Abstract
Nonvisual opsin (OPN) receptors have recently been implicated in blue light-mediated photorelaxation of smooth muscle in various organs. Since photorelaxation has not yet been demonstrated in airway smooth muscle (ASM) or in human tissues, we questioned whether functional OPN receptors are expressed in mouse and human ASM. mRNA, encoding the OPN 3 receptor, was detected in both human and mouse ASM. To demonstrate the functionality of the OPN receptors, we performed wire myography of ex vivo ASM from mouse and human upper airways. Blue light-mediated relaxation of ACh-preconstricted airways was intensity and wavelength dependent (maximum relaxation at 430-nm blue light) and was inhibited by blockade of the large-conductance calcium-activated potassium channels with iberiotoxin. We further implicated OPN receptors as key mediators in functional photorelaxation by demonstrating increased relaxation in the presence of a G protein receptor kinase 2 inhibitor or an OPN chromophore (9-cis retinal). We corroborated these responses in peripheral airways of murine precision-cut lung slices. This is the first demonstration of photorelaxation in ASM via an OPN receptor-mediated pathway.
Keywords: airway smooth muscle, blue light, G protein receptor kinase 2, opsin, relaxation
INTRODUCTION
Airway smooth muscle (ASM) is important for embryological lung development (41) and plays a role in the pathophysiology of reactive and inflammatory lung diseases. Smooth muscle constriction controls the aperture of large and small airways, creating resistance to air flow. It is well known that endogenous activation of G protein-coupled receptors (GPCRs; i.e., muscarinic, leukotriene, neurokinin) regulates ASM constriction. However, in the past decade, several GPCRs that mediate signaling functions in other organs have been suggested to have an additional role in ASM contraction. One example is the bitter taste receptors—sensory receptors normally found on the tongue that were found to have large effects on ASM tone (14). Beyond the underscoring of the complexity of signaling in normal ASM physiology, these novel signaling pathways hold promise for the understanding of the wide impact of atypical stimuli on ASM function.
The capacity of light to induce smooth muscle relaxation (termed photorelaxation) has been demonstrated in vascular (3, 10, 17, 27, 38, 44), urethral (46), and corpus cavernosum (28) tissues. This phenomenon appears to be wavelength dependent, with at least two wavelength regions (UV and blue spectra) contributing to photorelaxation via distinct mechanisms. Whereas it is thought that UV light promotes smooth muscle relaxation by increases in nitric oxide release from endogenous stores via a nitric oxide synthase-independent pathway (2, 4, 8), more recent studies have demonstrated that blue light (3, 44) induces relaxation by activating G protein-coupled signaling initiated by opsin (OPN) receptor activation.
OPNs are part of the seven-transmembrane GPCR superfamily of receptors that now includes >1,000 OPN subtypes. Photorelaxation has classically been attributed to the atypical members of the OPN family and includes encephalopsin (OPN3) and melanopsin (OPN4). OPN photoactivation classically involves a partner molecule (also known as chromophore) that enhances the photosensing properties of the receptor. Classically, photoactivation in vision-related OPNs involving 11-cis retinal activation leads to signaling through G transducin, increased phosphodiesterase activity, decreased cGMP, and reduced glutamate release by the photoreceptor cell. Whereas OPN3 and -4 have been described in an array of different tissues (1, 3, 6, 13, 15, 18, 21, 25, 26, 44, 49), the role of the atypical OPNs in extraocular human physiology has not been fully understood, and the associated G protein-coupled pathways of the atypical OPNs have yet to be elucidated.
OPN3 mRNA has been found in several tissues, including skin, lung, liver, brain, and white blood cells (1, 6, 15, 21, 25, 26, 49), and has been appropriately named encephalopsin/panopsin. With regard to its potential physiological role in the lung, OPN3 polymorphisms have recently been associated with an altered risk of developing asthma. A Danish genome-wide search (1,151 individuals) for associated genetic loci linked to asthma demonstrated a strong correlation between locus 1qter mutations (within the OPN3 gene) (49) and asthma. This Danish group confirmed its population finding by cross-referencing with the Genetics of Asthma International Network (1,551 individuals) and demonstrated a correlation of OPN3 single-nucleotide polymorphisms with asthma. Studies have also confirmed OPN3 protein expression in epithelial and immune cells within the lung and have demonstrated functional decreases in lymphocyte (Jurkat cell line) activation when treated with OPN3 small interfering RNA (49). Furthermore, 13-cis-retinoic acid, a derivative of the endogenous OPN ligand 11-cis retinal, has been prescribed to patients for the treatment of acne. Warnings of this medication have been noted for night blindness due to functional OPN inhibition, hypertension, and severe asthma exacerbations (23, 40). Given the relationship between the OPN3 mutation/inhibition and the development of asthma pathophysiology, we questioned if OPN3 activation would result in modulation of ASM tone.
Melanopsin (OPN4) is expressed in optic and nonoptic tissues and has been the best characterized of the atypical OPNs. OPN4 regulates numerous physiological responses, such as melatonin release from the pineal gland and circadian rhythm (5, 19, 47). With regard to smooth muscle effects, OPN4 activation has recently been implicated as mediating peripheral vascular relaxation (3, 44). This discovery by Sikka et al. (44) demonstrated that OPN4 is the key protein in light-mediated relaxation of mouse tail blood vessels. Studies by the same group also demonstrate the expression of OPN3 and OPN4 in the pulmonary vasculature, which displayed photorelaxation properties similar to that in the peripheral vasculature (3). In these studies, it was hypothesized that OPN receptor activation decreases cyclic nucleotides via the Gi pathway, decreasing activity of the cyclic nucleotide-gated channels leading to smooth muscle relaxation. The authors also hypothesized that the mechanism of action of vascular OPN relaxation is due to the opening of potassium channels, leading to smooth muscle hyperpolarization, due to the ineffectiveness of light in the relaxation of a potassium chloride-mediated constriction (3, 44).
In the current study, we demonstrate for the first time light-mediated ASM relaxation in tracheal ASM from human and mouse and in peripheral small airways of mice. We implicate OPN receptors as the receptors mediating ASM photorelaxation.
MATERIALS AND METHODS
Cell culture.
Primary cultures of human ASM cells were a kind gift from Dr. Panettieri (Rutgers, The State University of New Jersey) et al. (37) and have previously been characterized. The Columbia University Institutional Animal Care and Use Committee approved all mouse tissue protocols. Primary cultures of mouse ASM cells were established from ASM isolated from the posterior wall of murine trachea under a dissecting microscope. Mouse ASM was enzymatically digested using the Papain Dissociation System (Worthington, Lakewood, NJ) and collagenase type 4 (Sigma-Aldrich, St. Louis, MO), as previously described (33). The phenotype of these mouse ASM cell lines has also been previously characterized (33). Primary cultures of human and mouse ASM cells were grown to confluence in 75 cm2 flasks and used between passages 3 and 5 for isolation of total RNA for RT-PCR studies. All cells were maintained in M199 medium, supplemented with 10% fetal bovine serum, 10 units/ml penicillin, 10 µg/ml streptomycin, 25 pg/ml fungizone, 1 ng/ml human fibroblast growth factor, 0.25 ng/ml human epidermal growth factor, 1 µg/ml insulin, 0.55 µg/ml transferrin, and 0.67 ng/ml selenium (Thermo Fisher Scientific, Waltham, MA) at 37°C in 95% air-5% CO2.
Isolation of smooth muscle from human trachea and mouse.
All human airway tissue protocols were reviewed by the Columbia University Institutional Review Board and were deemed not human subject research under 45 CFR 46. Human tracheal tissue was from discarded airway tissue from healthy lung donors during transplantation surgery at Columbia University. All mouse tissue protocols have been approved by Columbia University Institutional Animal Care and Use Committee. Male and female C57BL/6 mice were euthanized, and the trachea was removed intact.
For both human and mouse trachea, extraluminal fibrous tissue and the luminal epithelial layer were removed either by gentle intraluminal abrasion or by fine dissection. For wire myography muscle force studies, mouse tracheal rings were kept intact, whereas human ASM strips were excised from the posterior wall of human trachea. Whole tracheal rings (mouse or human) were placed in an optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) for laser microdissection-assisted RNA extraction or immunofluorescence staining.
Isolation of RNA by laser capture microdissection and reverse transcription of cDNA.
Human and mouse tracheal rings were embedded in OCT and frozen using dry ice in isopentane. Serial sections (6 μm) were made under RNase-free conditions and were placed on a 1-mm polyethylene naphthalate membrane-coated slide (PALM Microlaser Technologies, Westchester, NY). Slides were processed for RNA preservation, and the cells were stained using a laser-capture microdissection (LCMD) kit (Ambion AM1935; Fisher Scientific). Smooth muscle was dissected and harvested, avoiding contamination from adjoining cells, nerve bundles, and vasculature, using a PALM MicroBeam laser microscope. Each sample represented 5–10 mm2 of excised ASM. An RNAqueous-micro kit (Fisher Scientific) was used to extract RNA from the harvested tissues, and reversed transcription of mRNA to cDNA was performed using SuperScript III (Thermo Fisher Scientific). RNA (10 μl) was transcribed into cDNA using random hexamer primers at 42°C for 1 h in 20 μl, according to the manufacturer’s recommendations.
RT-PCR.
PCR was performed on newly synthesized cDNA (5 μl) using RNA isolated from native mouse or human ASM by LCMD, mouse or human ASM-cultured cells, and whole mouse eye (mouse positive control; Advantage Polymerase Kit; Clontech, Mountain View, CA). Human retinal cDNA was purchased to use as positive controls for OPN expression (Takara, Mountain View, CA). Sense and antisense primers (0.4 μM) were used for corresponding OPN receptors and G transducin family members (Table 1) and were designed to anneal in exons that flanked at least one large intron to ensure that PCR products arose from cDNA and not contaminating genomic DNA. All cDNA samples were denatured at 94°C for 10 s. Annealing temperatures were all 68°C for 1 min. Each sample underwent 35 cycles of amplification in a PTC-200 Peltier thermal cycler (Bio-Rad, Hercules, CA). PCR products were separated for analysis on a 5% nondenaturing polyacrylamide gel in Tris acetate, EDTA buffer. PCR products were then stained with ethidium bromide (Molecular Probes, Eugene, OR) and digitally recorded (Biospectra UVP, Cambridge, UK) with Visionworks software (Biospectra UVP; n = 3 and represents samples obtained from three independent animals, humans, or cell lines).
Table 1.
Primer sequence and predicted product size
| Human | Primer Sequence and Accession Number | Product Size, bp | Mouse | Primer Sequence and Accession Number | Product Size, bp |
|---|---|---|---|---|---|
| RT-PCR OPN survey | |||||
| OPN3 | CGTACCTCTTTGCTAAATCGAACACTGTATACAAT | 154 | OPN3 | GCTAAATCGAGCACTGTGTACAACCCAGTTATCTA | 191 |
| ATTTCACTTCCAGCTGCTGGTAGGTCTT | TTTGGCCTGTCCCCATCTTTCTGTGACAT | ||||
| NM_014322 | NM_010098.3 | ||||
| OPN4 | CTTCACCCAGGCCCCTGTCTTCTT | 127 | OPN4 | TTCTTTGCCAGCAGCCTCTACAAGAAGT | 127 |
| CGTCAGGGTGATCATGGAGGAAATGCCAAA | CGTGTGATCACCAGATAGCGGTCCAT | ||||
| NM_033282 | NM_013887.2 | ||||
| RT-PCR GNAT survey | |||||
| GNAT1 | GAGAAGCACTCCAGGGAGCTGGAAA | 179 | GNAT1 | GAAGGACTCGGGTATCCAAGCTTGCTTT | 131 |
| ATGGCGATAAACTCGAGGCACTCTT | ACACGTCCTGCTCAGTGGGCACAT | ||||
| NM_144499.2 | NM_008140 | ||||
| GNAT2 | GCTAGAAAAGAAGCTGCAGGAGGATGCTGATAA | 140 | GNAT2 | TTCTCAACAAGAAGGACCTCTTTGAGGAAA | 137 |
| CTTCTGGTGAATAGCCATCCTGGTGAATGA | CATGTTGAGGTCAAGGAACTGACTCTTGATATAAT | ||||
| NM_005272 | NM_008141.3 | ||||
| GNAT3 | GGATGCTGAGCGAGATGCAAGAACCGTAAA | 209 | GNAT3 | CTAAGTGCCTATGACATGGTGCTTGTAGAAGATGA | 164 |
| AATCAATTCCAAGGGTAGTCATGGCTTTCACAA | ACCTTAGCCACTTTCTCCTGGAAGAGATCTTT | ||||
| NM_001102386 | NM_001081143.1 | ||||
All sequences are in the 5′-3′ direction. GNAT1/2/3, G protein subunit α transducin 1/2/3; OPN3/4, opsin 3/4.
Immunofluorescence histology.
Human tracheal rings were dehydrated using 30% sucrose and frozen and embedded in OCT for OPN immunostaining. Human tracheal rings were cut in 6 μm serial sections, placed on glass slides, and fixed in 4% paraformaldehyde for 15 min. Heat-mediated antigen retrieval was performed with 10 mM sodium citrate buffer, pH 6.0, for 30 min. Slides were rinsed with PBS, blocked with 10% goat serum in 0.1% Tween 20/PBS, and incubated overnight at 4°C with primary antibody against OPN3 (anti-OPN3; LS-C151367; LS Biosciences, Seattle, WA) at a concentration of 1:500 in 2% goat serum in PBS with 0.1% Tween 20. Tracheal ring sections were also incubated without primary antibody (as a negative control). Following overnight incubation at 4°C, slides were washed with PBS, and primary antibodies were detected using phycoerythrin (PE)-labeled anti-rabbit antibody at a 1:10,000 concentration in PBS with 2% goat serum and 0.1% Tween 20 (1 h at room temperature). Slides were also counterstained with 4,6-diamidino-2-phenylindole (DAPI) dihydrochloride to visualize cell nuclei, mounted with Vectashield (Vector, Burlingame, CA) mounting media, and covered with a coverslip (n = 3 and represents samples obtained from three independent humans).
Immunoblotting.
Denuded mouse trachea and eyes were place in 300 μl lysis buffer consisting of 150 mM NaCl, 1.0% Nonidet P-40 (Sigma-Aldrich), 50 mM Tris·HCl (pH 8.0), with complete Protease Inhibitor Cocktail (Sigma-Aldrich) on ice. Tissues were immediately homogenized and placed on an orbital shaker at 4°C for 2 h. Samples were then centrifuged at 500 g at 4°C for 20 min, and the supernatant was removed. Aliquots (10 μl) were removed to perform protein quantification using a bicinchoninic acid kit (Thermo Fisher Scientific) protocol, as indicated by the manufacturer. Laemmli buffer at final concentration of 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, 0.002% bromophenol blue, 0.0625 M Tris·HCl (pH 6.8) was added to the samples and placed in a boiling bath (100°C) for 5 min. Samples were loaded into premade Mini-PROTEAN TGX 4–15% acrylamide gradient gels (Bio-Rad). Equivalent sample loading was based on a bicinchoninic acid assay, where volumes were adjusted to load 20 μg protein in each lane of the gel. Eye samples were loaded at 10 μg per lane. Gels were run at 100 V for 1–2 h at room temperature (running buffer 25 mM Tris, 190 mM glycine, 0.1% SDS). Gels were then transferred in methanol-activated polyvinylidene difluoride (PVDF) using SDS-PAGE [Transfer buffer 25 mM Tris (pH 8.3), 192 mM glycine, 20% methanol) at 30 V overnight at 4°C. The PVDF was then blocked with 4% milk in Tris-buffered saline-Tween 20 (50 mM Tris, 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. The PVDF was then incubated overnight at 4°C in primary antibody against OPN3 or GAPDH (1:1,000; anti-OPN3; LS-C151367; LS Biosciences) and anti-GAPDH Ab-9484 (1:1,000; Abcam) in 1% milk Tris-buffered saline-Tween 20. Horseradish peroxidase-labeled secondary antibody in 1% milk was then applied for 1 h at room temperature. Visualization was obtained by SuperSignal Femto (Pierce, Rockford, IL) and was recorded in digital images (Biospectra UVP; n = 4 and represents samples obtained from four independent animals).
Force measurements of mouse ASM.
Tracheas from C57BL/6 mice were rapidly removed and placed in modified Krebs-Henseleit (KH) buffer of the following composition (in mM): 115 NaCl, 2.5 KCl, 1.91 CaCl2, 2.46 MgSO4, 1.38 NaH2PO4, 25 NaHCO3, and 5.56 d-glucose (pH 7.4). Connective tissue was removed under a dissecting microscope, and tracheas were cut in half axially. One-half trachea was used in each myograph bath (DMT, Ann Arbor, MI). The tissue was held at a resting tension of 0.5 g, and the buffer was exchanged every 15 min for 1 h with a continuous digital recording of muscle force. Following this equilibration period, three ACh dose-response curves were constructed (100 nM–1 mM). An ACh EC50 was determined for each tracheal ring based on these dose-response curves, and each ring was then contracted with ACh (EC50 concentration).
To demonstrate decreased desensitization of OPN-mediated blue-light photorelaxation, a combination of a G protein receptor kinase 2 (GRK2) inhibitor and normal ambient versus decreased ambient light (dark) pretreatments was performed in some mouse myograph studies. In indicated studies, dark pretreatments of airway tissues were achieved by covering the organ baths with foil, turning off all exogenous light sources within the room, and blocking windows with blackout curtains for 1 h before challenging the tissues with light exposure. Methyl 5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate (Santa Cruz Biotechnology, Dallas, TX) is a GRK2 inhibitor and is otherwise known as a β-adrenergic receptor kinase inhibitor with an IC50 of 128 μM. In indicated studies, a dose of 200 μM GRK2 inhibitor was used to inhibit GRK-mediated desensitization of the OPN receptors. Pretreatments with the GRK2 inhibitor were performed for 15 min before light exposure.
A halogen white-light source with adjustable power output was used to deliver light of varying brightness to test the intensity dependence of light-induced relaxation. Light intensity was measured using a handheld lux meter (UNI-T UT383; Signstek, Wilmington, DE). A monochromator (Ludl Electronic Products, Hawthorne, NY; CM 110, Spectral Products, Putnam, CT) was used to expose mouse ASM to light with increasing wavelengths (from 370 to 700 nm in 30-nm increments) to test the optimal wavelength at which relaxation occurred. A derivative of vitamin A, 9-cis retinal (0.28–28 μM), known to be a chromophore for human OPN receptors, was added as a pretreatment for 1 h before 405-nm light exposure to demonstrate the effect of the addition of an exogenous chromophore to photorelaxation. The buffer temperature was checked at 30 s and 1, 2, 5, and 10 min of light exposure to confirm that the buffer remained at 37°C.
In an attempt to elucidate the possible mechanism of light-mediated relaxation, some experiments were performed with a pretreatment of 100-nm iberiotoxin, in addition to 10 μM 9-cis retinal, 1 h before 405-nm light exposure. Iberiotoxin is a specific inhibitor of the large-conductance calcium-activated potassium (KCa) channel (also known as the BK channel). BK channel activation is, in part, regulated by PKA, which is well known to be activated by GPCRs. BK channel activation-mediated hyperpolarization induces ASM relaxation.
Amplified analog signals from the myograph (DMT) were digitized using a Biopac MP100 system. Digitized signals are analyzed using Acknowledge software (v. 3.9.1) and converted to muscle force in grams of tension (n = 3–6 and represents individual tracheal rings isolated from three to six independent animals).
Force measurements of human ASM.
Human tracheal smooth muscle strips in an organ bath were oxygenated in a KH buffer of the following composition (in mM): 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.236 MgSO4, 1.38 NaH2PO4, 25 NaHCO3, and 5.56 D-glucose (pH 7.4) at 37°C, as described previously (11, 51). In brief, tracheal strips were tied with silk in series with an FT03 force transducer (Grass Telefactor, West Warwick, RI). KH buffer was exchanged in the organ baths every 15 min, whereas resting tension was maintained at 1.5 g. The ASM strips were subjected to two cycles of increasing log concentrations of ACh (100 nM–100 μM). An ACh EC50 was determined for each ASM strip based on these dose-response curves, and each strip was then contracted with ACh (EC50 concentration). To eliminate the effects of airway nerves and histamine receptors, tetrodotoxin (1 μM) and pyrilamine (10 μM) were added to the buffers of all organ baths. In indicated experiments, the GRK2 inhibitor, methyl 5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate 200 μM was added to the buffer to inhibit GRK-mediated desensitization of the OPN receptors. A 15-min pretreatment was performed before 5 mW light-emitting diode (LED) lights were focused on the tissue from a distance of 10 cm. Tissues were exposed to specific wavelengths of light (i.e., green 532 nm or blue 405 nm) for 2.5–5 min. Amplified analog signals from the FT03 transducer (Grass Telefactor) were digitized using a Biopac MP100 system. Digitized signals were continuously captured and analyzed using Acknowledge software (v 3.9.1), converting volts to muscle force in grams of tension (n = 3–4 and represents individual ASM strips isolated from three to four independent human tracheal samples).
Precision-cut lung slice.
Mouse precision-cut lung slices (PCLSs) were prepared as previously described (7). In brief, mouse lungs were filled in situ with agarose, embedded in gelatin, and cut into 130 μm-thick slices using a tissue slicer (Precisionary Instruments, Greenville, NC). Lung slices were incubated (37°C, 10% CO2) overnight in DMEM with gentamycin and streptomycin. PCLSs were then placed and perfused (Hanks’ balanced salt solution) in between a 22 × 40-mm slide and a 11 × 30-mm coverslip. To prevent any movement of the lung slice during perfusion, a nylon mesh with a small opening was placed over the lung slice, such that the mesh holds the slice by the lung parenchyma, and the small opening was centered over the airway to allow imaging. Visualization was achieved with a phase-contrast microscope and charge-coupled device camera. Video Savant (IO Industries, London, ON, Canada) was used to capture and digitally analyze airway luminal area. Contraction of airways was induced by the switching of the perfusion buffer to Hanks’ balanced salt solution containing 0.2 μM ACh, and exposure of PCLS to other experimental drugs was made similarly by the switching of the appropriate perfusion solution. In indicated experiments, the GRK2 inhibitor, methyl 5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate was superfused at 200 μM for 5 min before blue-light exposure to inhibit OPN desensitization. PCLS imaging was performed under red light by the addition of a long-pass filter (600 nm cutoff; Edmund Optics, Barrington, NJ) between the microscope lamp housing and the condenser. To study the effect of blue light on changes in airway contractility, we customized the microscope’s fluorescence attachment with the following components: a Xenon Arc Lamp, iris, band-pass filter, electronic shutter, and fluorescence cube containing a dichroic mirror and barrier filter. The iris was used to create an illumination spot covering the small airway in the PCLS. The wavelength’s range of the blue light was set by the band-pass filter (86–652; 25 mm; 400 ± 12.5 nm; Edmund Optics). A 520-nm cutoff dichroic mirror (FF520, DiO2, 18 × 26; Semrock, Rochester NY) and a barrier filter (610 nm; Nikon, Tokyo, Japan) allowed the exposure of the airway to blue light without interfering with the continuous imaging of the PCLS. A 5-min exposure time to the blue light was controlled using the electronic shutter (n = 3–4 individual airways analyzed from lung slices obtained from three to four separate mice).
Statistical analysis.
Each experimental permutation included intraexperimental vehicle controls. We used one-way ANOVA with Bonferroni post-test comparisons between appropriate groups using Prism 4.0 software (GraphPad, San Diego, CA). For comparisons between two experimental groups, a Student's t test was used. Data are presented as means ± SE; P < 0.05, in all cases, was considered significant.
RESULTS
OPN mRNA expression.
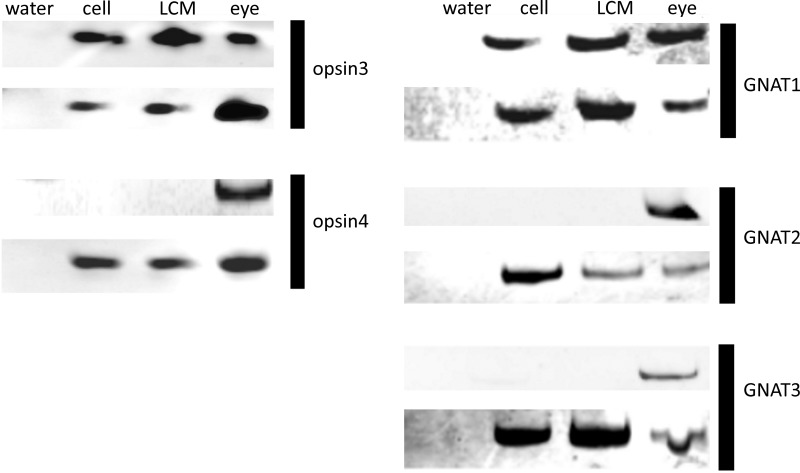
Representative gel images demonstrating the results of RT-PCR analysis of mRNA encoding OPN proteins are presented in Fig. 1. Analysis was performed on total RNA isolated from primary cultures of mouse and human ASM (cell) and from total RNA isolated from native mouse and human ASM using LCMD. Two members of the OPN family, found extraocularly (OPN3 and OPN4), were targeted for RT-PCR detection. mRNA encoding OPN3 was detected in all four sources of ASM (native and cultured human and mouse ASM). OPN4 was detected in native and cultured mouse ASM but not in human samples. Water blanks, negative control devoid of cDNA, did not demonstrate a PCR product. Human retinal (cDNA) and mouse eye (tissue), positive controls, demonstrated PCR products of expected size. In addition, an RT-PCR survey of the transducin family of G proteins (G proteins classically coupled to OPN receptors) demonstrated the conserved expression of G protein subunit α transducin 2 (GNAT2) in ASM from both native and cultured ASM of both mouse and human. GNAT1 and GNAT3 were found in both mouse ASM cells in culture and ASM obtained from LCMD (Fig. 1). All RT-PCR images are representative of three independent experiments using tissues or cells obtained from independent animals or humans.
Fig. 1.
Left: representative gel image of RT-PCR products from primers targeting mRNA encoding opsin (OPN) proteins, OPN3 and OPN4. Primary airway smooth muscle (ASM; cell) culture and discrete areas of histologically confirmed ASM tissue obtained by laser-capture microdissection (LCMD, or LCM) were sampled from both human and mouse sources. Eye tissue was used as a positive control (eye) for mouse, and purchased eye cDNA was used as a positive control (eye) for human. Water blank was used as a negative control (water). Human samples of ASM demonstrate positive mRNA expression of OPN3 but not for OPN4. Mouse ASM demonstrates mRNA expression of both OPN3 and OPN4. Image representative of 3 experiments from 3 independent tissue or cell sources. Right: representative gel image of RT-PCR products using primers targeting mRNA encoding G protein subunit α transducin 2 (GNAT) proteins GNAT1, GNAT2, and GNAT3. Primary airway smooth culture (cell) and discrete areas of histologically confirmed ASM tissue obtained by LCMD were sampled from both human and mouse sources. Eye tissue was used as a positive control (eye) for mouse, and purchased eye cDNA was used as a positive control (eye) for human. Water blank was used as a negative control (water). Human samples of ASM demonstrate positive mRNA expression of GNAT2, whereas mouse ASM demonstrates mRNA expression for all 3 GNAT subtypes. Image representative of 3 experiments from 3 independent tissue or cell sources.
OPN3 protein expression in human ASM and mouse trachea.
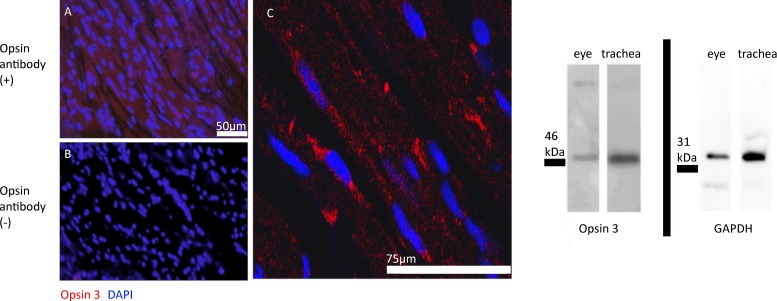
OPN3 protein was immunofluorescently detected in ASM of human trachea (Fig. 2). OPN3 stained positive (PE) in the smooth muscle region of human trachea, and cell nuclei were stained with DAPI. Immunoblot analysis of mouse epithelium-denuded trachea demonstrated positive immunostaining of a protein at 47 kDa (predicted 45 kDa) for both protein samples derived from mouse trachea and mouse eye (positive control). GAPDH immunoblotting was performed to demonstrate sufficient protein loading. GAPDH-targeted immunoblots demonstrated an immunoreactive band at 35 kDa (35 kDa predicted) for both mouse epithelium-denuded trachea and eye (Fig. 2). Immunoblotting (n = 4) and immunofluorescent (n = 3) images are representative of three to four independent experiments using samples from three to four independent humans or animals.
Fig. 2.
A–C: representative image of native human tracheal airway smooth muscle (ASM) treated with fluorescently labeled antibodies. Red fluorescent staining demonstrates positively labeled opsin (OPN)3 protein. Blue fluorescence represents positive nuclear staining using 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI). A: fluorescent microscope image with OPN3 antibody and red fluorescent phycoerythrin (PE)-labeled secondary antibody. B: fluorescent microscope image with only red fluorescent PE-labeled secondary antibody. C: confocal microscopy image at higher magnification with OPN3 antibody and red fluorescent PE-labeled secondary antibody. Images are representative of 3 independent experiments with tissues from 3 independent human samples. Right: representative image of an immunoblot from mouse epithelium-denuded trachea. OPN3-targeted antibodies identified a 45-kDa protein in both mouse trachea (20 μg/lane) and whole eye (10 μg/lane; positive control) tissue homogenates. GAPDH-targeted antibodies identified a 35-kDa protein in both mouse trachea (epithelium denuded) and whole eye (positive control) tissue homogenates, demonstrating relative protein loading of each sample. Images are representative of 3 independent experiments with tissues from 3 independent murine tracheal ring samples.
Light-mediated relaxation enhanced by dark and GRK2 inhibitor pretreatments.
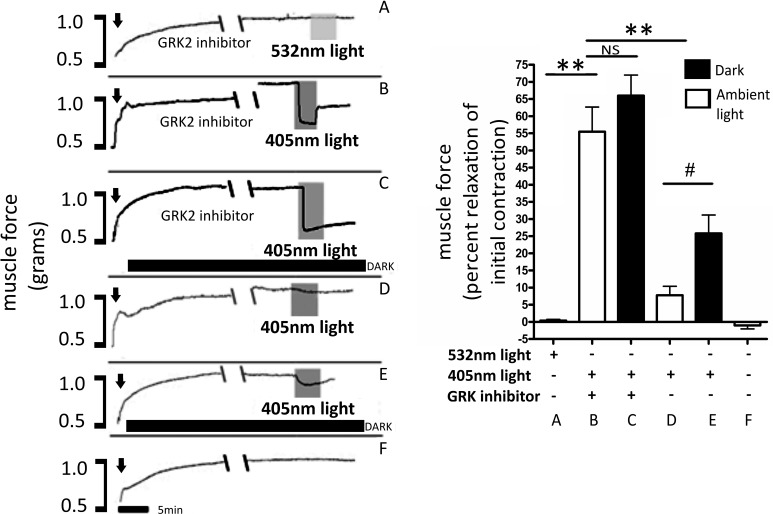
The OPN receptors are known to be rapidly desensitized by GRK-mediated phosphorylation. To demonstrate the effects of light desensitization on light-mediated ASM relaxation, combinations of dark, ambient light or GRK2 inhibition were evaluated as pretreatments before 405-nm blue light-mediated relaxation in mouse tracheal rings precontracted with an EC50 dose of ACh (Fig. 3). Light (532 nm; green light control), after exposure with GRK2 inhibitor pretreatment, had no effect on precontracted mouse trachea [0.4 ± 0.4 (percent relaxation mean ± SE); Fig. 3A]. However, 405-nm light treatments in the presence of the GRK2 inhibitor resulted in a >50% relaxation [56 ± 7.2 (percent relaxation mean ± SE), P < 0.001, n = 4 compared with green light treatment; Fig. 3B]. The combination of dark and GRK inhibitor pretreatment did not significantly increase 405-nm light relaxation compared with GRK inhibitor pretreatment alone [66 ± 6.0 (percent relaxation mean ± SE), P > 0.05, n = 3–4 compared with normal ambient light with GRK2 inhibitor and 405-nm treatment; Fig. 3C]. Under ambient laboratory lighting conditions, 405-nm light without GRK inhibitor demonstrated modest relaxation [7.8 ± 2.7 (percent relaxation mean ± SE); Fig. 3D]. When trachea was pretreated for 1 h in the dark, 405 nm-mediated relaxation was increased by over 300 percent [26 ± 5.3 (percent relaxation mean ± SE), P < 0.05, n = 4 compared with 405-nm treatment in ambient light; Fig. 3E]. A time control was performed where precontracted tracheal smooth muscle without any treatments demonstrated relaxation of −1.0 ± 1.0 (percent relaxation mean ± SE; Fig. 3F).
Fig. 3.
A–F, left: representative muscle force tracings of mouse tracheal rings in wire myographs measuring force in grams. All treatment groups were precontracted with an EC50 concentration of ACh (indicated by arrows). The black bars, labeled DARK, demonstrate tracings where the environmental ambient light was decreased for 1 h. Tracings labeled G protein receptor kinase 2 (GRK2) inhibitor received methyl 5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate at 200 μM. Double-backslash marks demonstrate a 30-min gap in the tracing. Shaded regions, labeled 405, demonstrate 405-nm blue-light treatment, and shaded region, labeled 532, demonstrates 532-nm green-light treatment. A–F, right: graphical analysis of mouse trachea rings during light treatments, with and without GRK2 inhibitor and with and without decreased ambient light. Relaxation was represented as decreased muscle force as a percent of the initial contraction. Decreased ambient light had a significant 3-fold increase in relaxation versus normal ambient light during the time of blue-light treatment in the absence of GRK inhibition. GRK inhibitor increased relaxation by 7-fold when compared within ambient light groups. The addition of decreased ambient light to the GRK2 inhibitor groups did not significantly change photorelaxation. Green light did not induce significant photorelaxation, with the magnitude of relaxation being significantly less than the relaxation caused by blue-light treatments within GRK2 inhibitor pretreatment groups (#P < 0.05, **P < 0.001, n = 3–4 independent experiments with tracheal rings from independent mice). NS, not significant.
Human ASM light-mediated relaxation.
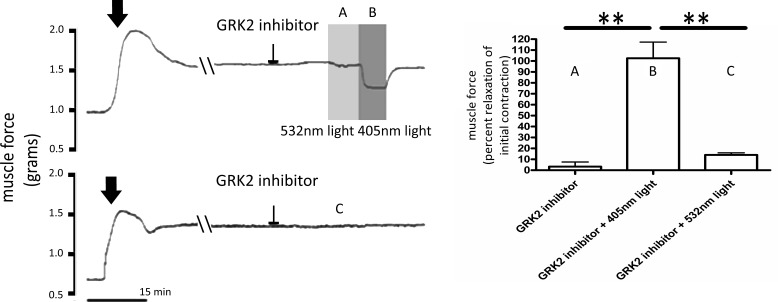
To demonstrate interspecies conservation of ASM photorelaxation, pretreatments of GRK inhibitor methyl-5-[9E0-2-(5-nitrofuran-2-yl)ethenyl] furan-2-carboxylate were performed before light treatments in human tracheal strips precontracted with an EC50 concentration of ACh (Fig. 4). Experiments were performed in ambient light. Blue light (405 nm) caused a significant relaxation of the ACh-induced contraction [Fig. 4; 103 ± 15 (percent relaxation mean ± SE), P < 0.001, n = 3–4 compared with GRK2 inhibitor alone (no-light control), which was 3.3 ± 4.3 (percent relaxation mean ± SE)]. There were nonsignificant changes in the ACh-induced muscle force when the tissue was exposed to green light (532 nm) in the presence of GRK2 inhibition [14 ± 1.9 (percent relaxation mean ± SE), P > 0.05, n = 3–4 compared with GRK2 inhibitor alone (no-light control), and P < 0.001, n = 4 compared with GRK2 inhibition and 405-nm light].
Fig. 4.
A–C, left: representative tracings of human tracheal airway smooth muscle (ASM) strips in wire myographs measuring force in grams. All treatment groups were precontracted with an EC50 concentration of ACh indicated by the arrows. All tracings were recorded where the environmental ambient light was decreased for 1 h. G protein receptor kinase 2 (GRK2) inhibitor received methyl 5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate at 200 μM treatments, as indicated by flattened arrows. Double-slash marks demonstrate a 30-min gap in the tracing. Shaded region, labeled 405, demonstrates 405-nm blue-light treatment, and shaded region, labeled 532, demonstrates 532-nm green-light treatment. A–C, right: graphical analysis of human tracheal strips during light treatments with the GRK2 inhibitor. Relaxation was measured as decreased muscle force as a percentage of the initial contraction. GRK2 inhibitor pretreatment with no-light treatment was not significantly different from green-light treatments. However, relaxation after blue-light treatments was significantly different from relaxation occurring after either no-light or green-light treatments, demonstrating a 3-fold increase in relaxation compared with no-light treatments (**P < 0.001, n = 3–4 independent experiments using independent samples from 3 to 4 humans).
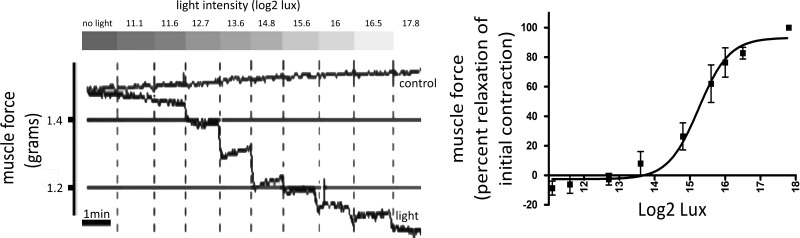
Photorelaxation in ASM is light-intensity dependent.
Precontracted mouse trachea were exposed to light at varying intensities following pretreatment with the GRK2 inhibitor to demonstrate the relationship between light intensity and ASM relaxation. Mouse tracheal ring relaxation increased as the white light intensity became more intense over the range of 0–228,000 lux units or 0–17.8 log2 lux units (Fig. 5). After curve fitting, the intensity of light that caused a 50% reduction in ACh-induced contractile force was ~39,000 lux (Fig. 5; n = 3).
Fig. 5.
Left: representative force tracing of mouse tracheal rings in an organ bath precontracted with an EC50 concentration of ACh. The tracheal ring was pretreated with a G protein receptor kinase 2 (GRK2) inhibitor {methyl 5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate 200 μM} and exposed to white light at increasing intensity (11.1–17.8 Log2 lux). Gray bars above indicate increasing light intensities. The tracing labeled light demonstrates increased relaxation when mouse trachea was exposed to increasing intensity of light. The tracing labeled control demonstrates a no-light treatment time control. Right: intensity response curve of mouse tracheal rings in an organ bath precontracted with an EC50 concentration of ACh, pretreated with GRK inhibitor, and exposed to increasing intensities of light (11.1–17.8 Log2 lux). Mean relaxation occurred at 39,000 lux (n = 3 independent experiments using tracheal rings from 3 different animals).
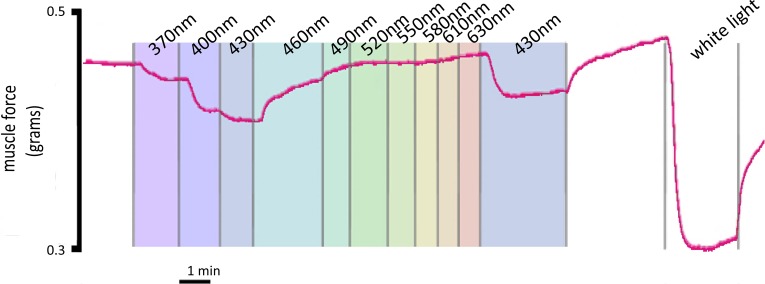
Light-mediated relaxation is wavelength dependent.
ACh-precontracted mouse trachea were pretreated with the GRK2 inhibitor to inhibit receptor desensitization and were exposed to light at varying wavelengths from 370 to 640 nm at 30-nm increments to identify the wavelength dependence for ASM relaxation. The representative tracing (Fig. 6) demonstrates a maximum relaxation at 430 nm and an activation range of 370–490 nm. A repeat 430-nm light treatment was performed to demonstrate that the relaxation mechanism was still intact, and a high-intensity white-light treatment was performed to demonstrate maximal light-induced relaxation. Figure 6 is a representative tracing of three independent experiments.
Fig. 6.
Representative force tracing of mouse tracheal rings in an organ bath precontracted with an EC50 concentration of ACh. The tracheal ring was pretreated with G protein receptor kinase {GRK2; methyl 5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate 200 μM} inhibitor and exposed to light at discreet wavelengths (370–640 nm) at 30-nm increments, as indicated by color-shaded regions. Maximal relaxation occurred at 430-nm light. A reexposure of 430-nm light was performed to demonstrate continued sensitivity. A white-light exposure at high intensity was performed to demonstrate maximal relaxation.
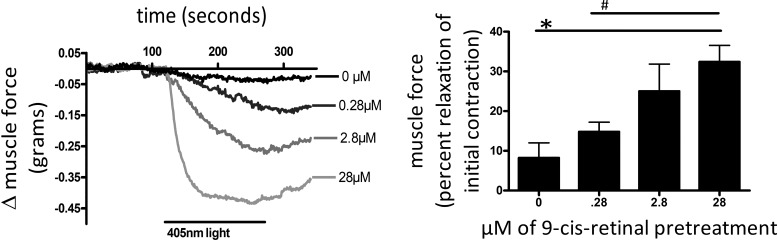
9-cis Retinal enhanced photorelaxation.
To demonstrate the effects of a known OPN ligand/chromophore, 9-cis retinal on photorelaxation, ACh-precontracted mouse trachea were pretreated with varying concentrations of 9-cis retinal (0–28 μM) for 1 h during dark pretreatment and then exposed to 405-nm blue light (Fig. 7). A 9-cis retinal dose-dependent increase in light-mediated relaxation was demonstrated with 8.3 ± 3.8, 14.8 ± 2.4, 25 ± 6.9, and 32 ± 4.2% relaxation (mean ± SE) at concentrations of 0, 0.28, 2.8, and 28 μM 9-cis retinal, respectively (Fig. 7). Pretreatment (28 μM) of 9-cis retinal demonstrated a significant difference in relaxation when compared with 0 μM (P < 0.01, n = 4–5) and with 0.28 μM (P < 0.05, n = 5) 9-cis retinal pretreatments.
Fig. 7.
Left: representative tracings of mouse tracheal rings in wire myographs measuring force in grams. All treatment groups were precontracted with an EC50 dose of ACh. The black bar labeled 405-nm light demonstrates the time course of blue-light treatments. The tracing labeled 0 μM was treated with 405-nm light alone. The other 3 tracings were pretreated for 1 h with 9-cis retinal at concentrations of 0.28, 2.8, and 28 μM, as indicated by their labeling. Tracings demonstrate a dose response to increasing 9-cis retinal pretreatments. Right: graphical analysis of mouse trachea rings during light treatments with varying 9-cis retinal pretreatments (0–28 μM) for a 1-h duration. Relaxation was measured as percent relaxation of the initial contraction. Pretreatments (9-cis retinal; 28 μM) had a significant 3-fold increase in relaxation versus blue-light treatment without 9-cis retinal pretreatment (*P < 0.01, n = 4–5). Furthermore, 28 μM 9-cis retinal pretreatment had a significant 2-fold increase in relaxation when compared with 0.28 μM 9-cis retinal pretreatment, demonstrating a dose response to 9-cis retinal pretreatments (#P < 0.05, n = 4–5 independent experiments with tracheal rings from 4 to 5 separate mice).
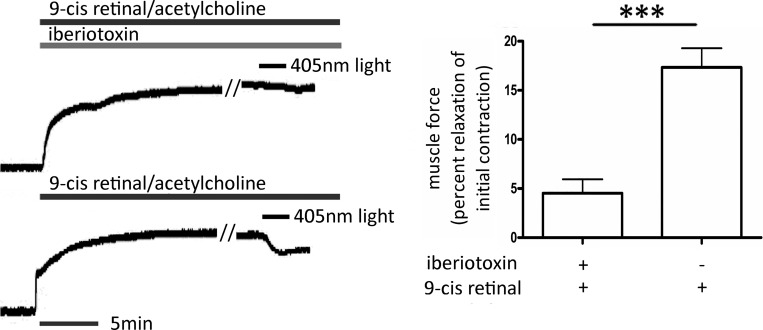
9-cis Retinal-enhanced photorelaxation is inhibited by iberiotoxin.
We questioned whether a component of light-mediated relaxation was mediated by the opening of the KCa (BK) channel, as has been described for relaxation mediated by bitter taste receptors (14). Mouse tracheal smooth muscle rings were contracted with an EC50 concentration of ACh and pretreated with 10 μM 9-cis retinal, with or without 100 nM iberiotoxin, before exposing the tissues to 405-nm light. Light induced a 4.5% ± 1.4% (mean ± SE) relaxation in tracheal rings pretreated with 9-cis retinal with iberiotoxin, whereas airways that received 9-cis retinal alone demonstrated a 17.3 ± 2.0% (mean ± SE) relaxation (P < 0.001, n = 6; Fig. 8).
Fig. 8.
Left: representative tracings of mouse tracheal rings in wire myograph measuring force in grams. The black bars labeled 405-nm light demonstrate the time course of blue-light treatments. The tracings labeled with black bar 9-cis retinal/acetylcholine represent tracheal rings pretreated for 1 h with 10 μM 9-cis retinal before an EC50 dose of ACh. The top tracing with the iberiotoxin-labeled gray bar represents a tracheal ring that was also pretreated with 100 nM iberiotoxin. These representative tracings of an n = 6 demonstrate significant inhibition of 405-nm light-mediated relaxation by iberiotoxin. Right: graphical analysis of blue light-induced relaxation of ACh-contracted mouse trachea rings in the presence of 10 μM 9-cis retinal, with or without iberiotoxin pretreatments. Relaxation was measured as a percent of relaxation of the initial ACh-induced contraction. Iberiotoxin pretreatment reduced blue light-mediated relaxation by ~80% (***P < 0.0001, n = 6 independent experiments with tracheal rings from 6 separate mice).
Peripheral airway.
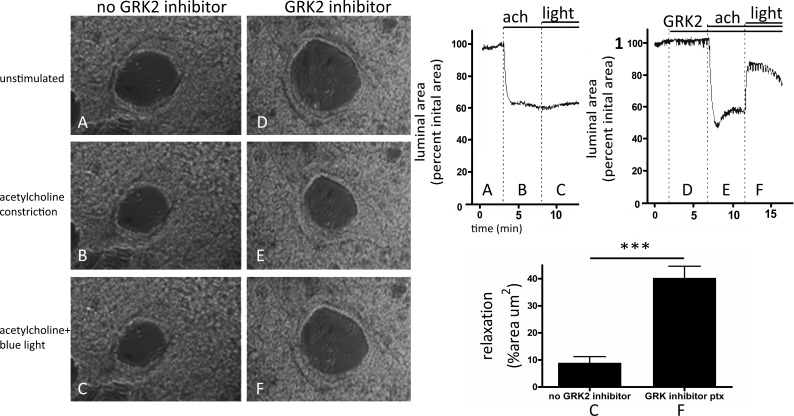
We used mouse PCLS to demonstrate blue light-mediated relaxation of the distal (peripheral) airways (Fig. 9). The airway was preconstricted with 200 nM ACh (Fig. 9, B and E), and the subsequent exposure to blue light (400-nm wavelength) for 5 min induced a modest relaxation of 9.0 ± 2.3% (mean ± SE) in the continuous presence of ACh (Fig. 9C). However, this blue light-induced airway relaxation increased to 40.3 ± 4.3% (mean ± SE) when the PCLSs were pretreated for 5 min with 200 μM GRK2 inhibitor methyl-5-[9E0-2-(5-nitrofuran-2-yl)ethenyl] furan-2-carboxylate (P < 0.0001 compared with control, n = 3–4; Fig. 9F).
Fig. 9.
A–F, left: representative microscopic images of mouse bronchioles in precision-cut lung slice. No G protein receptor kinase (GRK2) inhibitor group was performed and no GRK2 inhibitor pretreatment (A) before a 200-nM ACh precontraction (B) and blue-light treatment (C). The GRK2 inhibitor group was performed with a 5-min, 200-μM methyl 5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate pretreatment (D) before and during ACh precontraction (E) and 400-nm light treatment (F). A–F, right: representative tracings showing the temporal courses of the changes in the airway luminal area of the airways shown in A–F, left, in response to ACh (ach) and blue light in the absence (top, left) and presence (top, right) of GRK2 inhibitor. Graphical regions correlate to airway images of the same label (A–F, left). C and F, bottom right: Graphical analysis of mouse bronchioles in precision-cut lung slice before and after blue-light treatment. Airway contraction was recorded as a percent luminal area compared with initial area. The addition of the GRK2 inhibitor before 400-nm light [pretreatment (ptx)] demonstrates a significant 40.3% relaxation after a 200-nM ACh contraction compared with control. Control demonstrated a 9.0% relaxation with 400-nm light without GRK2 inhibitor pretreatment (***P < 0.0001, n = 3–4 independent experiments using lung slices from 3 to 4 separate mice).
DISCUSSION
The current study demonstrates for the first time that electromagnetic energy in the visible spectrum, applied directly to ASM, causes smooth muscle relaxation. The characterization of the light applied demonstrates that relaxation is dose dependent with a 50% response at 39,000 lux and demonstrates wavelength specificity with a maximal potency at the 430-nm wavelength. Prior animal studies have shown photorelaxation in sheep urethra, rabbit aorta, rat pulmonary artery, and mouse arteries (1, 3, 6, 13, 15, 18, 21, 25, 26, 44, 49); however, this is the first demonstration of photorelaxation in ASM and in human tissue. When surveying for possible receptor candidates in mouse and human ASM, we demonstrate that OPN3 mRNA expression was conserved between human and murine samples. We also confirmed in the human ASM that protein expression of the OPN3 receptor is present using fluorescent immunohistology and similarly show correlation in mice by immunoblot analysis.
We provide several layers of evidence that implicates that the OPN receptor is mediating this photorelaxation effect. First, we observed that selective light-mediated effects are restricted to the blue-wavelength bandwidth (405 nm), a finding that is consistent with OPN3 activation (29, 39). OPN3 is classically activated by 415–470-nm light, but wavelength specificity is determined by the OPN subtype, as well as the binding of specific chromophores. The chromophore 9-cis retinal induces a small shift or shortening of the wavelength sensitivity and retains sensitivity in the blue-light wavelength region, including 405 nm. Thus OPN3-specific activation should occur within the blue-light region, as we have demonstrated in Fig. 6. In addition, we demonstrate enhanced photorelaxation with the use of the β-adrenoceptor kinase (GRK2) inhibitor, illustrating the involvement of GPCR kinase signaling that has been reported for the OPN family (34, 43). Classically, β-adrenoceptor kinase is described as a key mediator of β-adrenoceptor desensitization via feedback mechanisms of the Gs pathway to decrease both activity and the available pool of membrane-associated β-adrenoceptors following receptor activation (32, 45). Notably, GRK2 has also been shown to mediate OPN4 desensitization, which is implicated in the desensitization of OPN4 receptors in vascular smooth muscle (3, 16, 42, 44). Our studies demonstrate enhanced relaxation via inhibition of GPCR phosphorylation-mediated desensitization, illustrating that ambient light mediates a desensitization of the OPN receptor. Indeed, this phenomenon could be minimized by removal of ambient light stimulation, since ex vivo dark pretreatment experiments demonstrate enhanced light-mediated photorelaxation without the use of a GRK2 inhibitor. Although not tested yet, we hypothesize that in vivo (inside the body), sufficient darkness exists, allowing for OPN receptor-mediated relaxation without the need for GRK2 inhibition. Future experiments will seek to address this important consideration, as well as to exploit other mechanisms of enhanced photorelaxation (e.g., targeted phosphorylation of the OPN receptor).
In addition to the role of GPCR-desensitization pathways, we explored the role of known OPN-associated chromophore molecules as they relate to ASM photorelaxation. A point of molecular convergence between these groups (OPN3 and OPN4) involves a predicted chromophore/retinal binding at a lysine residue located on the seventh helix of the protein receptor. The prototypical chromophore (in the retina), which allows for visual photo-mediated signal transduction, is 11-cis retinal (a metabolite of vitamin A). Photosensitivity is achieved as 11-cis retinal forms a covalent bond with the previously mentioned OPN receptor lysine residue to create a “Schiff base,” a retinylidene protein (also known as the retinal OPN complex). The resultant retinylidene protein exhibits differential activation parameters (excitation wavelength) and differential downstream G protein couplings (i.e., Gs vs. Gq), which are thought to be determined by variations in combination between different OPN receptor and chromophore subtypes (12, 22, 24, 30, 35). We found that an exogenously added 9-cis retinal pretreatment resulted in a dose-dependent enhancement of blue light-mediated relaxation. Whereas blue light has potential non-OPN biological effects (20, 36, 50), blue light is well established as an activator of OPN3 and OPN4. To implicate OPN receptors further in ASM photorelaxation, we applied exogenous 9-cis retinal to enhance OPN receptor light responsiveness. 9-cis Retinal has only been associated with light-sensitive biological activity through OPN receptors, suggesting that 9-cis retinal-mediated enhanced relaxation is due to its association with OPN receptors. Interestingly, the endogenous chromophore for the extraocular OPN receptors has yet to be discovered. 11-cis Retinal, the classic OPN chromophore, is found in the eye and is produced by retinal pigment epithelial cells via a series of specialized enzymes. Vitamin A is converted to all-trans retinol in the liver and is the circulating precursor to 11-cis retinal. It is unlikely that the 11-cis retinal isoform would be stable enough to be made from the retinal epithelium and then be delivered to the airway tissue. It may be possible that more stable forms of vitamin A could reach the lung and bind to the endogenous extraocular OPN receptors. It could also be possible that some isomerase enzymes could be produced by the airway to modify circulating forms of vitamin A to chromophores that activate the OPN receptor. Given that certain OPN themselves are known to be isomerases transforming all-trans retinal to cis retinals (9), it is possible that the ASM OPN receptor acts in an autocrine fashion to modulate its own signaling. Further studies in the endogenous signaling and activation of these receptors are warranted.
Our findings demonstrate that light-mediated airway relaxation involves potassium channel activation, as previously demonstrated, in vascular smooth muscle (3, 44). In the current study, we demonstrate that specific inhibition of KCa channels of the large conductance subtype (i.e., BK channels) significantly blocked 405-nm blue light-mediated ASM relaxation. BK channels are activated by increases in cellular calcium, which are mediated by myriad signaling events in ASM. Many of these signaling events are G protein coupled, most commonly attributed to receptors coupled to the Gq or Gi proteins, but members of the G transducin family are also expressed in ASM, as demonstrated in the present study. Additionally, multiple families of membrane channels and transporters are involved in the influx and efflux of calcium. The specific signaling events that link blue light-mediated relaxation to the opening of BK channels and relaxation of ASM are the topic of ongoing investigations. The involvement of BK channels in light-mediated ASM relaxation is consistent with the initially hypothesized mechanism by which bitter taste receptors relax ASM. The original studies suggested that localized increases in calcium (via the Gq pathway) activated BK channels, which were inhibited by iberiotoxin (14). It is important to note that BK channel activity can also be increased by PKA-mediated phosphorylation (48), a kinase activated by classic relaxing ligands coupled through β2-adrenoceptor/Gs/cAMP. Further studies are needed to determine whether OPN receptors in ASM may be activating this classic cAMP/PKA pathway to open BK channels, as well as multiple other known PKA prorelaxant pathways in ASM.
Visual OPN receptors activate heterotrimeric G proteins after three criteria are met: application of light at the correct wavelength, chromophore binding at the receptor, and homodimerization of the OPN receptor. Whether extraocular OPNs behave in the same manner and are associated to the same pathways as classic visual OPNs have yet to be determined. It is possible that the extraocular OPN receptors mediate nonclassical signaling pathways that are prorelaxant (such as Gs), which is supported by the GRK2 inhibitor enhancement of photorelaxation. Whereas OPN receptors of different subtypes have been associated with the activation of G proteins, such as Gt, Gi/Go, Gq, and Gs (31), the classical association of OPN3/4 has been linked to Gq. We demonstrate in airway that mRNA of GNAT2, a member of the G transducin family, is present in ASM (Fig. 1). However, alternative G protein pathways remain possible mechanisms of ASM OPN-mediated photorelaxation.
To determine if our results were applicable to all levels of ASM throughout the lung, we used PCLS. We found conservation of photorelaxation in small (peripheral) airways that were precontracted with the bronchoconstrictor ACh. Given the major role that these peripheral airways play as a determinant of airway resistance, the capacity to induce photorelaxation adds significant clinical relevance to our findings. Similar to our observations in tracheal specimens, bronchiolar relaxation occurred at the blue-light wavelengths with enhanced relaxation after pretreatments of the GRK2 inhibitor.
In summary, we have characterized the ASM physiological effects of ASM photorelaxation, demonstrating wavelength and signaling associations that implicate the OPN family. The use of light to relax the airway directly is novel and can be a very versatile means of treatment. LED technology has dramatically advanced recently, where light can be exposed to large areas or focused onto a single cell. Medical application of the technology may allow us to expose light to target cells, whereas sparing others’ decreasing systemic effects. The field of optogenetics has already begun this work, demonstrating micro-LED implantation into mouse brains in vivo and that OPN activation is feasible. Furthermore, the field of nanotechnology has produced not only cell-targeted nanoparticles but also light-emitting nanoparticles. If the field of “photophysiology” is able to answer some of the important mechanistic questions as technology advances, then the clinical use of phototherapy may be on the horizon.
GRANTS
This study was supported by a Foundation for Anesthesiology Education and Research, Mentored Research Training grant (to P. D. Yim); National Institute of General Medical Science Grant GM065281 (to C. W. Emala); National Heart, Lung, and Blood Institute Grants HL122340 (to C. W. Emala) and HL124213 (to D. E. Berkowitz); and National Institute of Child Health and Human Development Grant HD082251 (to G. Gallos).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.D.Y., G.G., J.F.P.-Z., D.E.B., and C.W.E. conceived and designed research; P.D.Y., J.F.P.-Z., Y.Z., D.X., A.W., and D.E.B. performed experiments; P.D.Y., G.G., D.X., A.W., and C.W.E. analyzed data; P.D.Y., G.G., J.F.P.-Z., Y.Z., D.X., A.W., D.E.B., and C.W.E. interpreted results of experiments; P.D.Y., J.F.P.-Z., D.X., A.W., and C.W.E. prepared figures; P.D.Y. and G.G. drafted manuscript; P.D.Y., G.G., J.F.P.-Z., D.E.B., and C.W.E. edited and revised manuscript; P.D.Y., G.G., J.F.P.-Z., Y.Z., D.X., A.W., D.E.B., and C.W.E. approved final version of manuscript.
REFERENCES
- 1.Agrawal DK, Shao Z. Pathogenesis of allergic airway inflammation. Curr Allergy Asthma Rep 10: 39–48, 2010. doi: 10.1007/s11882-009-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews KL, McGuire JJ, Triggle CR. A photosensitive vascular smooth muscle store of nitric oxide in mouse aorta: no dependence on expression of endothelial nitric oxide synthase. Br J Pharmacol 138: 932–940, 2003. doi: 10.1038/sj.bjp.0705115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreto Ortiz S, Hori D, Nomura Y, Yun X, Jiang H, Yong H, Chen J, Paek S, Pandey D, Sikka G, Bhatta A, Gillard A, Steppan J, Kim JH, Adachi H, Barodka VM, Romer L, An SS, Shimoda LA, Santhanam L, Berkowitz DE. Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition. Am J Physiol Lung Cell Mol Physiol 314: L93–L106, 2018. doi: 10.1152/ajplung.00091.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer JA, Fung HL. Photochemical generation of nitric oxide from nitro-containing compounds: possible relation to vascular photorelaxation phenomena. Life Sci 54: PL1–PL4, 1994. doi: 10.1016/0024-3205(94)00578-8. [DOI] [PubMed] [Google Scholar]
- 5.Buhr ED, Van Gelder RN. Local photic entrainment of the retinal circadian oscillator in the absence of rods, cones, and melanopsin. Proc Natl Acad Sci USA 111: 8625–8630, 2014. doi: 10.1073/pnas.1323350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buscone S, Mardaryev AN, Raafs B, Bikker JW, Sticht C, Gretz N, Farjo N, Uzunbajakava NE, Botchkareva NV. A new path in defining light parameters for hair growth: discovery and modulation of photoreceptors in human hair follicle. Lasers Surg Med 49: 705–718, 2017. doi: 10.1002/lsm.22673. [DOI] [PubMed] [Google Scholar]
- 7.Castro-Piedras I, Perez-Zoghbi JF. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J Physiol 591: 5999–6015, 2013. doi: 10.1113/jphysiol.2013.257790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang KC, Chong WS, Park BW, Seung BW, Chun GW, Lee IJ, Park PS. NO- and NO2-carrying molecules potentiate photorelaxation in rat trachea and aorta. Biochem Biophys Res Commun 191: 509–514, 1993. doi: 10.1006/bbrc.1993.1247. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M, Fong HK. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet 28: 256–260, 2001. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Gillis CN. Methylene blue enhanced photorelaxation in aorta, pulmonary artery and corpus cavernosum. Biochem Biophys Res Commun 190: 559–563, 1993. doi: 10.1006/bbrc.1993.1084. [DOI] [PubMed] [Google Scholar]
- 11.Danielsson J, Perez-Zoghbi J, Bernstein K, Barajas MB, Zhang Y, Kumar S, Sharma PK, Gallos G, Emala CW. Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology 123: 569–581, 2015. doi: 10.1097/ALN.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dartnall HJ, Bowmaker JK, Mollon JD. Human visual pigments: microspectrophotometric results from the eyes of seven persons. Proc R Soc Lond B Biol Sci 220: 115–130, 1983. doi: 10.1098/rspb.1983.0091. [DOI] [PubMed] [Google Scholar]
- 13.de Assis LV, Moraes MN, Magalhães-Marques KK, Castrucci AML. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: unravelling the photosensitive system of the skin. Eur J Cell Biol 97: 150–162, 2018. doi: 10.1016/j.ejcb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Massri N, Cullen KM, Stefani S, Moro C, Torres N, Benabid AL, Mitrofanis J. Evidence for encephalopsin immunoreactivity in interneurones and striosomes of the monkey striatum. Exp Brain Res 236: 955–961, 2018. doi: 10.1007/s00221-018-5191-9. [DOI] [PubMed] [Google Scholar]
- 16.Fahrenkrug J, Falktoft B, Georg B, Hannibal J, Kristiansen SB, Klausen TK. Phosphorylation of rat melanopsin at Ser-381 and Ser-398 by light/dark and its importance for intrinsically photosensitive ganglion cells (ipRGCs) cellular Ca2+ signaling. J Biol Chem 289: 35482–35493, 2014. doi: 10.1074/jbc.M114.586529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furchgott RF, Ehrreich SJ, Greenblatt E. The photoactivated relaxation of smooth muscle of rabbit aorta. J Gen Physiol 44: 499–519, 1961. doi: 10.1085/jgp.44.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295: 1065–1070, 2002. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higuchi S, Hida A, Tsujimura S, Mishima K, Yasukouchi A, Lee SI, Kinjyo Y, Miyahira M. Melanopsin gene polymorphism I394T is associated with pupillary light responses in a dose-dependent manner. PLoS One 8: e60310, 2013. doi: 10.1371/journal.pone.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ieda N, Hotta Y, Miyata N, Kimura K, Nakagawa H. Photomanipulation of vasodilation with a blue-light-controllable nitric oxide releaser. J Am Chem Soc 136: 7085–7091, 2014. doi: 10.1021/ja5020053. [DOI] [PubMed] [Google Scholar]
- 21.Jiao J, Hong S, Zhang J, Ma L, Sun Y, Zhang D, Shen B, Zhu C. Opsin3 sensitizes hepatocellular carcinoma cells to 5-fluorouracil treatment by regulating the apoptotic pathway. Cancer Lett 320: 96–103, 2012. doi: 10.1016/j.canlet.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Kanaho Y, Tsai SC, Adamik R, Hewlett EL, Moss J, Vaughan M. Rhodopsin-enhanced GTPase activity of the inhibitory GTP-binding protein of adenylate cyclase. J Biol Chem 259: 7378–7381, 1984. [PubMed] [Google Scholar]
- 23.Kapur N, Hughes JR, Rustin MH. Exacerbation of asthma by isotretinoin. Br J Dermatol 142: 388–389, 2000. doi: 10.1046/j.1365-2133.2000.03324.x. [DOI] [PubMed] [Google Scholar]
- 24.Karunarathne WK, Giri L, Patel AK, Venkatesh KV, Gautam N. Optical control demonstrates switch-like PIP3 dynamics underlying the initiation of immune cell migration. Proc Natl Acad Sci USA 110: E1575–E1583, 2013. doi: 10.1073/pnas.1220755110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasper G, Taudien S, Staub E, Mennerich D, Rieder M, Hinzmann B, Dahl E, Schwidetzky U, Rosenthal A, Rump A. Different structural organization of the encephalopsin gene in man and mouse. Gene 295: 27–32, 2002. doi: 10.1016/S0378-1119(02)00799-0. [DOI] [PubMed] [Google Scholar]
- 26.Kato M, Sugiyama T, Sakai K, Yamashita T, Fujita H, Sato K, Tomonari S, Shichida Y, Ohuchi H. Two opsin 3-related proteins in the chicken retina and brain: a TMT-type opsin 3 is a blue-light sensor in retinal horizontal cells, hypothalamus, and cerebellum. PLoS One 11: e0163925, 2016. doi: 10.1371/journal.pone.0163925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Hong Y, Shim CS. Mechanism of UV light-induced photorelaxation in isolated rat aorta. J Vet Sci 1: 81–86, 2000. [PubMed] [Google Scholar]
- 28.Kim SC, Oh CH, Park JK, Lee MY, Uhm DY. Effects of ultraviolet light on the tension of isolated human cavernosal smooth muscle from non-diabetic and diabetic impotent men. Urol Res 25: 149–152, 1997. doi: 10.1007/BF01037932. [DOI] [PubMed] [Google Scholar]
- 29.Koyanagi M, Takada E, Nagata T, Tsukamoto H, Terakita A. Homologs of vertebrate Opn3 potentially serve as a light sensor in nonphotoreceptive tissue. Proc Natl Acad Sci USA 110: 4998–5003, 2013. doi: 10.1073/pnas.1219416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyanagi M, Takano K, Tsukamoto H, Ohtsu K, Tokunaga F, Terakita A. Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc Natl Acad Sci USA 105: 15576–15580, 2008. doi: 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyanagi M, Terakita A. Diversity of animal opsin-based pigments and their optogenetic potential. Biochim Biophys Acta 1837: 710–716, 2014. doi: 10.1016/j.bbabio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG, Lefkowitz RJ. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J Biol Chem 267: 8558–8564, 1992. [PubMed] [Google Scholar]
- 33.Mikami M, Zhang Y, Danielsson J, Joell T, Yong HM, Townsend E, Khurana S, An SS, Emala CW. Impaired relaxation of airway smooth muscle in mice lacking the actin-binding protein gelsolin. Am J Respir Cell Mol Biol 56: 628–636, 2017. doi: 10.1165/rcmb.2016-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69: 451–482, 2007. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 35.Ockenfels A, Schapiro I, Gärtner W. Rhodopsins carrying modified chromophores–the ‘making of’, structural modelling and their light-induced reactivity. Photochem Photobiol Sci 15: 297–308, 2016. doi: 10.1039/C5PP00322a. [DOI] [PubMed] [Google Scholar]
- 36.Özgür S, Sancar A. Purification and properties of human blue-light photoreceptor cryptochrome 2. Biochemistry 42: 2926–2932, 2003. doi: 10.1021/bi026963n. [DOI] [PubMed] [Google Scholar]
- 37.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol Cell Physiol 256: C329–C335, 1989. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- 38.Plass CA, Loew HG, Podesser BK, Prusa AM. Light-induced vasodilation of coronary arteries and its possible clinical implication. Ann Thorac Surg 93: 1181–1186, 2012. doi: 10.1016/j.athoracsur.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 39.Regazzetti C, Sormani L, Debayle D, Bernerd F, Tulic MK, De Donatis GM, Chignon-Sicard B, Rocchi S, Passeron T. Melanocytes sense blue light and regulate pigmentation through opsin-3. J Invest Dermatol 138: 171–178, 2018. doi: 10.1016/j.jid.2017.07.833. [DOI] [PubMed] [Google Scholar]
- 40.Sabroe RA, Staughton RC, Bunker CB. Bronchospasm induced by isotretinoin. BMJ 312: 886, 1996. doi: 10.1136/bmj.312.7035.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schittny JC, Miserocchi G, Sparrow MP. Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am J Respir Cell Mol Biol 23: 11–18, 2000. doi: 10.1165/ajrcmb.23.1.3926. [DOI] [PubMed] [Google Scholar]
- 42.Sexton TJ, Van Gelder RN. G-protein coupled receptor kinase 2 minimally regulates melanopsin activity in intrinsically photosensitive retinal ganglion cells. PLoS One 10: e0128690, 2015. doi: 10.1371/journal.pone.0128690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shichi H, Somers RL. Light-dependent phosphorylation of rhodopsin. Purification and properties of rhodopsin kinase. J Biol Chem 253: 7040–7046, 1978. [PubMed] [Google Scholar]
- 44.Sikka G, Hussmann GP, Pandey D, Cao S, Hori D, Park JT, Steppan J, Kim JH, Barodka V, Myers AC, Santhanam L, Nyhan D, Halushka MK, Koehler RC, Snyder SH, Shimoda LA, Berkowitz DE. Melanopsin mediates light-dependent relaxation in blood vessels. Proc Natl Acad Sci USA 111: 17977–17982, 2014. doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stadel JM, Nambi P, Shorr RG, Sawyer DF, Caron MG, Lefkowitz RJ. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci USA 80: 3173–3177, 1983. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Triguero D, Costa G, Labadía A, Jiménez E, García-Pascual A. Spontaneous photo-relaxation of urethral smooth muscle from sheep, pig and rat and its relationship with nitrergic neurotransmission. J Physiol 522: 443–456, 2000. doi: 10.1111/j.1469-7793.2000.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchiya S, Buhr ED, Higashide T, Sugiyama K, Van Gelder RN. Light entrainment of the murine intraocular pressure circadian rhythm utilizes non-local mechanisms. PLoS One 12: e0184790, 2017. doi: 10.1371/journal.pone.0184790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang ZW, Kotlikoff MI. Activation of KCa channels in airway smooth muscle cells by endogenous protein kinase A. Am J Physiol Lung Cell Mol Physiol 271: L100–L105, 1996. doi: 10.1152/ajplung.1996.271.1.L100. [DOI] [PubMed] [Google Scholar]
- 49.White JH, Chiano M, Wigglesworth M, Geske R, Riley J, White N, Hall S, Zhu G, Maurio F, Savage T, Anderson W, Cordy J, Ducceschi M, Vestbo J, Pillai SG; GAIN Investigators . Identification of a novel asthma susceptibility gene on chromosome 1qter and its functional evaluation. Hum Mol Genet 17: 1890–1903, 2008. doi: 10.1093/hmg/ddn087. [DOI] [PubMed] [Google Scholar]
- 50.Yang MY, Chang CJ, Chen LY. Blue light induced reactive oxygen species from flavin mononucleotide and flavin adenine dinucleotide on lethality of HeLa cells. J Photochem Photobiol B 173: 325–332, 2017. doi: 10.1016/j.jphotobiol.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Yim PD, Gallos G, Perez-Zoghbi JF, Trice J, Zhang Y, Siviski M, Sonett J, Emala CW Sr. Chloride channel blockers promote relaxation of TEA-induced contraction in airway smooth muscle. J Smooth Muscle Res 49: 112–124, 2013. doi: 10.1540/jsmr.49.112. [DOI] [PMC free article] [PubMed] [Google Scholar]