Abstract
We have previously reported that mice genetically deficient in the actin binding protein gelsolin exhibit impaired airway smooth muscle (ASM) relaxation. Primary cultured ASM cells from these mice demonstrate enhanced inositol triphosphate (IP3) synthesis and increased intracellular calcium in response to Gq-coupled agonists. We hypothesized that this was due to increased intracellular availability of unbound phosphatidylinositol 4,5-bisphosphate (PIP2), based on the fact that gelsolin contains a short peptide region that binds PIP2, presumably making it a less available substrate. We now questioned whether a peptide that corresponds to the PIP2 binding region of gelsolin could modulate ASM signaling and contraction. The 10 amino acid sequence of the gelsolin peptide within the PIP2-binding region was incubated with primary cultures of human ASM cells, and IP3 synthesis was measured in response to a Gq-coupled agonist. Gelsolin peptide-treated cells generated less IP3 under basal and bradykinin or acetylcholine (Gq-coupled) conditions. Acetylcholine-induced contractile force measured in isolated tracheal rings from mice and human tracheal muscle strips in organ baths was attenuated in the presence of the gelsolin peptide. The gelsolin peptide also attenuated methacholine-induced airway constriction in murine precision-cut lung slices. Furthermore, this peptide fragment delivered to the respiratory system of mice via nebulization attenuated subsequent methacholine-induced increases in airway resistance in vivo. The current study demonstrates that introduction of this small gelsolin peptide into the airway may be a novel therapeutic option in bronchoconstrictive diseases.
Keywords: actin cytoskeleton, nebulization, precision-cut lung slice, small peptide, smooth muscle relaxation
INTRODUCTION
Asthma is characterized by reversible airway constriction and involves a complex interplay between multiple cell types in the airway including airway nerves, inflammatory cells, airway epithelium, and airway smooth muscle (ASM). ASM is hypersensitive and hypercontractile in asthmatic airways (2) and undergoes both hypertrophy and hyperplasia during asthmatic airway remodeling (1, 14). Acute ASM constriction during exacerbations of asthma is classically treated by inhaled β2-adrenoceptor agonists; however, some patients become refractory to the chronic or acute benefits of these therapies (10, 26). Thus there is a continuing need to identify novel targets and therapies in ASM that may augment or replace current therapies.
Contractile tone of ASM is modulated by contractile and relaxant signaling cascades that ultimately regulate the interaction of actin and myosin. The ability of actin to participate in contraction is in part regulated by the elongation of actin polymers into filaments. This process of actin filament elongation and shortening (polymerization and depolymerization) is regulated by a family of cytoskeletal regulatory proteins including gelsolin (17).
Gelsolin is a calcium-activated actin-capping and -severing protein belonging to the superfamily of gelsolin/villin proteins. An increase in intracellular calcium concentrations leads to gelsolin binding to actin filaments with subsequent cleavage of these filaments (25) favoring smooth muscle cell relaxation. Additionally, the full-length gelsolin protein contains a short peptide region that is a binding domain for phosphatidylinositol 4,5 bisphosphate (PIP2) (15), the critical substrate for phospholipase C-β (PLC-β). As such, it may play a homeostatic role of controlling and/or modulating G protein-coupled receptor-mediated generation of inositol triphosphate (IP3) and calcium release from the sarcoplasmic reticulum. We have previously reported that mice genetically deficient in the actin-binding protein gelsolin exhibit impaired ASM relaxation ex vivo and in vivo (18). We also reported that primary cultured ASM cells from gelsolin null mice demonstrated enhanced IP3 synthesis and increased intracellular calcium in response to Gq-coupled agonists (18). We hypothesized that this was due to increased intracellular availability of unbound PIP2, the substrate for Gq-activated PLC-β hydrolysis of PIP2 to IP3 and diacylglycerol (DAG). This was based on the fact that gelsolin includes a region that binds PIP2 (16) presumably making it a less available substrate for PLC-β-mediated hydrolysis. We now questioned whether this peptide region of the gelsolin protein (5) could modify Gq-coupled agonist-mediated signaling in ASM and attenuate airway constriction.
MATERIALS AND METHODS
Materials.
The 10 amino acid sequence of the gelsolin peptide within the PIP2-binding region (QRLFQVKGRR) and a control peptide (QRL) were synthesized and conjugated with rhodamine B by Biomatik (Wilmington, DE). Unless otherwise stated, all other chemicals were obtained from Millipore Sigma (St. Louis, MO).
Animals.
All animal studies were approved by the Institutional Animal Care and Use Committee at Columbia University. C57BL/6J or A/J wild-type male mice at 8–10 wk old were purchased from Jackson Laboratory (Bar Harbor, ME).
Human ASM culture.
Studies using de-identified human tissue were reviewed by the Columbia University Institutional Review Board and deemed not human subjects research. ASM was dissected from the posterior wall of the trachea or bronchi of discarded portions of healthy donor lungs for transplant. The tissue was enzymatically digested using the Papain Dissociation System (Worthington Biochemical, Lakewood, NJ) according to the manufacturer’s protocol. Immortalized human ASM cell lines modified to stably express human telomerase reverse transcriptase (8) were a kind gift from Dr. William Gerthoffer (University of Nevada, Reno, NV) and were stably transfected to express the human M3 muscarinic receptor (27). The cells were cultured in medium 199 supplemented with 10% fetal bovine serum, 10 units/ml penicillin, 10 µg/ml streptomycin, 25 pg/ml fungizone, 1 ng/ml human fibroblast growth factor, 0.25 ng/ml human epidermal growth factor, and insulin-transferrin-selenium (0.17 µM insulin, 6.9 nM transferrin, and 3.9 nM sodium selenite) (Gibco, Thermo Fisher Scientific, Waltham, MA).
Inositol phosphate assay.
Inositol phosphate synthesis was measured in confluent cultures of primary or immortalized human ASM cells as described previously (12). Briefly confluent cells in 24-well plates were loaded overnight with 5 μCi/ml myo-[3H]inositol (specific activity: 20 Ci/mmol) in inositol-free DMEM. After two buffer washes (37°C HBSS containing 10 mM LiCl), cells were either untreated or pretreated for 10 min with 50 µM of rhodamine B-conjugated control peptide or 10 amino acid gelsolin peptide followed by an incubation in 300 µl buffer/well containing no additives or 10 µM bradykinin or acetylcholine for 20 min at 37°C. Reactions were terminated by the addition of cold methanol, and newly synthesized [3H]inositol phosphates were separated from 3H-inositols by methanol/chloroform extraction and column chromatography and quantified by liquid scintillation counting as described previously (12). Human ASM cells stably expressing the human M3 muscarinic receptor were used to measure inositol phosphate synthesis in response to the Gq-coupled agonist acetylcholine.
Mouse myograph study.
A multiwire myograph system (DMT, Ann Arbor, MI) was used to measure tracheal ring ASM tension. C57Bl/6J male mice were euthanized after an intraperitoneal injection of 100 mg/kg sodium pentobarbital. The tracheas were dissected free of connective tissue and cut in half. Each half closed tracheal ring was mounted in an organ bath filled with Krebs-Henseleit (KH) buffer, between two pins with one side attached to a force transducer. The KH buffer was of the following composition (in mM): 115 NaCl, 2.5 KCl, 1.91 CaCl2, 2.46 MgSO4, 1.38 NaH2PO4, 25 NaHCO3, and 5.56 d-glucose, pH 7.4, and was maintained at 37°C and continuously bubbled with 95% O2-5% CO2. After equilibration at 5-mN resting tension for 1 h in KH buffer with a buffer exchanges every 15 min, contractile force was continuously recorded using Acknowledge 3.7.3 software (Biopac Systems, Goleta, CA). The rings were contracted two times with increasing concentrations of acetylcholine (0.1 µM to 1 mM) with extensive buffer exchanges between and after these two cycles. Each ring was then contracted to its individually calculated EC50 concentration with acetylcholine for three times. After the third contraction and subsequent buffer exchange, 50 µM of peptides or vehicle control (water) were added to the buffer and the rings were incubated for 45 min before the fourth contraction by the same EC50 concentration of acetylcholine. Tetrodotoxin (1 µM) was added to the KH buffer 5 min before each contraction with acetylcholine to eliminate the potential confounding effects of endogenous nerves to the contractions.
Human myograph study.
Tracheal ASM strips were isolated and suspended under resting tension (1.5 g) in oxygenated physiological buffer at 37°C. The buffer was of the following composition (in mM): 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.2 MgSO4, 1.3 NaH2PO4, 25 NaHCO3, and 5.6 d-glucose, with 10 µM indomethacin, pH 7.4, and was maintained at 37°C and continuously bubbled with 95% O2-5% CO2. A 4-ml water-jacketed organ bath was used to measure tracheal ASM tension in response to exogenous acetylcholine with or without pretreatment with the gelsolin peptide or control peptide. After three cycles of contractions with increasing concentrations of acetylcholine (0.1 µM to 1 mM), each ASM strip was contracted to its individually calculated EC50 concentration with acetylcholine. Following a buffer exchange and resetting of the resting tension to 1.5 g, ASM strips were pretreated with 50 µM of the gelsolin or control peptide conjugated to rhodamine B. The ASM strips were then challenged with a repeated exposure to an EC50 concentration of acetylcholine. The magnitude of the contraction induced by this second acetylcholine EC50 challenge was compared with the acetylcholine EC50 contraction before the peptide treatment.
Mouse precision-cut lung slices preparation.
Mouse precision-cut lung slices (PCLS) were prepared from C57Bl/6J wild-type male mice as described in detail previously (3, 21, 22). Briefly, mice were euthanized after an intraperitoneal injection of 100 mg/kg sodium pentobarbital and the trachea was cannulated. The lungs were inflated with 1.3 ml of 2% low-melting temperature agarose in HBSS at 37°C. Lung lobes were cut into 130-µm slices using a tissue slicer (Compresstom VF-300; Precisionary Instruments, San Jose, CA). The collected lung slices were incubated overnight at 37°C in low-glucose DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA) supplemented with antibiotics in a cell culture incubator with 10% CO2.
Peripheral lung luminal airway diameter measurements.
Contractile response of peripheral airways was measured as previously described (20). In brief, lung slices were mounted in a custom-made perfusion chamber and the airway luminal diameter change was observed using phase-contrast microscopy and images were recorded using a CCD camera and image acquisition software (Video Savant; IO Industries, London, ON, Canada). Lung slices were continuously superfused with HBSS buffer allowing for the introduction and removal of contractile mediators (i.e., methacholine) or pretreatments (i.e., gelsolin or control peptide) without halting perfusion.
Respiratory system mechanics measurements.
A/J male mice were anesthetized with an intraperitoneal injection of 50 mg/kg pentobarbital (Vortech Pharmaceuticals, Dearborn, MI), and a tracheotomy was performed. Mechanical ventilation was initiated through an 18-gauge metal cannula connected to a flexiVent FX1 module (SCIREQ, Montreal, QC, Canada). The animal was paralyzed with 10 mg/kg intraperitoneal succinylcholine and ventilated with room air at a constant volume of 10 ml/kg at a frequency of 150 breaths/min and a positive endo-expiratory pressure of 3 cmH2O. Three baseline forced oscillatory measurements were performed, and total respiratory system resistance and elastance were recorded. After 30 μl PBS were aerosolized using an ultrasonic nebulizer (Aeroneb Laboratory ANP-1000, pore size 4.0–6.0 μm), a standard lung volume history was established and measured for ~5 min. Following PBS, each animal was nebulized with either 5 mM control peptide or 10 amino acid gelsolin peptide, followed 5 min later by increasing concentrations (3.125, 6.25, 12.5, 25, and 37.5 mg/ml) of methacholine chloride in PBS with an administration time of 10 s. The change in response to methacholine aerosol challenge was recorded by the repeated force oscillatory technique every 20 s for 8 min for each concentration of methacholine. In separate experiments, PBS, gelsolin, or control peptide was nebulized to tracheotomized mice to measure the airway deposition of the peptide in vivo. After nebulization, mice were ventilated with room air for 30 min, then lungs were dissected and fixed in 4% paraformaldehyde, and frozen sections were made for histological analysis. The lung sections were permeabilized with 0.2% Triton X-100 in PBS, blocked in 5% normal goat serum and incubated with 1:100 anti-SM22 alpha antibody (ab 14106; Abcam, Cambridge, MA) in 2% normal goat serum followed by incubation with Alexa 488-conjugated secondary antibody.
Pyrene actin depolymerization and polymerization assay.
In vitro actin depolymerization and polymerization were measured using an Actin Polymerization Biochem Kit (Cytoskeleton, Denver, CO) according to the manufacturer’s instructions with minor modifications. For the depolymerization assay, pyrene F-actin was prepared by incubating pyrene labeled muscle actin (1 mg/ml) in 5 mM Tris·HCl, 0.2 mM CaCl2, 12.5 mM KCl, 0.5 mM MgCl2, 1.25 µM guanidine carbonate, and 0.25 mM ATP, pH 8.0, for 1 h at room temperature. The resulting actin stock was diluted to 0.04 mg/ml in 5 mM Tris·HCl buffer with 0.2 mM CaCl2 and 0.2 mM ATP and transferred to a 96-well black polystyrene assay plate, 200 µl/well. After baseline fluorescent measurements to ensure equal loading of the actin stock, 20 µl of buffer control (water), peptides, or full-length gelsolin protein (final concentration 0.1 µM) were added to the wells, and the fluorescent signal (excitation: 350 nm; emission: 407 nm) was read every 30 s for 20 min using a FlexStation 3 microplate reader (Molecular Devices, Sunnyvale, CA). For the polymerization assay, pyrene-labeled muscle actin (0.4 mg/ml) in 5 mM Tris·HCl buffer with 0.2 mM CaCl2 and 0.2 mM ATP was transferred to 96-well black polystyrene assay plates, 200 µl/well. After baseline fluorescent measurements, 20 µl of buffer control (water or 0.01% DMSO) or peptides (final 0.1 µM) were added and the fluorescent reading was continued to determine if the peptides themselves could enhance actin polymerization. The fluorescence reading was then paused, 20 µl of polymerization buffer (resulting concentration: 5 mM Tris·HCl, 0.2 mM CaCl2, 40 mM KCl, 1.6 mM MgCl2, 4 µM guanidine carbonate, and 0.8 mM ATP, pH 8.0) were added, and the fluorescence recording was continued every 1 min for 60 min. 0.1 µM of human recombinant plasma gelsolin (Cytoskeleton) was used as positive control for actin depolymerization, and 1 µM latrunculin A (Molecular Probes, Eugene, OR) was used as a control for the inhibition of actin polymerization.
Statistics.
Data are expressed as means ± SE. Statistical analysis was carried out using Graph Pad Prism software (GraphPad Software, La Jolla, CA). Comparisons between two groups (control peptide and gelsolin peptide) were tested using unpaired Student’s t-test, Mann-Whitney test, or Wilcoxon’s test as appropriate on the distribution of data. Multiple comparisons were tested using ANOVA followed by applying the Bonferroni post hoc comparisons. For the human myograph study, the responses of control peptide- and gelsolin peptide-treated tracheal strips from a single donor were considered paired observation, because each donor’s tracheal samples were harvested and stored at different times, which could possibly affect contractile responses. Two-sided P < 0.05 was considered significant.
RESULTS
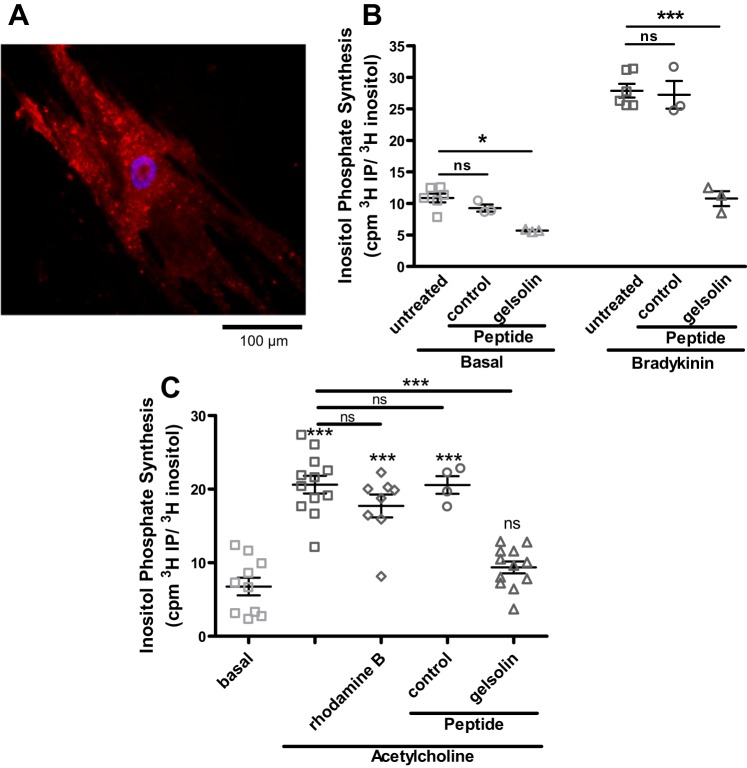
Rhodamine B-conjugated peptides were rapidly internalized into ASM cells. Preliminary time-course experiments showed that the peptides were visualized intracellularly within 5 min of the introduction of the peptides to cell culture media. The fluorescence seen in the ASM cells was distributed through the cell in a punctate pattern and was resistant to washing (Fig. 1A). We chose to use a 10-min preincubation time with the peptides to measure IP3 synthesis in primary cultures of human ASM cells in response to Gq-coupled receptor agonists. Cells that were pretreated with the 10 amino acid gelsolin peptide fragment synthesized less IP3 under both unstimulated basal conditions (P < 0.05; Fig. 1B, left) and when stimulated with the Gq-coupled agonist bradykinin (P < 0.001; Fig. 1B, right). Similar to the findings with bradykinin, pretreatment of immortalized human ASM cells stably expressing the M3 muscarinic receptor attenuated IP3 synthesis in response to another Gq-coupled receptor agonist, acetylcholine (P < 0.001; Fig. 1C, comparing acetylcholine stimulation with and without gelsolin).
Fig. 1.
Gelsolin peptide introduction into primary cultures of human airway smooth muscle (ASM) cells and inositol triphosphate (IP3) synthesis decreases in response to the Gq-coupled agonists bradykinin and acetylcholine. A: representative fluorescent micrograph of primary human ASM cell after 1 h incubation with rhodamine B-conjugated gelsolin peptide. Scale bar = 100 µm. B: IP3 synthesis at 20 min in response to bradykinin in primary cultures of human ASM cells. Gelsolin peptide-treated cells generated less IP3 under basal and bradykinin conditions compared with untreated or control peptide treated cells (n = 3–6). C: IP3 synthesis in response to another Gq-coupled agonist acetylcholine in human ASM cells stably expressing the M3 muscarinic receptor. Gelsolin peptide-treated cells again generated less IP3 compared with controls (n = 4–12 from 3 different sets of experiments). cpm, Counts/min; ns, not significant. *P < 0.05, ***P < 0.001, ANOVA with Bonferroni comparison.
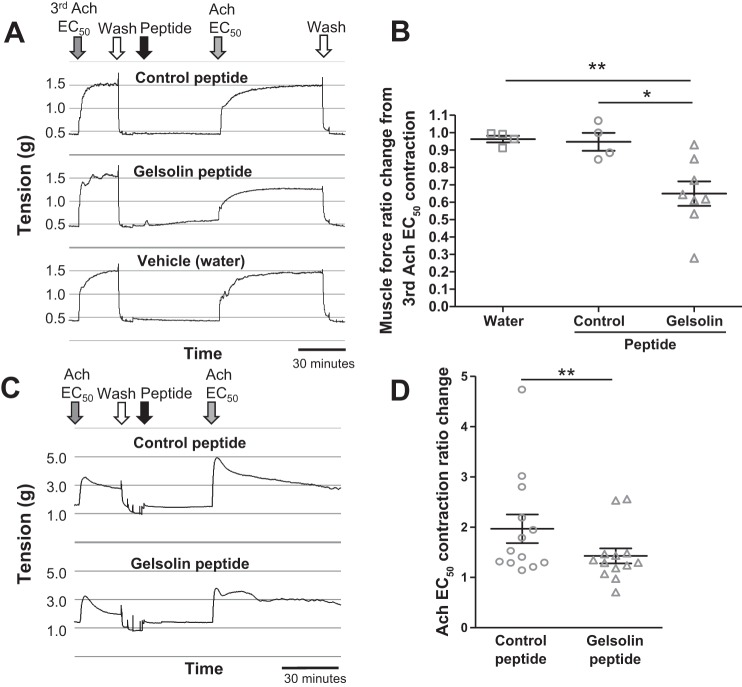
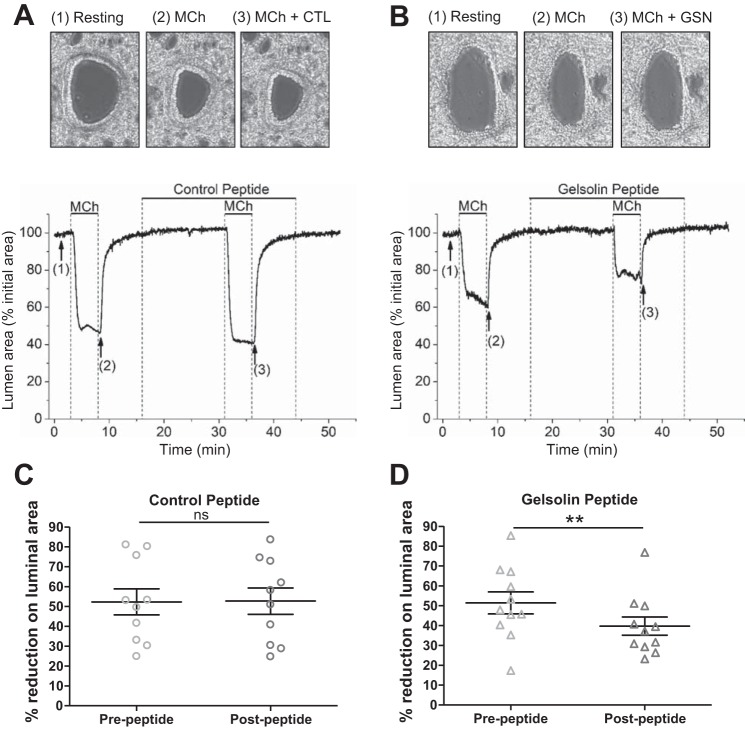
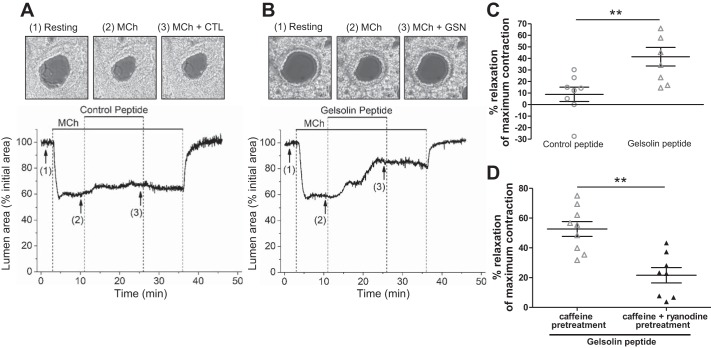
We next tested the effect of the peptides on ASM contraction. Muscle force from isolated mouse tracheal rings and isolated human tracheal muscle strips was measured ex vivo. A representative muscle force tracing of mouse tracheal rings (Fig. 2A) and human tracheal ASM strips (Fig. 2C) contracted with an EC50 concentration of acetylcholine before and after peptide exposure is shown. In response to acetylcholine, gelsolin peptide-treated mouse tracheal rings contracted significantly less than vehicle-treated (water) (P < 0.01; Fig. 2B) or control peptide-treated rings (P < 0.05; Fig. 2B). In human tracheal ASM strips, the contractile response after gelsolin peptide pretreatment was attenuated compared with the response after control peptide pretreatment (P = 0.005; Fig. 2D). These results of the attenuation of central ASM contraction by the gelsolin peptide led us to investigate the peptide’s effect on peripheral airway contractions, using PCLS. Changes in the luminal area of peripheral airways in response to methacholine with control or gelsolin peptide were recorded. We found that pretreatment with the gelsolin peptide resulted in a significantly smaller methacholine-induced decrease in airway luminal area (P = 0.003; Fig. 3) and that the gelsolin peptide relaxed a preestablished methacholine-induced airway contraction to a significantly greater degree than the control peptide (P = 0.005; Fig. 4, A–C).
Fig. 2.
Wire myograph study using isolated murine tracheal rings and isolated human tracheal airway smooth muscle. A: after 3 sets of contractions induced by cumulative concentrations of acetylcholine (Ach), mouse tracheal rings were washed and contracted with an EC50 concentration of Ach another 3 times. The 4th Ach EC50-induced contraction after gelsolin or control peptides was compared with the Ach EC50-induced contraction before the peptides. Representative wire myograph tracings are shown. B: gelsolin peptide-treated mouse tracheal rings contracted significantly less than water control- or control peptide-treated rings (n = 4 for water control or control peptide; n = 8 for gelsolin peptide; *P < 0.05, **P < 0.01, ANOVA with Bonferroni comparison). C: after 3 sets of contractions induced by cumulative concentrations of Ach, the human tracheal muscle strips were washed and contracted with an EC50 concentration of Ach. The magnitude of the following Ach EC50 contraction after peptide was compared with the previous Ach EC50 contraction before peptide treatment. Representative myograph tracings are shown. D: gelsolin peptide-treated human tracheal muscle strips also contracted significantly less than control peptide-treated rings (n = 13 paired tracheal airway smooth muscle strips from 7 organ donors; **P < 0.01, Wilcoxon signed rank test).
Fig. 3.
Mouse precision cut lung slices (PCLS) pretreated with a gelsolin (GSN) peptide demonstrated reduced methacholine (MCh)-induced airway constriction. A and B: peripheral airway was first contracted with 300 nM MCh, then incubated with 10 µM control (CTL; A) or GSN peptide (B) for 15 min. Subsequent MCh-induced contractions in the presence of peptides were compared with the 1st MCh-induced contractions. Representative images (top) from the data plotted in tracing (bottom) are shown, with corresponding numbered time points (1–3). corresponds to airway before stimulation (1: Resting), contraction in response to 300 nM MCh (2: MCh), and subsequent MCh contraction after pretreatment with peptides (3: MCh + control CTL peptide or GSN peptide). C: prepeptide MCh contraction (time point indicated as 2) was not different from postpeptide MCh contraction in control peptide treated lung slices. D: gelsolin peptide significantly attenuated postpeptide MCh-induced contraction (n = 10–11 slices; n = 3 mice for each group; **P < 0.01, Wilcoxon signed rank test to compare before and after peptide treatment).
Fig. 4.
Gelsolin (GSN) peptide attenuated methacholine (MCh)-induced peripheral airway constriction, and ryanodine and caffeine pretreatment abolished subsequent relaxation of MCh-induced contraction by GSN peptide. A and B: 8 min after initiation of airway constriction with MCh, peripheral airways were exposed to 10 µM gelsolin or control peptide. Representative micrograph images (top) from the data plotted in tracings (bottom) are shown (1–3). corresponds to airway before stimulation (1: Resting), contraction in response to 300 nM MCh (2: MCh) and airway after peptide application [3: MCh + control (CTL) peptide or GSN peptide]. C: airway relaxation measured at the end of peptide treatment was significantly more in gelsolin peptide-treated airways (n = 8 slices, n = 3 mice for each group). D: when 25 µM ryanodine were applied with 20 mM caffeine to deplete intracellular calcium stores, the subsequent MCh-induced constriction was not reversed by GSN peptide. GSN peptide-induced relaxation was compared between caffeine alone and caffeine plus ryanodine treatment (n = 8–9 slices, n = 3 mice for each group). **P < 0.01, Mann-Whitney.
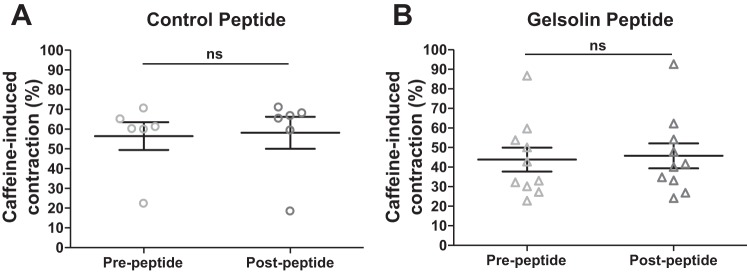
A central mechanistic hypothesis for the relaxing effect of the gelsolin peptide, is that the peptide binds to PIP2, making it an unavailable substrate for PLC, resulting in reduced IP3 synthesis and reduced release of intracellular calcium. Therefore, we treated murine lung slices with caffeine and ryanodine to deplete the intracellular stores targeted by IP3 and attempted to relax a preestablished methacholine contraction. The relaxation effect of the gelsolin peptide was no longer observed (P = 0.002; Fig. 4D), consistent with the hypothesis that the relaxing effect of gelsolin peptide is due to its PIP2 binding. In addition, we also confirmed that caffeine-induced contractions in PCLS, which result from a release of internally sequestered calcium in sarcoplasmic reticulum through the ryanodine receptor, were not affected by the gelsolin peptide (Fig. 5), further supporting the hypothesis that the site of action of the gelsolin peptide is at the binding of PIP2.
Fig. 5.
Caffeine-induced peripheral airway constriction was not affected by the gelsolin peptide. Peripheral airway in mouse precision cut lung slices was first contracted with 20 mM caffeine and then incubated with 10 µM control (A) or gelsolin (B) peptide for 15 min. Subsequent caffeine-induced contractions in the presence of peptides were compared with the prepeptide caffeine-induced contractions. Both control and gelsolin peptide had no effect on caffeine-induced contraction (n = 6–10 slices; n = 3 mice for each group; ns, not significant, Wilcoxon signed rank test to compare before and after peptide treatment).
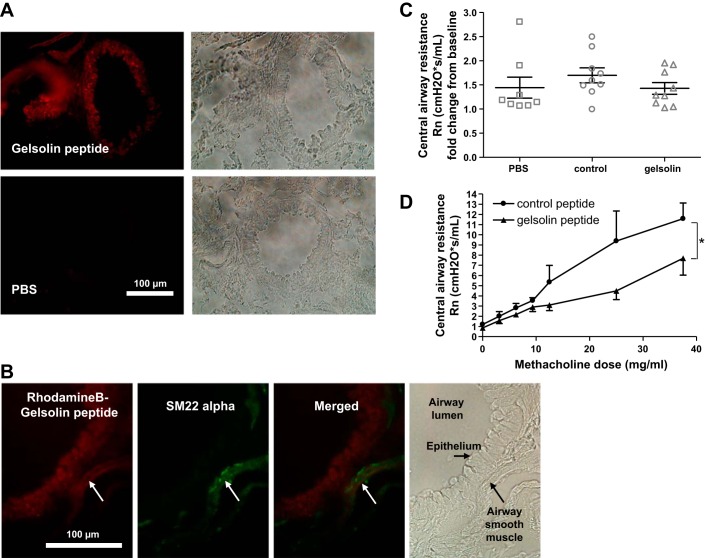
In an attempt to investigate the feasibility of the clinical usage of the gelsolin peptide, we delivered the peptide by nebulization in vivo to tracheotomized mice. Figure 6A shows successful deposition of the rhodamine B-conjugated gelsolin peptide in peripheral airways after nebulization. The red fluorescent signal was detected beyond the epithelial layers, suggesting good penetration of the peptide to the area around the ASM. Further immunofluorescent staining with a smooth muscle marker SM22 alpha demonstrated that the red fluorescent signal colocalized with SM22 alpha staining in ASM (Fig. 6B). Nebulization of control or gelsolin peptides alone did not affect baseline central airway resistance compared with PBS nebulization (Fig. 6C). Consistent with ex vivo studies, in vivo increases in central airway resistance in response to methacholine were significantly less in gelsolin peptide-nebulized mice, compared with control peptide nebulized mice (P < 0.04; Fig. 6D).
Fig. 6.
Gelsolin peptide prevented increases in airway resistance in response to escalating doses of inhaled methacholine. A: confirmation of the penetration of rhodamine B-conjugated peptides in peripheral airways. Representative mouse lung histology 30 min after nebulization of gelsolin peptide or PBS via a tracheostomy. B: costaining with a smooth muscle marker SM22 alpha (green) showed localization of rhodamine B-conjugated peptide (red) overlapping with the green signal. White arrow indicates airway smooth muscle layer. C: inhalation of control or gelsolin peptide itself did not affect central airway resistance (Rn) compared with PBS inhalation (n = 8–9 mice in each group, ANOVA with Bonferroni comparison). D: following inhalation of a control peptide or gelsolin peptide, A/J mice were exposed to increasing concentrations of methacholine via nebulization. An increase in Rn in response to an escalating dose of methacholine was significantly attenuated in mice pretreated with the gelsolin peptide compared with mice pretreated with the control peptide. n = 6–9 (up to 25 mg/ml dose) and n = 3–5 (up to 37.5 mg/ml dose), *P < 0.05, Mann-Whitney.
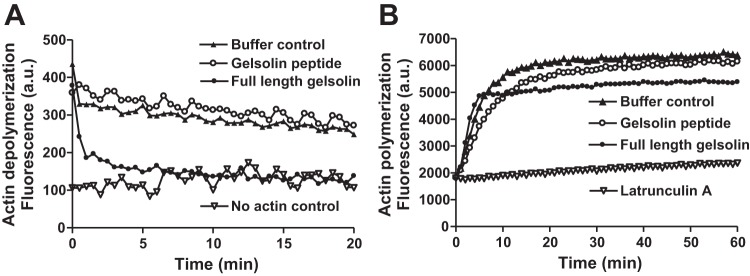
To further investigate the central hypothesis that the mechanism by which gelsolin peptide attenuates ASM contraction is via gelsolin’s PIP2 sequestration as opposed to a direct effect of the peptide on actin polymerization, cell-free actin polymerization and depolymerization were measured. In vitro actin depolymerization and polymerization assays using both full-length gelsolin and the peptide fragment of gelsolin were performed. As expected, full-length gelsolin was able to depolymerize actin filaments in vitro, but the gelsolin peptide tested in the current study did not induce actin depolymerization (Fig. 7A), supporting the hypothesis that the gelsolin peptide impairment of contraction is mediated by peptide sequestration of the PIP2 substrate of PLC-β. Both full-length gelsolin and the gelsolin peptide did not affect the initial rate of actin polymerization in vitro; however, full-length gelsolin reached a lower fluorescence plateau indicating less polymerized actin, possibly due to gelsolin’s capping effect, which is known to prevent prolongation of actin filaments (Fig. 7B).
Fig. 7.
The peptide fragment of gelsolin did not induce depolymerization of actin and did not affect actin polymerization. A: time courses for depolymerization of pyrene-F-actin after the addition of 0.1 µM gelsolin peptide fragment (open circle) or full-length gelsolin protein (closed circle). Gelsolin peptide did not cause actin depolymerization assessed as a decrease in pyrene-F-actin fluorescence, whereas full-length gelsolin protein acutely decreased the fluorescence as expected. B: time courses for polymerization of pyrene-F-actin over a period of 1 h. Buffer control (closed triangle) and gelsolin peptide (open circle) samples resulted in similar fluorescence intensity. Full-length gelsolin protein (closed circle) increased fluorescence initially but subsequently reached lower fluorescence plateau, consistent with gelsolin’s actin filament capping effect. Latrunculin A (open inverted triangle) served as a control for the prevention of polymerization. The data shown are representative of 3 experiments for both depolymerization and polymerization.
DISCUSSION
In the current study, we demonstrated that a 10 amino acid peptide of the actin-binding protein gelsolin, containing a PIP2-binding region, attenuates Gq-coupled receptor agonist-mediated ASM constriction ex vivo using isolated mouse and human upper ASM as well as murine peripheral airways. We were able to successfully deliver this peptide fragment of gelsolin into the mouse lung in vivo by nebulization and observed that the increase in central airway resistance of mice pretreated with the gelsolin peptide in response to increasing doses of methacholine was significantly reduced compared with control peptide-treated mice.
This is the first demonstration of a peptide attenuating Gq-coupled receptor agonist-mediated ASM constriction ex vivo and in vivo following nebulized delivery. Peptide-based approaches to treat asthma have been limited to allergen-derived peptide therapy to induce T-cell tolerance or targeting the reduction of the immune response (11). For example, signal transducer and activator of transcription 6 (STAT6) is activated by IL-4 and IL-13 and plays a role in promoting Th2-type responses (7), and it is reported that transnasal treatment with a STAT6 inhibitory peptide at the time of neonatal respiratory syncytial virus infection attenuated methacholine-induced airway hyperreactivity upon respiratory syncytial virus reinfection as adults (24). The intraperitoneal injection of a peptide inhibitor of the disintegrin and metalloproteinase domain-containing protein 8 (ADAM8) attenuated airway responsiveness to methacholine stimulation in the ovalbumin mouse model of asthma (4). In contrast, in the current study, we administered the gelsolin peptide via nebulization immediately before in vivo mouse lung resistance measurements and demonstrated a reduction in methacholine-induced airway constriction. It is likely that this gelsolin peptide effect was upon the ASM and not on inflammation since the methacholine challenge began 5 min after the nebulization of the gelsolin peptide. Our approach targeting ASM contraction may be an alternative therapeutic strategy to the use of immunomodulatory peptides and proteins in the management of allergic lung diseases.
The rhodamine B-conjugated gelsolin peptide has been studied in vitro for its binding activity on polyphosphoinositides, which include PIP and PIP2, and the biochemical properties of this peptide and its effect on actin assembly and structure have been reported in fibroblasts, platelets, and neutrophils (5). It was shown that the 10 amino acid gelsolin peptide blocked actin assembly in neutrophils and fibroblasts by measuring the relative decreases in F-actin or stress fibers in neutrophils and fibroblasts, respectively. This suggested direct effect of the gelsolin peptide on actin assembly is in contrast to the results of the current study in which the functional effects of the peptide were attributed to PIP2 binding and not due to a direct effect on actin. These prior findings in neutrophils and fibroblasts may be explained by the gelsolin peptide binding to PIP2 and effecting calcium homeostasis/signaling in these intact cells, which subsequently modulates actin assembly.
We demonstrated a beneficial effect of the gelsolin peptide on ASM contractile tone when it was administered before or after a contractile agonist. In our large airway ex vivo myograph studies and in vivo airway lung resistance measurements, the gelsolin peptide was administered before the contractile agonists to prevent contraction. Conversely, the effect of this peptide on an established contraction was tested using PCLS to explore the possibility of using this peptide as a therapeutic during acute bronchoconstriction. We observed an attenuation of existing methacholine-induced small airway constriction by the gelsolin peptide. This observation expands the potential therapeutic utility of this peptide to both the prevention and treatment of bronchoconstriction.
The inhalational delivery of a small peptide therapeutic has multiple potential advantages over full-length proteins or other routes of delivery. The small size of the peptide allows for better distribution in airway if nebulized (19). Small peptides are relatively inexpensive to synthesize compared with full-length proteins. In addition, inhaled protein/peptide therapies have the potential to treat many types of respiratory diseases because they can be delivered to the target organ directly without proteolytic degradation in the blood (6). For example, the anti-EGF receptor monoclonal antibody cetuximab was shown to accumulate rapidly in the lung after its delivery via the airway, at concentrations two times higher than those achieved after intravenous administration (9). In our mouse in vivo flexiVent experiment, the rhodamine-conjugated peptides distributed rapidly and extensively after nebulization and we were able to observe the penetration of the peptide beyond the epithelial layers into the region of the ASM. However, this delivery method may have effects on many other cell types in the airway. Because the current study was focused on the effects of the peptide on ASM, we did not evaluate acute or chronic effects on airway epithelial or immune cells. The rhodamine B carrier did not exhibit cell toxicity up to concentrations of 20 µM (5). Because the peptide could modify the homeostasis of calcium signaling in epithelial cells and immune cells in the lung, future studies will be needed to clarify the potential acute and chronic effects on immune cell modulation, including cell migration, macrophage phenotypic change, altered cytokine production, and airway remodeling (23). There could be additional beneficial effects of the gelsolin peptide on these other airway cells. For example, in airway epithelial cells, an environmental allergen such as house dust mite allergen induces sustained intracellular calcium elevations through the activation of store-operated calcium channels and downstream activation of nuclear factor of activated t-cells/calcineurin signaling to increase proinflammatory cytokine production (13). If the gelsolin peptide inhibited PIP2 hydrolysis in airway epithelial cells, intracellular store depletion of calcium would be decreased leading to reduced store-operated calcium entry, resulting in reduced proinflammatory cytokine production.
We performed two complementary studies that support the hypothesis that the major mechanism by which the gelsolin peptide relaxed ASM was via sequestration of PIP2, the substrate of PLC-β for the production of IP3 and DAG. In selected PCLS experiments, intracellular calcium stores were depleted by caffeine and ryanodine. This would deplete the intracellular calcium store targeted by IP3 following its synthesis from PIP2. Under these conditions the gelsolin peptide was no longer effective against a methacholine-induced constriction, which is consistent with its site of action being at the level of PIP2 sequestration. In addition, the gelsolin peptide did not prevent caffeine-induced transient contractions of airways in PCLS, which is the result of direct calcium release from endoplasmic reticulum/sarcoplasmic reticulum calcium stores through ryanodine receptors. A second level of evidence to support the hypothesis is the actin polymerization experiments. The full-length gelsolin protein induced actin depolymerization and impaired maximal actin polymerization consistent with its function as an actin-severing and -capping protein. In contrast, the gelsolin peptide did not affect either of these assays suggesting that its ASM relaxation effects do not involve direct effects on the actin cytoskeleton.
In summary, our study demonstrates that a 10 amino acid fragment of the gelsolin protein, containing a binding region for PIP2, attenuates contraction in response to Gq-coupled ligands in central mouse and human airways and in the mouse peripheral airway. The observed gelsolin peptide’s effect on airway contraction is likely due to the peptide binding to PIP2 rendering it a less available substrate for PLC-β-mediated hydrolysis of PIP2 to IP3 and DAG and not due to an actin-severing function, because the peptide itself did not have an effect on actin depolymerization and polymerization in vitro. The successful delivery of this small peptide fragment of gelsolin via nebulization and the reduction of lung resistance in mice suggests that local lung delivery of this peptide could be a novel therapeutic option for bronchoconstriction.
GRANTS
This work was supported by National Institutes of Health Grants GM-065281 (to C. W. Emala, Sr.), HL-122340 (to C. W. Emala, Sr.), and GM-008464 (to C. W. Emala), Foundation for Anesthesia Education and Research Mentored Research Training Grant (to M. Mikami), and Stony Wold-Herbert Fund (to M. Mikama).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M. conceived and designed research; M.M., J.F.P.-Z., Y.Z., and C.W.E. performed experiments; M.M., J.F.P.-Z., Y.Z., and C.W.E. analyzed data; M.M., J.F.P.-Z., and C.W.E. interpreted results of experiments; M.M., J.F.P.-Z., and C.W.E. prepared figures; M.M. and C.W.E. drafted manuscript; M.M., J.F.P.-Z., and C.W.E. edited and revised manuscript; M.M., J.F.P.-Z., Y.Z., and C.W.E. approved final version of manuscript.
REFERENCES
- 1.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 167: 1360–1368, 2003. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 2.Berair R, Hollins F, Brightling C. Airway smooth muscle hypercontractility in asthma. J Allergy (Cairo) 2013: 185971, 2013. doi: 10.1155/2013/185971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro-Piedras I, Perez-Zoghbi JF. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J Physiol 591: 5999–6015, 2013. doi: 10.1113/jphysiol.2013.257790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Deng L, Dreymüller D, Jiang X, Long J, Duan Y, Wang Y, Luo M, Lin F, Mao L, Müller B, Koller G, Bartsch JW. A novel peptide ADAM8 inhibitor attenuates bronchial hyperresponsiveness and Th2 cytokine mediated inflammation of murine asthmatic models. Sci Rep 6: 30451, 2016. doi: 10.1038/srep30451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham CC, Vegners R, Bucki R, Funaki M, Korde N, Hartwig JH, Stossel TP, Janmey PA. Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J Biol Chem 276: 43390–43399, 2001. doi: 10.1074/jbc.M105289200. [DOI] [PubMed] [Google Scholar]
- 6.Fellner RC, Terryah ST, Tarran R. Inhaled protein/peptide-based therapies for respiratory disease. Mol Cell Pediatr 3: 16, 2016. doi: 10.1186/s40348-016-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res 50: 87–96, 2011. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L523–L534, 2006. doi: 10.1152/ajplung.00013.2006. [DOI] [PubMed] [Google Scholar]
- 9.Guilleminault L, Azzopardi N, Arnoult C, Sobilo J, Hervé V, Montharu J, Guillon A, Andres C, Herault O, Le Pape A, Diot P, Lemarié E, Paintaud G, Gouilleux-Gruart V, Heuzé-Vourc’h N. Fate of inhaled monoclonal antibodies after the deposition of aerosolized particles in the respiratory system. J Control Release 196: 344–354, 2014. doi: 10.1016/j.jconrel.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Hancox RJ, Aldridge RE, Cowan JO, Flannery EM, Herbison GP, McLachlan CR, Town GI, Taylor DR. Tolerance to beta-agonists during acute bronchoconstriction. Eur Respir J 14: 283–287, 1999. doi: 10.1034/j.1399-3003.1999.14b08.x. [DOI] [PubMed] [Google Scholar]
- 11.Hauff K, Zamzow C, Law WJ, De Melo J, Kennedy K, Los M. Peptide-based approaches to treat asthma, arthritis, other autoimmune diseases and pathologies of the central nervous system. Arch Immunol Ther Exp (Warsz) 53: 308–320, 2005. [PubMed] [Google Scholar]
- 12.Hotta K, Emala CW, Hirshman CA. TNF-α upregulates Giα and Gqα protein expression and function in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 276: L405–L411, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Jairaman A, Maguire CH, Schleimer RP, Prakriya M. Allergens stimulate store-operated calcium entry and cytokine production in airway epithelial cells. Sci Rep 6: 32311, 2016. doi: 10.1038/srep32311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, Abramson MJ, McKay KO, Green FH. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med 185: 1058–1064, 2012. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 15.Janmey PA, Lamb J, Allen PG, Matsudaira PT. Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J Biol Chem 267: 11818–11823, 1992. [PubMed] [Google Scholar]
- 16.Janmey PA, Stossel TP. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature 325: 362–364, 1987. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells 29: 311–325, 2010. doi: 10.1007/s10059-010-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikami M, Zhang Y, Danielsson J, Joell T, Yong HM, Townsend E, Khurana S, An SS, Emala CW. Impaired relaxation of airway smooth muscle in mice lacking the actin-binding protein gelsolin. Am J Respir Cell Mol Biol 56: 628–636, 2017. doi: 10.1165/rcmb.2016-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov 6: 67–74, 2007. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Zoghbi JF, Bai Y, Sanderson MJ. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J Gen Physiol 135: 247–259, 2010. doi: 10.1085/jgp.200910365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Zoghbi JF, Sanderson MJ. Endothelin-induced contraction of bronchiole and pulmonary arteriole smooth muscle cells is regulated by intracellular Ca2+ oscillations and Ca2+ sensitization. Am J Physiol Lung Cell Mol Physiol 293: L1000–L1011, 2007. doi: 10.1152/ajplung.00184.2007. [DOI] [PubMed] [Google Scholar]
- 22.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 125: 535–553, 2005. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samanta K, Parekh AB. Store-operated Ca2+ channels in airway epithelial cell function and implications for asthma. Philos Trans R Soc Lond B Biol Sci 371: 20150424, 2016. doi: 10.1098/rstb.2015.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasa BT, Restori KH, Shan J, Cyr L, Xing L, Lee S, Ward BJ, Fixman ED. STAT6 inhibitory peptide given during RSV infection of neonatal mice reduces exacerbated airway responses upon adult reinfection. J Leukoc Biol 101: 519–529, 2017. doi: 10.1189/jlb.4A0215-062RR. [DOI] [PubMed] [Google Scholar]
- 25.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem 274: 33179–33182, 1999. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 26.Svedmyr NL, Larsson SA, Thiringer GK. Development of “resistance” in beta-adrenergic receptors of asthmatic patients. Chest 69: 479–483, 1976. doi: 10.1378/chest.69.4.479. [DOI] [PubMed] [Google Scholar]
- 27.Townsend EA, Zhang Y, Xu C, Wakita R, Emala CW. Active components of ginger potentiate β-agonist-induced relaxation of airway smooth muscle by modulating cytoskeletal regulatory proteins. Am J Respir Cell Mol Biol 50: 115–124, 2014. doi: 10.1165/rcmb.2013-0133OC. [DOI] [PMC free article] [PubMed] [Google Scholar]