Abstract
Oral epithelial cells (OECs) represent the first line of defense against viruses that are spread via saliva, including Kaposi’s sarcoma-associated herpesvirus (KSHV). Infection of humans by KSHV and viral pathogenesis begins by infecting OECs. One method OECs use to limit viral infections in the oral cavity is the production of antimicrobial peptides (AMPs), or host defense peptides (HDPs). However, no studies have investigated the antiviral activities of any HDP against KSHV. The goal of this study was to determine the antiviral activity of one HDP, LL-37, against KSHV in the context of infecting OECs. Our results show that LL-37 significantly decreased KSHV’s ability to infect OECs in both a structure- and dose-dependent manner. However, this activity does not stem from affecting OECs, but instead the virions themselves. We found that LL-37 exerts its antiviral activity against KSHV by disrupting the viral envelope, which can inhibit viral entry into OECs. Our data suggest that LL-37 exhibits a marked antiviral activity against KSHV during infection of oral epithelial cells, which can play an important role in host defense against oral KSHV infection. Thus, we propose that inducing LL-37 expression endogenously in oral epithelial cells, or potentially introducing as a therapy, may help restrict oral KSHV infection and ultimately KSHV-associated diseases.
Keywords: saliva, antimicrobial peptide, host defense peptide, gingival epithelium, KSHV, LL-37
1. Introduction
Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is one of seven human oncoviruses (Boshoff and Weiss, 2002). In addition to Kaposi’s sarcoma (KS) (Chang et al., 1994), KSHV infection is the etiological agent of a number of B cell disorders including primary effusion lymphoma (Cesarman et al., 1995) and several types of multicentric Castleman’s disease (Soulier et al., 1995). KS remains a threat to the health of people who suffer from AIDS as both the most common cancer (Cohen et al., 2005) and one of the overall leading direct cause of death (Ganem, 2010) in these patients. Similarly, primary effusion lymphoma is resistant to most chemotherapy treatments resulting in a median survival of only six months (Okada et al., 2014).
The current view is that saliva is the main route of transmission for KSHV (Pica and Volpi, 2007), though not due to shedding from the salivary gland (Corbellino et al., 1996; Corey et al.). KSHV infections in resident immune cells of the oral cavity have been studied (Campbell et al., 2014; Knowlton et al., 2012), as well as infections in oral epithelial cells (OECs) (Duus et al., 2004; Toth et al., 2013). Unlike in most other cell types (Bechtel et al., 2003), de novo KSHV infection in OECs from saliva transfer leads to a primarily lytic replication state and a subsequent viral amplification in the oral cavity (Duus et al., 2004; Toth et al., 2013). In contrast, KSHV has been shown to latently reside in B and T cells of the tonsils (Hassman et al., 2011; Myoung and Ganem, 2011a). The mechanism of how KSHV infection in OECs is transmitted to lymphatic endothelial cells and B cells in the host, which can ultimately lead to the development of KS, primary effusion lymphoma, or multicentric Castleman’s disease in immunocompromised people, has yet to be defined. Overall, the initial KSHV infection of OECs is a critical step in KSHV pathogenesis whose control by the innate immune system is still poorly understood.
One method organisms utilize to directly defend against infections is through the production of antimicrobial peptides (AMPs), also known as host defense peptides (HDPs). HDPs represent the most primitive form of immunity against infection, with expression of these peptides found in all forms of life, from bacteria to humans (Kumar et al., 2018). These small peptides (most less than 50 amino acids in length) exhibit activities against a wide range of microorganisms: fungi, Gram-positive and -negative bacteria, and enveloped and non-enveloped viruses (Hans and Madaan Hans, 2014). Most antibiotic and antiviral activities of these peptides stem from their overall cationic and amphipathic structure, leading to binding to microorganism surfaces and subsequent membrane perturbation (Lee and Lee, 2015). KSHV is transmitted via saliva, so we focused on antimicrobial peptides of the oral cavity that can potentially affect oral KSHV infection. The oral cavity is home to many different HDPs, including defensins, histatins, adrenomedullin, and the lone known human cathelicidin LL-37 (Khurshid et al., 2016). OECs, the site of initial KSHV infection, produce LL-37, beta-defensins 1-3, and adrenomedullin (Khurshid et al., 2016). While the antiviral activities of adrenomedullin have not been characterized, beta-defensins 1-3 and LL-37 were shown to exhibit antiviral activities (Barlow et al., 2014a; Klotman and Chang, 2006). Of these HDPs, LL-37, a 37-amino acid cationic peptide present in both saliva and gingival crevicular fluid (Murakami et al., 2002; Türkoğlu et al., 2009), has been the most widely studied in the context of antiviral activities, with defined mechanisms against dengue type 2 virus (DENV2) (Alagarasu et al., 2017), hepatitis C virus (HCV) (Matsumura et al., 2016), human immunodeficiency virus (HIV) (Wong et al., 2011), influenza A virus (Tripathi et al., 2015b, 2014, 2013), respiratory syncytial virus (RSV) (Currie et al., 2016, 2013), vaccinia virus (VV) (Dean et al., 2010; Ulaeto et al., 2016), and herpes simplex virus type 1 (HSV-1) (Lee et al., 2014; Takiguchi et al., 2014). In contrast, no HDP has been studied with respect to KSHV infection.
The goal of this study was to define potential antiviral activities of LL-37 against KSHV in the context of protecting OECs from initial KSHV infection. We found that LL-37 specifically inhibits KSHV infection of OECs in a sequence dependent manner. Our results show that LL-37 can prevent KSHV entry into OECs by disrupting the viral envelope indicating that LL-37 can function as an antiviral agent against KSHV during oral infection.
2. Materials and Methods
2.1. Cell lines
The oral mucosal keratinocyte cell line OKF6/Tert-1 (Dickson et al., 2000) was cultured in keratinocyte serum-free media (K-SFM) (Gibco) supplemented with 2 mM L-glutamine (Corning), 1 IU/ml penicillin (Corning), 100 μg/ml streptomycin (Corning), 75 μg/ml bovine pituitary extract (Gibco), 0.3 mM CaCl2 (Sigma-Aldrich), and 0.6 ng/ml epidermal growth factor (EGF) (Gibco). To produce KSHV, we used the iSLK-BAC16 cell line carrying the recombinant KSHV clone BAC16 (Brulois et al., 2012). The iSLK cell line is engineered to express the KSHV lytic switch gene RTA under doxycycline control (Myoung and Ganem, 2011b). The resulting iSLK cell line was then further engineered to carry the KSHV clone BAC16 expressing GFP under the constitutive EF-1α human promoter (Brulois et al., 2012). The iSLK-BAC16 cells were cultured in Dulbecco’s modified Eagle’s media (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco), 1 IU/ml penicillin (Corning), 100 μg/ml streptomycin (Corning), and 1 mg/ml hygromycin B (Sigma-Aldrich). Hygromycin B is used for the selection of cells with BAC16. HEK293 cells were grown in DMEM (Gibco) supplemented with 10% FBS (Gibco), 1 IU/ml penicillin (Corning), and 100 μg/ml streptomycin (Corning).
2.2. Production of KSHV and viral infection
KSHV production was performed as described previously (Brulois et al., 2012). Briefly, the iSLK-BAC16 cells were treated with 1 μg/ml doxycycline (Sigma-Aldrich), and 1 mM sodium butyrate (Sigma-Aldrich) for 4 days to induce virus production. The cell culture medium was collected and centrifuged at 400 × g for five minutes at 4°C. The resulting supernatant was passed through a 0.45 μm filter and then centrifuged at 24,000 rpm for three hours at 4°C. The virus pellets were resuspended in cell culture media and the virus was kept at either 4°C for shortterm storage or −80°C for long-term storage. To infect OECs, we used both non-spinning and spinning infection methods. In case of non-spinning infection, the virus was added to the cells in the cell culture media and incubated with the cells for the period of time as indicated at the experiments. For spinning infection, the cells with KSHV were centrifuged at 2000 × g for 45 minutes at 30°C.
2.3. Peptide inhibition of KSHV infection
LL-37 (Invivogen) (N-LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-C) and scrambled LL-37 (scrLL-37) (Genscript) (N-GLKLRFEFSKIKGEFLKTPEVRFRDIKLKDNRISVQR-C) were used in experiments testing peptide inhibition of KSHV infection. KSHV-BAC16 and various concentrations of LL-37 were mixed in PBS and rotated at room temperature for increasing amount of time. The resulting mixtures were then added to cells to determine the effect of LL-37 on KSHV binding to oral epithelial cells, entry into the cells, or infection of cells.
2.4. Quantification of infectious KSHV
The quantification of infectious KSHV-BAC16 has been described previously (Brulois et al., 2012). Briefly, 2 × 105 of HEK293 (for initial multiplicity of infection, MOI, calculations) or OKF6/Tert-1 cells/well in 12-well plate were inoculated with KSHV followed by spinning infection at 2000 × g for 45 minutes at 30°C. After one-hour incubation of the cells with KSHV at 37°C, 5% CO2, the cells were washed with PBS and fresh cell culture media was added to the cells. Twenty-four hours later, cells were prepared for flow cytometry: washed three times with cold PBS, detached via 0.25% trypsin, washed twice with PBS, fixed with 2% paraformaldehyde (Santa Cruz) at room temperature for 30 minutes, and then washed twice with PBS again. All wash steps occurred via centrifugation of the cells at 600 × g for three minutes at 4°C. At the end, the cells were resuspended in 500 μl of PBS and subject to flow cytometry using the CantoII fluorescence-activated cell sorter (FACS) (Becton Dickinson) to detect the presence of GFP-positive cells, which were considered to be infected by KSHV due to the expression of GFP from KSHV-BAC16. The GFP signal was quantified on the CantoII FACS and analyzed by FlowJo software to calculate multiplicity of infection (MOI).
2.5. Virus attachment and internalization assays
KSHV-BAC16 attachment and internalization assays were adapted from a quantitative polymerase chain reaction (qPCR)-based assay used for measuring attachment of adeno-associated virus to cells (Berry and Tse, 2017; Berry and Asokan, 2016). Attachment and internalization were each measured using both spinning non-spinning infection conditions, with spinning infection performed as described previously in this paper through the incubation at 37°C, 5% CO2 for one hour. For non-spinning infections, cells were incubated at 4°C for 30 minutes before infection. The cells were incubated with KSHV at 4°C for one hour to allow for viral attachment. After either spinning or non-spinning infections, cells were then washed three times with cold PBS. Samples used for attachment measurements were subjected to RNA extraction using the RNeasy Mini kit (Qiagen) without DNase. DNA was isolated from the membrane of the spin column after the elution of RNA using a method previously described(Martins, 2009). For those samples used for internalization measurements, medium was added to the cells after the three washes with cold PBS, followed by incubation at 37°C, 5% CO2 for 90 minutes. Cells were then again washed three times with cold PBS, detached with 0.25% trypsin, washed twice with PBS, and lysed with the RLT buffer of the RNeasy Mini kit. All wash steps occurred via centrifugation at 600 × g, 4°C for 3 minutes with resuspension in 500 μl of PBS. The DNA was then harvested in the same manner as the “attachment” samples. For all samples, qPCR was performed on the DNA using SsoAdvanced SYBR Green (Biorad, 172-5274) in the CFX96-C1000 Touch thermocycler (Biorad). Total KSHV DNA was measured using primers specific to the ORF11 gene (Forward: 5’-GGCACCCATACAGCTTCTACGA-3’; Reverse 5’-CGTTTACTACTGCACACTGCA-3’) with human HS1 as the reference gene (Forward: 5’-TTCCTATTTGCCAAGGCAGT-3’; Reverse: 5’-CTCTTCAGCCATCCCAAGAC-3’). The percentage of KSHV bound to cells was calculated using a standard curve of KSHV DNA from input virus alone and qPCR analysis.
2.6. Electron microscopy
Sample preparation for cryo-transmission electron microscopy (cyro-TEM) was performed in the EM Core of the University of Florida’s Interdisciplinary Center for Biotechnology Research. Three microliter aliquots of suspended virus were applied to C-flat holey carbon grids (Protochips, Inc.) and vitrified using a Vitrobot™ Mark IV (FEI Co.) operated at 4°C and with ~90% humidity in the control chamber. The vitrified sample was stored under liquid nitrogen and transferred into a Gatan cryo-holder (Model 626/70) for imaging. The sample was examined using a 4k × 4k CCD camera (Gatan, Inc.) on a Tecnai (FEI Co.) G2 F20-TWIN Transmission Electron Microscope operated at a voltage of 200 kV using low dose conditions (~20 e/Å2). Images were recorded with a defocus of approximately 3 μm to improve contrast.
2.7. Statistical Analyses
Oral epithelial cell treatment groups were analyzed using the Shapiro-Wilk normality test for Gaussian distributions. No statistical outliers were observed in any treatment groups. One-way ANOVA with Bonferroni multiple corrections (parametric) or one-way ANOVA Kruskal-Wallis test with Dunn’s multiple corrections (non-parametric) were conducted to compare more than two treatment groups. To verify ANOVA findings and compare LL-37 and scrLL-37 treatments, either a two-tailed, unpaired Student’s t-test (parametric) or a Mann-Whitney U test (non-parametric) were performed. Proportion comparisons were conducted using an N-1 chi-squared test. All statistical analyses except N-1 chi-squared test were performed using GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. N-1 chi-squared tests were conducted using MedCalc for Windows version 15.0 (Medcalc Software, Ostend, Belgium).
3. Results
3.1. LL-37 inhibits KSHV infection of oral epithelial cells
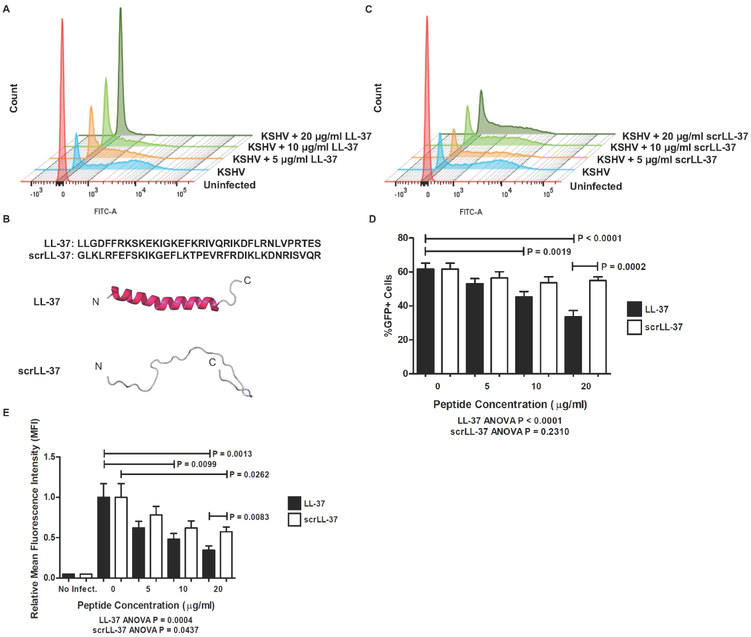
To determine whether LL-37 exhibits antiviral activity against KSHV in the context of OEC infection, KSHV was incubated with increasing concentrations of LL-37 before being added to oral epithelial cells. We found that the incubation of KSHV with LL-37 inhibited KSHV infection of OKF6/Tert-1 cells in a concentration-dependent manner (Fig. 1A). To test whether the antiviral activity of LL-37 arises from its overall cationic nature or to its amphipathic structure, KSHV was incubated with increasing concentrations of scrambled LL-37 (scrLL-37), a peptide that contains all amino acids present in LL-37, but in a different primary structured order (Fig. 1B). Unlike LL-37, scrLL-37 did not inhibit KSHV infection of OKF6/Tert-1 cells in percentage of cells infected (%GFP+) (Fig. 1 C,D). The native LL-37 significantly decreased degree of cell infection (mean fluorescence intensity, MFI) compared to scrLL-37 (Fig. 1 C,E). These data indicate that LL-37 possesses an antiviral activity against KSHV in a specific, structurally dependent manner.
Figure 1. LL-37 inhibits KSHV infection of oral epithelial cells.
KSHV was incubated for two hours with either LL-37 or scrambled LL-37 (scrLL-37) before being added to OKF6/Tert-1 cells at MOI of 1. After spinning infection and incubation, cells were prepared for flow cytometry and measured for GFP signal indicative of KSHV infection. (A) Representative FACS histograms of cells infected with KSHV pre-incubated with increasing concentrations of LL-37. (B) Primary amino acid sequence and overall structure comparison between LL-37 and scrLL-37 using RaptorX online software (University of Chicago). (C) Representative FACS histograms of cells infected with KSHV pre-incubated with increasing concentrations of scrLL-37. (D) Bar graphs showing the percentage of GFP+ cells for each experimental condition. (E) Bar graph of relative mean fluorescence intensity (MFI) of the histograms for each experimental condition. Bar graphs and error bars represent the means and standard error of measurement (SEM) of four independent experiments, each with three biological replicates per condition.
3.2. LL-37 exerts antiviral activity through direct effect on KSHV virion
To discern which aspect of the KSHV infection LL-37 was affecting, potential cellular cytotoxicity and proliferation effects by the peptide were tested with propidium iodide uptake and XTT cell proliferation assay, respectively. We found no evidence for LL-37 affecting cell viability or proliferation when OKF6/Tert-1 cells were incubated with up to 80 μg/ml LL-37 for 48 hours (data not shown). This observation is in agreement with previous studies of testing of LL-37 cytotoxicity in other cell types (Svensson et al., 2016).
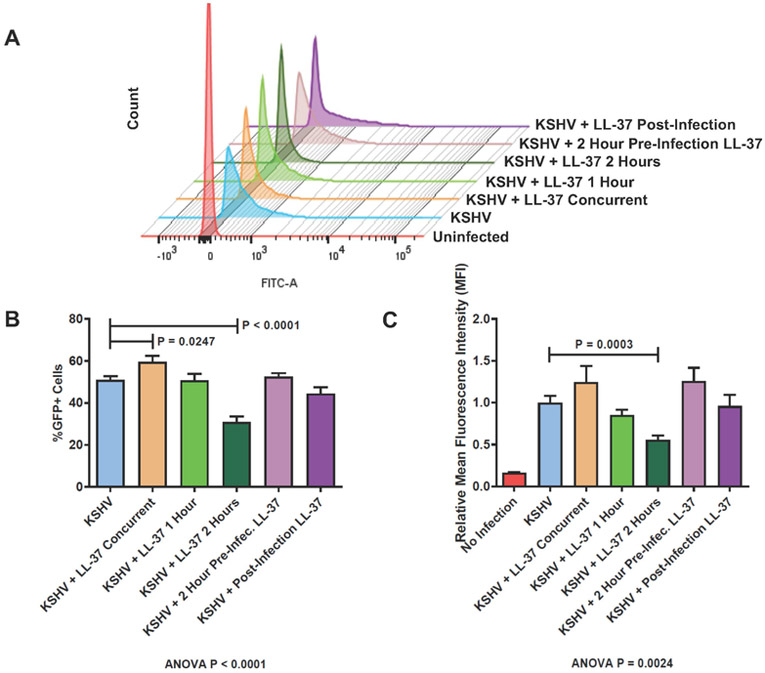
Next, to define the kinetics of the activity of LL-37 against KSHV, the virus was incubated with LL-37 for increasing time before being added to OKF6/Tert-1 cells. Co-incubation of LL-37 with KSHV before infection exhibited a time-dependent inhibitory effect of the peptide on KSHV infection, which was evident by the significantly reduced number of GFP+ cells and MFI (Fig. 2, labeld “KSHV + LL-37 2 Hours”). To test whether LL37 affects OECs to exert its antiviral activity on KSHV, we pre-treated OKF6/Tert-1 cells with LL-37 for two hours and then excess peptide was washed away before KSHV infection. This pre-treatment of cells with LL-37 did not affect the ability of KSHV to infect OKF6/Tert-1 cells, suggesting the mechanism of LL-37’s antiviral activity involves the interaction between the peptide and the virus (Fig. 2, labeled “KSHV + 2 Hour Pre-Infection LL-37”). The effect of LL-37 after infection was also measured, with LL-37 being added to cells only after the KSHV infection procedure. Similar to the preinfection addition of LL-37, the post-infection introduction of cells to LL-37 did not have a measured effect on KSHV infection (Fig. 2, labeled “KSHV + Post Infection LL-37”). Taken together, these data indicate that LL-37 is more likely to exert its antiviral activity via affecting the KSHV virion itself instead of interacting with OECs.
Figure 2. LL-37 exerts antiviral activity through direct effect on KSHV virion.
KSHV was incubated for up to two hours with 20 μg/ml LL-37 before being used to infect OKF6/Tert-1 cells at MOI of 1. The infected cells were analyzed by flow cytometry to measure the number of GFP infected cells as GFP+ cells are considered to be successfully infected with KSHV. (A) Representative FACS histograms of cells infected with either KSHV pre-incubated for up to two hours with LL-37 (KSHV + LL-37 Concurrent, 1 Hour, or 2 Hours), KSHV after LL-37 was added to cells for two hours (KSHV + 2 Hours Pre-Infection LL-37), or KSHV before addition of LL-37 (KSHV + Post-Infection LL-37). (B) Bar graph showing the percentage of GFP+ cells for each experimental condition. (C) Bar graph of relative MFI of the histograms for each experimental condition. Bar graphs and error bars represent the means and SEMs of three independent experiments, each with three biological replicates for each condition.
3.3. LL-37 inhibits KSHV internalization during infection of oral epithelial cells
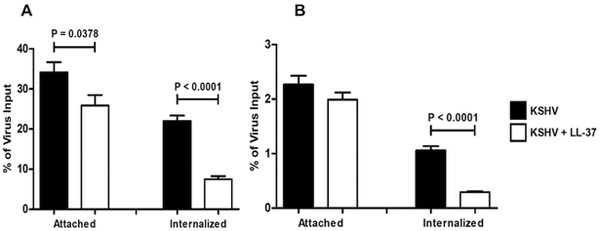
We next examined the effect of LL-37 on the attachment to and entry into host cells. A qPCR-based viral adhesion and internalization assay measuring the presence of viral DNA in OECs following KSHV infection was performed to determine the ability of LL-37 to affect KSHV binding to and internalization into OKF6/Tert-1 cells. KSHV was incubated with or without LL-37 for two hours before being added to OKF6/Tert-1 cells. Binding and internalization were first measured using spinning infections (the method used in previous experiments of this paper). We found that the incubation of KSHV with LL-37 significantly affected the ability of the virus to both bind to and enter OECs in the spinning infection assay (Fig. 3A). To test a more physiologically relevant manner of infection binding and internalization were again tested, but this time using a non-spinning infection assay. In the non-spinning assay, only the internalization of KSHV was affected by LL-37. Taken together these data suggest that, while the peptide might affect viral attachment to cells as seen in the spinning infection assay, the mechanism by which LL-37 most likely inhibits KSHV infection of OECs is through causing aberrant internalization dynamics of the virion into the host cell since this phenomenon was seen in both spinning and non-spinning infections.
Figure 3. LL-37 inhibits KSHV internalization during infection of oral epithelial cells.
KSHV was incubated for two hours with or without 20 μg/ml LL-37 before being added to OKF6/Tert-1 cells at MOI of 1. Cells were then subjected to either spinning (A) or non-spinning (B) infection procedures. The percentage of virus bound to or internalized into cells compared to the virus input was determined via viral attachment and uptake assays, using qPCR primers specific for the KSHV gene ORF11, and adjusted to control for the relative amount of OECs using qPCR primers specific for HS1 genomic region in the human genome. Bar graphs and error bars represent the means and SEMs of three independent experiments, each with three biological replicates for each condition.
3.4. LL-37 disrupts the envelope of KSHV
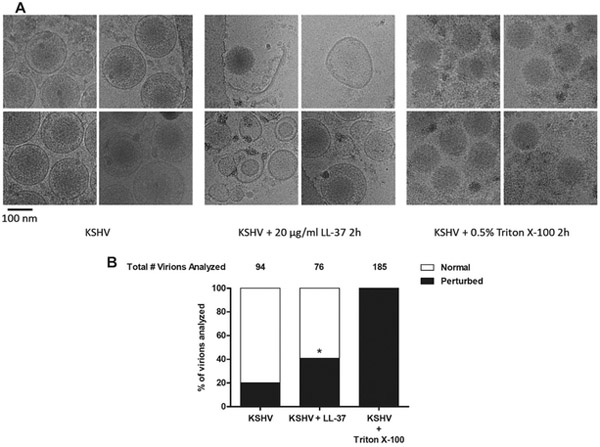
The next question we addressed was how LL-37 was affecting the ability of KSHV to be internalized into OECs. With virion envelope perturbation as one of the antiviral mechanisms of LL-37 (Currie et al., 2016), we investigated the potential KSHV envelope disruption capabilities of LL-37 via cryo-transmission electron microscopy (cryo-TEM). Upon incubating KSHV in PBS with or without LL-37 for two hours, we found that significantly more virions were perturbed in the presence of LL-37 compared to KSHV alone (Fig. 4). Viral envelope rips, free capsids, free envelope pieces, and empty envelopes were all more common in images obtained from samples where KSHV was incubated with LL-37. These data indicate that LL-37 can perturb KSHV envelope, which can inhibit viral internalization into OECs, subsequently leading to overall blocking of viral infection.
Figure 4. LL-37 disrupts KSHV envelope structure.
KSHV was incubated for two hours at room temperature alone or with 20 μg/ml LL-37 or 0.5% Triton X-100 as a control for solvent of the viral envelope. Suspended viral samples were prepared for cryo-transmission electron microscopy and subsequently visualized. The resulting images were analyzed for the presence of visible perturbations (free capsids, free envelope pieces, torn envelopes, empty envelopes, or released tegument). (A) Representative images of KSHV-BAC16 virions, under different experimental conditions, as visualized by cryo-TEM. (B) Stacked bar graphs of percentage of virions analyzed with one or more of the listed perturbations. *P < 0.005 compared to KSHV condition using an N-1 chi-squared test.
4. Discussion
Many pathogenic viruses are spread through saliva. These include herpesviruses, such as herpes simplex virus type 1, human cytomegalovirus, and Epstein-Barr virus, with around 98% of adults in the world infected by at least one of these viruses (Fülöp et al., 2013; Looker et al., 2015; Toussirot et al., 2008). The oral cavity is often the first mucosal environment pathogens encounter in our bodies and serves as an important line of defense against infection. One such pathogen is KSHV, which is known to spread via saliva and infect OECs before progressing to infect endothelial cells and B cells resulting in life-long infection of the host (Bechtel et al., 2003; Duus et al., 2004; Toth et al., 2013). However, little is known about the innate antiviral roles of the cells, which line the mucosal surface of the oral cavity, the oral epithelial cells. OECs are known to produce and secrete a number of antimicrobial peptides (AMPs), or host defense peptides (HDPs), like LL-37 and human beta-defensins (Hans and Madaan Hans, 2014). These HDPs can inhibit infection of not only bacteria and fungi, but also enveloped viruses through a variety of mechanisms, the most common of which is disrupting the viral envelope structure (Lee and Lee, 2015). However, no studies have tested the effects of any HDP against KSHV yet. This study represents the first evidence of LL-37 exhibiting antiviral activity against KSHV.
The ability of LL-37 to directly inhibit a number of viruses has been reviewed previously (Barlow et al., 2014b), with further activities found against Aichi virus A (Vilas Boas et al., 2017), human adenoviruses 8 and 19 (HAdV-8 and HAdV-19) (Gordon et al., 2005; Uchio et al., 2013), hepatitis C virus (HCV) (Matsumura et al., 2016), human papillomavirus 16 (HPV16) (Buck et al., 2006), human rhinovirus (HRV) (Findlay et al., 2017; Schögler et al., 2016; Sousa et al., 2017), and varicella zoster virus (VZV) (Crack et al., 2012). While LL-37 has only been measured at 0.5 μg/ml in saliva (Bachrach et al., 2006; Takeuchi et al., 2012) and 10 μg/ml in gingival crevicular fluid29, the actual physiological concentration may be much higher at sites closer to the oral epithelial cells themselves due to the diffusion of the peptides once released from cells. Similarly, LL-37 concentrations have been recorded in excess of the 20 μg/ml concentration used here in epithelial tissue during inflamed conditions (Currie et al., 2013). Our results demonstrate that concentrations as low as 10 μg/ml were sufficient to lead to a reduction in KSHV infection of the oral epithelial cell line OKF6/Tert-1. This finding fits within other studies of antiviral activities of LL-37, suggesting LL-37 expressed from OEC and resident immune cells could participate in the innate defense against KSHV infection of the oral cavity. The use of PBS for antiviral assays for LL-37 may also have resulted in a more diminished level of activity for the peptide in our studies than may be occurring naturally, as others have found LL-37 to be sensitive to salt in the context of antibacterial and antiviral testing (Dürr et al., 2006).
With many antimicrobial peptides exerting their antimicrobial activity through their overall charge (Bahar and Ren, 2013), we aimed to determine whether LL-37 possessed any antiviral activity outside of its cationicity. The activity of the native LL-37 was significantly greater than scrLL-37, leading us to conclude that LL-37 had a structure-dependent antiviral activity against KSHV. This ability of the native primary structure of LL-37 to inhibit viral infections as opposed to a scrambled version of the peptide has also been observed with a number of other viruses—including DENV (Alagarasu et al., 2017), HAdV19 (Gordon et al., 2005), HCV (Matsumura et al., 2016), HRV (Sousa et al., 2017), HSV-1 (Gordon et al., 2005; Lee et al., 2014), influenza A virus (Barlow et al., 2011; Tripathi et al., 2015a, 2013 White et al., 2017), and RSV (Currie et al., 2013; Harcourt et al., 2016)—suggesting the specific structure, and not simply the charge, of LL-37 is important in its antiviral activity.
Overall, in this study we demonstrate the ability of LL-37 to inhibit KSHV infection of OECs by disrupting the structure of the viral envelope, leading to decreased internalization into the host cell. While this disruption of the viral envelope by LL-37 has also been observed in HCV (Matsumura et al., 2016), influenza A virus (Tripathi et al., 2013), and VV (Dean et al., 2010; Ulaeto et al., 2016), this study represents the first example of LL-37 specifically inhibiting viral entry into target cells, as opposed to viral attachment (Currie et al., 2016; Lee et al., 2014). Since LL-37 has been shown to bind lipid bilayers (Wang et al., 2014) and even influenza A virus (Tripathi et al., 2013), interaction between LL-37 and the KSHV envelope may be the most likely explanation of how LL-37 can target the KSHV. This binding to the envelope may be the reason for the slight, but significant, increase in the percentage of GFP+ cells after the short timepoint of co-incubation of LL-37 and KSHV before infection (Fig. 2B). LL-37 may be binding to KSHV and disrupting KSHV aggregates at early timepoints, leading to a greater percentage of cells infected, but not an overall increase in total infection (we observed increased percentage of GFP+ cells, but not relative MFI). The reason why LL-37 binds to the lipid bilayers of microorganisms yet is not cytotoxic to human cells (Svensson et al., 2016) may be the key for determining the mechanism by which LL-37 exhibits antiviral activity against KSHV. One possible scenario involves binding of the peptide to viral glycoproteins on the surface of KSHV. LL-37 has been shown to bind to envelope glycoproteins (Alagarasu et al., 2017). Interaction with specific glycoproteins on the surface of KSHV may be responsible for LL-37’s inhibition of internalization (which is mediated by viral envelope glycoproteins gB and gpK8.1A), but not attachment (which is mediated by gB, gH/L, and gM/N) (Chakraborty et al., 2012). This binding of other HDPs to viral glycoproteins, leading to the inhibition of internalization, has been previously observed (Leikina et al., 2005). While we could demonstrate the antiviral activity of LL-37 against KSHV, additional studies will be required to determine the mechanism by which LL-37 reduce KSHV infection.
Taken together, our results suggest that the ability of LL-37 to inhibit infection of OECs can represent the first step of a possible method for the prevention of the initial oral KSHV infection, for which no therapies or treatments currently exist. LL-37 has been shown to be upregulated in KS lesions compared to healthy skin (Fathy et al., 2012), suggesting an inherent response of epithelial cells to combat the viral infection with LL-37. Similar upregulation of LL-37 and other antimicrobial peptides by vitamin D3 (Wang et al., 2004) in oral epithelial cells (McMahon et al., 2011) are thought to be the mechanisms by which the vitamin has exerted antiviral activity in previous studies (Beard et al., 2011). While LL-37 exerts its antiviral activity via a direct effect on the virion in the case of this paper and others, cathelicidins in general (He et al., 2018) and LL-37 specifically (Barlow et al., 2014a) have also been shown to enhance other methods of innate antiviral immunity, namely interferon expression. Current work in endogenous antimicrobial peptide expression (Dhawan et al., 2015; Klein-Patel et al., 2006; Rivas-Santiago et al., 2005; Ryan et al., 2011; Ryan and Diamond, 2017) and small molecule mimetics of antimicrobial peptides (Beckloff et al., 2007; Menzel et al., 2017; Ryan et al., 2014) would potentially serve as methods to prevent not only KSHV infection, but also other viral infections of the oral cavity.
Highlights.
LL-37 inhibits KSHV infection in oral epithelial cells
This inhibition occurs through a direct effect on the virion
LL-37 disrupts the KSHV envelope structure
Acknowledgements
The authors would like to thank to Rodolfo Alvarado and the University of Florida Interdisciplinary Center for Biotechnology Research (UF ICBR) Electron Microscopy Core Facility for their technical assistance in cryo-TEM imaging of KSHV virions. The authors would also like to thank Andria Doty, Ph.D. and Craig Moneypenny, Ph.D. of the UF ICBR Flow Cytometry Core Facility for their help in initial flow cytometry analysis and Theodore Harris for statistical consultation. Further thanks goes to a number of UF faculty members for their guidance on this project: Corwin Nelson, Ph.D. of the Department of Animal Sciences, and Stephanie Karst, Ph.D. of the Department of Microbiology and Molecular Genetics, as well as Shannon Wallet, Ph.D. of the Department of Foundational Sciences at East Carolina University. This study was supported by grants from the US Public Health Service, NIH 1R01DE22723 to GD, NIH R01AI132554, R03DE025562, UF Research Opportunity Seed Fund to ZT, and 5T90DE021990-07 to DCB at the University of Florida College of Dentistry, Department of Oral Biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alagarasu K, Patil PS, Shil P, Seervi M, Kakade MB, Tillu H, Salunke A, 2017. In-vitro effect of human cathelicidin antimicrobial peptide LL-37 on dengue virus type 2. Peptides 92, 23–30. 10.1016/j.peptides.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Bachrach G, Chaushu G, Zigmond M, Yefenof E, Stabholz A, Shapira J, Merrick J, Chaushu S, 2006. Salivary LL-37 Secretion in Individuals with Down Syndrome is Normal. J. Dent. Res. 85, 933–936. 10.1177/154405910608501012 [DOI] [PubMed] [Google Scholar]
- Bahar AA, Ren D, 2013. Antimicrobial peptides. Pharmaceuticals (Basel). 6, 1543–75. 10.3390/ph6121543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow PG, Findlay EG, Currie SM, Davidson DJ, 2014a. Antiviral potential of cathelicidins. Future Microbiol. 9, 55–73. 10.2217/fmb.13.135 [DOI] [PubMed] [Google Scholar]
- Barlow PG, Findlay EG, Currie SM, Davidson DJ, 2014b. Antiviral potential of cathelicidins. Future Microbiol. 9, 55–73. 10.2217/fmb.13.135 [DOI] [PubMed] [Google Scholar]
- Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, Davidson DJ, Donis RO, 2011. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One 6, e25333 10.1371/journal.pone.0025333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JA, Bearden A, Striker R, 2011. Vitamin D and the anti-viral state. J. Clin. Virol 50, 194–200. 10.1016/jjcv.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel JT, Liang Y, Hvidding J, Ganem D, 2003. Host range of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol 77, 6474–6481. 10.1128/JVI.77.11.6474-6481.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckloff N, Laube D, Castro T, Furgang D, Park S, Perlin D, Clements D, Tang H, Scott RW, Tew GN, Diamond G, 2007. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob. Agents Chemother. 51,4125–32. 10.1128/AAC.00208-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry G, Tse L, 2017. Virus Binding and Internalization Assay for Adeno-associated Virus. BIO-PROTOCOL 7. 10.21769/BioProtoc.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry GE, Asokan A, 2016. Chemical Modulation of Endocytic Sorting Augments Adeno-associated Viral Transduction. J. Biol. Chem. 291, 939–47. 10.1074/jbc.M115.687657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C, Weiss R, 2002. AIDS-related malignancies. Nat. Rev. Cancer 2, 373–82. 10.1038/nrc797 [DOI] [PubMed] [Google Scholar]
- Brulois KF, Chang H, Lee AS-Y, Ensser A, Wong L-Y, Toth Z, Lee SH, Lee H-R, Myoung J, Ganem D, Oh T-K, Kim JF, Gao S-J, Jung JU, 2012. Construction and manipulation of a new Kaposi’s sarcoma-associated herpesvirus bacterial artificial chromosome clone. J. Virol. 86, 9708–20. 10.1128/JVI.01019-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT, 2006. Human alpha-defensins block papillomavirus infection. Proc. Natl. Acad. Sci. U. S. A. 103, 1516–21. 10.1073/pnas.0508033103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DM, Rappocciolo G, Jenkins FJ, Rinaldo CR, 2014. Dendritic cells: key players in human herpesvirus 8 infection and pathogenesis. Front. Microbiol. 5, 452 10.3389/fmicb.2014.00452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM, 1995. Kaposi’s Sarcoma-Associated Herpesvirus-Like DNA Sequences in AIDS-Related Body-Cavity-Based Lymphomas. N. Engl. J. Med. 332, 1186–1191. 10.1056/NEJM199505043321802 [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Veettil MV, Chandran B, 2012. Kaposi’s Sarcoma Associated Herpesvirus Entry into Target Cells. Front. Microbiol. 3, 6 10.3389/fmicb.2012.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS, 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 10.1126/science.7997879 [DOI] [PubMed] [Google Scholar]
- Cohen A, Wolf DG, Guttman-Yassky E, Sarid R, 2005. Kaposi’s sarcoma-associated herpesvirus: clinical, diagnostic, and epidemiological aspects. Crit. Rev. Clin. Lab. Sci. 42, 101–53. 10.1080/10408360590913524 [DOI] [PubMed] [Google Scholar]
- Corbellino M, Poirel L, Bestetti G, Pizzuto M, Aubin JT, Capra M, Bifulco C, Berti E, Agut H, Rizzardini G, Galli M, Parravicini C, 1996. Restricted tissue distribution of extralesional Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS patients with Kaposi’s sarcoma. AIDS Res. Hum. Retroviruses 12, 651–7. 10.1089/aid.1996.12.651 [DOI] [PubMed] [Google Scholar]
- Corey L, Brodie S, Huang M-L, Koelle DM, Wald A,. HHV-8 infection: a model for reactivation and transmission. Rev. Med. Virol. 12, 47–63. 10.1002/rmv.341 [DOI] [PubMed] [Google Scholar]
- Crack LR, Jones L, Malavige GN, Patel V, Ogg GS, 2012. Human antimicrobial peptides LL-37 and human β-defensin-2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin. Exp. Dermatol. 37, 534–543. 10.1111/j.1365-2230.2012.04305.x [DOI] [PubMed] [Google Scholar]
- Currie SM, Findlay EG, McHugh BJ, Mackellar A, Man T, Macmillan D, Wang H, Fitch PM, Schwarze J, Davidson DJ, 2013. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One 8, e73659 10.1371/journal.pone.0073659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SM, Gwyer Findlay E, McFarlane AJ, Fitch PM, Bottcher B, Colegrave N, Paras A, Jozwik A, Chiu C, Schwarze J, Davidson DJ, 2016. Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans. J. Immunol. 196, 2699–710. 10.4049/jimmunol.1502478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RE, O’Brien LM, Thwaite JE, Fox MA, Atkins H, Ulaeto DO, 2010. A carpet-based mechanism for direct antimicrobial peptide activity against vaccinia virus membranes. Peptides 31, 1966–1972. https://doi.Org/10.1016/J.PEPTIDES.2010.07.028 [DOI] [PubMed] [Google Scholar]
- Dhawan P, Wei R, Sun C, Gombart AF, Koeffler HP, Diamond G, Christakos S, 2015. C/EBPα and the Vitamin D Receptor Cooperate in the Regulation of Cathelicidin in Lung Epithelial Cells. J. Cell. Physiol. 230, 464–472. 10.1002/jcp.24729 [DOI] [PubMed] [Google Scholar]
- Dickson M. a, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg R. a, Louis DN, Li FP, Rheinwald JG, 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20, 1436–1447. 10.1128/MCB.20.4.1436-1447.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr UHN, Sudheendra US, Ramamoorthy A, 2006. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta - Biomembr. 1758, 1408–1425. https://doi.org/10.1016ZJ.BBAMEM.2006.03.030 [DOI] [PubMed] [Google Scholar]
- Duus KM, Lentchitsky V, Wagenaar T, Grose C, Webster-cyriaque J, 2004. Wild-Type Kaposi ‘ s Sarcoma-Associated Herpesvirus Isolated from the Oropharynx of Immune-Competent Individuals Has Tropism for Cultured Oral Epithelial Cells Wild-Type Kaposi ‘ s Sarcoma-Associated Herpesvirus Isolated from the Oropharynx of Immune-Comp 78, 4074–4084. 10.1128/JVI.78.8.4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathy H, Amin MM, El-Gilany A-H, 2012. Upregulation of human β-defensin-3 and cathelicidin LL-37 in Kaposi’s sarcoma. F1000Research 1,38 10.12688/f1000research 1–38.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay F, Pohl J, Svoboda P, Shakamuri P, McLean K, Inglis NF, Proudfoot L, Barlow PG, 2017. Carbon Nanoparticles Inhibit the Antimicrobial Activities of the Human Cathelicidin LL-37 through Structural Alteration. J. Immunol. 199, 2483–2490. 10.4049/jimmunol.1700706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülöp T, Larbi A, Pawelec G, 2013. Human T Cell Aging and the Impact of Persistent Viral Infections. Front. Immunol. 4, 271 10.3389/fimmu.2013.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D, 2010. KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J. Clin. Invest. 120, 939–49. 10.1172/JCI40567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM, 2005. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 30, 385–94. 10.1080/02713680590934111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans M, Madaan Hans V, 2014. Epithelial antimicrobial peptides: guardian of the oral cavity. Int. J. Pept. 2014, 370297 10.1155/2014/370297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt JL, McDonald M, Svoboda P, Pohl J, Tatti K, Haynes LM, 2016. Human cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC Res. Notes 9, 11 10.1186/s13104-015-1836-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassman LM, Ellison TJ, Kedes DH, 2011. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. J. Clin. Invest. 121, 752–68. 10.1172/JCI44185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zhang H, Li Y, Wang G, Tang B, Zhao J, Huang Y, Zheng J, 2018. Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway. Front. Immunol. 9, 722 10.3389/fimmu.2018.00722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid Z, Naseem M, Sheikh Z, Najeeb S, Shahab S, Zafar MS, 2016. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 24, 515–524. 10.1016/jjsps.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Patel ME, Diamond G, Boniotto M, Saad S, Ryan LK, 2006. Inhibition of beta-defensin gene expression in airway epithelial cells by low doses of residual oil fly ash is mediated by vanadium. Toxicol. Sci. 92, 115–25. 10.1093/toxsci/kfj214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotman ME, Chang TL, 2006. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 6, 447–56. 10.1038/nri1860 [DOI] [PubMed] [Google Scholar]
- Knowlton ER, Lepone LM, Li J, Rappocciolo G, Jenkins FJ, Rinaldo CR, 2012. Professional antigen presenting cells in human herpesvirus 8 infection. Front. Immunol. 3, 427 10.3389/fimmu.2012.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Kizhakkedathu J, Straus S, 2018. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 8, 4 10.3390/biom8010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-J, Buznyk O, Kuffova L, Rajendran V, Forrester JV, Phopase J, Islam MM, Skog M, Ahlqvist J, Griffith M, 2014. Cathelicidin LL-37 and HSV-1 Corneal Infection: Peptide Versus Gene Therapy. Transl. Vis. Sci. Technol. 3,4 10.1167/tvst.3.3.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee DG, 2015. Antimicrobial Peptides (AMPs) with Dual Mechanisms: Membrane Disruption and Apoptosis. J. Microbiol. Biotechnol. 25, 759–64. 10.4014/jmb.1411.11058 [DOI] [PubMed] [Google Scholar]
- Leikina E, Delanoe-Ayari H, Melikov K, Cho M-S, Chen A, Waring AJ, Wang W, Xie Y, Loo JA, Lehrer RI, Chernomordik LV, 2005. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat. Immunol. 6, 995–1001. 10.1038/ni1248 [DOI] [PubMed] [Google Scholar]
- Looker KJ, Magaret AS, May MT, Turner KME, Vickerman P, Gottlieb SL, Newman LM, 2015. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One 10, e014075 10.1371/journal.pone.0140765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins WK, 2009. A useful procedure to isolate simultaneously DNA and RNA from a single tumor sample: Protocol Exchange. Protoc. Exch. 10.1038/nprot.2009.163 [DOI] [Google Scholar]
- Matsumura T, Sugiyama N, Murayama A, Yamada N, Shiina M, Asabe S, Wakita T, Imawari M, Kato T, 2016. Antimicrobial peptide LL-37 attenuates infection of hepatitis C virus. Hepatol. Res. 46, 924–932. 10.1111/hepr.12627 [DOI] [PubMed] [Google Scholar]
- McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, Diamond G, 2011. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect. Immun. 79, 2250–6. 10.1128/IAI.00099-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel LP, Chowdhury HM, Masso-Silva JA, Ruddick W, Falkovsky K, Vorona R, Malsbary A, Cherabuddi K, Ryan LK, DiFranco KM, Brice DC, Costanzo MJ, Weaver D, Freeman KB, Scott RW, Diamond G, 2017. Potent in vitro and in vivo antifungal activity of a small molecule host defense peptide mimic through a membrane-active mechanism. Sci. Rep. 7, 4353 10.1038/s41598-017-04462-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Ohtake T, Dorschner RA, Gallo RL, 2002. Cathelicidin Antimicrobial Peptides are Expressed in Salivary Glands and Saliva. J. Dent. Res. 81, 845–850. 10.1177/154405910208101210 [DOI] [PubMed] [Google Scholar]
- Myoung J, Ganem D, 2011a. Active lytic infection of human primary tonsillar B cells by KSHV and its noncytolytic control by activated CD4+ T cells. J. Clin. Invest. 121, 1130–40. 10.1172/JCI43755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoung J, Ganem D, 2011b. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: maintenance of tight latency with efficient reactivation upon induction. J. Virol. Methods 174, 12–21. 10.1016/jjviromet.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Goto H, Yotsumoto M, 2014. Current status of treatment for primary effusion lymphoma. Intractable rare Dis. Res. 3, 65–74. 10.5582/irdr.2014.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica F, Volpi A, 2007. Transmission of human herpesvirus 8: an update. Curr. Opin. Infect. Dis. 20, 152–6. 10.1097/QCO.0b013e3280143919 [DOI] [PubMed] [Google Scholar]
- Rivas-Santiago B, Schwander SK, Sarabia C, Diamond G, Klein-Patel ME, Hernandez-Pando R, Ellner JJ, Sada E, 2005. Human {beta}-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect. Immun. 73, 4505–11. 10.1128/IAI.73.8.4505-4511.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LK, Dai J, Yin Z, Megjugorac N, Uhlhorn V, Yim S, Schwartz KD, Abrahams JM, Diamond G, Fitzgerald-Bocarsly P, 2011. Modulation of human beta-defensin-1 (hBD-1) in plasmacytoid dendritic cells (PDC), monocytes, and epithelial cells by influenza virus, Herpes simplex virus, and Sendai virus and its possible role in innate immunity. J. Leukoc. Biol. 90, 343–56. 10.1189/jlb.0209079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LK, Diamond G, 2017. Modulation of Human β-Defensin-1 Production by Viruses. Viruses 9. 10.3390/v9060153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LK, Freeman KB, Masso-Silva JA, Falkovsky K, Aloyouny A, Markowitz K, Hise AG, Fatahzadeh M, Scott RW, Diamond G, 2014. Activity of potent and selective host defense peptide mimetics in mouse models of oral candidiasis. Antimicrob. Agents Chemother. 58, 3820–7. 10.1128/AAC.02649-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schögler A, Muster RJ, Kieninger E, Casaulta C, Tapparel C, Jung A, Moeller A, Geiser T, Regamey N, Alves MP, 2016. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur. Respir. J. 47, 520–30. 10.1183/13993003.00665-2015 [DOI] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86, 1276–80. 10.2169/internalmedicine.38.279 [DOI] [PubMed] [Google Scholar]
- Sousa FH, Casanova V, Findlay F, Stevens C, Svoboda P, Pohl J, Proudfoot L, Barlow PG, 2017. Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides 95, 76–83. 10.1016/j.peptides.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson D, Wilk L, Mörgelin M, Herwald H, Nilsson B-O, 2016. LL-37-induced host cell cytotoxicity depends on cellular expression of the globular C1q receptor (p33). Biochem. J. 473, 87–98. 10.1042/BJ20150798 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Nagasawa T, Katagiri S, Kitagawara S, Kobayashi H, Koyanagi T, Izumi Y, 2012. Salivary Levels of Antibacterial Peptide (LL-37/hCAP-18) and Cotinine in Patients With Chronic Periodontitis. J. Periodontol. 83, 766–772. https://doi.Org/10.1902/jop.2011.100767 [DOI] [PubMed] [Google Scholar]
- Takiguchi T, Morizane S, Yamamoto T, Kajita A, Ikeda K, Iwatsuki K, 2014. Cathelicidin antimicrobial peptide LL-37 augments interferon-β expression and antiviral activity induced by double-stranded RNA in keratinocytes. Br. J. Dermatol. 171,492–498. 10.1111/bjd.12942 [DOI] [PubMed] [Google Scholar]
- Toth Z, Brulois K, Lee HR, Izumiya Y, Tepper C, Kung HJ, Jung JU, 2013. Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following De Novo Infection. PLoS Pathog. 9, 1–14. 10.1371/journal.ppat.1003813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussirot É, Roudier J, Roudier C, et al. , 2008. Epstein-Barr virus in autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 22, 883–896. 10.1016/j.berh.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Tripathi S, Tecle T, Verma A, Crouch E, White M, Hartshorn KL, 2013. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 94, 40–9. 10.1099/vir.0.045013-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S, Verma A, Kim E-J, White MR, Hartshorn KL, 2014. LL-37 modulates human neutrophil responses to influenza A virus. J. Leukoc. Biol. 96, 931–8. 10.1189/jlb.4A1113-604RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S, Wang G, White M, Qi L, Taubenberger J, Hartshorn KL, 2015a. Antiviral Activity of the Human Cathelicidin, LL-37, and Derived Peptides on Seasonal and Pandemic Influenza A Viruses. PLoS One 10, e0124706 10.1371/journal.pone.0124706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S, Wang G, White M, Rynkiewicz M, Seaton B, Hartshorn K, 2015b. Identifying the Critical Domain of LL-37 Involved in Mediating Neutrophil Activation in the Presence of Influenza Virus: Functional and Structural Analysis. PLoS One 10, e0133454 10.1371/journal.pone.0133454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türkoğlu O, Emingil G, Kütükçüler N, Atilla G, 2009. Gingival Crevicular Fluid Levels of Cathelicidin LL-37 and Interleukin-18 in Patients With Chronic Periodontitis. J. Periodontol. 80, 969–976. 10.1902/jop.2009.080532 [DOI] [PubMed] [Google Scholar]
- Uchio E, Inoue H, Kadonosono K, 2013. Anti-adenoviral effects of human cationic antimicrobial protein-18/LL-37, an antimicrobial peptide, by quantitative polymerase chain reaction. Korean J. Ophthalmol. 27, 199–203. 10.3341/kjo.2013.27.3.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaeto DO, Morris CJ, Fox MA, Gumbleton M, Beck K, 2016. Destabilization of α-Helical Structure in Solution Improves Bactericidal Activity of Antimicrobial Peptides: Opposite Effects on Bacterial and Viral Targets. Antimicrob. Agents Chemother. 60, 1984–91. 10.1128/AAC.02146-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas Boas LCP, de Lima LMP, Migliolo L, Mendes G dos S, de Jesus MG, Franco OL, Silva PA, 2017. Linear antimicrobial peptides with activity against herpes simplex virus 1 and Aichi virus. Biopolymers 108, e22871 10.1002/bip.22871 [DOI] [PubMed] [Google Scholar]
- Wang G, Mishra B, Epand RF, Epand RM, 2014. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta 1838, 2160–72. 10.1016/j.bbamem.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH, Hanrahan JH, 2004. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 173, 2909–12. 10.4049/JIMMUNOL.173.5.2909 [DOI] [PubMed] [Google Scholar]
- White MR, Tripathi S, Verma A, Kingma P, Takahashi K, Jensenius J, Thiel S, Wang G, Crouch EC, Hartshorn KL, 2017. Collectins, H-ficolin and LL-37 reduce influence viral replication in human monocytes and modulate virus-induced cytokine production. Innate Immun. 23, 77–88. 10.1177/1753425916678470 [DOI] [PubMed] [Google Scholar]
- Wong JH, Legowska A, Rolka K, Ng TB, Hui M, Cho CH, Lam WWL, Au SWN, Gu OW, Wan DCC, 2011. Effects of cathelicidin and its fragments on three key enzymes of HIV-1. Peptides 32, 1117–1122. https://doi.Org/10.1016/J.PEPTIDES.2011.04.017 [DOI] [PubMed] [Google Scholar]