Abstract
Bronchopulmonary dysplasia (BPD) is a chronic lung disease of infants that is characterized by interrupted lung development. Postnatal sepsis causes BPD, yet the contributory mechanisms are unclear. To address this gap, studies have used lipopolysaccharide (LPS) during the alveolar phase of lung development. However, the lungs of infants who develop BPD are still in the saccular phase of development, and the effects of LPS during this phase are poorly characterized. We hypothesized that chronic LPS exposure during the saccular phase disrupts lung development by mechanisms that promote inflammation and prevent optimal lung development and repair. Wild-type C57BL6J mice were intraperitoneally administered 3, 6, or 10 mg/kg of LPS or a vehicle once daily on postnatal days (PNDs) 3–5. The lungs were collected for proteomic and genomic analyses and flow cytometric detection on PND6. The impact of LPS on lung development, cell proliferation, and apoptosis was determined on PND7. Finally, we determined differences in the LPS effects between the saccular and alveolar lungs. LPS decreased the survival and growth rate and lung development in a dose-dependent manner. These effects were associated with a decreased expression of proteins regulating cell proliferation and differentiation and increased expression of those mediating inflammation. While the lung macrophage population of LPS-treated mice increased, the T-regulatory cell population decreased. Furthermore, LPS-induced inflammatory and apoptotic response and interruption of cell proliferation and alveolarization was greater in alveolar than in saccular lungs. Collectively, the data support our hypothesis and reveal several potential therapeutic targets for sepsis-mediated BPD in infants.
Keywords: bronchopulmonary dysplasia, inflammation, lipopolysaccharide, proteomics, T-regulatory cells
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is a chronic developmental lung disorder of preterm infants that occurs secondarily to an imbalance between lung injury and repair (37). Recent management strategies for preterm infants with respiratory failure significantly reduce the risk of lung injury; however, the incidence of BPD has remained unchanged over the past few decades (53). Furthermore, this disorder increases the economic burden (3, 62) because 1) no specific therapies are available and 2) it is associated with significant long-term cardiopulmonary and neurological morbidities (35, 60, 69, 70). Indeed, BPD is the most expensive neonatal disease in the United States (38). These data emphasize the need for discovering specific therapies based on the underlying pathogenic mechanism of the disorder.
The pathogenesis of BPD is complex and multifactorial. Factors that contribute to BPD pathogenesis may be antenatal, natal, or postnatal in origin. In addition to mechanical ventilation and hyperoxia, neonatal sepsis is a major postnatal risk factor for developing BPD. Although there is no consensus-based definition of sepsis in neonates, sepsis in general is defined as a “life-threatening organ dysfunction caused by a dysregulated host response to infection” (71). Inflammation is a necessary biological process through which the host eliminates the pathogen. However, it is important that immune homeostasis is restored as soon as the pathogens are eliminated. Failure to achieve this complex homeostasis can lead to several disorders (33). A large body of evidence indicates that postnatal sepsis independently increases the incidence of BPD (42, 45, 51). In fact, data from previous studies suggest that postnatal infection is a more important predictor of BPD than antenatal infection (7, 36). It is unclear whether postnatal sepsis directly causes BPD or is only associated with BPD. Furthermore, the molecular mechanisms by which sepsis disrupts lung development are poorly understood.
Lipopolysaccharide (LPS) is the major biologically active component and primary recognition structure of gram-negative bacteria (31). Therefore, LPS has been widely used to model sepsis in experimental animals (27, 50). In most animal models of LPS-induced developmental lung injury, LPS was administered antenatally to pregnant animals (15, 18, 20, 22, 29, 41, 46, 49, 74); therefore, these studies have implications for lung disorders mediated by chorioamnionitis rather than by postnatal sepsis. In studies aimed at determining the effects of postnatal LPS exposure, LPS was mostly administered during the alveolar phase of lung development (28, 34, 57, 58). However, the lungs of most preterm infants who develop sepsis-associated BPD are still in the saccular phase of lung development. The effects of LPS on alveolarization and pulmonary vascular development during this phase of lung development are largely unknown. Furthermore, age-related differences in lung tissue responses to inflammation are well elucidated (4, 39, 54, 59); therefore, there is a need to determine the impact of early-life LPS exposure on the developing lungs.
The objectives of this study were to address the above-mentioned knowledge gaps and use a proteomics approach to determine the potential mechanisms by which LPS mediates its effects on the developing lungs. To this end, we used neonatal C57BL6J wild-type (WT) mice and tested the following hypothesis: chronic LPS exposure during the saccular phase of lung development disrupts alveolarization by mechanisms that promote lung inflammation and prevent optimal lung development and repair. Our findings demonstrate that chronic early-life (saccular phase of lung development) LPS exposure causes alveolar simplification in a dose-dependent manner. We also show that LPS similarly increases proinflammatory cells and cytokines/chemokines, decreases the anti-inflammatory T-regulatory cells (Tregs), and dysregulates the expression of proteins that are necessary for lung development and repair.
MATERIALS AND METHODS
Animals.
This study was approved and conducted in strict accordance with the federal guidelines for the humane care and use of laboratory animals by the Institutional Animal Care and Use Committee of Baylor College of Medicine. C57BL/6J WT mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Timed-pregnant mice raised in our animal facility were used for the experiments. The dams were fed standard mouse food and water ad libitum, and all experimental animals were maintained under 12-h:12-h day-night cycles.
LPS treatment.
LPS was used to induce experimental sepsis. Neonatal WT mice were injected intraperitoneally with 3, 6, or 10 mg/kg of Escherichia coli O55:B5 LPS (Sigma-Aldrich, St. Louis, MO; Cat. No. L2880) or an equivalent volume of the vehicle control (PBS) once daily on postnatal days (PNDs) 3–5. In a separate set of experiments, neonatal WT mice were injected intraperitoneally with a single dose of E. coli O55:B5 LPS (10 mg/kg) on PND3 or PND7 to investigate whether the responses of lung tissue to an inflammatory insult differed between lungs in the saccular and alveolar stages of development.
Tissue preparation for lung morphometry and immunohistochemistry studies.
Vehicle- and LPS-treated animals were euthanized on PND7 or PND14 (n = 6/treatment group), and their lungs were inflated and fixed via the trachea with 10% formalin at a pressure of 25 cmH2O for at least 10 min. Sections of the paraffin-embedded lungs were obtained for lung morphometry and immunohistochemistry studies (68).
Analyses of alveolarization.
Alveolar development was evaluated by measuring radial alveolar counts (RACs) and mean linear intercepts (MLIs). The observers performing the measurements were blinded to the slide identity. RACs were determined as described by Cooney and Thurlbeck (23). RAC measurements were performed by dropping a perpendicular line from the center of a respiratory bronchiole to the edge of the septum or pleura and counting the number of alveoli traversed by this line. MLIs were assessed as described previously (73). Briefly, grids of horizontal and vertical lines were superimposed on an image, and the number of times the lines intersected the tissue was counted. The total length of the grid lines was then divided by the number of intersections to provide the MLI in micrometers. Photographs from at least 10 random nonoverlapping lung fields (original magnification ×100) were taken of each animal for RAC and MLI measurements.
Analyses of lung vascularization.
Pulmonary blood vessel density was determined based on immunohistochemical staining for the von Willebrand factor (vWF), which is an endothelial-specific marker. At least 10 counts from 10 random nonoverlapping fields (original magnification ×20) were performed for each animal (n = 6/group). The observers performing the measurements were masked to the slide identity.
Reverse-phase protein array analyses.
In a separate set of experiments, neonatal WT mice were injected intraperitoneally with a single dose of E. coli 0127:B8 LPS (10 mg/kg) or the vehicle control on PND3. Twenty-four hours later, the lungs of these animals (n = 4/treatment group) were harvested and stored at −80°C for subsequent reverse-phase protein array (RPPA) assays, which were conducted as described previously (17), with minor modifications. Protein lysates were prepared using modified Tissue Protein Extraction Reagent (Pierce) and a cocktail of protease and phosphatase inhibitors (Roche Life Science) (32). The lysates were diluted into 0.5 mg/ml of total protein in sodium dodecyl sulfate sample buffer and denatured on the same day. An Aushon 2470 Arrayer (Aushon BioSystems) with a 40-pin (185 µm) configuration was used to spot samples and control lysates onto nitrocellulose-coated slides (Grace Bio-Laboratories) using an array format of 960 lysates/slide (2,880 spots/slide). The slides were processed as described (17) and probed with a set of 204 antibodies against total and phosphoprotein proteins using an automated slide stainer (Autolink 48, Dako). Each slide was incubated with one specific primary antibody, and each negative-control slide was incubated with the antibody diluent instead of the primary antibody. Primary antibody binding was detected using a biotinylated secondary antibody followed by staining with streptavidin conjugated with the IRDye680 fluorophore (LI-COR Biosciences). The total protein content of each spotted lysate was assessed by fluorescent staining with SYPRO Ruby Protein Blot Stain, according to the manufacturer's instructions (Molecular Probes).
Fluorescence-labeled slides were scanned on a GenePix 4400 AL scanner. Each slide, along with its accompanying negative-control slide, was scanned at an appropriate photomultiplier tube to obtain an optimal signal for this specific set of samples. The images were analyzed with GenePix Pro 7.0 Microarray Acquisition & Analysis Software (Molecular Devices). The total fluorescence signal intensities of each spot were obtained after subtraction of the local background signal for each slide and were normalized for variations in background and nonspecific labeling and total protein levels using a group-based normalization method (32). For each spot on the array, the background-subtracted foreground signal intensity was subtracted by the corresponding signal intensity of the negative-control slide (omission of primary antibody) and then normalized to the corresponding signal intensity for the total protein level for that spot. Each image, along with its normalized data, was carefully evaluated for quality through manual inspection and comparison with control samples. Antibody slides that failed the quality inspection were either repeated at the end of the staining runs or removed before data reporting. The remaining 182 antibodies were used for subsequent data analyses.
Differential protein expression analyses.
The heatmap of median-centered normalized expression values and unsupervised hierarchical clustering values [based on uncentered correlations of expression profiles of significantly different (P < 0.05) samples] was generated using the heatmap function in R statistical software. The mean of the triplicate experimental values (normalized signal intensity) was taken for each sample for subsequent statistical analysis. We determined significant differences (P < 0.05) in protein expression levels between experimental groups by employing Student’s t-test and requiring at least a 1.25-fold change in expression. Differentially expressed proteins (DEPs) were further investigated by enrichment analysis using Gene Ontology mapping. A gene set was considered significantly enriched when P < 0.05.
Immunohistochemistry studies.
The paraffin-embedded lung tissues were deparaffinized and subjected to antigen retrieval. The lung sections were then incubated with 7.5% normal donkey serum for 1 h to block nonspecific protein binding, after which they were incubated overnight at 4°C with the following primary antibodies: anti-vWF (Dako, Carpinteria, CA; Cat. No. A0082, dilution 1:750), anti-phospho-signal transducer and activator of transcription (STAT)3 (Cell Signaling, Danvers, MA; Cat. No. 9145, dilution 1:150), anti-fibroblast growth factor receptor 1 (FGFR1) (Abcam; Cat. No. ab63601, dilution 1:150), anti-cleaved caspase 3 (Biocare Medical, Pacheco, CA; Cat. No. PP 229 AA, prediluted), and anti-Ki67 (Abcam; Cat. No. ab15580, dilution 1:1,000). The Ki67 antibody was detected by incubation with biotinylated anti-rabbit secondary (Vector, Burlingame, CA, dilution 1:200), followed by horseradish peroxidase (Vector, Cat. No. PK-6100). All other primary antibodies were detected using an anti-rabbit polymer (Vector, Cat. No. MP-7401). The observers analyzing these slides were masked to the experimental conditions.
Analyses of lung inflammation.
The extent of lung inflammation was assessed by quantifying lung cytokine/chemokine protein expression. Total RNA extracted from frozen lung tissues using the Direct-zol RNA MiniPrep Kit (Zymo Research, Irvine, CA; R2052) was reverse transcribed to cDNA, as described previously (79). Real-time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis was then performed with a 7900HT Real-Time PCR System using TaqMan Gene Expression Master Mix (Grand Island, NY; 4369016) and TaqMan Gene-Expression Assays (Applied Biosystems, Foster City, CA) for the following genes: chemokine (C-C motif) ligand 2 (CCL2; Mm00441242_m1), CCL3 (Mm00441259_g1), chemokine (C-X-C motif) ligand 1 (CXCL1; Mm04207460_m1), intercellular adhesion molecule 1 (ICAM1; Mm00516023_m1), interleukin (IL)-1β (IL-1β; Mm00434228_m1), IL-10 (Mm01288386_m1), tumor necrosis factor-α (TNF-α; Mm00443258_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_g1). GAPDH was detected as the reference gene. The samples were denatured at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The ΔΔCt method was used to calculate fold changes in mRNA expression.
Flow cytometry studies.
Lymphocytes and other leukocytes from the lung tissue were isolated as described previously (78), with some modifications. Briefly, lung tissues were cut into small pieces in petri dishes containing Roswell Park Memorial Institute 1640 medium supplemented with 5% fetal calf serum, 100 U penicillin-streptomycin, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 20 mM l-glutamine, 100 mg of type IV collagenase/50 ml, and 2 U/ml of type I DNase. Collagenase digestion was performed for 60 min at 37°C on a rotary agitator, and any remaining undigested tissue was mechanically disrupted using a 70-μm cell strainer and a piston of a 3-ml syringe. Density-gradient centrifugation was used to isolate the lymphocytes, wherein the cell pellet obtained after collagenase digestion was resuspended in 40% Percoll, layered onto 70% Percoll, and centrifuged at 2,400 revolutions/min for 30 min at 4°C. These cells were initially stained for cell surface markers by incubation with antibodies against CD4 (100451, dilution 1:400), TCRβ (109219, dilution 1:200), and CD11b (101241, dilution 1:400) for 30 min in 1X PBS containing 0.05% sodium azide and 5% fetal bovine serum, after which the cells were fixed and permeabilized using Cytofix/Cytoperm buffer for 1 h and then incubated with an anti-forkhead box P3 (Foxp3) antibody (12-5773-82, dilution 1:100) in Perm/wash buffer for 1 h. All antibodies were obtained from BD Biosciences, Biolegend, or eBioscience (San Diego, CA). Data were obtained using an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar, Ashland, OR).
Lung tissue harvesting and protein extraction.
The lungs from a subset of study animals (n = 6/treatment group) were snap frozen in liquid nitrogen and stored at −80°C for subsequent isolation of total proteins. A mortar and pestle was used to homogenize the lung tissue with the same buffer that was used to prepare the protein lysates for the RPPA analyses. The homogenate was centrifuged at 2,400 × g for 5 min at 4°C. The supernatant (protein lysate) was stored at −80°C.
Immunoblot assays.
Protein lysates from the experimental animals were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were incubated overnight at 4°C with primary antibodies against annexin-1 (Invitrogen, Carlsbad, CA; 71-3400, dilution 1:1,000), β-actin (Santa Cruz Biotechnology; sc-47778, dilution 1:5,000), FGFR1 (Cell Signaling Technology, Danvers, MA; 9740, dilution 1:1,000), STAT1 (Cell Signaling Technology; 9172, dilution 1:1,000), phospho(p)-STAT1 [STAT1(Tyr701); Cell Signaling Technology; 7649, dilution 1:1,000], STAT3 (Cell Signaling Technology; 12640, dilution 1:1,000), and p-STAT3 [STAT3(Ser727); Cell Signaling Technology; 9134, dilution 1:1,000]. The primary antibodies were detected by incubation with appropriate horseradish peroxidase-conjugated secondary antibodies. The immunoreactive bands were detected by chemiluminescence methods, and the band densities were quantified by Image laboratory 5.2.1 software (Bio-Rad, Hercules, CA).
Statistical analyses.
The results were analyzed with GraphPad Prism 5 software. Data are expressed as means ± SD. At least two separate experiments were performed for each measurement, except for the RPPA analyses (described above). Log-rank (Mantel-Cox) testing was used to determine the effects of LPS on survival, and one-way analysis of variance (ANOVA) was used to determine the effects of LPS on alveolarization and lung inflammation. A post hoc Bonferroni's multiple comparison test was performed in cases where statistical significance of either variable was noted by ANOVA. An unpaired t-test was used to determine differences in cytokine mRNA expression levels between saccular and alveolar lungs, and two-way ANOVA was used to determine the effects of LPS and the stage of lung development on their associated interactions with the outcome variables, body weight, and STAT1 and STAT3 activation. Post hoc multiple t-tests with Bonferroni’s correction were performed when statistical significance of either variable or interaction was noted by two-way ANOVA. A P value < 0.05 was considered significant.
RESULTS
Survival and growth rates of mice exposed to early LPS.
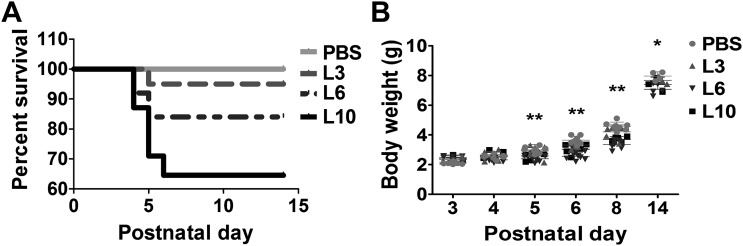
We initially investigated the dose-dependent effects of LPS on the survival and growth rates of neonatal mice. Early LPS exposure decreased the survival rate of neonatal mice in a dose-dependent manner. While the survival rate over the first 14 days of life was not statistically different in animals treated with the vehicle control or 3 mg/kg of LPS, it decreased by 16 and 35.5%, respectively, in animals treated with 6 mg/kg and 10 mg/kg of LPS (Fig. 1A). LPS exerted similar effects on the growth rates of mice. Body weights at PND3 were not statistically different between the vehicle- and LPS-treated mice. At PND14, the body weights of animals treated with 6 mg/kg and 10 mg/kg of LPS were significantly lower compared with vehicle-treated mice (Fig. 1B).
Fig. 1.
Survival rate and body weights of mice exposed to LPS early in life. Newborn mice treated intraperitoneally with LPS at doses of 3 (L3), 6 (L6), or 10 (L10) mg/kg or vehicle (PBS) on postnatal days 3–5 were monitored for survival and weight gain over the first 14 days of life. A: percent survival of PBS- and LPS-treated mice (n = 20–31/group). The data shown are representative of four independent experiments. Log-rank (Mantel-Cox) testing showed significant differences in the percent survival between mice treated with PBS and 6 or 10 mg/kg of LPS. B: body weight (in g) of PBS- and LPS-treated mice. The data shown are representative of four independent experiments. Values are presented as means ± SD (n = 20–31/group). Two-way ANOVA showed an effect of LPS and age and an interaction between them for the dependent variable, body weight. Significant differences between PBS- and LPS-treated animals are indicated by **P < 0.01 and *P < 0.05.
Alveolarization of mice exposed to early LPS.
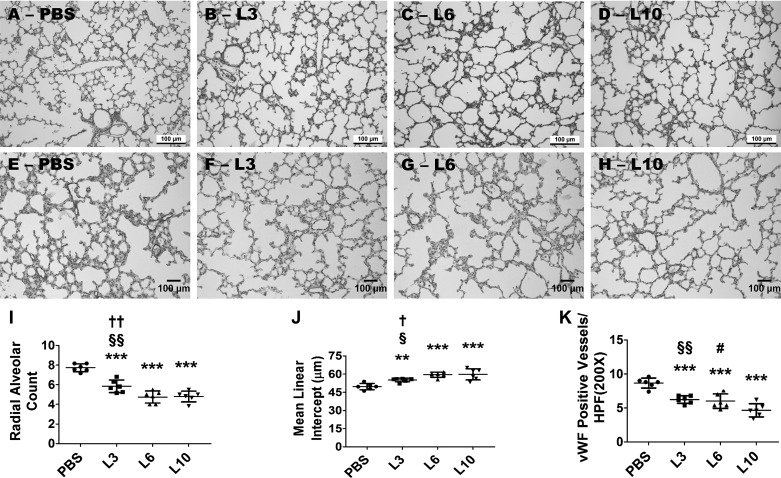
Alveolar development was determined by taking RAC and MLI measurements on PND7. LPS disrupted alveolar development (alveolar simplification) in a dose-dependent manner. Alveolar simplification was significantly greater in animals treated with 6 or 10 mg·kg−1·day−1 of LPS compared with those treated with 3 mg·kg−1·day−1 of LPS, whereas the severity of alveolar simplification was similar in animals treated with 6 and 10 mg·kg−1·day−1 of LPS (Fig. 2, A–D, I, and J). Exposure of neonatal mice to 3, 6, or 10 mg·kg−1·day−1 of LPS for 3 days significantly decreased RACs (Fig. 2, A–D and I), indicating that there were fewer alveoli compared with vehicle-treated animals. Additionally, the MLIs of these LPS-treated mice significantly increased (Fig. 2, A–D and J), indicating that their alveoli were also larger than those of vehicle-treated mice.
Fig. 2.
Effect of chronic LPS exposure on alveolarization. Newborn mice were treated intraperitoneally with LPS at doses of 3 (L3), 6 (L6), or 10 (L10) mg/kg or a vehicle control (PBS) on postnatal days (PNDs) 3–5, and the lung tissues were collected for morphometry on PND7. Representative hematoxylin-eosin-stained lung sections obtained from mice treated with PBS (A), L3 (B), L6 (C), and L10 (D). Representative von Willebrand factor (vWF)-immunostained lung sections obtained from mice treated with PBS (E), L3 (F), L6 (G), and L10 (H). Alveolarization was quantified by determining radial alveolar counts (RACs) (I) and mean linear intercepts (MLIs) (J), and pulmonary vascularization was quantified by counting the vWF-stained lung blood vessels (K). The data shown are representative of three independent experiments. Values are presented as means ± SD (n = 6/group). One-way ANOVA showed an effect of LPS for the dependent variables, RAC, MLI, and vWF-stained lung blood vessels. Significant differences between PBS- and LPS-exposed animals are indicated by ***P < 0.001 and **P < 0.01. Significant differences between L3- and L6-exposed animals are indicated by ††P < 0.01 and †P < 0.05. Significant differences between L3- and L10-exposed animals are indicated by §§P < 0.01 and §P < 0.05. Significant differences between L6- and L10-exposed animals are indicated by #P < 0.05. Scale bar = 100 µM.
Lung vascularization of mice exposed to early LPS.
We next examined the effects of early LPS on pulmonary blood vessel density by quantifying the vWF-stained lung blood vessels. LPS decreased vWF-stained lung blood vessels (Fig. 2, E–H and K), indicating that early LPS exposure causes pulmonary vascular simplification and recapitulates the pathology of BPD infants. Pulmonary vascular simplification was significantly greater in animals treated with 10 mg·kg−1·day−1 of LPS compared with those treated with 3 or 6 mg·kg−1·day−1 of LPS, whereas the severity of pulmonary vascular simplification was similar in animals treated with 3 and 6 mg·kg−1·day−1 of LPS (Fig. 2, E–H and K).
Lung proteomic analyses of mice exposed to early LPS.
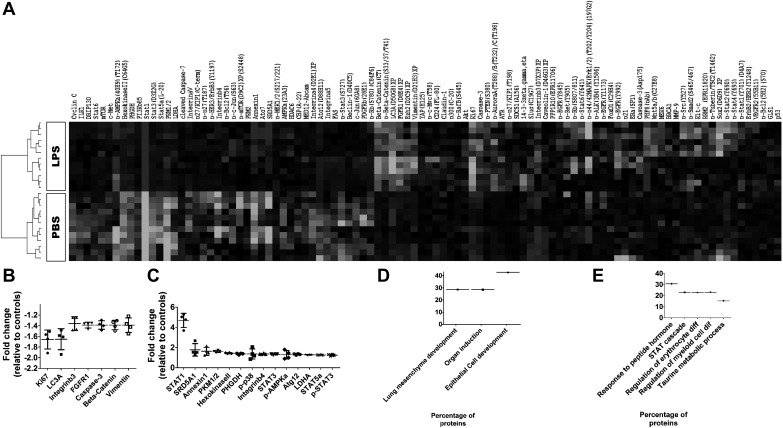
To examine proteins that were differentially regulated by LPS exposure, we performed RPPA analysis on lung samples obtained 24 h after intraperitoneal administration of a single LPS dose of 10 mg·kg−1·day−1 on PND3. A heatmap of the median-centered, normalized expression levels of the DEPs, based on LPS exposure (P < 0.05), demonstrated that the lung protein expression profiles of vehicle-treated mice were distinguishable from those of LPS-treated mice (Fig. 3A). Analysis of the DEPs between vehicle- and LPS-treated mice showed 21 out of 182 proteins studied that were significantly regulated (P < 0.05) by LPS with a fold change exceeding 1.25 times. Among the DEPs in LPS-treated mice, 7 proteins were downregulated (Fig. 3B) and 14 proteins were upregulated (Fig. 3C) compared with vehicle-treated mice. We next determined the biological processes affected by the DEP by performing enrichment analysis using the Gene Ontology database. Downregulated proteins were enriched for biological processes, such as epithelial cell development (β-catenin, FGFR1, and vimentin), organ induction (β-catenin and FGFR1), and lung-associated mesenchyme development (FGFR1 and β-catenin), indicating the repression of these pathways in LPS-treated animals (Fig. 3D). In contrast, the upregulated proteins were significantly enriched for biological processes specific to taurine metabolism (PHGDH and STAT5a), regulation of myeloid cell and erythrocyte differentiation (STAT1, STAT3, and STAT5a), STAT signaling (STAT1, STAT3, and STAT5a), and cellular responses to peptide hormones (p38, STAT1, STAT3, and STAT5a), indicating activation of these pathways in LPS-treated animals (Fig. 3E).
Fig. 3.
Lung proteomics of mice exposed to LPS early in life. The lung tissues of 4-day-old mice were collected 24 h after treatment with the vehicle control (PBS; n = 4) or one dose of LPS (10 mg/kg; n = 4) and analyzed in a reverse-phase protein array assay. Heatmap of unsupervised hierarchical clustering of LPS-treated mice inclusive of significantly different (P < 0.05) protein expression data (A). Significantly downregulated proteins (B) and significantly upregulated proteins (C) in the lungs of LPS-treated mice. Gene Ontology analysis of significant downregulated (D) and upregulated (E) biological processes in the lungs of LPS-treated mice. FGFR, fibroblast growth factor receptor; STAT, signal transducer and activator of transcription.
Validation of the RPPA results by performing immunoblot assays.
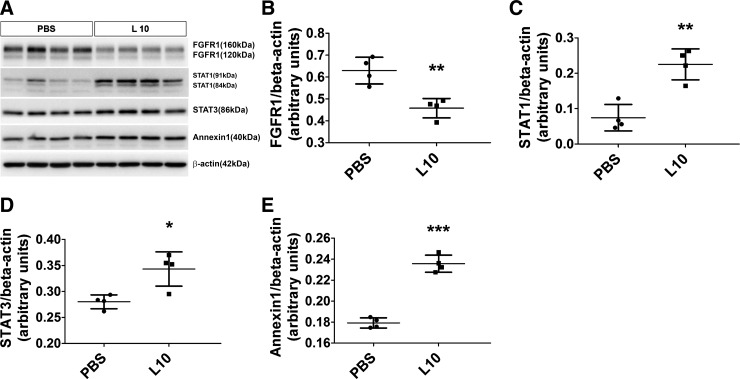
Based on their relevance to organ development and inflammation, we selected four DEPs (FGFR1, STAT1, STAT3, and annexin-1) identified by the RPPA for validation by immunoblotting analyses. The RPPA data were confirmed for all the proteins tested (Fig. 4). The magnitude change in protein expression identified by the RPPA experiments correlated with the immunoblotting results: FGFR1 (RPPA: 0.7-fold decrease; immunoblotting assay: 0.7-fold decrease; Fig. 4, A and B), STAT1 (RPPA: 4.7-fold increase; immunoblotting assay: 3-fold increase; Fig. 4, A and C), STAT3 (RPPA: 1.3-fold increase; immunoblotting assay: 1.2-fold increase; Fig. 4, A and D), and annexin-1 (RPPA: 1.6-fold increase; immunoblotting assay: 1.3-fold increase; Fig. 4, A and E).
Fig. 4.
Validation of the reverse-phase protein array results in immunoblot assays. The lung tissues of 4-day-old mice were collected 24 h after treatment with the vehicle control (PBS) or one 10 mg/kg dose of LPS (L10) and analyzed in immunoblot assays to determine the protein expression levels of fibroblast growth factor receptor 1 (FGFR1), signal transducer and activator of transcription (STAT)1, STAT3, and annexin-1 (A). Densitometric analyses wherein FGFR1 (B), STAT1 (C), STAT3 (D), and annexin-1 (E) band intensities were quantified and normalized to β-actin. Values shown are presented as means ± SD (n = 4/group). Significant differences between PBS- and L10-exposed animals are indicated by ***P < 0.001, **P < 0.01, and *P < 0.05 (t-test).
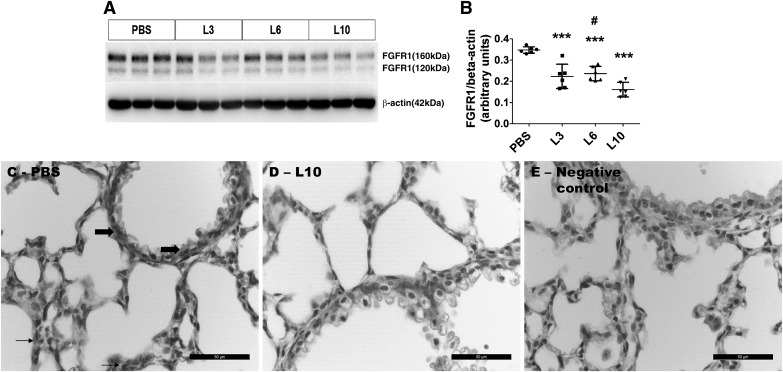
Dose-dependent effects of LPS on lung FGFR1 protein expression.
Fibroblast growth factors signal through FGFR and play a crucial role in alveolar development and regeneration. Thus, we determined the dose-dependent effects of LPS on FGFR1 protein expression to elucidate the mechanisms through which LPS disrupts lung development. Consistent with our proteomic data, LPS decreased lung FGFR1 protein expression in a dose-dependent manner when compared with control mice (Fig. 5, A and B), indicating that FGFR1 downregulation may represent one mechanism through which LPS disrupts lung development. To determine the cell-specific expression of FGFR1 protein in the lung, we performed immunohistochemistry studies on fixed lung sections using anti-FGFR1 antibody. The FGFR1 protein was expressed mainly in the alveolar interstitial cells (Fig. 5C, arrows) and bronchiolar epithelial cells (Fig. 5C, bold block arrows).
Fig. 5.
Expression of the fibroblast growth factor receptor 1 (FGFR1) protein in saccular lungs chronically exposed to LPS. Newborn mice were treated intraperitoneally with LPS at doses of 3 (L3), 6 (L6), or 10 (L10) mg/kg or a vehicle control (PBS) on postnatal days (PNDs) 3–5, and the lung tissues were collected on PND6 to determine FGFR1 protein expression (A). Densitometric analyses wherein FGFR1 band intensities were quantified and normalized to β-actin (B). The data shown are representative of two independent experiments. Values are presented as means ± SD (n = 6/group). One-way ANOVA showed an effect of LPS on FGFR1 protein expression. Significant differences between PBS- and LPS-exposed animals are indicated by ***P < 0.001. Significant differences between L6- and L10-exposed animals are indicated by #P < 0.05. Representative lung sections showing FGFR1-positive lung cells from animals treated intraperitoneally with a vehicle control [PBS; alveolar interstitial cells (arrows) and bronchiolar epithelial cells (bold block arrows)] (C) or 10 mg/kg of LPS (L10; D) on PNDs 3–5. Representative lung section from PBS-treated animals stained with secondary antibody only (negative control) (E). Scale bar = 50 µM.
Effects of early LPS on lung cell proliferation and apoptosis.
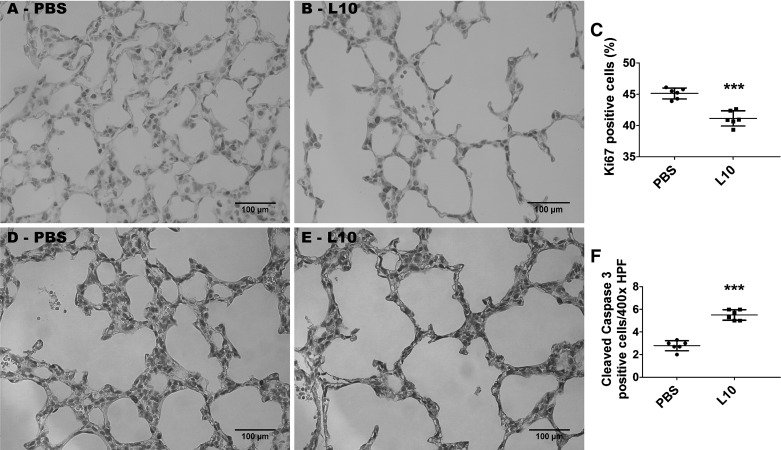
To determine the mechanisms of disrupted lung development in our model, we examined the effects of LPS on lung cell proliferation and apoptosis. Exposure of neonatal mice to 10 mg·kg−1·day−1 of LPS (L10) through PNDs 3–5 significantly decreased the percentage of Ki67-positive lung cells compared with controls (L10, 41 ± 1.5 vs. PBS, 45.2 ± 1.1; P < 0.001), suggesting that LPS inhibits cell proliferation in the saccular phase of lung development (Fig. 6, A–C). Furthermore, these LPS-exposed animals had increased cleaved caspase 3-positive lung cells (L10, 5.5 ± 0.9 vs. PBS, 2.8 ± 0.8; P < 0.001), indicating that LPS also promotes apoptosis in the saccular phase of lung development (Fig. 6, D–F).
Fig. 6.
Effect of chronic LPS exposure on lung cell proliferation and apoptosis. Newborn mice were treated intraperitoneally with 10 mg/kg of LPS (L10) or a vehicle control (PBS) on postnatal days (PNDs) 3–5, and the lung tissues were harvested for immunohistochemistry studies on PND7. Representative lung sections showing Ki67-stained cells from mice treated with PBS (A) and L10 (B). Quantification of the percentage of Ki67-positive lung cells (C). Representative lung sections showing cleaved caspase 3-positive cells from mice treated with PBS (D) and L10 (E). Quantification of cleaved caspase 3-positive lung cells (F). HPF, high-power field. Values are presented as means ± SD (n = 6/group). Significant differences between PBS- and L10-treated animals are indicated by ***P < 0.001 (t-test).
Lung inflammatory response of mice exposed to early LPS.
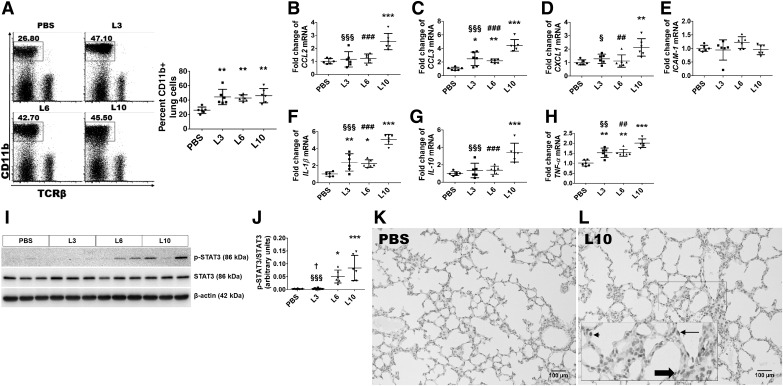
We evaluated the extent of lung inflammation 24 h after the third dose of the vehicle or LPS. To this end, we initially quantified the number of inflammatory macrophages that infiltrated the lungs. To compare the percentage of infiltrating macrophages between the treatment groups, leukocytes extracted from the lung tissues were stained with fluorescent antibodies against CD11b and analyzed by flow cytometry. Neonatal mice treated with all the three doses of LPS showed increased percentages of macrophages in their lung tissues compared with vehicle-treated mice (Fig. 7A).
Fig. 7.
Inflammatory response of saccular lung chronically exposed to LPS. Newborn mice were treated intraperitoneally with LPS at doses of 3 (L3), 6 (L6), or 10 (L10) mg/kg or a vehicle control (PBS) on postnatal days (PNDs) 3–5, and the lung tissues were collected on PND6 for flow cytometry, real-time RT-PCR, and immunoblot analyses. Flow cytometry-based quantification of CD11b+ cells (macrophages) (A). Real-time RT-PCR analyses-based determination of CCL2 (B), CCL3 (C), CXCL1 (D), ICAM-1 (E), IL-1β (F), IL-10 (G), and TNF-α (H) mRNA-expression levels. Determination of phosphorylated signal transducer and activator of transcription (p-STAT)3 and STAT3 protein levels by immunoblotting (I). Quantification and normalization of p-STAT3 band intensities to those of STAT3 (J). The data shown are representative of two independent experiments. Values are presented as means ± SD (n = 6/group). Significant differences between PBS- and LPS-treated animals are indicated by ***P < 0.001, **P < 0.01, and *P < 0.05. Significant differences between L3- and L6-exposed animals are indicated by †P < 0.05. Significant differences between L3- and L10-exposed animals are indicated by §§§P < 0.001, §§P < 0.01, and §P < 0.05. Significant differences between L6- and L10-exposed animals are indicated by ###P < 0.001 and ##P < 0.01 (one-way ANOVA). Representative lung sections showing p-STAT3-positive lung cells from animals treated intraperitoneally with a vehicle control (PBS; K) or 10 mg/kg of LPS [L10; rectangle showing the zoomed-in inset: alveolar interstitial cells (bold block arrow), alveolar macrophages (arrowhead), and vascular endothelial cell (arrow)] (L) on PNDs 3–5. Scale bar = 100 µM.
We next determined the extent of lung inflammation by quantifying production of the proinflammatory cytokines CCL2, CCL3, CXCL1, ICAM-1, IL-1β, and TNF-α in the lung tissues by real-time RT-PCR. LPS affected the expression of these cytokines in a dose-dependent manner. Whereas 10 mg·kg−1·day−1 of LPS increased the mRNA expression levels of CCL2 (Fig. 7B), CCL3 (Fig. 7C), CXCL1 (Fig. 7D), IL-1β (Fig. 7F), and TNF-α (Fig. 7H) by ≥2-fold, we found that lower doses of LPS [3 and 6 mg·kg−1·day−1] only increased the mRNA-expression levels of CCL3 (Fig. 7C), IL-1β (Fig. 7F), and TNF-α (Fig. 7H) by ≥1.5-fold. Early LPS exposure did not alter ICAM (Fig. 7E) mRNA expression in our experimental animals.
Furthermore, we quantified the mRNA expression of the anti-inflammatory cytokine IL-10 in the lung tissues. We observed that exposure to 10 mg·kg−1·day−1 LPS, but not 3 or 6 mg·kg−1·day−1 LPS, increased the lung IL-10 mRNA expression. Compared with vehicle-treated animals, IL-10 mRNA expression increased by >3.4-fold in animals exposed to 10 mg·kg−1·day−1 LPS (Fig. 7G). Our results indicate that the magnitude of IL-10 mRNA expression was directly proportional to the severity of lung inflammation.
We next determined the activation of transcription factors that regulate inflammation. Early LPS failed to activate STAT1, as evidenced by the lack of p-STAT1 production in our experimental animals (Fig. 9, H and I); however, early LPS treatment activated STAT3 in a dose-dependent manner. The p-STAT3/total STAT3 ratio increased by 18.4-fold and 30.2-fold, respectively, in animals treated with 6 or 10 mg·kg−1·day−1 LPS compared with vehicle-treated animals, although p-STAT3 expression was not statistically different between animals treated with the vehicle or 3 mg·kg−1·day−1 LPS (Fig. 7, I and J). Finally, we performed immunohistochemistry studies on fixed lung sections using anti-p-STAT3 antibody to determine the cell-specific expression of p-STAT3 protein in the lung. The p-STAT3 protein was expressed mainly in the alveolar interstitial cells (Fig. 7L, bold block arrow), alveolar macrophages (Fig. 7L, arrow head), and vascular endothelial cells (Fig. 7L, arrow).
Fig. 9.
Inflammatory responses of saccular and alveolar lungs exposed to a single dose of LPS. Newborn mice were injected intraperitoneally with a single dose of vehicle control (PBS) or 10 mg/kg LPS (L10) on postnatal day (PND)3 or PND7, and the lung tissues were collected 24 h after the injection for real-time RT-PCR and immunoblot analyses. Real-time RT-PCR analyses-based determination of CCL2 (A), CCL3 (B), CXCL1 (C), ICAM-1 (D), IL-1β (E), IL-10 (F), and TNF-α (G) mRNA-expression levels. Determination of phosphorylated signal transducer and activator of transcription (p-STAT)1, STAT1, p-STAT3, and STAT3 protein levels by immunoblotting (H). Quantification and normalization of p-STAT1 (I) and p-STAT3 (J) band intensities to those of STAT1 and STAT3, respectively. The data shown are representative of two independent experiments. Values are presented as means ± SD (n = 6/group). For mRNA expression, significant differences between LPS-treated animals in the saccular phase versus alveolar phase of lung development are indicated by ***P < 0.001, **P < 0.01, and *P < 0.05 (t-test). For protein expression, significant differences between PBS- and LPS-treated animals are indicated by ***P < 0.001 (two-way ANOVA).
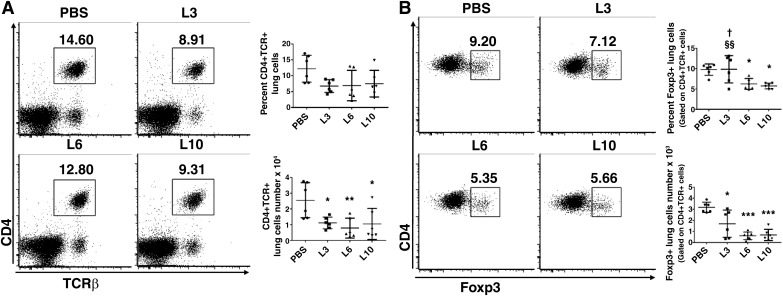
Tregs in mice exposed to early LPS.
Tregs modulate inflammatory responses and play crucial roles in maintaining immune homeostasis. Therefore, we investigated whether LPS exposure could potentiate lung inflammatory responses by decreasing the Tregs in our model of developmental lung injury. Using flow cytometry, Tregs were identified as CD4+TCRβ+ cells expressing the transcription factor FoxP3. Overall, LPS treatment significantly decreased the number of CD4+TCRβ+ cells in the lungs of our experimental animals (Fig. 8A). Compared with the controls, LPS treatment at doses of 3 (L3), 6 (L6), or 10 (L10) mg/(kg/d) decreased the absolute number (L3, 1.7 ± 1.2; L6, 0.6 ± 0.3; L10, 0.7 ± 0.5; PBS, 3.2 ± 0.5; P < 0.05), whereas LPS treatment at doses L6 or L10 mg·kg−1·day−1 decreased the percent (L3, 9.8 ± 3.3; L6, 6.3 ± 1.3; L10, 5.7 ± 0.7; PBS, 9.8 ± 1.4%; P < 0.05) of Tregs (Fig. 8B). These findings indicate that downregulation of anti-inflammatory Tregs may represent one of the mechanisms by which LPS causes an uninhibited proinflammatory state.
Fig. 8.
Effect of chronic LPS exposure on T-regulatory cells (Tregs) of saccular lungs. Newborn mice were treated intraperitoneally with LPS at doses of 3 (L3), 6 (L6), or 10 (L10) mg/kg or a vehicle control (PBS) on postnatal days (PNDs) 3–5, and the lung tissues were collected on PND6 to quantify CD4+TCRβ+ (A) and CD4+TCRβ+FoxP3+ (Tregs; B) cells using flow cytometry. The data shown are representative of two independent experiments. Values are presented as means ± SD (n = 6/group). Significant differences between PBS- and LPS-treated animals are indicated by ***P < 0.001, **P < 0.01, and *P < 0.05. Significant differences between L3- and L10-exposed animals are indicated by §§P < 0.01. Significant differences between L3- and L6-exposed animals are indicated by †P < 0.05 (one-way ANOVA).
LPS-mediated inflammatory responses of the lung in saccular versus alveolar phase of development.
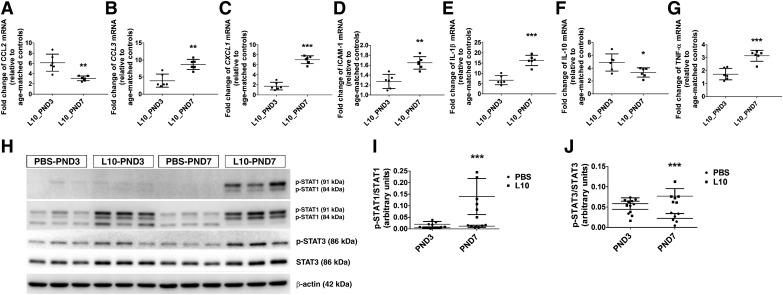
Age-related differences in lung inflammatory responses to LPS are well documented. Therefore, we sought to investigate whether the inflammatory responses differed during the saccular and alveolar phases of lung development. To this end, we quantified the extent of lung inflammation 24 h after intraperitoneal administration of a single LPS dose of 10 mg/kg on PND3 or PND7 by performing real-time RT-PCR and immunoblotting analyses. LPS exposure significantly increased the mRNA-expression levels of CCL3 (Fig. 9B), CXCL1 (Fig. 9C), IL-1β (Fig. 9E), and TNF-α (Fig. 9G) during both the saccular and alveolar phases of lung development compared with age matched controls; however, the expression of these cytokines was significantly greater during the alveolar than during the saccular phase. In contrast, LPS-induced expression of CCL2 (Fig. 9A) and IL-10 (Fig. 9F) mRNA was significantly greater in the saccular phase of lung development than in the alveolar phase. Furthermore, LPS increased ICAM-1 mRNA expression in the alveolar phase of lung development but not in the saccular phase, in our experimental animals (Fig. 9D). These results signify important age-related differences in lung cytokine expression following LPS exposure.
To investigate the regulators of lung inflammation at the protein level, we quantified the levels of total and p-STAT1 and STAT3 by immunoblotting analyses. The results of our immunoblotting experiments were similar to those of the real-time RT-PCR experiments. While a single intraperitoneal dose of 10 mg/kg LPS significantly increased the total lung protein levels of STAT1 and STAT3 in both saccular and alveolar lungs, it increased those of p-STAT1 and p-STAT3 only during the alveolar phase of development (Fig. 9, H–J). Our findings indicate that alveolar lungs mount a robust inflammatory response compared with saccular lungs when challenged with LPS.
LPS-mediated effects on alveolarization, lung vascularization, and lung cell proliferation and apoptosis in saccular versus alveolar phase of development.
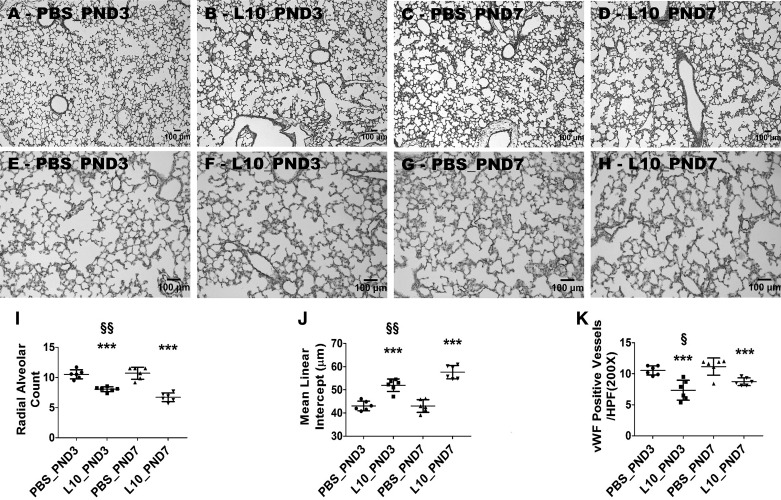
Having seen an age-related difference in the lung inflammatory response to LPS in our model, we sought to examine whether the disruptive effects of LPS on lung development differed during the saccular and alveolar phases of lung development. Therefore, we quantified the extent of alveolarization, lung vascularization, and lung cell proliferation and apoptosis on PND14 in animals exposed to a single dose of 10 mg/kg of LPS on PND3 or PND7. LPS exposure during both the saccular and alveolar phases of lung development caused alveolar and pulmonary vascular simplification compared with age matched controls (Fig. 10); however, the severity of disrupted alveolarization was modestly increased in animals exposed to LPS during the early alveolar phase of lung development compared with those exposed to LPS during the saccular phase of lung development (Fig. 10, A–D, I, and J). In contrast, the severity of pulmonary vascular simplification was modestly increased in animals exposed to LPS during the saccular phase of lung development than those exposed to LPS during the early alveolar phase of lung development (Fig. 10, E–H and K).
Fig. 10.
Deficits in development of saccular and alveolar lungs exposed to a single dose of LPS. Newborn mice were injected intraperitoneally with a single dose of vehicle control (PBS) or 10 mg/kg LPS (L10) on postnatal day (PND3) or PND7, and the lung tissues were harvested on PND14 for lung morphometry. Representative hematoxylin-eosin-stained lung sections obtained from mice treated on PND3 with PBS (A) and L10 (B) and on PND7 with PBS (C) and L10 (D). Representative von Willebrand factor (vWF)-immunostained lung sections obtained from mice treated on PND3 with PBS (E) and L10 (F) and on PND7 with PBS (G) and L10 (H). Alveolarization was quantified by determining radial alveolar counts (I) and mean linear intercepts (J), and pulmonary vascularization was quantified by counting the vWF-stained lung blood vessels (K). Values are presented as means ± SD (n = 6/group). Significant differences between age-matched PBS- and L10-exposed animals are indicated by ***P < 0.001. Significant differences between L10_PND3- and L10_PND7-exposed animals are indicated by §§P < 0.01 and §P < 0.05 (one-way ANOVA). Scale bar = 100 µM. L10_PND3, treated on PND3 with 10 mg/kg LPS; L10_PND7, treated on PND7 with 10 mg/kg LPS; PBS_PND3, treated on PND3 with PBS; PBS_PND7, treated on PND7 with PBS.
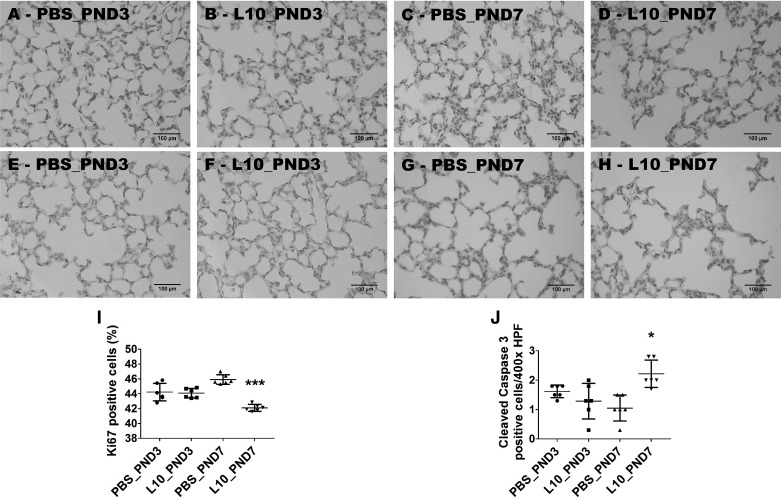
Finally, we examined the age-related effects of LPS on lung cell proliferation and apoptosis at PND14. Exposure of neonatal mice to 10 mg·kg−1·day−1 of LPS on PND7 (L10_PND7) significantly decreased the percentage of Ki67-positive lung cells compared with controls (L10, 42.1 ± 1.2 vs. PBS, 45.9 ± 1.4; P < 0.001); however, there were no significant differences in the percentage of Ki67-positive lung cells between animals treated with LPS (L10_PND3) and vehicle (PBS_PND3) on PND3 (L10, 44.1 ± 1.6 vs. PBS, 44.2 ± 2; P > 0.05) (Fig. 11, A–D and I). The age-related effects of LPS on lung apoptosis mirrored its effects on cell proliferation. When compared with age-matched controls, LPS exposure during the early alveolar phase of lung development increased cleaved caspase 3-positive lung cells (L10, 2.2 ± 1.6 vs. PBS, 1 ± 0.8; P < 0.05), whereas exposure during the saccular phase of lung development had no effect on the number of cleaved caspase 3-positive lung cells at PND14 (L10, 1.4 ± 1.2 vs. PBS, 1.6 ± 0.9; P > 0.05) (Fig. 11, E–H and J).
Fig. 11.
Deficits in proliferation and survival of the cells of saccular and alveolar lungs exposed to a single dose of LPS. Newborn mice were injected intraperitoneally with a single dose of vehicle control (PBS) or 10 mg/kg LPS (L10) on postnatal day (PND)3 or PND7, and the lung tissues were harvested on PND14 for lung immunohistochemistry studies. Representative lung sections showing Ki67-stained cells from mice treated on PND3 with PBS (A) and L10 (B) and on PND7 with PBS (C) and L10 (D). Representative lung sections showing cleaved caspase 3-positive cells from mice treated on PND3 with PBS (E) and L10 (F) and on PND7 with PBS (G) and L10 (H). Quantification of Ki67-positive (percentage) (I) and cleaved caspase 3-positive (number per high power field) (J) lung cells. Values are presented as means ± SD (n = 6/group). Significant differences between age-matched PBS- and L10-exposed animals are indicated by ***P < 0.001 and *P < 0.05 (one-way ANOVA). Scale bar = 100 µM. L10_PND3, treated on PND3 with 10 mg/kg LPS; L10_PND7, treated on PND7 with 10 mg/kg LPS; PBS_PND3, treated on PND3 with PBS; PBS_PND7, treated on PND7 with PBS.
DISCUSSION
In this study, we investigated the effects of systemic LPS exposure in murine lungs during the saccular phase of lung development. We demonstrate that repeated early-life systemic LPS exposure increased macrophages and decreased Tregs in the lungs and disrupted lung development. Additionally, we applied bioinformatics (RPPA) to understand the molecular mechanisms by which LPS disrupts lung development to inform future studies aimed at preventing and/or treating developmental lung disorders, such as BPD, in preterm infants. Finally, we showed the age-related differences in lung inflammatory responses to a single dose of LPS.
It is important to note that the developmental lung stage of a PND3–PND5 mouse resembles that of an infant > 30 wk postmenstrual age. While it is rare for these infants to develop BPD, our model has translational significance for the following reasons. First, this time period still represents an important window of murine lung development (63), where an injurious stimulus can disrupt alveolarization and lung vascularization (61). Second, extremely preterm infants (born between 23 and 28 wk gestational age) are commonly exposed at 30–34 wk postmenstrual age to systemic inflammatory insults, such as sepsis, necrotizing enterocolitis, and major surgeries, which can contribute to BPD pathogenesis. The dose of systemic LPS used in this study to model sepsis was comparable to those used in prior rodent studies (16, 25, 81). While a single dose of LPS is frequently used to model an acute injury, repetitive doses are necessary to model a chronic inflammatory disorder, such as BPD (76). Therefore, we used multiple doses of LPS to model sepsis-induced BPD. We initially examined the effects of LPS on mortality and observed an increased mortality rate in mice treated with either 6 or 10 mg/kg LPS (Fig. 1A). In a study conducted by Alvira et al. (4), the survival rates were similar between mice treated with a vehicle control or systemic LPS up to 10 mg/kg, with an increased mortality rate noted only in mice treated with 20 mg·kg−1·day−1 of LPS. One possibility for this discrepant finding is that we administered repetitive doses, whereas they administered a single dose of LPS. The mechanisms where LPS caused mortality were not investigated in this study; however, high-dose LPS was previously shown to increase mortality by inhibiting cardiorespiratory function secondarily to damaging vital organs, such as the brain, heart, and lungs (77). Failure to thrive is one of the morbidities of sepsis and BPD in infants (1, 65). Consequently, we studied the effects of LPS on body weight and found that animals treated with 6 or 10 mg/kg of LPS had significant weight loss (Fig. 1B), a finding consistent with previous rodent studies (16, 26).
The main function of the lungs is to promote effective gas exchange. To accomplish this function, it is necessary to develop an increased surface area (an increased number of alveoli and blood vessels and a thin alveolo-capillary barrier), both of which occur during the later stages (late saccular and alveolar stages) of lung development (14). Most preterm infants are born before completion of these stages and therefore require therapies, such as positive-pressure ventilatory support and supplemental oxygen therapy, to support their lung function. Unfortunately, these life-saving therapies, along with postnatal sepsis, can interrupt the final stages of lung maturation and lead to BPD (5, 48). Sepsis is associated with an increased risk of developing BPD in preterm infants (42, 45, 51), and microbial products such as LPS can disrupt lung development in experimental animals (19, 22, 34, 58). In agreement with these studies, postnatal systemic LPS exposure interrupted alveolarization and lung vascularization, i.e., lung development, in our experimental animals (Fig. 2). In a two-hit BPD model induced by a combined administration of low-dose prenatal and early postnatal LPS, Choi and colleagues (19) demonstrated that LPS administered during the saccular period interrupts alveolarization and pulmonary vascularization at PNDs 7 and 14. However, the findings of our study differ from those of other preclinical studies in two aspects: 1) we administered repetitive high-dose LPS to mimic a moderate-to-severe chronic inflammatory state and 2) we determined differences in the LPS effect between the saccular and alveolar lungs. Although these aspects closely align our model with sepsis-associated BPD in preterm infants, we recognize that the effects of LPS on alveolarization and pulmonary vascularization in our model were modest compared with BPD infants. However, infants with sepsis-associated BPD are exposed to additional insults, such as hyperoxia and mechanical ventilation, and our study signifies the independent effect of an inflammatory microbial product on lung development. Future studies elucidating the effects of LPS, in addition to other insults such as hyperoxia and mechanical ventilation, are necessary to accurately model infants with sepsis-associated BPD.
High-throughput gene-based assays, such as RNA microarrays and RNA-Seq, have been widely used to identify molecular mechanisms and novel therapies for BPD (2, 9–12, 24, 52, 75). Although genes and RNA-expression levels provide information regarding the status of a cell, the encoded proteins determine most cellular functions and, therefore, a high-throughput antibody-based protein assay may be more beneficial in discovering meaningful therapies. Moreover, most RNA high-throughput studies were performed using models of hyperoxia-induced lung injury. Consequently, we performed RPPA to determine the lung proteomic profile of LPS-treated mice and found that LPS deregulates several proteins and disrupts pathways (Fig. 3) that are essential for promoting the induction and development of organs and maintaining immune homeostasis. Immunoblotting assay was used to verify the RPPA data for four DEPs, revealing a good correlation between both assays (Fig. 4). These findings have important implications for the management of BPD, a developmental lung disease that is characterized by interrupted lung development and increased inflammation. In alignment with data from several studies (8, 34, 57, 58), we found that LPS increased p38 (Fig. 3C) and decreased FGF (Fig. 3B) signaling in the developing lungs. Consistent with the distribution pattern of other FGFRs, we observed that FGFR1 expression in the lungs was localized to alveolar interstitial cells and bronchiolar epithelial cells (Fig. 5C). Although costaining with cell-specific markers and FGFR1 is required to confirm the lung cell-specific expression, our observations are in agreement with other studies (43, 47). An environment characterized by increased inflammation and decreased growth factor signaling can adversely affect lung development and repair by altering cell proliferation and survival (30, 34, 72, 80). Our data (Fig. 6) reinforces this concept. In addition, we identified several other proteins (microtubule-associated proteins 1A/1B light chain 3A, 3-oxo-5α-steroid 4-dehydrogenase 1, pyruvate kinase 1/2, hexokinase II, and annexin-1) that were disrupted in the developing lungs by LPS that can potentially serve as biomarkers and therapeutic targets for infants with BPD.
Persistent inflammation inhibits lung development and causes BPD in preterm infants (6, 40). Consistent with these findings and the results of several preclinical studies of LPS-induced lung injury (34, 49, 54, 55, 57, 58), repeated LPS exposure increased the mRNA-expression levels of proinflammatory chemokines and cytokines (Fig. 7) in our animal model. These chemokines can recruit and activate granulocytes, monocyte/macrophages, and T cells to secrete additional cytokines, leading to an augmented inflammatory cascade. Furthermore, they can activate and alter the phenotype of resident lung epithelial and endothelial cells from one that promotes lung development to that which augments lung inflammation. For example, LPS exposure decreases the expression of VEGFR2 (34) while increasing the expression of proinflammatory mediators in lung cells (56). Similarly, increased expression of the proinflammatory cytokine IL-1β in lung epithelial cells attenuates VEGF expression and disrupts murine lung development (13).
Balanced and coordinated signaling of the innate and adaptive immune systems is needed to restore immune homeostasis and prevent tissue injury following an infection. Following exposure to such an insult, innate immune cells, such as neutrophils and macrophages, are activated, leading to a proinflammatory state that helps clear the infectious agent. Once the inciting insult is cleared, immune homeostasis is restored through activation of the adaptive immune cells such as Tregs, which functions to abrogate the proinflammatory signaling of innate immune cells and prevent tissue damage. In preterm neonates, this immune balance is perturbed by the anatomic and functional immaturity of the immune system, which predisposes them to develop infections and chronic inflammatory diseases (64). The preponderance of lung macrophages is an important marker of a chronic inflammatory state, such as BPD (21). Investigators (66) have suggested that macrophages may be important mediators of lung injury in mice. Our findings (Fig. 7A) are consistent with these observations. In experimental models of neonatal lung injury, the roles of the proinflammatory cells, such as neutrophils and macrophages, are well examined when compared with those of the anti-inflammatory cells, such as Tregs. Evidence suggests that there are age-related differences in the effects of LPS on Tregs. While LPS may increase the Treg population in adult animals (33), it may have the opposite effect in neonatal animals. In a chorioamnionitis model, Kunzmann and colleagues (44) showed that intra-ammonitic LPS administration caused thymic atrophy and reduced the percentage of thymic Tregs in fetal sheep. Recently, Rueda and colleagues (67) elegantly demonstrated that intra-ammonitic LPS administration decreased the Treg percentage and absolute number in the fetal spleen and peripheral blood cells in a rhesus macaque model of chorioamnionitis. Furthermore, they demonstrated that the decrease in Tregs was secondary to deficient generation of Tregs in the thymus. In a mouse model of acute lung injury, intrapharyngeal administration of LPS increased airway Tregs in juvenile but not neonatal mice, and an adoptive transfer of adult Tregs mitigated lung injury in these neonatal mice (54). In agreement with these studies, our findings (Fig. 8) highlight the mechanistic link between Tregs and inflammatory disorders such as BPD.
Our findings demonstrate significant differences in lung inflammatory responses during the saccular and alveolar stages of development. In particular, LPS increased ICAM-1 mRNA expression (Fig. 9D) and activated STAT1 (Fig. 9, H and I) only during the alveolar phase. Furthermore, while a single dose of LPS activated STAT3 during the alveolar phase (Fig. 9, H and J), repeated LPS administration was required to activate STAT3 in the saccular phase (Fig. 7, I and J) in our experimental model. The molecular mechanisms responsible for these differences are not currently well understood and warrant further investigation. p-STAT3 was enriched in the lung endothelial cells, alveolar interstitial cells, and macrophages in our model (Fig. 7L), indicating that the lung inflammatory response may be initiated via the vascular route after intraperitoneal administration of LPS. Future studies that entail costaining with cell-specific markers and p-STAT3 are essential to confirm the lung cell-specific expression of p-STAT3. Differences in lung inflammatory responses between neonatal and juvenile or adult mice have been well investigated (4, 54, 81). When challenged with LPS, juvenile and adult mice mount a more robust lung proinflammatory response than neonatal mice. Our data are consistent with this concept. Alvira and colleagues (4) elegantly showed that LPS differentially activated nuclear factor κ-light-chain enhancer of activated B cells in the lungs of neonatal and adult mice. They observed that this differential nuclear factor κ-light-chain enhancer of activated B cell activation was associated with increased lung inflammation in adult mice compared with neonatal mice. Likewise, McGrath-Morrow and colleagues (54) demonstrated that after LPS exposure, the airway inflammatory response was significantly greater in juvenile mice versus neonatal mice. Additionally, they showed that the chemokine profile differed between these mouse groups when challenged with LPS. While the IL-6 and TNF-α levels were comparable, CXCL1, CCL5, CCL2, and CXCL10 were significantly higher and CCL20 was significantly lower in juvenile lungs compared with neonatal lungs. Despite a more robust airway proinflammatory response, the juvenile mice also displayed a significant increase in the anti-inflammatory cytokine IL-10 and Tregs, as well as rapid resolution of lung inflammation and injury compared with neonatal mice. In contrast, Zhao and colleagues (81) observed that LPS induced a stronger inflammatory response in neonatal (1 or 7 day old) than adult (8 to 10 wk old) mice. These contrasting results may be due to differences in the dose and route of LPS administration.
Finally, we determined the differential effects of LPS on lung development, cell proliferation, and apoptosis during the saccular and alveolar stages of development. At PND14, a single dose of LPS administered during either the saccular or alveolar phase of lung development interrupted alveolar and lung vascular development (Fig. 10). However, the LPS effect on lung vascularization was greater in saccular than alveolar lungs (Fig. 10K), whereas its effect on alveolarization was greater in alveolar than saccular lungs (Fig. 10, I and J). Additionally, a single dose of LPS administered during the alveolar phase inhibited proliferation and promoted apoptosis of lung cells at PND14, whereas exposure of saccular lungs to a single dose of LPS did not alter cell proliferation and apoptosis at PND14 (Fig. 11). The mechanisms behind these differential effects of LPS are poorly understood at this time. It is possible that LPS exposure during the saccular phase disrupts lung development by preferentially affecting angiogenesis, whereas LPS exposure during the alveolar phase disrupts lung development mainly via inflammatory mechanisms. Future mechanistic studies are warranted to understand these differential effects of LPS.
The strength of our study is that we examined the dose-dependent effects of repetitive LPS exposure (chronic inflammatory state) during the saccular phase of lung development, which closely reflects the clinical scenario of preterm infants. Furthermore, we used high-throughput bioinformatics to identify the global impact of LPS on the cellular functions of the lungs. Lastly, we demonstrated differences in lung inflammatory responses between the saccular and alveolar phases of development. Despite these strengths, our study has a few limitations. First, RPPA analysis at a single time point could have failed to identify some dysregulated proteins and pathways. Second, this study was not designed to examine sex- or cell-specific effects of LPS in the developing lungs. Finally, we did not evaluate the effects of LPS on cardiopulmonary function. We will address these weaknesses in our future studies.
In summary, this study characterizes the dose-dependent effects of LPS on developing lungs and identifies potential mechanisms whereby LPS disrupts lung development. Specifically, we demonstrated that repetitive LPS exposure disrupts alveolar development, increases lung inflammation, and decreases Tregs and the expression of factors that promote lung development. Furthermore, we showed that the lungs mount a more robust inflammatory response during the alveolar phase than during the saccular phase of development. Finally, by applying bioinformatics, we identified biological molecules that could serve as biomarkers and therapeutic targets for infants with BPD.
GRANTS
This work was supported by National Institutes of Health Grants HD-073323 (to B. Shivanna), P30-CA-125123 (to D. P. Edwards and S. Huang), and P30-DK-056338 (to Digestive Disease Center Core at the Baylor College of Medicine); Cancer Prevention and Research Institute of Texas Proteomics and Metabolomics Core Facility Support Award RP170005 (to D. P. Edwards and S. Huang); American Heart Association Award BGIA-20190008 (to B. Shivanna); and American Lung Association Award RG-349917 (to B. Shivanna).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.S., M.L.B., R.T.M., V.Y.N.G, S.H., D.P.E., M.P., R.B., and B.S. conceived and designed research; A.K.S., M.L.B., R.T.M., and B.S. performed experiments; A.K.S., M.L.B., R.T.M., V.Y.N.G., S.H., D.P.E., M.P., R.B., and B.S. analyzed data; A.K.S., M.L.B., R.T.M., S.H., D.P.E., M.P., R.B., and B.S. interpreted results of experiments; M.L.B., V.Y.N.G., and B.S. prepared figures; A.K.S., D.P.E., and B.S. drafted manuscript; A.K.S., M.L.B., R.T.M., V.Y.N.G., S.H., D.P.E., M.P., R.B., and B.S. approved final version of manuscript; M.L.B., R.T.M., V.Y.N.G., S.H., D.P.E., M.P., R.B., and B.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Pamela Parsons for timely processing of histopathology slides and core technical personnel Fuli Jia and Danli Wu for helping with the reverse-phase protein array (RPPA) experiments. Finally, we thank statisticians Kimal Rajapakshe, Cristian Coarfa, and Qianxing Mo for processing, normalizing, and analyzing the RPPA data.
REFERENCES
- 1.Abman SH, Collaco JM, Shepherd EG, Keszler M, Cuevas-Guaman M, Welty SE, Truog WE, McGrath-Morrow SA, Moore PE, Rhein LM, Kirpalani H, Zhang H, Gratny LL, Lynch SK, Curtiss J, Stonestreet BS, McKinney RL, Dysart KC, Gien J, Baker CD, Donohue PK, Austin E, Fike C, Nelin LD; Bronchopulmonary Dysplasia Collaborative . Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr 181: 12–28.e1, 2017. doi: 10.1016/j.jpeds.2016.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad A, Bhattacharya S, Sridhar A, Iqbal AM, Mariani TJ. Recurrent copy number variants associated with bronchopulmonary dysplasia. Pediatr Res 79: 940–945, 2016. doi: 10.1038/pr.2016.23. [DOI] [PubMed] [Google Scholar]
- 3.Álvarez-Fuente M, Arruza L, Muro M, Zozaya C, Avila A, López-Ortego P, González-Armengod C, Torrent A, Gavilán JL, Del Cerro MJ. The economic impact of prematurity and bronchopulmonary dysplasia. Eur J Pediatr 176: 1587–1593, 2017. doi: 10.1007/s00431-017-3009-6. [DOI] [PubMed] [Google Scholar]
- 4.Alvira CM, Abate A, Yang G, Dennery PA, Rabinovitch M. Nuclear factor-kappaB activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation. Am J Respir Crit Care Med 175: 805–815, 2007. doi: 10.1164/rccm.200608-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker CD, Alvira CM. Disrupted lung development and bronchopulmonary dysplasia: opportunities for lung repair and regeneration. Curr Opin Pediatr 26: 306–314, 2014. doi: 10.1097/MOP.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balany J, Bhandari V. Understanding the impact of infection, inflammation, and their persistence in the pathogenesis of bronchopulmonary dysplasia. Front Med (Lausanne) 2: 90, 2015. doi: 10.3389/fmed.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballard AR, Mallett LH, Pruszynski JE, Cantey JB. Chorioamnionitis and subsequent bronchopulmonary dysplasia in very-low-birth weight infants: a 25-year cohort. J Perinatol 36: 1045–1048, 2016. doi: 10.1038/jp.2016.138. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin JT, Carver BJ, Plosa EJ, Yamamoto Y, Miller JD, Liu JH, van der Meer R, Blackwell TS, Prince LS. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol 185: 4896–4903, 2010. doi: 10.4049/jimmunol.1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, Pryhuber GS. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med 186: 349–358, 2012. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya S, Zhou Z, Yee M, Chu CY, Lopez AM, Lunger VA, Solleti SK, Resseguie E, Buczynski B, Mariani TJ, O’Reilly MA. The genome-wide transcriptional response to neonatal hyperoxia identifies Ahr as a key regulator. Am J Physiol Lung Cell Mol Physiol 307: L516–L523, 2014. doi: 10.1152/ajplung.00200.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucherat O, Franco-Montoya ML, Thibault C, Incitti R, Chailley-Heu B, Delacourt C, Bourbon JR. Gene expression profiling in lung fibroblasts reveals new players in alveolarization. Physiol Genomics 32: 128–141, 2007. doi: 10.1152/physiolgenomics.00108.2007. [DOI] [PubMed] [Google Scholar]
- 12.Bozyk PD, Popova AP, Bentley JK, Goldsmith AM, Linn MJ, Weiss DJ, Hershenson MB. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev 20: 1995–2007, 2011. doi: 10.1089/scd.2010.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bry K, Whitsett JA, Lappalainen U. IL-1beta disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol 36: 32–42, 2007. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burri PH. Structural aspects of postnatal lung development-alveolar formation and growth. Biol Neonate 89: 313–322, 2006. doi: 10.1159/000092868. [DOI] [PubMed] [Google Scholar]
- 15.Cao L, Wang J, Tseu I, Luo D, Post M. Maternal exposure to endotoxin delays alveolarization during postnatal rat lung development. Am J Physiol Lung Cell Mol Physiol 296: L726–L737, 2009. doi: 10.1152/ajplung.90405.2008. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso FL, Herz J, Fernandes A, Rocha J, Sepodes B, Brito MA, McGavern DB, Brites D. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J Neuroinflammation 12: 82, 2015. doi: 10.1186/s12974-015-0299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CH, Zhang M, Rajapakshe K, Coarfa C, Edwards D, Huang S, Rosen JM. Mammary stem cells and tumor-initiating cells are more resistant to apoptosis and exhibit increased DNA repair activity in response to DNA damage. Stem Cell Reports 5: 378–391, 2015. doi: 10.1016/j.stemcr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi CW, Kim BI, Hong JS, Kim EK, Kim HS, Choi JH. Bronchopulmonary dysplasia in a rat model induced by intra-amniotic inflammation and postnatal hyperoxia: morphometric aspects. Pediatr Res 65: 323–327, 2009. doi: 10.1203/PDR.0b013e318193f165. [DOI] [PubMed] [Google Scholar]
- 19.Choi CW, Lee J, Oh JY, Lee SH, Lee HJ, Kim BI. Protective effect of chorioamnionitis on the development of bronchopulmonary dysplasia triggered by postnatal systemic inflammation in neonatal rats. Pediatr Res 79: 287–294, 2016. doi: 10.1038/pr.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou HC, Li YT, Chen CM. Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Am J Transl Res 8: 342–353, 2016. [PMC free article] [PubMed] [Google Scholar]
- 21.Clement A, Chadelat K, Sardet A, Grimfeld A, Tournier G. Alveolar macrophage status in bronchopulmonary dysplasia. Pediatr Res 23: 470–473, 1988. doi: 10.1203/00006450-198805000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Collins JJ, Kuypers E, Nitsos I, Jane Pillow J, Polglase GR, Kemp MW, Newnham JP, Cleutjens JP, Frints SG, Kallapur SG, Jobe AH, Kramer BW. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 303: L778–L787, 2012. doi: 10.1152/ajplung.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax 37: 572–579, 1982. doi: 10.1136/thx.37.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuna A, Halloran B, Faye-Petersen O, Kelly D, Crossman DK, Cui X, Pandit K, Kaminski N, Bhattacharya S, Ahmad A, Mariani TJ, Ambalavanan N. Alterations in gene expression and DNA methylation during murine and human lung alveolar septation. Am J Respir Cell Mol Biol, 2015. doi: 10.1165/rcmb.2014-0160OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erickson MA, Hartvigson PE, Morofuji Y, Owen JB, Butterfield DA, Banks WA. Lipopolysaccharide impairs amyloid β efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood-brain barrier. J Neuroinflammation 9: 150, 2012. doi: 10.1186/1742-2094-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan LW, Kaizaki A, Tien LT, Pang Y, Tanaka S, Numazawa S, Bhatt AJ, Cai Z. Celecoxib attenuates systemic lipopolysaccharide-induced brain inflammation and white matter injury in the neonatal rats. Neuroscience 240: 27–38, 2013. doi: 10.1016/j.neuroscience.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink MP. Animal models of sepsis. Virulence 5: 143–153, 2014. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco ML, Waszak P, Banalec G, Levame M, Lafuma C, Harf A, Delacourt C. LPS-induced lung injury in neonatal rats: changes in gelatinase activities and consequences on lung growth. Am J Physiol Lung Cell Mol Physiol 282: L491–L500, 2002. doi: 10.1152/ajplung.00140.2001. [DOI] [PubMed] [Google Scholar]
- 29.Hanita T, Matsuda T, Saito M, Kitanishi R, Cho K, Harding R, Kobayashi Y. Potential role of prenatal inflammation in the impairment of lung development following mechanical ventilation of preterm lambs. Reprod Sci 24: 478–487, 2017. doi: 10.1177/1933719116660846. [DOI] [PubMed] [Google Scholar]
- 30.Hattori Y, Kotani T, Tsuda H, Mano Y, Tu L, Li H, Hirako S, Ushida T, Imai K, Nakano T, Sato Y, Miki R, Sumigama S, Iwase A, Toyokuni S, Kikkawa F. Maternal molecular hydrogen treatment attenuates lipopolysaccharide-induced rat fetal lung injury. Free Radic Res 49: 1026–1037, 2015. doi: 10.3109/10715762.2015.1038257. [DOI] [PubMed] [Google Scholar]
- 31.Heine H, Rietschel ET, Ulmer AJ. The biology of endotoxin. Mol Biotechnol 19: 279–296, 2001. doi: 10.1385/MB:19:3:279. [DOI] [PubMed] [Google Scholar]
- 32.Holdman XB, Welte T, Rajapakshe K, Pond A, Coarfa C, Mo Q, Huang S, Hilsenbeck SG, Edwards DP, Zhang X, Rosen JM. Upregulation of EGFR signaling is correlated with tumor stroma remodeling and tumor recurrence in FGFR1-driven breast cancer. Breast Cancer Res 17: 141, 2015. doi: 10.1186/s13058-015-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13: 862–874, 2013. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou Y, Liu M, Husted C, Chen C, Thiagarajan K, Johns JL, Rao SP, Alvira CM. Activation of the nuclear factor-κB pathway during postnatal lung inflammation preserves alveolarization by suppressing macrophage inflammatory protein-2. Am J Physiol Lung Cell Mol Physiol 309: L593–L604, 2015. doi: 10.1152/ajplung.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med 192: 134–156, 2015. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 100: 145–157, 2014. doi: 10.1002/bdra.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jobe AH. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med (Lausanne) 2: 49, 2015. doi: 10.3389/fmed.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr 162: 243–249.e1, 2013. doi: 10.1016/j.jpeds.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston CJ, Holm BA, Gelein R, Finkelstein JN. Postnatal lung development: immediate-early gene responses post ozone and LPS exposure. Inhal Toxicol 18: 875–883, 2006. doi: 10.1080/08958370600822466. [DOI] [PubMed] [Google Scholar]
- 40.Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed 91: F132–F135, 2006. doi: 10.1136/adc.2004.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, Kramer BW, Newnham JP, Ikegami M, Jobe AH. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med 179: 955–961, 2009. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B; Israel Neonatal Network . Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics 125: e736–e740, 2010. doi: 10.1542/peds.2009-2017. [DOI] [PubMed] [Google Scholar]
- 43.Kranenburg AR, Willems-Widyastuti A, Mooi WJ, Saxena PR, Sterk PJ, de Boer WI, Sharma HS. Chronic obstructive pulmonary disease is associated with enhanced bronchial expression of FGF-1, FGF-2, and FGFR-1. J Pathol 206: 28–38, 2005. doi: 10.1002/path.1748. [DOI] [PubMed] [Google Scholar]
- 44.Kunzmann S, Glogger K, Been JV, Kallapur SG, Nitsos I, Moss TJ, Speer CP, Newnham JP, Jobe AH, Kramer BW. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. Am J Obstet Gynecol 202: 476.e1–476.e9, 2010. doi: 10.1016/j.ajog.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics 123: 1314–1319, 2009. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 46.Lee HJ, Choi CW, Kim BI, Kim EK, Kim HS, Choi JH, Lee MJ, Yang EG. Serial changes of lung morphology and biochemical profiles in a rat model of bronchopulmonary dysplasia induced by intra-amniotic lipopolysaccharide and postnatal hyperoxia. J Perinat Med 38: 675–681, 2010. doi: 10.1515/jpm.2010.091. [DOI] [PubMed] [Google Scholar]
- 47.MacKenzie B, Korfei M, Henneke I, Sibinska Z, Tian X, Hezel S, Dilai S, Wasnick R, Schneider B, Wilhelm J, El Agha E, Klepetko W, Seeger W, Schermuly R, Günther A, Bellusci S. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir Res 16: 83, 2015. doi: 10.1186/s12931-015-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madurga A, Mizíková I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 305: L893–L905, 2013. doi: 10.1152/ajplung.00267.2013. [DOI] [PubMed] [Google Scholar]
- 49.Maneenil G, Kemp MW, Kannan PS, Kramer BW, Saito M, Newnham JP, Jobe AH, Kallapur SG. Oral, nasal and pharyngeal exposure to lipopolysaccharide causes a fetal inflammatory response in sheep. PLoS One 10: e0119281, 2015. doi: 10.1371/journal.pone.0119281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Männel DN. Advances in sepsis research derived from animal models. Int J Med Microbiol 297: 393–400, 2007. doi: 10.1016/j.ijmm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O’Shea TM; North Carolina Neonatologists Association . Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. Pediatrics 104: 1345–1350, 1999. doi: 10.1542/peds.104.6.1345. [DOI] [PubMed] [Google Scholar]
- 52.McAdams RM, Vanderhoeven J, Beyer RP, Bammler TK, Farin FM, Liggitt HD, Kapur RP, Gravett MG, Rubens CE, Adams Waldorf KM. Choriodecidual infection downregulates angiogenesis and morphogenesis pathways in fetal lungs from Macaca nemestrina. PLoS One 7: e46863, 2012. [Erratum in PLoS One 8, 2013]. doi: 10.1371/journal.pone.0046863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc 11, Suppl 3: S146–S153, 2014. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGrath-Morrow SA, Lee S, Gibbs K, Lopez A, Collaco JM, Neptune E, Soloski MJ, Scott A, D’Alessio F. Immune response to intrapharyngeal LPS in neonatal and juvenile mice. Am J Respir Cell Mol Biol 52: 323–331, 2015. doi: 10.1165/rcmb.2014-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKenna S, Wright CJ. Inhibiting IκBβ-NFκB signaling attenuates the expression of select pro-inflammatory genes. J Cell Sci 128: 2143–2155, 2015. doi: 10.1242/jcs.168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menden H, Tate E, Hogg N, Sampath V. LPS-mediated endothelial activation in pulmonary endothelial cells: role of Nox2-dependent IKK-β phosphorylation. Am J Physiol Lung Cell Mol Physiol 304: L445–L455, 2013. doi: 10.1152/ajplung.00261.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menden H, Welak S, Cossette S, Ramchandran R, Sampath V. Lipopolysaccharide (LPS)-mediated angiopoietin-2-dependent autocrine angiogenesis is regulated by NADPH oxidase 2 (Nox2) in human pulmonary microvascular endothelial cells. J Biol Chem 290: 5449–5461, 2015. doi: 10.1074/jbc.M114.600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menden HL, Xia S, Mabry SM, Navarro A, Nyp MF, Sampath V. Nicotinamide adenine dinucleotide phosphate oxidase 2 regulates LPS-induced inflammation and alveolar remodeling in the developing lung. Am J Respir Cell Mol Biol 55: 767–778, 2016. doi: 10.1165/rcmb.2016-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Metsola J, Maksimow M, Ojaniemi M, Metsola H, Marttila-Ichihara F, Vuolteenaho R, Yegutkin GG, Salmi M, Hallman M, Jalkanen S. Postnatal development and LPS responsiveness of pulmonary adenosine receptor expression and of adenosine-metabolizing enzymes in mice. Pediatr Res 76: 515–521, 2014. doi: 10.1038/pr.2014.132. [DOI] [PubMed] [Google Scholar]
- 60.Mourani PM, Abman SH. Pulmonary hypertension and vascular abnormalities in bronchopulmonary dysplasia. Clin Perinatol 42: 839–855, 2015. doi: 10.1016/j.clp.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nardiello C, Mižíková I, Silva DM, Ruiz-Camp J, Mayer K, Vadász I, Herold S, Seeger W, Morty RE. Standardisation of oxygen exposure in the development of mouse models for bronchopulmonary dysplasia. Dis Model Mech 10: 185–196, 2017. doi: 10.1242/dmm.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel AL, Johnson TJ, Robin B, Bigger HR, Buchanan A, Christian E, Nandhan V, Shroff A, Schoeny M, Engstrom JL, Meier PP. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch Dis Child Fetal Neonatal Ed 102: F256–F261, 2017. doi: 10.1136/archdischild-2016-310898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pozarska A, Rodríguez-Castillo JA, Surate Solaligue DE, Ntokou A, Rath P, Mižíková I, Madurga A, Mayer K, Vadász I, Herold S, Ahlbrecht K, Seeger W, Morty RE. Stereological monitoring of mouse lung alveolarization from the early postnatal period to adulthood. Am J Physiol Lung Cell Mol Physiol 312: L882–L895, 2017. doi: 10.1152/ajplung.00492.2016. [DOI] [PubMed] [Google Scholar]
- 64.Pryhuber GS. Postnatal infections and immunology affecting chronic lung disease of prematurity. Clin Perinatol 42: 697–718, 2015. doi: 10.1016/j.clp.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramel SE, Brown LD, Georgieff MK. The impact of neonatal illness on nutritional requirements-one size does not fit all. Curr Pediatr Rep 2: 248–254, 2014. doi: 10.1007/s40124-014-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogers LK, Valentine CJ, Pennell M, Velten M, Britt RD, Dingess K, Zhao X, Welty SE, Tipple TE. Maternal docosahexaenoic acid supplementation decreases lung inflammation in hyperoxia-exposed newborn mice. J Nutr 141: 214–222, 2011. doi: 10.3945/jn.110.129882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rueda CM, Presicce P, Jackson CM, Miller LA, Kallapur SG, Jobe AH, Chougnet CA. Lipopolysaccharide-induced chorioamnionitis promotes IL-1-dependent inflammatory FOXP3+ CD4+ T Cells in the fetal rhesus macaque. J Immunol 196: 3706–3715, 2016. doi: 10.4049/jimmunol.1502613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shivanna B, Zhang W, Jiang W, Welty SE, Couroucli XI, Wang L, Moorthy B. Functional deficiency of aryl hydrocarbon receptor augments oxygen toxicity-induced alveolar simplification in newborn mice. Toxicol Appl Pharmacol 267: 209–217, 2013. doi: 10.1016/j.taap.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, Baley J, Singer LT. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics 112: e359, 2003. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simpson SJ, Hall GL, Wilson AC. Lung function following very preterm birth in the era of ‘new’ bronchopulmonary dysplasia. Respirology 20: 535–540, 2015. doi: 10.1111/resp.12503. [DOI] [PubMed] [Google Scholar]
- 71.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 801–810, 2016. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sucre JM, Wilkinson D, Vijayaraj P, Paul M, Dunn B, Alva-Ornelas JA, Gomperts BN. A three-dimensional human model of the fibroblast activation that accompanies bronchopulmonary dysplasia identifies Notch-mediated pathophysiology. Am J Physiol Lung Cell Mol Physiol 310: L889–L898, 2016. doi: 10.1152/ajplung.00446.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Eijl S, Mortaz E, Versluis C, Nijkamp FP, Folkerts G, Bloksma N. A low vitamin A status increases the susceptibility to cigarette smoke-induced lung emphysema in C57BL/6J mice. J Physiol Pharmacol 62: 175–182, 2011. [PubMed] [Google Scholar]
- 74.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol (1985) 108: 1347–1356, 2010. doi: 10.1152/japplphysiol.01392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 36: 782–801, 2004. doi: 10.1016/j.freeradbiomed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Hellgren G, Löfqvist C, Li W, Hellström A, Hagberg H, Mallard C. White matter damage after chronic subclinical inflammation in newborn mice. J Child Neurol 24: 1171–1178, 2009. doi: 10.1177/0883073809338068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol 37: 439–479, 2010. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Dong Z, Zhou R, Luo D, Wei H, Tian Z. Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cell Mol Immunol 2: 271–280, 2005. [PubMed] [Google Scholar]
- 79.Zhang S, Patel A, Chu C, Jiang W, Wang L, Welty SE, Moorthy B, Shivanna B. Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: mechanistic roles of antioxidant enzymes and RelB. Toxicol Appl Pharmacol 286: 92–101, 2015. doi: 10.1016/j.taap.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao H, Dennery PA, Yao H. Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 314: L544–L554, 2018. doi: 10.1152/ajplung.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci USA 105: 7528–7533, 2008. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]