients undergoing laparoscopic colorectal cancer surgery[8] and laparoscopic cholecystectomy.[9] They concluded that dexmedetomidine as an adjuvant provides better postoperative analgesia and all these studies have used IP dexmedetomidine in a dose of 1 μg/kg in combination with 0.25% bupivacaine. We undertook this study with the aim to compare the postoperative analgesic efficacy of a combination of either a low bolus dose of IV dexmedetomidine (0.5 μg/kg) with IP bupivacaine, or an IP dexmedetomidine (0.5 μg/kg) with bupivacaine with IP bupivacaine alone in patients undergoing laparoscopic cholecystectomy.
METHODS
This randomised, double-blind, prospective controlled trial was undertaken following approval from the Institutional Ethics Committee-Human (IEC-H) between November 2016 to April 2018 in a tertiary care hospital in the city of Delhi. The trial is registered with the Clinical Trial Registry- India (CTRI) (trial registry number: 2017/11/015837).
In total, 75 patients aged 18–60 years of either sex with American Society of Anesthesiologists (ASA) physical status I or II undergoing laparoscopic cholecystectomy under general anaesthesia (GA) were included. Patients were excluded if the BMI was >30 kg/m2, allergic to any medication, renal or hepatic insufficiency, neurologic and psychiatric disease, with preoperative heart rate (HR) <45/min, on antihypertensive medication with any α2 adrenergic agonists, e.g., clonidine or if surgical procedure converted to open cholecystectomy. Written informed consent was taken from each patient included in the study, explaining that each one will be followed up for a minimum of 24 h after surgery.
Pre-anaesthetic medication was administered in the form of alprazolam 0.25 mg orally in the night prior and on the morning of surgery. In the preoperative room, patients were instructed on how to use a 10-cm visual analogue scale (VAS) (VAS: 0-10, where 0 = no pain and 10 = worst possible pain). Patients were randomly allocated into one of the three groups by using a computer-generated random numbers table. Allocation concealment was done by using sequentially numbered opaque envelopes; these were used to assign randomisation on the day of surgery. The study drug was prepared by a person not involved in the study. The patients, surgeons and anaesthesiologists were blinded to the patient allocation. Patients were randomly allocated into one of the three groups. In Group C, patients received 30 mL of normal saline (NS) IV over 10 min soon after removal of gall bladder and 40 mL of 0.25% bupivacaine IP. In Group IV, patients received 0.5 μg/kg dexmedetomidine infusion IV in 30-mL NS over 10 min soon after removal of gall bladder and 40 mL of 0.25% bupivacaine IP, whereas patients in Group IP received 30-mL NS IV soon after removal of gall bladder and 0.5 μg/kg dexmedetomidine in 40 mL of 0.25% bupivacaine IP. The IP injection of 40 mL of 0.25% bupivacaine is prepared by using 20 mL of 0.5% diluted with 20 mL of saline in a 50-mL syringe.
Standard anaesthetic technique for general anaesthesia (GA) was followed in all the patients. Baseline vital parameters were recorded. An IV line was established for the infusion of IV fluid. Anaesthesia was induced with propofol 2 mg/kg and morphine 0.1 mg/kg. Tracheal intubation was facilitated by vecuronium 0.1 mg/kg. Anaesthesia was maintained by isoflurane and 66% nitrous oxide in oxygen to achieve a MAC of 1-1.2. Patients were monitored by using continuous electrocardiogram (ECG), oxygen saturation (SpO2), end tidal CO2 and intermittent non-invasive blood pressure (NIBP). HR and NIBP were recorded at 3 min after intubation, soon after pneumoperitoneum and at 10-min interval thereafter (P0, P10, P20, P30, etc.). Soon after the removal of gall bladder along with the start of IV study drug, all the aforementioned monitoring was done at an interval of 5 min till the tracheal extubation. All patients received IV ondansetron 0.1 mg/kg to prevent the postoperative nausea and vomiting before the completion of surgery.
Hypotensive episode was defined as fall of ≥20% of baseline systolic blood pressure (SBP) and was treated with bolus of Lactated Ringer's solution or mephentermine 3–6 mg bolus IV, if required. Bradycardia was defined as HR ≤45 bpm and was treated with atropine 0.6 mg IV.
During laparoscopy, intra-abdominal pressure (IAP) was maintained at 10–12 mmHg. Following the removal of gall bladder, the study solution was administered via IV and IP routes. The study solution to be administered intravenously was infused over a period of 10 min. The study solution to be administered via IP route was instilled before removal of trocar in Trendelenburg's position, into the hepatodiaphragmatic space, on gall bladder bed and near and above hepatoduodenal ligament. The CO2 was carefully evacuated at the end of surgery by manual compression of abdomen with open trocars. At the end of surgery, residual neuromuscular block was reversed and trachea extubated on meeting the standard criteria for extubation.
In the postoperative period, all patients were administered oxygen therapy and were kept under observation for the next 6 h in the recovery room. HR, SBP, diastolic blood pressure (DBP) and mean arterial pressure (MAP) were recorded soon after shifting to the postoperative area and at an interval of 10 min thereafter till the end of first hour. The intensity of postoperative pain was recorded for all the patients using VAS pain score at various designated intervals, i.e., 0.5, 1, 2, 4, 6, 12, 24 h. The 'Time to first request of analgesia' was noted, considering the extubation as 'Time 0'. Rescue analgesia was considered in when VAS ≥4. Tramadol in boluses of 1 mg/kg IV to the maximum dose of 200 mg was administered for the same. The intensity and severity of sedation was assessed by using four-point categorical scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). The total consumption of tramadol in 24 h was calculated for each patient. Any side effects, such as hypotension, bradycardia, sedation, nausea and vomiting were recorded in the perioperative period. The primary outcome was the time to first request of analgesia and the secondary outcomes were total consumption of tramadol in 24 h, postoperative VAS pain score and the incidence and severity of adverse effects, i.e., hypotension, bradycardia, nausea/vomiting.
In a previous study (25), the time to first request of analgesia in patients receiving IP bupivacaine during laparoscopic cholecystectomy was found to be 55 ± 20 min. Therefore, we considered a 40% increase in the aforementioned the time to first request of analgesia, i.e., 22 min with the addition of IV or IP dexmedetomidine along with IP bupivacaine to be statistically significant. Since there were three groups, so to account for multiple comparisons, we consider the level of significance as α = 2% (i.e., Zα/2=2.33) and power = 90% (i.e., Zβ=1.28), we get the final sample size of 21 patients in each group. However, to compensate for any dropouts, i.e., when the laparoscopic procedure is converted to open cholecystectomy, 25 patients in each group were included.
Statistical analysis was performed using SPSS 20.0 version. Results were expressed as mean ± standard deviation, number and percentage (%). Data were analysed using posthoc analysis method. Intergroup P value was derived using Tukey's test and the cumulative P value was derived using ANOVA. Normally distributed data were assessed using unpaired Student's t-test for comparison of parameters among the three groups. Comparison of non-parametric data was carried out using Chi-square (χ2) test with a P value reported at 95% confidence level. A P value ≤0.02 was considered statistically significant.
RESULTS
Out of 102 patients who were assessed for inclusion, 79 patients were enrolled and 23 were excluded from the study. Out of 79, 2 in Group I and 1 each in Groups II and III were excluded due to the conversion of laparoscopic procedure to open cholecystectomy and finally, 75 patients were included with 25 patients in each group [Figure 1].
Figure 1.
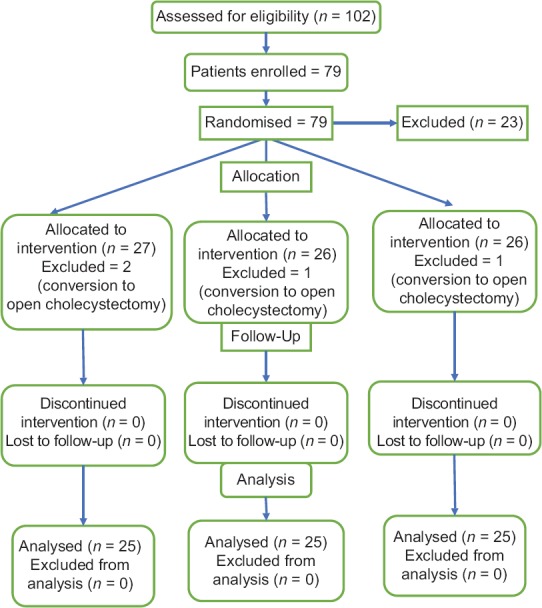
Consort flow diagram
All the groups were comparable with respect to the demographic profile, duration of surgery and mean baseline HR and SBP except the patients' age [Table 1].
Table 1.
Patients’ characteristics
| Parameters | Group I (NS) |
Group II (IV dexmedetomidine) | Group III (IP Dexmedetomidine) |
P | P cumulative | |||
|---|---|---|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | Mean±SD | Range | |||
| Age (years) | 42.84±11.49 | 25-60 | 34.56±10.42 | 19-55 | 33.56±9.18 | 18-53 | 0.02* 0.94† 0.017‡ |
0.004 |
| Weight (kg) | 55.60±9.66 | 42-80 | 53.56±9.42 | 40-72 | 53.28±10.30 | 40-70 | 0.74* 0.99† 0.68‡ |
0.660 |
| Duration of surgery (mean±SD) (in min) | 118.20±26.09 min | 109.20±18.64 min | 105.56±24.71 min | 0.34* 0.85† 0.14‡ |
0.16 | |||
| ASA I (%) | 23 (92%) | 24 (96%) | 23 (92%) | 0.855 | ||||
| ASAII (%) | 2 (8%) | 1 (4%) | 2 (8%) | 0.761 | ||||
NS – Normal saline group, IV – Intravenous, IP – Intraperitoneal dexmedetomidine, SD – Standard deviation. *Level of significance between Groups NS and IV. †Level of significance between Groups IV and IP. ‡Level of significance between group NS and IP. P<0.02 is considered statistically significant
The mean time to first request of analgesia was found to be highest in the IV dexmedetomidine group followed by IP group. On intergroup analysis, this difference was found to be statistically significant between control and IV dexmedetomidine group and between IV and IP dexmedetomidine groups [Table 2]. The mean tramadol consumption in 24 h was found to be significantly higher in the C group when compared with the IV and IP dexmedetomidine groups. The mean value of the IV dexmedetomidine group was found to be the lowest followed by IP group [Table 2].
Table 2.
Time to first request of analgesia and total tramadol consumption (mg) in 24 h
| Time interval | Group l (NS) (n=25) | Group-ll (IV dexmedetomidine) (n=25) |
Group-III (IP dexmedetomidine) (n=25) |
P | P cumulative |
|---|---|---|---|---|---|
| Mean time to first request of analgesia (in min) Median IQR 50th (25th to 75th) Min-Max |
59.68±71.05 35.00 (5.00-67.50) (2, 250) |
210.52±161.17 230 (52.50-340) (15, 700) |
90.80±80.46 35 (50.00-147.50) (10, 320) |
0.00* 0.001† 0.59‡ |
0.000 |
| Total tramadol consumption in 24 h (mg) | 198.80±81.216 | 137.64±52.41 | 152.40±60.96 | 0.005* 0.71† 0.04‡ |
0.004 |
NS – Normal saline group, IV – Intravenous, IP – Intraperitoneal dexmedetomidine. *Level of significance between Groups NS and IV. †Level of significance between Groups IV and IP. ‡Level of significance between Groups NS and IP. P<0.02 is considered statistically significant
There was statistically significant reduction in the mean VAS pain score in Groups IV and IP when compared with control group from 0.5 to 12 h except at the sixth hour following shifting of the patient to the postoperative area. Thereafter, no significant difference in mean VAS pain score was observed between the three groups at the end of 1 day [Table 3 and Figure 2].
Table 3.
Postoperative VAS score
| Time interval (h) | Group l (NS) (n=25) | Group ll (IV dexmedetomidine) (n=25) | Group III (IP dexmedetomidine (n=25) | P | P cumulative |
|---|---|---|---|---|---|
| 0.5 | 4.36±2.08 | 2.56±1.64 | 2.68±0.75 | 0.00* 0.96† 0.001‡ |
0.000 |
| 1 | 3.72±1.24 | 2.44±1.12 | 3.36±1.35 | 0.001* 0.03† 0.56‡ |
0.002 |
| 2 | 3.56±0.82 | 2.72±0.79 | 3.12±0.88 | 0.002* 0.22† 0.16‡ |
0.003 |
| 4 | 3.92±0.99 | 3.28±0.68 | 3.16±0.85 | 0.03* 0.88† 0.007‡ |
0.005 |
| 6 | 3.80±0.997 | 3.56±0.71 | 3.52±0.87 | 0.56* 0.99† 0.45‡ |
0.430 |
| 12 | 4.08±1.038 | 3.44±0.96 | 3.52±0.87 | 0.05* 0.95† 0.11‡ |
0.042 |
| 24 | 3.40±0.645 | 3.56±0.77 | 3.52±0.65 | 0.69* 0.98† 0.81‡ |
0.697 |
VAS – Visual analogue scale, NS – Normal saline group, IV – Intravenous, IP – Intraperitoneal dexmedetomidine. *Level of significance between Groups NS and IV. †Level of significance between Groups IV and IP. ‡Level of significance between Groups NS and IP. P<0.02 is considered statistically significant
Figure 2.
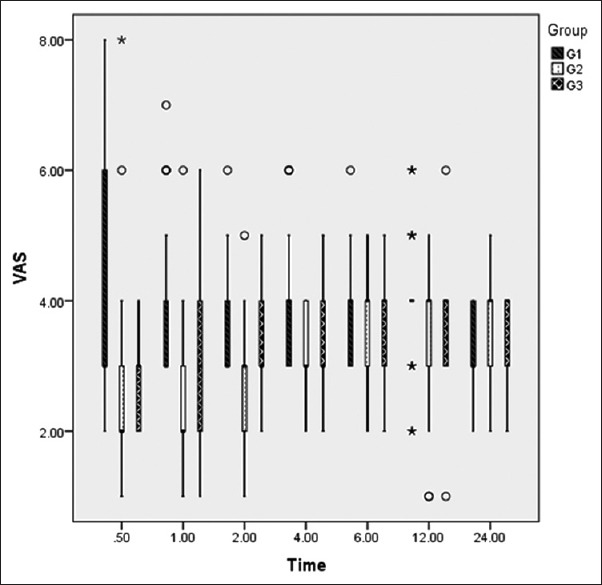
Visual analogue scale (VAS) (at rest). Box Whisker plot of VAS at different time period. Boxes indicate median with 25th and 75th percentile and Whisker caps indicate 10th and 90th percentiles (° representing the outliners and *representing the extremes). Group I - Normal saline group, Group II - intravenous dexmedetomidine, Group III - intraperitoneal dexmedetomidine
The HR and SBP were recorded after gall bladder removal (GB0) and at an interval of 5 min, thereafter till the release of pneumoperitoneum. No statistically significant difference in the mean HR and mean SBP was observed after the gall bladder removal or the start of study drug till the tracheal extubation. The mean HR in the postoperative period at various designated intervals was comparable between the three groups. No statistical significance was observed. On comparing the three groups, the mean SBP was observed to be significantly reduced in the dexmedetomidine groups (II and III) at all the designated time points except at 1 h in the postoperative period when compared with the control group.
On intergroup analysis, the mean sedation score in the postoperative period was found to be the highest in the IV dexmedetomidine followed by IP dexmedetomidine group in the initial 2 h. However, this difference in the initial 2 h was found to be statistically significant between IV and control group and between IV and IP group. However, the difference between control and IP group was not found to be statistically significant [Table 4]. On intergroup analysis, the mean sedation score was found to be significant till the end of 2nd hour and comparable at the end of 4th, 6th, 12th and 24th hour. Only one patient in the control group had nausea except that none of the patients had any of the side effects in the perioperative period, i.e., hypotension, bradycardia, desaturation spells, vomiting, etc.
Table 4.
Postoperative sedation score
| Time interval (h) | Group l (NS) (n=25) | Group ll (IV dexmedetomidine) (n=25) | Group-III (IP dexmedetomidine) (n=25) | P | P cumulative |
|---|---|---|---|---|---|
| 0.5 | 0.28±0.61 | 1.04±0.89 | 0.59±0.81 | 0.002* 0.02† 0.73‡ |
0.001 |
| 1 | 0.20±0.58 | 0.68±0.85 | 0.20±0.50 | 0.03* 0.03† 1.00‡ |
0.02 |
| 2 | 0.00±0.00 | 0.24±0.52 | 0.04±0.20 | 0.03* 0.08† 0.90‡ |
0.02 |
| 4 | 0.00±0.00 | 0.16±0.47 | 0.04±0.20 | 0.14* 0.33† 0.88‡ |
0.15 |
| 6 | 0.00±0.00 | 0.04±0.20 | 0.04±0.20 | 0.66* 1.00† 0.66‡ |
0.61 |
| 12 | 0.00±0.00 | 0.04±0.20 | 0.04±0.20 | 0.66* 1.00† 0.63‡ |
0.61 |
| 24 | 0.00±0.00 | 0.04±0.20 | 0.04±0.20 | 0.66* 1.00† 0.66‡ |
0.61 |
NS – Normal saline group, IV – Intravenous, IP – Intraperitoneal dexmedetomidine. *Level of significance between Groups NS and IV. †Level of significance between Groups IV and IP. ‡Level of significance between Groups NS and IP. P<0.02 is considered statistically significant
DISCUSSION
The result of the present study indicates that IP dexmedetomidine in a low dose of 0.5 μg/kg with bupivacaine is as efficacious as low bolus IV dexmedetomidine (0.5 μg/kg) along with IP bupivacaine and hence can be used as a part of multimodal analgesia techniquec in patients undergoing laparoscopic cholecystectomy.
The use of IV dexmedetomidine in patients undergoing laparoscopic cholecystectomy has shown many beneficial effects, such as reduced anaesthetic requirement,[10] reduced inflammatory response,[11] reduced postoperative pain,[12] reduced shivering[13] and better haemodynamic response to pneumoperitoneum,[14] and recently it has been found to benefit the postoperative cognitive function in elderly patients.[15] Most studies evaluating the haemodynamic response and postoperative analgesic efficacy have used IV dexmedetomidine in a loading dose of 1 μg/kg bolus followed by continuous infusion of 0.5–0.7 μg/kg/h.[10,12,16] Recently, few researches have evaluated the low infusion dose of dexmedetomidine. Bhattacharjee et al. and Park et al. evaluated the effect of IV dexmedetomidine in a low dose of 0.2 and 0.3 μg/kg/h, respectively on haemodynamics in patients undergoing laparoscopic cholecystectomy. The MAP and HR in the dexmedetomidine group were observed to be significantly less after intubation and throughout the period of pneumoperitoneum. However, both the studies did not evaluate the analgesic efficacy of dexmedetomidine.[17,18] All the aforementioned studies evaluating the low infusion doses of dexmedetomidine are associated with stable haemodynamics with minimal side effects. Manne et al.[7] evaluated the dexmedetomidine infusion doses of 0.2 and 0.4 μg/kg/h and found the dose of 0.4 μg/kg/h to be efficacious in the management of postoperative pain.
IP instillation of local anaesthetic agents has become an important method to control postoperative pain, nausea, vomiting and reduces hospital stay. Recently, few researchers have used IP dexmedetomidine for postoperative analgesia in laparoscopic procedures in a dose of 1 μg/kg along with bupivacaine.[8,9,16,18] This dose was found to reduce the postoperative pain and analgesic requirement in laparoscopic cholecystectomy.
Patients characteristics and duration of surgery was comparable except for age. A statistically significant difference was observed between the groups with respect to age; irrespective of the fact that the patients were randomised by using computer-generated random number tables and allocation concealment was done by using opaque sealed envelopes. This difference in the age was not found to be clinically significant.
Dexmedetomidine has antinociceptive action which has been extensively studied in laparoscopic cholecystectomy. Various authors have evaluated the analgesic efficacy of IV dexmedetomidine in a dose of 1 μg/kg bolus followed by 0.2–0.7 μg/kg/h infusion;[10,11,12] however, in context to the low dose, Manne et al. in the only study has evaluated the efficacy of low-dose IV dexmedetomidine, i.e., 0.2–0.4 μg/kg/h.[7]
IP administration of dexmedetomidine causes local analgesia by enhancement of the hyperpolarization-activated cation channels, which prevents the nerve from returning to resting membrane potential.[4] The studies evaluating the analgesic efficacy of IP dexmedetomidine have used the dose of 1 μg/kg.[5,8,9] Various outcomes to evaluate analgesic efficacy used in these studies are VAS pain score, time to first request for analgesia and total consumption of rescue analgesia. In the present study, we have used the time to first request for analgesia as the primary outcome and VAS pain score and total consumption of tramadol in 24 h postoperatively as secondary outcomes.
The mean time to first request of analgesia in this study was found to be highest in the IV dexmedetomidine group (210.52 ± 161.17 min) followed by IP group. This difference was found to be statistically significant between the control and IV group and between IV and IP group. The inference derived is that the IV dexmedetomidine is more efficacious for providing postoperative analgesia in comparison to the control group and IP dexmedetomidine group. Though, the mean time to first request of analgesia was more in IP dexmedetomidine group when compared with the control group; this difference was not statistically significant. The time to first request of analgesia in the IP group in our study is comparable to Shukla et al., where they have used IP dexmedetomidine in a dose of 1 μg/kg in laparoscopic cholecystectomy (90.80 ± 80.46 vs 120 ± 20 min).
In this study, we used Inj. Tramadol 50 mg IV as rescue analgesia if VAS pain score is ≥4. The mean tramadol consumption in 24 h was found to be significantly reduced in the IV (137.64 ± 52.41 mg) and IP (152.40 ± 60.96 mg) group when compared with the control group (198.80 ± 81.22 mg). IP dexmedetomidine could be a potential alternative to IV dexmedetomidine when administered in a low dose of 0.5 μg/kg. The mean tramadol consumption in 24 h in this study is comparable to Fares et al.,[8] where they had used IP dexmedetomidine in the double dose, i.e., 1 μg/kg. Similarly, the total rescue analgesia in 24 h in a study by Shukla et al. was found to be significantly reduced in the IP dexmedetomidine group when compared with the IP tramadol or control group in patients undergoing laparoscopic cholecystectomy.[9]
In this study, the intensity of postoperative pain was evaluated using VAS pain score at various designated intervals, i.e., 0.5, 1, 2, 4, 6, 12, 24 h postoperatively. We observed a statistically significant reduction in the mean VAS pain score in between the three groups till 4 h, postoperatively. On intergroup analysis, a statistically significant reduction in VAS pain score was observed in IV dexmedetomidine group when compared to the control group at all-time points till the end of 12th hour. However, mean VAS pain scores in IV dexmedetomidine group and IP dexmedetomidine group were found to be comparable at various time points except at the end of first hour. On intergroup analysis between control group and IP dexmedetomidine group, no particular trend was observed. Shukla et al. evaluated the mean VAS pain score in patients undergoing laparoscopic cholecystectomy and observed it to be significantly lower at all-time points in IP dexmedetomidine group till 24 h when compared with the tramadol or control group.[9] This difference in the mean VAS pain score of Shukla et al. from this study (24 vs 12 h) could be attributed to the use of low-dose dexmedetomidine (0.5 μg/kg) in this study.
On intergroup analysis of intraoperative haemodynamic parameters after the gall bladder removal and the start of the study drug either via IP or IV route, no fixed pattern was observed in HR and DBP. However, a decrease in the SBP was observed at all-time points; though, not statistically significant. This stable haemodynamics throughout the intraoperative period even after the start of study drug till the tracheal extubation could be attributed to the use of low-dose dexmedetomidine in this study. This finding is in concordance to the studies evaluating the low-dose dexmedetomidine in laparoscopic cholecystectomy[16] and in contrast to the various other studies evaluating the role of dexmedetomidine in the conventional dose of 1 μg/kg followed by 0.5–0.7 μg/kg/h in laparoscopic cholecystectomy where it was found to be associated with significant haemodynamic changes.[6,10,11] Similarly, in the postoperative period, the haemodynamic remained stable. None of the patients in any of the three groups had any episode of hypotension or bradycardia. This again could be attributed to the use of low-dose IV or IP dexmedetomidine in this study.
In this study, the mean sedation score in the postoperative period was found to be ≤1, at various time points in all the three groups. Although, the sedation score was observed to be statistically significant till the end of second hour in the postoperative period; it was not found to be significant clinically. The above finding could be explained with the elimination half-life of dexmedetomidine, i.e., 2–3 h.
Limitation of this study is that the postoperative pain is the subjective experience and we did not quantify it by objective assessment. There have been very few studies evaluating the efficacy of IP dexmedetomidine in laparoscopic cholecystectomy.
CONCLUSION
We conclude that IP dexmedetomidine in a low dose of 0.5 μg/kg with bupivacaine is as efficacious as low bolus IV dexmedetomidine (0.5 μg/kg) along with IP bupivacaine in terms of total tramadol consumption in 24 hrs and the time to first request of analgesia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Alexander JI. Pain after laparoscopy. Br J Anaesth. 1997;79:369–78. doi: 10.1093/bja/79.3.369. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Bogra J, Kothari N, Kohli M. Postoperative analgesia with intraperitoneal fentanyl and bupivacaine: A randomized control trial. Can Jr Med. 2010:11–11. [Google Scholar]
- 3.Ickeringill M, Shehabi Y, Adamson H, Rutteimann R. Dexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: Haemodynamic effects and efficacy. Anaesth Intensive Care. 2004;32:741–5. doi: 10.1177/0310057X0403200602. [DOI] [PubMed] [Google Scholar]
- 4.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anaesthesiology. 2011;115:836–43. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed B, Ashraf AE, Doaa R. Antinociceptive effect of adrenoceptor agonist dexmedetomidine vs meperidine topically, after laparoscopic gynecological surgery. J Med Sci. 2008;8:400–4. [Google Scholar]
- 6.Srivastava VK, Nagle V, Agrawal S, Kumar D, Verma A, Kedia S. Comparative evaluation of dexmedetomidine and esmolol on hemodynamic responses during laparoscopic cholecystectomy. J Clin Diagn Res. 2015;9:UC01–5. doi: 10.7860/JCDR/2015/11607.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manne GR, Upadhyas MR, Swadia VN. Effects of low dose dexmedetomidine infusion on haemodynamic stress response, sedation and post-operative analgesia requirement in patients undergoing laparoscopic cholecystectomy. Ind Jr Anaesth. 2014;58:726–31. doi: 10.4103/0019-5049.147164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fares KM, Mohamed SA, Abd EI-Rahman AM, Mohamed AA, Amin AT. Efficacy and safety of intraperitoneal dexmedetomidine with bupivacaine in laparoscopic colorectal surgery, a randomized trial. Pain Med. 2015;16:1186–94. doi: 10.1111/pme.12687. [DOI] [PubMed] [Google Scholar]
- 9.Shukla U, Prabhakar T, Malhotra K, Srivastav D. Intraperitoneal bupivacaine alone or with dexmedetomidine or tramadol for post-operative analgesia following laparoscopic cholecystectomy: A comparative evaluation. Ind Jr Anaesth. 2015;59:234–8. doi: 10.4103/0019-5049.155001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanduja S, Ohri A, Panwas M. Dexmedetomidine decreases requirement of thiopentone sodium and pentazocine followed with improved recovery in patients undergoing lapraroscopic cholecystectomy. J Anesthesiol Clin Pharmacol. 2014;30:208–12. doi: 10.4103/0970-9185.130022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang SH, Kim YS, Hong TH, Chaes MS, Cho ML, Her YM, et al. Effect of dexmedetomidine on inflammatory repair in patients undergoing laparoscopic cholecystectomy. Acta Anesthesiol Scand. 2013;57:480–7. doi: 10.1111/aas.12039. [DOI] [PubMed] [Google Scholar]
- 12.Park JK, Cheong SH, Lee KM, Lim SH, Lee JH, Cho K, et al. Does dexmedetomodine reduce postoperative pain after laparoscopic cholecystectomy with multimodal analgesia? Korean J Anesthesiol. 2012;63:436–40. doi: 10.4097/kjae.2012.63.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Huang H, Zeng J, Chen Y, Cao Y, Wang R, et al. Effect of dexmedetomidine in preventing shivering after general anesthesia on laparoscopic surgery: A randomized single blinded and placebo control study. Nan Fang Y; Ke Da Xue Bauov. 2013;33:611–14. [PubMed] [Google Scholar]
- 14.Kumar S, Kushwaha BR, Prakash R, Jafa S, Malik A, Wahal R, et al. Comparative study of effects of dexmedetomidine and clonidine premedication in perioperative hemodynamic stability and postoperative analgesia in laparoscopic cholecystectomy. Internet J Anesthesiol. 2014:33. [Google Scholar]
- 15.Chen I, Yan J, Han X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Exp Ther Med. 2013;5:489–94. doi: 10.3892/etm.2012.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahokehr A, Sammour T, Soop M, Hill AG. Intraperitoneal local anesthetic in abdominal surgery-A systemic review. ANZ J Surg. 2011;81:237–45. doi: 10.1111/j.1445-2197.2010.05573.x. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharjee DP, Nayek SK, Dawn S, Bandopadhyay G, Gupta K. Effects of dexmedetomidine on haemodynamics in patients undergoing laparoscopic cholecystectomy-A comparative study. J Anaesth Clin Pharmacol. 2010;2:45–8. [Google Scholar]
- 18.Park HY, Kim JY, Cho SH, Lee D, Kwak HJ. The effects of low-dose dexmedetomidine on hemodynamics and anesthetic requirements during bis-spectral index guided total intravenous anesthesia. J Clin Monit Comput. 2015;11:429–35. doi: 10.1007/s10877-015-9735-2. [DOI] [PubMed] [Google Scholar]


