Abstract
Premature male neonates are at a greater risk of developing bronchopulmonary dysplasia (BPD). The reasons underlying sexually dimorphic outcomes in premature neonates are not known. The role of miRNAs in mediating sex biases in BPD is understudied. Analysis of the pulmonary transcriptome revealed that a large percentage of angiogenesis-related differentially expressed genes are miR-30a targets. We tested the hypothesis that there is differential expression of miR-30a in vivo and in vitro in neonatal human pulmonary microvascular endothelial cells (HPMECs) upon exposure to hyperoxia. Neonatal male and female mice (C57BL/6) were exposed to hyperoxia [95% fraction of inspired oxygen (FiO2), postnatal day (PND) 1–5] and euthanized on PND 7 and 21. HPMECs (18–24-wk gestation donors) were subjected to hyperoxia (95% O2 and 5% CO2) or normoxia (air and 5% CO2) up to 72 h. miR-30a expression was increased in both males and females in the acute phase (PND 7) after hyperoxia exposure. However, at PND 21 (recovery phase), female mice showed significantly higher miR-30a expression in the lungs compared with male mice. Female HPMECs showed greater expression of miR-30a in vitro upon exposure to hyperoxia. Delta-like ligand 4 (Dll4) was an miR-30a target in HPMECs and showed sex-specific differential expression. miR-30a increased angiogenic sprouting in vitro in female HPMECs. Lastly, we show decreased expression of miR-30a and increased expression of DLL4 in human BPD lung samples compared with controls. These results support the hypothesis that miR-30a could, in part, contribute to the sex-specific molecular mechanisms in play that lead to the sexual dimorphism in BPD.
Keywords: angiogenesis, BPD, Dll4, hyperoxia, miR-30a, sex
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is a cause of significant morbidity in premature neonates (54). With increasing survival of extremely premature neonates, the incidence of this disease has plateaued (38). Though prematurity is the main causative factor, many other postnatal factors such as exposure to hyperoxia contribute to the pathogenesis of this disease (25, 51). Arrest in alveolarization and abnormal pulmonary vascular development are hallmarks of this disease (1). Neonatal mice exposed to postnatal hyperoxia develop features similar to human BPD (43, 59). Adult mice exposed to hyperoxia between postnatal days (PNDs) 0 and 4 develop pulmonary hypertension and display capillary rarefaction and cardiac failure (74).
Premature male neonates are at a higher risk of developing BPD (6, 11, 27, 45, 76) compared with females. The underlying molecular mechanisms behind the sexual dimorphism are not known (59). Our published data show that after exposure of male and female neonatal mice to hyperoxia [95% fraction of inspired oxygen (FiO2), PND 1–5, saccular stage of lung development] male mice had greater arrest in alveolarization and angiogenesis (32). This was accompanied by a significant decrease in the expression of proangiogenic genes (Pecam1 and Vegfr2) in males compared with females. We recently used RNA sequencing (RNA-Seq) to profile the pulmonary transcriptome during lung development in a mouse model of BPD (10). We exposed neonatal male and female mice (C57BL/6) to hyperoxia (95% FiO2, PND 1–5, saccular stage of lung development) or room air and euthanized on PND 7 (immediately after hyperoxia exposure) or PND 21 (recovery phase; alveolar stage of lung development). Analysis of the pulmonary transcriptome in male and female mice revealed angiogenesis as one of the crucial differentially modulated pathways.
microRNAs (miRs) are small noncoding RNAs involved in the posttranscriptional regulation of protein-coding genes mainly by decreasing mRNA stability of target genes. Males and females share a similar repertoire of genes, yet show sexual dimorphism in many diseases, which could be mediated though changes in gene expression by miRNAs. Sex-biased expression of microRNAs has been observed both in invertebrates and higher organisms (53). miRs can thus play a critical role in the sex-specific differences (40). Sex-based differential miRNA expression may be driven through sex hormones or through differential regulation by sex chromosome genes (53). miRNAs located on the X chromosome may also explain sexual dimorphism seen in some diseases (48). Studies have reported on the role of several putative miRNA targets in lung development and BPD (4, 13, 15, 46, 49, 52, 67, 71, 73, 75). However, sex-specific expression of these miRNAs has not been shown in these studies. The mechanistic role of miRNAs in mediating sex biases in BPD is thus understudied.
The proangiogenic role of miR-30 has been reported in previous studies (7, 56) (24). miR-30a stimulates arteriolar branching by downregulating Dll4 (delta-like ligand 4) expression, thereby controlling endothelial cell behavior (24, 35). In clinical studies, miR-30a was downregulated in preterm infants with BPD (70). These studies thus suggest that decreased miR-30a expression may be associated with compromised lung development in neonates. During miRNA biogenesis, the pre-miRNA is cleaved by Dicer in the cytoplasm to yield the miRNA/miRNA duplex (28); one of these strands gives rise to the mature miRNA either from the 5′ or the 3′ arm of the duplex and is denoted with a -5p or -3p suffix, respectively. One of the strands becomes functional, whereas the other arm is typically degraded. However, recent studies have shown that both the -3p and -5p strands can have distinct mRNA targets and biological function depending on the cell type (39) and therefore, it may be relevant to study both the -3p and -5p strand expression.
Dll4, an miR-30a target, encodes a transmembrane ligand for the Notch family of cell surface receptors and is largely restricted to the vascular endothelium, suggesting Dll4 is a key ligand for Notch receptors in the developing vasculature. Haploinsufficiency of dll4 results in embryonic lethality because of major defects in arterial and vascular development (18). However, DLL4 can act as a negative regulator of sprouting angiogenesis both during normal development and in pathological states (35). Dll4-Notch1 signaling during sprouting angiogenesis restricts endothelial tip-cell formation in response to VEGF (20). Dll4 expression in endothelial tip cells activates Notch signaling and suppresses sprouting in adjacent endothelial cells (3). Dll4/Notch1 signaling is thus very tightly regulated for an appropriate ratio between tip and stalk cells leading to proper angiogenesis (20).
In this investigation, we tested the hypothesis that there is sex-specific differential expression of miR-30a and subsequently of its target DLL4 in vivo in a murine model of BPD, in vitro in neonatal human pulmonary vascular endothelial cells upon exposure to hyperoxia, and in human BPD lung samples. We also tested the hypothesis that miR-30a inhibits DLL4 expression in pulmonary microvascular endothelial cells
METHODS
Animals.
The Institutional Animal Care and Use Committee of Baylor College of Medicine (protocol no. AN-6474) provided the approval for this study. All experiments were performed in accordance with relevant guidelines and regulations. Care of animals in research met the highest contemporary standards as per the 8th edition of the guide for the care and use of laboratory animals and other Institutional Animal Care and Use Committee protocols. Timed pregnant C57BL/6J wild-type (WT) mice were obtained from Charles River Laboratories (Wilmington, MA). The sex in neonatal mouse pups was determined by both the anogenital distance and pigmentation in the anogenital region method (68) and with PCR analysis for the Sry gene as described before (32).
Mouse model of BPD.
Mouse pups from multiple litters were pooled before being randomly and equally redistributed to 2 groups, with 1 group exposed to normoxia (21% O2) and the other group exposed to hyperoxia (95% O2), within 12 h of birth for 5 days. Animals at this stage of development were chosen because neonatal mice are at the saccular stage of lung development during this period, which is equivalent to 26–36 wk in human neonates. The dams were rotated between air- and hyperoxia-exposed litters every 24 h to prevent oxygen toxicity in the dams and to eliminate maternal effects between the groups. Oxygen exposure was conducted in plexiglass chambers (55 × 40 × 50 cm), into which O2 was delivered through an oxygen blender to achieve a constant level of 95% O2. Soda lime was used to remove excess CO2. Mice were euthanized on PND 7 and PND 21 (after recovery in room air) as most of postnatal lung development in mice is completed by this age. The control group was kept at room air for the same duration of time (PND 7 and PND 21).
Analysis of miR-30a targets and angiogenic genes.
We used the hyperoxia RNA-Seq signatures for male and female, respectively, at PND 7 and PND 21 as inferred in our previous report (10). We determined miR-30a targets in the mouse genome using the mirWalk database (16). mirWalk is a database that aggregates miRNA/gene target predictions from multiple established miRNA/mRNA targets resources, such as TargetScan, mirDB (66), and mirTarBase (9) and augments them with experimentally validated predictions and also a novel machine learning target prediction algorithms. The RNA-Seq results highlighting sex-specific differences in neonatal hyperoxic lung injury were previously reported (10). Briefly, data were mapped using TopHat2 on the mm10 mouse genome and quantified against the Gencode gene model (19) using Cufflinks 2.0 (41). Pathway enrichment was carried out using Gene Set Enrichment Analysis (58) against an extensive and widely used pathway and gene signature compendium, Molecular Signatures Database (31). Significance was achieved for Q value < 0.25. As reported previously, our analysis highlighted angiogenesis as a key molecular process dysregulated by hyperoxic exposure. We further determined angiogenic targets using the Molecular Signatures Database pathway compendium (31). Finally, we determined overlaps between the RNA-Seq signatures, miR-30a targets, and angiogenic genes using the Python programming system. We used the TargetScan mouse database of predicted gene targets of miRs to identify the binding site of miR-30a on the 3′ untranslated region (UTR) of Dll4 (2).
Cell culture and hyperoxia treatment.
Neonatal human pulmonary microvascular endothelial cells (HPMECs) were purchased from ScienCell (female lot nos: 5016, 10169, 10160, 15900 and 17799; male lot nos. 11367, 10899, 10885, 16021, 11816, 11422) and maintained in endothelial cell medium (lot no. 1001, ScienCell) at 37°C in 5% CO2. The gestational age of the donors varied from 18 to 24 wk. Per ScienCell, the tissue is obtained from nonprofit tissue providers who strictly adhere to the guidelines for tissue collection and distribution according to established protocols in compliance with local, state, and federal laws and regulations governing the procurement and distribution of human tissue after informed consent. Male and female HPMECs were used from passages 3–6 to ensure their endothelial characteristics. Male or female HPMECs (1 × 105) were seeded in a 6-mm dish. Twenty-four hours later, these cells were incubated at 37°C in room air condition (21% O2, 5% CO2) or in hyperoxia (95% O2, 5% CO2) as described before for 72 h (62).
miRNA mimic and miRNA inhibitor transfection in HPMECs.
mir30a-3p mimic (HMI0455), mir30a-5p mimic (HMI0454), microRNA negative control (HMC0002), synthetic mir30a-3p inhibitor (HLTUD0455), and synthetic mir30a-5p inhibitor (HSTUD0454) were purchased from Sigma-Aldrich. miRNA target protector (catalog no. 219000) was purchased from Qiagen. miRNA negative control (30 pmol), miRNA mimic (30 pmol), or miRNA inhibitor (30 pmol) were used for HPMEC transfection separately using lipofectamine RNAiMAX reagent (4.5 µl per well of 6-well plate). mir30a-5p target protector (30 pmol) and mir30a-5p mimic (30 pmol) were transfected together using lipofectamine RNAiMAX reagent (4.5 µl per well of 6-well plate). After 6-h transfection in room air (21% O2, 5% CO2), old medium was removed and fresh medium was added to the plate, and cells were subjected to normoxic or hyperoxic conditions.
Quantitative PCR.
Total RNA and miRNA was extracted from the lung tissues and cell lines using TRIzol-chloroform and then treated with DNase I (Invitrogen). cDNA was prepared using RevertAid Reverse Transcriptase (ThermoFisher). microRNA cDNA was generated using MystiCq microRNA cDNA Synthesis Mix (Millipore-Sigma). Quantitative PCR was performed using the QuantStudio 7 Flex real-time PCR detection system (ThermoFIsher) and SYBR Green (Bio-Rad). The thermal cycling conditions used were as follows: 1 cycle at 95°C for 1 min, 40 cycles at 95°C for 15 s, and 1 cycle at 60°C for 15 s. The primers used in the real-time PCR test were listed as follows: DLL4 (human) forward primer: CCCATGCCTCCAACTACTGT, reverse primer: CCCTCTGCCTGTCTGCTTAC; DLL4 (mice) forward primer: CCTCTCGAACTTGGACTTGC, reverse primer: TGGAAATACAGATGCCCACA; β-actin (human) forward primer: CATCGAGCACGGCATCGTCA, reverse primer: TAGCACAGCCTGGATAGCAAC; β-actin (mice) forward primer: GATCTGGCACCACACCTTCT, reverse primer: GGGGTGTTGAAGGTCTCAAA. mir30a-3p (MIRAP00080), mir30a-5p (MIRAP00079), MystiCq Universal PCR primer (MIRUP), and U6 (MIRCP00001) primer were purchased from Millipore-Sigma. Relative mRNA levels were calculated using the 2−ΔΔCT method and normalized by β-actin in the same sample.
Western immunoblotting.
Protein was isolated from mice lung tissue and HPMECs using radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher) containing protease mixture inhibitors (Thermo Fisher). Proteins were separated by 4%–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, then transferred to a PVDF membrane using a mini-PROTEAN tetra cell system (Bio-Rad). The following primary antibodies were used: rabbit anti-DLL4 (1:1,000, Abcam, cat. no. ab-7280) and rabbit anti-β-actin (1:5,000, Cell Signaling Technology, Beverly, MA). The anti-Dll4 antibody has been reported for its specificity in many previous publications (23, 29, 65). Pierce ECL plus Western blotting substrate (Thermo Fisher) was used for visualizing immunoreactive protein bands. The protein bands were normalized by β-actin on the same membrane.
Angiogenesis assay.
HPMECs were incubated with lipofectamine RNAiMax and either miRNA mimic or miRNA negative control (45 pmol). After a 6-h incubation, fresh medium was added and cells were given 48 h to recover. Angiogenesis potential was measured using HPMEC-coated cytodex-3 microcarrier beads (Sigma-Aldrich) suspended in a fibrin gel as previously described (42). Each bead is an independent replicate, and 7–15 beads were analyzed per donor. Briefly, cytodex-3 beads were coated with HPMECs over a 4-h incubation at 37°C with periodic agitation. Coated beads were then incubated in medium overnight to ensure confluence before resuspension in 2 mg/ml fibrin gels (Millipore) supplemented with 0.15 U/ml of aprotinin (Sigma-Aldrich) at a concentration of 250 beads/ml. The gels were maintained in extracellular matrix for 4 days with medium changes on alternating days. After culture, the gels were fixed in 4% paraformaldehyde supplemented with 0.1% Triton-X for 2 h at 4°C, washed three times with 1X PBS, and stained overnight with CF594-conjugated phalloidin (Cell Signaling Technologies, Danvers, MA). Gels were again washed three times with 1X PBS and stored hydrated until they were imaged. Sprout length was determined with ImageJ (NIH, Bethesda, MD) by generating a circle (centered on the bead center) whose radius intersects the longest angiogenic sprout and thereby contains the full sprouted construct. Maximum sprout distance was calculated by subtracting the radius of the bead from the radius of the overlaid circle. We chose max sprout length as a quantitative metric of angiogenic potential. This was a quantitative metric that represented the differences in angiogenesis between beads/donors. A student’s t-test was used to calculate the significance.
Human samples.
Lung protein and mRNA samples from patients with BPD and age-matched controls were kindly provided by Dr. Gloria Pryhuber (University of Rochester Medical Center, Rochester). Samples were obtained under protocols approved by the institutional review board of the University of Rochester after obtaining informed consent. All tissues are deidentified. Pathological diagnosis of BPD was made after examination of lung samples under microscopy. As controls, lung samples were collected from term and preterm infants who died from nonlung causes and who had brief exposures to supplemental oxygen and mechanical ventilation. This collection of human lung BPD and control samples (with details of inclusion/exclusion criteria, sample collection/storage methodology) has been reported in many previous publications (5, 30, 49). Nine samples per group were obtained from patients with BPD and no lung disease controls. Gestational age at birth and postconceptional age at death has been provided in Table 1.
Table 1.
Characteristics of patient lung samples
| Sample No. | Disease State | GA at Birth | GA at Death |
|---|---|---|---|
| 1 | No BPD | 32.3 | 32.7 |
| 2 | No BPD | 26.6 | 28.5 |
| 3 | No BPD | 32 | 32.3 |
| 4 | No BPD | 23 | 23.9 |
| 5 | No BPD | 41 | 41.4 |
| 6 | No BPD | 24.5 | 26.1 |
| 7 | No BPD | 40 | 40.6 |
| 8 | No BPD | 36 | 36.1 |
| 9 | No BPD | 41 | 41.4 |
| 10 | BPD | 25 | 34.1 |
| 11 | BPD | 27 | 40.7 |
| 12 | BPD | 28 | 45.0 |
| 13 | BPD | 30 | 33.9 |
| 14 | BPD | 25.4 | 34.0 |
| 15 | BPD | 24.7 | 31.8 |
| 16 | BPD | 29.1 | 46.5 |
| 17 | BPD | 29.2 | 43.1 |
| 18 | BPD | 26 | 34.1 |
BPD, bronchopulmonary dysplasia; GA, gestational age.
Statistical analysis.
GraphPad version 7 was used for the analysis of our data. Data are expressed as means ± SE. Data were analyzed by two-way ANOVA to test for the independent effects of sex and hyperoxia and to look for any interaction (sex × hyperoxia) or by student’s t-test. Multiple-comparison testing (Bonferroni) was performed if statistical significance (P < 0.05) was noted by ANOVA.
RESULTS
Identification of miR-30a as a potential driver of sex-specific differences in the pulmonary transcriptome in a murine model of BPD.
Pulmonary gene expression was studied using RNA-Seq on the Illumina HiSeq 2500 platform. Using Gene Set Enrichment Analysis we identified angiogenesis as a key process altered by hyperoxic exposure in both males and females at PND 7 and PND 21 (10). Focusing on this biological pathway, we next identified the number of angiogenesis related differentially expressed genes (DEGs) in this model and we also determined how many of them were miR-30a targets. These results are shown in Fig. 1. Figure 1A shows percentage of miR-30a targets among the differentially regulated angiogenesis-related genes at PND 7 and PND 21 in both males and females. We further show the distribution of miR-30a targets as a subset of angiogenesis-related DEGs in males (Fig. 1B) and females (Fig. 1C). The overall number of angiogenic DEGs for each signature is represented in the center of each circle diagram.
Fig. 1.
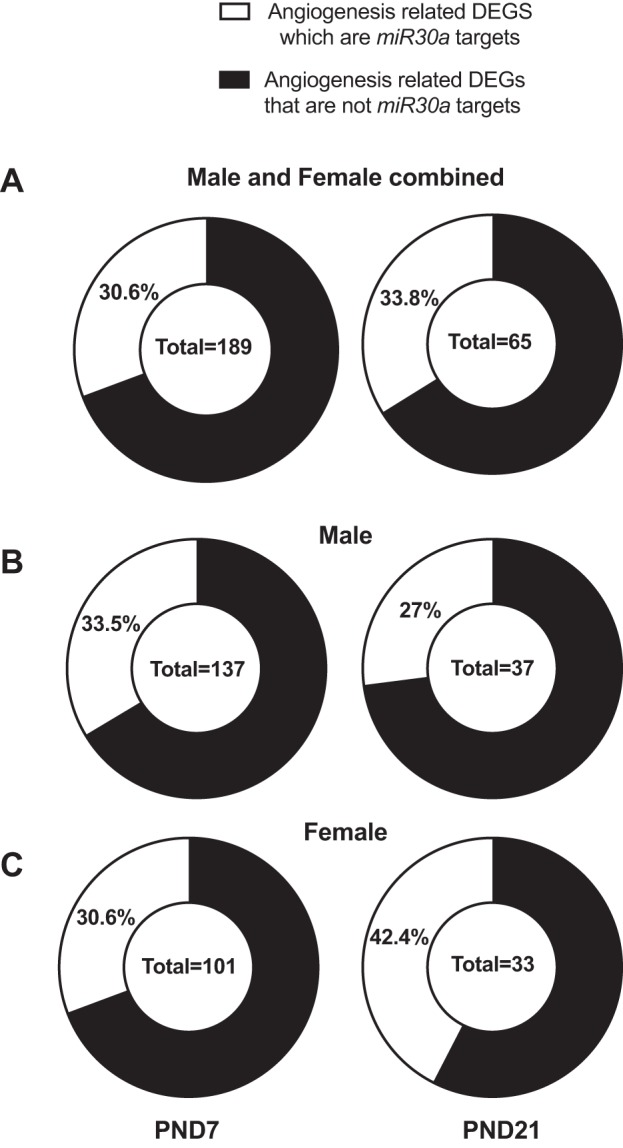
miR-30a targets among differentially expressed angiogenesis genes. We interrogated the hyperoxia RNA sequencing signatures in the lung for male and female neonatal mice, at PND 7 and PND 21 as inferred in our previous report (10). We further determined angiogenic targets using the Molecular Signatures Database (MSigDB) pathway compendium. We determined miR-30a targets in the mouse genome using the mirWalk database. The overall number of angiogenic DEGs for each signature is represented in the center of each circle diagram. Fig. 1 shows percentage of miR-30a targets among the differentially regulated angiogenesis related genes at PND 7 and PND 21 in both males and females (A). We further show the distribution of miR-30a targets as a subset of angiogenesis related DEGs in males (B) and females (C). DEG, differentially expressed gene; PND, postnatal day.
Overall in both sexes, on PND 7, 30.6% of angiogenesis-related DEGs were identified as miR-30a targets, with almost equal percentages in males (33.5%) and females (30.6%). At PND 21, males and females exhibit overall 65 distinct angiogenic DEGs in response to hyperoxia, with 22 of them reported miR-30a targets in the mirWalk compendium for an overall ratio of 33.8%. However, by separately analyzing the males’ and females’ angiogenic DEGs at PND 21, we observed a stark contrast. Whereas in males a total of 10 genes are an miR-30a target out of total 37 angiogenic DEGs for a ratio of 27%, in females we observed 14 miR-30a targets out of a total 33 angiogenic DEGs. The top up- and downregulated angiogenic DEGs for males and similarly the top up- and downregulated angiogenic DEGs for females are represented in Supplemental Tables S1 and S2 (Supplemental Material for this article is available online at the Journal website). We have further indicated which of those DEGs are miR-30a targets as indicated by the mirWalk microRNAs targets compendium.
Differential sex-specific expression of miR-30a in vivo and in vitro.
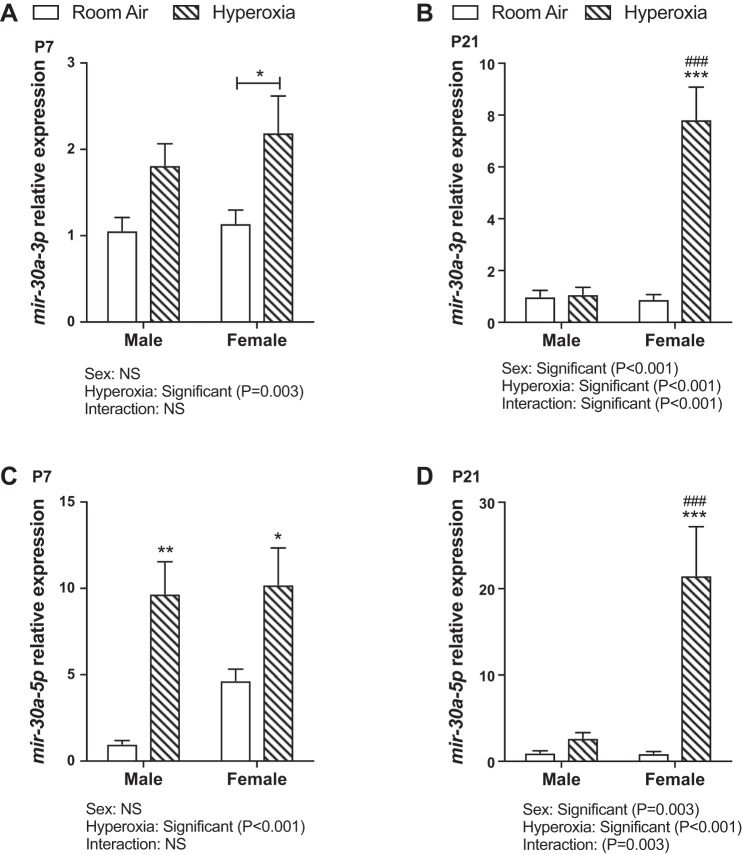
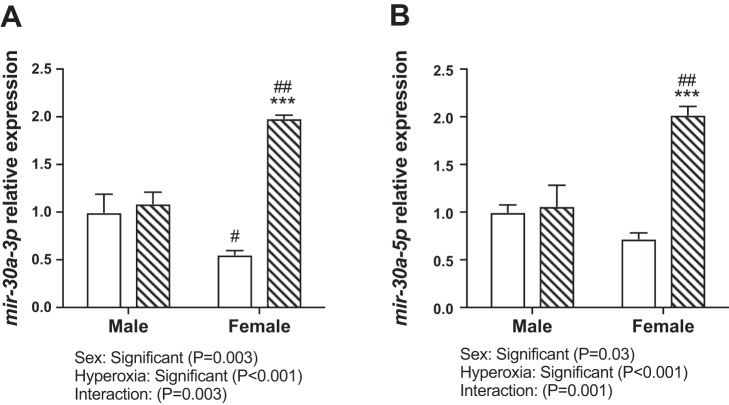
In WT mice, miR-30a expression was increased in the lungs in both males and females in the acute phase (PND 7) after hyperoxia exposure, with no difference between male and female mice (Fig. 2, A and C). By statistical analysis by two-way ANOVA, hyperoxia exposure was statistically significant but not sex or the interaction term (sex × hyperoxia). At PND 21 (recovery phase), both the independent variables (hyperoxia treatment and sex) as well as the interaction term (sex × hyperoxia) were significant in the statistical analysis by two-way ANOVA. Interestingly, female mice showed significantly higher miR-30a-3p and -5p expression in the lungs compared with male mice (Fig. 2, B and D). In our previous publication, following hyperoxia exposure from PND 1–5, at PND 21 pulmonary angiogenesis was better preserved in hyperoxia-exposed female mice (32). We also determined expression of mir-30a-3p (Fig. 3A) and -5p (Fig. 3B) in male and female neonatal (18–24-wk gestation) HPMECs exposed to hyperoxic or normoxic conditions. Both independent variables (sex, hyperoxia exposure) and the interaction term (sex × hyperoxia) were significant. Similar to the results in vivo, female HPMECs showed a significant increase in miR30a-5p and -3p expression upon exposure to hyperoxia.
Fig. 2.
Differential sex-specific expression on miR-30a in vivo. A and B: miR-30a-3p expression in male and female neonatal mice exposed to hyperoxia (95% FiO2, PND 1–5) at PND 7 and PND 21 (n = 6 animals/group). C and D: miR-30a-5p expression in male and female neonatal mice exposed to hyperoxia at PND 7 and PND 21 (n = 6 animals/group). Values are means ± SE. Significant differences between room air and hyperoxia within each sex are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001. Significant differences between male and female mice in normoxia or hyperoxia are indicated by ###P < 0.001. FiO2, fraction of inspired oxygen; NS, not significant; PND, postnatal day.
Fig. 3.
Differential sex-specific expression on miR-30a in vitro. A and B: miR-30a-3p and miR-30a-5p expression in human pulmonary microvascular endothelial cells (HPMECs) exposed to room air (RA) (RA-5% CO2) and 72 h of hyperoxia (95% O2-5% CO2) (n = 3/group). Values are means ± SE. Significant differences between RA and hyperoxia within each sex are indicated by ***P < 0.001. Significant differences between male and female mice in normoxia or hyperoxia are indicated by #P < 0.05 and ##P < 0.01.
Dll4 expression in male and female murine lung and neonatal HPMECs upon exposure to hyperoxia.
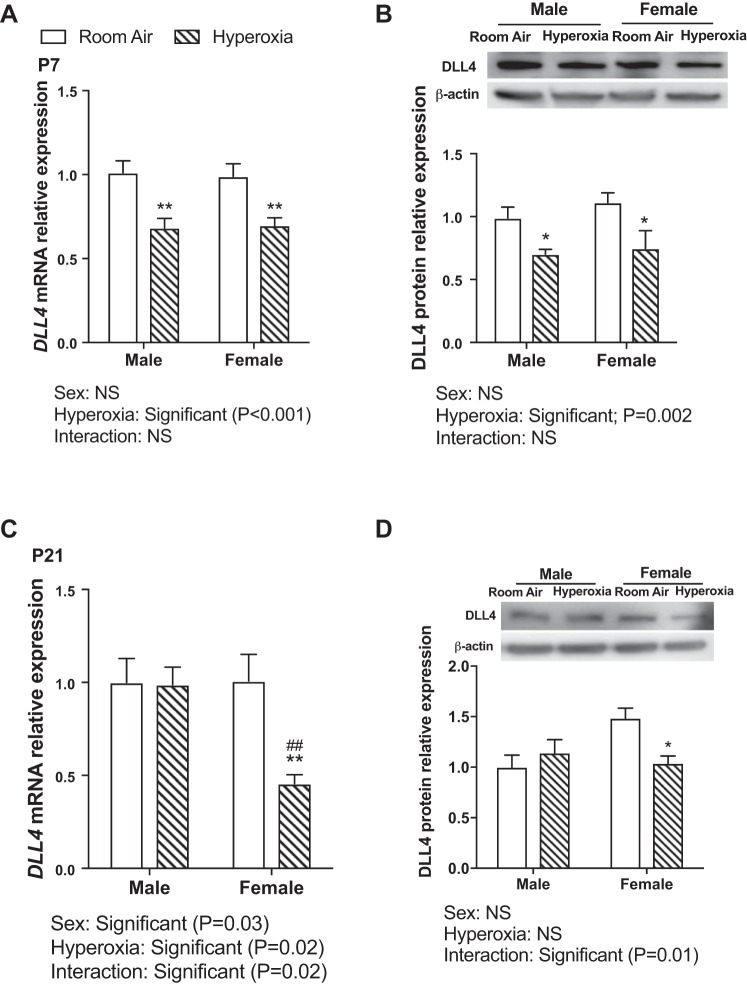
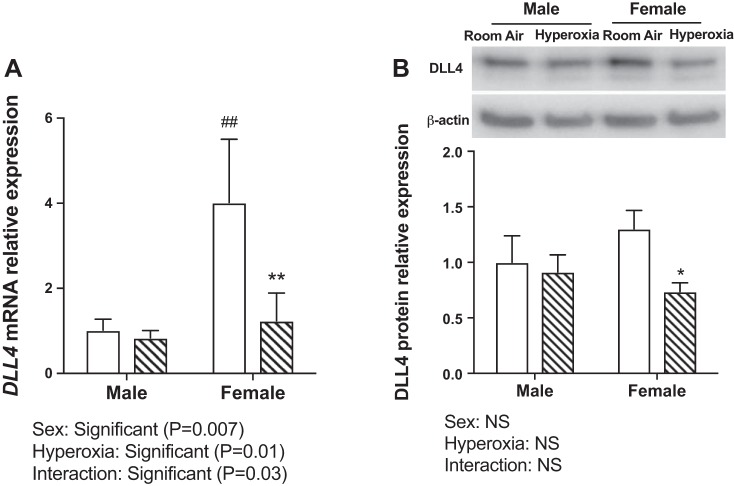
DLL4 is a membrane-bound ligand belonging to the Notch signaling family and plays an important role in vascular development and angiogenesis. We found an inverse relationship between miR-30a expression and Dll4 protein and mRNA expression in vivo and in vitro. Following hyperoxia exposure in vivo (PND 1–5; 95% FiO2), Dll4 mRNA (Fig. 4A) and protein expression (Fig. 4B) was decreased in both sexes at PND 7 (early), with just the hyperoxia exposure variable being significant at this time point. At PND 21, both sex and hyperoxia and the interaction term (sex × hyperoxia) were significant (Fig. 4C) for Dll4 mRNA expression, and the interaction term was significant for DLL4 protein expression (Fig. 4D). Females showed significantly decreased expression of Dll4 mRNA and protein expression in vivo at PND 21. Similarly, female HPMECs showed decreased expression of DLL4 mRNA (Fig. 5A; both sex and hyperoxia and the interaction term were significant) and protein expression (Fig. 5B) after exposure to hyperoxia.
Fig. 4.
Delta-like ligand (Dll) 4 expression in male and female murine lung upon exposure to hyperoxia in vivo: DLL4 mRNA (A and C) and protein (B and D) expression in male and female neonatal mice exposed to hyperoxia (95% FiO2, PND 1–5) at PND 7 (A and B) and PND 21 (C and D) (n = 6 animals /group). Values are means ± SE. Significant differences between room air and hyperoxia within each sex are indicated by *P < 0.05 and **P < 0.01. Significant differences between male and female mice in normoxia or hyperoxia are indicated by ##P < 0.01. FiO2, fraction of inspired oxygen; NS, not significant; PND, postnatal day.
Fig. 5.
Delta-like ligand 4 (Dll4) expression in male and female human neonatal pulmonary microvascular endothelial cells upon exposure to hyperoxia in vitro: DLL4 mRNA (A) and protein (B) expression in male and female neonatal human pulmonary microvascular endothelial cells (HPMECs) exposed to room air (RA) (RA-5% CO2) and 72 h of hyperoxia (95% O2-5% CO2) (n = 3/group). Values are means ± SE. Significant differences between RA and hyperoxia within each sex are indicated by *P < 0.05 and **P < 0.01. Significant differences between male and female mice in normoxia or hyperoxia are indicated by ##P < 0.01. NS, not significant.
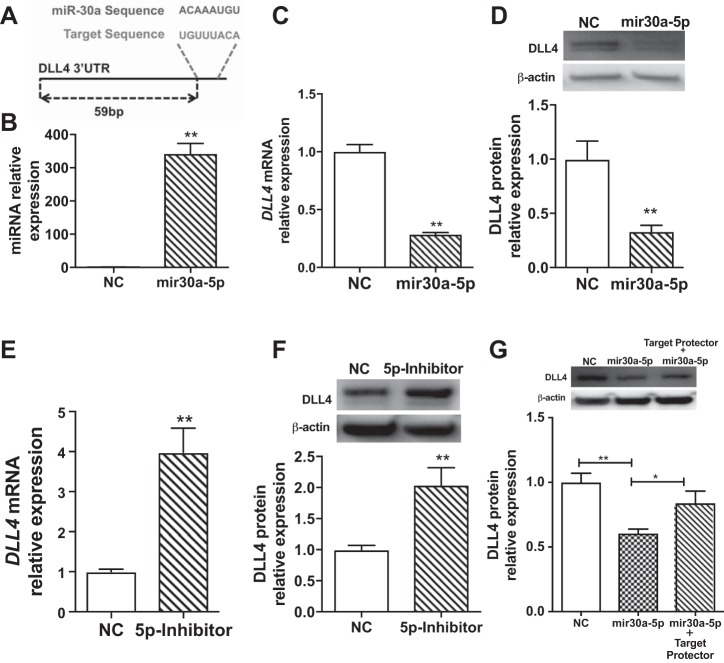
Dll4 is an miR-30a target in neonatal HPMECs.
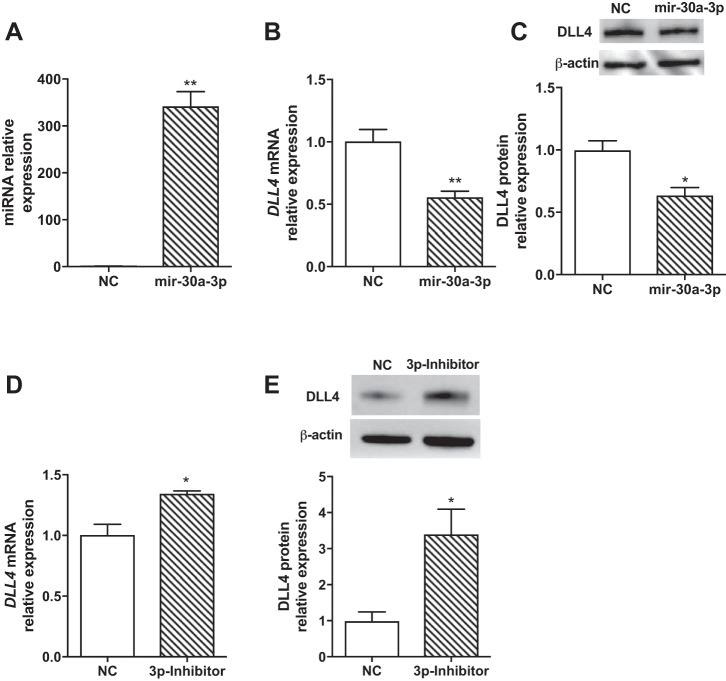
We predicted DLL4 as a target of miR-30a (Fig. 6A). We measured DLL4 expression after overexpression and inhibition of mir30a-5p. Treatment with mir30a-5p mimic increased mir30a-5p expression in HPMECs (Fig. 6B). mir30a-5p overexpression decreased DLL4 mRNA (Fig. 6C) and protein (Fig. 6D) levels significantly. Inhibition of mir30a-5p significantly increased DLL4 mRNA (Fig. 6E) and protein (Fig. 6F) levels in HPMECs. miRs can potentially regulate several targets. To address the relative functional contribution of a specific target (Dll4) to miR-30a, we used oligonucleotides complementary to Dll4 that compete with the binding of miR-30a (e.g., “target protectors”) (57). HPMECs treated with target protectors specific for miR30a binding sequence in the 3′ untranslated region of Dll4 showed no decrease in DLL4 expression despite treatment with miR30a-5p mimic. Collectively, these data together show that DLL4 is a specific and direct target of mir30a-5p in neonatal HMPECs. Similar results were seen with mir30a-3p overexpression (Fig. 7A, B, and C) and inhibition (Fig. 7, D and E) in HPMECs. Treatment with mir30a-3p mimic increased mir30a-3p expression (Fig. 7A) and decreased DLL4 mRNA (Fig. 7B) and protein (Fig. 7C) levels significantly. mir30a-3p inhibition significantly increased DLL4 mRNA (Fig. 7D) and protein (Fig. 7F) levels in HPMECs.
Fig. 6.
Delta-like ligand 4 (Dll4) is an miR-30a-5p target in neonatal human pulmonary microvascular endothelial cells. TargetScan reported miR-30a binding sites 59–66bp in the 3′UTR of DLL4 (A). Increased mir30a-5p expression after mir30a-5p mimic transfection in HPMECs (B). DLL4 mRNA (C) and protein (D) expression after mir30a-5p mimic transfection in HPMECs. DLL4 mRNA (E) and protein (F) expression after mir30a-5p inhibitor transfection in HPMECs. DLL4 protein expression (G) after mir30a-5p mimic transfection with and without target protector in HPMECs. Values are means ± SE from 3 independent experiments (n = 3/group). Significant differences between indicated groups are indicated by *P < 0.05 and **P < 0.01. HPMEC, human pulmonary microvascular endothelial cell; UTR, untranslated region; NC, normal control.
Fig. 7.
Effect of miR30a-3p overexpression and inhibition on DLL4 expression in HPMECs. Increased mir30a-3p expression after mir30a-3p mimic transfection in HPMECs (A). DLL4 mRNA (B) and protein (C) expression after mir30a-3p mimic transfection in HPMECs. DLL4 mRNA (D) and protein (E) expression after mir30a-3p inhibitor transfection in HPMECs. Values are means ± SE from three independent experiments (n = 3/group). Significant differences between indicated groups are indicated by *P < 0.05 and **P < 0.01. DLL4, delta-like ligand 4; HPMEC, human pulmonary microvascular endothelial cell. NC, normal control.
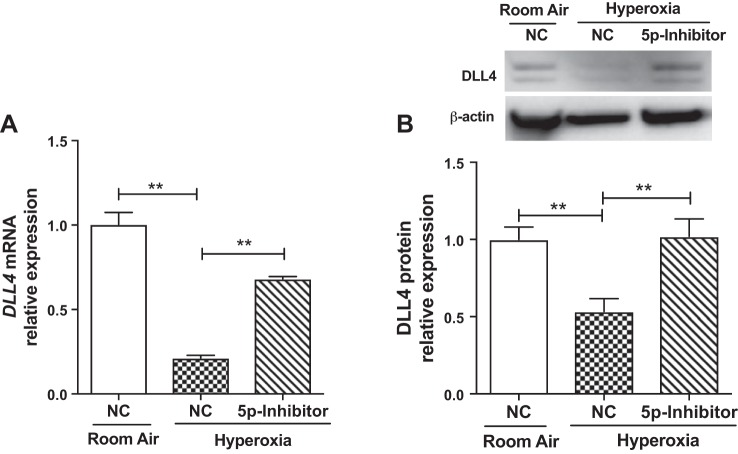
miR30a-5p inhibition under hyperoxic conditions increases Dll4 expression in female HPMECs.
In the context of in vitro hyperoxia exposure, we wanted to elucidate the effect of miR30–5p inhibition on DLL4 expression in female HPMECs. These results are shown in Fig. 8. Treatment with miR30a-5p inhibitor prevented the decrease in DLL4 mRNA (Fig. 8A) and protein (Fig. 8B) expression in female HPMECs upon exposure to hyperoxia.
Fig. 8.
miR30a-5p inhibition under hyperoxic conditions decreases increases Dll4 expression in female HPMECs. DLL4 mRNA (A) and protein (B) expression after inhibition of mir30a-5p in HPMECs upon exposure to hyperoxia. Values are means ± SE from three independent experiments (n = 3/group). Significant differences between indicated groups are indicated by **P < 0.01. DLL4, delta-like ligand 4; HPMEC, human pulmonary microvascular endothelial cell. NC, normal control.
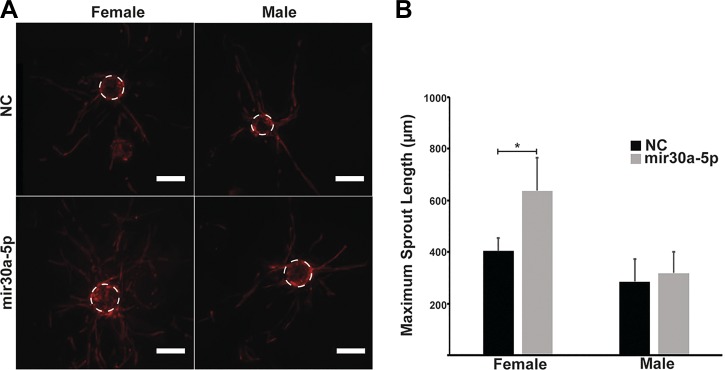
miR30a-5p mimic increases sprouting angiogenesis in female HPMECs.
Because miR30a-5p has a higher expression in our experimental model, we chose to focus on miR30a-5p for the in vitro angiogenesis experiment. Sprouting angiogenesis was measured using miR30a-5p mimic and negative control-treated male and female HPMEC-coated cytodex-3 microcarrier beads suspended in a fibrin gel. Representative images of the assay are shown in Fig. 9A. Measurements from male and female donors are shown in Fig. 9B. The sprout distance in female HPMECs treated with miR30–5p mimic (maximum sprout distance of 637 μm) was significantly greater than respective negative control-treated HPMECs (maximum sprout distance of 403 μm). There was no response seen in male HPMECs treated with miR30–5p mimic.
Fig. 9.
miR30a-5p mimic increases sprouting angiogenesis in female HPMECs. Angiogenic potential was quantified based on the maximum length of sprouts protruding from HPMEC-coated cytodex-3 microcarrier beads suspended in a fibrin gel (n = 3/group). A: representative images from male and female HPMECs subjected to sprouting angiogenesis assay treated with miR30a-5p mimic or negative control. Scale bar represents 200 μm. B: maximum sprouting distance in male and female HPMECs. Values are means ± SE. Significant differences between treatment and control groups is indicated by *P < 0.05. HPMEC, human pulmonary microvascular endothelial cell.
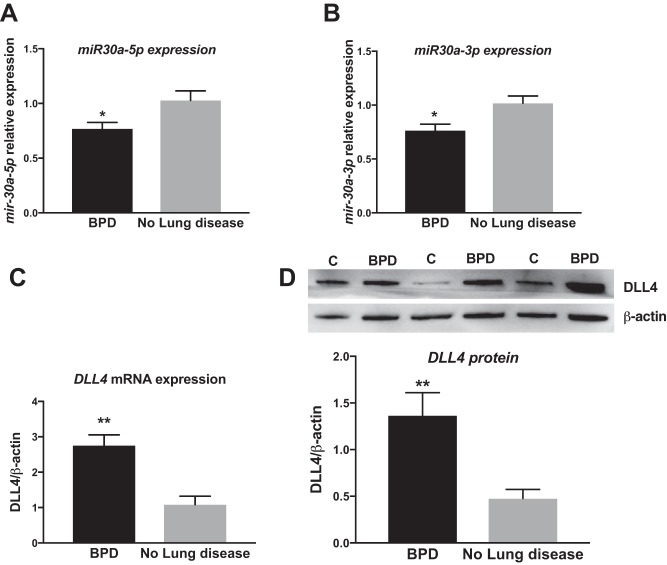
Expression of miR-30a and DLL4 in human BPD and control lung samples.
miR-30a and DLL4 expression was measured in human patients with BPD and was compared with expression levels in term and preterm infants with no lung disease. Expression levels of miR-30a-3p (Fig. 10A) and of -5p (Fig. 10B), respectively, were decreased in patients with BPD. Expression levels of the miR-30a target DLL4 were increased in patients with BPD at the mRNA (Fig. 10C) and protein levels (Fig. 10D).
Fig. 10.
miR30a-5p (A), miR30a-3p (B), and DLL4 mRNA (C) and protein (D) expression in human BPD and control (C) lung samples. Significant differences between BPD and no lung disease are indicated by *P < 0.05 and **P < 0.01. BPD, bronchopulmonary dysplasia; DLL4, delta-like ligand 4.
DISCUSSION
In this study, we determined the scope for miR-30a as a modulator of angiogenesis-related genes in the murine BPD model and underlying sex-specific differences. In a previous study, unbiased transcriptomic analysis of the neonatal murine lung revealed angiogenesis as one of the differentially modulated pathways between neonatal male and female mice (10). Pulmonary vascular development is better preserved in hyperoxia-exposed female neonatal mice compared with males (32). miR-30a is a known proangiogenic miR (7, 24, 56), and a large percentage of angiogenesis-related genes in the neonatal hyperoxia-induced BPD model were miR-30a targets both at PND 7 (early phase) and PND 21 (recovery phase) as shown in Fig. 1. Taken together, these results suggested that sex-specific differential miR-30a expression could modulate pulmonary vascular development in the neonatal lung exposed to hyperoxia.
miR-30a stimulates arteriolar branching by downregulating Dll4 expression, thereby controlling endothelial cell behavior (24), (35). We measured the expression of both the -3p and -5p strands of miR-30a in vivo and in vitro. Interestingly, both -3p and -5p strands of miR-30a have sexually dimorphic but concordant expression in this study. Expression levels were higher in female mice in vivo (Fig. 2) and in female HPMECs (Fig. 3) in vitro. In contrast to miR30a expression, Dll4 expression was decreased in female mice in vivo (Fig. 4) and in female HPMECs in vitro (Fig. 5). We further showed that Dll4 was a direct target of mir-30a in HPMECs (Fig. 6) and that miR-30a inhibition increased Dll4 expression in HPMECs upon exposure to hyperoxia (Fig. 8). We also showed that miR30a-5p may have a sex-specific proangiogenic function as it increased sprouting angiogenesis in female HPMECs but had no effect on male cells (Fig. 9).
The proangiogenic role of miR-30 has been shown in many previous studies (7, 24, 56). Jiang et al. (24) showed that miR30a stimulates angiogenesis in a zebrafish model and identified Dll4 as a direct target of miR30a in zebrafish and in human umbilical vein endothelial cells. However, miR-30a can also modulate other biological pathways apart from angiogenesis, which may be relevant in the context of neonatal hyperoxic lung injury and BPD. For example, miR30a-5p is also known to have anti-inflammatory effects, as shown by Demolli et al. (12) in a model of shear stress by decreasing angiopoietin-2. It is also known to have antiapoptotic (64) and antifibrotic (63) properties and is known to inhibit epithelial to mesenchymal transition (34) and autophagy (55). Thus, we speculate that overexpression of miR-30a in the recovering neonatal lung may be beneficial through its effects on multiple biological pathways.
The miR-30 families of miRNAs were found to be differentially expressed in many lung diseases. miR-30 was among the downregulated miRs in murine lungs exposed to cigarette smoke (22) and during the development of pulmonary hypertension (8). The miR-30a family was also associated with lung repair after influenza infection in the late repair phase (15 days after infection) (60). In a study by Wu et al. (70), decreased expression of miR30a-3p was identified as a blood biomarker for the development of BPD in preterm infants. Interestingly, glucocorticoids, which are used in the prevention of respiratory distress syndrome and used in the prevention or treatment of BPD, have been shown to maintain tissue miR-30a levels (69). Though we show increased expression of miR-30a in the HPMECs in vitro, the expression of miR-30a by other lung cell types in vivo cannot be ruled out. It is possible that miR30a expression from other lung cells has paracrine effects on the developing endothelium. In fact, exosomal transfer of miR-30a between cells has been reported (72). The upstream mechanisms leading to sex-specific differential expression of miR-30a still need to be elucidated. Sex hormones may modulate mir-30a expression. Androgen-induced androgen receptor activation has been shown to decrease miR-30a expression (36).
DLL4 is a membrane-bound ligand belonging to the Notch signaling family and plays an important role in vascular development and angiogenesis. Haploinsufficiency of Dll4 results in embryonic lethality because of defects in vascular development (18). Increased expression of DLL4 is associated with pathological angiogenesis (21, 26). Increased DLL4/Notch activity leads to decreased sprouting and branching of the vascular network, whereas blockade is associated with enhanced angiogenic sprouting and branching (44).
Optimal DLL4 expression is critical for the maintenance of endothelial cell function. Lobov et al. (35) showed that in the postnatal retina, deletion of a single Dll4 allele or pharmacological inhibition of Dll4 enhanced angiogenic sprouting and endothelial cell proliferation. Dll4 has been identified as a target of miR-30a in vitro (21). Inhibition of miR-30 blocks angiogenesis by lifting its inhibition on Dll4 (7). Bridge et al. (7) showed that Notch signaling through DLL4 decreases the expression of VEGFR2, one of the key receptors for VEGFA mediated angiogenic signaling (61). We had previously shown decreased expression of VEGFR2 in neonatal male mice following exposure to hyperoxia during the saccular stage of lung development (32). We thus speculate that miR-30a promotes angiogenesis by decreasing Dll4-mediated suppression of VEGFR2 in female mice. DLL4 also regulates the ratio of tip cells to stalk cells in the developing endothelium. DLL4 expression by the tip cells increases Notch signaling in the adjacent cells and promotes stalk-cell identity and prevents the formation of tip cells (20) (37). DLL4/Notch 1 signaling may also contribute to endothelial cell quiescence by preventing sprouting angiogenesis (50). Apart from its effects on angiogenesis, DLL4 triggered Notch signaling may also mediate macrophage mediated proinflammatory effects (17) (47) and thus by decreasing DLL4 expression, miR-30a expression may also have an anti-inflammatory effect in females.
Strengths of our study include the identification of miR-30a based on the unbiased transcriptomic analysis of the murine lung and its validation in a human patient cohort of both the miRNA and its target DLL4 (Fig. 10). We show the sexually dimorphic expression of miR-30a and its target Dll4 in vivo in a murine BPD model as well as in neonatal HPMECs from canalicular stage of lung development exposed to hyperoxia in vitro. Specificity of DLL4 as an miR30a-5p target in HPMECs was established using a target protector. We recognize limitations of our study as well. It is possible that miR-30a may modulate hyperoxic lung injury through other targets. Few such recognized targets in the literature are CTGF (14), IGF-1R (33), and p53 (69), all of which are known to modulate hyperoxic lung injury. Other genes or miRNAs could modulate Dll4. Our findings in the murine model may not translate to humans but our findings in HPMECs and in human BPD samples increase their translational relevance.
The control group in the human samples is not the perfect comparison for the BPD group (Fig. 10). Ideally, this group should comprise of premature infants born at similar gestational age as the disease group but did not develop BPD and then died because of nonlung-related causes at comparable postconceptional ages. Such controls are unfortunately very rare. Our study compared premature infants who died with BPD with term and preterm infants who died from nonlung causes and who had brief exposures to supplemental oxygen and mechanical ventilation. This comparison may be justified as it compares the diseased BPD lung with nondiseased lungs at similar postconceptional ages at death. The no lung disease group thus represents the gene expression if the patients had not developed the disease. The number of samples is underpowered to answer the question of whether there was sex-specific differential expression of miR-30a and Dll4 in the human samples. Even though we provided the sex and gestational age of the endothelial cell lots, we do not have detailed information on donor health or any maternal data. Also, the media used in the in vitro studies contained hormones, though both male and female cells were exposed to the same culture conditions. We only used a single hyperoxia concentration (95% ) for our experiments. A dose-dependent effect of different hyperoxia concentrations was not studied.
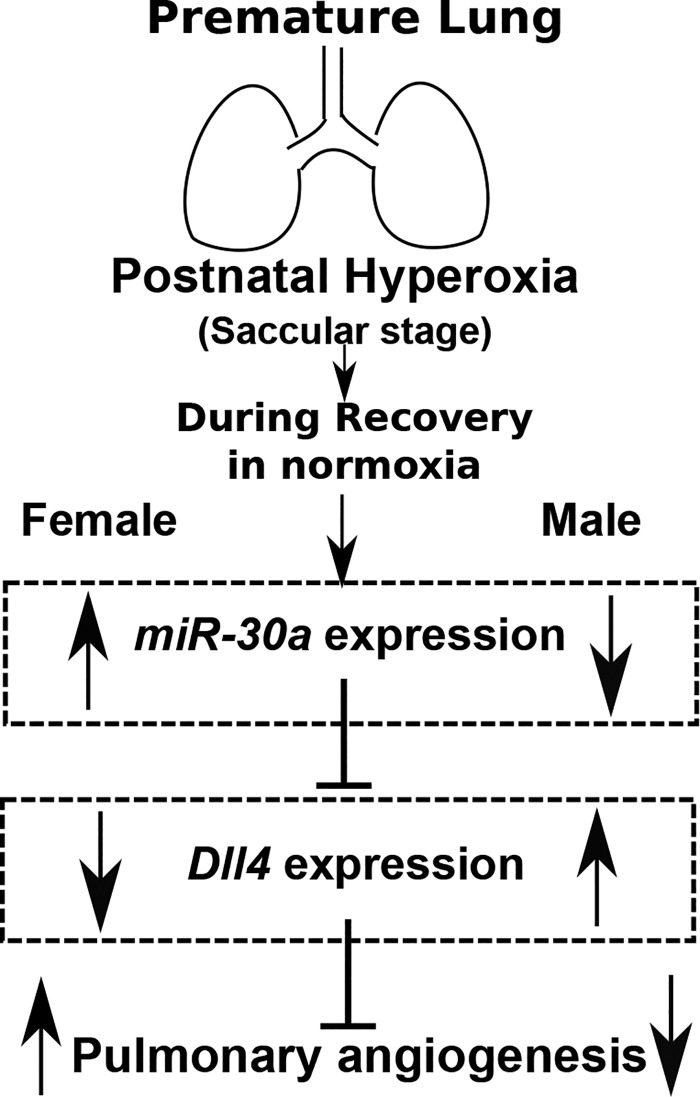
The collective results of these experiments show sexual dimorphism in miR-30a expression in vivo and in vitro and propose Dll4 as one of the downstream target genes in pulmonary endothelial cells through which miR-30a exerts its proangiogenic effects (Fig. 11). Future studies need to address the cell-specific expression of mir-30a in the animal models as well as in human tissues, and the ability to deliver mir-30a in a cell- and organ-specific manner needs to be addressed. The study highlights that biologic mediators and therapeutic effects vary based on biological sex, and this needs to be taken into consideration both in basic science and clinical studies.
Fig. 11.
Overall schema supporting a hypothesized role of sexually dimorphic miR-30a expression in neonatal hyperoxic lung injury: increased miR-30a expression in the female neonatal lung preserves lung vascular development through miR-30a-mediated downregulation of Dll4 expression in females. DLL4, delta-like ligand 4.
GRANTS
This work was supported in part by grants from NIH [Grant Nos. K08-HL-127103 and R03-HL-141572 to K. Lingappan, Grant Nos. R01-HL-133163 and R21-ES-027962 to J. Gleghorn, and Grant Nos. S10-OD-016361 (confocal microscope) and T32-GM-008550 Chemistry-Biology Interface training program to B. Hayward-Piatkovskyi], the American Lung Association (Grant No. RG-418067 to K. Lingappan), the National Science Foundation (Grant No. 1537256 to J. Gleghorn), and the March of Dimes Basil O’Connor Award (5-FY16–33 to J. Gleghorn).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.L. conceived and designed research; Y.Z., X.D., W.J., B.H.-P., J.P.G., and K.L. performed experiments; Y.Z., C.C., X.D., W.J., B.H.-P., J.P.G., and K.L. analyzed data; Y.Z., B.H.-P., J.P.G., and K.L. interpreted results of experiments; Y.Z., C.C., B.H.-P., J.P.G., and K.L. prepared figures; Y.Z., X.D., B.H.-P., J.P.G., and K.L. drafted manuscript; Y.Z., C.C., X.D., B.H.-P., J.P.G., and K.L. edited and revised manuscript; Y.Z., C.C., X.D., B.H.-P., J.P.G., and K.L. approved final version of manuscript.
Supplemental Data
REFERENCES
- 1.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med 164: 1755–1756, 2001. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4: e05005, 2015. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137: 1124–1135, 2009. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskaran M, Xi D, Wang Y, Huang C, Narasaraju T, Shu W, Zhao C, Xiao X, More S, Breshears M, Liu L. Identification of microRNAs changed in the neonatal lungs in response to hyperoxia exposure. Physiol Genomics 44: 970–980, 2012. doi: 10.1152/physiolgenomics.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya S, Go D, Krenitsky DLD, Huyck HLH, Solleti SKS, Lunger VAV, Metlay L, Srisuma S, Wert SES, Mariani TJT, Pryhuber GSG. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med 186: 349–358, 2012. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binet M-E, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B; Canadian Neonatal Network . Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol 29: 159–166, 2012. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]
- 7.Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, Patient R, Boshoff C. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood 120: 5063–5072, 2012. doi: 10.1182/blood-2012-04-423004. [DOI] [PubMed] [Google Scholar]
- 8.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 30: 716–723, 2010. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 9.Chou C-H, Shrestha S, Yang C-D, Chang N-W, Lin Y-L, Liao K-W, Huang W-C, Sun T-H, Tu S-J, Lee W-H, Chiew M-Y, Tai C-S, Wei T-Y, Tsai T-R, Huang H-T, Wang C-Y, Wu H-Y, Ho S-Y, Chen P-R, Chuang C-H, Hsieh P-J, Wu Y-S, Chen W-L, Li M-J, Wu Y-C, Huang X-Y, Ng FL, Buddhakosai W, Huang P-C, Lan K-C, Huang C-Y, Weng S-L, Cheng Y-N, Liang C, Hsu W-L, Huang H-D. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res 46: D296–D302, 2018. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coarfa C, Zhang Y, Maity S, Perera DN, Jiang W, Wang L, Couroucli X, Moorthy B, Lingappan K. Sexual dimorphism of the pulmonary transcriptome in neonatal hyperoxic lung injury: identification of angiogenesis as a key pathway. Am J Physiol Lung Cell Mol Physiol 313: L991–L1005, 2017. doi: 10.1152/ajplung.00230.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR; EPICure Study Group . The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 106: 659–671, 2000. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- 12.Demolli S, Doebele C, Doddaballapur A, Lang V, Fisslthaler B, Chavakis E, Vinciguerra M, Sciacca S, Henschler R, Hecker M, Savant S, Augustin HG, Kaluza D, Dimmeler S, Boon RA. MicroRNA-30 mediates anti-inflammatory effects of shear stress and KLF2 via repression of angiopoietin 2. J Mol Cell Cardiol 88: 111–119, 2015. doi: 10.1016/j.yjmcc.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Dong J, Carey WA, Abel S, Collura C, Jiang G, Tomaszek S, Sutor S, Roden AC, Asmann YW, Prakash YS, Wigle DA. MicroRNA-mRNA interactions in a murine model of hyperoxia-induced bronchopulmonary dysplasia. BMC Genomics 13: 204, 2012. doi: 10.1186/1471-2164-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res 104: 170–178, 2009. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]
- 15.Durrani-Kolarik S, Pool CA, Gray A, Heyob KM, Cismowski MJ, Pryhuber G, Lee LJ, Yang Z, Tipple TE, Rogers LK. miR-29b supplementation decreases expression of matrix proteins and improves alveolarization in mice exposed to maternal inflammation and neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 313: L339–L349, 2017. doi: 10.1152/ajplung.00273.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dweep H, Gretz N. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat Methods 12: 697, 2015. doi: 10.1038/nmeth.3485. [DOI] [PubMed] [Google Scholar]
- 17.Fung E, Tang S-MT, Canner JP, Morishige K, Arboleda-Velasquez JF, Cardoso AA, Carlesso N, Aster JC, Aikawa M. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation 115: 2948–2956, 2007. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 18.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA 101: 15949–15954, 2004. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrow J, Denoeud F, Frankish A, Reymond A, Chen C-K, Chrast J, Lagarde J, Gilbert JGR, Storey R, Swarbreck D, Rossier C, Ucla C, Hubbard T, Antonarakis SE, Guigó R. GENCODE: producing a reference annotation for ENCODE. Genome Biol 7, Suppl 1: S4.1–9, 2006. doi: 10.1186/gb-2006-7-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellström M, Phng L-K, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson A-K, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780, 2007. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 21.Huang QB, Ma X, Zhang X, Liu SW, Ai Q, Shi TP, Zhang Y, Gao Y, Fan Y, Ni D, Wang BJ, Li HZ, Zheng T. Down-regulated miR-30a in clear cell renal cell carcinoma correlated with tumor hematogenous metastasis by targeting angiogenesis-specific DLL4. PLoS One 8: e67294, 2013. doi: 10.1371/journal.pone.0067294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 23: 806–812, 2009. doi: 10.1096/fj.08-121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarad M, Kuczynski EA, Morrison J, Viloria-Petit AM, Coomber BL. Release of endothelial cell associated VEGFR2 during TGF-β modulated angiogenesis in vitro. BMC Cell Biol 18: 10, 2017. doi: 10.1186/s12860-017-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Q, Lagos-Quintana M, Liu D, Shi Y, Helker C, Herzog W, le Noble F. miR-30a regulates endothelial tip cell formation and arteriolar branching. Hypertension 62: 592–598, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01767. [DOI] [PubMed] [Google Scholar]
- 25.Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol 33: 1076–1078, 2016. doi: 10.1055/s-0036-1586107. [DOI] [PubMed] [Google Scholar]
- 26.Kangsamaksin T, Murtomaki A, Kofler NM, Cuervo H, Chaudhri RA, Tattersall IW, Rosenstiel PE, Shawber CJ, Kitajewski J. NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer Discov 5: 182–197, 2015. doi: 10.1158/2159-8290.CD-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraybill EN, Runyan DK, Bose CL, Khan JH. Risk factors for chronic lung disease in infants with birth weights of 751 to 1000 grams. J Pediatr 115: 115–120, 1989. doi: 10.1016/S0022-3476(89)80345-2. [DOI] [PubMed] [Google Scholar]
- 28.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597–610, 2010. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 29.Lanier V, Gillespie C, Leffers M, Daley-Brown D, Milner J, Lipsey C, Webb N, Anderson LM, Newman G, Waltenberger J, Gonzalez-Perez RR. Leptin-induced transphosphorylation of vascular endothelial growth factor receptor increases Notch and stimulates endothelial cell angiogenic transformation. Int J Biochem Cell Biol 79: 139–150, 2016. doi: 10.1016/j.biocel.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MK, Pryhuber GS, Schwarz MA, Smith SM, Pavlova Z, Sunday ME. Developmental regulation of p66Shc is altered by bronchopulmonary dysplasia in baboons and humans. Am J Respir Crit Care Med 171: 1384–1394, 2005. doi: 10.1164/rccm.200406-776OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27: 1739–1740, 2011. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 311: L481–L493, 2016. doi: 10.1152/ajplung.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Zhou Y, Gong X, Zhang C. MicroRNA-30a-5p inhibits the proliferation and invasion of gastric cancer cells by targeting insulin-like growth factor 1 receptor. Exp Ther Med 14: 173–180, 2017. doi: 10.3892/etm.2017.4477. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Liu Z, Tu K, Liu Q. Effects of microRNA-30a on migration, invasion and prognosis of hepatocellular carcinoma. FEBS Lett 588: 3089–3097, 2014. doi: 10.1016/j.febslet.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 35.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104: 3219–3224, 2007. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyu S, Liu H, Liu X, Liu S, Wang Y, Yu Q, Niu Y. Interrelation of androgen receptor and miR-30a and miR-30a function in ER−, PR−, AR+ MDA-MB-453 breast cancer cells. Oncol Lett 14: 4930–4936, 2017. doi: 10.3892/ol.2017.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majumder S, Zhu G, Xu X, Senchanthisai S, Jiang D, Liu H, Xue C, Wang X, Coia H, Cui Z, Smolock EM, Libby RT, Berk BC, Pang J. G-protein-coupled receptor-2-interacting protein-1 controls stalk cell fate by inhibiting delta-like 4-Notch1 signaling. Cell Rep 17: 2532–2541, 2016. doi: 10.1016/j.celrep.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malleske DT, Chorna O, Maitre NL. Pulmonary sequelae and functional limitations in children and adults with bronchopulmonary dysplasia. Paediatr Respir Rev 26: 55–59, 2018. doi: 10.1016/j.prrv.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Mitra R, Lin C-C, Eischen CM, Bandyopadhyay S, Zhao Z. Concordant dysregulation of miR-5p and miR-3p arms of the same precursor microRNA may be a mechanism in inducing cell proliferation and tumorigenesis: a lung cancer study. RNA 21: 1055–1065, 2015. doi: 10.1261/rna.048132.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan CP, Bale TL. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol Sex Differ 3: 22, 2012. doi: 10.1186/2042-6410-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mudge JM, Harrow J. Creating reference gene annotation for the mouse C57BL6/J genome assembly. Mamm Genome 26: 366–378, 2015. doi: 10.1007/s00335-015-9583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakatsu MN, Davis J, Hughes CCW. Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp 186: 186, 2007. doi: 10.3791/186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nardiello C, Mižíková I, Morty RE. Looking ahead: where to next for animal models of bronchopulmonary dysplasia? Cell Tissue Res 367: 457–468, 2017. doi: 10.1007/s00441-016-2534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444: 1032–1037, 2006. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 45.O’Shea JE, Davis PG, Doyle LW; Victorian Infant Collaborative Study Group . Maternal preeclampsia and risk of bronchopulmonary dysplasia in preterm infants. Pediatr Res 71: 210–214, 2012. doi: 10.1038/pr.2011.27. [DOI] [PubMed] [Google Scholar]
- 46.Olave N, Lal CV, Halloran B, Pandit K, Cuna AC, Faye-Petersen OM, Kelly DR, Nicola T, Benos PV, Kaminski N, Ambalavanan N. Regulation of alveolar septation by microRNA-489. Am J Physiol Lung Cell Mol Physiol 310: L476–L487, 2016. doi: 10.1152/ajplung.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagie S, Gérard N, Charreau B. Notch signaling triggered via the ligand DLL4 impedes M2 macrophage differentiation and promotes their apoptosis. Cell Commun Signal 16: 4, 2018. doi: 10.1186/s12964-017-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays 33: 791–802, 2011. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 49.Rogers LK, Robbins M, Dakhlallah D, Yang Z, Lee LJ, Mikhail M, Nuovo G, Pryhuber GS, McGwin G, Marsh CB, Tipple TE. Attenuation of miR-17-92 cluster in bronchopulmonary dysplasia. Ann Am Thorac Soc 12: 1506–1513, 2015. doi: 10.1513/AnnalsATS.201501-058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rostama B, Turner JE, Seavey GT, Norton CR, Gridley T, Vary CPH, Liaw L. DLL4/Notch1 and BMP9 interdependent signaling induces human endothelial cell quiescence via P27KIP1 and Thrombospondin-1. Arterioscler Thromb Vasc Biol 35: 2626–2637, 2015. doi: 10.1161/ATVBAHA.115.306541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 8: 39–49, 2003. doi: 10.1016/S1084-2756(02)00194-X. [DOI] [PubMed] [Google Scholar]
- 52.Schulz MH, Pandit KV, Lino Cardenas CL, Ambalavanan N, Kaminski N, Bar-Joseph Z. Reconstructing dynamic microRNA-regulated interaction networks. Proc Natl Acad Sci USA 110: 15686–15691, 2013. doi: 10.1073/pnas.1303236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma S, Eghbali M. Influence of sex differences on microRNA gene regulation in disease. Biol Sex Differ 5: 3, 2014. doi: 10.1186/2042-6410-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva DMG, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 309: L1239–L1272, 2015. doi: 10.1152/ajplung.00268.2015. [DOI] [PubMed] [Google Scholar]
- 55.Singh SV, Dakhole AN, Deogharkar A, Kazi S, Kshirsagar R, Goel A, Moiyadi A, Jalali R, Sridhar E, Gupta T, Shetty P, Gadewal N, Shirsat NV. Restoration of miR-30a expression inhibits growth, tumorigenicity of medulloblastoma cells accompanied by autophagy inhibition. Biochem Biophys Res Commun 491: 946–952, 2017. doi: 10.1016/j.bbrc.2017.07.140. [DOI] [PubMed] [Google Scholar]
- 56.Song M-S, Rossi JJ. The anti-miR21 antagomir, a therapeutic tool for colorectal cancer, has a potential synergistic effect by perturbing an angiogenesis-associated miR30. Front Genet 4: 301, 2014. doi: 10.3389/fgene.2013.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staton AA, Giraldez AJ. Use of target protector morpholinos to analyze the physiological roles of specific miRNA-mRNA pairs in vivo. Nat Protoc 6: 2035–2049, 2011. doi: 10.1038/nprot.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 313: L1101–L1153, 2017. doi: 10.1152/ajplung.00343.2017. [DOI] [PubMed] [Google Scholar]
- 60.Tan KS, Choi H, Jiang X, Yin L, Seet JE, Patzel V, Engelward BP, Chow VT. Micro-RNAs in regenerating lungs: an integrative systems biology analysis of murine influenza pneumonia. BMC Genomics 15: 587, 2014. doi: 10.1186/1471-2164-15-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thurston G, Kitajewski J. VEGF and Delta-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer 99: 1204–1209, 2008. doi: 10.1038/sj.bjc.6604484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiwari KK, Moorthy B, Lingappan K. Role of GDF15 (growth and differentiation factor 15) in pulmonary oxygen toxicity. Toxicol In Vitro 29: 1369–1376, 2015. doi: 10.1016/j.tiv.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tu X, Zhang Y, Zheng X, Deng J, Li H, Kang Z, Cao Z, Huang Z, Ding Z, Dong L, Chen J, Zang Y, Zhang J. TGF-β-induced hepatocyte lincRNA-p21 contributes to liver fibrosis in mice. Sci Rep 7: 2957, 2017. doi: 10.1038/s41598-017-03175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Jiao Y, Cui L, Jiang L. miR-30 functions as an oncomiR in gastric cancer cells through regulation of P53-mediated mitochondrial apoptotic pathway. Biosci Biotechnol Biochem 81: 119–126, 2017. doi: 10.1080/09168451.2016.1238294. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Yu Y, Wang Y, Li X, Bao J, Wu G, Chang H, Shi T, Yue Z. Delta-like ligand 4: a predictor of poor prognosis in clear cell renal cell carcinoma. Oncol Lett 8: 2627–2633, 2014. doi: 10.3892/ol.2014.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X. Improving microRNA target prediction by modeling with unambiguously identified microRNA-target pairs from CLIP-ligation studies. Bioinformatics 32: 1316–1322, 2016. doi: 10.1093/bioinformatics/btw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams AE, Moschos SA, Perry MM, Barnes PJ, Lindsay MA. Maternally imprinted microRNAs are differentially expressed during mouse and human lung development. Dev Dyn 236: 572–580, 2007. doi: 10.1002/dvdy.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolterink-Donselaar IG, Meerding JM, Fernandes C. A method for gender determination in newborn dark pigmented mice. Lab Anim (NY) 38: 35–38, 2009. doi: 10.1038/laban0109-35. [DOI] [PubMed] [Google Scholar]
- 69.Wu J, Zheng C, Fan Y, Zeng C, Chen Z, Qin W, Zhang C, Zhang W, Wang X, Zhu X, Zhang M, Zen K, Liu Z. Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J Am Soc Nephrol 25: 92–104, 2014. doi: 10.1681/ASN.2012111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Y-T, Chen WJ, Hsieh W-S, Tsao P-N, Yu S-L, Lai C-Y, Lee W-C, Jeng S-F. MicroRNA expression aberration associated with bronchopulmonary dysplasia in preterm infants: a preliminary study. Respir Care 58: 1527–1535, 2013. doi: 10.4187/respcare.02166. [DOI] [PubMed] [Google Scholar]
- 71.Xing Y, Fu J, Yang H, Yao L, Qiao L, Du Y, Xue X. MicroRNA expression profiles and target prediction in neonatal Wistar rat lungs during the development of bronchopulmonary dysplasia. Int J Mol Med 36: 1253–1263, 2015. doi: 10.3892/ijmm.2015.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y, Li Y, Chen X, Cheng X, Liao Y, Yu X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J Mol Med (Berl) 94: 711–724, 2016. doi: 10.1007/s00109-016-1387-2. [DOI] [PubMed] [Google Scholar]
- 73.Yang Y, Qiu J, Kan Q, Zhou X-G, Zhou X-Y. MicroRNA expression profiling studies on bronchopulmonary dysplasia: a systematic review and meta-analysis. Genet Mol Res 12: 5195–5206, 2013. doi: 10.4238/2013.October.30.4. [DOI] [PubMed] [Google Scholar]
- 74.Yee M, Cohen ED, Domm W, Porter GA JR, McDavid AN, O’Reilly MA. Neonatal hyperoxia depletes pulmonary vein cardiomyocytes in adult mice via mitochondrial oxidation. Am J Physiol Lung Cell Mol Physiol 314: L846–L859, 2018. doi: 10.1152/ajplung.00409.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Peng W, Zhang S, Wang C, He X, Zhang Z, Zhu L, Wang Y, Feng Z. MicroRNA expression profile in hyperoxia-exposed newborn mice during the development of bronchopulmonary dysplasia. Respir Care 56: 1009–1015, 2011. doi: 10.4187/respcare.01032. [DOI] [PubMed] [Google Scholar]
- 76.Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia - trends over three decades. Paediatr Child Health 18: 86–90, 2013. doi: 10.1093/pch/18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.