Abstract
Background
Cystoscopy is commonly performed for diagnostic purposes to inspect the interior lining of the bladder. One disadvantage of cystoscopy is the risk of symptomatic urinary tract infection (UTI) due to pre‐existing colonization or by introduction of bacteria at the time of the procedure. However, the incidence of symptomatic UTI following cystoscopy is low. Currently, there is no consensus on whether antimicrobial agents should be used to prevent symptomatic UTI for cystoscopy.
Objectives
To assess the effects of antimicrobial agents compared with placebo or no treatment for prevention of UTI in adults undergoing cystoscopy.
Search methods
We comprehensively searched electronic databases of the Cochrane Library, PubMed, Embase, LILACS, and CINAHL. We searched the WHO ICTRP and ClinicalTrials.gov for ongoing trials. We used no language or date restrictions in the electronic searches. We searched the reference lists of identified articles and contacted authors for related information. The last search of the electronic databases was 4 February 2019.
Selection criteria
We included randomized controlled trials (RCTs) or quasi‐RCTs that compared any prophylactic antibiotic versus placebo, no treatment, or other non‐antibiotic prophylaxis in adults undergoing cystoscopy. There was no restriction on the dose, frequency, formulation, duration, or mode of administration of the antibiotics.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Our primary outcomes were systemic UTI, symptomatic UTI (composite of systemic and/or localized UTI), and serious adverse events. Secondary outcomes were minor adverse events, localized UTI, asymptomatic bacteriuria, and bacterial resistance. We assessed the quality of evidence using GRADE.
Main results
We included 20 RCTs and two quasi‐RCTs with 7711 participants, all of which compared antibiotic prophylaxis with placebo or no treatment control. We found no studies comparing antibiotic prophylaxis with non‐antibiotic prophylaxis.
Primary outcomes
Systemic UTI: antibiotic prophylaxis may have little or no effect on the risk of systemic UTI compared with placebo or no treatment (risk ratio (RR) 1.12, 95% confidence interval (CI) 0.38 to 3.32; 5 RCTs; 504 participants; low‐quality evidence); this corresponds to two more people (95% CI 12 fewer to 46 more) per 1000 people developing a systemic UTI. We downgraded the quality of the evidence for study limitations and imprecision.
Symptomatic UTI: antibiotic prophylaxis may reduce the risk of symptomatic UTI (RR 0.49, 95% CI 0.28 to 0.86; 11 RCTs; 5441 participants; low‐quality evidence); this corresponds to 30 fewer people (95% CI 42 fewer to 8 fewer) per 1000 people developing a symptomatic UTI when provided with antibiotic prophylaxis. We downgraded the quality of the evidence for study limitations and potential publication bias.
Serious adverse events: the studies reported no serious adverse events in either the intervention group or control group and no effect size could be calculated. Antibiotic prophylaxis may have little or no effect on serious adverse events (4 RCTs, 630 participants; very low‐quality evidence), but we are very uncertain of this finding. We downgraded the quality of the evidence for study limitations and very serious imprecision.
Secondary outcomes
Minor adverse events: prophylactic antibiotics may have little or no effect on minor adverse events when compared with placebo or no treatment (RR 2.82, 95% CI 0.54 to 14.80; 4 RCTs; 630 participants; low‐quality evidence). We downgraded the quality of the evidence for study limitations and imprecision.
Localized UTI: prophylactic antibiotics may have little or no effect on the risk of localized UTI (RR 1.0, 95% CI 0.06 to 15.77; 1 RCT; 200 participants; very low‐quality evidence), but we were very uncertain of this finding. We downgraded the quality of the evidence for study limitations and very serious imprecision.
Bacterial resistance: prophylactic antibiotics may increase bacterial resistance (RR 1.73, 95% CI 1.04 to 2.87; 38 participants; 2 RCTs; very low‐quality evidence), but we were uncertain of this finding. We downgraded the quality of the evidence for study limitations, indirectness, and imprecision.
We were able to perform few secondary analyses; these did not suggest any subgroup effects.
Authors' conclusions
Antibiotic prophylaxis may reduce the risk of symptomatic UTI but not systemic UTIs. Serious and minor adverse events may not be increased with the use of antibiotic prophylaxis. The findings are informed by low‐ and very low‐quality evidence ratings for all outcomes.
Keywords: Adult; Humans; Antibiotic Prophylaxis; Antibiotic Prophylaxis/adverse effects; Anti‐Infective Agents, Urinary; Anti‐Infective Agents, Urinary/adverse effects; Anti‐Infective Agents, Urinary/therapeutic use; Cystoscopy; Cystoscopy/adverse effects; Drug Resistance, Bacterial; Placebos; Placebos/therapeutic use; Randomized Controlled Trials as Topic; Urinary Tract Infections; Urinary Tract Infections/etiology; Urinary Tract Infections/prevention & control
Plain language summary
Antimicrobial agents for preventing urinary tract infections in adults undergoing cystoscopy
Review question
We reviewed the evidence for the benefits and harms of using antibiotics for cystoscopy (an examination of the inside of bladder) to prevent urinary tract infections (UTI).
Background
Cystoscopy may cause UTIs. This may cause bothersome symptoms like burning with urination due to an infection limited to the bladder or fevers and chills due to a more serious infection that has goes to the bloodstream, or a combination of burning, fevers, and chills. Antibiotics may prevent infections and reduce these symptoms but can also cause unwanted effects. It is uncertain whether people should be given antibiotics before this procedure.
Study characteristics
We found 22 studies with 7711 participants. These studies were published from 1971 to 2017. In these studies, chance decided whether people received an antibiotic or a placebo/no treatment. The evidence is current to 4 February 2019.
Key results
Antibiotics given for UTI prevention before cystoscopy may have had little or no effect on the risk of developing a more serious infection that went into the bloodstream.
They may have reduced the risk of infection when both serious infection that went into the bloodstream and infections limited to the bladder were taken together.
None of the people included in the trials had serious unwanted effects. Therefore, we concluded that antibiotics given for prevention of UTIs may not cause serious unwanted effects but we are very uncertain of this finding.
Antibiotics may also have had little or no effect on minor unwanted effects. They may also have had little or no effect on infections limited to the bladder taken alone, but we were very uncertain of this finding. People treated with antibiotics may have been more likely to have bacteria that were more resistant to antibiotics, but we are very uncertain of this finding.
Quality of the evidence
We rated the quality of the evidence as low or very low meaning that our confidence in the results was limited or very limited. The true effect of antibiotics for prevention of UTIs before cystoscopy may be quite different from what this review found.
Summary of findings
Summary of findings for the main comparison. Antimicrobial compared to placebo or no antibiotics for preventing urinary tract infections in adults undergoing cystoscopy.
| Antimicrobial compared to placebo or no antibiotics for preventing urinary tract infections in adults undergoing cystoscopy | |||||
| Patient or population: people undergoing cystoscopy Setting: various Intervention: antimicrobial Comparison: placebo or no antibiotics | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo or no antibiotics | Risk difference with antimicrobial | ||||
| Systemic UTI Follow‐up: range 1–30 days | 504 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | RR 1.12 (0.38 to 3.32) | Study population | |
| 20 per 1000 | 2 more per 1000 (12 fewer to 46 more) | ||||
| Symptomatic UTI Follow‐up: range 1–30 days | 5441 (11 RCTs) | ⊕⊕⊝⊝ Lowa,c | RR 0.49 (0.28 to 0.86) | Study population | |
| 58 per 1000 | 30 fewer per 1000 (42 fewer to 8 fewer) | ||||
| Serious adverse events Follow‐up: range 1–30 days | 630 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,d | RR approximately 1 | 0/326 participants receiving prophylactic antibiotics and 0/304 participants receiving control had a serious adverse event. | |
| Minor adverse events Follow‐up: range 1–30 days | 630 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | RR 2.82 (0.54 to 14.80) | Study population | |
| 3 per 1000 | 6 more per 1000 (2 fewer to 46 more) | ||||
| Localized UTI Follow‐up: range 1–30 days | 200 (1 RCT) | ⊕⊝⊝⊝ Very lowa,d | RR 1.00 (0.06 to 15.77) | Study population | |
| 10 per 1000 | 0 fewer per 1000 (9 fewer to 152 more) | ||||
| Bacterial resistance Follow‐up: range 1–30 days | 38 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,d,e | RR 1.73 (1.04 to 2.87) | Study population | |
| 406 per 1000 | 297 more per 1000 (16 more to 760 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; UTI: urinary tract infection. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded for study limitations (–1) related to unclear or high risk of selection, performance, detection, and selective reporting bias.
bDowngraded for imprecision (–1) due to wide confidence intervals that included both no effect and increased risk.
cDowngraded for publication bias (–1) detected by asymmetry funnel plot.
dDowngraded for imprecision (–2) due to wide confidence interval around the pooled estimate which included no effect, small sample size, and few events.
eDowngraded for indirectness (–2) due to urine culture being performed after cystoscopy, and antibiotic prophylaxis would kill sensitive bacteria, thus leaving the percentage of bacterial resistance higher than the control group. As a result, even the pooled result could not deduce that antibiotic prophylaxis may have increased bacterial resistance from current results.
Background
Description of the condition
Cystoscopy is a diagnostic technique which allows urologists to inspect the interior lining of the bladder. It is usually performed in the outpatient clinic for the evaluation of haematuria (blood in the urine), the diagnosis of tumours of bladder, and assessment of urinary tract benign diseases. It was estimated that more than one million cystoscopies were performed from 2009 to 2015 in the USA (Henry 2018). Two types of cystoscopes are now currently used in daily clinical practice in urology (i.e. rigid cystoscope and flexible cystoscope). The main and most concerning disadvantage of cystoscopy is the risk of urinary tract infection (UTI) due to pre‐existing colonization or by introduction of bacteria at the time of the procedure, even with appropriate periprocedural preparation (Schaeffer 2012). The most frequently implicated uropathogens in UTIs after cytoscopy are Escherichia coli (E coli) (58%), Enterococcus (17.6%), and Klebsiella (8.8%) (Jimenez‐Pacheco 2012). UTI symptoms reflect an inflammatory response of the urothelium to bacterial invasion, which is associated with bacteriuria and pyuria (pus in the urine). Bacteriuria can be asymptomatic or symptomatic, which describes the absence or presence of symptoms such as fever, dysuria, urinary frequency, and suprapubic pain (Schaeffer 2012). The incidence of asymptomatic bacteriuria after cystoscopy ranges from 2.8% to 21% (Garcia‐Perdomo 2013). In contrast, symptomatic UTI is less common after cystoscopy (Herr 2014). Whether antimicrobial agents should be used to prevent a less than 5% mean risk of symptomatic UTI after cystoscopy is controversial (Garcia‐Perdomo 2013; Herr 2012; Herr 2014; Herr 2015; Johnson 2007; Rané 2001).
Description of the intervention
Antimicrobial prophylaxis is a brief course antibiotics before intervention, intended to minimize the risk of postprocedural infections resulting from diagnostic and therapeutic interventions. Fluoroquinolones, cephalosporins, and aminoglycosides are generally considered efficacious and ideal antibiotics for prophylaxis praxis in the urinary tract (Wolf 2008). People with risk factors (i.e. advanced age, anatomic anomalies of the urinary tract, poor nutritional status, smoking, chronic corticosteroid use, and immunodeficiency) are reported to be inclined to have UTI after transurethral procedures (Burke 2002; Wolf 2008). Since the majority of people with bladder cancer have one or more of these risk factors, antibiotics are usually given before each outpatient cystoscopy (Herr 2014). However, unnecessary antimicrobial prophylaxis should be avoided as overuse of antibiotics prior to cystoscopy may contribute to adverse effects and multidrug bacterial resistance (Gross 2007). Antibiotics are associated with adverse events, including nausea, emesis, diarrhoea, headache, delirium, hallucinations, convulsions, rash, and pruritus (Wolf 2008). Meanwhile, given the numerous cystoscopies performed every year worldwide, and people with bladder tumours need to undergo repetitive cystoscopy for surveillance, there are concerns about the development of antibiotic‐resistant bacteria when routine antimicrobial prophylaxis is used (Herr 2014). For example, for ciprofloxacin, the most widely used antibiotic before urological procedures for preventing UTI, resistant infections are reported to be more than 30% (Bootsma 2008; Fillon 2012). The frequency of infectious complications after cystoscopy in healthy people with sterile preoperative urine is low (Garcia‐Perdomo 2013; Herr 2014). In view of the very large number of cystoscopic examinations, the low infectious risk, and the high risk of contributing to increasing antimicrobial resistance, latest practice guidelines of the European Association of Urology (EAU) strongly recommend that antibiotic prophylaxis should not be considered for people undergoing cystoscopy (flexible or rigid) (Bonkat 2018). Despite evidence‐based recommendations against routine prophylactic antibiotics for cystoscopy, antibiotic use has increased over time, with implications for antibiotic resistance and changes in normal microbial flora (Henry 2018).
How the intervention might work
Prophylactic antibiotics in urological procedures should meet the following requirements: long half‐life, high renal elimination, no hepatic biotransformation, broad‐spectrum coverage for the most commonly encountered organisms, and good tolerance. They can be classified as bactericidal drugs (e.g. fluoroquinolones) and bacteriostatic drugs (e.g. sulphonamides) (Sorlozano 2014; Wolf 2008). The mechanism of action for antibiotics commonly used for the urinary system varies: those that inhibit DNA replication (fluoroquinolones), or cell wall synthesis (cephalosporins), or essential bacterial enzymes (aminoglycoside) have bactericidal activities; those that inhibit folate synthesis (trimethoprim and sulphonamides) are usually bacteriostatic (Finberg 2004). Oral administration is as effective as intravenous antibiotics with sufficient bioavailability. The EAU proposed that oral antibiotic prophylaxis be given approximately one hour before the intervention, which allows antibiotic prophylaxis to reach peak concentration at the time of procedure (Grabe 2014).
Why it is important to do this review
Previous studies have shown that a single dose of prophylactic antibiotic can significantly reduce the risk of bacteriuria after cystoscopy (Johnson 2007; Rané 2001). Johnson 2007 also suggested that one dose of oral antibiotic could not only lower costs, but also reduce the risks of drug resistance. In contrast, Garcia‐Perdomo 2013 found prophylactic antibiotics did not significantly reduce UTIs of people undergoing cystoscopy compared with placebo. Even for people with asymptomatic bacteriuria before cystoscopy, the rate of symptomatic UTI after cytoscopy was just 3.7% (Herr 2015). Herr 2014 indicated that urologists may need to accept a less than 5% risk of symptomatic UTI after cystoscopy and avoid routine antibiotic prophylaxis, which might help to reduce the percentage of resistant bacteria. Antimicrobial prophylaxis should be recommended in clinical practice when the potential benefit outweighs the risks and anticipated costs. Injudicious use of the antibiotics may cause adverse effects, as well as multidrug bacterial resistance, result in treatment failure, and increase healthcare costs. At present, there is an epidemic of bacterial resistance due to overuse of antibiotics, with the susceptibility rates of antibiotics to E coli ranging from about 60% to nearly 70% (cefuroxime 67.8% to 86.4%, ciprofloxacin 61.2% to 69.8%, and cotrimoxazole 55.0% to 65.5%) (Bakken 2004; Sorlozano 2014). To preserve the continued antibacterial activity of these antibiotic drugs, urologists need to ensure that antibiotic prophylaxis is given to participants who need to be treated. As a result, a comprehensive and rigorous Cochrane systematic review is needed to assess the benefits and adverse events of using antimicrobial agents before cystoscopy for prevention of symptomatic UTI. We used GRADE to assess the quality of evidence, which will help inform future guidelines on this topic.
Objectives
To assess the effects of antimicrobial agents compared with placebo or no treatment for prevention of UTI in adults undergoing cystoscopy.
Methods
Criteria for considering studies for this review
Types of studies
We included prospective randomized controlled trials (RCTs) or quasi‐RCTs. We did not consider cluster‐randomized or cross‐over design trials as they were not directly applicable to this topic. We did not consider non‐randomized studies given that these would likely provide only low or very low quality of evidence and we knew of the existence of many relevant RCTs.
Types of participants
We included adults (age 18 years or greater) who underwent outpatient rigid or flexible cystoscopy, with or without manipulation (e.g. biopsy, fulguration).
Exclusion criteria
We excluded studies of people:
with a symptomatic UTI on the day of cystoscopy;
taking antibiotic prophylaxis for other health conditions (e.g. prosthetic cardiac valve, vascular);
currently taking other medication that may interact with antibiotics;
with allergies to antibiotics;
who are immunocompromised.
Types of interventions
We included the comparison of any prophylactic antibiotic versus placebo, no treatment, or other non‐antibiotic prophylaxis. There were no restrictions on the dose, frequency, formulation, duration, or mode of administration of the antibiotics. We investigated the following comparisons of experimental intervention versus comparator intervention.
Experimental intervention
Antibiotic prophylaxis.
Comparator interventions
Placebo.
No treatment.
Other non‐antibiotic prophylaxis.
Comparisons
Antibiotic prophylaxis versus placebo or no treatment or other non‐antibiotic prophylaxis.
We allowed concomitant interventions that were the same in the experimental and comparator groups to establish fair comparisons.
Types of outcome measures
Measurement of outcomes assessed in this review was not used as an eligibility criterion.
Primary outcomes
Systemic UTI (sepsis, fever 38 °C or greater, and documented bacteruria). Bacteruria was defined as midstream urine culture with more than 105 colony‐forming units (CFU)/mL of uropathogens, or greater than 104 CFU/mL of a single organism cultured, or greater than 104 CFU/mL uropathogens in a midstream sample of urine in men, catheterized urine culture 102 CFU/mL or greater.
Symptomatic UTI defined as a composite of both systemic and localized UTI.
Serious adverse events (e.g. Stevens‐Johnson syndrome, anaphylaxis, renal toxicity, and hepatotoxicity).
Secondary outcomes
Minor adverse events (nausea, vomiting, dizziness).
Localized UTI (local symptoms such as urinary irritative symptoms, dysuria, suprapubic pain, and documented bacteruria).
Asymptomatic bacteruria (documented bacteruria with no local or systemic symptoms).
Bacterial resistance (urine bacteria that was resistant to primary antibiotic treatment).
Method and timing of outcome measurements
In routine clinical practice, methods and criteria of urine collection and culture may vary. In general, a urine culture before cystoscopy should be taken within one week and urine culture after cytoscopy should be performed within one month, except for participants who were required to have urine culture at the discretion of physician during follow‐up.
Postcystoscopy, a follow‐up questionnaire, telephone call, or appointment should have occurred within three months to determine if a participant was symptomatic or experiencing adverse effects.
Main outcomes for 'Summary of findings' table
We presented a 'Summary of findings' table reporting the following outcomes listed according to priority.
Systemic UTI.
Symptomatic UTI.
Serious adverse events.
Minor adverse events.
Localized UTI.
Bacterial resistance.
Search methods for identification of studies
We performed a comprehensive search with no restrictions on the language of publication or publication status. Studies reported in different languages were translated by review authors with the help of Google (https://translate.google.com/). We reran all searches within three months prior to publication and screened the results for eligible studies.
Electronic searches
We searched the following sources from inception of each database to 4 February 2019.
-
The Cochrane Library (see Appendix 1 for search strategy):
Cochrane Database of Systematic Reviews (CDSR);
Cochrane Central Register of Controlled Trials (CENTRAL);
Database of Abstracts of Reviews of Effects (DARE);
Health Technology Assessment Database (HTA).
MEDLINE (PubMed; www.ncbi.nlm.nih.gov/pubmed; see Appendix 2 for search strategy).
Embase (Elsevier; see Appendix 3 for search strategy).
LILACS (lilacs.bvsalud.org/en/; see Appendix 4 for search strategy).
CINAHL (EBSCOhost; see Appendix 5 for search strategy).
We searched the following.
ClinicalTrials.gov (www.clinicaltrials.gov/; see Appendix 6 for search strategy).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/), a meta‐register of studies with links to the numerous other trials registers (see Appendix 7 for search strategy).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses, and health technology assessment reports. We searched the proceedings of meetings from the American Urological Association (AUA; www.auanet.org/) and EAU (www.europeanurology.com/search/advanced) from April 2009 to May 2018.
Data collection and analysis
Selection of studies
We used the reference management software EndNote to identify and remove potential duplicate records. Two review authors (SXZ, ZSZ) independently scanned the abstract, title, or both, of remaining records retrieved, to determine which studies should be further assessed. Two review authors (SXZ, ZSZ) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any discrepancies through consensus or recourse to a third review author (YB). If resolution of a disagreement was not possible, we designated the study as 'awaiting classification' and we contacted study authors for clarification. We documented reasons for exclusion of studies that might have reasonably been expected to be included in the review in the Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009; Figure 1).
1.
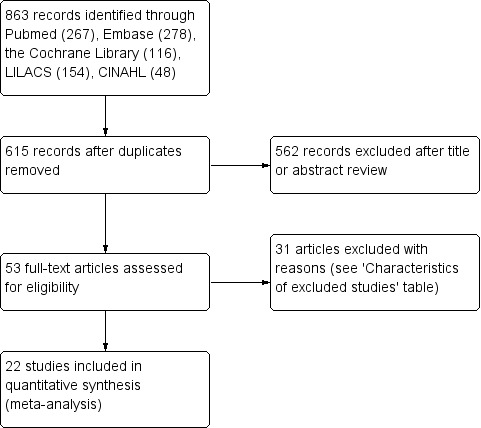
Study flow diagram.
Data extraction and management
We developed a dedicated data abstraction form that we have pilot tested.
For studies that fulfilled the inclusion criteria, two review authors (SXZ, ZSZ) independently extracted the following information, which we provided in the Characteristics of included studies table.
Study design.
Accrual dates.
Study settings and country.
Participant inclusion and exclusion criteria.
Participant details, baseline demographics.
Number of participants by study and by study arm.
Details of antibiotic prophylaxis and comparator interventions such as dose, route, frequency, and duration.
Definitions of relevant outcomes such as bacteriuria, symptomatic UTI, and method and timing of outcome measurement, as well as any relevant subgroups.
Details of outcomes relevant to this review, including the incidence of symptomatic UTI, asymptomatic bacteruria, adverse effects of antibiotics, bacterial resistance.
Study funding sources.
Declarations of interest by primary investigators.
We extracted outcome data relevant to this review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals for population of a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or data necessary to calculate this information.
We resolved any disagreements by discussion, or, if required, by consultation with a third review author (YB).
We provided information, including trial identifiers, about potentially relevant ongoing studies in the Characteristics of ongoing studies table.
We attempted to contact authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data and used the most complete data‐set aggregated across all known publications. If there was any uncertainty, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (SXZ, ZSZ) independently assessed the risk of bias of each included study. We resolved disagreements by consensus, or by consultation with a third review author (YB).
We assessed risk of bias using the Cochrane tool (Higgins 2011b). We assessed the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other sources of bias.
We judged 'Risk of bias' domains as 'low risk', 'high risk', or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We presented a 'Risk of bias' summary figure to illustrate these findings.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome, and we grouped outcomes according to whether measured subjectively or objectively when reporting our findings in the 'Risk of bias' table.
We also assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and grouped outcomes with similar judgements when reporting our findings in the 'Risk of bias' tables.
We further summarized the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome.
We defined the following endpoints as subjective outcomes.
Symptomatic UTI (systemic UTI or localized UTI), asymptomatic bacteruria, and adverse events.
We defined the following endpoint as objective outcomes.
Bacterial resistance defined as urine culture.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis was the individual participant. Should we have identified trials with more than two intervention groups for inclusion in the review, we planned to handle these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
We attempted to obtain missing data from study authors to perform intention‐to‐treat analyses; if data were not available, we performed available‐case analyses. We tried to contact study authors of included trials to obtain critical missing data (e.g. dropouts, losses to follow‐up and withdrawals, randomization method). We received replies from Garcia‐Perdomo 2013 and Johnson 2007, and we received no reply or no email address available for contacting the corresponding author for further information in other studies, details were shown in the notes of Characteristics of included studies. We did not impute missing data.
Assessment of heterogeneity
In the event of excessive heterogeneity unexplained by subgroup analyses, we did not report outcome results as the pooled effect estimate in a meta‐analysis, but provided a narrative description of the results of each study.
We identified heterogeneity (inconsistency) through visual inspection of forest plots to assess the amount of overlap of CIs, and the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); we interpreted the I2 statistic as follows:
0% to 40%: may not be important;
30% to 60%: may indicate moderate heterogeneity;
50% to 90%: may indicate substantial heterogeneity;
75% to 100%: considerable heterogeneity.
If we found heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We attempted to obtain study protocols to assess for selective outcome reporting.
We used funnel plots to assess small‐study effects when we included 10 or more studies investigating a particular outcome. Several explanations could be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials), and publication bias (Kicinski 2015). Therefore, we interpreted results carefully.
Data synthesis
We summarized data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For dichotomous outcomes, we used the Mantel‐Haenszel method. We used Review Manager 5 (RevMan 5) software to perform analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We had expected the following characteristics to introduce clinical heterogeneity, and planned to carry out subgroup analyses with investigation of interactions for the primary outcomes.
Rigid cystoscopy versus flexible cystoscopy, as they may be associated with different degrees of mucosal trauma.
Participants with manipulation (biopsy, fulguration, etc.) at cystoscopy versus those without manipulation.
Participants with presence of asymptomatic bacteriuria before cystoscopy versus those with no presence.
Men versus women.
We used the test for subgroup differences in Review Manager 5 to compare subgroup analyses (Review Manager 2014).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect sizes.
Restricting the analysis by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk'.
'Summary of findings' table
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which took into account five criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias), and external validity (such as directness of results) (Guyatt 2008). For each comparison, two review authors (SXZ, ZSZ) independently rated the quality of evidence for each outcome as 'high', 'moderate', 'low', or 'very low' using GRADEproGDT; we resolved discrepancies by consensus, or, if needed, by arbitration by a third review author (YB). For each comparison, we presented a summary of the evidence for the main outcomes in a 'Summary of findings' table, which provided key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2011). We presented results in a narrative 'Summary of findings' table when meta‐analysis was not possible.
Results
Description of studies
Results of the search
The flow of literature through the assessment process is shown in Figure 1. The electronic database search identified 615 citations after removal of duplicates, of which we selected 53 studies for full‐text review (searched 4 February 2019). We finally included 22 trials in the review (see Characteristics of included studies table) and excluded 31 trials that did not meet the inclusion criteria (see Characteristics of excluded studies table). We identified no unpublished studies that met the criteria for inclusion.
Included studies
The review included 22 studies, 20 RCTs and two quasi‐RCTs (Rané 2001; Vasanthakumar 1990). The trials published between 1971 to 2017 in five languages (English, Spanish, Portuguese, French, Chinese), and took place in 11 countries: the UK (Hares 1981; Hart 1980; Johnson 2007; MacDermott 1988; Rané 2001; Vasanthakumar 1990), the USA (Blackard 1972; Manson 1988; Mendoza 1971), Spain (Asuero 1989; Jimenez 1993; Jimenez‐Pacheco 2012; Martinez Rodriguez 2017), Turkey (Cam 2009; Soydan 2012), Colombia (Garcia‐Perdomo 2013), Singapore (Goh 1982), France (Karmouni 2001), Brazil (Rodrigues 1994), China (Si 1997), Japan (Tsugawa 1998), and New Zealand (Wilson 2005). We have provided further details of the included studies in the Characteristics of included studies table.
Eight out of 22 trials included participants with asymptomatic bacteriuria or negative urine culture before cystoscopy for analysis (Asuero 1989; Blackard 1972; Hart 1980; Johnson 2007; Martinez Rodriguez 2017; Rané 2001; Soydan 2012; Wilson 2005), 13 trials excluded participants with asymptomatic bacteriuria before cystoscopy for analysis, and the information was unclear in one trial due to lack of information (Vasanthakumar 1990).
Five trials used flexible cystoscope for examination (Jimenez‐Pacheco 2012; Johnson 2007; Martinez Rodriguez 2017; Rané 2001; Wilson 2005); five trials used rigid cystoscope for examination (Cam 2009; Garcia‐Perdomo 2013; Karmouni 2001; Si 1997; Tsugawa 1998). We contacted the author of one trial for information about the type of cystoscope (Garcia‐Perdomo 2013). Twelve trials did not describe the type of cystoscope for examination and we were unable to contact these authors to get further information because we could not find their email contact address. The trials that did not describe the type of cystoscope were published between 1971 to 1994, and probably used rigid cystoscope.
Eight trials included participants with or without manipulation (biopsy, fulguration, etc.) during cystoscopy (Asuero 1989; Cam 2009; Garcia‐Perdomo 2013; Hares 1981; Johnson 2007; MacDermott 1988; Si 1997; Soydan 2012), five trials only included participants without manipulation during cystoscopy (Blackard 1972; Manson 1988; Martinez Rodriguez 2017; Rané 2001; Wilson 2005), and there was no information about manipulation during cystoscopy in the remaining nine trials.
Excluded studies
The most common reasons for exclusion was trial design (retrospective studies and non‐randomized trials). Details of excluded studies are given in the Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2 for a summary of the risk of bias assessments for each trial and Figure 3 for a summary of the risk of bias assessments for the trials together. The 'Risk of bias' table within the Characteristics of included studies table gives detailed information.
2.
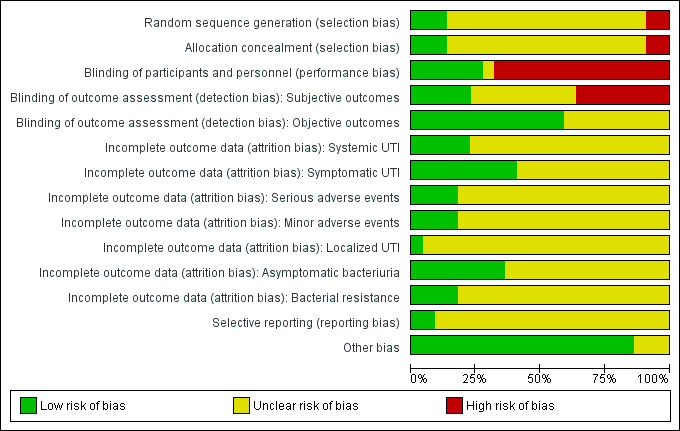
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
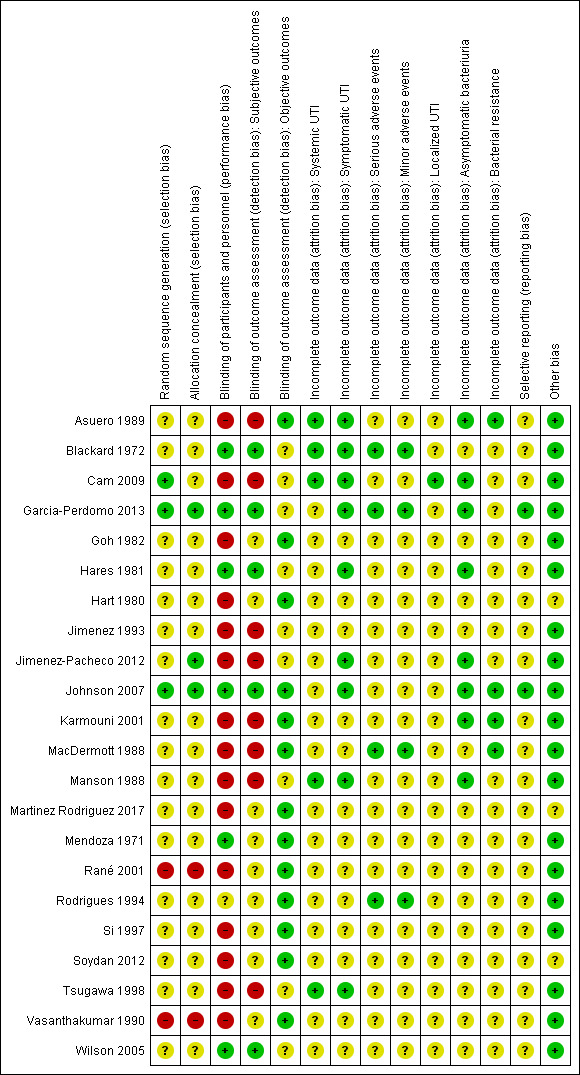
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Seventeen out of 22 trials were at unclear risk of bias for random sequence generation, three out of the 22 trials were at low risk for random sequence generation (Cam 2009; Garcia‐Perdomo 2013; Johnson 2007), and two trials used a quasi‐randomized method of sequence generation and were at high risk for random sequence generation (Rané 2001; Vasanthakumar 1990).
Allocation concealment
Seventeen out of 22 trials were at unclear risk of allocation concealment, three trials were at low risk of allocation concealment (Garcia‐Perdomo 2013; Jimenez‐Pacheco 2012; Johnson 2007), two trials used a quasi‐randomized method of sequence generation and were at high risk of allocation concealment (Rané 2001; Vasanthakumar 1990).
Blinding
Blinding of participants and personnel
Fifteen out of 22 trials were at high risk of performance bias due to non‐blinded study design, six trials had low risk of performance bias by blinding both participants and personnel (Blackard 1972; Garcia‐Perdomo 2013; Hares 1981; Johnson 2007; Mendoza 1971; Wilson 2005), and one trial was at unclear risk based on the data provided in the manuscript (Rodrigues 1994).
Blinding of outcome assessment
Subjective outcomes
Subjective outcomes were more likely to be influenced by lack of blinding. Due to non‐blinded study design, eight out of 22 trials were at high risk of detection bias for subjective outcomes such as symptomatic (systemic or localized, or both) UTI, adverse events caused by antibiotics, and asymptomatic bacteriuria (Asuero 1989; Cam 2009; Jimenez 1993; Jimenez‐Pacheco 2012; Karmouni 2001; MacDermott 1988; Manson 1988; Tsugawa 1998). Five trials showed low risk of detection bias for subjective outcomes due to proper blinding (Blackard 1972; Garcia‐Perdomo 2013; Hares 1981; Johnson 2007; Wilson 2005). The remaining trials were at unclear risk of bias because these subjective outcomes were not reported.
Objective outcomes
Four trials reported bacterial resistance assessed by urine culture (Asuero 1989; Johnson 2007; Karmouni 2001; MacDermott 1988). Ten trials reported bacteriuria (simply assessed by urine examination but without differentiating whether participants had symptoms or not) as their primary outcome (Goh 1982; Hart 1980; MacDermott 1988; Martinez Rodriguez 2017; Mendoza 1971; Rané 2001; Rodrigues 1994; Si 1997; Soydan 2012; Vasanthakumar 1990). We considered risk of detection bias for these objective outcomes to be low.
Incomplete outcome data
We assessed risk of bias for incomplete outcome data on an outcome‐specific basis (see Characteristics of included studies table).
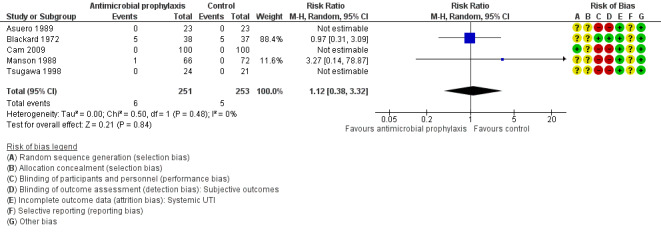
Systemic UTI: five trials reported systemic UTIs and the risk of attrition bias was low, because these trials had no postrandomization losses or few participants were excluded postrandomization, and the exclusions and reasons for exclusions were balanced between groups (Asuero 1989; Blackard 1972; Cam 2009; Manson 1988; Tsugawa 1998).
Symptomatic UTI: nine trials investigated symptomatic UTI and were at low risk of attrition bias (Asuero 1989; Blackard 1972; Cam 2009; Garcia‐Perdomo 2013; Hares 1981; Jimenez‐Pacheco 2012; Johnson 2007; Manson 1988; Tsugawa 1998). These trials had no postrandomization losses or few participants were excluded postrandomization, and the exclusions and reasons for exclusions were balanced between groups. We judged Wilson 2005 at unclear risk of attrition bias for symptomatic UTI, because 29 out of 263 participants were excluded because of incomplete data acquisition. There was no information about whether these withdrawals were before or after randomization or from which group the withdrawals came from. The study was stopped and interim analysis was performed because of low recruitment rate. We judged Jimenez 1993 at unclear risk of attrition bias for symptomatic UTI, because 2284 participants were randomized, 2172 participants were finally included for analysis of this outcome, 105 participants were excluded after randomization due to failure to meet inclusion criteria, and the reason for seven participants that were not included for analysis was not given.
Serious adverse events: four trials reported adverse events (serious and minor adverse events). The risk of attrition bias was low, because these trials had no postrandomization losses or few participants were excluded postrandomization, and the exclusions and reasons for exclusions were balanced between groups (Blackard 1972; Garcia‐Perdomo 2013; MacDermott 1988; Rodrigues 1994).
Minor adverse events: four trials reported minor adverse events (Blackard 1972; Garcia‐Perdomo 2013; MacDermott 1988; Rodrigues 1994). The risk of attrition bias was low, because these trials had no postrandomization losses or few participants were excluded postrandomization, and the exclusions and reasons for exclusions were balanced between groups.
Localized UTI: one trial reported localized UTI (Cam 2009). All participants included were analyzed for this outcome and the risk of attrition bias was low.
Asymptomatic bacteriuria: eight trials were at low risk of attrition bias for asymptomatic bacteriuria (Asuero 1989; Cam 2009; Garcia‐Perdomo 2013; Hares 1981; Jimenez‐Pacheco 2012; Johnson 2007; Karmouni 2001; Manson 1988). Two trials were at unclear risk of attrition bias for asymptomatic bacteriuria (Jimenez 1993; Wilson 2005) (reasons were mentioned above).
Bacterial resistance: four trials reported bacterial resistance. The risk of attrition bias was low because these trials had no postrandomization losses or few participants were excluded postrandomization, and the exclusions and reasons for exclusions were balanced between groups (Asuero 1989; Johnson 2007; Karmouni 2001; MacDermott 1988).
Selective reporting
We assessed 20 out of 22 trials to be at an unclear risk of reporting bias, although data reported on all outcomes specified in methods section, there was no access to trial protocol/registration to further assess selective reporting in these trials. Protocol documents of two trials were available for analysis, and their outcomes were reported in line with the protocol (Garcia‐Perdomo 2013; Johnson 2007).
The publication bias was tested by funnel plots for outcomes of symptomatic UTI (Figure 4) and asymptomatic bacteriuria (Figure 5), there appear to have asymmetry which suggested publication bias.
4.
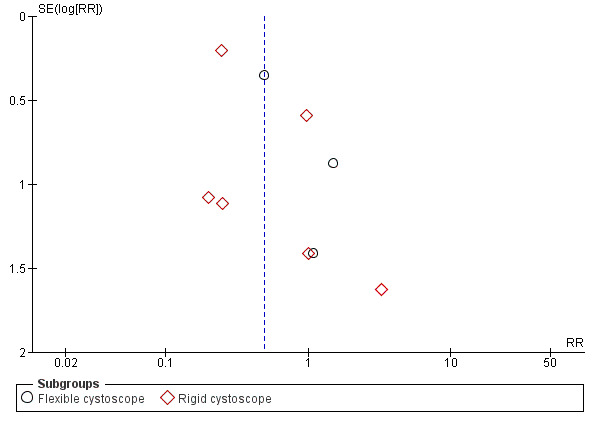
Funnel plot of comparison: 1 Antimicrobial versus placebo or no antibiotics, outcome: 1.2 Symptomatic urinary tract infection.
5.
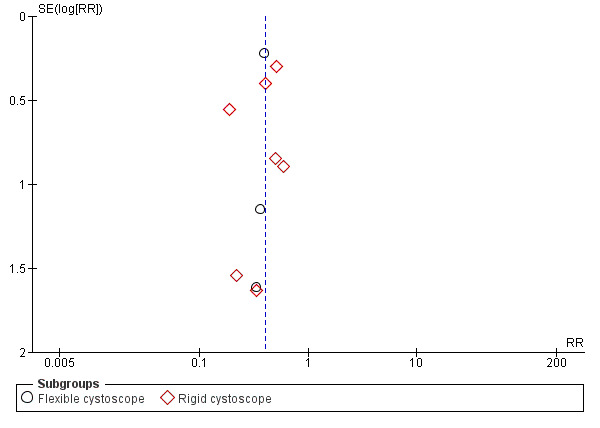
Funnel plot of comparison: 1 Antimicrobial versus placebo or no antibiotics, outcome: 1.5 Asymptomatic bacteriuria.
Other potential sources of bias
There were no other potential sources of bias.
Effects of interventions
See: Table 1
Antibiotic prophylaxis versus placebo or no treatment or other non‐antibiotic prophylaxis
Primary outcomes
See Table 1.
Systemic urinary tract infection
Five trials with 504 participants contributed to the analysis of systemic UTI (Asuero 1989; Blackard 1972; Cam 2009; Manson 1988; Tsugawa 1998). The incidence of systemic UTI was low in both antibiotic prophylaxis group (6/251, 2.39%) and control group (5/253, 1.98%). We found low‐quality evidence that antibiotic prophylaxis may have little or no effect on the risk of systemic UTI compared with the control group (RR 1.12, 95% CI 0.38 to 3.32; Analysis 1.1; Figure 6). We downgraded the quality of evidence by one level for study limitations and by one level for imprecision. This corresponds to two more people (95% CI 12 fewer to 46 more) per 1000 people having a systemic UTI when provided with antibiotic prophylaxis.
1.1. Analysis.
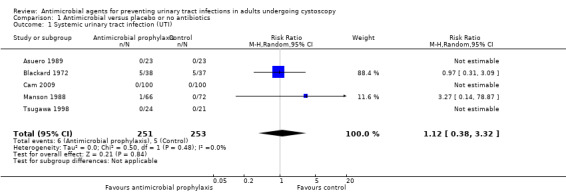
Comparison 1 Antimicrobial versus placebo or no antibiotics, Outcome 1 Systemic urinary tract infection (UTI).
6.
Forest plot of comparison: 1 Antimicrobial versus placebo or no antibiotics, outcome: 1.1 Systemic urinary tract infection.
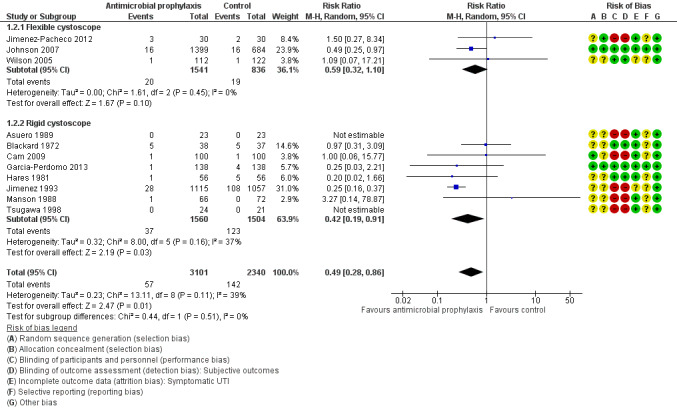
Symptomatic urinary tract infection
Six trials contributed to the analysis of symptomatic UTI (the six trials reported symptomatic UTI as their primary outcome without distinguishing systemic or localized UTI) (Garcia‐Perdomo 2013; Hares 1981; Jimenez 1993; Jimenez‐Pacheco 2012; Johnson 2007; Wilson 2005). Five trials reported systemic UTI or localized UTI, or both, separately as mentioned above (Asuero 1989; Blackard 1972; Cam 2009; Manson 1988; Tsugawa 1998). We pooled data from the 11 trials, with 5441 participants, for the analysis of symptomatic UTI. When compared to control group, participants receiving prophylactic antibiotics had fewer symptomatic UTIs (57/3101, 1.84%) than control group (142/2340, 6.07%).
Antibiotic prophylaxis may reduce the incidence of symptomatic UTI (RR 0.49, 95% CI 0.28 to 0.86; low‐quality evidence; Analysis 1.2; Figure 7). We downgraded one level for study limitations and one level for publication bias. This corresponds to 30 fewer people (95% CI 42 fewer to 8 fewer) per 1000 people having a symptomatic UTI when provided with antibiotic prophylaxis.
1.2. Analysis.
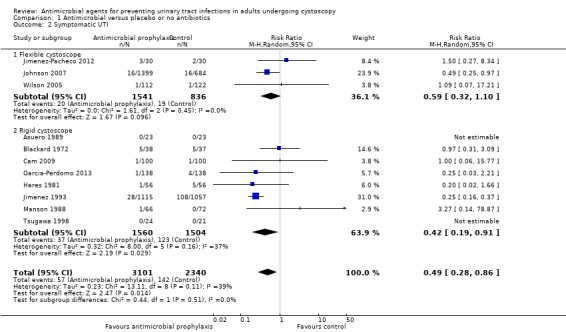
Comparison 1 Antimicrobial versus placebo or no antibiotics, Outcome 2 Symptomatic UTI.
7.
Forest plot of comparison: 1 Antimicrobial versus placebo or no antibiotics, outcome: 1.2 Symptomatic urinary tract infection.
Serious adverse events
Four trials with 630 participants reported adverse effects caused by antibiotics prophylaxis, but all of them were minor adverse events, that is, 0/326 participants in the antibiotic prophylaxis group and 0/304 participants in the control group had serious adverse events (Blackard 1972; Garcia‐Perdomo 2013; MacDermott 1988; Rodrigues 1994). We could not calculate an absolute effect estimate but our best estimate is that there may be no difference (RR approximately 1; very low‐quality evidence), but we were very uncertain of this finding. We downgraded the quality of evidence one level for study limitations and one level for imprecision. We were unable to calculate an absolute effect size measure.
Secondary outcomes
See Table 1.
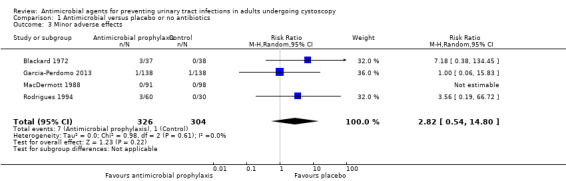
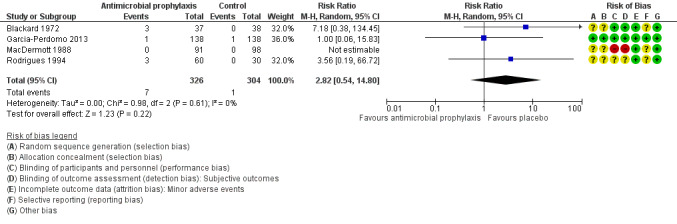
Minor adverse events
Four trials with 630 participants contributed to the analysis of minor adverse events caused by prophylactic antibiotic (Blackard 1972; Garcia‐Perdomo 2013; MacDermott 1988; Rodrigues 1994). We found low‐quality evidence that prophylactic antibiotic may result in little or no difference in minor adverse events compared with placebo (RR 2.82, 95% CI 0.54 to 14.80; Analysis 1.3; Figure 8). We downgraded the quality of evidence one level for study limitations and one level for imprecision. This corresponds to six more (95% CI 2 fewer to 46 more) people with minor adverse events.
1.3. Analysis.
Comparison 1 Antimicrobial versus placebo or no antibiotics, Outcome 3 Minor adverse effects.
8.
Forest plot of comparison: 1 Antimicrobial versus placebo or no antibiotics, outcome: 1.3 Minor adverse effects.
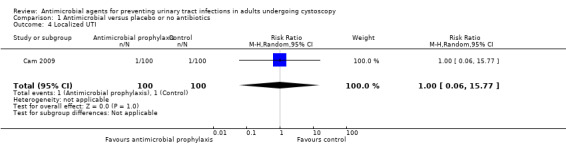
Localized urinary tract infection
We found one trial that contributed to the analysis of localized UTI (Cam 2009). Cam 2009 reported 1/100 localized UTI in the antibiotic prophylaxis group and 1/100 in the control group, Very low‐quality evidence suggests that antibiotic prophylaxis have little or no effect on localized UTI compared with control group (RR 1.00, 95% CI 0.06 to 15.77; Analysis 1.4; Figure 9), but we are very uncertain of this finding. We downgraded the quality of evidence one level for study limitations and two levels for imprecision. This corresponds to zero more people (95% CI 9 fewer to 152 more) per 1000 people having a localized UTI when provided with antibiotic prophylaxis.
1.4. Analysis.
Comparison 1 Antimicrobial versus placebo or no antibiotics, Outcome 4 Localized UTI.
9.
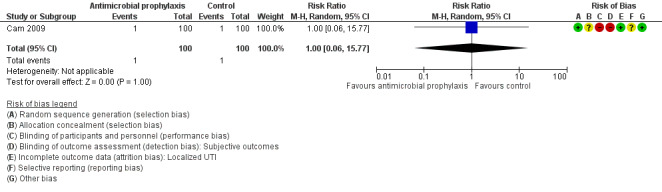
Forest plot of comparison: 1 Antimicrobial versus placebo or no antibiotics, outcome: 1.4 Localized urinary tract infection.
Asymptomatic bacteriuria
Ten trials with 5447 participants contributed to the analysis of asymptomatic bacteriuria (Asuero 1989; Cam 2009; Garcia‐Perdomo 2013; Hares 1981; Jimenez 1993; Jimenez‐Pacheco 2012; Johnson 2007; Karmouni 2001; MacDermott 1988; Wilson 2005). Asymptomatic bacteriuria was less frequent in the antibiotic prophylaxis group (68/3106, 2.19%) compared to control group (126/2341, 5.38%). Based on low‐quality evidence, antibiotic prophylaxis may reduce asymptomatic bacteriuria (RR 0.40, 95% CI 0.30 to 0.53; Analysis 1.5; Figure 10). We downgraded the quality of evidence one level for study limitations and one level for publication bias.
1.5. Analysis.
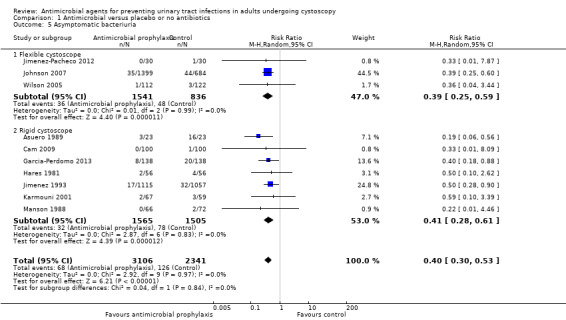
Comparison 1 Antimicrobial versus placebo or no antibiotics, Outcome 5 Asymptomatic bacteriuria.
10.
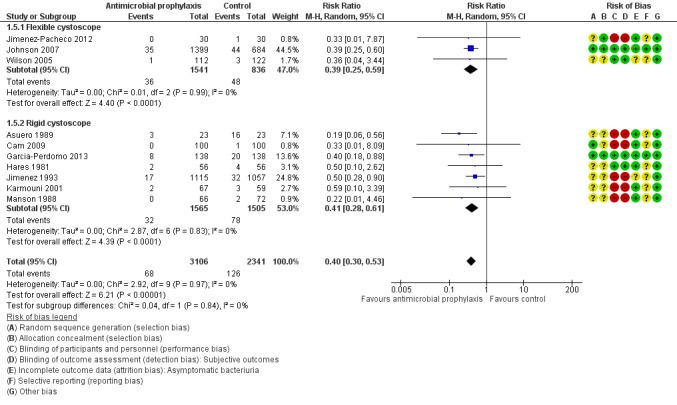
Forest plot of comparison: 1 Antimicrobial versus placebo or no antibiotics, outcome: 1.5 Asymptomatic bacteriuria.
Bacterial resistance
Four trials with 2444 participants reported bacterial resistance; however, only 89 participants contributed to the analysis (Asuero 1989; Johnson 2007; Karmouni 2001; MacDermott 1988). Only results from two studies were suitable for pooling (Asuero 1989; MacDermott 1988).
In Asuero 1989, 9/16 (56.3%) participants with bacteriuria in the control group showed no sensitivity to antibiotic, and 3/3 (100%) participants with bacteriuria showed no sensitivity to antibiotic in the treatment group post cystoscopy. Johnson 2007 reported organism was resistant to the antibiotic in 9/22 (40.1%) participants given trimethoprim and in 4/28 (14.3%) participants given ciprofloxacin post cystoscopy; however, the incidence of bacterial resistance in the control group was not available. Karmouni 2001 found one participant had multiresistant bacteria post cystoscopy, but did not specify whether they were in the intervention or control group. MacDermott 1988 reported 4/16 (25.0%) participants with bacteriuria in the control group, and 2/3 (66.7%) participants with bacteriuria in the treatment group showed no sensitivity to antibiotic post cystoscopy.
The results from Asuero 1989 and MacDermott 1988 were able to be pooled. Antibiotic prophylaxis may increase bacterial resistance (RR 1.73, 95% CI 1.04 to 2.87; very low‐quality evidence; Analysis 1.6; Figure 11), but we are very uncertain of this finding. We downgraded the quality of evidence one level for study limitations, and two levels for indirectness and imprecision (Table 1). We downgraded for indirectness because urine cultures were performed after cystoscopy, and antibiotic prophylaxis would kill sensitive bacteria, thus leaving the percentage of bacterial resistance rate higher than that of the control group. This finding corresponds to 297 more people (16 more to 760 more) with bacterial resistance.
1.6. Analysis.
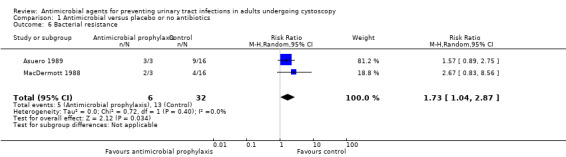
Comparison 1 Antimicrobial versus placebo or no antibiotics, Outcome 6 Bacterial resistance.
11.
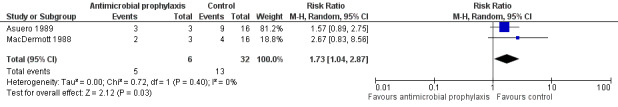
Forest plot of comparison: 1 Antimicrobial versus placebo or no antibiotics, outcome: 1.6 Bacterial resistance.
Antibiotic prophylaxis versus other non‐antibiotic prophylaxis
We found no studies comparing antibiotic prophylaxis versus other non‐antibiotic prophylaxis.
Subgroup analysis
Rigid cystoscopy versus flexible cystoscopy
For systemic UTI and serious adverse events, we were unable to perform other planned subgroup analyses due to the limited number of studies included and paucity of data for primary outcomes.
For symptomatic UTI, eight trials with 3064 participants underwent rigid cystoscopy (Asuero 1989; Blackard 1972; Cam 2009; Garcia‐Perdomo 2013; Hares 1981; Jimenez 1993; Manson 1988; Tsugawa 1998), and we found a reduction in symptomatic UTI in the antibiotic prophylaxis group in studies using rigid cystoscopy (RR 0.42, 95% CI 0.19 to 0.91; P = 0.03; Analysis 1.2; Figure 7). Three trials with 2377 participants underwent flexible cystoscopy (Jimenez‐Pacheco 2012; Johnson 2007; Wilson 2005), but the difference regarding symptomatic UTI was not observed in these studies using flexible cystoscopy (RR 0.59, 95% CI 0.32 to 1.10; P = 0.10; Figure 7). However, the subgroup interaction test indicated no evidence of a subgroup effect (Chi2 = 0.44, P = 0.51, I2 = 0%).
Participants with manipulation at cystoscopy versus those without manipulation
We were unable to perform the subgroup analyses due to paucity of data for this comparison.
Participants with presence of asymptomatic bacteriuria before cystoscopy versus those with no presence
We were unable to perform the subgroup analyses due to paucity of data for this comparison.
Men versus women
We were unable to perform the subgroup analyses due to paucity of data for this comparison.
Sensitivity analysis
We did not conduct sensitivity analyses for systemic UTI and serious adverse events, as we judged none of studies included in these comparison to be at low risk of bias overall.
We performed sensitivity analysis for symptomatic UTI in which we included only two studies with low risk of bias (Garcia‐Perdomo 2013; Johnson 2007). The pooled result was similar, indicating that antibiotic prophylaxis may have reduced the incidence of symptomatic UTI compared with control group (RR 0.46, 95% CI 0.24 to 0.89; P = 0.02).
Discussion
Summary of main results
All findings of this review were limited to the comparison of antibiotics prophylaxis versus placebo or no prophylaxis (without use of placebo). We found no comparisons of antibiotic prophylaxis versus other forms of non‐antibiotic prophylaxis.
We found that antibiotic prophylaxis may reduce UTIs when analyzed as symptomatic UTI (defined as the composite of systematic UTI and localized UTI) based on low‐quality evidence. It may have little to no effect on each of these outcomes when analyzed in isolation based on low‐quality evidence (systemic UTI) and very low‐quality evidence (localized UTI). Antibiotics prophylaxis may have little or no effect on serious and minor adverse events, based on low‐quality evidence (serious) and very low‐quality evidence (minor). Antibiotic prophylaxis may increase bacterial resistance but we are very uncertain of this finding based on very low‐quality evidence.
Overall completeness and applicability of evidence
Most trials pertained to antibiotics and regimens that are no longer used in current daily clinical practice, and were performed in the 1980s and 1990s; this limits the applicability of our findings. Although the settings of included trials varied, they do reflect common situations in the clinical practice and therefore the evidence appears applicable in that regard.
There was considerable clinical heterogeneity meaning that the studies used different types of cystoscopes (flexible versus rigid), included manipulation or not, were performed in men and women with their different anatomy, and assessed for asymptomatic bacteriuria before cystoscopy or not. We were unable to complete many of our preplanned subgroup analysis to explore the observed heterogeneity because there were insufficient data from the included studies and most of the studies did not analyze these subgroups individually. We attempted to contact authors for additional clarifying information but only received replies from two of them (Garcia‐Perdomo 2013; Johnson 2007). Eleven trials that were published between 1971 to 1994 did not specify the type of cystoscope for examination; they were classified as rigid cystoscope in the subgroup analysis because flexible cystoscope was not widely used then, and some trials used spinal or general anaesthesia during procedure which was uncommon for flexible cystoscopy.
Although no serious adverse event was reported among studies, it should be noted that it was not possible to determine severe adverse events from such relatively small sample sizes, and the incidence and types of severe adverse events also varied between different antibiotics. Meropol 2008 reported that the incidence of any serious adverse events of ciprofloxacin ranged from 3.6 per 100,000 person‐days to 16.9 per 100,000 person‐days. Thornhill 2015 found that with amoxicillin there were no fatal reactions per million prescriptions and 22.62 non‐fatal reactions per million prescriptions.
Bacterial resistance analysis was only performed for few participants with positive urine culture in included studies, as a result the included trials were not well suited to show the association between antibiotic prophylaxis and bacterial resistance.
Quality of the evidence
We graded the quality of the evidence based on the GRADE approach (Table 1). We found that the level of evidence ranged from very low to low for all outcomes. The most common reasons for downgrading the quality of evidence was risk of bias due to study limitations and imprecision of data due to wide CI and low event rates. Figure 2 and Figure 3 showed that unclear risk of biases were often due to lack of reporting methodology and high risk of biases were often due to non‐blinded study design.
Potential biases in the review process
We reduced potential biases by using a comprehensive search strategy; however, it is possible that we could have missed trials that were not indexed in the commonly used databases. Should any such studies be identified, we will include them in updates of this review. We considered only RCTs for inclusion in this review. Eleven trials reported bacteriuria as the primary outcome without distinguishing whether they were accompanied by symptoms or not (Blackard 1972; Goh 1982; Hart 1980; MacDermott 1988; Martinez Rodriguez 2017; Mendoza 1971; Rané 2001; Rodrigues 1994; Si 1997; Soydan 2012; Vasanthakumar 1990). Bacteriuria was not consistently defined as prespecified for our primary and secondary outcomes. We considered pooling these trials that reported bacteriuria in such unspecified manner with symptomatic UTI or asymptomatic bacteriuria but concluded that this might result in misleading results. As a result, although these 11 trials were included for analysis of methodological quality, their data were not included for meta‐analysis. Two RCTs were published as conference abstracts (Martinez Rodriguez 2017; Soydan 2012), and we were unable to obtain additional information from the authors to better evaluate their methodological quality and to extract useful data. Since the boundaries between a localized UTI and a systemic UTI may be fluid in clinical practice and both matter to participants and their providers, we added symptomatic UTI as a primary outcome post hoc, because we considered this was also a patient‐important outcome.
Agreements and disagreements with other studies or reviews
We identified two published systematic reviews that addressed the topic of antibiotic prophylaxis in cystoscopy (Carey 2015; Garcia‐Perdomo 2015).
Carey 2015 pooled results from nine studies and found that the control group was more likely to have symptomatic UTIs post flexible cystoscopy than the antibiotic group. The number needed to treat to prevent one episode was 26. In the present review, we found antibiotic prophylaxis was unlikely to reduce the incidence of symptomatic UTI compared with the control group post flexible cystoscopy (RR 0.59, 95% CI 0.32 to 1.10; P = 0.10; Figure 7). Carey 2015 considered three trials using flexible cystoscope for cystoscopy in their review (Garcia‐Perdomo 2013; Jimenez 1993; Mendoza 1971). However, we classified the trials conducted by Jimenez 1993 and Mendoza 1971 as rigid cystoscope in the present study because flexible cystoscope was not widely used at that time, and we obtained the information from the author of Garcia‐Perdomo 2013 that they used a rigid cystoscope in their study.
Garcia‐Perdomo 2015 included five trials for the analysis of symptomatic UTI and found that antibiotic prophylaxis might have reduced the incidence of symptomatic UTI (RR 0.52, 95% CI 0.31 to 0.89; P = 0.02). In the present review, we found 11 trials contributed to the analysis of symptomatic UTI, and results also showed that antibiotic prophylaxis might have reduced symptomatic UTI, subgroup analysis suggested that antibiotic prophylaxis might have been effective in participants undergoing rigid cystoscopy, but appeared to be not effective for flexible cystoscopy; however, the subgroup interaction test indicated no evidence of a subgroup effect. We identified two prospective non‐randomized trials by Cano‐Garcia 2016 and Herr 2014 that suggested that antibiotic prophylaxis before flexible cystoscopy did not appear necessary for participants who had no clinical signs or symptoms of acute UTI.
The present systematic review associated with this topic was the only one with an a priori protocol and performed strictly along with the PRISMA principle, and a comprehensive search that included studies irrespective of language and publication status. We also use GRADE to rate the quality of the evidence.
Authors' conclusions
Implications for practice.
Antibiotic prophylaxis may lead to a small reduction of urinary tract infections (UTIs) but only when considering systemic and localized UTIs together. This corresponds to 30 fewer (95% confidence interval (CI) 42 fewer to 8 fewer) symptomatic UTIs per 1000 people. Antibiotic prophylaxis does not appear to increase serious adverse events or minor adverse events, although we are very uncertain about the latter finding. We are also very uncertain whether it increases bacterial resistance.
Implications for research.
Additional high‐quality, adequately powered randomized controlled trials (RCTs) using proper blinding method, reporting outcomes in subgroups stratified by different types of cystoscope (rigid versus flexible), and by different risk of UTI (e.g. with asymptomatic bacteriuria before cystoscopy, manipulation is needed during cystoscopy) may help to clarify the ideal strategy of antibiotic prophylaxis to prevent symptomatic UTI (systemic or localized UTI, or both) post cystoscopy, and provide a more definitive and robust evidence for this comparison. The incidence of severe adverse events of antibiotics is low (Meropol 2008; Thornhill 2015). Although there was no severe adverse event caused by antibiotic prophylaxis in the current review, future prospective observational studies with large sample size and standardized adverse reporting criteria will better inform this issue.
Notes
Parts of the Methods section and Appendix 1 of this review were based on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group that has been modified and adapted for use by the Cochrane Urology Group.
Acknowledgements
We are grateful to Professor Chunbo Li, Director of the Chinese Satellite of Cochrane Schizophrenia Group, who kindly gave us advice on how to perform this systematic review and meta‐analysis. We wish to thank the staff and editors (Philipp Dahm, Molly Neuberger, Alea Miller, Robert Lane, Jim Tacklind, and Kourosh Afshar) of the Cochrane Urology Group for feedback to the draft protocol and review. We would like to thank Tommaso Cai, Magnus Grabe, and Harry Herr for their valuable comments to improve the draft protocol. We would also like to thank Franck Bruyere, Harry Herr, and Robert Pickard for their valuable comments to improve the manuscript.
Appendices
Appendix 1. The Cochrane Library search strategy
#1 MeSH descriptor: [Cystoscopy] explode all trees
#2 cystoscop* or cystourethroscop* or urethrocystoscop*
#3 #1 or #2
#4 MeSH descriptor: [Antibiotic Prophylaxis] explode all trees
#5 Antibioti* or anti‐bacterial* or antibacterial* or Probioti* or ofloxacin or levofloxacin or ciprofloxacin or metronidazole or azithromycin or clarithromycin or erythromycin or amoxicillin or penicillin or loracarbef or ceph* or trimethoprim or vancomycin or augmentin or chemoprophylaxis or Quinolone*
#6 #4 or #5
#7 #3 and #6
Appendix 2. MEDLINE (PubMed) search strategy
((((((((((("Controlled Clinical Trial" [Publication Type]) OR "Randomized Controlled Trial" [Publication Type]) OR (((((groups[Title/Abstract]) OR trial[Title/Abstract]) OR drug therapy[MeSH Subheading]) OR placebo[Title/Abstract]) OR random*[Title/Abstract])))) OR controll*[Title/Abstract]) OR blind*[Title/Abstract]) OR allocate*[Title/Abstract]) OR assign*[Title/Abstract]) OR volunteer*[Title/Abstract])) AND ((((((((((((((((((((((((((((Antibioti*) OR anti‐bacterial*) OR antibacterial*) OR Probioti*) OR ofloxacin) OR levofloxacin) OR ciprofloxacin) OR metronidazole) OR azithromycin) OR clarithromycin) OR erythromycin) OR amoxicillin) OR penicillin) OR loracarbef) OR ceph*) OR trimethoprim) OR vancomycin) OR augmentin) OR chemoprophylaxis) OR Quinolone*)) OR "Antibiotic Prophylaxis"[Mesh])) OR antimicrobial*) OR anti‐microbial*)) AND (((((cystoscop*) OR Cystourethroscop*) OR urethrocystoscop*)) OR "Cystoscopy"[Mesh])))
Appendix 3. Embase (Elsevier) search strategy
#1 antibacterial*
#2 antimicrobial*
#3 antibioti*
#4 premedication
#5 probioti*
#6 ofloxacin
#7 levofloxacin
#8 ciprofloxacin
#9 metronidazole
#10 azithromycin
#11 clarithromycin
#12 erythromycin
#13 amoxicillin
#14 penicillin
#15 loracarbef
#16 ceph*
#17 trimethoprim
#18 vancomycin
#19 augmentin
#20 chemoprophylaxis
#21 'antibiotic prophylaxis'/exp
#22 or/1‐21
#23 'cystoscopy'/exp
#24 cystoscop*
#25 cystourethroscop*
#26 urethrocystoscop*
#27 or/23‐26
#28 random*:ab,ti
#29 groups:ab,ti
#30 trial:ab,ti
#31 placebo:ab,ti
#32 controll*:ab,ti
#33 blind*:ab,ti
#34 allocate*:ab,ti
#35 assign*:ab,ti
#36 volunteer*:ab,ti
#37 randomized controlled trial
#38 controlled clinical trial
#39 'controlled clinical trial'/exp
#40 randomized controlled trial
#41 'randomized controlled trial'/exp
#42 or/28‐41
#43 #22 and #27 and #42
Appendix 4. LILACS search strategy
(tw:((tw:(groups OR trial OR placebo OR random* OR assign* OR allocate* OR blind* OR controll* OR volunteer*)))) AND (tw:((tw:( (tw:(Antibioti* or anti‐bacterial* or antibacterial* or Probioti* or ofloxacin or levofloxacin or ciprofloxacin or metronidazole or azithromycin or clarithromycin or erythromycin or amoxicillin or penicillin or loracarbef or ceph* or trimethoprim or vancomycin or augmentin or chemoprophylaxis or Quinolone*)))) AND (tw:(cystoscop* OR Cystourethroscop* OR urethrocystoscop*))))
Appendix 5. CINAHL (EBSCOhost) search strategy
TX ( Antibioti* or anti‐bacterial* or antibacterial* or Probioti* or ofloxacin or levofloxacin or ciprofloxacin or metronidazole or azithromycin or clarithromycin or erythromycin or amoxicillin or penicillin or loracarbef or ceph* or trimethoprim or vancomycin or augmentin or chemoprophylaxis or Quinolone* ) AND TX ( cystoscop* OR Cystourethroscop* OR urethrocystoscop* ) AND AB ( randomized controlled trial OR controlled clinical trial OR groups OR trial OR placebo OR random* OR assign* OR allocate* OR blind* OR controll* OR volunteer* )
Appendix 6. ClinicalTrials.gov search strategy
cystoscopy OR cystoscopic OR Cystourethroscopy OR Cystourethroscopic OR urethrocystoscopy OR urethrocystoscopic
Appendix 7. WHO ICTRP search strategy
cystoscop* OR Cystourethroscop* OR urethrocystoscop*
Data and analyses
Comparison 1. Antimicrobial versus placebo or no antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systemic urinary tract infection (UTI) | 5 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.38, 3.32] |
| 2 Symptomatic UTI | 11 | 5441 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.86] |
| 2.1 Flexible cystoscope | 3 | 2377 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.32, 1.10] |
| 2.2 Rigid cystoscope | 8 | 3064 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.91] |
| 3 Minor adverse effects | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.54, 14.80] |
| 4 Localized UTI | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.77] |
| 5 Asymptomatic bacteriuria | 10 | 5447 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.53] |
| 5.1 Flexible cystoscope | 3 | 2377 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.59] |
| 5.2 Rigid cystoscope | 7 | 3070 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.61] |
| 6 Bacterial resistance | 2 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.04, 2.87] |
| 7 Bacteriuria | 10 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.16, 0.33] |
| 7.1 Flexible cystoscope | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.08, 0.65] |
| 7.2 Rigid cystoscope | 9 | 1691 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.14, 0.35] |
1.7. Analysis.
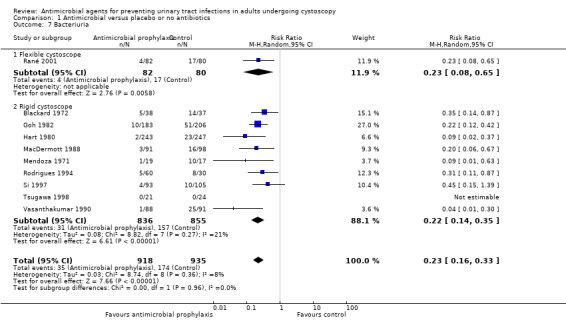
Comparison 1 Antimicrobial versus placebo or no antibiotics, Outcome 7 Bacteriuria.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asuero 1989.
| Methods |
Study design: prospective randomized control study Study dates: not available Setting: 1 hospital Country: Spain |
|
| Participants |
Inclusion criteria: people with negative preoperative urine cultures Exclusion criteria: allergy to penicillins, indwelling catheter, easily bleeding during manipulation Sample size: 46 Age (years): overall median: 65 (51–78) Sex: not available |
|
| Interventions |
Group 1 (n = 23): no antibiotic prophylaxis Group 2 (n = 23): cefuroxime 750 mg intravenously 1 hour preoperatively and 2 more doses administered at 12 and 24 hours after surgery |
|
| Outcomes |
Systemic UTI How measured: not reported Time points measured: not reported Time points reported: not reported Outcomes: no participant had symptoms suggestive of a UTI Asymptomatic bacteriuria How measured: not reported Time points measured: urine cultures performed on 5th day and 1 month postoperatively Time points reported: not reported Outcomes: control group: 16/23 participants had bacteriuria 5 days after cystoscopy, and 2/23 had bacteriuria 1 month after cystoscopy; treatment group: 3/23 had bacteriuria 5 days after cystoscopy, and 5/23 had bacteriuria 1 month after cystoscopy Bacterial resistance How measured: not reported Time points measured: bacteria cultures from urine performed 5 days after cystoscopy Time points reported: not reported Outcomes: control group: 16 participants with positive urine cultures, 9 of them they showed no sensitivity to cefuroxime; treatment group, 3 participants were all resistant |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | In the antibiotic prophylaxis group, all participants with negative blood cultures in the 3rd postoperative day, and 4 participants in the control group had bacteraemia. 3 participants showed an acute epididymitis after cystoscopy, but this study did not report which group they came from. No email address available for contacting the corresponding author for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We have carried out a prospective study on 46 patients, these 46 patients were randomly divided into two groups." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding the concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A first control group of 23 patients who were not administered antibiotic prophylaxis, a second group of 23 patients who were administered 750 mg." Comment: participants in control group were not administered antibiotic prophylaxis, while the treatment group receive intravenous antibiotic prophylaxis. Unlikely that participants and personnel were blinded to intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "A first control group of 23 patients who were not administered antibiotic prophylaxis, a second group of 23 patients who were administered 750 mg." Comment: participants were not blinded to their treatment, the risk of detection bias for subjective outcomes, i.e. symptoms suggestive of UTI after cystoscopy was high. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "When cultures were positive postoperative urine, the sensitivity of the germ to cefuroxime titrated were tested." Comment: since bacterial resistance was evaluated by urine culture from laboratory, results were objective and probably not influenced by blinding or not. |
| Incomplete outcome data (attrition bias) Systemic UTI | Low risk | Quote: "no participant had symptoms suggestive of a urinary tract infection." Comment: 46/46 participants (23 participants in each arm) included for analysis of this outcome, this risk of bias was low. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "no participant had symptoms suggestive of urinary tract infection." Comment: 46/46 participants (23 participants in each arm) included for analysis of this outcome, this risk of bias was low. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Low risk | Quote: "Urine cultures performed the 5th postoperative day were positive in three patients in the prophylaxis group (13%) remain negative in the remaining 20 (87%). By contrast, in the control group, these cultures were positive in 16 (69.6%) and negative in 7 of them (30.4%)." Comment: 46/46 participants (23 participants in each arm) included for analysis of this outcome, this risk of bias was low. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Low risk | Quote: "Three patients in the prophylaxis, isolated germs were resistant, whereas in the control group of 16 patients with positive urine cultures 9 of them they showed no sensitivity." Comment: all included participants were included for analysis of this outcome. 46/46 participants (23 participants in each arm) included for analysis of this outcome, this risk of bias was low. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Blackard 1972.
| Methods |
Study design: parallel‐group, double‐blind randomized trial Study dates: 1 January 1969 to 31 December 1969 Setting: 1 hospital Country: USA |
|
| Participants |
Inclusion criteria: no fever or clinical UTI; required no antibacterial agents during the preceding 2 weeks; no retention type urethral catheter; no need for immediate operation; no allergy to sulphonamide Exclusion criteria: not available Sample size: 75 men Age (years): overall median 74 (44–82) Sex: all men |
|
| Interventions |
Group 1 (n = 37): placebo, oral, started 2 days before cystoscopy and maintained 10 days following cystoscopy Group 2 (n = 38): sulphamethoxazole 500 mg + phenazopyridine 100 mg, oral, started 2 days before cystoscopy and maintained 10 days following cystoscopy |
|
| Outcomes |
Systemic UTI How measured: body temperature ≥ 38 °C Time points measured: within 10 days after cystoscopy Time points reported: within 10 days after cystoscopy Outcomes: placebo group: 0/37 participants had fever 1 day after cystoscopy, and 5/37 had fever within 2–10 days after cystoscopy; treatment group: 4/38 had fever 1 day after cystoscopy, and 5/38 had fever within 2–10 days after cystoscopy Bacteriuria How measured: urine culture yielded > 104 CFU/mL Time points measured: within 10 days after cystoscopy Time points reported: within 10 days after cystoscopy Outcomes: placebo group: 17/37 participants had bacteriuria 1 day after cystoscopy, and 14/37 had bacteriuria within 2–10 days after cystoscopy; treatment group: 6/38 had bacteriuria 1 day after cystoscopy, and 5/38 had bacteriuria within 2–10 days after cystoscopy Minor adverse events How measured: decided by physician Time points measured: within 10 days after cystoscopy Time points reported: within 10 days after cystoscopy Outcomes: treatment group: 1 participants had drug eruption and 2 participants had sulphonamide crystals in the urine 1 day after cystoscopy |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | For participants with sterile urine before cystoscopy, in the placebo group, 7/27 had bacteriuria 1 day after cystoscopy, and 6/27 had bacteriuria within 2–10 days after cystoscopy. In the treatment group, 3/27 had bacteriuria 1 day after cystoscopy, and 3/27 had bacteriuria within 2–10 days after cystoscopy Localized symptom How measured: burning or painful urination usually accompanied by frequency and urgency Time points measured: within 10 days after cystoscopy Time points reported: within 10 days after cystoscopy Outcomes: placebo group: 16/37 participants had localized symptom 1 day after cystoscopy, and 9/37 had localized symptom within 2–10 days after cystoscopy; treatment group: 16/38 had localized symptom 1 day after cystoscopy, and 11/38 had localized symptom within 2–10 days after cystoscopy No email address available for contacting the corresponding author for further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Drugs were randomly assigned and administered in a double‐blind fashion." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding the concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Drugs and placebo looked like the same and they were randomly assigned to participants, principle investigator and participants were not able to identify the active drugs or placebo." Comment: treatment providers and participants adequately blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Drugs were randomly assigned and administered in a double‐blind fashion." Comment: double‐blind study, and this probably done. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Low risk | Quote: "In the placebo group, none of 37 had fever 1 day after cystoscopy, and 5 out of 37 had fever within 2 to 10 days after cystoscopy. in the treatment group, 4 out of 38 had fever 1 day after cystoscopy, and 5 out of 38 had fever within 2 to 10 days after cystoscopy (rephrased from table)." Comment: 75/75 randomized participants (37 participants in the control arm, 38 participants in the intervention arm) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "In the placebo group, 17 out of 37 had bacteriuria 1 day after cystoscopy, and 14 out of 37 had bacteriuria within 2 to 10 days after cystoscopy. In the treatment group, 6 out of 38 had bacteriuria 1 day after cystoscopy, and 5 out of 38 had bacteriuria within 2 to 10 days after cystoscopy (rephrased from table)." Comment: 75/75 randomized participants (37 participants in control arm, 38 participants in intervention arm) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Serious adverse events | Low risk | Quote: "In the treatment group, 1 participants had drug eruption and 2 participants had sulfenamide crystals in the urine 1 day after cystoscopy (rephrased from table)." Comment: 75/75 randomized participants (37 participants in control arm, 38 participants in intervention arm) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Minor adverse events | Low risk | Quote: "In the treatment group, 1 participants had drug eruption and 2 participants had sulfenamide crystals in the urine 1 day after cystoscopy (rephrased from table)." Comment: 75/75 randomized participants (37 participants in control arm, 38 participants in intervention arm) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Cam 2009.
| Methods |
Study design: parallel‐group, prospective randomized trial Study dates: not available Setting: 1 hospital Country: Turkey |
|
| Participants |
Inclusion criteria: people undergoing diagnostic cystoscopy for check‐up of a superficial bladder tumour; people with subsequent incidental interventions, including punch biopsy and transurethral resection of a small bladder tumour; people with initial negative urine cultures Exclusion criteria: used antibiotics for any reason during last month. Moreover, people requiring antibiotic prophylaxis for infective endocarditis or those with positive urine cultures were excluded Sample size: 200 participants randomized Age (years): mean control group: 56.3 (SD 5.4); mean prophylaxis group: 58.9 (SD 5.2) Sex: control group: 59 men and 41 women; prophylaxis group: 62 men and 38 women |
|
| Interventions |
Group 1 (n = 100): antibiotic prophylaxis, intravenous, single dose 1 g, at the time of induction of anaesthesia Group 2 (n = 100): no antibiotic prophylaxis |
|
| Outcomes |
Systemic UTI How measured: participants had a follow‐up visit at the first month after cystoscopy; clinical parameters including fever, dysuria, and frequency evaluated Time points measured: within 30 days after cystoscopy Time points reported: within 30 days after cystoscopy Outcomes: no fever or any other severe symptom was detected in any participant Localized UTI How measured: participants with positive urine cultures had complaints of dysuria and frequency Time points measured: urine culture tested 1 day after cystoscopy Time points reported: not available Outcomes: placebo group: 1/100 had localized symptom and positive urine culture 1 day after cystoscopy; treatment group: 1/100 had localized symptom and positive urine culture 1 day after cystoscopy Asymptomatic bacteriuria How measured: participants with positive urine culture and no symptoms Time points measured: urine culture tested 1 day after cystoscopy Time points reported: not reported Outcomes: control group: 1/100 had asymptomatic bacteriuria; treatment group: 0/100 had asymptomatic bacteriuria |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | Used rigid cystoscopes. 2 participants, 1 from each group, had dysuria without associated positive culture results. We tried to contact corresponding author about allocation concealment, but we received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using tables of random numbers and using a block randomization." Comment: random sequence generation performed adequately. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The first group received no antibiotic prophylaxis, the second group had a single dose of intravenous cefoperazone (1 g)." Comment: participants in control group were not administered antibiotic prophylaxis, while the treatment group received intravenously antibiotic prophylaxis. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "The first group received no antibiotic prophylaxis, the second group had a single dose of intravenous cefoperazone (1 g)." Comment: participants were not blinded to their treatment. Risk of detection bias for systemic UTI, localized UTI and asymptomatic bacteriuria was high. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Low risk | Quote: "No statistical difference was detected regarding age and gender between the groups, two groups were similar with regard to the distribution of cystoscopy indications." Comment: 200/200 randomized participants (100 participants in each arm) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "No statistical difference was detected regarding age and gender between the groups, two groups were similar with regard to the distribution of cystoscopy indications." Comment: 200/200 randomized participants (100 participants in each arm) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Low risk | Quote: "No statistical difference was detected regarding age and gender between the groups, two groups were similar with regard to the distribution of cystoscopy indications." Comment: 200/200 randomized participants (100 participants in each arm) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Low risk | Quote: "No statistical difference was detected regarding age and gender between the groups, two groups were similar with regard to the distribution of cystoscopy indications." Comment: 200/200 randomized participants (100 participants in each arm) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Garcia‐Perdomo 2013.
| Methods |
Study design: multicentre randomized controlled trial Study dates: 1 March 2011 to 30 April 2012 Setting: performed in 2 cities in Colombia (Cali and Bogota). The participating centres were: Urological Salus Clinic (Cali), Hospital Universitario San Ignacio (Bogota), ESENSA Foundation (Cali), Farallones Maternal and Child Clinic (Cali), and Colsubsidio Clinic (Bogota) Country: Colombia |
|
| Participants |
Inclusion criteria: men and women aged ≥ 18 years undergoing cystoscopy for any non‐urgent indication on an outpatient basis; negative urine culture results before the procedure and provided written informed consent prior to participation. Exclusion criteria: participants who could not be followed up; allergy to antibiotics; taking other medications which could interact with the study drugs or for the purpose of prophylaxis for other health conditions (e.g. prosthetic heart valve, heart murmur, prosthetic orthopaedic, or vascular); taking antibiotics at the time of the procedure; history of permanent urethral catheter; immunosuppression; spinal cord injury requiring intermittent catheterization; or required a urethral catheter after the study procedure. Sample size: 290 participants included and 285 participants randomized. Age (years): mean placebo group: 59 (SD 14.8): mean treatment group: 58 (SD 15.4). Sex: placebo group: 94 men and 44 women; treatment group: 90 men and 48 women. |
|
| Interventions |
Group 1 (n = 138): placebo tablet similar in appearance to antibiotic administered to the treatment group administered 30–60 minutes before procedure Group 2 (n = 138): oral levofloxacin 500 mg administered 30–60 minutes before procedure |
|
| Outcomes |
Symptomatic UTI How measured: presence of irritative symptoms of UTI with a positive urine culture > 105 CFU/mL Time points measured: 3rd–10th day after procedure Time points reported: not available Outcomes: placebo group: 4/138 had UTI after cystoscopy; treatment group: 1/138 had UTI after cystoscopy Asymptomatic bacteriuria How measured: positive urine culture > 105 CFU/mL for 1 micro‐organism in a midstream sample of urine, without systemic symptoms or irritative symptoms of the urinary tract Time points measured: 3rd–10th day after procedure Time points reported: not reported Outcomes: placebo group: 20/138 had bacteriuria after cystoscopy; treatment group: 8/138 had bacteriuria after cystoscopy Minor adverse events How measured: questionnaire Time points measured: 3rd–10th day after procedure Time points reported: not reported Outcomes: control group: 1/138 participants had pruritus; treatment group: 1/138 participants had nausea |
|
| Funding sources | No information about funding | |
| Declarations of interest | No conflict of interest with any of the researchers involved in study. | |
| Notes | Information that rigid cystoscope was used for examination and some participants required manipulation during cystoscope were also included was obtained from corresponding author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Permuted block randomization with variable sized blocks to ensure a similar number of participants in each group." Comment: random sequence generation performed adequately. |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment assignments were kept in sealed, opaque, consecutively numbered envelopes, which were opened in the order of participant arrival at each center in order to conceal the allocation to which study group each patient would be assigned." Comment: allocation concealment performed adequately. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, researchers, and treating physicians were blinded to whether or not participants received antibiotics or placebo. The placebo tablet of identical presentation and weight to levofloxacin 500 mg tablet." Comment: blinding of participants and personnel performed adequately. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Principal investigator could not identify participants received the active drug or not, and participants were blinded about their treatments." Comment: detection bias for symptomatic UTI, asymptomatic bacteriuria, and minor adverse events low due to proper blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "285 patients were randomized. No urine culture was performed for 9 (3.2 %) patients after the procedure (3 patients in the antibiotic group and 6 patients in the placebo group). There were no significant differences between the nine patients lost to follow‐up and those who remained in the study. The analyses include 138 patients in each study arm." Comment: overall, loss to follow‐up was less and their reasons given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Serious adverse events | Low risk | Quote: "285 patients were randomized. No urine culture was performed for 9 (3.2 %) patients after the procedure (3 patients in the antibiotic group and 6 patients in the placebo group). There were no significant differences between the nine patients lost to follow‐up and those who remained in the study. The analyses include 138 patients in each study arm." Comment: overall, loss to follow‐up was less and their reasons given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Minor adverse events | Low risk | Quote: "285 patients were randomized. No urine culture was performed for 9 (3.2 %) patients after the procedure (3 patients in the antibiotic group and 6 patients in the placebo group). There were no significant differences between the nine patients lost to follow‐up and those who remained in the study. The analyses include 138 patients in each study arm." Comment: overall, loss to follow‐up was less and their reasons given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Low risk | Quote: "285 patients were randomized. No urine culture was performed for 9 (3.2 %) patients after the procedure (3 patients in the antibiotic group and 6 patients in the placebo group). There were no significant differences between the nine patients lost to follow‐up and those who remained in the study. The analyses include 138 patients in each study arm." Comment: overall, loss to follow‐up was less and their reasons given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: trial was publicly registered in the Australian New Zealand Clinical Trials Registry ACTRN12611000750987. All prespecified outcomes in protocol were reported. |
| Other bias | Low risk | Comment: no other bias detected. |
Goh 1982.
| Methods |
Study design: randomized controlled trial Study dates: not reported Setting: not reported Country: Singapore |
|
| Participants |
Inclusion criteria: attending for check cystoscopy for previous bladder neoplasms, or for primary investigation of haematuria Exclusion criteria: not reported Sample size: 420 participants randomized and 31 participants had bacteriuria present at cystoscopy and were excluded. Age (years): mean control group: 66.5 (SD 15.64); mean treatment group: 63.2 (SD 14.43) Sex: not reported |
|
| Interventions |
Trial A Group 1 (n = 111): no antibiotic prophylaxis Group 2 (n = 93): 2 tablets each containing trimethoprim 80 mg + sulphamethoxazole 400 mg twice daily for 2 days after cystoscopy Trial B Group 3 (n = 95): no antibiotic prophylaxis Group 4 (n = 90): 1 tablet containing trimethoprim 160 mg + sulphamethoxazole 800 mg, taken once after cystoscopy |
|
| Outcomes |
[Bacteriuria] How measured: urine samples with count of organisms > 105 CFU/mL Time points measured: midstream urine samples 5 days after cystoscopy Time points reported: not reported Outcomes: trial A: control group: 34/111 participants had bacteriuria after cystoscopy; treatment group: 5/93 had bacteriuria after cystoscopy; trial B: control group: 17/95 had bacteriuria after cystoscopy; treatment group: 5/90 had bacteriuria after cystoscopy |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | 1 participant developed Escherichia coli septicaemia, an incidence of 0.2%, from the trial B control group with no existing bacteriuria. No email address available for contacting the corresponding author for further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In both trials patients were randomly allocated into a control group and a study group." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study group took a standard preparation of co‐trimoxazole, two tablets each containing trimethoprim 80 mg and sulphamethoxazole 400 mg twice daily for two days post‐cystoscopy. The control group received no antibiotics." Comment: participants in the control group were not administered antibiotic prophylaxis, while the treatment group receive tablets. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: systemic and localized symptoms after cystoscopy; adverse events not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was primary outcome. Result obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Hares 1981.
| Methods |
Study design: randomized control study Study dates: not reported Setting: 1 centre Country: UK |
|
| Participants |
Inclusion criteria: all participants entering hospital for cystoscopy over the trial period Exclusion criteria: indwelling catheter left in situ following cystoscopy, urine infection on admission Sample size: 122 cystoscopies were performed on 112 participants, 10 cases were excluded from analysis, 112 cystoscopies included for analysis Age (years): median: control group: 66; treatment group: 64 Sex: 79 men and 33 women |
|
| Interventions |
Group 1 (n = 56): bladder irrigation with no antibiotic solution added Group 2 (n = 56): bladder irrigation with 6 vials of Polybactrin Soluble GU containing polymyxin B sulphate 450,000 units, neomycin sulphate 120,000 units, and bacitracin 6000 units to each bag of irrigating fluid |
|
| Outcomes |
Symptomatic UTI How measured: urine samples with a count of micro‐organisms > 105 CFU/mL, or confluent or semi‐confluent growth of micro‐organisms on the dip slides, together with symptoms of cystitis and a sterile pyuria on a subsequent midstream specimen of urine Time points measured: on morning after the cystoscopy, a mid‐stream sample of urine was taken. After discharge, the participant was asked to provide dip slides on the 3rd, 7th and 14th day after cystoscopy. These were sent to the laboratory by first class post Time points reported: not reported Outcomes: control group: 5/56 participants had symptomatic UTI after cystoscopy; treatment group: 1/56 had symptomatic UTI after cystoscopy Asymptomatic bacteriuria How measured: urine samples with a count of micro‐organisms > 105 CFU/mL, or confluent or semi‐confluent growth of organisms on the dip slides, but without any symptoms Time points measured: midstream urine samples 5 days after cystoscopy Time points reported: not reported Outcomes: control group: 4/56 participants had asymptomatic bacteriuria after cystoscopy; treatment group: 2/56 had asymptomatic bacteriuria UTI after cystoscopy |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | No email address available for contacting the corresponding author for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to either the control group or the treatment group by the theatre sister." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding the concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The composition of fluid was only known by the pharmacists, participants were randomly allocated to either the control or the treatment group." Comment: participants and personnel blinded to intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "The composition of fluid was only known by the pharmacists, participants were randomly allocated to either the control or the treatment group." Comment: blinding of participants and personnel performed adequately. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "122 cystoscopies were randomized, six of the cystoscopy results were removed from the trial because the participants failed to return sufficient dip slides or urine specimens, 4 cases where the urine was infected on admission were also excluded but their follow up results were reported, 112 cases (56 participants in each group) were analyzed." Comment: overall, loss to follow‐up was less and their reasons given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Low risk | . Quote: "122 cystoscopies were randomized, six of the cystoscopy results were removed from the trial because the participants failed to return sufficient dip slides or urine specimens, 4 cases where the urine was infected on admission were also excluded but their follow up results were reported, 112 cases (56 participants in each group) were analyzed." Comment: overall, loss to follow‐up was less and their reasons given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Hart 1980.
| Methods |
Study design: randomized control study Study dates: not reported Setting: not reported Country: UK |
|
| Participants |
Inclusion criteria: consecutive participants in day‐bed unit for urological endoscopy Exclusion criteria: history of allergy or drug reaction, and required antibiotic treatment for confirmed existing infection Sample size: 690 participants who were consecutive admissions to day‐bed unit for urological endoscopy were randomized Age: not reported Sex: trial A: control group: 105 men and 38 women; treatment group: 85 men and 46 women; trial B: control group: 125 men and 55 women; treatment group: 108 men and 60 women |
|
| Interventions |
Trial A Group 1 (n = 131): cephazolin sodium 1 g in 10 mL water intravenously following induction of anaesthesia Group 2 (n = 143): no treatment Trial B Group 3 (n = 168): cephazolin sodium 1 g in 2 mL water intramuscularly at time of procedure Group 4 (n = 180): inert placebo intramuscularly |
|
| Outcomes |
Bacteriuria How measured: culturing a mid‐stream specimen of urine by the dip‐slide (Oxoid) technique before their discharge from the unit Time points measured: about 4 hours after endoscopy Time points reported: not reported Outcomes: trial A: 5/68 participants in control group and 1/66 in treatment group had bacteriuria; trial B: 18/179 in control group and 1/177 in treatment group had bacteriuria |
|
| Funding sources | Drug and financial assistance received from Eli LiIly and Co Ltd | |
| Declarations of interest | No information about conflict and interest | |
| Notes | The overall incidence of rigors after cystoscopy was reported. In trial A, 17/143 participants in control group and 18/131 in treatment group had rigors after cystoscopy. In trial B, 29/180 participants in control group and 12/168 in treatment group had rigors after cystoscopy. Medical practitioner call‐out after cystoscopy: trial A: 6/143 participants in control group and 5/131 in treatment group needed medical practitioner call‐out; trial B: 12/180 in control group and 6/168 in treatment group need medical practitioner call‐out. No email address available for contacting the corresponding author for further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In Group I, 300 patients were randomly assigned to receive…(Group II) were randomly assigned to receive either…" Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "patients were randomly assigned to receive either 1 g cephazolin sodium in 10 ml water intravenously following induction of anaesthesia, or no treatment." Comment: participants in control group did not receive antibiotics, while the treatment group received intravenous antibiotic. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: systemic and localized symptoms after cystoscopy; adverse events not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was regarded as the primary outcome, the result was obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Unclear risk | Quote: "Evaluation of bacteriuria and symptoms post cystoscopy were conducted in the first 24 hours after cystoscopy." Comment: bacteriuria evaluated just within 24 hours; unsure whether this would introduce any bias. |
Jimenez 1993.
| Methods |
Study design: multicenter, prospective, comparative, randomized study Study dates: not reported Setting: 9 hospitals Country: Spain |
|
| Participants |
Inclusion criteria: participants > 16 years of age and had prescanning, negative urine culture, undergoing diagnostic cystoscopy Exclusion criteria: microbiological UTI (treated or antimicrobial) showed, with catheter before exploration or post cystoscopy 24–48 hours; and need for concomitant therapy with other antimicrobial Sample size: 2284 outpatients from 9 hospitals randomized and 105 participants excluded due to failure to meet inclusion criteria Age: not reported Sex: 2172 participants included, 70% men and 30% women |
|
| Interventions |
Group 1 (n = 1087): no antimicrobial prophylaxis before implementation Group 2 (n = 1197): intramuscular ceftriaxone 1 g prior to endoscopic examination |
|
| Outcomes |
Symptomatic UTI How measured: not reported Time points measured: urine culture, clinical and microbiological response assessed 48–72 hours and 4 weeks after instrumentation Time points reported: not reported Outcomes: 108/1057 participants in control group and 28/1115 in treatment group had symptomatic bacteriuria Asymptomatic bacteriuria How measured: not reported Time points measured: urine culture, clinical and microbiological response assessed 48–72 and 4 weeks after instrumentation Time points reported: not reported Outcomes: 32/1057 participants in control group and 17/1115 in treatment group had asymptomatic bacteriuria |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | Irritative syndrome with sterile urine found in 31/1057 participants in control group and 29/1115 in treatment group. No email address available for contacting the corresponding author for further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized into two groups." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Group 1 received no antimicrobial prophylaxis before implementation, being considered as a control. Group 2 were given an intramuscular dose of I gr [gram], prior to endoscopic examination ceftriaxone, constituting these the prophylaxis group." Comment: participants in the control group did not receive antibiotics, while participants in the treatment group received intramuscular ceftriaxone. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "Group 1 received no antimicrobial prophylaxis before implementation, being considered as a control. Group 2 were given an intramuscular dose of I gr, prior to endoscopic examination ceftriaxone, constituting these the prophylaxis group." Comment: participants were not blinded to their intervention. Risk of detection bias for symptomatic UTI and asymptomatic bacteriuria was high. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Quote: "2,284 out‐participants were randomized (1087 participants in control group, 1197 participants in intervention group), 2172 participants were finally included for analysis of this outcome, 105 participants were excluded after randomization due to failure to meet inclusion criteria." Comment: overall, loss to follow‐up was less and balanced between groups; however, the reason for 7 participants who were not included in the analysis was not given. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Quote: "2,284 out‐participants were randomized (1087 participants in control group, 1197 participants in intervention group), 2172 participants were finally included for analysis of this outcome, 105 participants were excluded after randomization due to failure to meet inclusion criteria." Comment: overall, loss to follow‐up was less and balanced between groups; however, the reason for 7 participants who were not included for analysis was not given. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Jimenez‐Pacheco 2012.
| Methods |
Study design: unblinded, randomized, controlled study Study dates: March–August 2011 Setting: Urology Department, Santa Ana Hospital de Motril Country: Spain |
|
| Participants |
Inclusion criteria: diagnostic flexible cystoscopy indication, aged ≥ 18 years Exclusion criteria: antibiotic administration for any reason during the previous month; urethral catheterization during previous month or at the moment of intervention; history of UTI during previous month; positive culture; pregnancy; ≥ 2 UTI episodes during last 3 months; obstructive uropathy diagnosis with residual urine > 100 mL; unilateral or bilateral vesicoureteral reflux; neurogenic bladder or any lower urinary system malformation; intermittent or urethral permanent catheterization; risk of endocarditis (participants with prosthetic cardiac or vascular valves, etc); and hypersensitivity to fosfomycin Sample size: 60 participants Age (years): mean: control group: 65.4; treatment group: 64.6 Sex: 27 men and 3 women in control group; 25 men and 5 women in treatment group |
|
| Interventions |
Group 1 (n = 30): no antibiotics after flexible cystoscopy Group 2 (n = 30): oral single dose of fosfomycin trometamol 3 g during 2 hours prior to test |
|
| Outcomes |
Symptomatic UTI How measured: 10 days after cystoscopy, urine culture and urinalysis performed, bacteriuria considered when > 105 CFU/mL were recorded in urinalysis. 1 month later, a telephonic questionnaire performed to evaluate lower urinary tract symptoms regardless of bacteriuria Time points measured: urine culture performed 10 days after cystoscopy, symptoms evaluated 1 month after cystoscopy Time points reported: not reported Outcomes: 2/30 participants in control group and 3/30 in treatment group had symptomatic bacteriuria Asymptomatic bacteriuria How measured: 10 days after cystoscopy, urine culture and urinalysis performed, bacteriuria considered when > 105 CFU/mL were recorded in urinalysis. 1 month later, a telephonic questionnaire performed to evaluate lower urinary tract symptoms regardless of bacteriuria Time points measured: urine culture performed 10 days after cystoscopy, symptoms evaluated 1 month after cystoscopy Time points reported: not reported Outcomes: 1/30 participants in control group and 0/30 in treatment group had asymptomatic bacteriuria |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | We tried to contact corresponding author regarding random sequence generation method, but received no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sixty patients were distributed in two groups by random assignment." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequence was kept hidden to the responsible conductor of assignments just before the moment of intervention." Comment: allocation concealment performed adequately. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Control did not receive any dose of antibiotics after the test, treatment was given antibiotic prophylaxis: three grams as oral single‐dose of fosfomycin trometamol, during the first two hours previous to the test." Comment: participants in control group did not receive antibiotics before cystoscopy, while participants in the treatment group receive oral single dose of fosfomycin trometamol 3 g. Unlikely that participants and personnel were blinded to intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "Control did not receive any dose of antibiotics after the test, treatment was given antibiotic prophylaxis: three grams as oral single‐dose of fosfomycin trometamol, during the first two hours previous to the test." Comment: participants not blinded to their intervention. Risk of detection bias for symptomatic UTI and asymptomatic bacteriuria was high. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "No statistically significant differences were observed regarding the distribution of most baseline variables between both groups." Comment: 60/60 randomized participants (30 participants in each group) were included for analysis of this outcome, 5 participants were lost to follow‐up 1 month later for evaluation of lower urinary tract symptoms, intention‐to‐treat analysis performed for symptom analysis. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | .Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Low risk | Quote: "No statistically significant differences were observed regarding the distribution of most baseline variables between both groups." Comment: 60/60 randomized participants (30 participants in each group) were included for analysis of this outcome, 5 participants were lost to follow‐up 1 month later for evaluation of lower urinary tract symptoms, intention‐to‐treat analysis performed for symptom analysis |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Johnson 2007.
| Methods |
Study design: 3‐arm placebo randomized controlled trial Study dates: July 1999 to September 2002 Setting: Freeman Hospital, Newcastle Country: UK |
|
| Participants |
Inclusion criteria: adults undergoing cystoscopy for diagnostic or surveillance. Exclusion criteria: symptoms of UTI on day of investigation; hypersensitivity to ciprofloxacin or trimethoprim, potential interaction with other drugs or contraindications; specific indication for parenteral prophylaxis; presence of a urethral catheter. Sample size: 2481 participants entered study; 2083 completed study; 398 were randomized but did not complete study. Age: not reported Sex: not reported |
|
| Interventions |
Group 1 (n = 684): placebo, oral, 1 hour before the planned procedure Group 2 (n = 712): trimethoprim 200 mg, oral, 1 hour before the planned procedure Group 3 (n = 687): ciprofloxacin 500 mg, oral, 1 hour before the planned procedure |
|
| Outcomes |
[Symptomatic UTI] How measured: midstream specimen of urine returned for analysis 5 days after cystoscopy. Significant bacteriuria defined as pure growth of > 105 CFU/mL. Participants completed a questionnaire to determine the presence of symptoms that were associated with bacteriuria. These were then classified as: 0 = asymptomatic significant bacteriuria; 1 = mild (presence of dysuria plus significant bacteriuria); 2 = moderate (presence of dysuria and loin pain plus significant bacteriuria); 3 = severe (any combination of the above plus rigors or admission to hospital for infection) Time points measured: 5 days after cystoscopy Time points reported: not reported Outcomes: 16/684 participants in placebo group, 10/712 in the trimethoprim group, and 6/687 in ciprofloxacin group had symptomatic bacteriuria Asymptomatic bacteriuria How measured: midstream specimen of urine returned for analysis 5 days after cystoscopy. Significant bacteriuria defined as pure growth of > 105 CFU/mL. Participants completed a questionnaire to determine the presence of symptoms that were associated with bacteriuria. These were then classified as: 0 = asymptomatic significant bacteriuria; 1 = mild (presence of dysuria plus significant bacteriuria); 2 = moderate (presence of dysuria and loin pain plus significant bacteriuria); 3 = severe (any combination of the above plus rigors or admission to hospital for infection). Time points measured: 5 days after cystoscopy Time points reported: not reported Outcomes: 44/684 participants in placebo group, 23/712 in trimethoprim group, and 12/687 in ciprofloxacin group had asymptomatic bacteriuria Bacterial resistance How measured: midstream specimen of urine returned for analysis 5 days after cystoscopy Time points measured: bacteria cultures from urine performed 5 days after cystoscopy Time points reported: not reported Outcomes: organism was resistant to the antibiotic in 9/22 (41%) participants who received trimethoprim and in 4/28 (14%) who received ciprofloxacin. |
|
| Funding sources | Trial funded by NHS R&D programme (Northern and Yorkshire) and the Newcastle upon Tyne Trustees. | |
| Declarations of interest | None of the authors had a financial or other conflict of interest. | |
| Notes | Information about randomization and allocation method were obtained by contacting corresponding author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done in the pharmacy using random‐number tables." Comment: method for generation of random sequence was performed adequately. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was allocated by cards in plain envelopes." Comment: allocation concealment was performed adequately. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were given a container by nurse, each container was numbered and held an oral preparation of either placebo, trimethoprim 200 mg or ciprofloxacin 500 mg. The hospital pharmacy alone held the code allowing identification of the contents." Comment: blinding of participants and personnel performed adequately. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "The hospital pharmacy alone held the code allowing identification of the contents." Comment: participants and personnel were blinded to the intervention. Risk of detection bias for symptomatic UTI and asymptomatic bacteriuria was low. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: since bacterial resistance was evaluated by urine culture from laboratory, results were objective and probably not influenced by blinding or not. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "2083 out of 2481 randomized participants (684/830 in the placebo group, 712/829 in the trimethoprim group, and 687/822 in the ciprofloxacin group) completed the study and included for analysis of this outcome. 398 participants were randomized but did not complete the study, the reasons for lost to follow up or discontinued intervention were given." Comment: overall, the loss to follow‐up was less and their reasons were given; therefore judged adequate. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Low risk | Quote: "2083 out of 2481 randomized participants (684/830 in the placebo group, 712/829 in the trimethoprim group, and 687/822 in the ciprofloxacin group) completed the study and included for analysis of this outcome. 398 participants were randomized but did not complete the study, the reasons for lost to follow up or discontinued intervention were given." Comment: overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Low risk | Quote: "2083 out of 2481 randomized participants (684/830 in the placebo group, 712/829 in the trimethoprim group, and 687/822 in the ciprofloxacin group) completed the study and included for analysis of this outcome. 398 participants were randomized but did not complete the study, the reasons for lost to follow up or discontinued intervention were given." Comment: overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Selective reporting (reporting bias) | Low risk | Comment: trial performed according to the registered protocol (ISRCTN37802560); all prespecified outcomes in the protocol were reported in the published paper. |
| Other bias | Low risk | Comment: no other bias detected. |
Karmouni 2001.
| Methods |
Study design: randomized controlled study Study dates: not reported Setting: not reported Country: France |
|
| Participants |
Inclusion criteria: absence of infection before cystoscopy (negative urine dipstick), and patient consent. Exclusion criteria: need to use antibiotics for reducing the risk of endocarditis; having a J stent or catheter due to increased risk of infection related to the presence of the catheter; reason for undergoing cystoscopy was to monitor bladder tumours; balance of unexplained urinary tract symptoms, haematuria, and incontinence. Sample size: 126 participants Age (years): mean: 66 (range 23–81) Sex: 74 men and 52 women |
|
| Interventions |
Group 1 (n = 67): norfloxacin 400 when preparing for cystoscopy Group 2 (n = 59): no drugs |
|
| Outcomes |
Asymptomatic bacteriuria How measured: infection was retained when the bacteriuria ≥ 105 CFU/mL Time points measured: urine culture 3 days after the examination Time points reported: not reported Outcomes: 3/59 participants in control group and 2/67 in treatment group had asymptomatic bacteriuria Bacterial resistance How measured: midstream specimen of urine returned for analysis 5 days after cystoscopy Time points measured: bacteria cultures from urine performed 5 days after cystoscopy Time points reported: not reported Outcomes: 1 multiresistant Citrobacter was hospital‐type |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | We tried to contact corresponding author regarding random sequence generation and allocation method, and some further results about drug resistance, but received no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 126 patients were randomized into 2 groups." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding the concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Group 1 receiving 400 mg of norfloxacin when preparing to cystoscopy, Group 2 not receiving." Comment: participants in control group did not receive antibiotics, while participants in treatment group received norfloxacin. Considered unlikely that participants and personnel were blinded to intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "Group 1 receiving 400 mg of norfloxacin when preparing to cystoscopy, Group 2 not receiving." Comment: participants not blinded to their treatment. Risk of detection bias for asymptomatic bacteriuria was high. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: since bacterial resistance was evaluated by urine culture from laboratory, results were objective and probably not influenced by blinding. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | .Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Low risk | Quote: "The overall incidence of infection after cystoscopy was 4% (5/126). In all cases it was asymptomatic bacteriuria." Comment: 126/126 randomized participants (59 participants in control group, 67 participants in intervention group) were included for analysis of this outcome, the risk of attrition bias was thus low. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Low risk | Quote: "The bacteria isolated were four times from the Community, only one multiresistant citrobacter was hospital‐type." Comment: 126/126 randomized participants (59 participants in control group, 67 participants in intervention group) were included for analysis of this outcomes, the risk of attrition bias was thus low. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
MacDermott 1988.
| Methods |
Study design: randomized controlled study Study dates: not reported Setting: not reported Country: UK |
|
| Participants |
Inclusion criteria: participants undergoing cystoscopy for the diagnosis, transurethral resection, or follow‐up of transitional cell carcinoma of the bladder Exclusion criteria: history of sensitivity to cephalosporins or penicillins, antibiotics or indwelling catheters in the 7 days preoperatively Sample size: 243 participants entered trial, 26 excluded. Preoperative urine specimens were infected in 28 participants and these were studied as 1 group. Remaining 189 participants divided into 4 groups, depending on preoperative randomization and findings at cystoscopy Age (years): mean: group 1: 68.4 (range 44–81); group 2: 67.7 (range 25–84); group 3: 69.7 (range 52–83); group 4: 67.4 (range 52–82) Sex: not reported |
|
| Interventions |
Group 1 (n = 47): cystoscopy showed no bladder tumour recurrence, and participants received cephradine 1 g intramuscularly 6 hours preoperatively, 1 g intravenously on induction of the general anaesthetic, and 1 g orally 12 hours postoperatively Group 2 (n = 51): no antibiotic Group 3 (n = 44): cystoscopy showed new or recurrent bladder tumour recurrence, and participants received cephradine 1 g intramuscularly 6 hours preoperatively, 1 g intravenously on induction of the general anaesthetic, and 1 g orally 12 hours postoperatively. Participants had either cystodiathermy or transurethral resection of their bladder tumours Group 4 (n = 47): no antibiotic. Participants had either cystodiathermy or transurethral resection of their bladder tumours |
|
| Outcomes |
Bacteriuria How measured: infection defined as a pure culture > 105 CFU/mL Time points measured: urine specimens collected prior to first dose of antibiotic, on passing the cystoscope and at 5 days postoperatively Time points reported: not reported Outcomes: group 1: 1/47 participants had bacteriuria; group 2: 8/51 had bacteriuria; group 3: 2/44 had bacteriuria; group 4: 8/47 had bacteriuria Bacterial resistance How measured: not reported Time points measured: urine specimens collected prior to the first dose of antibiotic, on passing the cystoscope and at 5 days postoperatively Time points reported: not reported Outcomes: group 1: 0 participants with bacteriuria were resistant to cephradine; group 2: 2/8 with bacteriuria were resistant to cephradine; group 3: 2 participants with bacteriuria were resistant to cephradine; group 4: 2/8 participants with bacteriuria were resistant to cephradine Adverse events How measured: not reported Time points measured: not reported Time points reported: not reported Outcomes: no adverse effects reported |
|
| Funding sources | E. R. Squibb and Sons Ltd for assistance with the funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | 28 participants had infection preoperatively,13 were randomized to receive cephradine and 15 to receive no antibiotics. The postoperative urine specimens showed the infection had cleared in 3 participants who had received only the protocol doses of cephradine. All of the other participants in this group required further treatment for their infections. No email address available for contacting the corresponding author for further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated into two groups." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding the concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Control group received no antibiotic, the trial group received cephradine 1 g intramuscularly 6 h [hours] pre‐operatively, 1 g intravenously on induction of the general anaesthetic and 1 g orally 12 h post‐operatively." Comment: participants in the control group received no antibiotic prophylaxis, while the treatment group received intravenously antibiotic prophylaxis. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "Control group received no antibiotic, the trial group received cephradine 1 g intramuscularly 6 h pre‐operatively, 1 g intravenously on induction of the general anaesthetic and 1 g orally 12 h post‐operatively." Comment: participants not blinded to their treatment. Risk of detection bias for adverse effects was high. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacterial resistance and bacteriuria results were obtained from laboratory. Detection bias for these outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Low risk | Quote: "Of the 243 patients entering the trial, 26 were excluded (Table 1). Pre‐operative urine specimens were found to be infected in 28 patients and these were studied as one group. The remaining 189 patients were divided into four groups, depending on the pre‐operative randomisation and the findings at cystoscopy." Comment: 189/243 randomized participants (91 participants in intervention group, 98 in control group) were included for analysis of this outcome. 26 participants excluded with reasons, preoperative urine specimens infected in 28 participants and these were studied as 1 group and analyzed. Overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Minor adverse events | Low risk | Quote: "Of the 243 patients entering the trial, 26 were excluded (Table 1). Pre‐operative urine specimens were found to be infected in 28 patients and these were studied as one group. The remaining 189 patients were divided into four groups, depending on the pre‐operative randomisation and the findings at cystoscopy." Comment: 189/243 randomized participants (91 participants in intervention group, 98 participants in control group) were included for analysis of this outcome. 26 participants excluded with reasons, preoperative urine specimens infected in 28 participants and these were studied as 1 group and analyzed. Overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Low risk | Quote: "Of the 243 patients entering the trial, 26 were excluded (Table 1). Pre‐operative urine specimens were found to be infected in 28 patients and these were studied as one group. The remaining 189 patients were divided into four groups, depending on the pre‐operative randomisation and the findings at cystoscopy." Comment: 189/243 randomized participants (91 participants in intervention group, 98 participants in control group) were included for analysis of this outcome. 26 participants excluded with reasons, preoperative urine specimens infected in 28 participants and these were studied as 1 group and analyzed. Overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Manson 1988.
| Methods |
Study design: randomized controlled study Study dates: May 1986 to June 1987 Setting: outpatient clinic Country: USA |
|
| Participants |
Inclusion criteria: participants undergoing diagnostic cystoscopy Exclusion criteria: requiring therapeutic intervention (i.e. resection of bladder tumour), indwelling catheters or preoperatively infected urine, at risk for subacute bacterial endocarditis Sample size: 168 participants entered study, 138 returned for necessary follow‐up cultures, and others were excluded. Age: not reported Sex: 78 men and 60 women |
|
| Interventions |
Group 1 (n = 72): no antibiotics Group 2 (n = 66): 3‐day course of oral antibiotic. 65 received trimethoprim 160 mg + sulphamethoxazole 800 mg twice a day, 17 received nitrofurantoin 100 mg 4 times a day, and 2 received cephalosporins (owing to known drug sensitivity or allergy) |
|
| Outcomes |
Systemic UTI How measured: participants with symptoms and positive urine cultures, which were considered positive if contained ≥ 105 CFU/mL Time points measured: participants underwent urinalysis and urine culture before the procedure. All participants were instructed to return in 2 weeks for repeat urine cultures. Time points reported: not reported Outcomes: 0 participants in control group had systemic UTI, 1/66 participants in treatment group had symptomatic infection (irritative voiding symptoms and fever) and the urine culture in this participant was positive for Pseudomonas Asymptomatic bacteriuria How measured: urine cultures considered positive if contained ≥ 105 CFU/mL Time points measured: participants underwent urinalysis and urine culture before the procedure. All participants were instructed to return in 2 weeks for repeat urine cultures. Time points reported: not reported Outcomes: 2/72 participants in control group and 0/66 in treatment group had asymptomatic bacteriuria |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | No email address available for contacting the corresponding author for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 168 patients were placed into 2 groups in a prospective randomized fashion." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Group 1 (84 patients) was not given any antibiotics and is presented as the control group. Group 2 (84 patients) received a 3‐day course of an oral antibiotic." Comment: participants in control group did not receive antibiotics, while participants in treatment group received antibiotics. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "Group 1 (84 patients) was not given any antibiotics and is presented as the control group. Group 2 (84 patients) received a 3‐day course of an oral antibiotic." Comment: participants not blinded to their treatment. Risk of detection bias for systemic UTI and asymptomatic bacteriuria after cystoscopy was high. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Low risk | Quote: "Of the 168 patients entered in the study 138 returned for the necessary follow up cultures and the others were excluded from the study." Comment: 138/168 randomized participants (72 participants in control group, 66 participants in intervention group) returned samples for the necessary follow‐up cultures and were included for analysis of all outcomes. 30 participants (12 in control group and 18 in intervention group) failed to return enough samples for evaluation and were excluded from the study. Overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "Of the 168 patients entered in the study 138 returned for the necessary follow up cultures and the others were excluded from the study." Comment: 138/168 randomized participants (72 participants in control group, 66 participants in intervention group) returned samples for the necessary follow‐up cultures and were included for analysis of all outcomes. 30 participants (12 in control group and 18 in intervention group) failed to return enough samples for evaluation and were excluded from the study. Overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Low risk | Quote: "Of the 168 patients entered in the study 138 returned for the necessary follow up cultures and the others were excluded from the study." Comment: 138/168 randomized participants (72 participants in control group, 66 participants in intervention group) returned samples for the necessary follow‐up cultures and were included for analysis of all outcomes. 30 participants (12 in control group and 18 in intervention group) failed to return enough samples for evaluation and were excluded from the study. Overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Martinez Rodriguez 2017.
| Methods |
Study design: randomized controlled study Study dates: June 2015 to May 2016 Setting: not reported Country: Spain |
|
| Participants |
Inclusion criteria: people undergoing diagnostic cystoscopy Exclusion criteria: temporary or permanent urinary stents, procedures that involved bladder biopsies. Sample size: 251 people were recruited, 129 participants in group 1 (no antibiotic treatment) and 117 in group 2 (antibiotic prophylaxis). Remaining 6 participants excluded. Age: not reported Sex: not reported |
|
| Interventions |
Group 1 (n = 129): no antibiotics Group 2 (n = 117): 4 doses norfloxacin 400 mg twice a day for 2 days |
|
| Outcomes |
Positive urine culture How measured: not reported Time points measured: not reported Time points reported: not reported Outcomes: 14/129 participants in control group had positive urine culture, and 8/117 participants in treatment group had positive urine culture |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Conference abstract, and we could not contact authors for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective, randomized, aleatory study was performed." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were aleatoryzaded in two groups: Antibiotic prophylaxis Vs nothing." Comment: participants in control group did not receive antibiotics, while participants in treatment group received antibiotics. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: subjective outcomes of systemic and localized symptoms after cystoscopy; adverse events not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was regarded as the primary outcome; the result was obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Unclear risk | Comment: we could not obtain the full text of the abstract, and we were unable to assess any other bias. |
Mendoza 1971.
| Methods |
Study design: double‐blind, randomized controlled study Study dates: not reported Setting: all participants hospitalized and examined daily Country: USA |
|
| Participants |
Inclusion criteria: not reported Exclusion criteria: severe renal impairment, active severe cystitis, or known allergy to the active drug used Sample size: 2 trials of men were studied. In each trial, 30 participants were treated with an active drug and 30 with a placebo Age: not reported Sex: men |
|
| Interventions |
Trial A Group 1 (n = 30): sulphamethoxypyridazine‐pyridazine, initial dose 1 g, and then 0.5 g, daily for 3 days Group 2 (n = 30): placebo Trial B Group 3 (n = 30): demeclocycline hydrochloride 150 mg 4 times a day for 4 days Group 4 (n = 30): placebo |
|
| Outcomes |
Bacteriuria How measured: positive culture indicating presence of an organism usually considered pathogenic Time points measured: prior to cystoscopic examination and 1, 3, and 4 days after instrumentation each participant had a urine culture Time points reported: not reported Outcomes: in trial A, no statistically significant difference in any category between participants in terms of bacteriuria. In trial B, more cultures remained negative after demeclocycline hydrochloride than after placebo (20/22 vs 11/21). For participants who were initially totally asymptomatic, i.e. had no abnormal clinical symptoms or laboratory findings. In trial A, 6/8 participants in placebo group and 1/11 participants in treatment group had bacteriuria. In trial B, 4/9 participants in placebo group and 0 participants in treatment group had bacteriuria |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | No email address available for contacting the corresponding author for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Active drugs and placebos were administered in capsule form, coded and assigned randomly to patients." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Active drugs and placebos were administered in capsule form, coded and assigned randomly to participants, participants and personnel were likely to be blinded." Comment: blinding of participants and personnel performed adequately. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: subjective outcomes of systemic and localized symptoms after cystoscopy; adverse events not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was regarded as the primary outcome, the result was obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Rané 2001.
| Methods |
Study design: prospective controlled study Study dates: January 1998 to September 1998 Setting: not reported Country: UK |
|
| Participants |
Inclusion criteria: not reported Exclusion criteria: positive precystoscopy urine culture; underwent a biopsy; indwelling catheters; at specific risk of endocarditis; receiving antibiotics for any other reason Sample size: 253 participants Age: not reported Sex: 152 men, 101 women |
|
| Interventions |
Group 1 (n = 82): intramuscular gentamicin 120 mg just prior to commencing cystoscopy Group 2 (n = 80): no antibiotic |
|
| Outcomes |
Bacteriuria How measured: positive culture with ≥ 105 CFU/mL and > 10 white cells/mm3 Time points measured: 1 week following cystoscopy Time points reported: not reported Outcomes: 17/80 participants in control group and 4/82 participants in treatment group had bacteriuria |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | No email address available for contacting the corresponding author for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Participants were prospectively grouped according to their consultant's practice." Comment: detailed method for group classification not given, but group classification according to consultants' practice was at high risk of selection bias. |
| Allocation concealment (selection bias) | High risk | Quote: "Participants were prospectively grouped according to their consultant's practice." Comment: quasi‐randomized method of sequence generation; therefore, at high risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Group A received 120 mg gentamicin (Roussel Laboratories, Uxbridge, UK) intramuscularly just prior to commencing the cystoscopy; group B received no antibiotic." Comment: participants in control group did not receive antibiotics, while participants in treatment group received antibiotics. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: subjective outcomes of systemic and localized symptoms after cystoscopy; adverse events not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was regarded as the primary outcome, the result was obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Rodrigues 1994.
| Methods |
Study design: prospective, randomized study Study dates: February 1992 to February 1993 Setting: outpatient Country: Brazil |
|
| Participants |
Inclusion criteria: negative urine culture harvested 72 hours prior to cystoscopy Exclusion criteria: history of recurrent urinary infections or factors related to urinary infection, such as those with urolithiasis, vesicoureteral reflux, or use of bladder catheters Sample size: 90 participants Age (years): median 58 (range 24–84) Sex: 59 men, 31 women |
|
| Interventions |
Group 1 (n = 30): single‐dose fosfomycin trometamol 3000 mg, 2 hours before procedure Group 2 (n = 30): sulphamethoxazole 800 mg + trimethoprim 160 mg every 12 hours starting 2 hours before procedure and continuing for 3 days Group 3 (n = 30): placebo tablet 2 hours before procedure |
|
| Outcomes |
Bacteriuria How measured: positive urine cultures ≥ 105 CFU/mL in asymptomatic men and women or 103 CFU/mL in symptomatic women Time points measured: all participants underwent clinical evaluation and urine cultures of control 30 days after procedure Time points reported: not reported Outcomes: 2/30 participants in group 1, 3/30 participants in group 2, and 8/30 participants in group 3 had bacteriuria Adverse events How measured: not reported Time points measured: not reported Time points reported: not reported Outcomes: adverse effects were identified in 3 participants, all belonging to group 2, 2 cases with epigastric pain and 1 case of hives, in the latter being necessary to interrupt the antibiotic prophylaxis and administration of antihistamine |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | No email address available for contacting the corresponding author for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized into three equal groups." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "3,000 mg of fosfomycin trometamol in single dose (Group I); 800 mg of sulfamethoxazole and trimethoprim 160 mg every 12 hours continuing for three days (Group II); a tablet of placebo (Group III)." Comment: no information about whether the placebo and antibiotics had the same appearance or whether participants could see a difference between them. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no information about whether investigators were blinded to the investigation. Detection bias for adverse events was unclear. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was regarded as the primary outcome, the result was obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Low risk | Quote: "Side effects were identified in three patients, all belonging to Group II." Comment: 90/90 randomized participants (30 in control group, 60 in intervention group) included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Minor adverse events | Low risk | Quote: "Side effects were identified in three patients, all belonging to Group II." Comment: 90/90 randomized participants (30 in control group, 60 in intervention group) were included for analysis of this outcome. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Si 1997.
| Methods |
Study design: randomized controlled study Study dates: January 1990 to December 1994 Setting: not reported Country: China |
|
| Participants |
Inclusion criteria: not reported Exclusion criteria: not reported Sample size: 252 cystoscopes performed on 206 participants Age: not reported Sex: 140 men, 61 women |
|
| Interventions |
Group 1 (n = 64): simple cystoscopy examination without other manipulation. No antibiotic Group 2 (n = 52): simple cystoscopy examination without other manipulation. Norfloxacin 400 mg 2 hours before cystoscopy, then 200 mg twice after cystoscopy, interval 6 hours Group 3 (n = 41): cystoscopy examination with manipulations, e.g. biopsy. No antibiotic Group 4 (n = 44): cystoscopy examination with manipulations, e.g. biopsy. Norfloxacin, 400 mg 2 hours before cystoscopy, then 200 mg twice after cystoscopy, interval 6 hours |
|
| Outcomes |
Bacteriuria How measured: positive urine cultures ≥ 105 CFU/mL or > 5 white blood cells/high power field in microscope Time points measured: urine cultures before cystoscopy and 3 days after procedure Time points reported: not reported Outcomes: 3/64 participants in group 1, 2/52 in group 2, 7/41 in group 3, and 2/41 in group 4 had bacteriuria |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | No email address available for contacting the corresponding author for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned into groups." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding the concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants in the treatment group received 400mg norfloxacin, while the control group received no antibiotic." Comment: participants in control group did not receive antibiotics, while participants in treatment group received antibiotics. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: subjective outcomes of systemic and localized symptoms after cystoscopy; adverse events not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was regarded as the primary outcome, the result was obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Soydan 2012.
| Methods |
Study design: randomized controlled study Study dates: not reported Setting: not reported Country: Turkey |
|
| Participants |
Inclusion criteria: cystoscopy examination for any reason Exclusion criteria: not reported Sample size: 90 cases were included to study, but 65 cases who had urine culture results before and after cystoscopy were evaluated. Age: not reported Sex: not reported |
|
| Interventions |
Group 1 (n = 20): gentamycin 80 mg Group 2 (n = 28): fosfomycin 3 g Group 3 (n = 17): no antibiotic |
|
| Outcomes |
Positive urine culture How measured: not reported Time points measured: not reported Time points reported: not reported Outcomes: before cystoscopy in 3 cases urine culture was positive: 1 Escherichia coli (received gentamycin), 2 Klebsiella pneumonia (received fosfomycin). After cystoscopy in 1 case, no positive urine culture and received gentamycin before cystoscopy, urine culture was positive. 5 participants had manipulation during cystoscopy, and 0 of these cases had positive urine culture after cystoscopy. |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Conference abstract, and we could not contact authors for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who will be having cystoscopy for any reason randomised to 3 groups." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding the concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Before cystoscopy 80mg gentamycin and 3 gr fosfomycin were given to first and second group. Any medication was given to last group." Comment: participants in control group did not receive antibiotics, while participants in treatment group received antibiotics. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: outcome not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was regarded as the primary outcome, the result was obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Unclear risk | Comment: we could not obtain the full text of the abstract, and we were unable to assess any other bias. |
Tsugawa 1998.
| Methods |
Study design: prospective, randomized controlled study Study dates: October 1995 to November 1996 Setting: 1 hospital Country: Japan |
|
| Participants |
Inclusion criteria: undergoing urethrocystoscopy or urethrocystography based on clinical symptoms or a urinalysis in outpatient clinic; no pyuria (≥ 5 white blood cells/high power field) or bacteriuria (bacterial count > l04 CFU/mL) Exclusion criteria: not reported Sample size: 47 participants Age (years): mean: 69.0 (range 48–86) in control group; 63.1 (range 38–86) in treatment group Sex: control group: 10 men and 14 women; treatment group: 11 men and 10 women |
|
| Interventions |
Group 1 (n = 21): sparfloxacin 200 mg within 1‐hour period before cystoscopy Group 2 (n = 24): no drugs |
|
| Outcomes |
Systemic UTI How measured: bacteriuria (bacterial count > l04 CFU/mL) Time points measured: participants returned to the outpatient clinic within 1 month after the examination and were examined for subjective symptoms and urinalysis Time points reported: not reported Outcomes: no pyuria or bacteriuria, or febrile infection after cystoscopy |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | No email address available for contacting the corresponding author for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who underwent urethrocystoscopy or urethrocystography and did not have pyuria and bacteriuria were included and divided randomly into 2 groups." Comment: method for generation of random sequence not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Either receiving a prophylactic antibiotic or no antibiotic." Comment: participants in control group did not receive antibiotics, while participants in treatment group received antibiotics. Unlikely that participants and personnel were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "Either receiving a prophylactic antibiotic or no antibiotic." Comment: participants not blinded to their treatment. Risk of detection bias for subjective outcomes, e.g. symptoms after cystoscopy, was high. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Low risk | Quote: "21 in the treatment group and 24 in the non treatment group returned to the outpatient clinic within 1 month after the examination and were examined for subjective symptoms and a urinalysis." Comment: 45/47 randomized participants were included for analysis, 2 were excluded since they did not return samples within 1 month and not available for analysis. Overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Low risk | Quote: "21 in the treatment group and 24 in the non treatment group returned to the outpatient clinic within 1 month after the examination and were examined for subjective symptoms and a urinalysis." Comment: 45/47 randomized participants were included for analysis, 2 were excluded since they did not return samples within 1 month and not available for analysis. Overall, loss to follow‐up was less and their reasons were given; therefore, judged adequate. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Vasanthakumar 1990.
| Methods |
Study design: prospective controlled study Study dates: not reported Setting: not reported Country: UK |
|
| Participants |
Inclusion criteria: aged ≥ 60 years and undergoing planned endoscopic procedures Exclusion criteria: clinical evidence of infection; pyrexia in week before procedure; indwelling urinary catheter, nasogastric tube, venous catheter, or line; history of catheterization, dental procedure, endoscopy, or barium study in preceding week; history of antimicrobial therapy in preceding month; allergy to penicillins or gentamicin; valvular heart disease or valve prostheses; history of endocarditis Sample size: 179 cystoscopy examinations Age (years): mean: 74.5 (range 60–93 years) Sex: not reported |
|
| Interventions |
Group 1 (n = 88): amoxicillin 1 g in 2.5 mL 1% lignocaine + gentamicin 120 mg intramuscularly up to 15 minutes before procedure Group 2 (n = 91): no antibiotic |
|
| Outcomes |
Bacteriuria How measured: not reported Time points measured: outpatients instructed to report back if they developed any symptoms in the following 2 weeks. Inpatients observed for pyrexia or symptoms. Time points reported: not reported Outcomes: 25/91 participants in control group and 1/88 in treatment group had cultured organisms |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | No email address available for contacting the corresponding author for further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Alternate participants, regardless of sex, were allocated to chemoprophylaxis or no chemoprophylaxis (control) groups." Comment: group classification assigned alternatively, not randomly. |
| Allocation concealment (selection bias) | High risk | Quote: "Alternate participants, regardless of sex, were allocated to chemoprophylaxis or no chemoprophylaxis (control) groups." Comment: alternate participants received chemoprophylaxis or no chemoprophylaxis; thus, at high risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Alternate patients, regardless of sex, were allocated to chemoprophylaxis or no chemoprophylaxis (control) groups." Comment: participants in control group did not receive antibiotics, while participants in treatment group received antibiotics. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: subjective outcomes of systemic and localized symptoms after cystoscopy; adverse events not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: bacteriuria evaluated by urine culture was regarded as the primary outcome, the result was obtained from laboratory. Detection bias for this outcome was unlikely to be influenced by the unblinded design. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Comment: outcome not reported (bacteriuria was reported, but without differentiating whether participants had symptoms or not). |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
Wilson 2005.
| Methods |
Study design: prospective, randomized controlled study Study dates: not reported Setting: Auckland Hospital Country: New Zealand |
|
| Participants |
Inclusion criteria: undergoing diagnostic flexible cystoscopy Exclusion criteria: undergoing therapeutic intervention; e.g. stent removal or bladder biopsy; requiring intravenous antibiotic prophylaxis or already taking antibiotics Sample size: 263 participants recruited, but 29 excluded because of incomplete data acquisition, leaving 234 for analysis Age: not reported Sex: control group: 91 men and 31 women; treatment group: 85 men and 27 women |
|
| Interventions |
Group 1 (n = 122): placebo 20–60 minutes before flexible cystoscopy Group 2 (n = 112): norfloxacin 400 mg orally 20–60 minutes before flexible cystoscopy |
|
| Outcomes |
Symptomatic UTI How measured: participants were questioned by a nurse by telephone regarding symptoms of UTI, UTI was defined as urinary symptoms associated with significant growth (> 102 CFU/mL) on urine culture Time points measured: urine culture performed at days 3 and 7 after flexible cystoscopy Time points reported: not reported Outcomes: 1/122 participants in control group and 1/112 in treatment group had symptomatic UTI. The infection in the placebo group occurred de novo, while the participant in the norfloxacin group had bacteriuria before the procedure Asymptomatic bacteriuria How measured: not reported Time points measured: urine culture performed at days 3 and 7 after flexible cystoscopy Time points reported: not reported Outcomes: 3/122 participants in control group and 1/112 participants in treatment group had asymptomatic bacteriuria |
|
| Funding sources | No information about funding | |
| Declarations of interest | No information about conflict and interest | |
| Notes | We tried to contact corresponding author regarding the random sequence generation and allocation method, but received no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The pharmacy department of Auckland Hospital provided both antibiotic and placebo in randomized numbered packs, which were blinded to the patient, clinic nurse, and the physician performing the procedure." Comment: method for generation of random sequence was not given. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding concealment of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The pharmacy department of Auckland Hospital provided both antibiotic and placebo in randomized numbered packs, which were blinded to the patient, clinic nurse, and the physician performing the procedure." Comment: blinding of participants and personnel performed adequately. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Principal investigator could not identify participants received the active drug or not, and participants were blinded about their treatments." Comment: double‐blind study. Risk of detection bias for symptomatic UTI and asymptomatic bacteriuria was low. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not applicable, since the objective outcome of drug resistance was not reported. |
| Incomplete outcome data (attrition bias) Systemic UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Symptomatic UTI | Unclear risk | Quote: "263 patients were recruited, but 29 were excluded because of incomplete data acquisition, leaving 234 for analysis." Comment: 234/ 263 recruited participants (122 in control group, 112 in intervention group) were included for analysis of this outcome, 29 excluded because of incomplete data acquisition. No information about whether these dropouts were before or after randomization or to which group dropouts belonged. Study was stopped and interim analysis performed because of low recruitment rate. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Localized UTI | Unclear risk | Comment: outcome not reported. |
| Incomplete outcome data (attrition bias) Asymptomatic bacteriuria | Unclear risk | Quote: "263 patients were recruited, but 29 were excluded because of incomplete data acquisition, leaving 234 for analysis." Comment: 234/263 recruited participants (122 in control group, 112 in intervention group) were included for analysis of this outcome, 29 excluded because of incomplete data acquisition. No information about whether these dropouts were before or after randomization or to which group these dropouts belonged. Study was stopped and interim analysis performed because of low recruitment rate. |
| Incomplete outcome data (attrition bias) Bacterial resistance | Unclear risk | Comment: outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data reported on all outcomes specified in methods section, but there was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | Comment: no other bias detected. |
CFU: colony‐forming units; n: number of participants; SD: standard deviation; UTI: urinary tract infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott 2016 | Retrospective review of 70 participants undergoing cystoscopic ureteral stent removal following kidney stone treatment (35 per group): with and without prophylactic antibiotics. Antimicrobial prophylaxis at time of cystoscopic stent removal did not appear to provide a significant benefit in UTI prevention. |
| Alsaywid 2013 | Not a randomized controlled study; systematic review of whether antibiotic prophylaxis should be used for transurethral urological surgeries. |
| Batura 2012 | Retrospective review. The purpose was to evaluate whether antimicrobial prophylaxis should be used for preventing symptomatic UTI after outpatient flexible cystoscopy. 359 participants with antimicrobial prophylaxis and 243 participants without antimicrobial prophylaxis were retrospectively selected. There was no significant difference in occurrence of symptomatic UTI in participants who received antibiotic prophylaxis and those who did not. |
| Bhatia 1992 | Number of participants undergoing urethrocystoscopy and efficacy of antimicrobial prophylaxis with regard to urethrocystoscopy were not individually analysed. Randomized controlled study comparing the efficacy of different antibiotics for preventing UTI with placebo in women undergoing lower urinary tract instrumentation procedures, including urethrocystoscopy, urethral dilations, or simultaneous urethrocystometric urodynamic studies. Cefadroxil and nitrofurantoin were both significantly more effective in preventing postinstrumentation UTI than placebo (P < 0.003). |
| Cano‐Garcia 2015 | Prospective observational non‐randomized study evaluating efficacy of antibiotic prophylaxis with ciprofloxacin to reduce incidence of UTIs. 30 participants received antibiotic prophylaxis (group 1) and 30 (group 2) did not. They found that 4 participants in group 1 and 1 in group 2 had a positive urine culture. Only 1 participant in group 1 consulted in primary care for symptoms. They concluded that antibiotic prophylaxis with ciprofloxacin 500 mg prior to cystoscopy had no benefit. |
| Carey 2015 | Not a randomized controlled study, but a systematic review of whether antibiotic prophylaxis should be used for flexible cystoscopy. |
| Ciudin 2015 | Prospective non‐randomized study comparing the efficacy of 2 different ways of antibiotics prophylaxis, i.e. fosfomycin and fosfomycin with cranberry extract, for UTI after outpatient flexible cystoscopy. No placebo or no antibiotic treatment group. Prophylaxis with cranberry extract and antibiotics was superior to antibiotics alone for preventing urinary infections in participants undergoing outpatient flexible cystoscopy. |
| Cundiff 1999 | Number of participants undergoing urethrocystoscopy and the efficacy of antimicrobial prophylaxis with regard to urethrocystoscopy was not individually analysed. Randomized controlled study comparing the efficacy of nitrofurantoin vs placebo for preventing UTI in women undergoing urethrocystoscopy and urodynamic examinations. Bacteriuria after combined urodynamics and cystourethroscopy was not improved by a 1‐day course of nitrofurantoin. |
| Dicker 2000 | Non‐randomized study. 125 participants undergoing transperineal interstitial permanent prostate brachytherapy in conjunction with cystoscopy. All participants received intravenous perioperative antibiotic prophylaxis. No placebo or no treatment control group. 125 participants undergoing brachytherapy and cystoscopy, 1 participant (1%) developed a symptomatic UTI. |
| Ersev 1992 | Randomized controlled study comparing the efficacy of gentamicin for preventing UTI with placebo in participants undergoing endoscopic procedures of urinary tract, including transurethral resection of the prostate, transurethral resection of bladder tumour, ureteroscopy, urethrocystoscopy, etc. Efficacy of antimicrobial prophylaxis with regard to urethrocystoscopy was not individually analysed. |
| Escandon‐Vargas 2015 | Non‐randomized study. 13 participants with positive urine culture before cystoscopy were allocated to antimicrobial prophylaxis, while 76 participants without positive urine culture before cystoscopy were allocated to no antimicrobial prophylaxis. |
| Foon 2012 | Systematic review of prophylactic antibiotics to reduce the risk of UTIs after urodynamic studies. |
| Fujita 1994 | Retrospective study with 1249 participants undergoing urethral manipulations including urethral dilation, retrograde urethrography, and cystography. |
| Garcia‐Perdomo 2015 | Systematic review of antibiotic prophylaxis to prevent UTIs in men and women undergoing cystoscopy. |
| Grabe 2001 | Review regarding antibiotic prophylaxis for different urological interventions. |
| Gregg 2016 | Retrospective study to identify groups at increased risk for UTI after cystoscopy. 5488 participants underwent cystoscopy, of whom 29 (0.53%) had a UTI. They found that recent antibiotic exposure, infection, or hospitalization was associated with an increased risk of UTI after cystoscopy. |
| Herr 2012 | Non‐randomized study. All 1017 participants underwent cystoscopy or Bacillus Calmette‐Guerin (BCG) treatment received no antibiotic prophylaxis. No treatment group received antibiotic prophylaxis. |
| Herr 2014 | Prospective non‐randomized study including 2010 participants undergoing outpatient flexible cystoscopy. Antibiotic prophylaxis was not used for all participants, there was no antibiotic prophylaxis treatment group. |
| Higgins 1966 | Blind controlled study for evaluating the efficacy of antibiotic prophylaxis for participants undergoing transurethral procedures (cystoscopy, anterior dilation, closed cystodiathermy, retrograde pyelogram, or ureterogram). Efficacy of antimicrobial prophylaxis with regard to cystoscopy was not individually analysed. |
| Hosoglu 2003 | Cross‐sectional, countrywide survey to assess the quality of antibiotic prophylaxis for clean and clean‐contaminated elective surgical procedures. Antibiotic prophylaxis for cystoscopy was not investigated. |
| Klimberg 1992 | Randomized study comparing the efficacy of oral lomefloxacin vs parenteral cefotaxime as prophylactic agents in transurethral surgery. Participants in both groups took antibiotics for prophylaxis. Participants with simple cystoscopy examination were excluded. |
| Leyton 1993 | Randomized study for evaluating the efficacy of antibiotic prophylaxis for participants undergoing transurethral procedures (cystoscopy, urodynamic, internal urethrotomy). Number of participants undergoing urethrocystoscopy and the efficacy of antimicrobial prophylaxis with regard to urethrocystoscopy were not individually analysed. |
| Poppel 1990 | Randomized study comparing different doses of ciprofloxacin for preventing UTI in people with neurogenic bladder. Participants in both groups took antibiotics and underwent different transurethral manipulations. |
| Pozzi 1984 | Randomized study to evaluate the efficacy of cephalosporins and aminoglycosides for prophylaxis in urological surgery. Participants undergoing cystoscopy were not individually reported. |
| Proskurin 2010 | Non‐randomized study. All participants received antibiotic prophylaxis for cystoscopy. No placebo or no treatment group. |
| Ravichandraprakash 2011 | Retrospective study to evaluate the risk factors for UTI after urinary tract procedures. No control group. |
| Reilly 1981 | Randomized study comparing different types of antibiotics (cephazolin and gentamicin) for preventing UTI in participants undergoing urological endoscopy. Participants in both groups received antibiotics. |
| Scarpa 1990 | Non‐randomized study with no placebo or no antibiotic prophylaxis group. All participants received netilmicin prophylaxis. |
| Siracusano 2008 | Randomized study to evaluate the efficacy of antibiotic prophylaxis before invasive urodynamics in women. No cystoscopy examinations. |
| Sommers 1983 | Review of pharmacological principles in the treatment of UTIs. |
| Wooster 1990 | Prospective non‐randomized study. 200 participants undergoing cystoscopy or prostatectomy. Participants who received antibiotics prophylaxis or not were based on the discretion of the physician. 89 participants received antibiotics, 10 (12%) had positive urinary culture; of the 111 participants not receiving antibiotics, 32 (28%) had positive urinary culture. |
UTI: urinary tract infection.
Differences between protocol and review
This review is based on a published protocol (Zeng 2016), with differences as described here.
Symptomatic UTI was added as a primary outcome post hoc, because this was a patient‐important outcome and in clinical practice the boundaries between a localized UTI and a systematic UTI could be fluid and both matter to patients and doctors.
Localized UTI was moved to the secondary outcomes due to the limitation of primary outcomes and localized UTI was lesser importance compared to the other three primary outcomes.
Contributions of authors
SXZ: protocol drafting, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and review updates.
ZSZ: protocol drafting, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and review updates.
YB: search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, and review drafting.
YHS: data interpretation, review drafting.
CLX: data interpretation, review drafting.
SXZ and ZSZ contributed equally to the paper.
Sources of support
Internal sources
Department of Urology, Changhai Hospital, Second Military Medical University, China.
External sources
University of Minnesota, Cochrane Urology, USA.
Declarations of interest
SXZ: none known.
ZSZ: none known.
YB: none known.
YHS: none known.
CLX: none known.
New
References
References to studies included in this review
Asuero 1989 {published data only}
- Asuero Mantero M, Gomez Velazquez M, Leal Arenas J. Antibiotic prophylaxis in endoscopic urologic surgery. Actas Urológicas Españolas 1989;13:353‐6. [PubMed] [Google Scholar]
Blackard 1972 {published data only}
- Blackard CE, Nicolaidis AN. Use of azo‐sulfonamide for the prevention of urinary tract symptoms and bacteriuria following cystourethroscopic examination. Journal of Urology 1972;107:650‐1. [DOI] [PubMed] [Google Scholar]
Cam 2009 {published data only}
- Cam K, Kayikci A, Erol A. Prospective evaluation of the efficacy of antibiotic prophylaxis before cystoscopy. Indian Journal of Urology 2009;25:203‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Perdomo 2013 {published data only}
- Garcia‐Perdomo HA, Lopez H, Carbonell J, Castillo D, Catano JG, Seron P. Efficacy of antibiotic prophylaxis in patients undergoing cystoscopy: a randomized clinical trial. World Journal of Urology 2013;31:1433‐9. [DOI] [PubMed] [Google Scholar]
Goh 1982 {published data only}
- Goh HS, Burge DM, Bramble JF. A simple method of reducing bacteriuria after out‐patient cystoscopy. Singapore Medical Journal 1982;23(6):315‐7. [PubMed] [Google Scholar]
Hares 1981 {published data only}
- Hares MM. A double‐blind trial of half‐strength Polybactrin Soluble GU bladder irrigation in cystoscopy. British Journal of Urology 1981;53:62‐7. [DOI] [PubMed] [Google Scholar]
Hart 1980 {published data only}
- Hart AJ, Miles RS, Varnam DJ, Edmond P. Assessment of the morbidity and value of prophylactic cephazolin sodium in urinary tract instrumentation in out‐patients. Current Medical Research and Opinion 1980;6:658‐62. [DOI] [PubMed] [Google Scholar]
Jimenez 1993 {published data only}
- Jimenez Cruz JF, Sanz Chinesta S, Otero G, Diaz Gonzalez R, Alvarez Ruiz F, Flores N, et al. Antimicrobial prophylaxis in urethrocystoscopy. Comparative study. Actas Urologicas Españolas 1993;17(3):172‐5. [PubMed] [Google Scholar]
Jimenez‐Pacheco 2012 {published data only}
- Jimenez‐Pacheco A, Lardelli Claret P, Lopez Luque A, Lahoz‐Garcia C, Angel Arrabal Polo M, Nogueras Ocaña M. Randomized clinical trial on antimicrobial prophylaxis for flexible urethrocystoscopy. Actas Urologicas Españolas 2012;65:542‐9. [PubMed] [Google Scholar]
Johnson 2007 {published data only}
- Johnson MI, Merrilees D, Robson WA, Lennon T, Masters J, Orr KE, et al. Oral ciprofloxacin or trimethoprim reduces bacteriuria after flexible cystoscopy. BJU International 2007;100:826‐9. [DOI] [PubMed] [Google Scholar]
Karmouni 2001 {published data only}
- Karmouni T, Bensalah K, Alva A, Patard JJ, Lobel B, Guille F. Role of antibiotic prophylaxis in ambulatory cystoscopy. Progrès en Urologie 2001;11:1239‐41. [PubMed] [Google Scholar]
MacDermott 1988 {published data only}
- MacDermott JP, Ewing RE, Somerville JF, Gray BK. Cephradine prophylaxis in transurethral procedures for carcinoma of the bladder. British Journal of Urology 1988;62:136‐9. [DOI] [PubMed] [Google Scholar]
Manson 1988 {published data only}
- Manson AL. Is antibiotic administration indicated after outpatient cystoscopy. Journal of Urology 1988;140:316‐7. [DOI] [PubMed] [Google Scholar]
Martinez Rodriguez 2017 {published data only}
- Martinez Rodriguez RH, Felip E, Arzoz Fabregas M, Juventeny N, Ibarz Servio L. Efficacy of antibiotic prophylaxis and cleaning/disinfection devices inflexible cystoscopy to prevent positive urinary culture after procedure. European Urology 2017;16(3):e236. [Google Scholar]
Mendoza 1971 {published data only}
- Mendoza GB Jr, Gerwig WH Jr, Stackhouse KL, Easley GW. Prophylactic use of antibacterial drugs following cystoscopy: a double‐blind controlled study of demeclocycline hydrochloride and sulfamethoxypyridazine. Journal of Urology 1971;106:682‐4. [DOI] [PubMed] [Google Scholar]
Rané 2001 {published data only}
- Rané A, Cahill D, Saleemi A, Montgomery B, Palfrey E. The issue of prophylactic antibiotics prior to flexible cystoscopy. European Urology 2001;39:212‐4. [DOI] [PubMed] [Google Scholar]
Rodrigues 1994 {published data only}
- Rodrigues Palma PC, Zanettini Riccetto CL, Fregonesi A, Rodrigues Netto N Jr. Fosfomycin trometamol versus sulfametoxazole‐trimetoprim in prophylaxis of urinary tract infection following urologic endoscopy. Jornal Brasileiro de Ginecologia 1994;104:295‐8. [Google Scholar]
Si 1997 {published data only}
- Si JF. Cystoscopy with local administration of antibiotics. China Journal of Endoscopy 1997; Vol. 2, issue 2:31.
Soydan 2012 {published data only}
- Soydan H, Yilmaz Ö, Ates F, Okçelik S, Yesildal C, Senkul T. Is antibiotic prophylaxis necessary prior to cystoscopy?. Journal of Endourology 2012;26(S1):A422. [Google Scholar]
Tsugawa 1998 {published data only}
- Tsugawa M, Monden K, Nasu Y, Kumon H, Ohmori H. Prospective randomized comparative study of antibiotic prophylaxis in urethrocystoscopy and urethrocystography. International Journal of Urology 1998;5:441‐3. [DOI] [PubMed] [Google Scholar]
Vasanthakumar 1990 {published data only}
- Vasanthakumar V, Bhan GL, Perera BS, Taft P. A study to assess the efficacy of chemoprophylaxis in the prevention of endoscopy‐related bacteraemia in patients aged 60 and over. Quarterly Journal of Medicine 1990;75:647‐53. [PubMed] [Google Scholar]
Wilson 2005 {published data only}
- Wilson L, Ryan J, Thelning C, Masters J, Tuckey J. Is antibiotic prophylaxis required for flexible cystoscopy? A truncated randomized double‐blind controlled trial. Journal of Endourology 2005;19:1006‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbott 2016 {published data only}
- Abbott JE, McDonald M, Han A, Lakin C, Sur RL. Are antibiotics necessary during routine cystoscopic stent removal?. Journal of Urology 2016;195:e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alsaywid 2013 {published data only}
- Alsaywid B, Smith G. Antibiotic prophylaxis for transurethral urological surgeries: systematic review. Urology Annals 2013;5:61‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batura 2012 {published data only}
- Batura D, Sahibzada I, Gopal Rao G. The role of antibiotic prophylaxis in the prevention of symptomatic UTI after outpatient flexible cystoscopy. European Urology 2012;11:e294. [Google Scholar]
Bhatia 1992 {published data only}
- Bhatia NN, Karram MM, Bergman A, Evans RP. Antibiotic prophylaxis following lower urinary tract instrumentation. Urology 1992;39:583‐5. [DOI] [PubMed] [Google Scholar]
Cano‐Garcia 2015 {published data only}
- Cano‐Garcia Mdel C, Casares‐Perez R, Castillo‐Gallardo E, Merino‐Salas S, Arrabal‐Martin M, Arrabal‐Polo MA. Usefulness of antimicrobial prophylaxis with ciprofloxacin prior to flexible cystoscopy. Revista Médica de Chile 2015;143:1001‐4. [DOI] [PubMed] [Google Scholar]
Carey 2015 {published data only}
- Carey MM, Zreik A, Fenn NJ, Chlosta PL, Aboumarzouk OM. Should we use antibiotic prophylaxis for flexible cystoscopy? A systematic review and meta‐analysis. Urologia Internationalis 2015;95:249‐59. [DOI] [PubMed] [Google Scholar]
Ciudin 2015 {published data only}
- Ciudin A, Sanchez J, Wahab A, Mando S, Alcaraz A. Adding cranberry extract to flexible cystoscopy profilaxis decreases urinary infection rates. European Urology 2015;14:e262. [Google Scholar]
Cundiff 1999 {published data only}
- Cundiff GW, McLennan MT, Bent AE. Randomized trial of antibiotic prophylaxis for combined urodynamics and cystourethroscopy. Obstetrics and Gynecology 1999;93:749‐52. [DOI] [PubMed] [Google Scholar]
Dicker 2000 {published data only}
- Dicker AP, Figura AT, Waterman FM, Valicenti RK, Strup SE, Gomella LG. Is there a role for antibiotic prophylaxis in transperineal interstitial permanent prostate brachytherapy?. Techniques in Urology 2000;6:104‐8. [PubMed] [Google Scholar]
Ersev 1992 {published data only}
Escandon‐Vargas 2015 {published data only}
- Escandon‐Vargas K, Garcia‐Perdomo HA, Echeverria F, Osorio J D. Risk of urinary tract infection in patients with positive urine culture and antibiotic therapy undergoing cystoscopy in a third‐level hospital. Le Infezioni in Medicina 2015;23:336‐42. [PubMed] [Google Scholar]
Foon 2012 {published data only}
- Foon R, Toozs‐Hobson P, Latthe P. Prophylactic antibiotics to reduce the risk of urinary tract infections after urodynamic studies. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008224.pub2] [DOI] [PubMed] [Google Scholar]
Fujita 1994 {published data only}
- Fujita K, Matsushima H, Nakano M, Kaneko M, Munakata A. Prophylactic oral antibiotics in urethral instrumentation. Japanese Journal of Urology 1994;85:802‐5. [DOI] [PubMed] [Google Scholar]
Garcia‐Perdomo 2015 {published data only}
- Garcia‐Perdomo HA, Jimenez‐Mejias E, Lopez‐Ramos H. Efficacy of antibiotic prophylaxis in cystoscopy to prevent urinary tract infection: a systematic review and meta‐analysis. International Brazilian Journal of Urology 2015;41:412‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grabe 2001 {published data only}
- Grabe M. Perioperative antibiotic prophylaxis in urology. Current Opinion in Urology 2001;11:81‐5. [DOI] [PubMed] [Google Scholar]
Gregg 2016 {published data only}
- Gregg JR, Lai C, Dmochowski R, Talbot TR, Barocas DA. Recent antibiotic treatment increases the risk of urinary tract infection after outpatient cystoscopy. Urology Practice 2016;3:90‐6. [DOI] [PubMed] [Google Scholar]
Herr 2012 {published data only}
- Herr HW. Outpatient urological procedures in antibiotic‐naive patients with bladder cancer with asymptomatic bacteriuria. BJU International 2012;110:E658‐60. [DOI] [PubMed] [Google Scholar]
Herr 2014 {published data only}
- Herr HW. Should antibiotics be given prior to outpatient cystoscopy? A plea to urologists to practice antibiotic stewardship. European Urology 2014;65:839‐42. [DOI] [PubMed] [Google Scholar]
Higgins 1966 {published data only}
- Higgins PM. Value of prophylactic antibacterial therapy in instrumentation of urinary tract. BMJ 1966;1:26‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosoglu 2003 {published data only}
- Hosoglu S, Sunbul M, Erol S, Altindis M, Caylan R, Demirdag K, et al. A national survey of surgical antibiotic prophylaxis in Turkey. Infection Control and Hospital Epidemiology 2003;24:758‐61. [DOI] [PubMed] [Google Scholar]
Klimberg 1992 {published data only}
- Klimberg IW, Childs SJ, Madore RJ, Klimberg SR. A multicenter comparison of oral lomefloxacin versus parenteral cefotaxime as prophylactic agents in transurethral surgery. American Journal of Medicine 1992;92:121S‐5S. [DOI] [PubMed] [Google Scholar]
Leyton 1993 {published data only}
- Leyton NR, Valenzuela AA, Cabezón FA, Navarrete HA, Zúñiga SC. Profilaxis antibiótica en procedimientos transuretrales (PTU). Revista Chilena de Urologia 1993;57:51‐3. [Google Scholar]
Poppel 1990 {published data only}
Pozzi 1984 {published data only}
- Pozzi E, Bocciardi A, Roggia A. A randomized trial with cephalosphorines and aminoglicosides for the antibiotic prophilaxis in urology. Archivio Italiano di Urologia e Nefrologia 1984;56:209‐13. [Google Scholar]
Proskurin 2010 {published data only}
- Proskurin AA, Asfandiiarov FR, Kalashnikov ES, Miroshnikov VM. Using safocid for antibiotic prophylaxis in minimally‐invasive endoscopic operations and manipulations. Urologiia (Moscow, Russia : 1999) 2010;10(6):37‐40. [PubMed] [Google Scholar]
Ravichandraprakash 2011 {published data only}
- Ravichandraprakash H, Ravikumar R, Krishna S, Mariraj J, Vijayanath V, Gadigi S, et al. A clinical and microbiological study of urinary tract infection in patients following instrumentation. Journal of Pure and Applied Microbiology 2011;5:669‐82. [Google Scholar]
Reilly 1981 {published data only}
- Reilly CS, Hart AJ, McAllister TA. Comparison of cephazolin and gentamicin in the prophylactic treatment of infection in out‐patient urinary tract endoscopy. British Journal of Urology 1981;53:138‐40. [DOI] [PubMed] [Google Scholar]
Scarpa 1990 {published data only}
- Scarpa RM, Cossu FM, Ambus G, Migliari R, Lisa A, Campus G, et al. Antibiotic prophylaxis with netilmicin in patients undergoing cystoscopic study. Minerva Urologica e Nefrologica [Italian Journal of Urology and Nephrology] 1990;42:167‐71. [PubMed] [Google Scholar]
Siracusano 2008 {published data only}
- Siracusano S, Knez R, Tiberio A, Alfano V, Giannantoni A, Pappagallo G. The usefulness of antibiotic prophylaxis in invasive urodynamics in postmenopausal female subjects. International Urogynecology Journal and Pelvic Floor Dysfunction 2008;19:939‐42. [DOI] [PubMed] [Google Scholar]
Sommers 1983 {published data only}
- Sommers DK. Pharmacological principles in the treatment of urinary tract infections. South African Medical Journal 1983;64:123‐6. [PubMed] [Google Scholar]
Wooster 1990 {published data only}
- Wooster DL, Krajden S. Selection of antibiotic coverage in vascular patients undergoing cystoscopy. Journal of Cardiovascular Surgery 1990;31:469‐73. [PubMed] [Google Scholar]
Additional references
Bakken 2004
- Bakken JS. The fluoroquinolones: how long will their utility last?. Scandinavian Journal of Infectious Diseases 2004;36(2):85‐92. [DOI] [PubMed] [Google Scholar]
Bonkat 2018
- Bonkat G, Pickard R, Bartoletti R, Bruyère F, Geerlings SE, Köves B, et al. Guidelines on urological infections, 2018. Available from uroweb.org/guideline/urological‐infections/. European Association of Urology, (accessed prior to 29 January 2019).
Bootsma 2008
- Bootsma AM, Laguna Pes MP, Geerlings SE, Goossens A. Antibiotic prophylaxis in urologic procedures: a systematic review. European Urology 2008;54(6):1270‐86. [DOI] [PubMed] [Google Scholar]
Burke 2002
- Burke DM, Shackley DC, O'Reilly PH. The community‐based morbidity of flexible cystoscopy. BJU International 2002;89(4):347‐9. [DOI] [PubMed] [Google Scholar]
Cano‐Garcia 2016
- Cano‐Garcia MC, Casares‐Perez R, Arrabal‐Martin M, Merino‐Salas S, Arrabal‐Polo MA. Prospective non‐randomized study on the use of antibiotic prophylaxis with ciprofloxacin in flexible urethrocystoscopy. Archivos Espanoles de Urologia 2016;69(9):648‐53. [PubMed] [Google Scholar]
Fillon 2012
- Fillon M. CDC launches infection awareness initiative for cancer patients. Journal of the National Cancer Institute 2012;104(3):170‐2. [DOI] [PubMed] [Google Scholar]
Finberg 2004
- Finberg RW, Moellering RC, Tally FP, Craig WA, Pankey GA, Dellinger EP, et al. The importance of bactericidal drugs: future directions in infectious disease. Clinical Infectious Diseases 2004;39(9):1314‐20. [DOI] [PubMed] [Google Scholar]
Grabe 2014
- Grabe M, Bartoletti R, Bjerklund‐Johansen TE, Çek HM, Pickard RS, Tenke P, et al. Guidelines on urological infections. European Association of Urology Guidelines. European Association of Urology, 2014:77‐85. [Google Scholar]
GRADEproGDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEproGDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014.
Gross 2007
- Gross PA, Patel B. Reducing antibiotic overuse: a call for a national performance measure for not treating asymptomatic bacteriuria. Clinical Infectious Diseases 2007;45(10):1335‐7. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Henry 2018
- Henry M, Patil D, Filson C, Mehta A. Antibiotic stewardship for ambulatory urologic procedures: cystoscopy and vasectomy. Journal of Urology 2018;199(4S):e12‐13. [DOI] [PubMed] [Google Scholar]
Herr 2015
- Herr HW. The risk of urinary tract infection after flexible cystoscopy in patients with bladder tumor who did not receive prophylactic antibiotics. Journal of Urology 2015;193(2):548‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kicinski 2015
- Kicinski M, Springate DA, Kontopantelis E. Publication bias in meta‐analyses from the Cochrane Database of Systematic Reviews. Statistics in Medicine 2015;34:2781‐93. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meropol 2008
- Meropol SB, Chan KA, Chen Z, Finkelstein JA, Hennessy S, Lautenbach E, et al. Adverse events associated with prolonged antibiotic use. Pharmacoepidemiology and Drug Safety 2008;17(5):523‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schaeffer 2012
- Schaeffer AJ, Schaeffer EM. Infections of the urinary tract. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA editor(s). Campbell‐Walsh Urology. 10th Edition. Philadelphia (PA): Saunders, 2012:257‐326. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sorlozano 2014
- Sorlozano A, Jimenez‐Pacheco A, Dios Luna Del Castillo J, Sampedro A, Martinez‐Brocal A, Miranda‐Casas C, et al. Evolution of the resistance to antibiotics of bacteria involved in urinary tract infections: a 7‐year surveillance study. American Journal of Infection Control 2014;42(10):1033‐8. [DOI] [PubMed] [Google Scholar]
Thornhill 2015
- Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. Journal of Antimicrobial Chemotherapy 2015;70(8):2382‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2008
- Wolf JS Jr, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ. Best practice policy statement on urologic surgery antimicrobial prophylaxis. Journal of Urology 2008;179(4):1379‐90. [DOI] [PubMed] [Google Scholar]
Zeng 2016
- Zeng S, Zhang Z, Bai Y, Sun Y, Xu C. Antimicrobial agents for preventing urinary tract infections in patients undergoing cystoscopy. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012305] [DOI] [PMC free article] [PubMed] [Google Scholar]


