Abstract
Acute airway acidification is a potent stimulus of sensory nerves and occurs commonly with gastroesophageal reflux disease, cystic fibrosis, and asthma. In infants and adults, airway acidification can acutely precipitate asthma-like symptoms, and treatment-resistant asthma can be associated with gastroesophageal reflux disease. Airway protective behaviors, such as mucus secretion and airway smooth muscle contraction, are often exaggerated in asthma. These behaviors are manifested through activation of neural circuits. In some populations, the neural response to acid might be particularly important. For example, the immune response in infants is relatively immature compared with adults. Infants also have a high frequency of gastroesophageal reflux. Thus, in the current study, we compared the transcriptomes of an airway-nervous system circuit (e.g., tracheal epithelia, nodose ganglia, and brain stem) in neonatal piglets challenged with intra-airway acid. We hypothesized that the identification of parallel changes in the transcriptomes of two neutrally connected tissues might reveal the circuit response, and, hence, molecules important for the manifestation of asthma-like features. Intra-airway acid induced airway hyperreactivity and airway obstruction in male piglets. In contrast, female piglets displayed airway obstruction without airway hyperreactivity. Pairwise comparisons revealed parallel changes in genes directly implicated in airway hyperreactivity (scn10a) in male acid-challenged piglets, whereas acid-challenged females exhibited parallel changes in genes associated with mild asthma (stat 1 and isg15). These findings reveal sex-specific responses to acute airway acidification and highlight distinct molecules within a neural circuit that might be critical for the manifestation of asthma-like symptoms in pediatric populations.
INTRODUCTION
Asthma is a common airway disease characterized by wheezing, chest tightness, cough, and variable airflow obstruction. Airway hyperreactivity (AHR) and airway obstruction are key features of asthma (11, 48). Several studies suggest that the airway becomes acidic in asthma via immune cell infiltration, inflammation, and oxidative stress (9, 29, 36, 39). Aspiration of acidic gastric contents can also acidify the airways (58). In adults, airway acidification through aspiration of gastric contents causes an acute airway injury characterized by inflammation and neutrophil accumulation (35). In infants, gastroesophageal reflux (the passage of gastric materials into the esophagus) is common and often evokes no symptoms (17, 32, 69). However, in some infants and children, gastroesophageal reflux becomes gastroesophageal reflux disease (GERD). GERD in infants can precipitate acute asthma-like symptoms (19). In children with GERD, the onset of asthma symptoms can also occur and may involve inflammation (e.g., IL-8) and neutrophilic infiltration (59). Although airway inflammation has been proposed to mediate asthma-like symptoms in infants with GERD, the data to support this statement are not apparent and appear to be extrapolated from adult studies, as well as those from children. Given that infants display “immature” inflammatory responses compared with adults (16), such extrapolations merit caution.
One proposed mediator of acid effects on airway function is airway sensory nerves (20, 25, 31, 42, 43, 57, 75). For example, using an adult guinea pig model of gastroesophageal reflux, Hamamoto et al. (27) found that esophageal acid caused release of substance P in the lung. Consistent with this, a critical role for afferent and efferent airway nerves in the manifestation of asthma features has been increasingly established (6, 55, 56, 62, 63). Specific afferent contributors include the transient receptor potential vanilloid 1 (TRPV1) (65), the acid-sensing ion channel (ASIC) 1a (55), the sodium voltage-gated α-subunit 10 (scn10a/Nav1.8) (63), and the transient receptor potential cation channel, subfamily A, member 1 (TRPA1) (10). A major efferent contributor is cholinergic transmission (56). Additional contributors include serotonergic (50), GABAergic (6, 62), and neurotrophin 4 signaling (4). Given that acid is a potent activator of airway sensory nerves (37) and that adult therapies that target inflammation are less effective in infants (67), it is possible that acid-induced asthma-like symptoms in infants might involve neural components and/or neural remodeling.
In the current study, we hypothesized that the acute airway acidification might evoke asthma-like symptoms in neonatal piglets. Further, we hypothesized that these symptoms might be accompanied by alterations in a defined airway-nervous system circuit. Given that several studies indicate that male children/infants suffer greater asthma-like symptoms compared with female children/infants (23, 68), we also hypothesized that male piglets would display greater symptoms. We predicted that the information revealed by these studies would provide key insight into molecules that might be important for asthma-like symptoms in infants and toddlers, and, thus, novel therapeutic targets.
MATERIALS AND METHODS
Animals.
A total of 44 piglets (Yorkshire Landrace breed, 2–3 days of age) were fed commercial milk replacer (Soweena Litter Life) and allowed a 36–48-h acclimation period before interventions began. The University of Florida Animal Care and Use Committee approved all procedures. Procedures were completed in accordance with federal policies and guidelines. A total of 24 piglets were used for flexiVent experiments, 12 were reserved for RNA sequencing experiments, and 8 were used to generate airway cultures.
Airway instillation.
After acclimation, piglets were anesthetized with 8% SevoThesia (Henry Schein). The piglets’ airways were accessed with a laryngoscope; a laryngotracheal atomizer (MADgic) was passed directly beyond the vocal folds, as previously described (56) to aerosolize either a 500 μl 0.9% saline control or 1% acetic acid in 0.9% saline solution to the airway. This procedure results in widespread distribution of aerosolized solutions throughout the piglet airway (13, 14), including the lung. Consistent with acid as a cough-evoking stimulus (37, 73), acetic acid induced cough in 90% or greater of the piglets.
FlexiVent.
We have previously described the procedure for flexiVent in neonatal piglets (56). Briefly, 48 h postinstillation, animals were anesthetized with ketamine (20 mg/kg) and xylazine (2.0 mg/kg) and intravenous propofol (2 mg/kg) (Henry Schein Animal Health). A tracheostomy was performed, and a cuffless endotracheal tube (Coviden, 3.5–4.0 mm OD) was placed. Piglets were connected to a flexiVent system (SCIREQ); paralytic (rocuronium bromide, Novaplus) was administered. Piglets were ventilated at 60 breaths/min at a volume of 10 ml/kg body mass. Increasing doses of methacholine were administered intravenously. Measurements for each dose were taken at 10-s intervals over the course of ~3 min. Airway resistance in response to a single frequency was assessed.
Tracheal epithelia, nodose ganglia, and brain stem isolation.
Piglets used for whole transcriptome sequencing were not subjected to flexiVent because of the potential confound that methacholine and mechanical stimulation of the airways (e.g., methacholine-induced bronchoconstriction) might impart. Forty-eight hours after instillations, piglets were euthanized with a 90 mg/kg intravenous Euthasol solution (Henry Schein). Three rings of trachea were removed just proximal to the accessory lobe. Tracheas were then cut along the posterior edge (28) and pinned to a RNase/DNase free surface. The entire epithelium was delicately peeled from the surface of the trachea using a fine-forceps and immediately placed in TRIzol for storage at −80°C. Any nerve endings innervating the epithelia, glands that lifted with the epithelia, immune cells, blood cells, or connective tissues were not removed and were included in the extraction process. Nodose ganglia were carefully dissected using methods that we previously developed (44). Ganglia were removed, placed in TRIzol, and stored at −80°C until RNA extraction. Any connective tissues, inflammatory cells, satellite cells, or blood cells covering the ganglia were not removed. A region of the brain stem encompassing the nucleus tractus solitarius, nucleus ambiguous, and dorsal motor nucleus was removed. Briefly, a blunt scissor was used to cut through the sagittal suture of the skull post mortem, and the brain was delicately excised. The cerebellum was removed, and the obex was identified. A section of the brain stem encompassing 2 mm rostral and 2 mm caudal to the obex (5) was then removed and placed in RNAlater (Thermo Fisher Scientific). The block was further trimmed to extend 2 mm in depth and 2 mm lateral from the obex, while still submerged in RNAlater.
RNA isolation and quantitative RT-PCR.
RNA from the whole trachea, tracheal epithelia, whole lung, nodose ganglia, and brain stem were isolated using RNeasy Lipid Tissue kit (Qiagen) with optional DNase digestion (Qiagen). RNA concentrations were assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific). RNA was reverse transcribed for the whole trachea and whole lung (1,000 ng) using Superscript VILO Master Mix (Thermo Fisher Scientific). Briefly, RNA and master mix were incubated for 10 min at 25°C, followed by 60 min at 42°C, followed by 5 min at 85°C. Inflammation-directed quantitative RT-PCR arrays (Qiagen, PASS-011ZF), muc5AC, muc5B, and foxj1 transcript abundance were measured as previously described (56). All quantitative RT-PCR data were acquired using fast SYBR Green master mix (Applied Biosystems) and a LightCycler 480 (Roche). Standard ΔΔCT methods were used for analysis (56).
Chemicals.
USP grade acetic acid (Fisher Scientific) was dissolved 0.9% saline to final concentration of 1% and sterilized with a 0.22-µm filter (Millex GP). Acetyl-β-methacholine-chloride (Sigma) was dissolved in 0.9% saline for flexiVent studies. The pH of the 1% acetic acid solution measured 2.6 using an Accumet AE150 pH probe (Fisher Scientific).
Bronchoalveolar lavage, ELISAs, and cell count analysis.
The caudal left lung of each piglet was excised and the main bronchus cannulated; three sequential 5-ml lavages of 0.9% sterile saline were administered, as previously described (56). The recovered material was pooled, spun at 500 g, and the supernatant was removed. Cells were counted on a hemocytometer. A porcine IL-17A ELISA (ThermoFisher; ESIL17A), IL-13 (ThermoFisher; ESIL13), TNF-α (Life Technologies; KSC3011), IL-10, (Eagle BioSciences; IL1051-K01), IL-2 (Eagle BioSciences; IL251-K01), IL-4 (Eagle BioSciences; IL451-K01), IL-1β (ThermoFisher; ESIL1B), IL-6 (ThermoFisher, ESIL6), and IL-8 (ThermoFisher, KSC0081) were performed according to the manufacturer’s instructions. ELISAs were read using a filter-based accuSkan FC microphotometer (Fisher Scientific). The limits of sensitivity are as follows: IL-17A, 14 pg/ml; IL-13, 16 pg/ml; TNF-α, <4 pg/ml; IL-4, 39 pg/ml; IL-2, 12 pg/ml; IL-10, 7 pg/ml; IL-6, 45 pg/ml; IL-1β, 6 pg/ml; IL-8, <4 pg/ml.
Histology.
Lung tissues were fixed in 10% neutral buffered formalin (~7–10 days), processed, paraffin-embedded, sectioned (~4 µm), and stained with Periodic-acid-Schiff stain (PAS) to detect glycoproteins (56). Digital images were collected with a Zeiss Axio Zoom V16 microscope. Indices of obstruction were assigned as previously described (56). Histological examination included 24 piglets that underwent flexiVent experiments and 12 piglets that did not undergo flexiVent but were used for RNA sequencing. Two lung sections per piglet were assessed. Scorers were masked to treatment.
Whole mount immunostaining of the tracheal epithelia.
Epithelial were peeled away from the trachea as described above (tissue isolation procedures). Epithelia were fixed in 4% paraformaldehyde overnight at room temperature. Epithelia were washed, permeabilized, and blocked, as previously described (54). Permeabilization and blocking were extended to 30 min and 4 h, respectively. Sections were then incubated in mouse anti-peripherin (Millipore; MAB1527) at a 1:1,000 dilution for 4 h at 37°C. Following additional washes, epithelia were incubated with a goat anti-mouse Alexa Fluor 568 secondary (ThermoFisher Scientific; A-11045) at 1:1,000 dilution for 1.5 h at room temperature. A Hoechst stain was performed using a 1:1,000 dilution (ThermoFisher Scientific). Digital images were collected with a Zeiss Axio Zoom V16 microscope. Epithelia from two control male and two control female tracheas were examined.
RNA sequencing.
RNA concentration was determined on Qubit 2.0 Fluorometer (ThermoFisher/Invitrogen), RNA quality was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies). Total RNA with 28S/18S > 1 and RNA integrity number (RIN) ≥ 7 were used for RNAseq library construction. RNAseq library were constructed using NEBNext Ultra Directional RNA library prep kit for Illumina (New England Biolabs) following the manufacturer’s recommendations. Briefly, 1,000 ng of total RNA was used for mRNA isolation using NEBNext Ploy(A) mRNA magnetic isolation module (New England Biolabs, cat. no. E7490). RNA library construction was achieved using NEBNext Ultra Directional RNA library prep kit for Illumina (New England Biolabs; cat. no. E7420). Thirty-six barcoded libraries were sized on the bioanalyzer and quantitated by QUBIT. Libraries were pooled in an equimolar ratio and sequenced by Illumina HiSeq 3000 2X100 cycles run for total of six runs (Illumina). RNA library construction was performed at the Interdisciplinary Center for Biotechnology Research (ICBR) Gene Expression & Genotyping Core, University of Florida (UF). HiSeq 3000 sequencing run was performed at the ICBR NextGen DNA Sequencing core, UF. The tracheal epithelium, nodose ganglia, and brain stem of three individual piglets for each condition were prepared separately and used.
Mapping and differential gene expression.
The quality of the RNA-Seq sequence data was first evaluated using FastQC before further downstream analysis. Low-quality sequences were removed, and the poor-quality part of the reads were trimmed using Trimmomatic. Star Aligner was used to map high-quality paired-end reads to Sus scrofa genome, Sscrofa11.1. Expression was obtained using RSEM. The expected read counts and fragments per kilobase of transcript per million-mapped reads (FPKM) were extracted for further analysis. The estimated read counts were taken as input for edgeR to perform differential expression analysis using generalized linear models. The threshold for calling significantly differentially expressed genes was set at FDR 0.05 with the average FPKM for at least one of each comparison group being higher than 0. Additional criteria using P < 0.05 were also implemented (55).
Pathway analysis.
Transcripts with P < 0.05 were examined using PANTHER (45) overrepresentation test. PANTHER GO-biological processes were reported.
Primary cultures of differentiated airway epithelia.
Epithelial cells were isolated from piglet tracheas by enzymatic digestion (Pronase, Roche; DNase, Sigma) seeded onto collagen (Corning collagen I, rat tail)-coated permeable filter supports (Corning Transwell polycarbonate membrane inserts, area = 0.33 cm2, pore size = 4 μM), and grown at the air-liquid interface using previously described procedures (34). Differentiated epithelia were studied at a minimum of 14 days after seeding.
Airway culture pH measurements.
pH measurements were achieved using methods previously described (8, 53, 60). Briefly, a pH-sensitive foil (PreSens, Regensburg, Germany) was applied directly to the surface of the tracheal culture. Optical measurement of the pH-sensitive foil was accomplished by an optical fiber (PreSens, Regensburg, Germany). Excitation and emission light transmitted and received by the optical fiber required a single-channel pH meter (pH-1 mini; PreSens). Calibration curves were constructed by using the optical fiber and pH foil to measure the pH of filter paper soaked in standard pH buffers. Measurements were taken in a humidified chamber containing 95% oxygen and 5% carbon dioxide at 37°C. When determining the amount of acid to apply, we considered the following. The volume of airway surface liquid (ASL) in vitro in a 0.33 cm2 area is expected to be 0.33 μl. This is based on the fluid excretion rate of surface epithelia, which is estimated to be 1 μl/cm2 (71). In contrast, in vivo ASL volumes are the sum of the secretion of surface epithelial cells and submucosal glands (71). Submucosal glands are estimated to secrete fluid corresponding to 10 μl/cm2 (71). On the basis of computed tomography, the surface area of the trachea and major airways in the piglet is ~14.6 cm2 (1), and the ASL volume is estimated to be the sum of the volume secreted from surface and submucosal gland secretions (1 + 10 μl per cm2) (71). Therefore, the total ASL volume in the piglet trachea and major airways is ~154 μl. Thus, to mimic aerosolization of acid or saline in vivo, we applied 1 μl of saline or 1 μl of 1% acetic acid to the apical surface of the epithelia cultures. This results in approximately the same ratio of acid to ASL volume in both conditions (1 μl to 0.33 μl vs. 500 μl to 154 μl).
Statistical analysis.
A two-way ANOVA was performed to assess basal total airway resistance, total airway resistance in response to intravenous methacholine, cytokine concentrations, airway pH, and inflammation via quantitative RT-PCR (qRT-PCR) arrays. For the qRT-PCR arrays, a false discovery rate (FDR) using the two-stage step-up method of Benjamin, Krieger, and Yekutieli was applied (77). For airway pH, time was a repeated measure, and a Sidak’s multiple-comparison test was performed post hoc. An unpaired two-tailed Student’s t-test was used to compare muc5AC, muc5B, and foxJ1 mRNA within a single sex and a single tissue. A nonparametric one-way ANOVA (Kruskal-Wallis) test followed by a nonparametric two-tailed Mann-Whitney U-test was used to examine airway obstruction. All tests were carried out using GraphPad Prism 7.0a. Statistical significance was determined as P < 0.05.
Study design.
Piglet number for airway hyperreactivity was based on previous data, indicating that a “n” of 5 was required to achieve statistical significance (56). Therefore, we planned for one additional animal per group. Data were collected across six separate experiments, with both sexes and saline and acid animals being balanced across experiments. Tissues collected for RNA sequencing were from three separate experiments, consisting of both sexes, with one saline and one acid-treated animal per sex per experiment. An n of 3 was selected on the basis of our previous data (55). Our previous work for histological scoring indicated an n of 8 was required to achieve statistical significance (56); therefore, we planned for one additional animal per group. Similarly, for qRT-PCR experiments and ELISA, our previous data indicated that an n of 5 was required (56); therefore, we planned for one additional animal per group.
RESULTS
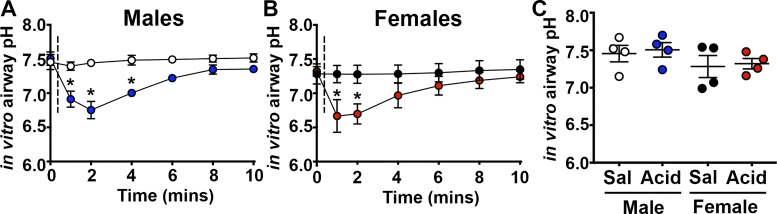
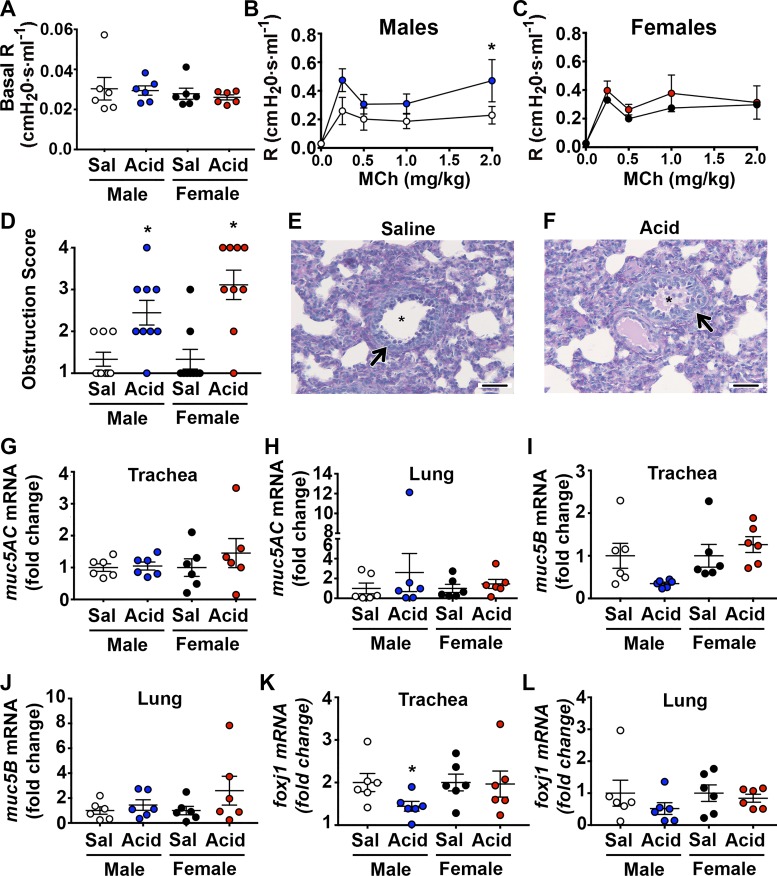
We atomized 1% acetic acid to the airways of neonatal piglets. Acid is a potent stimulus of airway afferents (37) and a proposed contributor to asthma (36). Our in vitro measurements suggested that 1% acetic acid decreases airway surface pH to ~6.6 (Fig. 1, A and B). There were no obvious differences in baseline pH measurements between treatment groups or sexes (Fig. 1C). Forty-eight hours later, we measured airway mechanics using a specialized flexiVent ventilator equipped for piglets (56). Basal airway resistance was similar among treatment groups (Fig. 2A). However, upon delivery of intravenous methacholine, male acid-challenged piglets displayed increased airway resistance compared with their saline-treated controls (Fig. 2B). Female acid-challenged piglets were unaffected (Fig. 2C). Thus, intra-airway acid induced a hallmark feature of asthma, notably, AHR, in male but not female piglets.
Fig. 1.
Rapid and acute acidification of the airway epithelia in vitro. pH time course of a 1% acetic acid/saline solution or saline solution was applied directly to cultured tracheal airway epithelia derived from male (A) or female (B) piglets. Acid or saline were applied directly after baseline measurements (indicated by dashed line), and pH was recorded within 1 min of application. C: basal pH measurements were not different among groups or sexes before the addition of saline or acid. For all groups, n = 4 cultures derived from four piglets. For A, treatment (F6,36 = 9.9; P < 0.0001), time (F1,6 = 26.2; P = 0.002), and interaction (F6,36 = 7.1; P < 0.0001). For B, treatment (F6,36 = 15.8; P < 0.0001), time (F1,6 = 2.0; P = 0.20), and interaction (F6, 36 = 12.3; P < 0.0001). For C, treatment (F1,12 = 0.16; P = 0.69) and sex (F1,12 = 2.7; P = 0.13). For A and B, *P < 0.05 compared with saline-treated controls. Sal, saline.
Fig. 2.
Intra-airway acid induces key features of asthma in neonatal piglets. A: basal airway resistance, treatment (F1,20 = 0.16; P = 0.69) and sex (F1,20 = 0.75; P = 0.89. B: maximal airway resistance in response to increasing doses of intravenous methacholine in male [treatment (F1,50 = 7.89; P = 0.0071) and dose (F4,50 = 6.11; P = 0.0004)] and female (C) acid-challenged piglets [treatment (F1,50 = 1.19; P = 0.28) and dose (F4,50 = 7.05; P = 0.0001)]. D: mean airway obstruction score is shown; Kruskal-Wallis statistic = 17.22; P = 0.006. E: representative photomicrograph of PAS-stained male saline-challenged piglet and male acid-challenged piglet lung tissue (F) The arrow highlights an airway, whereas the asterisk highlights airway lumen. Scale bar: 1,000 µm. G: muc5AC mRNA in the whole trachea. For males, t = 0.28, df = 10; P = 0.78; for females, t = 0.85, df = 10; P = 0.41. H: muc5AC mRNA in the whole lung. For males, t = 0.80, df = 10; P = 0.44; for females, t = 0.73, df = 10; P = 0.48. I: muc5B mRNA in the whole trachea. For males, t = 2.12, df = 10; P = 0.051; for females, t = 0.81, df = 10; P = 0.44. J: muc5B mRNA in the whole lung. For males, t = 0.86, df = 10; P = 0.41; for females, t = 1.32, df = 10; P = 0.21. K: foxj1 mRNA in the whole trachea. For males, t = 2.31, df = 10; P = 0.043; for females, t = 0.09, df = 10; P = 0.92. L: foxj1 mRNA in the whole lung. For males, t = 1.08, df = 10; P = 0.30; for females, t = 0.55, df = 10; P = 0.59. Data are expressed relative to sex-matched controls, and values are expressed as means ± SE. n = 6 piglets/group. R, resistance; Sal, saline.
Airway obstruction is also key feature of asthma and can contribute to AHR (48). To assess obstruction, we performed histological scoring of the intrapulmonary airways that were stained with Periodic-acid Schiff (PAS) and found increased glycoproteins in both male and female acid-challenged piglets (Fig. 2, D–F). The increased glycoproteins were not associated with increased transcript abundance of the two major secreted mucin glycoproteins, muc5AC and muc5B (64) (Fig. 2, G–J). In contrast, a trend (P = 0.0514) for decreased muc5B mRNA was observed in the trachea of male acid-challenged pigs (Fig. 1I). A tendency for decreased muc5B was consistent with literature reporting decreased muc5B in the sputum of people with asthma (41). Similarly, transcript abundance of foxJ1, a marker of goblet cell progenitors (66), revealed a decrease in the trachea of acid-challenged male piglets (Fig. 2K).
In asthma, both AHR and airway obstruction have been attributed to inflammation. However, it has been suggested that the immune system of infants is immature (16). Therefore, we investigated inflammation through several assays (Fig. 3, A–I; Supplemental Tables S1–S4 can be found online on the Journal website.). Interestingly, we noted a statistically significant main effect of sex on the percentage of granulocytes in the bronchoalveolar lavage, although there was no main effect of acid (treatment, F1,20 = 1.40; P = 0.25; sex F1,20 = 5.0; P = 0.037, Fig. 3B). Examination of several key cytokines revealed a statistically significant main effect of acid on IL-1β levels (treatment, F1,20 = 4.61; P = 0.043, Fig. 3I), but no sex effect (F1,20 = 0.91; P = 0.35, Fig. 3I). Inflammation-directed qRT-PCR arrays also revealed significantly elevated transcripts for several proinflammatory markers in the tracheas and lungs of male (see Supplemental Table S1 and S2), but not female (see Supplemental Tables S3 and S4) acid-challenged piglets. Thus, male piglets showed the greatest evidence for inflammation.
Fig. 3.
Inflammation assessment in neonatal piglets challenged with acid. A: number of cells per milliliter in bronchoalveolar lavage fluid is shown. Treatment (F1,20 = 0.038; P = 0.85) and sex (F1,20 = 1.04; P = 0.32). B: percentage (%) of cells of granulocytes is given. Treatment (F1,20 = 1.40; P = 0.25) and sex (F1,20 = 5.0; P = 0.037). C: bronchoalveolar lavage concentrations of TNF-α. Treatment (F1,20 = 1.67; P = 0.21) and sex (F1,20 = 2.52; P = 0.13). D: bronchoalveolar lavage concentrations of IL-13. Treatment (F1, 20 = 1.61; P = 0.22) and sex (F1,20 = 0.37; P = 0.55). E: bronchoalveolar lavage concentrations of IL-17A. Treatment (F1,20 = 1.29; P = 0.27) and sex (F1,20 = 0.37; P = 0.13). F: bronchoalveolar lavage concentrations of IL-2 and treatment (F1,20 = 0.89; P = 0.35) and sex (F1,20 = 2.54; P = 0.12). G: bronchoalveolar lavage concentrations of IL-10. Treatment (F1,20 = 1.14; P = 0.29) and sex (F1,20 = 1.2; P = 0.29). H: bronchoalveolar lavage concentrations of IL-4. Treatment (F1,20 = 0.032; P = 0.86) and sex (F1,20 = 4.03; P = 0.0585). I: bronchoalveolar lavage concentrations of IL-1β. Treatment (F1,20 = 4.61; P = 0.043) and sex (F1,20 = 0.91; P = 0.35). J: bronchoalveolar lavage concentrations of IL-8. Treatment (F1,20 = 1.39; P = 0.25) and sex (F1,20 = 3.58; P = 0.073). K: bronchoalveolar lavage concentrations of IL-6. Treatment (F1,20 = 3.11; P = 0.0.093) and sex (F1,20 = 3.79; P = 0.066). n = 6 piglets/group Sal, saline.
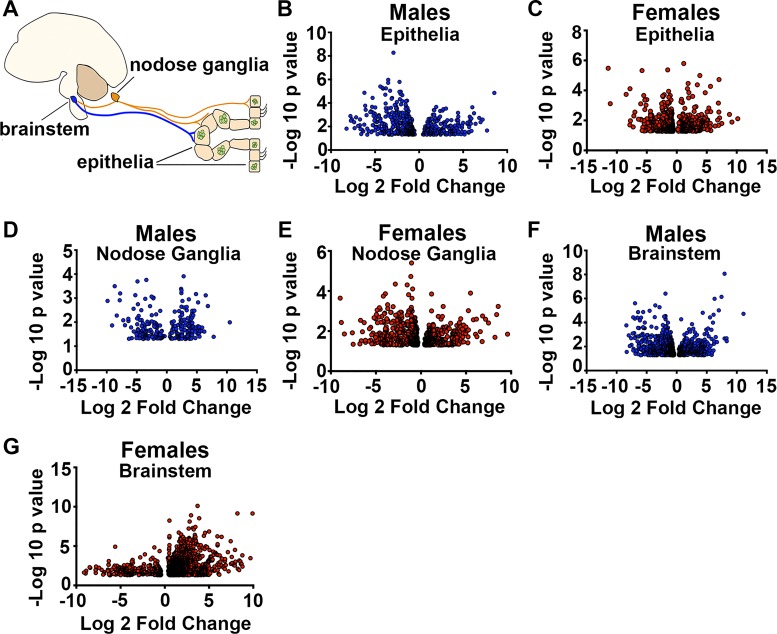
We next used whole transcriptome sequencing to probe the circuit response of the tracheal epithelia, nodose ganglia, and brain stem (Fig. 4A). With stringent criteria (e.g., a FDR of 0.05), we discovered 20 transcripts that were differentially expressed in the tracheal epithelium of male acid-challenged piglets (see Supplemental Table S5), and only five transcripts that were differentially expressed in the tracheal epithelium of female acid-challenged piglets. Using these criteria, we found no differentially expressed transcripts in the nodose ganglia of either sex. In the brain stem, we identified 26 differentially expressed transcripts in acid-challenged male piglets and 234 differentially expressed transcripts in acid-challenged female piglets. These results further supported a tissue and sex-dependent response to acute airway acidification.
Fig. 4.
Sex- and tissue-specific transcriptional responses in acid-challenged piglets. A: illustration demonstrating a simple airway-nervous system circuit. Airway sensory nerves (orange) from the nodose ganglia innervate the airway epithelia and relay information to the brain stem. Upon additional processing of that the information, the parasympathetic nervous system is engaged (blue). Volcano plots demonstrate differentially expressed transcripts using P < 0.05 criteria in acid-challenged piglets relative to sex-matched controls for the male epithelia (B), female epithelia (C), male nodose ganglia (D), female nodose ganglia (E), male brain stem (F), and female brain stem (G). n = 3 piglets each group.
In our previous work (55), loosening the criteria for differential expression by examining transcripts that were significant by P < 0.05 provided new and unexpected insight into asthma therapeutics. Therefore, we implemented a similar approach and found additional sex-dependent and tissue-dependent responses (Figs. 4, B–G and 5, A–C; see Supplemental Tables S5–S7). Shared responses (see Supplemental Table S8) and divergent responses (see Supplemental Table S9) between sexes were noted. One such transcript (chat) (see Supplemental Table S9), which encodes the enzyme responsible for synthesis of ACh (24), was increased in males, but decreased in females (see Supplemental Table S9). Notably, cholinergic signaling is a key mediator of AHR (56), and male piglets challenged with intra-airway acid displayed AHR.
Fig. 5.
Shared and unique transcriptional responses between male and female acid-challenged piglets. Venn diagram demonstrates unique and shared transcripts that increased or decreased in the epithelial transcriptome of male and female piglets (A), in the nodose ganglia transcriptome of male and female piglets (B), and in the brain stem of male and female piglets (C). Venn diagram demonstrates unique and shared biological pathways from overrepresented transcripts that increased or decreased in the epithelia of male and female piglets (D) in the nodose ganglia of male and female piglets (E), and in the brain stem of male and female piglets (G). For all panels, n = 3 piglets each group. M, male; F, female.
To gain insight into the differentially expressed transcripts, we performed PANTHER analysis (45). We found significant overrepresentation of several biological processes (Fig. 5, D–F; see Supplemental Tables S10–S16). Male acid-challenged epithelia were characterized by significant overrepresentation of cell adhesion pathways, both in transcripts that increased and decreased (see Supplemental Table S10). Female acid-challenged epithelia also displayed significant overrepresentation of cell adhesion pathways; however, only for transcripts that decreased (see Supplemental Table S11). Examination of the nodose ganglia of acid-challenged males revealed significant overrepresentation of one single pathway (muscle contraction; see Supplemental Table S12). In contrast, the most significantly overrepresented pathway among transcripts that increased in the nodose ganglia of acid-challenged females was cell differentiation (see Supplemental Table S13). The most significantly overrepresented pathways among transcripts that decreased in the nodose ganglia of acid-challenged females were those involved in cell cycle and DNA metabolic processes (see Supplemental Table S13). Finally, in the brain stem of male acid-challenged piglets, the most significantly overrepresented pathway among transcripts that increased was cholesterol metabolic process (see Supplemental Table S14), and the two most significantly overrepresented pathways among transcripts that decreased were neurological system and sensory perception (see Supplemental Table S14). By comparison, the three most significantly overrepresented pathways among transcripts that increased in the brain stems of female acid-challenged piglets were sensory perception of chemical stimulus, sensory perception of smell, and neurological system (see Supplemental Table S15). No overrepresentation of pathways among transcripts that decreased in the brain stems of female acid-challenged piglets was found (see Supplemental Table S15). These findings further reiterated sex-dependent responses to intra-airway acid and suggested that detection of acid at the level of the epithelia was partially similar and partially divergent, whereas downstream processing of acid at the level of the brain stem appeared to be partially divergent.
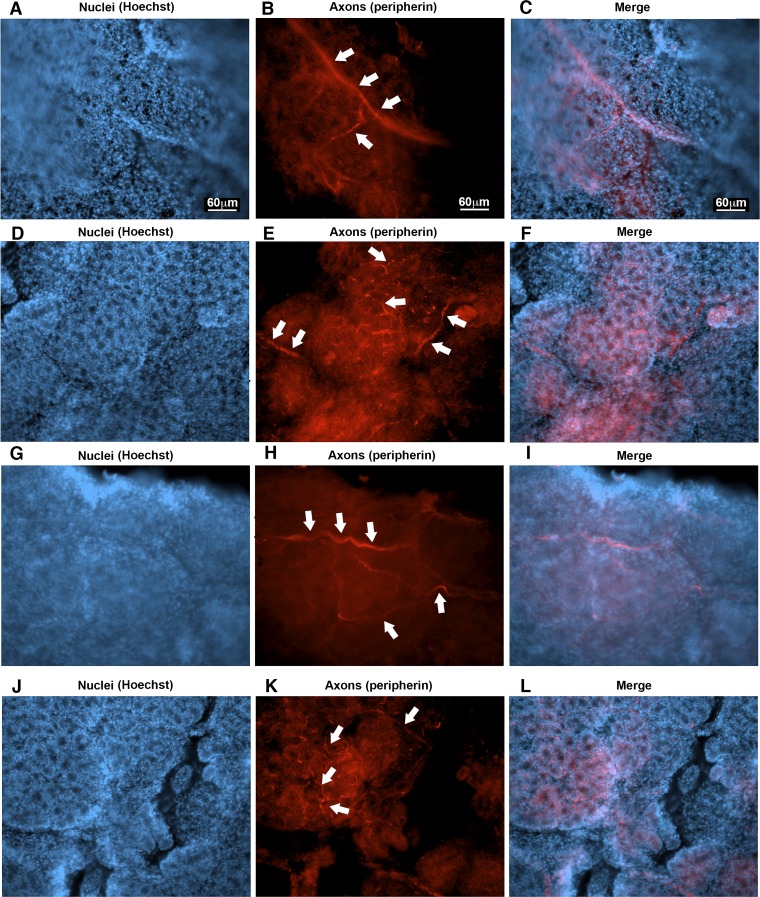
One biological process that was overrepresented in the epithelia of both male and female acid-challenged piglets that was intriguing was synaptic transmission (see Supplemental Tables S10, S11, and S16). This finding suggested that a portion of the transcripts detected in the epithelia likely represented mRNA that was axonal in origin (33), predictably due to nerves innervating the epithelia (Fig. 4A). For example, it is well known that neuronal mRNAs are transported to axons to facilitate local translation in stimulus-dependent fashion (38). In peripheral neurons, where there is often a long geographical separation between the cell body and the axon, this might be especially important (72). This means that an RNA molecule might be detected in the neuron cell body (e.g., the nucleus), as well as in the axonal projection of that neuron. Indeed, several transcripts of axon neurofilaments (nefl, nef) (76), as well as of myelinating proteins (pmp, mbp, plp, mpz) (46), were observed in the epithelia transcriptome (see Supplemental Table S5). Therefore, we peeled the epithelia from the tracheal surface similar to the RNA-sequencing studies and performed whole-mount staining. We observed several axons innervating the epithelia (Fig. 6, A–L), especially within and throughout the submucosal glands. Thus, these findings were supportive of the idea that some transcripts in the epithelial transcriptome might be axonal in origin.
Fig. 6.
Nerves in the tracheal airway epithelia in neonatal piglets. Representative photomicrographs of the tracheal epithelia demonstrating nerves (shown in red and highlighted with white arrows) in the tracheal epithelium. The serosal side is shown. n = two female control piglets (A–F) and two male control piglets (G–L). Scale bar: 60 μm and applies to all panels. The epithelial layer is thick and undulates due to the glands attached. This creates extra background and difficulty imaging.
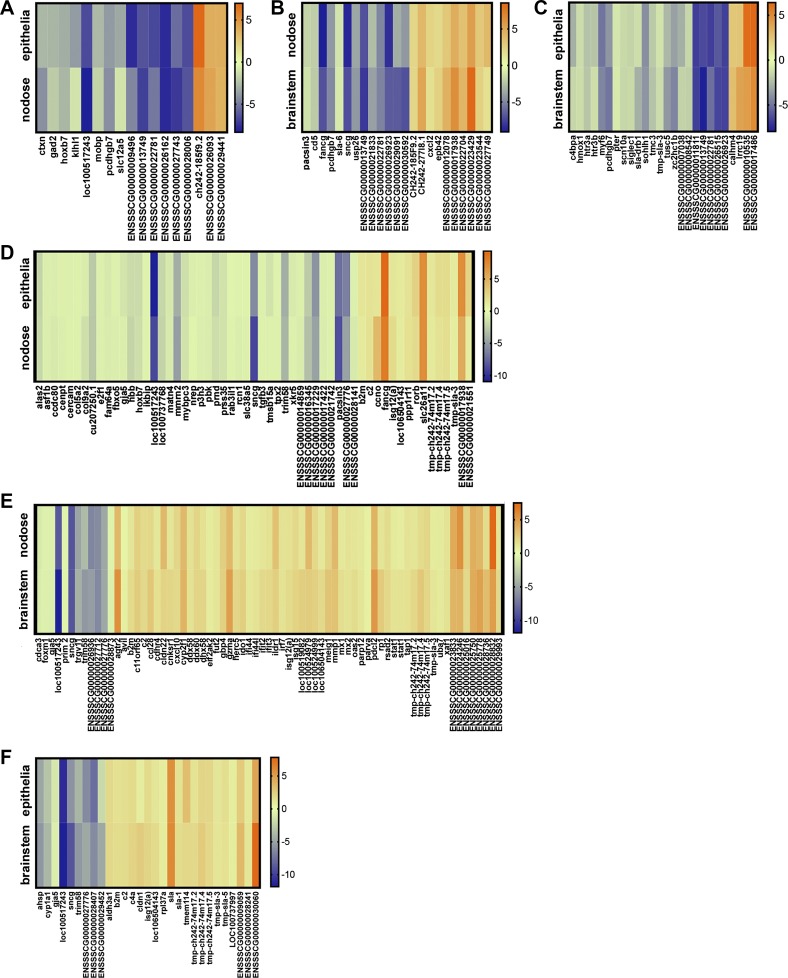
With this new insight, we reasoned that detection of parallel changes in the transcriptomes of neutrally connected tissues might reflect the response of a neuron-axon pair. Thus, identification of such transcripts might enable the discovery of specific molecules within afferent and efferent branches of the circuit. Using this approach uncovered several overlapping and parallel changes (Fig. 7, A–F). Some transcripts were of known importance either directly, or by association, in asthma. For example, gad2 decreased in parallel in the epithelia and nodose ganglia of male acid-challenged piglets (Fig. 7A). This transcript encodes glutamic acid decarboxylase, which is critical for GABA synthesis (30). Importantly, GABA induces goblet cell hyperplasia (62) and muc5AC expression (6), key components of asthma pathogenesis. Other transcripts of known asthma significance that showed parallel changes in acid-challenged male piglets included cxcl2 (2) (Fig. 7B) and scn10a (63) (Fig. 7C). Males also showed overlapping changes in transcripts implicated in neuronal excitability (slc12a5) (30) (Fig. 7A) and serotonergic pathways (hrt3a, hrt3b) (65, 70) (Fig. 7C). In contrast, acid-challenged females showed parallel changes in stat1 and isg15 (Fig. 7E), thus mimicking data obtained from airway transcriptomes of mild human asthmatics (7). Parallel changes in transcripts important for neuroprotection (isg12a) (Fig. 7, D–F) (40), as well as modulation of the asthma mediator TRPV4 (74) (pacsin3) (Fig. 7D) (15), were observed across neurally connected compartments of acid-challenged females. Therefore, pairwise comparisons enabled the identification of molecular targets of known asthma significance, as well as other molecular targets that might be of unknown or undiscovered importance.
Fig. 7.
Parallel transcriptional responses across tissue compartments in acid-challenged piglets. Heat map demonstrating transcripts that changed in both the airway epithelia and nodose ganglia of male acid-challenged piglets (A) the nodose ganglia and brain stem of male acid-challenged piglets (B), the brain stem and airway epithelia of male acid-challenged piglets (C), the airway epithelia and nodose ganglia of female acid-challenged piglets (D), the nodose ganglia and brain stem of female acid-challenged piglets (E), and the brain stem and airway epithelia of female acid-challenged piglets (F). For all panels, the scale shows log2 fold change. n = 3 piglets each group. For E, there are two stat1 molecules shown because there were two separate ensemble identifiers that changed in parallel, both matching to stat1 (ENSSSCG00000027918 and ENSSSCG00000016057).
DISCUSSION
We provide the first report describing sex-dependent manifestations of acute airway acidification in neonatal piglets. We identified unique airway physiological responses, as well as unique transcript signatures, in male and female piglets challenged with acid. Importantly, some transcripts that changed following acute airway acidification were of known asthma significance. Thus, our studies might offer new insight into early asthma pathogenesis.
Reports suggest that the frequency of asthma is higher in male children compared with female children (3, 23). For example, between the years of 0 and10, males are admitted twice as often to the hospital for asthma compared with females (61). This relationship flips around puberty into adulthood, at which time females tend to have a higher incidence of asthma (61). Interestingly, the frequency of GERD is slightly higher in male infants compared with female infants (47, 52), although female infants might have a greater number of reflux episodes. In our studies, we found that intra-airway acid induced AHR, transcriptional inflammation, and airway obstruction in male piglets, whereas females only showed airway obstruction. Consistent with a less severe asthma phenotype, females showed transcriptional responses that mimicked mild human asthmatics (7). Thus, it is possible that our findings offer new insight concerning why the males are at a higher risk for the development of childhood asthma.
Although our studies were not designed to determine why males and females respond differently, it is possible to speculate that intrinsic differences in the nervous system may account for this. For example, studies suggest that there are sex-dependent differences in the expression of pain responses that are already manifest in neonates (26). It has also been reported that the male sex is considered a risk factor for poor neurodevelopmental outcomes in premature babies and that perhaps the male brain is more susceptible to injury (51). We observed that female acid-challenged piglets had many more transcripts increase in the brain stem compared with the male piglet, and many fewer transcripts decrease in the brain stem compared with the male piglet. Again, although speculative, it is possible that some of the transcriptional changes in females were protective, thus accounting for a lack of a more severe physiological response to acid. It is also feasible that intrinsic differences in airway biology contribute to the sex-dependent responses observed. For example, it has been reported that males have a relatively smaller airway caliber per lung volume compared with females (18). Therefore, neonatal males might exhibit more severe phenotypes when compromised or obstructed. Consistent with this, male piglets challenged with acid displayed airway hyperreactivity, whereas females did not.
We also found regional differences in the male airways, with the trachea being more profoundly affected than the lungs. The most likely explanation for this is the relative heterogeneity of the lung tissue compared with the tracheal tissue. For example, when we administer acid to the airways, we deliver it through the trachea. The mist is then spread throughout the airway (13, 14). However, the probability that the trachea receives a higher and more homogeneous exposure to the acidic mist is greater compared with the lung simply because the trachea is a single tube located more proximally to the site of the initial acid administration. Conversely, the lung has many airways that are significantly smaller and more distal. Acidification of the proximal airway is also more common in GERD (22).
We found airway obstruction without corresponding increases in transcripts for the major airway mucus glycoproteins, muc5AC and muc5B. In contrast, we found a strong trend for decreased transcription of muc5B in the trachea, as well as decreased transcription of foxJ1, a marker of goblet cell progenitors (66). Previous work suggests that in neonatal piglets, muc5AC and muc5B are expressed in goblet cells, whereas muc5B (but not muc5AC), is expressed in glandular cells (49). Therefore, a decrease in muc5B could be due to a decrease in transcription at the level of the gland or the goblet cell. Because foxJ1 was also decreased, one might anticipate that muc5AC would similarly be decreased. However, we did not observe this. It is possible that muc5AC expression was upregulated in one cell population (e.g., existing goblet cells), and decreased in another (e.g., goblet cell progenitors), resulting in a net “no change” expression pattern. It is also important to note that transcription does not necessarily provide insight into secretion properties. For example, there are substantial intracellular reserves of mucins, and it is well known that hypersecretion of mucus occurs in disease states, including asthma (21). Thus, it is possible that acid-challenged piglets exhibited enhanced secretion of mucus, despite decreased or unchanged transcript levels of muc5B and muc5AC, respectively. Our ongoing studies further address this possibility.
Our studies also revealed subtle changes in inflammation at the transcriptional level in male piglet airways. It is possible that release of ACh secondary to acid-mediated activation of sensory afferents (20, 25, 31, 42, 43, 57, 75) contributed to the transcriptional inflammation. Indeed, our recent work suggests that a single cholinergic challenge in neonatal piglets induces transcriptional inflammation in the airway (56). Although our histological analyses do not suggest an overt injury response to intra-airway acid challenge, it is possible that local injury exists and contributes to inflammation. Injury could be due to the acid per se, or to mechanical injury following bronchoconstriction and/or mucus obstruction (12).
It is important to note that the transcriptome changes we observe are presumed to be due to an acidic pH in the airways. Consistent with this, acid challenge induced an acute cough response in piglets. This response is compatible with human and rodent studies, in which acid challenge is used to evoke cough (37, 73). However, there are limitations to this assumption. Specifically, we do not know the pH of the airways in vivo following acid challenge in the piglets, and data from human studies that could inform or provide an estimate of airway pH are lacking. However, on the basis of studies in rodents, as well as our in vitro data, we anticipate that the acid challenge protocol we used might be less robust than protocols used in rodents. For example, Kollarik and Undem (37) applied 500 μl of acid to a mechanically receptive airway field in the guinea pig, whereas in our studies, 500 μl of 1% acid was aerosolized to an entire piglet airway. In Kollarik studies, a modest decrease in pH leads to activation of nodose Aδ fibers and jugular c-fibers. Therefore, it is likely that similar fiber populations were activated in the piglet airway. In addition, because piglets cough in response to acid, there is a chance that some of the acid is aspirated into the esophagus. While we did not investigate this possibility, it is conceivable that some of the transcript changes observed in the nodose ganglia and brain stem were due to aspiration of acid into the esophagus.
We found several neural transcripts in the epithelial transcriptome. It is possible that some of the changes in the neurally relevant transcripts in the epithelial cell transcriptome were due to pulmonary neuroendocrine cells (62). However, given that some transcripts for myelinating proteins were differentially expressed suggests that at least a portion of the epithelial transcriptome contained mRNA from innervating nerves. Consistent with that, we observed numerous nerves throughout the airway epithelia when the epithelial layer was “peeled” off. Undoubtedly, finding parallel changes in transcripts between the epithelial transcriptome and the nodose and brain stem transcriptomes suggests common mediators across tissue compartments, independent of origin. This is additionally important to consider, given that axonal contamination of the epithelia might necessitate common expression patterns that do not necessarily represent neuron-axon pairs.
In summary, our data offer the first molecular map delineating probable targets within a defined airway-nervous system circuit following acute airway acidification. Although they provide limited insight into the mechanism, given the largely observational nature of the transcriptome data and inherent limitation of the computational biology applied, these studies should form the basis for more focused hypothesis testing. Thus, we surmise that these findings will advance our understanding of the physiological and biological outcomes of neonatal airway exposure to acid. This might be particularly relevant for the identification of new therapeutics that could mitigate asthma-like symptoms in a vulnerable and often understudied population.
GRANTS
This work was funded by NIH grants R00HL119560-03 (to principal investigator, L. R. Reznikov) and 10T2TR001983-01 (coinvestigator, L. R. Reznikov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.R.R. conceived and designed research; L.R.R., Y.-S.J.L., K.M.D., S.-P.K., J.S.D., E.N.C., M.V.G., and K.V. performed experiments; L.R.R., T.G., and K.R.A. analyzed data; L.R.R., T.G., and K.R.A. interpreted results of experiments; L.R.R. prepared figures; L.R.R. and T.G. drafted manuscript; L.R.R., Y.-S.J.L., T.G., K.M.D., S.-P.K., K.R.A., J.S.D., E.N.C., M.V.G., and K.V. edited and revised manuscript; L.R.R., Y.-S.J.L., T.G., K.M.D., S.-P.K., K.R.A., J.S.D., E.N.C., M.V.G., and K.V. approved final version of manuscript.
Supplemental Data
List of transcripts queried through inflammatory-directed PCR arrays in male piglet tracheal tissues - .docx (27 KB)
List of transcripts queried through inflammatory-directed PCR arrays in male piglet lung tissues - .docx (34 KB)
List of transcripts queried through inflammatory-directed PCR arrays in female piglet tracheal tissues - .docx (33 KB)
List of transcripts queried through inflammatory-directed PCR arrays in female piglet lung tissues - .docx (33 KB)
Transcripts differentially expressed in epithelia of male and female acid-challenged piglets - .xls (244 KB)
Transcripts differentially expressed in nodose ganglia of male and female acid-challenged piglets - .xls (198 KB)
Transcripts differentially expressed in brainstem of male and female acid-challenged piglets - .xls (449 KB)
Shared transcriptional responses of male and female acid-challenged piglets in the epithelia, nodose ganglia, and brainstem - .xls (37 KB)
Transcripts showing opposite regulation in the epithelia, nodose ganglia, and brainstem of male and female acid-challenged piglets - .xls (40 KB)
Biological processes overrepresented in epithelia of male acid-challenged piglets - .xls (57 KB)
Biological processes overrepresented in epithelia of female acid-challenged piglets - .xls (84 KB)
Biological processes overrepresented in nodose ganglia of male acid-challenged piglets - .xls (28 KB)
Biological processes overrepresented in nodose ganglia of female acid-challenged piglets - .xls (57 KB)
Biological processes overrepresented in brainstem of male acid-challenged piglets - .xls (37 KB)
Biological processes overrepresented in brainstem of female acid-challenged piglets - .xls (123 KB)
Shared biological pathways among decreased epithelial trascripts in male and female acid-challenged piglets. - .xls (27 KB)
ACKNOWLEDGMENTS
The authors thank Dr. Don Bolser, Dr. Mahmoud Abou Alaiwa, and Dr. Linda Hayward for helpful comments and suggestions in the preparation of this manuscript. The authors also thank Dr. Igancio Aguirre for providing technical support and resources.
All data are available in the article or in the supplemental material, which is available online at the Journal website.
REFERENCES
- 1.Adam RJ, Michalski AS, Bauer C, Abou Alaiwa MH, Gross TJ, Awadalla MS, Bouzek DC, Gansemer ND, Taft PJ, Hoegger MJ, Diwakar A, Ochs M, Reinhardt JM, Hoffman EA, Beichel RR, Meyerholz DK, Stoltz DA. Air trapping and airflow obstruction in newborn cystic fibrosis piglets. Am J Respir Crit Care Med 188: 1434–1441, 2013. doi: 10.1164/rccm.201307-1268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Alwan LA, Chang Y, Mogas A, Halayko AJ, Baglole CJ, Martin JG, Rousseau S, Eidelman DH, Hamid Q. Differential roles of CXCL2 and CXCL3 and their receptors in regulating normal and asthmatic airway smooth muscle cell migration. J Immunol 191: 2731–2741, 2013. doi: 10.4049/jimmunol.1203421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy 63: 47–57, 2008. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 4.Aven L, Paez-Cortez J, Achey R, Krishnan R, Ram-Mohan S, Cruikshank WW, Fine A, Ai X. An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity. FASEB J 28: 897–907, 2014. doi: 10.1096/fj.13-238212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes KL, Ferrario CM, Conomy JP. Comparison of the hemodynamic changes produced by electrical stimulation of the area postrema and nucleus tractus solitarii in the dog. Circ Res 45: 136–143, 1979. doi: 10.1161/01.RES.45.1.136. [DOI] [PubMed] [Google Scholar]
- 6.Barrios J, Patel KR, Aven L, Achey R, Minns MS, Lee Y, Trinkaus-Randall VE, Ai X. Early life allergen-induced mucus overproduction requires augmented neural stimulation of pulmonary neuroendocrine cell secretion. FASEB J 31: 4117–4128, 2017. doi: 10.1096/fj.201700115R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhakta NR, Christenson SA, Nerella S, Solberg OD, Nguyen CP, Choy DF, Jung KL, Garudadri S, Bonser LR, Pollack JL, Zlock LT, Erle DJ, Langelier C, Derisi JL, Arron JR, Fahy JV, Woodruff PG. IFN-stimulated gene expression, type 2 inflammation, and endoplasmic reticulum stress in asthma. Am J Respir Crit Care Med 197: 313–324, 2018. doi: 10.1164/rccm.201706-1070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blossfeld S, Gansert D. A novel non-invasive optical method for quantitative visualization of pH dynamics in the rhizosphere of plants. Plant Cell Environ 30: 176–186, 2007. doi: 10.1111/j.1365-3040.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 9.Brunetti L, Francavilla R, Tesse R, Fiermonte P, Fiore FP, Loré M, Margiotta M, Armenio L. Exhaled breath condensate cytokines and pH in pediatric asthma and atopic dermatitis. Allergy Asthma Proc 29: 461–467, 2008. doi: 10.2500/aap.2008.29.3152. [DOI] [PubMed] [Google Scholar]
- 10.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA 106: 9099–9104, 2009. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman DG, Irvin CG. Mechanisms of airway hyper-responsiveness in asthma: the past, present and yet to come. Clin Exp Allergy 45: 706–719, 2015. doi: 10.1111/cea.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu EK, Cheng J, Foley JS, Mecham BH, Owen CA, Haley KJ, Mariani TJ, Kohane IS, Tschumperlin DJ, Drazen JM. Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells. Am J Respir Cell Mol Biol 35: 628–638, 2006. doi: 10.1165/rcmb.2006-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooney AL, Abou Alaiwa MH, Shah VS, Bouzek DC, Stroik MR, Powers LS, Gansemer ND, Meyerholz DK, Welsh MJ, Stoltz DA, Sinn PL, McCray PB Jr. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight 1: e88730, 2016. doi: 10.1172/jci.insight.88730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooney AL, Singh BK, Loza LM, Thornell IM, Hippee CE, Powers LS, Ostedgaard LS, Meyerholz DK, Wohlford-Lenane C, Stoltz DA, B McCray P Jr, Sinn PL. Widespread airway distribution and short-term phenotypic correction of cystic fibrosis pigs following aerosol delivery of piggyBac/adenovirus. Nucleic Acids Res 46: 9591–9600, 2018. doi: 10.1093/nar/gky773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuajungco MP, Grimm C, Oshima K, D’hoedt D, Nilius B, Mensenkamp AR, Bindels RJ, Plomann M, Heller S. PACSINs bind to the TRPV4 cation channel. PACSIN 3 modulates the subcellular localization of TRPV4. J Biol Chem 281: 18753–18762, 2006. doi: 10.1074/jbc.M602452200. [DOI] [PubMed] [Google Scholar]
- 16.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol 30: 105–112, 2013. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czinn SJ, Blanchard S. Gastroesophageal reflux disease in neonates and infants: when and how to treat. Paediatr Drugs 15: 19–27, 2013. doi: 10.1007/s40272-012-0004-2. [DOI] [PubMed] [Google Scholar]
- 18.Dezateux C, Stocks J. Lung development and early origins of childhood respiratory illness. Br Med Bull 53: 40–57, 1997. doi: 10.1093/oxfordjournals.bmb.a011605. [DOI] [PubMed] [Google Scholar]
- 19.Eid NS, Shepherd RW, Thomson MA. Persistent wheezing and gastroesophageal reflux in infants. Pediatr Pulmonol 18: 39–44, 1994. doi: 10.1002/ppul.1950180110. [DOI] [PubMed] [Google Scholar]
- 20.El-Hashim AZ, Amine SA. The role of substance P and bradykinin in the cough reflex and bronchoconstriction in guinea-pigs. Eur J Pharmacol 513: 125–133, 2005. doi: 10.1016/j.ejphar.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 15: 4–11, 2009. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaude GS. Pulmonary manifestations of gastroesophageal reflux disease. Ann Thorac Med 4: 115–123, 2009. doi: 10.4103/1817-1737.53347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geier DA, Kern JK, Geier MR. Demographic and neonatal risk factors for childhood asthma in the USA. J Matern Fetal Neonatal Med 1–5, 2017. doi: 10.1080/14767058.2017.1393068. [DOI] [PubMed] [Google Scholar]
- 24.Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7: 73, 2006. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu Q, Lee LY. Airway irritation and cough evoked by acid: from human to ion channel. Curr Opin Pharmacol 11: 238–247, 2011. doi: 10.1016/j.coph.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guinsburg R, de Araújo Peres C, Branco de Almeida MF, de Cássia Xavier Balda R, Cássia Berenguel R, Tonelotto J, Kopelman BI. Differences in pain expression between male and female newborn infants. Pain 85: 127–133, 2000. doi: 10.1016/S0304-3959(99)00258-4. [DOI] [PubMed] [Google Scholar]
- 27.Hamamoto J, Kohrogi H, Kawano O, Iwagoe H, Fujii K, Hirata N, Ando M. Esophageal stimulation by hydrochloric acid causes neurogenic inflammation in the airways in guinea pigs. J Appl Physiol (1985) 82: 738–745, 1997. doi: 10.1152/jappl.1997.82.3.738. [DOI] [PubMed] [Google Scholar]
- 28.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA, Welsh MJ. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345: 818–822, 2014. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 161: 694–699, 2000. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 30.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci 31: 11088–11095, 2011. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta 1772: 915–927, 2007. doi: 10.1016/j.bbadis.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Jung AD. Gastroesophageal reflux in infants and children. Am Fam Physician 64: 1853–1860, 2001. [PubMed] [Google Scholar]
- 33.Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci 13: 308–324, 2012. [Errata in Nat Rev Neurosci 13: 445, 2012 and Nat Rev Neurosci 13: 597, 2012]. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188: 115–137, 2002. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy TP, Johnson KJ, Kunkel RG, Ward PA, Knight PR, Finch JS. Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesth Analg 69: 87–92, 1989. doi: 10.1213/00000539-198907000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Kodric M, Shah AN, Fabbri LM, Confalonieri M. An investigation of airway acidification in asthma using induced sputum: a study of feasibility and correlation. Am J Respir Crit Care Med 175: 905–910, 2007. doi: 10.1164/rccm.200607-940OC. [DOI] [PubMed] [Google Scholar]
- 37.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543: 591–600, 2002. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korsak LI, Mitchell ME, Shepard KA, Akins MR. Regulation of neuronal gene expression by local axonal translation. Curr Genet Med Rep 4: 16–25, 2016. doi: 10.1007/s40142-016-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med 165: 1364–1370, 2002. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 40.Labrada L, Liang XH, Zheng W, Johnston C, Levine B. Age-dependent resistance to lethal alphavirus encephalitis in mice: analysis of gene expression in the central nervous system and identification of a novel interferon-inducible protective gene, mouse ISG12. J Virol 76: 11688–11703, 2002. doi: 10.1128/JVI.76.22.11688-11703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD, Fahy JV. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med 194: 1296–1299, 2016. doi: 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee LY, Gu Q, Xu F, Hong JL. Acid-sensing by airway afferent nerves. Pulm Pharmacol Ther 26: 491–497, 2013. doi: 10.1016/j.pupt.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopes FD, Alvarenga GS, Quiles R, Dorna MB, Vieira JE, Dolhnikoff M, Martins MA. Pulmonary responses to tracheal or esophageal acidification in guinea pigs with airway inflammation. J Appl Physiol (1985) 93: 842–847, 2002. doi: 10.1152/japplphysiol.00013.2002. [DOI] [PubMed] [Google Scholar]
- 44.Meyerholz DK, Reznikov LR. Simple and reproducible approaches for the collection of select porcine ganglia. J Neurosci Methods 289: 93–98, 2017. doi: 10.1016/j.jneumeth.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res 45, D1: D183–D189, 2017. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittendorf KF, Marinko JT, Hampton CM, Ke Z, Hadziselimovic A, Schlebach JP, Law CL, Li J, Wright ER, Sanders CR, Ohi MD. Peripheral myelin protein 22 alters membrane architecture. Sci Adv 3: e1700220, 2017. doi: 10.1126/sciadv.1700220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazer D, Thomas R, Tolia V. Ethnicity and gender-related differences in extended intraesophageal pH monitoring parameters in infants: a retrospective study. BMC Pediatr 5: 24, 2005. doi: 10.1186/1471-2431-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ordoñez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 163: 517–523, 2001. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 49.Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, Powers LS, Gansemer ND, Bouzek DC, Cook DP, Meyerholz DK, Abou Alaiwa MH, Stoltz DA, Welsh MJ. Gel-forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci USA 114: 6842–6847, 2017. doi: 10.1073/pnas.1703228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel KR, Aven L, Shao F, Krishnamoorthy N, Duvall MG, Levy BD, Ai X. Mast cell-derived neurotrophin 4 mediates allergen-induced airway hyperinnervation in early life. Mucosal Immunol 9: 1466–1476, 2016. doi: 10.1038/mi.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res 71: 305–310, 2012. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 52.Peeters S, Vandenplas Y. Sex ratio of gastroesophageal reflux in infancy. J Pediatr Gastroenterol Nutr 13: 314, 1991. doi: 10.1097/00005176-199110000-00015. [DOI] [PubMed] [Google Scholar]
- 53.Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Bánfi B, Horswill AR, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reznikov LR, Dong Q, Chen JH, Moninger TO, Park JM, Zhang Y, Du J, Hildebrand MS, Smith RJ, Randak CO, Stoltz DA, Welsh MJ. CFTR-deficient pigs display peripheral nervous system defects at birth. Proc Natl Acad Sci USA 110: 3083–3088, 2013. doi: 10.1073/pnas.1222729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reznikov LR, Meyerholz DK, Abou Alaiwa M, Kuan SP, Liao YJ, Bormann NL, Bair TB, Price M, Stoltz DA, Welsh MJ. The vagal ganglia transcriptome identifies candidate therapeutics for airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol 315: L133–L148, 2018. doi: 10.1152/ajplung.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reznikov LR, Meyerholz DK, Kuan SP, Guevara MV, Atanasova KR, Abou Alaiwa MH. Solitary cholinergic stimulation induces airway hyperreactivity and transcription of distinct pro-inflammatory pathways. Hai 196: 219–229, 2018. doi: 10.1007/s00408-018-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricciardolo FL. Mechanisms of citric acid-induced bronchoconstriction. Am J Med 111, Suppl 8A: 18S–24S, 2001. doi: 10.1016/S0002-9343(01)00816-6. [DOI] [PubMed] [Google Scholar]
- 58.Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol 113: 610–619, 2004. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 59.Sacco O, Silvestri M, Sabatini F, Sale R, Moscato G, Pignatti P, Mattioli G, Rossi GA. IL-8 and airway neutrophilia in children with gastroesophageal reflux and asthma-like symptoms. Respir Med 100: 307–315, 2006. doi: 10.1016/j.rmed.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, Karp PH, Wohlford-Lenane CL, Heilmann KP, Leidinger MR, Allen PD, Zabner J, McCray PB Jr, Ostedgaard LS, Stoltz DA, Randak CO, Welsh MJ. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351: 503–507, 2016. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA 268: 3437–3440, 1992. doi: 10.1001/jama.1992.03490240045034. [DOI] [PubMed] [Google Scholar]
- 62.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, Deutsch G, Sun X. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360: eaan8546, 2018. doi: 10.1126/science.aan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, Laedermann C, Foster SL, Tran JV, Lai N, Chiu IM, Ghasemlou N, DiBiase M, Roberson D, Von Hehn C, Agac B, Haworth O, Seki H, Penninger JM, Kuchroo VK, Bean BP, Levy BD, Woolf CJ. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87: 341–354, 2015. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 70: 459–486, 2008. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 65.Tränkner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci USA 111: 11515–11520, 2014. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turner J, Roger J, Fitau J, Combe D, Giddings J, Heeke GV, Jones CE. Goblet cells are derived from a FOXJ1-expressing progenitor in a human airway epithelium. Am J Respir Cell Mol Biol 44: 276–284, 2011. doi: 10.1165/rcmb.2009-0304OC. [DOI] [PubMed] [Google Scholar]
- 67.van Aalderen WM. Childhood asthma: diagnosis and treatment. Scientifica (Cairo) 2012: 674204, 2012. doi: 10.6064/2012/674204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Merode T, Maas T, Twellaar M, Kester A, van Schayck CP. Gender-specific differences in the prevention of asthma-like symptoms in high-risk infants. Pediatr Allergy Immunol 18: 196–200, 2007. doi: 10.1111/j.1399-3038.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 69.Vandenplas Y, Goyvaerts H, Helven R, Sacre L. Gastroesophageal reflux, as measured by 24-hour pH monitoring, in 509 healthy infants screened for risk of sudden infant death syndrome. Pediatrics 88: 834–840, 1991. [PubMed] [Google Scholar]
- 70.Weigand LA, Myers AC, Meeker S, Undem BJ. Mast cell-cholinergic nerve interaction in mouse airways. J Physiol 587: 3355–3362, 2009. doi: 10.1113/jphysiol.2009.173054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Widdicombe JH. Regulation of the depth and composition of airway surface liquid. J Anat 201: 313–318, 2002. doi: 10.1046/j.1469-7580.2002.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willis DE, Twiss JL. Profiling axonal mRNA transport. Methods Mol Biol 714: 335–352, 2011. doi: 10.1007/978-1-61779-005-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong CH, Matai R, Morice AH. Cough induced by low pH. Respir Med 93: 58–61, 1999. doi: 10.1016/S0954-6111(99)90078-1. [DOI] [PubMed] [Google Scholar]
- 74.Wortley MA, Birrell MA, Belvisi MG. Drugs affecting TRP channels. Handb Exp Pharmacol 237: 213–241, 2017. doi: 10.1007/164_2016_63. [DOI] [PubMed] [Google Scholar]
- 75.Yasumitsu R, Hirayama Y, Imai T, Miyayasu K, Hiroi J. Effects of specific tachykinin receptor antagonists on citric acid-induced cough and bronchoconstriction in unanesthetized guinea pigs. Eur J Pharmacol 300: 215–219, 1996. doi: 10.1016/0014-2999(95)00881-0. [DOI] [PubMed] [Google Scholar]
- 76.Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments at a glance. J Cell Sci 125: 3257–3263, 2012. doi: 10.1242/jcs.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zehetmayer S, Posch M.. False discovery rate control in two-stage designs. BMC Bioinformatics 13: 81, 2012. doi: 10.1186/1471-2105-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of transcripts queried through inflammatory-directed PCR arrays in male piglet tracheal tissues - .docx (27 KB)
List of transcripts queried through inflammatory-directed PCR arrays in male piglet lung tissues - .docx (34 KB)
List of transcripts queried through inflammatory-directed PCR arrays in female piglet tracheal tissues - .docx (33 KB)
List of transcripts queried through inflammatory-directed PCR arrays in female piglet lung tissues - .docx (33 KB)
Transcripts differentially expressed in epithelia of male and female acid-challenged piglets - .xls (244 KB)
Transcripts differentially expressed in nodose ganglia of male and female acid-challenged piglets - .xls (198 KB)
Transcripts differentially expressed in brainstem of male and female acid-challenged piglets - .xls (449 KB)
Shared transcriptional responses of male and female acid-challenged piglets in the epithelia, nodose ganglia, and brainstem - .xls (37 KB)
Transcripts showing opposite regulation in the epithelia, nodose ganglia, and brainstem of male and female acid-challenged piglets - .xls (40 KB)
Biological processes overrepresented in epithelia of male acid-challenged piglets - .xls (57 KB)
Biological processes overrepresented in epithelia of female acid-challenged piglets - .xls (84 KB)
Biological processes overrepresented in nodose ganglia of male acid-challenged piglets - .xls (28 KB)
Biological processes overrepresented in nodose ganglia of female acid-challenged piglets - .xls (57 KB)
Biological processes overrepresented in brainstem of male acid-challenged piglets - .xls (37 KB)
Biological processes overrepresented in brainstem of female acid-challenged piglets - .xls (123 KB)
Shared biological pathways among decreased epithelial trascripts in male and female acid-challenged piglets. - .xls (27 KB)