Abstract
The gastrointestinal (GI) system is vital in its capacities for nutrient and water uptake, immune function, metabolism and detoxification, and stem-cell derived regeneration. Of significance to human health are a myriad of GI disorders associated with aging that integrate with the circadian clock. Here we present data from three groups of mice: young (3 mo old), middle aged (12 mo old), and old aged (24 mo old). Small intestine and colon samples taken every 4 h under light-dark (LD) conditions were assayed for gene expression related to molecular circadian rhythmicity, transcription, cell signaling, and immune function. Transcripts related to melatonin biosynthesis and signaling, as well as melatonin content from stool, were also included, as GI melatonin and aging have been associated in contexts outside of the circadian clock. With respect to circadian genes, the data here are congruent with data from other peripheral tissues: age does not affect the rhythmic expression of core clock genes in the gut. The same can be said for several clock-controlled transcripts. In contrast, diurnal patterns in the expression of nitric oxide synthase 1 and of immune factors irak4 and interleukin-8 were observed in the colon of young mice that were lost in middle-aged and aged animals. Furthermore, the diurnal pattern of melatonin synthesis genes was altered by age, and stool melatonin levels showed significant decline between young mice and aged cohorts. These data expand the evidence for the persistence of the circadian clock throughout the aging process and highlight its importance to health.
Keywords: aging, circadian, gastrointestinal, melatonin
INTRODUCTION
Aging is known to affect gastrointestinal function. Esophageal contractions and tension decrease, the stomach loses much of its elasticity and is less resistant to damage, and the microbiome within the intestinal lumen can show marked changes over time. In addition, pancreatic and hepatic fibrosis increases during aging, decreasing digestive enzyme and bile salt secretion (8, 9, 28). Other, perhaps indirect, factors associated with age can profoundly upset gastrointestinal homeostasis. These include secondary effects associated with decreases in locomotor activity and increases in the use of several pain medications, such as nonsteroidal anti-inflammatory drugs, which can cause bleeding and diarrhea, and opiates, which decrease intestinal motility and can cause constipation (30).
Among these factors, age-related changes in the circadian clock have been hypothesized to influence gastrointestinal function. Circadian clocks are ubiquitous components of all eukaryotic and at least a few prokaryotic organisms that synchronize molecular, biochemical, physiological, and behavioral processes to the 24 h cycle of night and day, as well as coordinating complex processes within organisms, providing efficient timing of function (2). These circadian rhythms, which express periods of approximately (circa) a day (diem), are entrained to the 24 h cycle through rhythmic sensitivity to timing cues, called Zeitgebers, such as the light-dark cycle, the most powerful Zeitgeber, daily changes in temperature, and/or timed meals.
In mammals, circadian rhythms are generated and regulated at multiple levels of biological organization. At the molecular level, circadian rhythms are generated through a gene network of “clock genes” whose products interact via a transcription-translation feedback loop (TTFL) in which “positive element” proteins CLOCK and BMAL1 dimerize and enter the nucleus where they enhance expression of genes carrying an E-box promoter region. These genes include “clock-controlled genes,” which form direct output pathways for cellular clocks, and the “negative elements” period (mPer), of which there are three paralogs mPer1, mPer2 and mPer3, and cryptochrome (mCry), of which there at least two paralogs mCry1 and mCry2. There are several other critical components of the circadian TTFL; these are reviewed extensively elsewhere (2, 10).
At the physiological level, mammalian circadian organization is hierarchical (10). At its core, the hypothalamic suprachiasmatic nuclei (SCN) are the system’s master pacemaker, which is synchronized to the light-dark cycle (LD) via a retinohypothalamic tract in every mammalian species studied thus far (2). The SCN express circadian rhythms in metabolic, transcriptional, and electrical activity in vivo and in vitro. Moreover, surgical destruction of the SCN abolishes circadian rhythms in locomotor behavior, sleep-wake, body temperature, and many metabolic and endocrine outputs (26). Finally, transplantation of fetal SCN tissue or cells restores behavioral rhythmicity to a previously arrhythmic SCN-lesioned rodent (25).
The SCN influences downstream processes through humoral and multisynaptic neuronal pathways via entrainment of peripheral oscillators in the central nervous system (CNS) and peripheral tissues (18). Among the CNS sites of SCN activity is the pineal gland, which synthesizes and releases the neurohormone melatonin via a multisynaptic circadian control of sympathetic activity in the pineal gland (6). Among the peripheral oscillators at least partially controlled by the SCN is the gastrointestinal system (16).
We have previously shown that the mouse gastrointestinal tract possesses a functional circadian clock as well as a subset of rhythmically expressed genes which may directly impact on colonic motility and disease (12–14). First and most obviously, stool number and weight are rhythmically produced in vivo such that stools are produced predominantly during the night in mice held in LD and during the subjective night (when they are active) in mice maintained in constant darkness (DD) (12, 13, 16). Furthermore, colonic contractility in vivo exhibits slow-wave rhythms of colonic pressure during the day and subjective day that transitions into higher frequency contractions during the night and subjective night (13). In vitro, colonic tissue’s contractile responses to varying concentrations (10−6 to 10−4 M) of acetylcholine (ACh) are also rhythmic, such that colonic circular muscle contractility in response to ACh is greater during the night and subjective night than during the day and subjective day (13).
The mouse gastrointestinal system expresses clock genes in a rhythmic fashion both in vivo and in vitro. mClock, mBmal1, mPer1, mPer2, mPer3, mCry1, and mCry2 mRNA are all expressed in the murine stomach, proximal, middle, and distal colonic tissues. mBMAL1, mPER1, and mPER2 protein expression is also present throughout the tissues but is most intensely stained immunohistochemically in epithelial cells along colonic crypts, especially in the bases of the crypts. Staining is also present in the myenteric plexus of the stomach and throughout the colon. Expression of clock gene mRNA and protein is rhythmic in the gastrointestinal system with mBmal1, mPer2, and mCry1 most consistently rhythmic in all tissues, while no rhythmicity in clock expression is apparent in any tissue (14). Western blot analysis of mBMAL1 and mPER2 protein confirms this pattern. Interestingly, timed feeding regimes in which food is present during the subjective day, when mice normally do not eat, phase-shift rhythmic mRNA and protein 12 h, while expression of mBmal1 and mPer2 mRNA in the SCN is unaffected by the restricted feeding. Cultured intestinal tissues from transgenic mice carrying a fusion construct of MPER2::luciferase exhibit robust circadian rhythmicity, punctuating the view that the mouse gastrointestinal system contains a circadian clock.
This is not to say that the gastrointestinal clock is independent of the SCN clock, however. We have shown that SCN lesions abolishes rhythms of stool number and weight in mice fed ad libitum, although timed meals restore these daily rhythms (16). It is likely that the SCN regulates gastrointestinal rhythms via control of sympathetic activity, analogous to the situation with the pineal, since sympathectomy also abolishes circadian rhythms in stool number and weight in SCN-lesioned or intact mice. As with SCN-lesioned mice, timed meals in sympathectomized mice restore patterns of defecation (16).
In the present study, we ask whether measures of the circadian clock or its molecular outputs are diminished or otherwise affected by the process of aging. The data indicate that, while the core clock components are remarkably unaffected by the progress of advanced age, several of the clock’s outputs, particularly in the production of melatonin, are diminished as mice age.
MATERIALS AND METHODS
Animal Procedures
CBA mice (n = 30/age group) were obtained from the National Institutes of Aging at 4 wk, 10 mo, and 22 mo of age. These mice were maintained in the University of Kentucky Department of Laboratory Animal Resources (DLAR) in LD 14:10 for 2 mo before sacrifice. At 3, 12, and 24 mo, respectively, mice were anesthetized by CO2 asphyxiation and killed by decapitation. Anesthesia and sacrifice were done in the dark during the dark period time points. After decapitation, intestines were removed separately using the stomach, cecum, and rectum as boundaries for the small intestine and colon. Small intestines and colons were flash-frozen in isopentane on dry ice and stored at −80°C Stool was collected from each mouse and stored at −80°C. All procedures were approved by University of Kentucky DLAR and the Institutional Animal Care and Use Committee.
RNA Quantification
Total RNA was extracted by ZYMO Direct-zol RNA mini Prep kit with DNase treatment (cat. R2052; ZYMO Research, Seattle, WA) from 20 mg fragments of each tissue, which we determined was sufficient to collect enough total RNA needed for NanoString analysis. The mRNA expression was measured by gene expression quantification using multiplexed, color-coded probe pairs (NanoString nCounter; Nanostring Technologies, Seattle, WA) and validated by semiquantitative PCR. For NanoString, each of the 40 genes including clock genes and genes related to cAMP signaling pathway were targeted for designing and synthesis of probe sets. Probes sets of 100 bp in length were designed to hybridize specifically to each mRNA target and be identified by the unique color code of each probe (23). Samples were subjected to Nanostring nCounter analysis by the University of Kentucky Genomics Core Facility (https://ukhealthcare.uky.edu/genomics-core-laboratory). Briefly, for each sample 10 μl of the Reporter CodeSet and 10 μl of hybridization buffer were mixed with 5 μl of RNA (100 ng). We added 5 μl of Capture ProbeSet to the mixture, and then the mixture was placed in a 65°C thermocycler for a 12 h incubation. Once removed from the thermocycler, samples were processed with the nCounter Prep Station (NanoString nCounter, Nanostring Technologies). The data were normalized first by subtracting the average of the negative controls to eliminate background and calibrated by the standard curve from positive controls, and then the data were normalized to the internal controls (mRplp0, mTubb4b, mPpib, mHmbs, and mEif2a) using the R package NanoStringNorm (29).
Melatonin ELISA of Stool Samples
Stools (3–5) were weighed and diluted in 0.1 M PO4 phosphate-buffered saline. Samples were homogenized in lysing matrix in a Thermo FP 120 FastPrep disruptor. Samples were centrifuged for 20 min at 4°C at 12,000 g. Supernatant was removed, and melatonin was extracted for assay according to the IBL protocol (Melatonin ELISA RE54021; IBL International, Hamburg, Germany). Briefly, supernatants were passed through IBL preconditioned extraction columns at 120 g and eluted with methanol. Methanol eluents were evaporated in a Savant SpeedVac concentrator (Cole-Parmer, Vernon Hills, CA) and reconstituted in 150 μl H2O. Aliquants (50 μl) of standards and unknowns were incubated with biotinylated melatonin and anti-melatonin serum for 24 h at 4°C in a 96-well plate. The samples were washed, blotted, and then exposed to alkaline phosphatase enzyme conjugate and p-nitrophenyl phosphate chromogenic substrate for 40 min before receiving the stop reaction. Samples were recorded on a Bio-Rad iMark microplate reader set at 405 nm.
To validate the melatonin ELISA for mouse stool, stool samples were pooled and disrupted in the Thermo FP 120 FastPrep in 500 μl H2O. Samples were serially diluted with H2O in triplicate at 100, 50, 20, 10, 5, 1, and 0.5%, extracted, and analyzed for melatonin in the ELISA to determine whether the dilution curve was parallel to the standard curves conducted in duplicate.
Statistical Analyses
Daily patterns of gene expression and of stool melatonin content were analyzed with ANOVA with a Newman Keuls post hoc test and by Circwave, a cosinor-based analysis software that incorporates linear harmonic regression (20). Age differences in the patterns of gene expression and of stool melatonin content were determined by three-way ANOVA. Slopes of melatonin standard curves were compared with unknown sample dilutions by regressing the slopes and comparing them. All ANOVA tests and regression analyses were performed using SigmaPlot software (Systat, San Jose, CA).
RESULTS
Melatonin, Melatonin Biosynthesis, and Receptors
Melatonin biosynthesis.
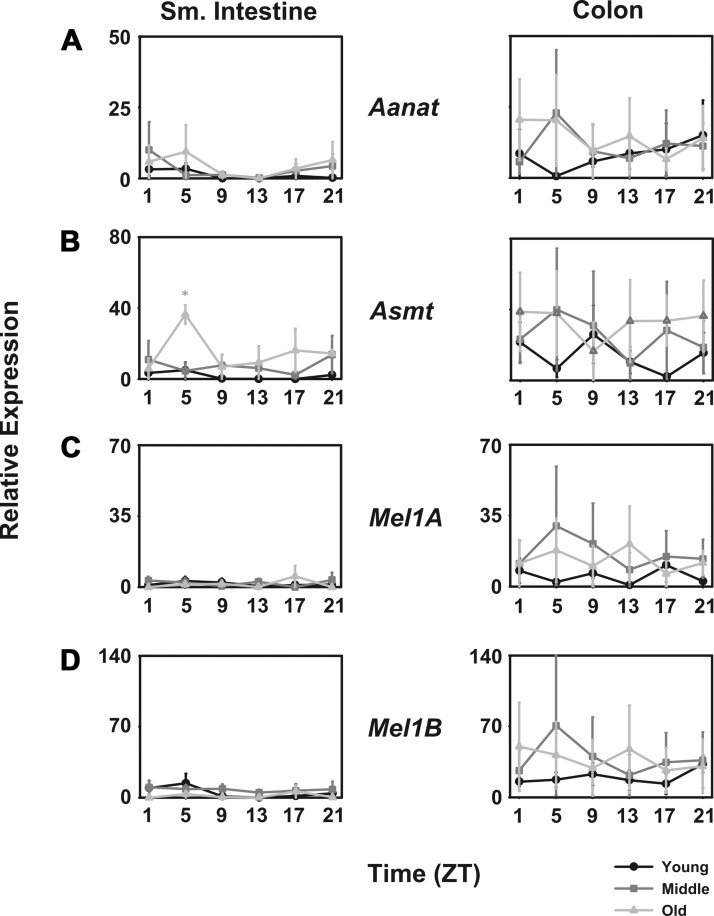
In the small intestine, mRNA encoding arylalkylamine-N-acetyltransferase (mAanat) was detected at very low levels, but it was not rhythmic. In the colon, higher levels of mAanat were detected. In young mice, aanat levels were higher during the night than during the day, but in middle-aged and aged mice, levels were higher during the day than during the night (Fig. 1A). The mRNA for acetylserotonin O-methyltransferase (mAsmt) in the small intestine was expressed with a daily pattern in which levels were higher during the day in old mice, but not rhythmic in young or middle-aged mice. In the colon, mAsmt levels were highly variable, but there were no statistically significant changes over the course of the day or among age groups (Fig. 1B).
Fig. 1.
Melatonin biosynthesis and receptor transcripts are expressed in both small intestine and colon. Comparison of young (black lines with circles), middle-aged (dark gray lines with squares), and old-aged (light gray lines with triangles) mice (n = 5 per age per time point) of relative abundance in mAanat (A), mAsmt (B), mMel1a (C), and mMel1b transcripts (D). Shade-matched asterisks indicate significant difference as determined by 2-way ANOVA. x-Axes are plotted as Zeitgeber time (ZT), where ZT0 corresponds to lights on and ZT14 corresponds to lights off in a 14:10 light-dark cycle.
Melatonin receptors.
No statistically significant rhythm in either the mMel1a (mMt1) or mMel1b (mMt2) melatonin receptor mRNA could be determined. Further, no differences among the age groups could be determined due at least in part to the very high variability among samples (Fig. 1, C and D).
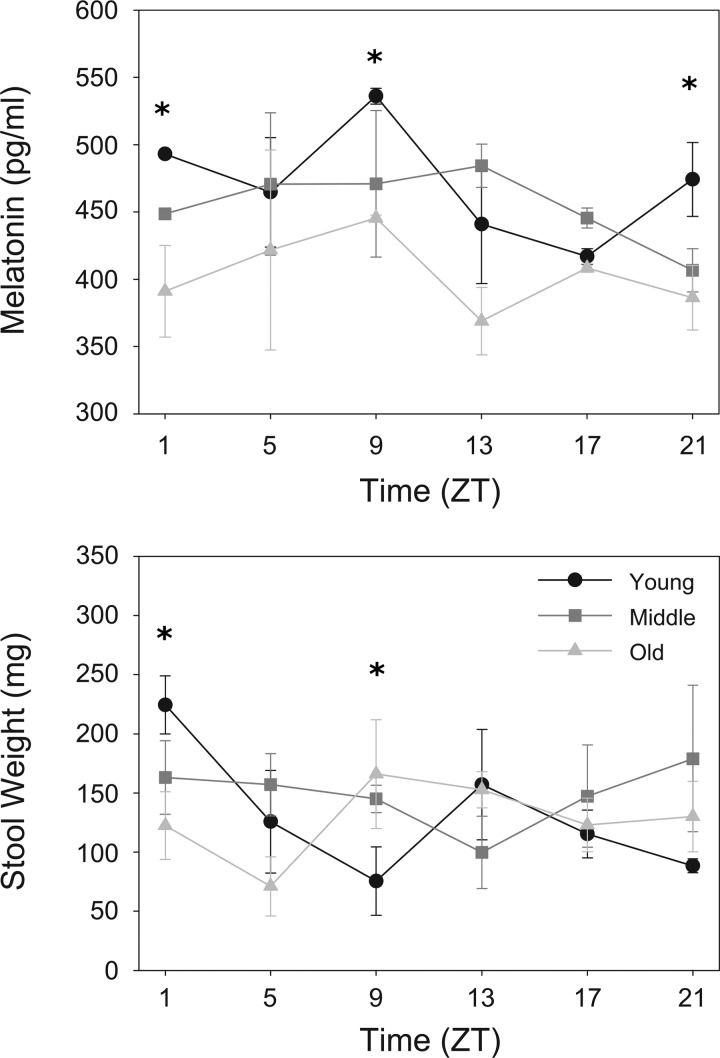
Stool melatonin content.
In contrast to the modest or nonexistent differences across the day and among age groups in mRNA encoding melatonin biosynthesis enzymes and receptors, there are profound differences in melatonin content in mouse feces (Fig. 2, top). In young mice, there is a high amplitude pattern of melatonin content that peaks between late day [Zeitgeber time (ZT)9] and the beginning of the night (ZT13). In middle-aged mice, the amplitude of the rhythm is diminished albeit significant, peaking in the middle of the night at approximately the same time. In aged mice, no daily patterns in melatonin content was determined.
Fig. 2.
Rhythms of melatonin in stool and stool weight diminishes with age. Top: melatonin content in stool taken from young (black lines with circles), middle-aged (dark gray lines with squares), and old-aged (light gray lines with triangles) was assayed by ELISA (n = 3–5 samples per time point per mouse) and reported as % of minimum value. Bottom: stool weights measured from timed collections (20 min for n = 5 mice per time point). Shade-matched asterisks indicate significant difference determined by 2-way ANOVA. x-Axis is plotted as Zeitgeber time (ZT), where ZT0 corresponds to lights on and ZT14 corresponds to lights off in a 14:10 light-dark cycle.
Stool weight measurement.
Stool weight, a measure of colonic motility, showed similar age-related decline in amplitude as stool melatonin content (Fig. 2, bottom). In young mice, stool weight was highest at ZT1 and decreased until ZT13. In middle-aged mice, peak stool weight was measured at ZT9, and no discernible oscillation was found in old mice.
Clock Genes
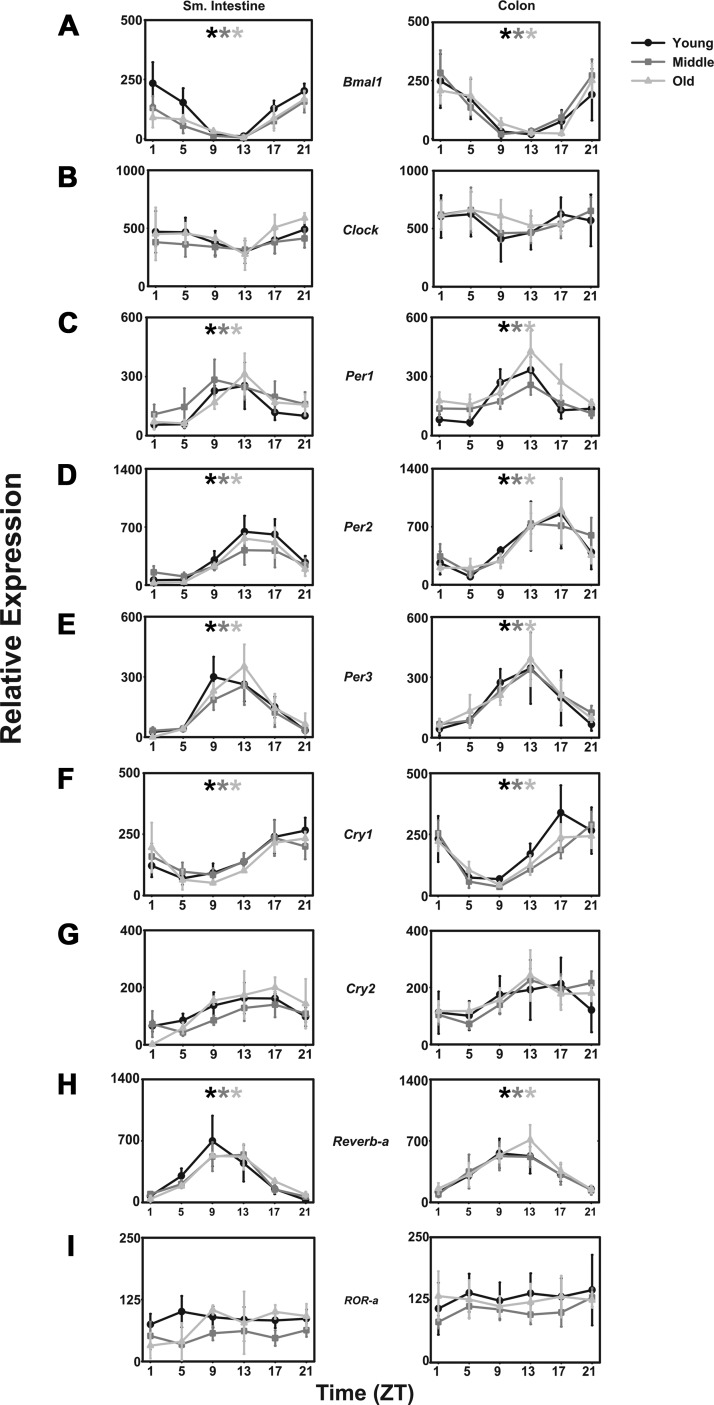
Positive elements.
Transcript levels for mBmal1 varied significantly over the course of the day (F = 10.539, P < 0.001), peaking from ZT21 to ZT24/1 with a nadir at ZT13 in both small intestine and colon (Fig. 3A). No differences were detected among young, middle-aged, or aged mice. mClock mRNA was not rhythmic in either tissue, and no differences were detected in either tissue at any age (Fig. 3B).
Fig. 3.
Intestinal clock gene expression in light-dark cycles is not affected by age in CBA/J mice. Comparison of young (black lines with circles), middle-aged (dark gray lines with squares), and old-aged (light gray lines with triangles) mice (n = 5 per age per time point) of relative abundance in mBmal1 (A), mClock (B), mPer1 (C), mPer2 (D), mPer3 (E), mCry1 (F), mCry2 (G), mReverb-α (H), and mRor-α transcripts (I). x-Axes are as in previous figures. Shade-matched asterisks indicate rhythmic expression as determined by Circwave software.
Negative elements.
Transcript levels for mPer1, mPer2, and mPer3 all varied significantly over the course of the day (F = 8.523, P < 0.001; F = 6.918, P < 0.001; and F = 9.083, P < 0.001) peaking at ZT13, ZT17, and ZT13, respectively, with nadirs between ZT1 and ZT5 in both small intestines and colon. As with the positive elements, no differences among young, middle-aged, and aged mice were detected (Fig. 3C mPer1; Fig. 3D mPer2; Fig. 3E mPer3). Transcript levels of mCry1 also varied depending on the time of day (F = 14.104, P < 0.001), peaking at ZT 17 with a nadir from ZT 5 to ZT 9 (Fig. 3F). No differences were determined among the young, middle-aged, and aged mice. No significant daily changes in mCry2 mRNA could be detected, nor could any differences be determined among the ages of mice (Fig. 3G).
Bmal1 regulators.
In addition to the core positive and negative elements, mReverb-α and the RAR-related orphan receptor mROR-α, which are known to share the same response regulator in the mBmal1 promoter region, positively and negatively regulate mBmal1 transcription. Transcript levels of mReverb-α vary depending on the time of day (F = 10.709, P < 0.001). However, this pattern is not affected by age (Fig. 3H). In contrast, no rhythm or effect of age is apparent in mRor-α levels (Fig. 3I).
Transcription Factors
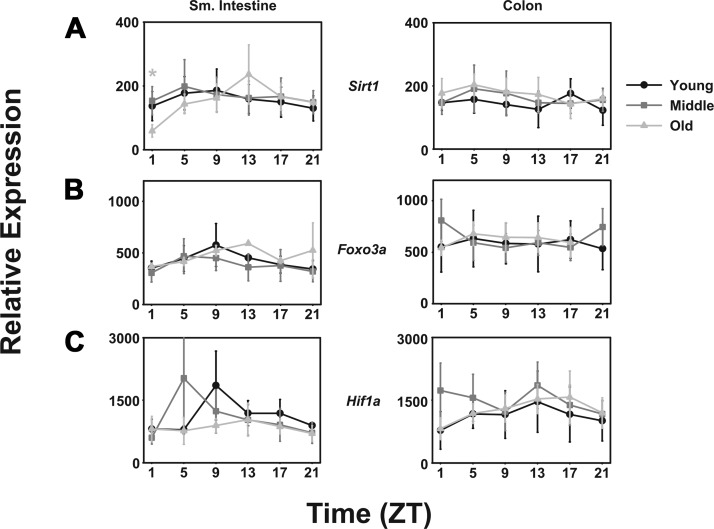
Sirtuin 1 (mSirt1) is a member of the sirtuin family of transcription factors that has been shown to deacetylate and affect the activity a variety of factors, including p53, PGC1-α, estrogen receptor ERR-α, and Bmal1. It has been associated with progeria and extension of life in both mice and Caenorhabditis elegans. In spite of this, no daily pattern in mSirt1 could be detected in either small intestine or colon, and no differences could be determined among differing ages (Fig. 4A).
Fig. 4.
Transcription factors expressed in the small intestine and colon are not affected by age in CBA/J mice. Transcription factor expression of mSirt1 (A), mFoxo3a (B), and mHif1-α (C) in young (black lines with circles), middle-aged (dark gray lines with squares), and old-aged (light gray lines with triangles) mice (n = 5 per age per time point). Shade-matched asterisks indicate rhythmic expression as determined by Circwave software. x-Axes are as in previous figures.
Forkhead box O3a (mFoxo3a) is a transcription factor that is characterized by a fork head DNA-binding domain and has been associated with longevity in humans as well as with several cancers. Similar to the situation with mSirt1, no daily rhythm in expression was detected, and no differences among the ages of mice was determined (Fig. 4B).
Hypoxia-inducible factor-1α (mHif1α) is a subunit of mHif-1, which encodes a basic helix loop helix PAS domain transcriptional regulator of cellular responses to hypoxia. mHif-1 mediates regeneration and repair responses. Neither tissue exhibited significant daily patterns of mHif1α expression, but in the small intestine, expression is lower in aged mice relative to middle-aged and young mice. No significant differences were detected in the colon (Fig. 4C).
Cell Signaling
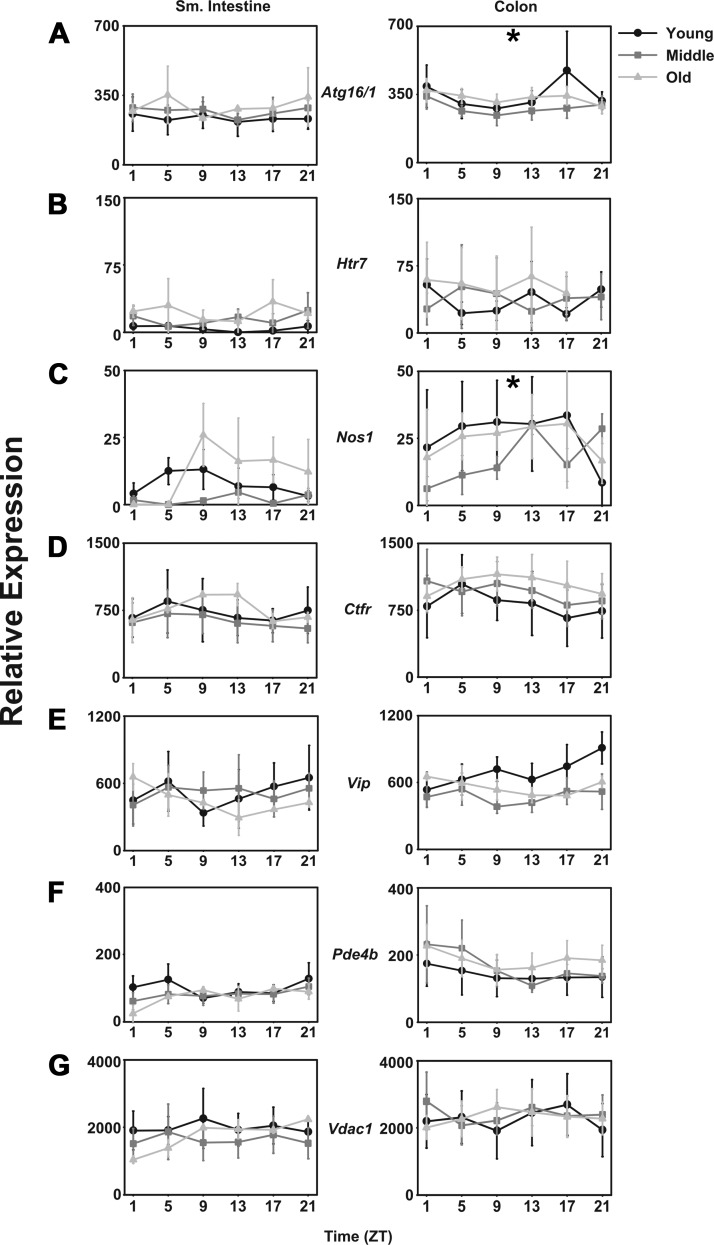
Autophagy-related protein 16-1 (mAtg16/1) is a member of the protein complex involved in autophagy through sequestration of cytoplasmic components into autophagosomes, which are in turn fused with lysosomes for degradation. The mRNA for mAtg16/1 is expressed rhythmically in young mice such that levels are higher during the night in the colon, but not in small intestine (Fig. 5A). In middle-aged and aged mice, there was no daily rhythm in mAtg16/1.
Fig. 5.
Cell signaling transcripts are not affected by aging in CBA/J mice. Relative expression levels in young (black lines with circles), middle-aged (dark gray lines with squares), and old-aged (light gray lines with triangles) mice (n = 5 per age per time point) of mAtg16/1 (A), mHtr7 (B), mNos1 (C), mCtfr (D), mVip (E), mPde4b (F), and mVdac1 (G) in small intestine and colon. Shade-matched asterisks indicate rhythmic expression as determined by Circwave software. x-Axes are as in previous figures.
In the small intestine, mRNA for the 5-hydroxytryptamine receptor 7 (mHtr7) is expressed at higher levels in middle-aged and aged mice than in young mice (Fig. 5B) and exhibits a bimodal pattern of expression in aged mice, peaking at ZT5 and ZT18. No daily patterns and no age differences in mHtr7 expression were determined in the colon (Fig. 5B).
While no nitric oxide synthase (mNos1) rhythm was detected in the small intestine of any age group, mNos1 expression was rhythmic in the colon of young mice. No daily pattern of expression was detected in the colon of middle-aged and aged mice, but the expression levels were similar in all age groups (Fig. 5C).
Cystic fibrosis transmembrane regulator (mCftr) is a membrane protein chloride channel in epithelial tissues, including the gastrointestinal tract. In this study, no daily changes nor any differences among age groups were detected (Fig. 5D) in either tissue. Similarly, the mRNA encoding vasoactive intestinal polypeptide (mVip), phosphodiesterase 4B (mPde4b), and voltage-dependent anion channel 1 (mVdac1) showed no daily patterns nor any effect of age (Fig. 5, E, F, and G, respectively).
Immune Function
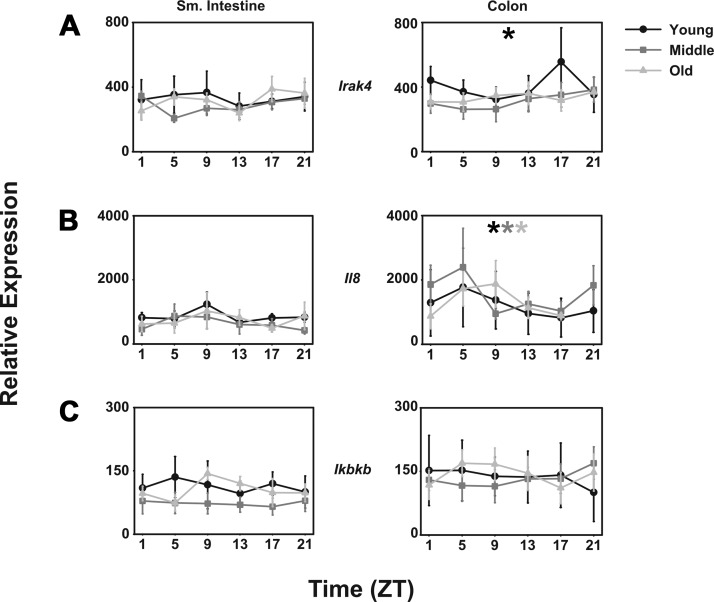
Interleukin-1 receptor-associated kinase 4 (mIrak4) is a protein kinase linked to innate immune responses through Toll-like receptors (TLRs) and mediating T cell responses. mIrak4 is expressed rhythmically in the colon of young mice such that levels are high during the night. However, this pattern is lost in middle-aged and aged mice and is not seen in the small intestine (Fig. 6A).
Fig. 6.
Immune-related transcripts are unaffected by aging in CBA/J mice. Comparison of young (black lines with circles), middle-aged (dark gray lines with squares), and old-aged (light gray lines with triangles) mice (n = 5 per age per time point) of relative abundance in mIrak4 (A), mIl-8 (B), and mIкbkΒ transcripts (C). Shade-matched asterisks indicate rhythmic expression as determined by Circwave software. x-Axes are as in previous figures.
Interleukin 8 (mIl8) is a chemokine that induces chemotaxis in neutrophils and macrophages. It also induces cells that express TLRs, including gastrointestinal epithelial cells, to release histamine. Expression of mIl8 is rhythmic such that levels are higher during the day than during the night. However, no effect of age could be determined on this pattern (Fig. 6B).
Inhibitor of kappa light polypeptide gene enhancer in B cells, kinase beta (mIкbkΒ) is a kinase essential for the activation of the immune regulator nuclear factor-кΒ (mNf-кΒ), another signal induced by several immunogenic signals, including TLRs and lipopolysaccharide. mIкbkΒ is not rhythmic in either small intestine or colon, and no significant effect of age is apparent (Fig. 6C).
DISCUSSION
In addition to the vast body of evidence showing that the rodent gastrointestinal system expresses a semi-independent circadian clock, there is ample evidence that human gastrointestinal function changes depending on the time of day (15), suggesting similar clock functions. Ambulatory colonic pressure recordings from healthy individuals exhibit maximal colonic activity during the day, especially following awakening and following a meal, and minimal activity during the night (22). Regulation of colonic motility is complex and involves the CNS, the peripheral nervous system, the enteric nervous system, as well as the muscle itself (11). Furthermore, clinical manifestations of gastrointestinal disease emerge when circadian clocks are disrupted. Gastrointestinal symptoms such as abdominal pain, constipation, and diarrhea are more common in shift workers and time zone travelers than in the general population (15). Although there are no objective clinical studies to date confirming that these symptoms are indeed due to abnormal gastrointestinal dysmotility in this population, both shift work and time zone traveling will disrupt the molecular clock network (27). Finally, as stated above, there is a large body of evidence showing that gastrointestinal function is deleteriously altered as animals and humans age (3, 8, 9, 28, 30).
Similar to previous transcriptomics studies of the colon, clock genes were rhythmically expressed in this study. It was, however, somewhat surprising then that the present study shows no systematic changes in the molecular clockworks as mice age from 2 mo to 2 yr of age, even as the aged mice were dying. Positive element mBmal1, negative elements mPer1, mPer2, mPer3, mCry1, and mCry2, as well as mReverbα, express daily rhythms in all age groups with undiminished amplitudes even into very old age in both small intestines and colon. In addition, although mClock and mRor-α are not expressed rhythmically in the gastrointestinal tissues studied here, there was no systematic changes during aging.
Even so, these data are consistent with the few published studies on clock gene expression during aging. Using transgenic rat tissue from the brain and peripheral tissues with a luciferase reporter construct and the mPer1 promoter, Yamazaki et al. (31) found that rhythms in bioluminescence in many explanted tissues were unaffected by age. These tissues included hypothalamic nuclei, cornea, kidney, liver, and lung. These authors do describe a “small but significant age-related shortening of the free-running period” in SCN explants and advanced phases of bioluminescence rhythms from explanted retrochiasmatic areas and hypothalamic paraventricular nuclei. Claustrat and colleagues (7) were able to find that the amplitudes of rhythms of expression of mPer1, mPer2, and mPer3 were reduced in the hearts and livers of aged mice. Unfortunately neither of the studies reported data from gastrointestinal tissues.
In contrast, our data show that age alters the temporal dynamics of mRNA encoding genes associated with melatonin biosynthesis and of fecal melatonin content. In young mice, expression of aanat mRNA is higher during the night than during the day, but in middle-aged and aged mice, colonic aanat mRNA is highest during the day. No significant daily pattern or age differences could be detected in mAsmt expression or in expression of melatonin receptor mRNA. There was a high amplitude rhythm of fecal melatonin content in young mice such that levels were highest in the early evening. In middle-aged mice, a significant daily pattern was retained, but the amplitude was much reduced, and the peak levels were delayed to midnight. This pattern was completely lost in the aged mice.
Melatonin has been implicated in aging as well as gastrointestinal health. In humans, there is a dramatic decrease in the amplitude of serum melatonin levels, presumably of pineal origin, as they age from 21–25 yr of age to 82–86 yr of age (24). The most dramatic decrease typically occurs between the ages of 50 and 60 yr. These decreases in amplitude have been suggested to contribute to aging itself through a decrease in purported antioxidant properties of the hormone, either through receptor-mediated actions or through direct chemical hydroxyl free radical scavenging properties of the hormone. Melatonin is also synthesized within the gastrointestinal system, although it is not clear whether gastrointestinal melatonin biosynthesis is rhythmic as it is in the pineal gland and retina (4). The data presented in this study are consistent with the previous studies showing decreased melatonin secretion associated with age (5). However, it is not clear what the source of the gastrointestinal melatonin is, nor is the mechanism of gastrointestinal melatonin biosynthesis altogether clear. The temporal pattern of mAanat mRNA in young mice is consistent with the increase in fecal melatonin during the night, but the presence of mAanat mRNA during the day in the middle-aged and aged mice is difficult to explain. Some authors have suggested that gastrointestinal melatonin may derive in part from dietary sources, but the diet was identical among age groups in this study.
Another question these observations raise is why should the gastrointestinal system secrete a neurohormone such as melatonin into the lumen of the gut? Aware of these data, our laboratory sought to ask whether any gastrointestinal microbes expressed sequences that were similar to known binding sites for the hormone. We queried the microbiome data set for sequence similarity with the melatonin binding sites in the MT1 and MT2 receptors and found several bacterial sequences with high identity. We then asked whether bacteria expressing these sequences were affected by physiological concentrations of the hormone. We were surprised to find that one, Enterobacter aerogenes, swarmed at a greater rate in the presence of nM melatonin concentrations. This effect was specific to melatonin, since other indoles did not elicit this response and not universally to all gut bacteria, since Escherichia coli does not respond to the hormone. This study also revealed a low-amplitude circadian rhythm of motility and gene expression in these bacteria (21). Thus, it is possible that at least one role for gastrointestinal melatonin may be as an exocrine signal to the microbiome. Ongoing research in our laboratory is addressing this and other questions associated with melatonin and the gastrointestinal microbiome.
Several species of mRNA exhibit a daily pattern in young mice, which is lost as the mice age. Among these, mAtg16/1, autophagy-related protein 16/1, is expressed more highly during the night than during the day in the colon of young mice. These data are consistent with a previous study in transcriptional profiling of the colon (14). However, this pattern is lost in middle-aged and aged mice, in which expression is constitutively low. Similarly, nos1 expression is rhythmically expressed in young mice, again consistent with Hoogerwerf et al. (14), but that pattern is lost in middle-aged and aged mice, this time with constitutively high levels of expression. Finally, mIrak-4, a protein kinase involved in signaling innate immune responses from TLRs, is expressed rhythmically with high levels of expression at night in young mice, but the night-time peak of expression is lost in middle-aged and aged mice.
It is interesting to note that rhythmic expression of mRrak4 in young mice and of il8 in the colons of all age groups has been associated with circadian regulation of microbiome complexity through their interaction with TLRs (19). It punctuates the view that gastrointestinal immune function is dynamically regulated. It may also point to age-related deficits, along with age-related decline in mAtg16/1, in gastrointestinal xenobiotic responses.
The most surprising aspect of this study is that, with the exception of melatonin biosynthesis, mNos1 and mIrak4, there is relatively little decline in the rhythmic expression of gastrointestinal genes. One possibility for this could be that this study was conducted in an LD cycle, so that the expression of free-running patterns of gene expression may be masked by the entrained clock system in the brain and gastrointestinal systems. This may be so, but, by and large, aging occurs normally in an LD cycle, not in DD. Second, it may be that because laboratory mice are maintained in very protected environments with an absence of predators, highly refined food ad libitum, and largely devoid of disease, these mice may not experience gastrointestinal distress associated with human age. Third, it may be that since the gastrointestinal system is replete with gastrointestinal stem cells (1), a high turnover of multiple cell types protects the tissue from the deleterious effects of age. Fourth, it is important to point out that this study focuses on just 40 genes that were selected based on our previous transcriptomics study (14) using a very different platform (Affymetrix DNA chips). It is possible that other genes expressed in the gastrointestinal system will show more pronounced effects of aging than shown here. In any case, the present study provides an important first step defining the cyclic patterns of gene expression and melatonin content in the laboratory mouse.
GRANTS
This project was funded by the National Institute of Health/National Institute of Aging RO1 AG-045833 awarded to V. M. Cassone.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.P. and V.M.C. conception and design of research; J.K.P. and C.V.C. performed experiments; J.K.P. and V.M.C. analyzed data; J.K.P. and V.M.C. interpreted results of experiments; J.K.P. prepared figures; J.K.P. and V.M.C. drafted manuscript; J.K.P. and V.M.C. edited and revised manuscript; J.K.P., C.V.C., and V.M.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Clifford Harpole, Kinga Graniczkowska, and Jacob Gunnell for helpful discussion.
REFERENCES
- 1.Andersson-Rolf A, Zilbauer M, Koo B-K, Clevers H. Stem Cells in Repair of Gastrointestinal Epithelia. Physiology (Bethesda) 32: 278–289, 2017. doi: 10.1152/physiol.00005.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 6: 544–556, 2005. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitar K, Greenwood-Van Meerveld B, Saad R, Wiley JW. Aging and gastrointestinal neuromuscular function: insights from within and outside the gut. Neurogastroenterol Motil 23: 490–501, 2011. doi: 10.1111/j.1365-2982.2011.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 47: 2336–2348, 2002. doi: 10.1023/A:1020107915919. [DOI] [PubMed] [Google Scholar]
- 5.Bubenik GA, Konturek SJ. Melatonin and aging: prospects for human treatment. J Physiol Pharmacol 62: 13–19, 2011. [PubMed] [Google Scholar]
- 6.Cassone VM. Melatonin’s role in vertebrate circadian rhythms. Chronobiol Int 15: 457–473, 1998. doi: 10.3109/07420529808998702. [DOI] [PubMed] [Google Scholar]
- 7.Claustrat F, Fournier I, Geelen G, Brun J, Corman B, Claustrat B. [Aging and circadian clock gene expression in peripheral tissues in rats]. Pathol Biol (Paris) 53: 257–260, 2005. doi: 10.1016/j.patbio.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Geokas MC, Conteas CN, Majumdar AP. The aging gastrointestinal tract, liver, and pancreas. Clin Geriatr Med 1: 177–205, 1985. doi: 10.1016/S0749-0690(18)30964-9. [DOI] [PubMed] [Google Scholar]
- 9.Geokas MC, Haverback BJ. The aging gastrointestinal tract. Am J Surg 117: 881–892, 1969. doi: 10.1016/0002-9610(69)90078-6. [DOI] [PubMed] [Google Scholar]
- 10.Honma S. The mammalian circadian system: a hierarchical multi-oscillator structure for generating circadian rhythm. J Physiol Sci 68: 207–219, 2018. doi: 10.1007/s12576-018-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoogerwerf WA. Role of clock genes in gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol 299: G549–G555, 2010. doi: 10.1152/ajpgi.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogerwerf WA, Hellmich HL, Cornélissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133: 1250–1260, 2007. doi: 10.1053/j.gastro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Hoogerwerf WA, Shahinian VB, Cornélissen G, Halberg F, Bostwick J, Timm J, Bartell PA, Cassone VM. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol 298: G143–G150, 2010. doi: 10.1152/ajpgi.00402.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogerwerf WA, Sinha M, Conesa A, Luxon BA, Shahinian VB, Cornélissen G, Halberg F, Bostwick J, Timm J, Cassone VM. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology 135: 2019–2029, 2008. doi: 10.1053/j.gastro.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 62: 139–150, 2011. [PubMed] [Google Scholar]
- 16.Malloy JN, Paulose JK, Li Y, Cassone VM. Circadian rhythms of gastrointestinal function are regulated by both central and peripheral oscillators. Am J Physiol Gastrointest Liver Physiol 303: G461–G473, 2012. doi: 10.1152/ajpgi.00369.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35: 445–462, 2012. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 153: 812–827, 2013. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms 21: 350–361, 2006. doi: 10.1177/0748730406293053. [DOI] [PubMed] [Google Scholar]
- 21.Paulose JK, Wright JM, Patel AG, Cassone VM. Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. PLoS One 11: e0146643, 2016. doi: 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao SSC, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol 280: G629–G639, 2001. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 23.Reis PP, Waldron L, Goswami RS, Xu W, Xuan Y, Perez-Ordonez B, Gullane P, Irish J, Jurisica I, Kamel-Reid S. mRNA transcript quantification in archival samples using multiplexed, color-coded probes. BMC Biotechnol 11: 46, 2011. doi: 10.1186/1472-6750-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiter RJ. The pineal gland and melatonin in relation to aging: a summary of the theories and of the data. Exp Gerontol 30: 199–212, 1995. doi: 10.1016/0531-5565(94)00045-5. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh Y, Nihonmatsu I, Kawamura H. Transplantation of the suprachiasmatic nucleus in the rat. Acta Neurochir Suppl (Wien) 41: 41–45, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159: 514–529, 2014. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 28.Thomson AB, Keelan M. The aging gut. Can J Physiol Pharmacol 64: 30–38, 1986. doi: 10.1139/y86-004. [DOI] [PubMed] [Google Scholar]
- 29.Waggott D, Chu K, Yin S, Wouters BG, Liu F-F, Boutros PC. NanoStringNorm: an extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics 28: 1546–1548, 2012. doi: 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiskur B, Greenwood-Van Meerveld B. The aging colon: the role of enteric neurodegeneration in constipation. Curr Gastroenterol Rep 12: 507–512, 2010. doi: 10.1007/s11894-010-0139-7. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA 99: 10801–10806, 2002. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]