Abstract
The tumor-suppressive role of p53, a transcription factor that regulates the expression of many genes, has been linked to cell cycle arrest, apoptosis, and senescence. The noncanonical function or the pathogenic role of p53 has more recently been implicated in pulmonary vascular disease. We previously reported that rapid nuclear accumulation of hypoxia-inducible factor (HIF)-1α in pulmonary arterial smooth muscle cells (PASMCs) upregulates transient receptor potential channels and enhances Ca2+ entry to increase cytosolic Ca2+ concentration ([Ca2+]cyt). Also, we observed differences in HIF-1α/2α expression in PASMCs and pulmonary arterial endothelial cells (PAECs). Here we report that p53 is increased in PAECs, but decreased in PASMCs, isolated from mice with hypoxia-induced pulmonary hypertension (PH) and rats with monocrotaline (MCT)-induced PH (MCT-PH). The increased p53 in PAECs from rats with MCT-PH is associated with an increased ratio of Bax/Bcl-2, while the decreased p53 in PASMCs is associated with an increased HIF-1α. Furthermore, p53 is downregulated in PASMCs isolated from patients with idiopathic pulmonary arterial hypertension compared with PASMCs from normal subjects. Overexpression of p53 in normal PASMCs inhibits store-operated Ca2+ entry (SOCE) induced by passive depletion of intracellularly stored Ca2+ in the sarcoplasmic reticulum, while downregulation of p53 enhances SOCE. These data indicate that differentially regulated expression of p53 and HIF-1α/2α in PASMCs and PAECs and the cross talk between p53 and HIF-1α/2α in PASMCs and PAECs may play an important role in the development of PH via, at least in part, induction of PAEC apoptosis and PASMC proliferation.
Keywords: Bcl-2 proteins, endothelial cell apoptosis, p53, smooth muscle cell proliferation, tumor-suppressor gene
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a complex and progressive disorder characterized by elevation of pulmonary vascular resistance (PVR) primarily due to sustained vasoconstriction, concentric vascular wall thickening, and occlusive intimal lesions (19, 58, 59). Increased PVR leads to increased pulmonary arterial (PA) pressure and right ventricular afterload in patients with PAH and animals with severe pulmonary hypertension (PH). One of the major causes for the concentric pulmonary vascular wall thickening is the increased proliferation and/or decreased apoptosis of pulmonary arterial smooth muscle cells (PASMCs) (37, 41, 57, 69). Moreover, PA endothelial dysfunction due to increased apoptosis of pulmonary arterial endothelial cells (PAECs) has also been demonstrated to contribute to sustained pulmonary vasoconstriction leading to increases in PVR and PA pressure in patients with PAH and animals with experimental PH (25, 44, 66). The cellular and molecular mechanisms of increased proliferation and decreased apoptosis of PASMCs and increased apoptosis of PAECs, however, remain unclear.
It has been demonstrated that cross talk and reciprocal regulation between p53 and hypoxia-inducible factors (HIFs) signaling cascades may play an essential role in the regulation of cell proliferation and apoptosis (31, 72). The transcription factor p53, which serves as a tumor suppressor, regulates a variety of cellular responses pertaining to stress signals. The tumor-suppressive function of p53 has been extensively investigated in malignant conditions, leading to the deduction of its crucial roles in cell-cycle arrest, apoptosis, and senescence (32, 48, 67). Recently, its noncanonical functions have also been implicated in pulmonary vascular disease (16, 28, 39, 42). HIFs, including HIF-1 and HIF-2, are a family of transcription factors that play a vital role in regulating cell function and gene expression under hypoxic conditions (24, 65). Several studies prove that both HIF-1 and HIF-2 coordinate the regulation of cell proliferation, migration, angiogenesis, and differentiation (49, 50, 52, 54). More recently, our group and other investigators have reported that HIF-α, particularly HIF-2α in PAECs, plays a major role in the development of PAH, which suggests different HIFs expressed in various cell types may have distinct functions in regulating cell proliferation and other cell processes (4, 14, 29, 30, 55). Although the hypoxia-induced HIF-1α increase in PASMCs has been implicated in the progression of pulmonary vascular remodeling (4, 34), it is still controversial if inhibition or downregulation of HIF is sufficient to inhibit hypoxic pulmonary vasoconstriction and/or PAH (55). Both p53 and HIFs are maintained at low or undetectable levels during normoxic conditions but function as mediators of cell adaptation to hypoxia and various stresses.
In this study, we observed the divergent expression of p53 and HIF-1α/2α in pulmonary vascular endothelial cells (ECs) and smooth muscle cells (SMCs) in animals with experimental PH. We show that the hypoxia-induced p53 decrease in PASMCs contributes to pulmonary vascular remodeling by stimulation of PASMC proliferation and store-operated Ca2+ entry (SOCE). We find that the hypoxia-induced p53 increase in PAECs contributes to sustained pulmonary vasoconstriction by induction of PAECs apoptosis. The data from this study link an oncological regulatory signaling pathway via p53 and its cross talk with the HIF signaling pathway to the development and progression of PAH or experimental PH. The study also reveals novel targets that can be used for developing new drugs for the treatment of PAH and other forms of PH.
METHODS AND MATERIALS
Animal experiments.
All experimental procedures on animals were approved by the Institutional Animal Care and Use Committee of the University of Arizona and Guangzhou Medical University. For the establishment of hypoxia-induced PH (HPH) in mice, adult male C57BL/6 mice (body weight, 22–25 g) were exposed to normobaric hypoxia (10% O2) in a BioSpherix A chamber (BioSpherix) for 21–28 days (3–4 wk). The hypoxic chamber was consistently maintained at a level of 10% O2 and low CO2 (<0.5%) during the induction period of 28 days (4 wk). Normoxic control mice were housed in the same room as the hypoxic chambers and were maintained under normoxic conditions. Male Sprague-Dawley rats (body weight, 100–150 g) were randomly divided into two groups: a control group and monocrotaline (MCT)-injected group. In the MCT group, a single intraperitoneal injection of MCT (60 mg/kg; Sigma-Aldrich, St. Louis, MO) was given at day 1 to establish the MCT-induced PH. In control rats, a single intraperitoneal injection of saline was used as vehicle control. Mice in hypoxic chambers were exposed to room air for 10 min twice a week for animal care and cage cleaning. All animals had free access to food and water.
Assessment of hemodynamics, right ventricular hypertrophy, and vascular remodeling.
Mice and rats were anesthetized, and a Millar catheter was inserted into the right ventricle via the right jugular vein for live monitoring of right ventricular systolic pressure. After measurement of right ventricular systolic pressure, the heart was excised to assess right ventricular hypertrophy, which was determined by the Fulton index, i.e., the ratio of the weight of right ventricle (RV) to the weight of left ventricle (LV) and septum (S) [RV/(LV + S)] (53). To determine the histological changes of PAs or PA wall, lung tissues embedded in paraffin were sectioned at 5-μm thickness and fixed in a 3% paraformaldehyde solution. Lung tissue samples were stained with antibodies for HIF-1α, HIF-2α, and p53 to examine the vascular distribution and expression level. For the quantification of pulmonary artery (PA) wall thickness, we used the Image-Pro Plus software to measure the thickness of the medial layer (smooth muscle) of small distal PA.
Culture of PASMCs and PAECs.
Use of human cells was approved by the University of Arizona Institutional Review Board. Normal human PASMCs were obtained from Lonza and Cleveland Clinic and maintained in smooth muscle growth (SmGM-2) medium (Lonza, Walkersville, MD) according to the manufacturer’s instruction, while normal human PAECs obtained from Lonza, Cleveland Clinic, and PHBI were cultured in EC growth (EGM-2) media (Lonza). The detailed demographic information of the patients that provide the PASMCs and PAECs are listed in Tables 1 and 2, respectively. For the normoxic group, cells were maintained in a humidified atmosphere of 5% CO2-95% air (21% O2) at 37°C, while cells from the hypoxic group were cultured in 3% O2 at 37°C for 48–72 h. For these studies, all cells used were between 5 and 8 passages. To study the effect of FG-4592, an inhibitor of prolyl hydroxylase domain proteins (PHDs), on the protein level of p53 in human PAECs, we treated the cells with FG-4592 (Cat. No. 10338; Advanced ChemBlocks) for 48 h.
Table 1.
Demographic information of human subjects from whom PASMCs were isolated for the study
| Subjects | Sex | Age | Race | Type of Cells |
|---|---|---|---|---|
| Failed donor 1 | Woman | 51 | Hispanic | PASMCs |
| Failed donor 2 | Man | 57 | Caucasian | PASMCs |
| Failed donor 3 | Man | 35 | Hispanic | PASMCs |
| Failed donor 4 | Woman | 56 | Caucasian | PASMCs |
| Failed donor 5 | Woman | 56 | White | PASMCs |
| IPAH patient 1 | Woman | 57 | Caucasian | PASMCs |
| IPAH patient 2 | Woman | 32 | Caucasian | PASMCs |
| IPAH patient 3 | Man | 41 | White | PASMCs |
| IPAH patient 4 | Man | 45 | Caucasian | PASMCs |
| IPAH patient 5 | Man | 51 | White | PASMCs |
PASMCs, pulmonary arterial smooth muscle cells; IPAH, idiopathic pulmonary arterial hypertension.
Table 2.
Demographic information of human subjects from whom PAEC were isolated for the study
| Subjects | Sex | Age | Race | Type of Cells |
|---|---|---|---|---|
| Failed donor 1 | Woman | 67 | Caucasian | PAECs |
| Failed donor 2 | Woman | 65 | Black | PAECs |
| Failed donor 3 | Woman | 54 | Caucasian | PAECs |
| Failed donor 4 | Woman | 49 | White | PAECs |
| Failed donor 5 | Man | 51 | White | PAECs |
| Failed donor 6 | Man | 49 | White | PAECs |
| IPAH patient 1 | Woman | 32 | Caucasian | PAECs |
| IPAH patient 2 | Man | 45 | Caucasian | PAECs |
| IPAH patient 3 | Man | 41 | White | PAECs |
| IPAH patient 4 | Woman | 34 | Caucasian | PAECs |
| IPAH patient 5 | Man | 51 | White | PAECs |
| IPAH patient 6 | Woman | 22 | White | PAECs |
PAECs, pulmonary arterial endothelial cells; IPAH, idiopathic pulmonary arterial hypertension.
Isolation of pulmonary artery.
As shown in our previous works (62–64), we dissected the distal (>4th generation) intra-PAs from lungs of male Sprague-Dawley rats or C57BL/6 mice under anesthesia. Briefly, the whole lung and heart were removed from the mouse or rat and placed in HBSS (Life Technologies, Carlsbad, CA). The branches of the intrapulmonary arteries were isolated from the whole lung with forceps under a dissecting microscope. The adventitia was removed, the vessel was dissected longitudinally, and the luminal surface was rubbed carefully with a cotton swab to remove the epithelium. The isolated pulmonary artery was used to extract total protein for Western blot experiments.
Western blotting.
PASMCs and PAECs were washed with ice-cold PBS, scraped, placed into an Eppendorf tube, and centrifuged. The pelleted cells were resuspended in 1× RIPA (Bio-Rad, Hercules, CA) supplemented with a protease inhibitor cocktail (Roche). Cells were incubated in lysis buffer on ice for 30 min. The lysates were centrifuged at 12,000 rpm for 30 min at 4°C. The pellet was discarded, and from the supernatant, protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Cell lysates with equal quantities of protein were mixed and boiled in 6 × SDS-sample buffer (Boston BioProducts). Protein lysates were resolved by SDS-PAGE and transferred onto 0.45-µm nitrocellulose membranes (Bio-Rad). Membranes were incubated for 1 h at 22–24°C in a blocking buffer [0.1% Tween 20 in TBS (TBST)] containing 5% nonfat dry milk powder. The membranes were then incubated with primary antibodies diluted in TBST containing 5% BSA, with shaking overnight at 4°C. Membranes were washed three times with TBST for 5 min each, followed by incubation in secondary antibody conjugated to horseradish peroxidase for 2 h at room temperature in TBST containing 5% milk. Membranes were washed three times for 5 min each, and peroxidase activity was visualized with a Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific). Antibodies specifically recognizing p53 (Cat. No. sc-126; Santa Cruz Biotechnology), HIF-1α (Cat. No. NB100-479; Novus), HIF-2α (Cat. No. NB100-122; Novus), Bax (Cat. No. 2772; Cell Signaling Technology), Bcl-2 (Cat. No. BS1511; Bioworld Technology), transient receptor potential channel 1 (TRPC1; Cat. No. ACC-118; Alomone Laboratories), and TRPC6 (Cat. No. ACC-120, Alomone Laboratories) were used at dilutions of 1:1,000. The protein levels of p53, HIF-1α, HIF-2α, Bax, Bcl-2, TRPC1, and TRPC6 were normalized to those of β-actin (1:2,000; Santa Cruz Biotechnology). Band intensity was quantified with ImageJ (National Institutes of Health, Bethesda, MD), and expressed as arbitrary units.
Cell proliferation assay.
Human PASMCs and PAECs were grown on 12-mm diameter coverslips at a density of 5 × 104 cells. Cells were maintained in either normoxic (21% O2) or hypoxic (3% O2) conditions for 48–72 h. During the final 6 h of incubation, 10 µM 5-ethynyl-2′-deoxyuridine (EdU; Life Technologies, Eugene, OR) was added to the cell culture media. Cells were fixed with 4% paraformaldehyde (EM Sciences; Cat. No. 15714) and permeabilized with 0.5% Triton-X (Sigma-Aldrich, St. Louis, MO). Detection of EdU incorporation into newly synthesized DNA was performed using the Click-iT EdU Alexa Fluor 594 imaging kit (Thermo Fisher Scientific, Waltham, MA). Hoescht (1:2,000) was used for staining nuclei. Coverslips were mounted on slides with ProLong Diamond Antifade Mountant (Life Technologies). All reagents were prepared according to manufacturer’s instructions. Fluorescently stained cells were imaged with a Nikon inverted fluorescence microscope (Eclipse Ti-E; Nikon, Tokyo, Japan) with a ×10 objective.
Measurement of cytosolic free Ca2+ concentration in PASMCs.
Fura-2 fluorescence intensity was measured in single human PASMCs as described previously (56, 73) using a Nikon inverted fluorescent microscopy system (Eclipse Ti-E; Nikon). Briefly, cells were grown to 50–60% confluence on 25-mm diameter circular glass coverslips and were incubated with 4 µM fura-2 acetoxymethyl ester (fura-2/AM; Invitrogen/Molecular Probes, Eugene, OR) in HEPES-buffered solution for 60 min at room temperature (22–24°C). Cells were alternatively illuminated at 340 and 380 nm by a Xenon lamp (Hamamatsu Photonics, Hamamatsu, Japan), and the 340/380 ratio was used to calculate the Ca2+ concentration ([Ca2+]cyt) in nanomolar concentration within a region of interest (5 × 5 m) in a cell recorded every 2 s. The fluorescence intensity emitted at 520 nm in cells was captured with an EM-CCD camera (Evolve; Photometrics, Tucson, AZ) and NIS Elements 3.2 software (Nikon). The HEPES-buffered solution contained the following (in mM): 137 NaCl, 5.9 KCl, 1.8 CaCl2, 1.2 MgCl2, 14 glucose, and 10 HEPES (pH was adjusted to 7.4 with 10 N NaOH). The Ca2+-free solution was prepared by replacing 1.8 mM CaCl2 with equimolar MgCl2 and addition of 0.1 mM EGTA to chelate residual Ca2+. All experiments for measurement of [Ca2+]cyt were carried out at room temperature (22–24°C).
Transfection of small interfering RNA.
Human PASMCs were transiently transfected with control small interfering RNA (siRNA) (10 μM; sc-37007; Santa Cruz Biotechnology) and p53 siRNA (10 μM; Santa Cruz Biotechnology) using Xfect siRNA transfection reagent (Clontech Laboratories) according to the manufacturer’s instructions. [Ca2+]cyt measurement and Western blot experiments using p53 siRNA-transfected cells were performed 48 h after transfection.
Statistical analysis.
Data are expressed as means ± SE with the number (n) of experiments performed. Statistical significance between two or more groups was performed by Student’s t-test (paired or unpaired as applicable) or One-way ANOVA using SigmaPlot software. P < 0.05 was accepted as statistically significant. Significant difference is expressed in the figures or figure legends as *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
p53 is decreased in PASMCs but increased in PAECs from animals with experimental PH.
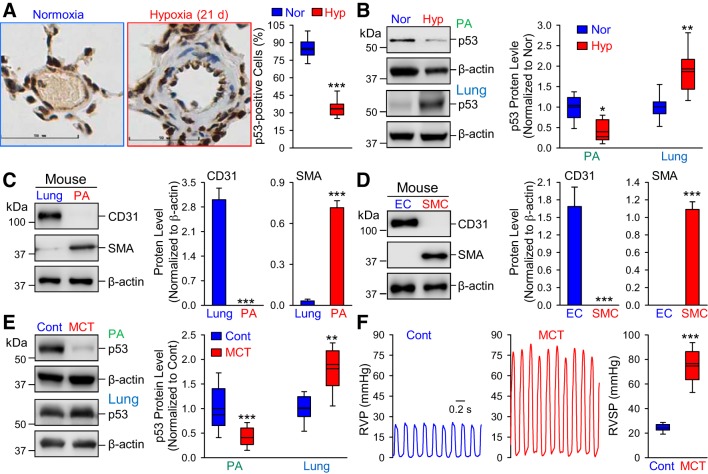
To examine the potential role of p53 in the development of PH, we first measured and compared the protein expression level of p53 in the PA using immunohistochemistry. As illustrated in Fig. 1A, the p53 expression level (brown staining) in the cross section of PA in the lung tissue isolated from normoxic mice was significantly higher than in PA from the mice with HPH (exposed to 10% O2 for 14 days). More than 80% of cells in PA were p53-positive (brown staining) in normoxic control mice, but only 40% of the cells in PA were p53-positive in HPH mice (Fig. 1A, right). The immunohistochemistry experiments were performed in five mice for each group. Western blot analysis further confirmed these results in both the isolated PA and whole lung tissue. The protein level of p53 in the isolated PA (mainly composed of PASMCs) was significantly lower in HPH mice than in normoxic control mice (Fig. 1B). In contrast, the protein level of p53 in the whole lung tissue (mainly composed of lung ECs) was significantly higher in HPH mice than in normoxic control mice (Fig. 1B). The experiments were performed in five normoxic control mice and five HPH mice.
Fig. 1.
The protein level of p53 is downregulated in pulmonary arteries (PA) [mainly composed of PA smooth muscle cells (PASMCs)] but upregulated in lung tissue [mainly consisting of lung endothelial cells (ECs)] in mice with hypoxia-induced pulmonary hypertension (PH) and rats with monocrotaline-induced PH (MCT-PH). A: representative immunohistochemistry images (left) showing p53 level (shown in brown) in PA from normoxic (Nor) and chronically hypoxic (Hyp, 10% O2 for 14 days) mice. Right: summarized data (means ± SE) showing the percentage of p53-positive cells from Nor and Hyp mice. Scale bar = 50 µm. B: representative images (left) and summarized data (means ± SE, n = 5; right) showing Western blot analysis on p53 in isolated PA (PA, mainly containing PASMCs) and whole lung tissue (Lung, mainly containing ECs) in Nor and Hyp mice. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. Nor. C and D: representative images (left) and summarized data (means ± SE, n = 5; right) showing Western blot analyses of CD31 (an EC marker) and α-smooth muscle actin (SMA; a SMC marker) in the whole lung tissue (Lung) and isolated PA (PA) from normal mice (C), as well as in mouse ECs and SMCs (D). E: representative images (left) and summarized data (means ± SE, n = 5; right) showing Western blot analysis on p53 in the isolated PA (PA) and whole lung tissue (Lung) from control (Cont) rats and rats with MCT-PH (MCT). **P < 0.01 and ***P < 0.001 vs. Cont. F: representative record of right ventricular pressure (RVP; left) and summarized data (means ± SE; right) showing right ventricular systolic pressure (RVSP) in Cont and MCT rats. ***P < 0.001 vs. Cont.
To confirm that the isolated PA ring is mostly composed of PASMCs, while the whole lung tissue mainly consists of lung ECs, we examined and compared the expressions level of the EC marker CD31 (or Pecam1) and the SMC marker α-smooth muscle actin (SMA; Acta2) in the whole lung tissue and the isolated PA ring. As shown in Fig. 1C, the protein expression level of CD31 (EC marker) was very high in the whole lung tissue of normal mice, while CD31 was not even detectable in isolated PA. The protein level of SMA (SMC marker), however, was significantly higher in isolated PA than in the whole lung tissue. These data indicate that the total protein extracted from the isolated PA is mainly composed of protein from PASMCs, while the total protein isolated from the whole lung tissue consists mostly of protein from lung vascular ECs.
Furthermore, the divergent changes of p53 in the PA (mainly PASMCs) and the lung tissue (mainly lung ECs) were also observed in control and MCT-PH rats (Fig. 1E). The protein expression level of p53 in the isolated PA from MCT-PH rats was significantly lower than in the PA from control rats, while the protein level of p53 in the whole lung tissue from MCT-PH rats (n = 5) was much higher than in the lung tissue of control rats (n = 5) (Fig. 1E).
These data show that endothelial and smooth muscle p53 demonstrate different responses to hypoxia and MCT injection, suggesting that p53 may play different roles in the regulation of proliferation and apoptosis of PASMC and lung vascular ECs.
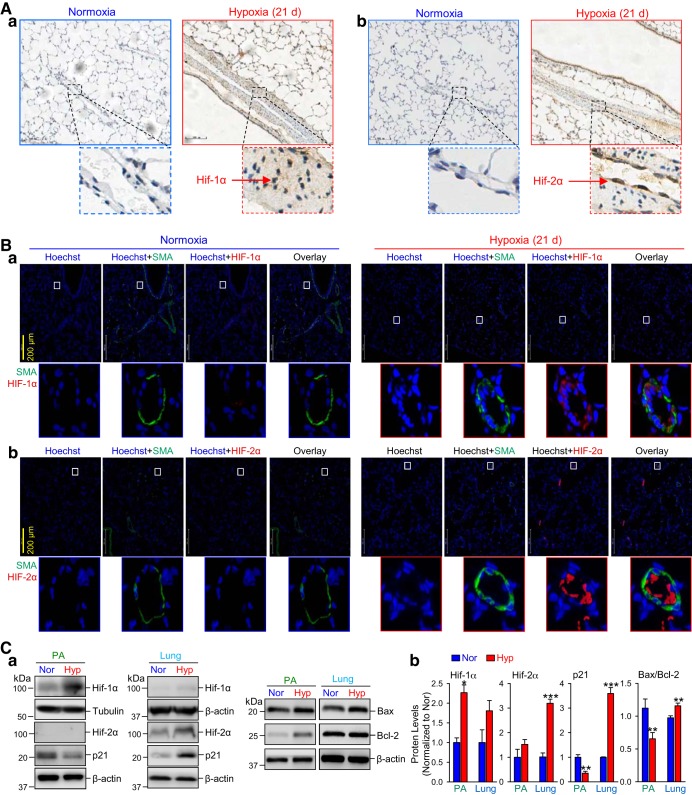
Hypoxia selectively increases HIF-1α in PASMCs and HIF-2α in PAECs.
Immunohistochemical analysis of lung tissues from HPH mice (exposed to 10% O2 for 21 days) and normoxic mice revealed that upon hypoxic exposure, HIF-1α (brown staining) was mostly increased in the medial layer (smooth muscle), while HIF-2α (brown staining) seemed to be only increased in the intima (endothelium) (Fig. 2A), in HPH mice compared with normoxic control mice. Similarly, immunofluorescence staining also revealed a dominant colocalization of HIF-1α (red) with the smooth muscle marker SMA (green) in the medial layer, while an marked increase of HIF-2α (red) staining in the intimal endothelial layer that is inside of the media positively stained with SMA (green) in HPH mice (Fig. 2B). Western blot experiments on HIF-1α and HIF-2α in the isolated PA (mainly contains PASMCs) and the whole lung tissue (mainly contains lung ECs) further confirm the differential expression levels of HIF-1α and HIF-2α in PASMCs and PAECs in HPH mice. As shown in Fig. 2C, the HIF-1α protein level was significantly increased in the isolated PA (mainly contains PASMCs), while HIF-1α was only increased in the whole lung tissue (mostly lung ECs) (Fig. 2Ca). The HIF-2α protein expression was too low to be detected in most of the isolated PA preparations. However, there was a slight, but statistically significant, increase in HIF-2α as well in HPH mice compared with normoxic control mice (Fig. 2Cb).
Fig. 2.
Chronic hypoxia selectively increases hypoxia-inducible factor-1α (HIF-1α) in pulmonary arterial smooth muscle cells (PASMCs) and HIF-2α in pulmonary arterial endothelial cells (PAECs). A: representative immunohistochemistry images showing HIF-1α (a) and HIF-2α (b) levels (shown in brown) in PA from normoxic (Nor) and chronically hypoxic (Hyp, 10% O2 for 21 days) mice. Scale bar = 100 µm. B: representative immunofluorescence images showing the colocalization of HIF-1α (a) and HIF-2α (b) levels (shown in red) together with the smooth muscle marker α-smooth muscle actin (SMA; shown in green) in PA from normoxic (Normoxia) and chronically hypoxic (Hypoxia, 10% O2 for 21 days) mice. Scale bar = 200 µm. C: representative images (a) and summarized data (means ± SE; n = 5; b) showing Western blot analysis on HIF-1α, HIF-2α, p21, Bax, and Bcl-2 in isolated PA (PA, mainly containing PASMCs) and whole lung tissue (Lung, mainly containing endothelial cells) in Nor and Hyp mice. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. Nor.
Taken together, these data imply that chronic hypoxia 1) increases HIF-1α with a greater degree in PASMCs and a less greater degree in PAECs but predominantly increases HIF-2α only in PAECs, and 2) decreases p53 in PASMCs, but increases p53 in PAECs. The decreased p53 is correlated with increased HIF-1α in PASMCs, while the increased p53 is associated with increased HIF-2α in PAECs.
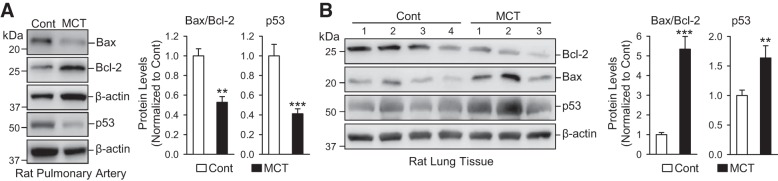
Divergent changes of p53 in PASMCs and PAECs are associated with divergent changes of the Bax/Bcl-2 ratio in MCT-induced PH.
To investigate whether the decreased p53 in PASMCs and increased p53 in PAECs are a general phenomenon that occurred in PH, we also compared p53 expression level in PASMCs and PAECs in control rats and rats with MCT-PH. The results indicated that p53 was decreased in isolated PA (mainly composed of PASMCs), but increased in whole lung tissue (primarily consisting of lung EC), isolated from MCT-PH rats compared with control rats (Fig. 3). Furthermore, the decreased p53 in isolated PA from MCT-PH rats was associated with a decrease of Bax, a proapoptotic protein, and an increase of Bcl-2, an antiapoptotic protein (Fig. 3A). The increased p53 in whole lung tissues from MCT-PH rats was associated with an increase of Bax and a decrease of Bcl-2 (Fig. 3B). Bax and Bcl-2 are both considered target proteins of p53 (26, 38), and the results shown in Fig. 3 indicate that p53 is involved in regulating Bax and Bcl-2 in PASMCs and PAECs.
Fig. 3.
Decreased p53 in isolated pulmonary arteries (PA) [mainly composed of PA smooth muscle cells (PASMCs)] is associated with the decreased Bax/Bcl-2 ratio, while increased p53 in whole lung tissue (mainly containing ECs) is associated with increased Bax/Bcl-2, in rats with monocrotaline-mediated PH (MCT-PH) compared with normal control (Cont) rats. A: Western blot analysis of Bax, Bcl-2, and p53 in isolated PAs from control and MCT rats (left). Summarized data (means ± SE; n = 5; right) showing the ratio of Bax to Bcl-2 and protein level of p53 in PA from control and MCT rats. B: Western blot analysis on Bax, Bcl-2, and p53 in whole lung tissues from control and MCT rats (left). Summarized data (means ± SE; right) showing the ratio of Bax to Bcl-2 and protein level of p53 in lung tissues from control and MCT rats. **P < 0.01 and ***P < 0.001 vs. Cont.
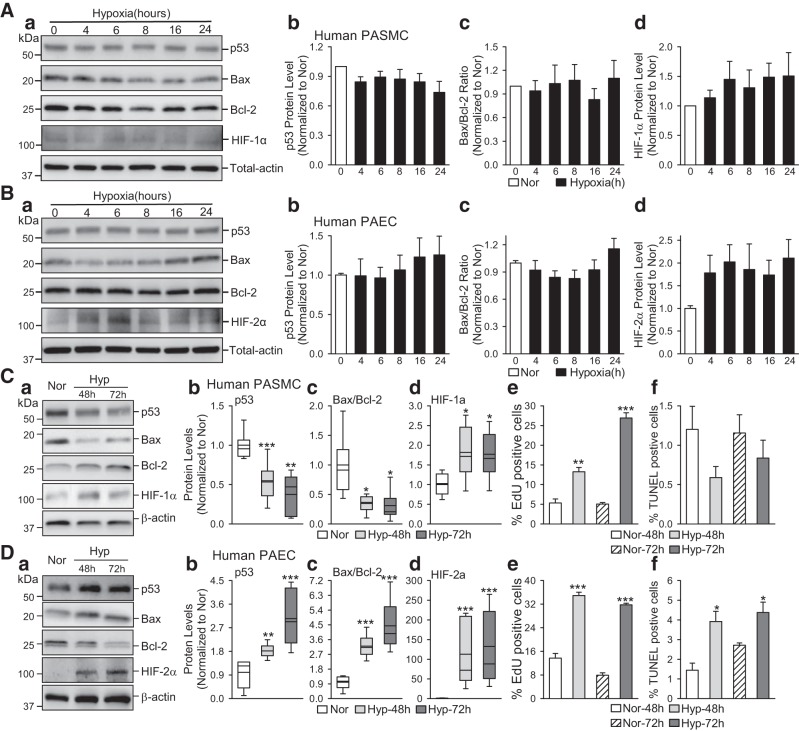
Hypoxia decreases p53 in human PASMCs but increases p53 in human PAECs.
To examine if hypoxia directly affects HIF and p53 in PASMCs, we also conducted experiments to compare HIF-1α/HIF-2α in human PASMCs and PAECs incubated under normoxic and hypoxic conditions. To begin with different time points, we measured the protein levels of p53, Bax, Bcl-2, and HIF-1α/HIF-2α in human PASMCs and PAECs at the early stage for hypoxia conditions (3% O2 for 4, 6, 8, 16, and 24 h). As shown in Fig. 4, A and B, the protein levels of p53 was gradually decreased in human PASMCs (Fig. 4Ab), while increased in human PAECs (Fig. 4Bb) under hypoxic conditions, compared with normoxic cells. The ratio of Bax/ Bcl-2 remained unchanged in human PASMCs (Fig. 4Ac) and PAECs (Fig. 4Bc) under short-time hypoxia condition. As shown in Fig. 4, C and D, incubation of human PASMCs in a hypoxic incubator (3% O2, for 48–72 h) significantly decreased the protein levels of p53 and Bax (Fig. 4C), whereas incubation of human PAECs in a hypoxic incubator (3% O2 for 48–72 h) significantly increased p53 and Bax (Fig. 4D). The hypoxia-induced decreases in p53 and the ratio of Bax/Bcl-2 in human PASMCs (Fig. 4C) were also associated with an increase in HIF-1α protein level and a significant increase of PASMCs proliferation, determined by EdU incorporation. The hypoxia-induced increases in p53 and the ratio of Bax/Bcl-2 were associated with an increase in HIF-2α in human PAECs. Moreover, we found significant increases of PAEC proliferation and apoptosis under hypoxic conditions (3% O2 for 48–72 h) (Fig. 4, De and Df). The decreased ratio of Bax, a pro-apoptotic protein, to Bcl-2, an antiapoptotic protein, in PASMCs would inhibit apoptosis and contribute to the hypoxia-induced cell proliferation and PA medial hypertrophy. Moreover, the increased Bax/Bcl-2 ratio in PAECs would increase apoptosis and cause endothelial dysfunction or injury.
Fig. 4.
Hypoxia decreases p53 and Bax/Bcl-2 ratio in human pulmonary arterial smooth muscle cells (PASMCs) and increases p53 and Bax/Bcl-2 ratio in human pulmonary arterial endothelial cells (PAECs). A: representative images (a) and summarized data (means ± SE, n = 5; b–d) showing Western blot analysis of p53 (b), Bax/Bcl-2 (c), and hypoxia-inducible factor (HIF)-1α (d) in human PASMCs incubated under normoxic (Nor) and hypoxic (Hyp, 3% O2 for 4, 6, 8, 16, and 24 h) conditions. B: representative images (a) and summarized data (means ± SE; n = 5; b–d) showing Western blot analysis of p53 (b), Bax/Bcl-2 (c), and HIF-2α (d) in human PAECs incubated under normoxic (Nor) and hypoxic (Hyp, (3% O2 for 4, 6, 8, 16 and 24 h) conditions. C: representative images (a) and summarized data (means ± SE, n = 5; b–f) showing Western blot analyses of p53 (b), Bax/Bcl-2 (c), and HIF-1α (d) in human PASMCs incubated under normoxic (Nor) and hypoxic (Hyp, (3% O2 for 48 and 72 h) conditions. Summarized data on cell proliferation and cell apoptosis, determined by 5-ethynyl-2′-deoxyuridine (EdU) incorporation (e) and terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay (f) in Nor and Hyp PASMCs is also shown. D: representative images (a) and summarized data (means ± SE, n = 5; b–f) showing Western blot analysis of p53 (b), Bax/Bcl-2 (c), and HIF-2α (d) in human PAECs incubated under normoxic (Nor) and hypoxic (Hyp, 3% O2 for 48 and 72 h) conditions. Summarized data on cell proliferation and cell apoptosis, determined by EdU incorporation (e) and TUNEL assay (f) in Nor and Hyp PAECs, are also shown. For these studies, all cells used were between 5 and 8 passages. We compare the same passage number of cells for each experiment. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. Nor.
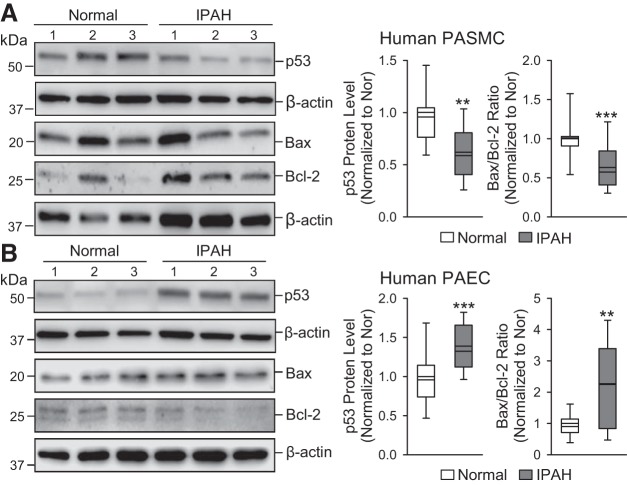
p53 is decreased in PASMCs and increased in PAECs from idiopathic PAH patients.
Similar to the changes of p53 in PASMCs and PAECs from animals with HPH and MCT-PH, PASMCs from idiopathic PAH (iPAH) patients exhibited significant decreases in the protein expression level of p53 and the Bax/Bcl-2 ratio compared with normal control PASMCs (Fig. 5A). In contrast, PAECs from IPAH patients demonstrated significant elevation of p53 and Bax/Bcl-2 ratio (Fig. 5B). The data obtained from PASMCs and PAECs in animal models and IPAH patients all indicate that p53 is decreased in PASMCs and increased in PAECs in animals and patients with PH.
Fig. 5.
Decreased p53 in human pulmonary arterial smooth muscle cells (PASMCs) is associated with the decreased Bax/Bcl-2 ratio, while increased p53 in human pulmonary arterial endothelial cells (PAECs) is associated with increased Bax/Bcl-2, in patients with idiopathic pulmonary arterial hypertension (IPAH), compared with normal controls. A: Western blot analysis of p53, Bax, and Bcl-2 in normal and IPAH PASMCs (left). Summarized data (means ± SE, n = 5; right) showing the protein level of p53 and the ratio of Bax/Bcl-2 in normal and IPAH PASMCs. B: Western blot analysis of p53, Bax, and Bcl-2 in normal and IPAH PAECs (left). Summarized data (means ± SE; right) showing the protein levels of p53 and the ratio of Bax/Bcl-2 in normal and IPAH PAECs. For these studies, all cells used were between 5 and 8 passages. We compare the same passage number of cells for each experiment. **P < 0.01 and ***P < 0.001 vs. Normal.
p53 inhibits PASMC proliferation by decreasing SOCE.
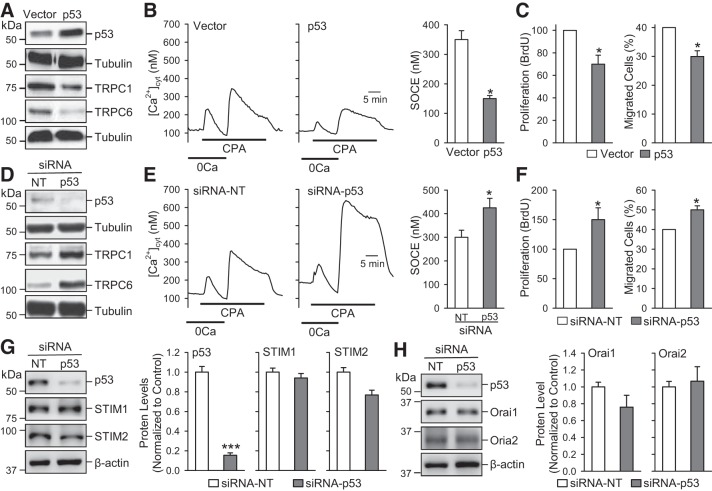
Our group and other investigators demonstrated that upregulation of selective Ca2+-permeable channels, such as TRPC channels, is involved in the enhancement of Ca2+ signaling in PASMCs contributing to sustained pulmonary vasoconstriction and pulmonary vascular remodeling (71). To investigate whether p53 is involved in functional and transcriptional regulation of TRPC channels and Ca2+ signaling, we conducted a series of in vitro experiments in human PASMCs using p53-siRNA (to decrease p53 in PASMCs) and p53-plasmid (to overexpress p53 expression level in PASMCs). As indicated in Fig. 6, overexpression of p53 in human PASMCs downregulated protein expression of TRPC1 and TRPC6 (Fig. 6A) and attenuated the amplitude of SOCE induced by cyclopiazonic acid-mediated passive store depletion (Fig. 6B). In contrast, downregulation of p53 in human PASMCs using siRNA upregulated TRPC1/TRPC6 (Fig. 6D) and significantly enhanced the amplitude of SOCE (Fig. 6E). Moreover, overexpression of p53 also resulted in substantially less proliferation and migration of human PASMCs compared with control transfected cells (Fig. 6C), whereas depletion of p53 in human PASMCs showed significantly higher proliferation and migration compared with control transfected cells (Fig. 6F).
Fig. 6.
Overexpression of p53 downregulates Transient receptor potential channel (TRPC) attenuates SOCE and inhibits pulmonary arterial smooth muscle cell (PASMC) proliferation and migration, while downregulation of p53 upregulates TRPC channels, enhances SOCE, and increases PASMC proliferation and migration. A: Western blot analysis of p53, TRPC1, and TRPC6 in human PASMC transfected with an empty vector and p53. B: representative record showing changes in cytosolic Ca2+ concentration ([Ca2+]cyt) before, during, and after application of 10 µM cyclopiazonic acid (CPA) in the absence (0Ca) or presence of extracellular Ca2+ in human PASMC transfected with an empty vector and p53. The bar graph shows the amplitude of [Ca2+]cyt increases due to CPA-induced SOCE (means ± SE; n = 5). C: cell proliferation [bromodeoxyuridine (BrdU) incorporation; means ± SE; n = 5] and migration (%cells migrated; means ± SE; n = 5) in PASMCs transfected with vector and p53. D: Western blot analyses of p53, TRPC1, and TRPC6 in human PASMCs transfected with siRNA-NT and siRNA-p53. E: representative record showing changes in [Ca2+]cyt before, during and after application of CPA in the absence (0Ca) or presence of extracellular Ca2+ in human PASMCs transfected with siRNA-NT and siRNA-p53. The bar graph shows the amplitude of [Ca2+]cyt increases due to CPA-induced SOCE (means ± SE; n = 5). F: cell proliferation (BrdU incorporation; means ± SE, n = 5) and migration (% cells migrated; means ± SE; n = 5) in human PASMCs transfected with siRNA-NT and siRNA-p53. G: Western blot analysis of p53, STIM1, and STIM2 in human PASMC transfected with siRNA-NT and siRNA-p53 (left). Summarized data (means ± SE; right) showing the protein levels of p53, STIM1, and STIM2 in control PASMCs and siRNA-p53 treated PASMCs. H: Western blot analysis of p53, Orai1, and Orai2 in human PASMC transfected with siRNA-NT and siRNA-p53 (left). Summarized data (means ± SE; n = 5; right) showing the protein levels of p53, Orai1, and Orai2 in control PASMCs and siRNA-p53-treated PASMCs. For these studies, all cells used were between 5 and 8 passages. We compared the same passage number of cells for each experiment. *P < 0.05 and ***P < 0.001 vs. vector or siRNA-NT.
Since TRPC channels have been demonstrated to participate mainly in forming receptor-operated channels, we also examined the effect of p53 on STIM1 and STIM2, the Ca2+ sensors in the sarcoplasmic/endoplasmic reticulum that recruit Orai in the plasma membrane to form store-operated Ca2+ channels in PASMCs. Downregulation of p53 with siRNA in human PASMCs significantly decreased the protein level of p53 but had a negligible effect on protein expression levels of STIM1/STIM2 (Fig. 6G) and Orai1/Orai2 (Fig. 6H). These data indicate that p53 selectively downregulates TRPC1 and TRPC6 channels in PASMCs to inhibit Ca2+ influx and attenuate PASMC proliferation. The antiproliferative effect of p53 in PASMCs is, at least in part, due to its inhibitory effect on TRPC1 and TRPC6 channels.
Hif is involved in upregulating p53 and increasing Bax/Bcl-2 ratio in PAECs.
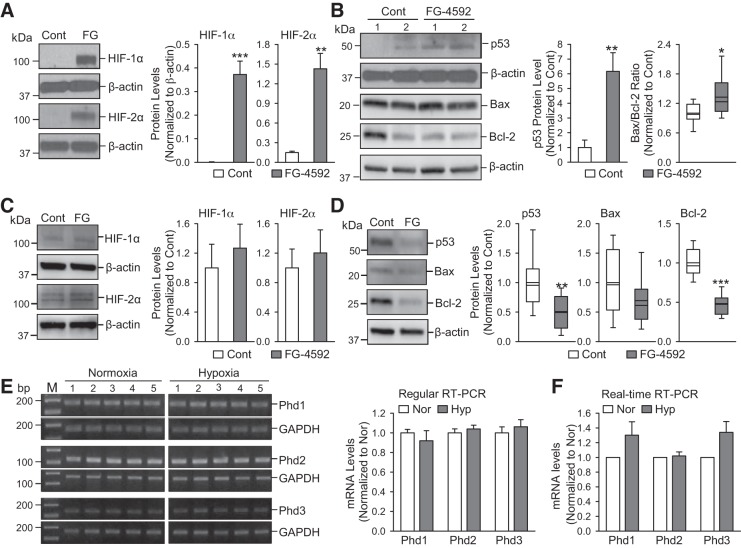
To examine whether increased HIF in PAECs is involved in upregulating p53, we conducted a series in vitro experiments using normal PAECs. Under normoxic conditions, Hif is hydroxylated by PHDs. Hydroxylation of Hif facilitates the binding of von Hippel-Lindau protein (VHL) to Hif, which turns Hif into a substrate for E3 ubiquitin ligase and undergoes polyubiquitylation and proteasomal degradation. Hypoxia inhibits PHD activity and increases Hif by attenuating its degradation. Using a specific inhibitor of PHD, we conducted in vitro experiments to examine whether increasing HIF in normal human PAECs and PASMCs upregulates or downregulates p53. Inhibition of PHD with FG-4592 (100 µM for 48 h) increased protein level of HIF-1α/HIF-2α (Fig. 7A) and, indeed, upregulated p53 and the ratio of Bax/Bcl-2 (Fig. 7B) in normal PAECs. However, the same dosage and time of FG-4592 did not markedly affect the expression of HIF-1α/HIF-2α (Fig. 7C) but downregulated p53, Bax and Bcl-2 in normal PASMCs. Our regular (Fig. 7E) and real-time (Fig. 7F) RT-PCR experiments showed that the mRNA expression levels of all Phd isoforms (Phd1, Phd2, and Phd3) were not changed in whole lung tissues (mainly contain lung ECs) in chronically hypoxic mice compared with normoxic mice. These results also suggest that hypoxia-induced increase of HIF-2α in PAECs is potentially an upstream transcriptional activator of p53 in the lung vascular endothelium.
Fig. 7.
Increasing hypoxia-inducible factor (Hif) by pharmacologically blocking prolyl hydroxylase domain proteins (PHDs) upregulates p53 in human pulmonary arterial endothelial cells (PAECs) and mRNA expression level of PHDs is comparable in lung tissues from normoxic and chronically hypoxic mice. A: Western blot analysis of HIF-1α and HIF-2α in normal human PAECs treated with vehicle (Cont) and FG-4592 (FG, 100 µM for 48 h), a selective blocker of PHDs. Summarized data (means ± SE; n = 5; right) showing protein levels of HIF-1α and HIF-2α in control PAECs and FG-treated PAECs. B: Western blot analysis of p53, Bax and Bcl-2 in control PAECs and FG-treated PAECs. Summarized data (means ± SE, n = 5; right) showing the protein levels of p53 and the ratio of Bax/Bcl-2 in control PAECs and FG-treated PAECs. C: Western blot analysis of HIF-1α and HIF-2α in normal human PASMCs treated with vehicle (Cont) and FG-4592 (FG, 100 µM for 48 h). Summarized data (means ± SE, n = 5; right) showing protein levels of HIF-1α and HIF-2α in control pulmonary arterial smooth muscle cells (PASMCs) and FG-treated PASMCs. D: Western blot analysis of p53, Bax and Bcl-2 in control PASMCs and FG-treated PASMCs. Summarized data (means ± SE, n = 5; right) showing the protein levels of p53, Bax, and Bcl-2 in control PASMCs and FG-treated PASMCs. E: RNA was extracted and regular quantitative RT-PCR was performed to determine mRNA expression levels for Phd1, Phd2, and Phd3 (left). Summarized data (means ± SE, n = 5; right) showing the mRNA levels of Phd1, Phd2, and Phd3 in whole lung tissues from normoxia and hypoxia (10% O2 for 21 days) mice. F: summarized data (means ± SE, n = 5) represented mRNA levels for Phd1, Phd2, and Phd3 in whole lung tissues from normoxia and hypoxia (10% O2 for 21 days) mice, using real-time quantitative RT-PCR. For these studies, all cells used were between 5 and 8 passages. We compared the same passage number of cells for each experiment. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. Cont or Nor.
DISCUSSION
Here we report that 1) p53 is increased in lung vascular ECs but decreased in PASMCs from animals with experimental PH (e.g., mice with HPH and rats with MCT-PH) and patients with IPAH in comparison to respective normal and normoxic controls; 2) chronic hypoxia increases HIF-1α in PASMCs but increases HIF-2α in PAECs (where HIF-1α expression level is very low); 3) the decreased p53 in PASMCs is associated with increased HIF-1α in mice with HPH and rats with MCT-PH compared with normoxic and normal controls; 4) the increased p53 in PAECs is associated with an increased ratio of Bax/Bcl-2 in cells from rats with MCT-PH; 5) hypoxia decreases p53 in normal human PASMCs and increases p53 in human PAECs, and the differential changes of p53 in pulmonary vascular SMCs and ECs correlates with the changes in HIF-1α and HIF-2α, respectively; and 6) overexpression of p53 in normal human PASMCs downregulates TRPC1 and TRPC6 channels and inhibits SOCE, while downregulation of p53 with siRNA upregulates TRPC1/C6 and enhances SOCE. These observations imply that differentially regulated p53 and HIF-1α/2α expression in PASMCs and PAECs and the cross talk between p53 and HIF-1α/2α in PASMCs and PAECs may play an important role in the development and progression of PAH in humans and experimental PH in animals. These experimental data also help highlight the importance of p53 in the regulation of cell proliferation and apoptosis of human PASMCs and PAECs under normoxic and hypoxic conditions.
Sustained pulmonary vasoconstriction and concentric PA wall thickening are two of the major causes of the elevated pulmonary vascular resistance in patients with PAH and animals with experimental PH. This study identifies p53 as a potential pathogenic transcriptional factor in PASMCs and PAECs that plays an important role in the development and progression of PH and PAH through its effects on PASMCs/PAECs proliferation and apoptosis.
Progressive pulmonary vascular remodeling, characterized by concentric arterial wall thickening and neointimal/plexiform lesions in small arteries and arterioles, in patients with IPAH shares similar features with cancer. The similarities between IPAH and cancer lie not only in the mechanisms of development of the diseases but also in the treatment strategies (2, 3, 6, 40). Although IPAH does not emulate all of the characteristics of cancer, it exhibits similar features of cancer pathogenesis, namely, self-sufficiency in growth signals, cellular metabolic disorder, resistance to apoptosis, angiogenesis, insensitivity to antiproliferative signals, and limitless replicative potential (21). However, IPAH is not cancer, because it does not adhere to the primary characteristics of cancer: tissue invasion and metastasis. IPAH still has multiple cancer-like pathways and processes in its development. Of note, pulmonary vascular remodeling due to the abnormal growth, the excess cellular proliferation, and the apoptosis resistance of PASMCs (and other types of vascular cells) lead IPAH to be a cancer-like pulmonary vascular disease (8).
The tumor suppressor p53 is activated in response to oncogenic signaling, DNA damage, and cell stress (7, 9, 35, 45). It prevents the propagation of transformed cells via induction of cell-cycle arrest, senescence, and/or apoptosis, and it also regulates intercellular communications within the tumor microenvironment (32, 48, 67). Given its specific roles in cancer, p53 has received attention from the pulmonary vascular disease community, and its noncanonical functions have been implicated in IPAH and PAH in general (16, 28, 42). Mice deficient for p53 developed more severe PH under hypoxic conditions, indicating a greater degree of RV hypertrophy and pulmonary vascular remodeling (1, 39). The potential role of p53 in driving proliferation and apoptosis imbalance in pulmonary vascular SMCs and ECs may provide an explanation of its pathogenic involved in the development and progression of pulmonary vascular remodeling and sustained vasoconstriction.
There are some conflicting reports on whether p53 is increased or decreased in lung tissues of animals with experimental PH. Some studies found that the p53 protein expression was significantly increased in lungs from HPH mice (39), while others revealed that its protein level was lower in MCT-PH and HPH animal models (1, 46). Our group and other investigators found that the expression of p53 was upregulated in PAECs from patients with IPAH, leading to PAEC apoptosis (16). Also, p53 was decreased in rat PASMCs incubated under hypoxic (5% O2-5% CO2-90% N2) conditions (for 24 h), leading to increased PASMCs proliferation and inhibited PASMC apoptosis (11). One obvious explanation for the widely variable findings is that p53 may be differentially regulated among the various specific cell populations within the lung.
It is thus important to examine p53 levels in PASMCs and PAECs and to determine whether hypoxia-induced changes of p53 are different in PASMCs and PAECs. Interestingly, as the results show in this study, we provide novel and robust evidence that p53 is decreased in PASMCs or the isolated PA (which mainly contains PASMCs), but increased in PAECs or the whole lung tissues (which mainly contain ECs), from patients with IPAH and animals with experimental PH. Decreased p53 in PASMCs from patients with IPAH and animals with HPH and MCT-PH leads to downregulation of Bax or reduction of the Bax/Bcl-2 ratio and contributes to pulmonary vascular medial hypertrophy. Increased p53 in PAECs from patients with IPAH and animals with HPH and MCT-PH leads to upregulation of Bax or increase of the Bax/Bcl-2 ratio and contributes to pulmonary vascular endothelial dysfunction (which can also subsequently result in sustained pulmonary vasoconstriction) and intimal lesions observed in patients with IPAH and animals with severe PH. These observations suggest a distinct and differential function of p53 signaling in PASMCs and PAECs during the development of PH. It is reasonable to speculate that decreased p53 in PASMCs inhibits cell apoptosis and increases cell proliferation and migration, leading to pulmonary vascular remodeling. Hypoxia-induced increase in p53 in PAECs, however, would result in PAECs apoptosis and lead to endothelial dysfunction. The mouse double minute 2 homology (MDM2) protein is an E3 ubiquitin ligase that promotes p53 degradation and inhibits p53 transcriptional activation. The half-life (t½) of p53 is short because of MDM2-mediated p53 degradation. Posttranslational modifications, including phosphorylation, acetylation, and methylation, of MDM2 prevent MDM2 from binding to p53 for degradation, thereby increasing the level of p53 (13, 15, 23). In PAECs, hypoxia may cause posttranslational regulations of MDM2 and inhibit MDM2-associated p53 degradation, therefore increasing the level of p53 in PAECs.
HIFs play a significant role in modulating hypoxia-induced pulmonary vasoconstriction and PH (30). It is, however, still controversial whether inhibition or downregulation of HIFs is sufficient to inhibit hypoxic pulmonary vasoconstriction and/or HPH (55). Under normoxic conditions, PHD enzymes hydroxylate conserved proline residues within HIF (5). Hydroxylation of HIF facilitates the binding of VHL to HIF, turning HIF into a substrate for VHL, and results in HIF polyubiquitylation and proteasome degradation (68, 70). Under hypoxic conditions, VHL cannot bind to HIFs that are not hydroxylated because of hypoxia-mediated inhibition of PHDs, so HIF is accumulated and increased in hypoxic cells or hypoxic PASMCs (5, 27, 68). VHL, an E3 ubiquitin ligase, also participates in the ubiquitination and degradation of HIF-α and has a decisive role on MDM2-p53. Also, VHL/HIF interact with MDM2/p53 to regulate downstream signaling cascades involved in cell proliferation and apoptosis (22, 33). The HIF-p53 interaction depends on the phosphorylation status of HIF-1α/2α, with dephosphorylated HIF-1α deviating from classical HIF-1α signaling to bind p53 (12). Direct binding of HIF-1α to MDM2 leads to p53 stabilization because HIF-bound MDM2 can no longer efficiently induce p53 degradation (10, 43); this mechanism may explain why hypoxia causes p53 stabilization and subsequently raises p53 level (18, 20) in PAECs. This may also be another mechanism by which hypoxia increases the level of p53 in PAECs.
On the other hand, the accumulated VHL under hypoxic conditions in PASMCs would bind to MDM2-p53 to enhance MDM2-mediated degradation of p53. Thus this mechanism may explain why hypoxia decreases p53 in PASMCs. It has been proposed that there obviously exist a tightly interconnected and functional yin-yang relationship between MDM2-p53 and VHL-HIF-α complexes (36, 47, 51). These findings indicate that a divergent change of p53 in PASMCs and PAECs may link to the development of pulmonary vascular remodeling and vasoconstriction. In addition to the effect of HIF on p53 accumulation and transcriptional activation, p53 may also play a pivotal role in the regulation of HIF expression and transcription activity. The balance between these two signaling pathways, along with the functional interaction, likely contributes to the development of PAH.
Our data also indicate that p53 downregulates TRPC1 and TRPC6 channels in PASMCs, which contributes to enhancing Ca2+ influx and increasing [Ca2+]cyt. As a transcription factor, p53 can mediate both transcriptional activation and repression and plays an important functional role in the regulation of the cell cycle, apoptosis and senescence (17). The p53-response element (p53RE) contains two repeats of a decamer motif “RRRCWWGYYY” separated by a spacer of 0–13 bp. The p53RE is the binding site or regulatory region on the target genes that p53 binds to for transcriptional activation and repression (60, 61). There are several thousands of direct p53 target genes identified by high-throughput studies and individual gene analyses (61). Evaluation of p53 target genes based on the meta-analysis data shows that high-confidence p53 target genes are involved in multiple cellular responses and functions including cell cycle arrest, DNA repair, apoptosis, metabolism, autophagy, and mRNA translation. It has been demonstrated that p53 is a transcription activator and the p53-mediated downregulation of gene expression is often indirect and requires p21 (17), while Wang et al. (60) has proposed different models for p53-mediated transcription repression. There are multiple p53RE in the promoter regions of TRPC1 and TRPC6 genes; however, it is still unclear whether p53 directly downregulates TRPC1 and TRPC6 by binding to the p53RE or indirectly downregulates TRPC1/C6 via upregulation of an intermediate transcription repressor.
In summary, divergently changed p53 in PASMCs (p53 is decreased to enhance PASMC proliferation) and PAECs (p53 is increased to cause PAEC apoptosis) may play an important role in the development and progression of sustained vasoconstriction and vascular remodeling in patients with PAH and animals with experimental PH. The unique cross talk between the p53 and HIF signaling pathways may represent a novel pathogenic mechanism involved in the development and progression of PAH. In this scenario, a hypoxia-mediated decrease of p53 in PASMCs and increase of p53 in PAECs are one of the key pathways to induce pulmonary vascular remodeling and sustained pulmonary vasoconstriction. Therapeutically upregulating p53 in PASMCs and downregulating PAECs, respectively, will probably prove to be an important strategy to develop novel drugs for PAH and PAH-associated RV dysfunction and failure.
GRANTS
This work was supported in part by National Natural Science Foundation of China Grants 81630004, 81470246, 81220108001, 81520108001, and 81770043; Department of Science and Technology of China Grants 2016YFC0903700 and 2016YFC1304102; Changjiang Scholars and Innovative Research Team in University Grant IRT0961; Guangdong Department of Science and Technology Grants 2016A030311020, 2016A030313606, and 2017A020215114; Guangzhou Department of Education Yangcheng Scholarship 12A001S; Guangzhou Department of Education Scholarship 1201630095; Guangzhou Department of Science and Technology Grants 2014Y2-00167 and 201607010358; Guangdong Province Universities; Colleges Pearl River Scholar Funded Scheme of China; and Inner Mongolia Autonomous Region Science and Technology Innovation Guidance Project and Inner Mongolia Autonomous Region Science and Technology Project 20160298. This work was also supported in part by National Heart, Lung, and Blood Institute Grants R35-HL-135807 and U01-HL-125208 and an Actelion ENTELLIGENCE Young Investigator Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W., W.L., K.Y., and J.X.-J.Y. conceived and designed research; Z.W., K.Y., Q. Zheng, C.Z., H.T., A. Babicheva, Q.J., M.L., Y.C., K.W., Q. Zhang, A. Balistrieri, C.W., S.S., R.J.A., and A.A.D. performed experiments; Z.W., Q. Zheng, Q.J., and J.W. analyzed data; Z.W., Q. Zheng, and J.W. interpreted results of experiments; Z.W., K.Y., Q. Zheng, H.T., and J.W. prepared figures; Z.W. and J.W. drafted manuscript; Z.W., K.Y., H.T., S.G.C., J.X.-J.Y., W.L., and J.W. edited and revised manuscript; Z.W., K.Y., Q. Zheng, C.Z., H.T., A. Babicheva, Q.J., M.L., Y.C., K.W., Q. Zhang, A. Balistrieri, C.W., S.S., R.J.A., A.A.D., S.M.B., J.G.G., A.M., J.X.-J.Y., W.L., and J.W. approved final version of manuscript.
REFERENCES
- 1.Abid S, Houssaïni A, Mouraret N, Marcos E, Amsellem V, Wan F, Dubois-Randé JL, Derumeaux G, Boczkowski J, Motterlini R, Adnot S. P21-dependent protective effects of a carbon monoxide-releasing molecule-3 in pulmonary hypertension. Arterioscler Thromb Vasc Biol 34: 304–312, 2014. doi: 10.1161/ATVBAHA.113.302302. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294: H570–H578, 2008. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 3.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 121: 2045–2066, 2010. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, Shah SJ, Schumacker PT. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med 189: 314–324, 2014. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 22: 4082–4090, 2003. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA 104: 11418–11423, 2007. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282: 1497–1501, 1998. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 8.Calvier L, Chouvarine P, Legchenko E, Hoffmann N, Geldner J, Borchert P, Jonigk D, Mozes MM, Hansmann G. PPARγ Links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab 25: 1118–1134.e7, 2017. doi: 10.1016/j.cmet.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev 14: 278–288, 2000. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 α and Mdm2 modulate p53 function. J Biol Chem 278: 13595–13598, 2003. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Cai H, Yu C, Wu P, Fu Y, Xu X, Fan R, Xu C, Chen Y, Wang L, Huang X. Salidroside exerts protective effects against chronic hypoxia-induced pulmonary arterial hypertension via AMPKα1-dependent pathways. Am J Transl Res 8: 12–27, 2016. [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury AR, Long A, Fuchs SY, Rustgi A, Avadhani NG. Mitochondrial stress-induced p53 attenuates HIF-1α activity by physical association and enhanced ubiquitination. Oncogene 36: 397–409, 2017. doi: 10.1038/onc.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, Barlev NA, Reinberg D. Regulation of p53 activity through lysine methylation. Nature 432: 353–360, 2004. doi: 10.1038/nature03117. [DOI] [PubMed] [Google Scholar]
- 14.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. PHD2 deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through HIF-2α. Circulation 133: 2447–2458, 2016. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeHart CJ, Chahal JS, Flint SJ, Perlman DH. Extensive post-translational modification of active and inactivated forms of endogenous p53. Mol Cell Proteomics 13: 1–17, 2014. doi: 10.1074/mcp.M113.030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, Nakahira K, Alcazar MA, Hopper RK, Ji L, Feldman BJ, Rabinovitch M. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab 21: 596–608, 2015. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer M. Census and evaluation of p53 target genes. Oncogene 36: 3943–3956, 2017. doi: 10.1038/onc.2016.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graeber TG, Peterson JF, Tsai M, Monica K, Fornace AJ Jr, Giaccia AJ. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol 14: 6264–6277, 1994. doi: 10.1128/MCB.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev 22: 543–551, 2013. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond EM, Denko NC, Dorie MJ, Abraham RT, Giaccia AJ. Hypoxia links ATR and p53 through replication arrest. Mol Cell Biol 22: 1834–1843, 2002. doi: 10.1128/MCB.22.6.1834-1843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 22.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299, 1997. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 23.Hay TJ, Meek DW. Multiple sites of in vivo phosphorylation in the MDM2 oncoprotein cluster within two important functional domains. FEBS Lett 478: 183–186, 2000. doi: 10.1016/S0014-5793(00)01850-0. [DOI] [PubMed] [Google Scholar]
- 24.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol 23: 9361–9374, 2003. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmüller P, Guignabert C, Humbert M. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation 129: 1332–1340, 2014. doi: 10.1161/CIRCULATIONAHA.113.004555. [DOI] [PubMed] [Google Scholar]
- 26.Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med 7: 1111–1117, 2001. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 28.Jacquin S, Rincheval V, Mignotte B, Richard S, Humbert M, Mercier O, Londoño-Vallejo A, Fadel E, Eddahibi S. Inactivation of p53 is sufficient to induce development of pulmonary hypertension in rats. PLoS One 10: e0131940, 2015. doi: 10.1371/journal.pone.0131940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, Shay S, French JL, West J, Haase VH. The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol 36: 1584–1594, 2016. doi: 10.1128/MCB.01055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YM, Barnes EA, Alvira CM, Ying L, Reddy S, Cornfield DN. Hypoxia-inducible factor-1α in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ Res 112: 1230–1233, 2013. doi: 10.1161/CIRCRESAHA.112.300646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korkolopoulou P, Patsouris E, Konstantinidou AE, Pavlopoulos PM, Kavantzas N, Boviatsis E, Thymara I, Perdiki M, Thomas-Tsagli E, Angelidakis D, Rologis D, Sakkas D. Hypoxia-inducible factor 1α/vascular endothelial growth factor axis in astrocytomas. Associations with microvessel morphometry, proliferation and prognosis. Neuropathol Appl Neurobiol 30: 267–278, 2004. doi: 10.1111/j.1365-2990.2003.00535.x. [DOI] [PubMed] [Google Scholar]
- 32.Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol 16: 393–405, 2015. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 33.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature 387: 299–303, 1997. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Shi B, Huang L, Wang X, Yu X, Guo B, Ren W. Suppression of the expression of hypoxia-inducible factor-1α by RNA interference alleviates hypoxia-induced pulmonary hypertension in adult rats. Int J Mol Med 38: 1786–1794, 2016. doi: 10.3892/ijmm.2016.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74: 957–967, 1993. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 36.Lu H. p53 and MDM2: their Yin-Yang intimacy. J Mol Cell Biol 9: 1–2, 2017. doi: 10.1093/jmcb/mjx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandegar M, Remillard CV, Yuan JX. Ion channels in pulmonary arterial hypertension. Prog Cardiovasc Dis 45: 81–114, 2002. doi: 10.1053/pcad.2002.127491. [DOI] [PubMed] [Google Scholar]
- 38.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11: 577–590, 2003. doi: 10.1016/S1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 39.Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez-Arroyo J, Voelkel NF, Ishizaki T. p53 Gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol 300: L753–L761, 2011. doi: 10.1152/ajplung.00286.2010. [DOI] [PubMed] [Google Scholar]
- 40.Moreno-Vinasco L, Gomberg-Maitland M, Maitland ML, Desai AA, Singleton PA, Sammani S, Sam L, Liu Y, Husain AN, Lang RM, Ratain MJ, Lussier YA, Garcia JG. Genomic assessment of a multikinase inhibitor, sorafenib, in a rodent model of pulmonary hypertension. Physiol Genomics 33: 278–291, 2008. doi: 10.1152/physiolgenomics.00169.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54, Suppl: S20–S31, 2009. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouraret N, Marcos E, Abid S, Gary-Bobo G, Saker M, Houssaini A, Dubois-Rande JL, Boyer L, Boczkowski J, Derumeaux G, Amsellem V, Adnot S. Activation of lung p53 by Nutlin-3a prevents and reverses experimental pulmonary hypertension. Circulation 127: 1664–1676, 2013. doi: 10.1161/CIRCULATIONAHA.113.002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieminen AL, Qanungo S, Schneider EA, Jiang BH, Agani FH. Mdm2 and HIF-1α interaction in tumor cells during hypoxia. J Cell Physiol 204: 364–369, 2005. doi: 10.1002/jcp.20406. [DOI] [PubMed] [Google Scholar]
- 44.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 45.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature 389: 300–305, 1997. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 46.Ravi Y, Selvendiran K, Meduru S, Citro L, Naidu S, Khan M, Rivera BK, Sai-Sudhakar CB, Kuppusamy P. Dysregulation of PTEN in cardiopulmonary vascular remodeling induced by pulmonary hypertension. Cell Biochem Biophys 67: 363–372, 2013. doi: 10.1007/s12013-011-9332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roe JS, Youn HD. The positive regulation of p53 by the tumor suppressor VHL. Cell Cycle 5: 2054–2056, 2006. doi: 10.4161/cc.5.18.3247. [DOI] [PubMed] [Google Scholar]
- 48.Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res 64: 2627–2633, 2004. doi: 10.1158/0008-5472.CAN-03-0846. [DOI] [PubMed] [Google Scholar]
- 49.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123: 3664–3671, 2013. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis 2: e164, 2011. doi: 10.1038/cddis.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimoda LA, Laurie SS. HIF and pulmonary vascular responses to hypoxia. J Appl Physiol (1985) 116: 867–874, 2014. doi: 10.1152/japplphysiol.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimoda LA, Semenza GL. Functional analysis of the role of hypoxia-inducible factor 1 in the pathogenesis of hypoxic pulmonary hypertension. Methods Enzymol 381: 121–129, 2004. doi: 10.1016/S0076-6879(04)81007-3. [DOI] [PubMed] [Google Scholar]
- 54.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med 183: 152–156, 2011. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2α (HIF-2α) alters vascular function and tumor angiogenesis. Blood 114: 469–477, 2009. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song S, Li J, Zhu L, Cai L, Xu Q, Ling C, Su Y, Hu Q. Irregular Ca(2+) oscillations regulate transcription via cumulative spike duration and spike amplitude. J Biol Chem 287: 40246–40255, 2012. doi: 10.1074/jbc.M112.417154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D4–D12, 2013. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 58: 2511–2519, 2011. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 59.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J 40: 1555–1565, 2012. doi: 10.1183/09031936.00046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang B, Xiao Z, Ko HL, Ren EC. The p53 response element and transcriptional repression. Cell Cycle 9: 870–879, 2010. doi: 10.4161/cc.9.5.10825. [DOI] [PubMed] [Google Scholar]
- 61.Wang B, Xiao Z, Ren EC. Redefining the p53 response element. Proc Natl Acad Sci USA 106: 14373–14378, 2009. doi: 10.1073/pnas.0903284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004. doi: 10.1152/ajplung.00319.2003. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 288: L1059–L1069, 2005. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 65.Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1α and hypoxia-inducible factor-2α in HEK293T cells. Cancer Res 65: 3299–3306, 2005. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- 66.Xu W, Erzurum SC. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr Physiol 1: 357–372, 2011. doi: 10.1002/cphy.c090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yee KS, Wilkinson S, James J, Ryan KM, Vousden KH. PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ 16: 1135–1145, 2009. doi: 10.1038/cdd.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu F, White SB, Zhao Q, Lee FS. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA 98: 9630–9635, 2001. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11: 407–420, 2007. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 292: L1202–L1210, 2007. doi: 10.1152/ajplung.00214.2006. [DOI] [PubMed] [Google Scholar]
- 72.Zhou J, Schmid T, Brüne B. HIF-1α and p53 as targets of NO in affecting cell proliferation, death and adaptation. Curr Mol Med 4: 741–751, 2004. doi: 10.2174/1566524043359926. [DOI] [PubMed] [Google Scholar]
- 73.Zhu L, Song S, Pi Y, Yu Y, She W, Ye H, Su Y, Hu Q. Cumulated Ca2+ spike duration underlies Ca2+ oscillation frequency-regulated NFκB transcriptional activity. J Cell Sci 124: 2591–2601, 2011. doi: 10.1242/jcs.082727. [DOI] [PubMed] [Google Scholar]