Abstract
The Translational Research Working Group (TRWG) was created as a national initiative to evaluate the current status of the investment of National Cancer Institute in translational research and envision its future. TheTranslational Research Working Group conceptualized translational research as a set of six developmental processes or pathways focused on various clinical goals. One of those pathways describes the development of immune response modifiers such as vaccines and cytokines. A hallmark of the Immune Response Modifier Developmental Pathway is the coordinated development of multiple components. The Immune Response Modifier Pathway was conceived not as a comprehensive description of the corresponding real-world processes but rather as a tool designed to facilitate movement of a candidate assay through the translational process to the point where it can be handed off for definitive clinical testing. This paper discusses key challenges associated with the immune response modifier agent development process in light of the pathway.
Immune response modifiers can be defined as immunotherapy agents that mimic, augment, or require participation of host immune cells for optimal effectiveness. Immune response modifier agents are either already approved or in pivotal trials for all major cancers and are currently a vibrant part of the anticancer armamentarium in the clinic. There are currently 13 approved immune response modifier agents that require host participation for optimal efficacy, including the following:
Cytokines requiring host participation: aldesleukin [interleukin (IL)-2] and IFN ∝ 2;
Antibodies requiring host participation—to the extent that they invoke antibody dependent cellular cytotoxicity for optimal efficacy: alemtuzumab, tositumomab, cetuximab, ibritumomab, rituximab, and trastuzumab;
Immunostimulants known to require host participation: Bacillus Calmette-Guerin, levamisole, and imiquimod; and
Immunostimulants with unknown host participation: lenalidomide and thalidomide.
A substantial number of immune response modifier agents with known ability to activate, augment, or enhance specific immune responses are currently in translational stages of development and are highly likely to have a profound effect on cancer therapy including the following:
T-cell growth factors to increase the number and repertoire of naive T cells;
T-cell growth factors to increase the growth and survival of immune T cells;
Agonists to activate and stimulate T cells;
Inhibitors of T-cell checkpoint blockade;
Growth factors to increase the number of dendritic cells;
Agonists to activate dendritic cells and other antigen- presenting cells;
Agents to inhibit, block, or neutralize cancer cell and immune cell-derived immunosuppressive cytokines;
Cancer antigen-specific monoclonal antibodies that require host effector cells for optimal efficacy, e.g., antibody dependent cellular cytotoxicity;
Cancer antigen vaccines for prevention and therapy; and
Adjuvants to allow, facilitate, and augment cancer vaccines.
The Tumor Immunology Think Tank,8 convened by the National Cancer Institute (NCI) Division of Cancer Biology in 2003, highlighted the therapeutic promise of advances in this field, as well as key obstacles that stood in the way of progress:
Unequivocal evidence has emerged from a number of sources of the capacity of the immune system, alone and in combination with other modalities, to effect clinically meaningful antitumor immune responses.
Recent advances in basic cellular and molecular immunology have been truly revolutionary, and have given us an unprecedented framework for understanding how the immune response is initiated and regulated... Already, these insights are leading to the conclusion that the most effective immunotherapies will utilize combinatorial approaches that impact the antitumor immune response at multiple points.
Infrastructure limitation with respect to preclinical models of cancer, production of immune cells for adoptive therapy in patients, vaccine generation and availability of clinical grade recombinant molecules (i.e., cytokines, antibodies, etc.) for early phase clinical testing are severely limiting progress in the translation of the most promising immunotherapeutic combination strategies.
Additionally, the growing regulatory burden for biologic therapies threatens to destroy even the current ongoing progress toward clinical translation.
Facilitation of the development and translation of rationally designed combination immunotherapy strategies should be the major NCI mandate in this area. This will require the dual approaches of empowering academically based groups for independent early stage translation as well as proactive promotion of effective public-private partnerships in this area.
Five years later, the obstacles highlighted by the Tumor Immunology Think Tank remain valid; progress in delivering workable therapies based on immune response modifier agents to the clinic remains frustratingly slow. The Immune Response Modifier Developmental Pathway (IRM) created by the NCI Translational Research Working Group provides a framework for conveying to a broader audience the challenges of the translational development of immune response modifier agents and facilitates the understanding of policy issues critical to continued progress. An introduction and overview of the developmental pathways of Translational Research Working Group is provided in Hawk and colleagues (1). The IRM Pathway is depicted in Fig. 1.
Fig. 1.
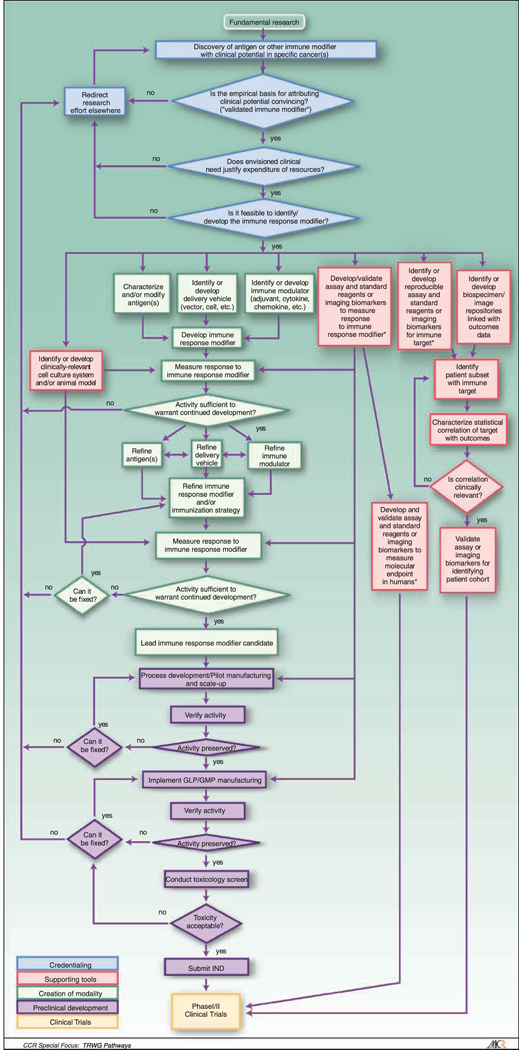
Immune Response Modifiers (IRM) Pathway. The IRM pathway is depicted as a flowchart, a schematic process representation widely used in engineering. Rounded rectangle at the top, origin of the process. Square-cornered rectangles, activity steps. Diamonds, conditional tests or decision steps. Unidirectional arrows, the direction of the activity sequence, and the direction of transfer of supporting tools from their parallel development paths to the main path of modality development. Bidirectional arrows, codevelopment or concurrent, interactive refinement. The initial steps of the pathway (blue) are required to proceed through the pathway, with the blue diamonds representing the credentialing steps of scientific validation, clinical need, and feasibility. The pathway proceeds to multiple parallel paths representing development of multiple components of the modality itself (green) as well as development of different classes of supporting tools (red). The three green boxes at the top of the creation of the modality path acknowledges the parallel and interactive development of a multicompent formulation and/or regimen, such as the need for an antigen, a delivery vehicle, and an immune modulator for the development of a cancer vaccine. The red boxes at the top of the supporting tools path depict the development of assays for characterizing and evaluating the effects of the modality and for defining the cohort for which the modality is appropriate. The development of these tools is further detailed in the Biospecimen-based or Imaging-based Assessment Modality pathway. Parallel paths have been made explicit to acknowledge that some of the required tools may not exist, and their parallel or codevelopment will be prerequisite for the viability of the new modality. Subsequent steps include preclinical development (purple) and early stage clinical trials (yellow). For each activity, decision point, parallel path, or feedback loop, it is understood that there are many more variations that can occur, and that not all steps may occur in each instance. The pathway does not address the ways in which insights gained from late-stage clinical trials can influence the development process. Immune response modifier agent interventions may be used for treatment or for primary, secondary, or tertiary prevention. The pathways are conceived not as comprehensive descriptions of the corresponding real-world processes but as tools designed to serve specific purposes, including research program and project management, coordination of research efforts, and professional and lay education and communication.
This paper provides a brief introduction to the field in the context of this framework, emphasizing those developmental elements and challenges that are distinctive to immune response modifier agents and highlighting key issues for policymakers.
Creation of Modality
Complexity of immune response modifier agents.
There are multiple categories of immune response modifier agents, each of which has unique complexities. To simply the presentation, this article will focus on cancer vaccines. Many of the principles discussed can be extrapolated to the translational development of other categories of immune response modifing agents. Conventional anticancer agents are most commonly developed as monotherapies and function as such, although many will eventually be used in combination therapy regimens. Vaccines, on the other hand, are a key element in the immune response modifier agent armamentarium, and most often emerge as multicomponent systems, consisting of a tumor-associated antigen (TAA), a delivery vehicle and an adjuvant to enhance immunity. The TAA can be in the form of a simple peptide, a protein, killed whole-tumor cells, or an expression vector coding the TAA gene coding the TAA. The delivery expression vector can be a viral, bacterial, or yeast vector, a DNA plasmid, or a dendritic cell. The adjuvants can be agents to sustain a depot effect for the antigen at the vaccination site, and/or to induce an inflammatory response at the vaccination site. Vaccines intended for prophylaxis of infectious diseases can be formulated with single nontoxic or nonreactogenic adjuvants. By contrast, cancer vaccines are often formulated with multiple adjuvants to achieve greater efficacy with a greater leeway allowed for reactogenicity. A further dimension of complexity extends across time: Immune response modifier agent-based regimens may need to be administered in precisely choreographed sequences of treatments over an extended period.
Immune response modifier agent-based therapeutic strategies under investigation include the following:
Vaccine plus conventional therapy.
To achieve additive or synergistic effects, a vaccine can be used in combination with conventional agents such as chemotherapy, small molecule-targeted therapeutics, or hormonal agents (2–5).
Vaccine plus other immune-potentiating agents.
Vaccines are most often—and possibly best—administered in combination with agents that can influence the immune system (6). The agents can be (a) cytokines such as granulocyte macrophage colony-stimulating factor, IL-2, IL-15, IL-12, or IL-7; (b) immune stimulants such as CpG motifs or a lipid-based adjuvant such as MPL; (c) agents such as Flt-3L, which induces proliferation of dendritic cellss (7); or (d) monoclonal antibodies or other agent that reduce immune inhibitory functions, such as anti-CTLA-4, denileukin diftitox, or cyclophosphamide (5, 8). There are a plethora of agents that either stimulate the immune system or reduce immune suppressive function.
Multiple vaccine therapies.
Vaccines are commonly used in diversified prime-and-boost regimens. The major reason is to alleviate host-induced antivector immune responses, and also to focus the immune response on the designated TAA. Diversified prime-and-boost regimens using different pox vectors, DNA, adenovirus, as well as other vectors, have been shown to increase immune responses (4, 9).
Scheduling of vaccine with other therapies.
Some standard therapies such as high-dose cytotoxic chemotherapy and radiation therapy can induce lymphopenia and compromise the ability of vaccines to induce immune responses (10). In many experimental models, however, vaccine-induced immune responses and standard therapies are synergistic. Evidence from several clinical studies shows that patients who first receive vaccine and develop an immune response, and then receive a subsequent therapy, can have enhanced clinical benefit compared with patients who receive the same therapy with no prior vaccine therapy (11, 12).
Phenotypic alteration.
Numerous preclinical studies have shown that certain chemotherapeutic agents and/or radiation can alter the phenotype of tumor cells to render them more susceptible to vaccine-induced, antibody, and T-cell-mediat- ed lysis (13, 14). As one example, many chemotherapy agents are mutagens and can induce expression of mutated proteins that can serve as cancer-specific proteins. Chemotherapy-treated tumors, if killed, can induce T-cell responses, and, if not killed, can have increased susceptibility to T-cell- mediated lysis.
Multiple antigens.
Many vaccine formulations in trials contain a series of peptide epitopes or multiple antigens. Thus, one of the key elements of a vaccine—the antigen—can itself be a composite. The immunogenicity and toxicity of each antigenic component need to be explored as well as the interactions of each in the vaccine.
The IRM Pathway acknowledges the composite character of vaccines, as well as other immune response modifier agents, by splitting the main line of development, on the left side of the diagram, into three parallel elements for selected, earlier portions of the development process. It is important to note that although antigens, delivery vehicles, and adjuvants or other supporting immune modulators are represented as independent elements with their own development challenges, creation of a vaccine often requires successful integration of these components, which in turn may require substantial interaction among the parallel courses. Where an initial approach to integration fails, substitution of alternative components may be required. Coordination of component development to achieve timely and successful integration is a key scientific and management challenge.
Appropriate standards to develop individual components of these composite systems are essential as well. For example, adjuvants by definition are agents expected and intended to potentiate the effects of another agent, rather than necessarily to have therapeutic effects in themselves. Recently, however, it has become evident that the development of effective immune response modifier agent-based treatment strategies has been hampered because many effective immune response modifier agents were judged failures when used alone as monotherapy, i.e., against the clinical efficacy benchmarks that are used for the development of single-agent therapeutics. As a result, development has been slowed or stopped on many agents with substantial proven ability to activate, induce, or augment immune responses. It is highly likely that many of the agents would be effective when used in combination with other immune response modifier agents, such as for use as vaccine adjuvants. A recent NCI Immunotherapy Agent Workshop9 with participants from the NCI, academia, and industry developed a priority list of immunotherapy agents with high potential to serve as immunotherapeutic drugs, some of which now might be made available for additional translational development with the assistance of the NCI Rapid Access to Intervention Development program.
Supporting Tools
As documented by the pathway, a key task at several stages of the development process is to assess the biological—and later the clinical—effects of the candidate agent, to determine whether development should proceed, and what modifications, if any, are needed to achieve acceptable performance. There is a substantial need for improved supporting tools in this area.
Measurement of response to immune response modifier agents.
Assessment of biological and clinical effects of immune response modifier agents is especially challenging because the immune system of the host is in effect a part of the intervention. Effects on the host immune response need to be measured as well as the effect the host immune response has on the tumor. Although conventional agents are typically evaluated by assessing their effects on tumor cells in vitro, or using human tumor xenografts in athymic mice, evaluation of immune response modifier agents requires both a living host and an intact immune system.
A further challenge at the preclinical stage is that many vaccines and cytokines are host species specific. An antigen that is immunogenic in a human may be nonimmunogenic or hyperimmunogenic in a primate or rodent model, or vice versa. Even if the TAA or cytokine shares a great deal of homology between humans and the test species, it has been shown that a difference in only a single amino acid can greatly alter immunogenicity among species. Indeed, there are examples where evaluating a human TAA or human cytokine in a nonhuman species may lead to a “false positive” toxicity profile for that agent. Moreover, in many cases, human TAAs are self-antigens and are expressed in very different tissues in humans compared with other species. Different levels and distribution of expression can lead to differences in the level of immune tolerance as well as a different spectrum of toxicities, especially those mediated by autoimmunity against organs or tissues expressing the TAA. Thus, the preferred way to evaluate the toxicity and/or efficacy profile of a given vaccine or cytokine is in early human phase I trials. Thus, reiterative testing of agents and combinations of agents is often an essential component of the IRM Pathway.
Pharmacokinetics.
A key stage in the development of a conventional agent is the assessment of its pharmacokinetic properties—the absorption, distribution, metabolism, and elimination of the agent. Clinical efficacy requires not only a suitable effect on the target cells but also that the agent can be delivered to the target in biologically active form and adequate dose. However, classic pharmacokinetic studies of the distribution of an agent among body compartments are not relevant for vaccines because a vaccine is administered locally and then induces a host immune response—which can be local, regional, or systemic. The concept that host immune cells mediate distant effects is similar for many other categories of immune response modifier agents.
Because of the active role of the host, measurement of immune responses may be considered the counterpart of pharmacokinetics in the immune response modifier agent context. However, there are multiple arms of the immune response, including cytotoxic and helper T cells, functional subsets of cytotoxic and helper T cells, antibodies of a variety of immune classes and functions, as well as a multiplicity of induced cytokines. Each might be related to efficacy and toxicity and each can be measured, albeit frequently with difficulty. Although the most important immune response is the response in the tumor, it is difficult to measure immune responses at sites other than the peripheral blood. In addition, what specific or nonspecific immune responses should be measured is not always clear. It is possible that a panel of measures addressing an array of immune functions will be required, along with statistical methods that enable assessment of trends across the immune system, with the ultimate goal of linking markers of biological activity to downstream clinical response.
There are further challenges in assessing the effects of cytokines. Administration of any immune response modifier agent such as a cytokine can often induce a panoply of secondary cytokines, which may in turn provide the predominant biological effect. In addition, it is well-known that the biological effects of cytokines have a bell-shaped curve; thus, more cytokine is not always better and, in many cases, can have a deleterious effect. There are clear cases, such as cytokine IL-12, where systemic delivery at certain doses can lead to toxicity and provide little clinical benefit. However, when used at lower doses or administered locally with a vaccine, a cytokine such as IL-12 or IL-15 may have positive biological effects with minimal toxicity.
Clinical Trials
The time dimension poses challenges in assessing the efficacy of immune response modifier agent-based regimens. It is a well-established phenomenon of both vaccine-mediated immunoprevention and immunotherapy that booster vaccinations are required to obtain optimal biological effects. Because the TAAs used in cancer vaccines are usually weakly immunogenic, multiple vaccinations administered over a period of weeks or months may be required to see the desired effect. Indeed, tumor progression may occur in patients who have begun vaccine therapy, before the biological manifestations of multiple booster vaccinations take effect. At the same time, the purpose of the cancer vaccine is to induce a dynamic process in the host immune system that can be sustained for months, and perhaps years, after administration, keeping tumor growth and/or invasion in check. Thus, vaccination may have prolonged effects that remain during the administration of subsequent therapies. The immune responses present at times distant from the administration of the vaccine can have additive or synergistic effects on subsequent therapies. Even where study designs provide for long-term follow-up— and where funding is available to implement it—patients who have undergone immune response modifier therapy may advance to additional treatments, which confounds evaluating the specific reasons for extended survival. Thus, ideally the effect of immune response modifier agents need to be monitored during the time of testing as well as long-term after cessation of active intervention. Long-term effects of autoimmune phenomena must also be monitored.
Finally, Response Evaluation Criteria in Solid Tumors criteria, a hallmark of activity of conventional therapeutics, measure “tumor response,” i.e., the degree of tumor shrinkage, might not be the best measure of immune response modifier agent effectiveness. However, as discussed above, immune response modifiers may produce prolonged beneficial effects even when no tumor shrinkage is apparent in the short run. To enable continued progress along the IRM Pathway, a robust system of measures is needed, beyond the simple progression free survival measure, to characterize stable and progressive disease, as well as the kinetics of disease growth, and which can account for active remodeling of the disease as well as cytotoxicity.
Development of a HER-2/neu Breast Cancer Vaccine as an Example of the IRM Pathway
Development of the HER-2/neu vaccine followed the IRM pathway through multiple iterations to finally achieve an immune response that was deemed adequate to justify testing of the vaccine in larger scale trials. Fundamental research studies showed that the tumor antigen HER-2/neu is overexpressed in a subset of breast cancer, and that some breast cancer patients had existent immune responses against the HER-2/neu protein, although the responses fell short of therapeutic levels (15). Investigators at the University of Washington credentialed the finding by demonstrating it was feasible to elicit an immune response in mouse models using portions of the HER-2/neu protein as an immunogen (16, 17). University of Washington scientists cofounded a start-up biotech company, Corixa, which licensed the patent and commenced creation of the modality and preclinical development, including GMP manufacturing, of a series of HER-2/neu peptide vaccines. Supporting tools included various assays of immune response (18, 19). The initial phase I trial was conducted by University of Washington with Corixa funding. This initial trial led to multiple Corixa-funded phase I trials in collaboration with University of Washington translational scientists to refine the vaccine construct and optimize the immune response, an iterative process from creation of the modality through phase I clinical trials (16–19). Corixa partnered with GlaxoSmithKline, which was essential for financing, manufacturing the vaccine, and providing unique combinations of adjuvants available to GlaxoSmithKline and not available to academic investigators. Phase I/II testing of the improved vaccine formulation was initiated by GlaxoSmithKline and Corixa in collaboration with multiple academic translational researchers (20).
Optimizing the Productivity of IRM Pathway Research
Methodologic challenges.
The immense complexity and subtlety of the immune response poses great demands on researchers in this field. Failure to identify and consistently apply the best available tools in the most methodologically rigorous and consistent manner can result in a failure to extract critical information from study results. Different methodologies can also impede comparison of results and prevent replication and further development of findings. Without standardization and a clear definition of the performance characteristics of the assays used to assess potency, it can be difficult or impossible to determine which of several competing strategies for achieving a given objective is most promising for further development. A reported result that is dependent on a particular assay has no value if that assay cannot be reproduced outside of the originating laboratory. Without an adequate toolkit of measures of effect, including both intermediate biological responses and ultimate clinical responses, potentially promising interventions may be dismissed as ineffective. Assuming the use of rigorous methodology in the design, implementation, and analysis of IRM Pathway studies, it is essential that negative as well as positive results be valued and disseminated, as these are equally important to advances in understanding, and essential to enable the research community to deploy scarce resources productively.
Opportunities for Progress in IRM Pathway Translational Research
Despite substantial public and private sector investment in basic immunology research and the development of agents with profound effects on the immune system, no translational research structure presently exists that is capable of taking advantage of both the knowledge base that has been created, and also the innovative immune response modifier agents available now—and those expected to be available in the near future. IRM Pathway translational research would benefit from the following:
The development of an administrative structure to efficiently develop innovative and biologically dictated regimens using several investigative immune response modifier agents.
Continued support by programs such as the NCI Rapid Access to Intervention Development (RAID) program for the development of immune response modifier regimen components that may require potentially complex or nontraditional methods of assessment, toxicology, formulation, manufacture, and clinical development.
Support for the development of panels of biomarkers that can provide a global view of immune response; more sophisticated measures of stable and progressive disease to complement existing Response Evaluation Criteria in Solid Tumors criteria.
A concerted effort by sponsors of immune response modifier agent translational research to promote standardization in the use of assays and biomarkers, as well as requiring complete and clear reporting on their use in the methodology sections of published papers.
Development of approaches for cost-effective tracking— and follow-up over an extended period—of patients who have received investigational immune response modifier therapies.
The cultivation of a research culture in which negative results obtained via well-conceived research strategies executed with rigorous methodology will be considered “productive failures,” encouraged and accepted for publication, and credited in assessments of research quality and productivity.
Conclusions
The Translational Research Working Group conceptualized the Developmental Pathway for Immune Response Modifiers to assist the translational development of immune response modifier agents. The agents are unique as their primary mode of action is to modulate host responses. The host response in turn mediates cancer therapy. In many instances, the agents do not directly contact or directly affect the tumor. The agents can be exquisitely targeted to particular components of the immune system and the subsequent immune response can mediate specifically targeted killing of cancer cells. A major confounding issue for the translational development of immune response modifier agents is the biological requirement for regimens containing multiple agents. It is extraordinarily difficult to develop multiple, novel agents in parallel. It is expected that the development of multiple novel agents in parallel will require multiple reiterative steps with different regimens at the phase I/II clinical trial level to optimize the effect on modulating host responses. The IRM Pathway was conceived as a tool to track the movement of candidate immune response modulators through the translational process to the point where they can be handed off for definitive clinical testing, and is anticipated to facilitate and accelerate that process.
KEY POINTS.
The Immune Response Modifiers Pathway demonstrates the complexity of codevelopment of multiple novel agents and highlights coordination of component development as a key scientific and management challenge.
The Immune Response Modifiers Pathway indicates the need for tools to determine the effect of the agent on the host immune response as well as the effect of the immune response on the tumor.
Development of immune response modifier agents and supporting tools often requires iterative clinical studies based on the species specificity of agents and variability of patient immune systems.
The flexibility of the Immune Response Modifiers Pathway enables the multiple, iterative modifications required for optimal advancement of effective immune response modifier agent combinations.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
NCI ThinkTanks in Cancer Biology: Tumor immunology think tank [cited January 18, 2008]. Available from: http://www.cancer.gov/think-tanks-cancer-biology/page2.
Cheever MA, Creekmore S eds. National Cancer Institute AgentWorkshop Proceedings: July12, 2007. http://web.ncifcrf.gov/research/brb/workshops.asp.
Note: Information on theTRWG is available at http://www.cancer.gov/trwg.
References
- 1.Hawk ET, Matrisian LM, Nelson WG, et al. Translational Research Working Group developmental pathways: introduction and overview. Clin Cancer Res 2008;14: 5664–71. [DOI] [PubMed] [Google Scholar]
- 2.Mihich E Combined effects of chemotherapy and immunity against Leukemia L1210 in DBA/2 Mice. Cancer Res 1969;29:848–54. [PubMed] [Google Scholar]
- 3.Arlen PM, Madan RA, Hodge JW, Schlom J. Combining vaccines with conventional therapies for cancer. Update CancerTher 2007;1:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arlen PM, Gulley JL, Madan RA, Hodge JW, Schlom J. Preclinical and clinical studies of recombinant poxvirus vaccines for carcinoma therapy. Crit Rev Immunol 2007;27:451–62. [DOI] [PubMed] [Google Scholar]
- 5.Finn OJ. Cancer Immunology. N Engl J Med 2008; 58:2704–15. [DOI] [PubMed] [Google Scholar]
- 6.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev 2008;22:357–68. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Chan AS, Dawson AJ, Liang X, Blazar BR, Miller JS FLT3 ligand administration after hematopoietic cell transplantation increases circulating dendritic cell precursors that can be activated by CpG oligodeoxy-nucleotides to enhance T cell and natural killer cell function. Biol Blood MarrowTransplant 2005;11:23–34. [DOI] [PubMed] [Google Scholar]
- 8.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006;90: 297–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irvine KR, Chamberlain RS, Shulman EP, Surman DR, Rosenberg SA, Restifo NP. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J Natl Cancer Inst 1997;89:1595–601. [DOI] [PubMed] [Google Scholar]
- 10.Mackall CL, Fleisher TA, Brown MR, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994;84:2221–8. [PubMed] [Google Scholar]
- 11.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res 2005;11:4430–6. [DOI] [PubMed] [Google Scholar]
- 12.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med (Maywood) 2008;233:522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolin A, Canti G, Marelli O,Veronese F, Goldin A. Chemotherapy and immunotherapy of L1210 leukemic mice with antigenic tumor sublines. Cancer Res 1981; 41:1358–62. [PubMed] [Google Scholar]
- 14.Lake RA, Robinson BW. Immunotherapy and chemotherapy-a practical partnership. Nat Rev Cancer 2005;5:397–405. [DOI] [PubMed] [Google Scholar]
- 15.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol 1997;15:3363–7. [DOI] [PubMed] [Google Scholar]
- 16.Disis ML, Gralow JR, Bernhard H, Hand SL, Rubin WD, Cheever MA. Peptide-based, but not whole protein, vaccines elicit immunity to HER-2/ neu, oncogenic self-protein. J Immunol 1996;156: 3151–8. [PubMed] [Google Scholar]
- 17.Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res 1999;5: 1289–97. [PubMed] [Google Scholar]
- 18.Disis ML, Gooley TA, Rinn K, et al. Generation of T- cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 2002;20:2624–32. [DOI] [PubMed] [Google Scholar]
- 19.Disis ML, Schiffman K, Guthrie K, et al. Effect of dose on immune response in patients vaccinated with an HER-2/neu intracellular domain protein-based vaccine. J Clin Oncol 2004;22:1916–25. [DOI] [PubMed] [Google Scholar]
- 20.Limentani S, Dorval T, White S, et al. Phase I dose- escalation trial of a recombinant HER2 vaccine in patients with Stage II/III HER2+ breast cancer. J Clin Oncol 2005;23:2520. [Google Scholar]


