Ischemic heart disease is the leading cause of death globally that accounts for approximately 7.4 million deaths every year (1). It kills cardiomyocytes due to the limited blood supply from obstruction of coronary arteries. Although reperfusion therapy is routinely used to restore the blood supply for ischemic myocardium, this treatment may paradoxically worsen cardiomyocyte death through reperfusion-induced inflammation and oxidative damage (2). When the ischemia-reperfusion injury (IRI) occurs, the loss of cardiomyocytes is usually initiated by apoptosis, a programmed death of cells associated with various cardiovascular diseases (3). Studies showed that inhibiting apoptosis could reduce reperfusion injury and attenuate the following ventricular remodeling and heart failure (4,5). To achieve better outcomes, therapeutic interventions need to be performed in a timely manner with the assistance of early apoptosis detection. To this end, molecular imaging has played an indispensable role in detecting myocardial apoptosis in vivo (6). In addition, molecular imaging also allows for noninvasive and systematic assessments on these therapeutic interventions.
Molecular imaging of apoptosis commonly uses radiolabeling to target molecular markers associated with apoptosis process, including externalized phospholipid, activated caspases, and dissipated mitochondrial transmembrane potential (Δψm) (Figure 1) (7–12). After administrating radiotracers, radionuclide imaging is used to detect the radiolabeled apoptosis cells in vivo. For the detection of myocardial apoptosis, the typical approach combines single photon emission computed tomography (SPECT) imaging with Technetium-99m-labeled annexin V (99mTc-Annexin-V), which has been proven to detect IRI and acute cardiac allograft rejection in several clinical trials (13,14). It uses annexin V to bind to the externalized phosphatidylserine (PS) on the outer leaflet of apoptotic cell membrane, one of the phospholipid alterations of cell membrane associated with apoptosis. However, as a large molecule with positive charges, 99mTc-Annexin-V inflicts significant radiation burden to non-target organs, especially to the kidney (14,15). Recently, minimizing exposure to radiation burden has received increasing attention in cardiovascular imaging (16,17), particularly for serial monitoring of cardiac therapies. In the last decade, the problem of significant radiation burden has in part slowed down the clinical applications of 99mTc-Annexin-V. Our recent studies indicated that radiation burden from cardiac radionuclide imaging could increase the DNA damage and gene activations for apoptosis and DNA repair (18,19). This shows that to facilitate apoptosis imaging for cardiovascular diseases, a more advanced imaging approach with sensitive detection of myocardial apoptosis but reduced radiation burden is needed.
Figure 1. Central pathways and imaging targets for apoptosis.
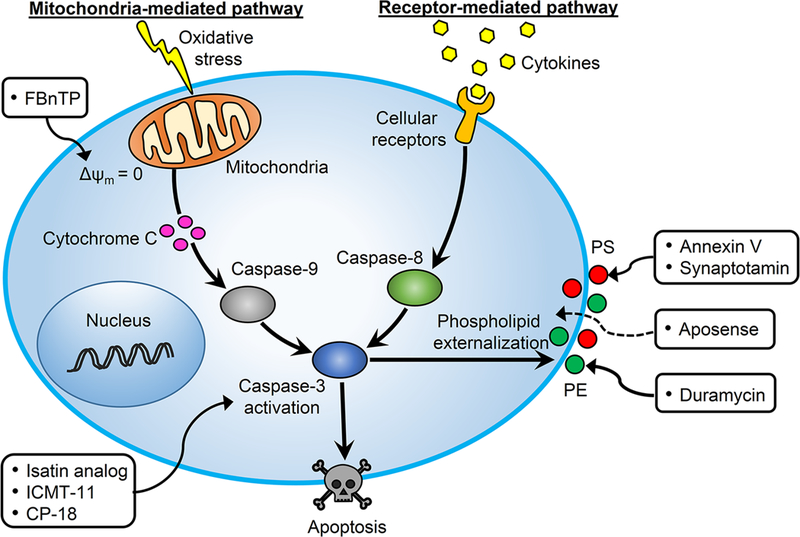
Both extrinsic pathway (cell-surface receptors) and intrinsic pathway (mitochondria) activate caspase-3 to finally result in apoptosis. The externalized phospholipid (i.e., PS and PE), activated caspase-3, and dissipated Δψm can be targeted by various radiotracers for noninvasive imaging of apoptosis. PS: phosphatidylserine; PE: phosphatidylethanolamine; Δψm: mitochondrial transmembrane potential.
To provide an alternative approach, Kawai et al. validated the feasibility of using radiolabeled Duramycin to detect myocardial apoptosis post-IRI with effective target uptake but reduced radiation burden for non-target organs (20). 99mTc-Duramycin was developed to bind to the externalized phosphatidylethanolamine (PE), another phospholipid maker on apoptotic cell membrane (21). Similar to PS, PE is a rich phospholipid in the inner leaflet of the cell membrane but will externalize onto the outer cell surface during apoptosis. By employing 99mTc-Duramycin to target PE, SPECT can detect the apoptotic cardiomyocytes post-IRI (22). In this study, Kawai et al. made several important findings on imaging myocardial apoptosis with 99mTc-Duramycin. First, using IRI animal models, they demonstrated that similar to 99mTc-Annexin-V, 99mTc-Duramycin was also an effective radiotracer to target myocardial apoptosis. In vivo microSPECT imaging showed that targeting PE with 99mTc-Duramycin detected similar myocardial apoptosis compared to targeting PS with 99mTc-Annexin-V. Significantly higher uptake of both tracers was found in the infarct region compared to the remote zone, demonstrating the specific uptake of 99mTc-Duramycin for the myocardium with elevated apoptosis. Additionally, simultaneous administration of fluorescent Duramycin and Annexin V showed that both tracers co-localized in the same infarcted regions. Second, they found an important advantage of 99mTc-Duramycin over 99mTc-Annexin-V in that 99mTc-Duramycin led to significantly lower radiation burden to the kidney (Duramycin: 0.358±0.210 %ID/g versus Annexin V: 1.58±0.316 %ID/g, P<0.001). Moreover, as Duramycin was a small 19-amino-acid peptide that could be rapidly cleared from the kidney, its overall off-target radiation was also less than that of 99mTc-Annexin-V. Lastly, besides detecting IRI, 99mTc-Duramycin could also detect the decreased apoptosis in minocycline-treated acute myocardial infarction, making it possible to use 99mTc-Duramycin for serial monitoring of therapeutic interventions for IRI.
The aforementioned advantages of 99mTc-Duramycin highlight its translational potential for clinical applications. Compared to the well-established apoptosis imaging of 99mTc-Annexin-V, 99mTc-Duramycin imaging is equally effective at detecting myocardial apoptosis but with less radiation burden, which overcomes an important clinical limitation of current apoptosis imaging. Moreover, as an antibiotic molecule, Duramycin has been used safely in humans, thus could be translated rapidly into clinics. In addition, its favorable dynamic range for detecting apoptosis allows 99mTc-Duramycin imaging to monitor therapeutic interventions by tracking the diminishing levels of apoptosis after anti-apoptotic therapies. However, before its clinical translation, 99mTc-Duramycin still needs more comprehensive validation on long-term safety and effective detection of myocardial apoptosis in patients. Future efforts may also involve exploring the clinical application of 99mTc-Duramycin imaging in other cardiovascular diseases, including heart failure, atherosclerosis, and cardiac allograft rejection.
ACKNOWLEDGEMENT
This publication was supported in part by research grants from National Institutes of Health (NIH) R01 HL133272, R01 HL132875, T32 EB009035, California Institute of Regenerative Medicine (CIRM) DR2A-05394 and RT3–07798 (J.C.W.), and the American Heart Association 17SDG33460212 (X.Q.).
Footnotes
Disclosures
None.
REFERENCES
- 1.Cardiovascular diseases fact sheet. World Health Organization 2017, Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
- 2.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eefting F, Rensing B, Wigman J et al. Role of apoptosis in reperfusion injury. Cardiovasc Res 2004;61:414–26. [DOI] [PubMed] [Google Scholar]
- 4.Garg S, Hofstra L, Reutelingsperger C, Narula J. Apoptosis as a therapeutic target in acutely ischemic myocardium. Curr Opin Cardiol 2003;18:372–377. [DOI] [PubMed] [Google Scholar]
- 5.Chandrashekhar Y, Sen S, Anway R, Shuros A, Anand I. Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol 2004;43:295–301. [DOI] [PubMed] [Google Scholar]
- 6.Chen IY, Wu JC. Cardiovascular molecular imaging: focus on clinical translation. Circulation 2011;123:425–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korngold EC, Jaffer FA, Weissleder R, Sosnovik DE. Noninvasive imaging of apoptosis in cardiovascular disease. Heart Fail Rev 2008;13:163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challapalli A, Kenny LM, Hallett WA et al. 18F-ICMT-11, a caspase-3-specific PET tracer for apoptosis: biodistribution and radiation dosimetry. J Nucl Med 2013;54:1551–6. [DOI] [PubMed] [Google Scholar]
- 9.Doss M, Kolb HC, Walsh JC et al. Biodistribution and radiation dosimetry of 18F-CP-18, a potential apoptosis imaging agent, as determined from PET/CT scans in healthy volunteers. J Nucl Med 2013;54:2087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Chu WH, Rothfuss J et al. Synthesis, radiolabeling, and in vivo evaluation of an F-18-labeled isatin analog for imaging caspase-3 activation in apoptosis. Bioorg Med Chem Lett 2006;16:5041–5046. [DOI] [PubMed] [Google Scholar]
- 11.Madar I, Huang Y, Ravert H et al. Detection and quantification of the evolution dynamics of apoptosis using the PET voltage sensor 18F-fluorobenzyl triphenyl phosphonium. J Nucl Med 2009;50:774–80. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A, Shirvan A, Levin G, Grimberg H, Reshef A, Ziv I. From the Gla domain to a novel small-molecule detector of apoptosis. Cell Res 2009;19:625–37. [DOI] [PubMed] [Google Scholar]
- 13.Hofstra L, Liem IH, Dumont EA et al. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet 2000;356:209–12. [DOI] [PubMed] [Google Scholar]
- 14.Narula J, Acio ER, Narula N et al. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med 2001;7:1347–52. [DOI] [PubMed] [Google Scholar]
- 15.Kemerink GJ, Liu X, Kieffer D et al. Safety, biodistribution, and dosimetry of 99mTc-HYNIC-annexin V, a novel human recombinant annexin V for human application. J Nucl Med 2003;44:947–52. [PubMed] [Google Scholar]
- 16.Nguyen PK, Wu JC. Radiation exposure from imaging tests: is there an increased cancer risk? Expert Rev Cardiovasc Ther 2011;9:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrashekhar Y, Shaw LJ, Narula J. Diagnostic imaging, radiation exposure, and carcinogenic risk: let’s be realistic, reasonable, and rational. JACC Cardiovasc imaging 2015;8:885–7. [DOI] [PubMed] [Google Scholar]
- 18.Lee WH, Nguyen P, Hu SJ et al. Variable activation of the DNA damage response pathways in patients undergoing single-photon emission computed tomography myocardial perfusion imaging. Circ Cardiovasc Imag 2015;8:e002851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen PK, Lee WH, Li YF et al. Assessment of the radiation effects of cardiac CT angiography using protein and genetic biomarkers. JACC Cardiovasc Imaging 2015;8:873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai H, Chaudhry F, Shekhar A et al. Molecular imaging of apoptosis in ischemia-reperfusion injury with radiolabeled duramycin targeting surface exposed phosphatidylethanolamine: effective target uptake and reduced non-target organ radiation burden. JACC Cardiovasc imaging 2017. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Li ZX, Bugenhagen S. Tc-99m-labeled duramycin as a novel phosphatidylethanolamine-binding molecular probe. J Nucl Med 2008;49:1345–1352. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Wang F, Fang W et al. The feasibility of imaging myocardial ischemic/reperfusion injury using Tc-99m-labeled duramycin in a porcine model. Nucl Med Biol 2015;42:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]


