Abstract
Cardiovascular diseases such as heart failure and metabolic syndrome have high prevalence in the elderly population and are leading causes of death, disability, hospitalization, driving high healthcare costs worldwide. To reduce this social and economic burden there is urgency to find effective therapeutic targets. Several studies have linked the dysfunction of the Sympathetic Nervous System and β-adrenergic receptor signaling with the pathogenesis of age-related cardiovascular diseases. Therapeutic treatments that restore their functions have been shown to be effective in subjects with cardiovascular comorbidities. In fact, lifestyle interventions (such as exercise training and diet) as well as pharmacologic treatments (e.g. β-blockers or moxonidine) and mini-invasive interventions (renal sympathetic denervation) have beneficial effects on age-related cardiovascular diseases. In the current “Medicine in focus” article we will discuss the pathogenic role of the Sympathetic Nervous System in age-related cardiovascular diseases as well as current and new therapeutic approaches.
Keywords: adrenergic nervous system, β-adrenergic signaling, cardiovascular diseases, aging
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death, disability, hospitalization and responsible for rising healthcare costs worldwide. CVDs have dramatically high prevalence in the elderly population and include conditions that affect the structures/function of the heart and blood vessels such as heart failure (HF), hypertension, diabetes and peripheral arterial disease (PAD). Based on improved survival and age-expectancy, it is predicted that 40% of the US population will have some form of CVD by 2030(Heidenreich et al., 2011). Hence, to reduce this social and economic burden there is urgency to find new effective therapeutic targets. The risk of developing CVD is mainly determined by the presence of traditional cardiovascular (CV) risk factors such as hypertension, diabetes, dyslipidemia, metabolic syndrome (MS), sedentary lifestyle, smoking and aging. Small improvements in risk factors earlier in life can have a huge impact(Heidenreich et al., 2011). However, when preventive medicine and measures such as lifestyle changes are not rigorously followed CVDs are the result, especially in the aged. Therefore, it is crucial to use efficient therapeutic treatments to fight multiple comorbidities in the same time.
Accordingly, the Sympathetic Nervous System (SNS) is an interesting therapeutic target as it is activated and dysfunctional in presence of CVDs as well as during aging (Figure 1). The Autonomic Nervous System plays a central role on CV system regulation during both basal physiological states as well as during disease and is divided into two branches: the SNS and the parasympathetic nervous system. The SNS is composed by postganglionic nerves originating from the paravertebral and prevertebral ganglia, and by the sympathetic endings that are responsible for innervation of several organs including heart and vessels (Figure 2)(Lymperopoulos et al., 2013). Importantly, the adrenal medulla (AM) is the inner portion of adrenal gland and contains chromaffin cells that act as modified post-ganglionic nerves (Figure 2).
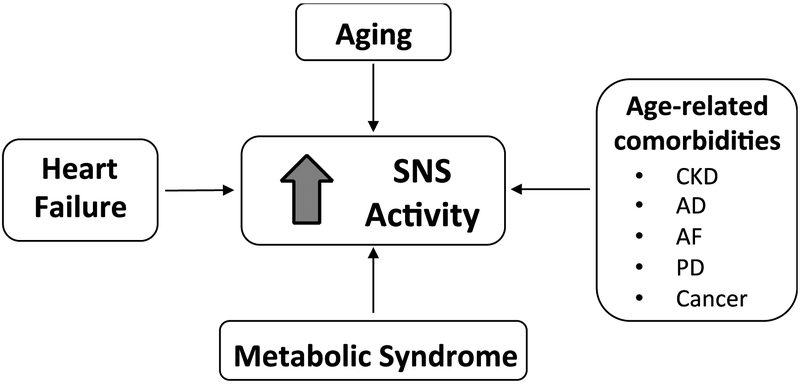
Figure 1: Sympathetic nervous system overactivity.
Sympathetic overdrive is typical of several age-related cardiovascular (heart failure, metabolic syndrome, AF) and non-cardiovascular diseases (CKD, AD, PD, Cancer) as well as aging per se. Acronyms: SNS, Sympathetic Nervous System, CKD, chronic kidney disease; AD, Alzheimer’s disease; AF, atrial fibrillation; PD, Parkinson’s disease.
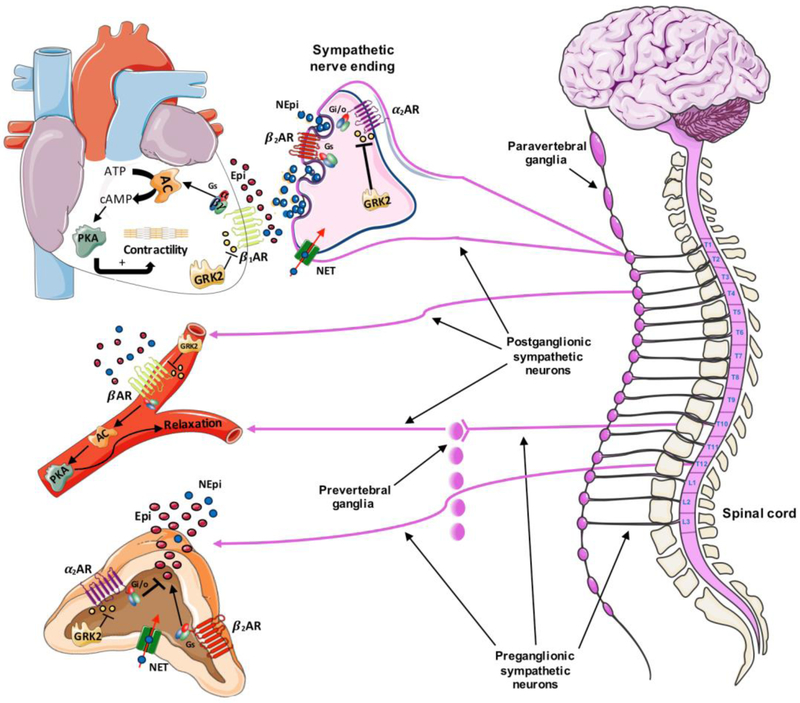
Figure 2: The Sympathetic Nervous System.
The Sympathetic Nervous System (SNS) includes 2 groups of neurons: the preganglionic neurons originate in the lateral horns of the 12 thoracic and the first 2 or 3 lumbar segments of the spinal cord and the post-ganglionic neurons originate in sympathetic ganglia (paravertebral or prevertebral). The preganglionic axons terminate either in paravertebral/prevertebral ganglia or in the chromaffin cells of the adrenal gland. The main neurotransmitter of postganglionic axons is norepinephrine (NEpi) (blue dots) while chromaffin cells release approximately 80% epinephrine (EPI) (red dots) and 20% NEpi in physiological conditions. NEpi and Epi bind the adrenergic receptor (AR) subtypes, (α-ARs and β-ARs). Catecholamines (CATs) binding to β-ARs (with β1-ARs being predominant in cardiomyocytes) activate the Gαs protein (stimulatory G protein) stimulating the adenylyl cyclase (AC) which converts the adenosine triphosphate (ATP) to the second messenger cyclic AMP (cAMP), ultimately activating the protein kinase A (PKA). PKA phosphorylates a variety of substrates (including phospholamban, L-type calcium channels and contractile proteins in the cardiomyocytes) leading to cardiomyocyte contractility or blood vessel relaxation. GRK2 (G protein-coupled receptor kinase 2) phosphorylation of the β-AR induces receptor desensitization, downregulation and recycling and, represents a cellular homeostatic mechanism. Of note, β2-AR and β3-AR can be also coupled to inhibitory Gi protein. In pathological conditions when SNS is overactive, GRK2 overexpression leads to β-AR dysregulation. In the chromaffin cells of adrenal medulla as well as the sympathetic nerve endings, the release of CATs is mainly regulated by the inhibitory α2-AR (acting via the Gi protein o other GO proteins) and the facilitatory β2-AR (via the Gs protein). Adrenal GRK2 phosphorylates and regulates the α2ARS in physiological conditions. During SNS hyperactive state such as heart failure (HF), adrenal GRK2 upregulation induces α2-AR dysregulation and increased CA secretion. The norepinephrine transporter (NET) is a transporter that is crucial for the clearance/reuptake of extracellular NEpi in both the synaptic cleft and the chromaffin cells. NET activity is impaired during HF as well as aging contributing to β-AR desensitization. Acronyms: NEpi, Norepinephrine; Epi, Epinephrine; AR, Adrenergic Receptor; GRK2, G protein-coupled Receptor Kinase 2; Gs, stimulatory G protein; Gi, inhibitory G protein; Go, other G protein; ATP, Adenosine Tri-Phosphate; AC, Adenylyl Cyclase; cAMP, cyclic Adenosine Mono-Phosphate; PKA, Protein Kinase A; NET, norepinephrine transporter. Adapted from Circ Res. 2013 Aug 30; 113(6):739-53.
SNS activity is regulated by several mechanisms including the aortic arch/carotid sinus (SNS inhibition) and cardiopulmonary (SNS inhibition) baroreceptor reflexes, peripheral chemoreceptors (SNS activation) and noradrenergic neurons in subcortical areas(Lymperopoulos et al., 2013). SNS is crucial for the so-called “fight or flight response” and is activated in stress conditions such as hypovolemia, hypoglycemia, hypoxia, as well as CV disease. The action of the SNS is carried out through the release of catecholamines (CATs): norepinephrine (NEpi, released by sympathetic nerve terminals and in a lesser amount by adrenal medulla) and epinephrine (Epi, released into the blood stream by the AM). The norepinephrine transporter (NET) is crucial for NEpi reuptake at the synaptic terminals/chromaffin cells and its function can be evaluated with a scintigraphy with radiolabeled metaiodobenzylguanidine (MIBG), a guanethidine analog of NEpi (Figure 2)(Rengo et al., 2016). CATs bind adrenergic receptors (ARs) that are members of the G protein-coupled receptor (GPCR) family. β-AR stimulation induces contractility in the cardiomyocytes and relaxation in the vasculature stimulating the smooth muscle cells (with β1-AR being dominant) (Figure 2)(de Lucia et al., 2018a;Schutzer et al., 2011). In chromaffin cells and sympathetic nerve endings, CA exocytosis is promoted by β2-ARs, while α2-ARs provide negative feedback(Lymperopoulos et al., 2013). In the cardiomyocytes, β1-ARs are prominent and after CAT simulation, triggers the stimulatory Gs protein with cAMP adenylylcyclase-mediated production followed by the activation of protein kinase A (PKA). Then, PKA phosphorylates several proteins including L-type Calcium channels and phospholamban, ultimately increasing cardiac contractility(de Lucia et al., 2018a). Of note, β2-AR and β3-AR can also couple to inhibitory Gi protein(de Lucia et al., 2018a).
β-AR function is regulated by homeostatic mechanisms including GPCR kinase (GRK)-mediated receptor phosphorylation, β-arrestin recruitment and eventually the receptor internalization, down-regulation and recycling(de Lucia et al., 2018a). Interestingly, one GRK, GRK2, found in the heart where it desensitizes β-ARs is also important in the SNS as it can mediate α2-AR down-regulation in adrenal medulla (Figure 2)(Lymperopoulos et al., 2013).
Several tools have been utilized to assess SNS activity/derangement including measurements of plasma CATs, cardiac or renal NEpi spillover, Ewing’ s battery tests, cold pressor test, heart rate variability (HRV), radiolabeled analogs of NEpi (e.g. MIBG imaging), and muscle and renal sympathetic nerve activity (MSNA, RSNA)(Lymperopoulos et al., 2013; Rengo et al., 2016). However, there is no gold standard tool for SNS assessment so far. In the current “Medicine in focus” article we will discuss the pathogenic role of SNS in age-related CVDs as well its potential as therapeutic target.
2. Pathogenesis
2.1. Aging
Aging is associated to impaired CV function together with SNS deregulation including cardiac and vascular β-AR signaling. With age plasma NEpi levels increase (due to increased spillover rate together with reduced clearance) and both MSNA (regardless of gender) and NEpi turnover in suprabulbar subcortical are augmented(de Lucia et al., 2018a; Esler et al., 2002). At cardiac level, increased NEpi spillover from sympathetic nerve endings and decreased NEpi metabolic clearance, has been found while Epi secretion from the adrenal medulla has been shown to be unchanged or reduced in older compared with young healthy subjects(Esler et al., 1995). Cardiac sympathetic innervation (evaluated with MIBG imaging) as well as the density/activity of the neuronal NEpi transporter are reduced with age(Leineweber et al., 2002; Rengo et al., 2016). Suprabulbar subcortical NEpi turnover is higher in elderly subjects and is correlated to cardiac NEpi spillover; data on arterial baroreflexes during aging are not consistent, instead(Esler et al., 2002).
Chronic CAT stimulation results in decreased β-AR density as well as impaired exercise tolerance and LV inotropic reserve in aged subjects(de Lucia et al., 2018a). Differently from HF, cardiac GRK expression and activity are unchanged with age and the cellular mechanism involved in the age-related β-AR desensitization is still unclear(de Lucia et al., 2018a). In addition, chronic CAT stimulation induces not only cardiac inflammation but also cardiac expression of p53, a pivotal regulator of cellular senescence in the endothelial cells(Katsuumi et al., 2018). Age-related vascular dysfunction has been associated to CV events (e.g. myocardial ischemia or PAD) as well as augmented risk of frailty/disability(Nappi et al., 2018). Of note, several studies have demonstrated that β-AR-induced vasorelaxation declines with age in with a GRK2-mediated mechanism (β-AR desensitization rather than downregulation)(Schutzer et al., 2011). Protein kinase A and the receptor coupling to Gαi proteins are not involved in the age-related vascular β2-AR desensitization, instead(Schutzer et al., 2006).
2.2. Heart failure
Cardiac dysfunction and HF result from several etiologies (ischemic injury, hypertension, valve disease, etc.) and are very common in the elderly population, drastically affecting survival rate(de Lucia et al., 2018a). SNS overactivity is a peculiarity of HF and is characterized by increased circulating CATs (secreted from the AM) as well as augmented NEpi spillover from cardiac sympathetic nerve endings(de Lucia et al., 2018a; Lymperopoulos et al., 2013). SNS activation is a compensatory response to counteract reduced contractility however, in the long-term, decisively contributes to cardiac dysfunction. Chronic CA stimulation triggers cardiac GRK2 upregulation impairing β-AR density/signaling, cardiac contractility as well as left ventricular (LV) remodeling and angiogenesis(de Lucia et al., 2018a). Interestingly, GRK2 overexpression is also involved in adrenal α2-AR-mediated CAT-secretion during HF. The crucial contributing role of GRK2 in chronic HF has been widely demonstrated in both animal models and humans(de Lucia et al., 2018a). Lymphocyte GRK2 protein levels (surrogate of GRK2 cardiac levels) and HRV have been proposed as a useful marker for SNS derangement and HF progression in failing patients(de Lucia et al., 2018a). In addition, HF patients show decreased NET and impaired sympathetic innervation within the heart(Lymperopoulos et al., 2013; Rengo et al., 2016). SNS tools such as cardiac MIBG parameters, CAT levels, HRV and MSNA carry independent prognostic information for stratifying the risk of mortality, arrhythmias as well as HF progression in patients with HF(Barretto et al., 2009; Patel et al., 2017; Rengo et al., 2016). Elderly HF patients, show a further reduction in MIBG uptake suggesting additional sympathetic dysfunction in the heart compared to adult HF patients(Rengo et al., 2016).
2.3. Metabolic Syndrome
MS, characterized by central obesity, dyslipidemia, elevated fasting glucose and hypertension, is common in older people and predisposes the affected to develop other CVDs(Sumner et al., 2012). Components of MS diverged between young and old adults with the latter showing higher glucose intolerance and blood pressure (BP)(Sumner et al., 2012). Interestingly, several constituents of MS have been associated to SNS hyperactivity (both in patients and animal models) that has a crucial pathophysiological role and contribute to the tremendous CV complications(Lambert et al., 2010). Numerous prospective studies have shown SNS overactivity as well as polymorphisms in the β2- and β3-AR to predict the development of MS features(Lambert et al., 2010; Masuo et al., 2005). In fact, obese as well hypertensive subjects show elevated plasma and urinary CAT levels, increased RSNA and MSNA and, SNS measures predict dietary weight loss in obese MS patients(Lambert et al., 2010; Straznicky et al., 2012).
Several factors have been implicated in the sympathetic activation that occurs in obesity such as hyperinsulinemia, hyperleptinemia, non-esterified fatty acids, proinflammatory cytokines and obstructive sleep apnea while during hypertension the main drivers are arterial baroreceptor/baroreflex and cardiopulmonary receptor dysfunction and renin–angiotensin–aldosterone system activation(Lambert et al., 2010). Interestingly, the presence of hypertension further increases sympathetic activation in obese patients(Huggett et al., 2004). MS is associated with early cardiac autonomic dysfunction and diabetes has been shown to reduce cardiac β1-AR and sympathetic innervation in HF patients(Olivieri et al., 2014; Paolillo et al., 2013). Moreover, insulinresistance has been shown in HF patients and GRK2 appears to be a crucial hub in crossregulation of insulin receptor signaling and β-AR signaling as it can negative-regulate both pathways(de Lucia et al., 2018a). Interestingly, chronic β-AR stimulation plays a crucial role in the pathogenesis of insulin resistance in numerous tissues including the heart(Heather et al., 2009; Mangmool et al., 2017; Mangmool et al., 2016; Morisco et al., 2005). Long-term stimulation of β-ARs impairs insulin signaling (insulin-induced glucose uptake, insulin-induced auto-phosphorylation of the insulin receptor and Glucose transporter type 4 -GLUT4- expression) via Akt- and cAMP/PKA-dependent pathways in cardiomyocytes(Mangmool et al., 2016; Morisco et al., 2005). β2-AR has been shown as the main subtype involved and β2-AR stimulation inhibit insulin-induced translocation of GLUT4 to the plasma membrane in cardiomyocytes(Mangmool et al., 2016). Importantly, β-blocker treatment rescue isoproterenol-induced cardiac insulin resistance strongly suggesting the use of these drugs in diabetic patients with heart failure(Giles, 2002; Mangmool et al., 2016).
3. Therapy
Numerous studies have linked the dysfunction of the SNS and β-AR signaling with the pathogenesis of both age-related CVDs as well as aging. Hence, therapeutic treatments that restore their functions are effective in subjects with CV comorbidities. Exercise training and β-blockers have beneficial effects on both cardiac and vascular function in patients with CVDs(de Lucia et al., 2018a). Both these treatments can modulate SNS overactivity and restore β-AR signaling (mediated primarily through GRK2 lowering) in the heart and arteries (as shown in animal models of HF as well cardiac/vascular aging)(Leosco et al., 2003). In addition, adrenal GRK2 down-regulation, leading to restored α2-AR density and decreased CAT-secretion, has been shown as a crucial mechanism for both the treatments, too(de Lucia et al., 2018a; Lymperopoulos et al., 2013). Therefore, cardiac and adrenal GRK2 inhibition (via gene therapy with a peptide inhibitor known as the βARKct or with emerging pharmacological approaches) has been proposed as an effective treatment to counteract SNS overactivity during HF(de Lucia et al., 2018a). Importantly, β-blocker use in the elderly patients should be started at a low dose and then up-titrated slowly. In addition, it has been demonstrated that β-blockers are associated with reduced risks of death and rehospitalization in elderly patients with HF and reduced systolic function(Hernandez et al., 2009).
Lifestyle interventions (e.g. dietary treatments and physical activity) are commonly recommended in patients with MS to reduce BP as well a lose weight and, are associated to sympathetic inhibition (reduced MSNA and whole-body NEpi spillover)(Straznicky et al., 2010). Exercise training decreases SNS overdrive normalizing levels of sympathetic activity markers such HRV or plasma CAT levels in patients with cardiac dysfunction(Besnier et al., 2017; Leosco et al., 2013). Importantly, physical activity positively affects prognosis in healthy aged patients as well as elderly subjects with CVDs, improving cardiac and vascular performance(Nobre et al., 2016; Sarmento et al., 2017; Seals et al., 1994; Wisløff et al., 2007). Exercise training has additive effects to pharmacological and resynchronization therapy in patients with HF(Nobre et al., 2016; Wisløff et al., 2007). Recently, our group has demonstrated that a dietary restriction is also effective in an animal model of ischemic HF through SNS modulation (restored cardiac β-AR density and sympathetic innervation)(de Lucia et al., 2018b).
In addition, pharmacological inhibition of central sympathetic activity (e.g. the imidazoline I1 agonist Moxonidine) is effective in MS patients in reducing blood pressure and body weight as well metabolic parameters such as insulin-sensitivity and dyslipidemia(Chazova and Schlaich, 2013). Renal sympathetic denervation (RSD) has emerged as an invasive but safe approach to reduce sympathetic activation (reduced renal and whole-body SNA as well as MSNA)(Hering et al., 2013). This treatment is currently recommended in patients with resistant hypertension and has shown beneficial effects on glucose metabolism, insulin sensitivity and waist circumference in MS subjects(Tsioufis et al., 2017). Variable results have been published on the effects of RSD in HF patients due to small sample sizes. However, recent results from a meta-analysis of human studies and a large-animal HF model, suggest RDN to improve LV function in HF setting increasing the interest in the ongoing human studies evaluating the safety and efficacy of RSD in HF(Fukuta et al., 2017; Liao et al., 2017).
The SNS plays a crucial role in regulating several body’s responses. Dysautonomia is a common feature in age-associated CVDs and during ageing. Chronic stimulation of the SNS is particularly detrimental in case of multiple-comorbidities and negatively influences clinical phenotype and outcomes. Sympatho-inhibition (through lifestyle changes, pharmacological/minimally-invasive approaches and in a near future gene therapy) is an effective therapeutic strategy to counteract functional decline in elderly patient with CV comorbidities.
Acknowledgments:
Figures were created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Uniported License; https://smart.servier.com.
Funding:
This work was supported by National Institutes of Health (NIH P01 HL091799, NIH R37 HL061690, NIH P01 HL075443 [Project 2]) to W.J.K. and American Heart Association (postdoctoral fellowship 17POST33660942) to C.d.L..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE, 2009. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135(3), 302–307. [DOI] [PubMed] [Google Scholar]
- Besnier F, Labrunée M, Pathak A, Pavy-Le Traon A, Galès C, Sénard JM, Guiraud T, 2017. Exercise training-induced modification in autonomic nervous system: An update for cardiac patients. Ann Phys Rehabil Med 60(1), 27–35. [DOI] [PubMed] [Google Scholar]
- Chazova I, Schlaich MP, 2013. Improved Hypertension Control with the Imidazoline Agonist Moxonidine in a Multinational Metabolic Syndrome Population: Principal Results of the MERSY Study. Int J Hypertens 2013, 541689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucia C, Eguchi A, Koch WJ, 2018a. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front Pharmacol 9, 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucia C, Gambino G, Petraglia L, Elia A, Komici K, Femminella GD, D'Amico ML, Formisano R, Borghetti G, Liccardo D, Nolano M, Houser SR, Leosco D, Ferrara N, Koch WJ, Rengo G, 2018b. Long-Term Caloric Restriction Improves Cardiac Function, Remodeling, Adrenergic Responsiveness, and Sympathetic Innervation in a Model of Postischemic Heart Failure. Circ Heart Fail 11(3), e004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals DR, 2002. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol 282(3), R909–916. [DOI] [PubMed] [Google Scholar]
- Esler M, Kaye D, Thompson J, Jennings G, Cox H, Turner A, Lambert G, Seals D, 1995. Effects of aging on epinephrine secretion and regional release of epinephrine from the human heart. J Clin Endocrinol Metab 80(2), 435–442. [DOI] [PubMed] [Google Scholar]
- Fukuta H, Goto T, Wakami K, Ohte N, 2017. Effects of catheter-based renal denervation on heart failure with reduced ejection fraction: a systematic review and meta-analysis. Heart Fail Rev 22(6), 657–664. [DOI] [PubMed] [Google Scholar]
- Giles TD, 2002. Use of beta-blockers for heart failure in patients with diabetes mellitus. Postgrad Med 112(5 Suppl Unanswered), 32–37. [DOI] [PubMed] [Google Scholar]
- Heather LC, Catchpole AF, Stuckey DJ, Cole MA, Carr CA, Clarke K, 2009. Isoproterenol induces in vivo functional and metabolic abnormalities: similar to those found in the infarcted rat heart. J Physiol Pharmacol 60(3), 31–39. [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, Committee AHAAC, Council S, Intervention C o.C.R.a., Cardiology, C.o.C., Prevention, C.o.E.a., Arteriosclerosis, C.o., Biology, T.a.V., Cardiopulmonary, C.o., Care, C., Resuscitation, P.a., Nursing, C.o.C., Disease, C.o.t.K.i.C., Council on Cardiovascular Surgery and Anesthesia, a.I.C.o.Q.o.C.a.O.R. 2011. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123(8), 933–944. [DOI] [PubMed] [Google Scholar]
- Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP, 2013. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 61(2), 457–464. [DOI] [PubMed] [Google Scholar]
- Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC, 2009. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol 53(2), 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett RJ, Burns J, Mackintosh AF, Mary DA, 2004. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension 44(6), 847–852. [DOI] [PubMed] [Google Scholar]
- Katsuumi G, Shimizu I, Yoshida Y, Hayashi Y, Ikegami R, Suda M, Wakasugi T, Nakao M, Minamino T, 2018. Catecholamine-Induced Senescence of Endothelial Cells and Bone Marrow Cells Promotes Cardiac Dysfunction in Mice. Int Heart J 59(4), 837–844. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP, 2010. Sympathetic nervous activation in obesity and the metabolic syndrome--causes, consequences and therapeutic implications. Pharmacol Ther 126(2), 159–172. [DOI] [PubMed] [Google Scholar]
- Leineweber K, Wangemann T, Giessler C, Bruck H, Dhein S, Kostelka M, Mohr FW, Silber RE, Brodde OE, 2002. Age-dependent changes of cardiac neuronal noradrenaline reuptake transporter (uptake1) in the human heart. J Am Coll Cardiol 40(8), 1459. [DOI] [PubMed] [Google Scholar]
- Leosco D, Iaccarino G, Cipolletta E, De Santis D, Pisani E, Trimarco V, Ferrara N, Abete P, Sorriento D, Rengo F, Trimarco B, 2003. Exercise restores beta-adrenergic vasorelaxation in aged rat carotid arteries. Am J Physiol Heart Circ Physiol 285(1), H369–374. [DOI] [PubMed] [Google Scholar]
- Leosco D, Parisi V, Femminella GD, Formisano R, Petraglia L, Allocca E, Bonaduce D, 2013. Effects of exercise training on cardiovascular adrenergic system. Front Physiol 4, 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SY, Zhen Z, Liu Y, Au KW, Lai WH, Tsang A, Tse HF, 2017. Improvement of Myocardial Function Following Catheter-Based Renal Denervation in Heart Failure. JACC Basic Transl Sci 2(3), 270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Koch WJ, 2013. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 113(6), 739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangmool S, Denkaew T, Parichatikanond W, Kurose H, 2017. β-Adrenergic Receptor and Insulin Resistance in the Heart. Biomol Ther (Seoul) 25(1), 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangmool S, Denkaew T, Phosri S, Pinthong D, Parichatikanond W, Shimauchi T, Nishida M, 2016. Sustained βAR Stimulation Mediates Cardiac Insulin Resistance in a PKA-Dependent Manner. Mol Endocrinol 30(1), 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo K, Katsuya T, Fu Y, Rakugi H, Ogihara T, Tuck ML, 2005. Beta2- and beta3-adrenergic receptor polymorphisms are related to the onset of weight gain and blood pressure elevation over 5 years. Circulation 111(25), 3429–3434. [DOI] [PubMed] [Google Scholar]
- Morisco C, Condorelli G, Trimarco V, Beilis A, Marrone C, Sadoshima J, Trimarco B, 2005. Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ Res 96(2), 180–188. [DOI] [PubMed] [Google Scholar]
- Nappi C, Gaudieri V, Acampa W, Arumugam P, Assante R, Zampella E, Mannarino T, Mainolfi CG, Imbriaco M, Petretta M, Cuocolo A, 2018. Coronary vascular age: An alternate means for predicting stress-induced myocardial ischemia in patients with suspected coronary artery disease. J Nucl Cardiol. [DOI] [PubMed] [Google Scholar]
- Nobre TS, Antunes-Correa LM, Groehs RV, Alves MJ, Sarmento AO, Bacurau AV, Urias U, Alves GB, Rondon MU, Brum PC, Martinelli M, Middlekauff HR, Negrao CE, 2016. Exercise training improves neurovascular control and calcium cycling gene expression in patients with heart failure with cardiac resynchronization therapy. Am J Physiol Heart Circ Physiol 311(5), H1180–H1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri F, Bonafè M, Spazzafumo L, Gobbi M, Prattichizzo F, Recchioni R, Marcheselli F, La Sala L, Galeazzi R, Rippo MR, Fulgenzi G, Angelini S, Lazzarini R, Bonfigli AR, Brugè F, Tiano L, Genovese S, Ceriello A, Boemi M, Franceschi C, Procopio AD, Testa R, 2014. Age- and glycemia-related miR-126-3p levels in plasma and endothelial cells. Aging (Albany NY) 6(9), 771–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolillo S, Rengo G, Pagano G, Pellegrino T, Savarese G, Femminella GD, Tuccillo M, Boemio A, Attena E, Formisano R, Petraglia L, Scopacasa F, Galasso G, Leosco D, Trimarco B, Cuocolo A, Perrone-Filardi P, 2013. Impact of diabetes on cardiac sympathetic innervation in patients with heart failure: a 123I meta-iodobenzylguanidine (123IMIBG) scintigraphic study. Diabetes Care 36(8), 2395–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Pierce BR, Bodapati RK, Brown DL, Ives DG, Stein PK, 2017. Association of Holter-Derived Heart Rate Variability Parameters With the Development of Congestive Heart Failure in the Cardiovascular Health Study. JACC Heart Fail 5(6), 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengo G, Pagano G, Vitale DF, Formisano R, Komici K, Petraglia L, Parisi V, Femminella GD, de Lucia C, Paolillo S, Cannavo A, Attena E, Pellegrino T, Dellegrottaglie S, Memmi A, Trimarco B, Cuocolo A, Filardi PP, Leosco D, Ferrara N, 2016. Impact of aging on cardiac sympathetic innervation measured by. Eur J Nucl Med Mol Imaging 43(13), 2392–2400. [DOI] [PubMed] [Google Scholar]
- Sarmento AO, Santos ADC, Trombetta IC, Dantas MM, Oliveira Marques AC, do Nascimento LS, Barbosa BT, Dos Santos MR, Andrade MDA, Jaguaribe-Lima AM, Brasileiro-Santos MDS, 2017. Regular physical exercise improves cardiac autonomic and muscle vasodilatory responses to isometric exercise in healthy elderly. Clin Interv Aging 12, 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutzer WE, Xue H, Reed J, Oyama T, Beard DR, Anderson S, Mader SL, 2011. Age-related β-adrenergic receptor-mediated vasorelaxation is changed by altering G protein receptor kinase 2 expression. Vascul Pharmacol 55(5-6), 178–188. [DOI] [PubMed] [Google Scholar]
- Schutzer WE, Xue H, Reed JF, Mader SL, 2006. Effect of age on vascular beta2-adrenergic receptor desensitization is not mediated by the receptor coupling to Galphai proteins. J Gerontol A Biol Sci Med Sci 61(9), 899–906. [DOI] [PubMed] [Google Scholar]
- Seals DR, Taylor JA, Ng AV, Esler MD, 1994. Exercise and aging: autonomic control of the circulation. Med Sci Sports Exerc 26(5), 568–576. [PubMed] [Google Scholar]
- Straznicky NE, Eikelis N, Nestel PJ, Dixon JB, Dawood T, Grima MT, Sari CI, Schlaich MP, Esler MD, Tilbrook AJ, Lambert GW, Lambert EA, 2012. Baseline sympathetic nervous system activity predicts dietary weight loss in obese metabolic syndrome subjects. J Clin Endocrinol Metab 97(2), 605–613. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Lambert EA, Nestel PJ, McGrane MT, Dawood T, Schlaich MP, Masuo K, Eikelis N, de Courten B, Mariani JA, Esler MD, Socratous F, Chopra R, Sari CI, Paul E, Lambert GW, 2010. Sympathetic neural adaptation to hypocaloric diet with or without exercise training in obese metabolic syndrome subjects. Diabetes 59(1), 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner AD, Sardi GL, Reed JF, 2012. Components of the metabolic syndrome differ between young and old adults in the US population. J Clin Hypertens (Greenwich) 14(8), 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsioufis C, Dimitriadis K, Kasiakogias A, Kalos T, Liatakis I, Koutra E, Nikolopoulou L, Kordalis A, Ella RO, Lau EO, Grassi G, Papademetriou V, Tousoulis D, 2017. Effects of multielectrode renal denervation on elevated sympathetic nerve activity and insulin resistance in metabolic syndrome. J Hypertens 35(5), 1100–1108. [DOI] [PubMed] [Google Scholar]
- Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T, 2007. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115(24), 3086–3094. [DOI] [PubMed] [Google Scholar]