Abstract
Aims: This study compared facilitators and barriers to genetic testing and determined awareness about the Genetic Information Nondiscrimination Act (GINA) across young Hispanic and non-Hispanic white (NHW) breast cancer (BC) survivors.
Materials and Methods: Women diagnosed with BC of age ≤50 years in 2009–2012 were recruited through the Florida State Cancer Registry to complete a questionnaire.
Results: There were 1182 participants of which 61% (174/285) of Hispanic patients, and 65% (580/897) of NHW patients had BC testing. Among untested participants, the most common barriers were lack of testing recommendation (44% Hispanics, 32% NHW; p = 0.02) and cost-related concerns (41% Hispanics, 40% NHW; p = 0.83). Among tested participants, the top facilitators were as follows: (1) “To benefit my family's future” (70% Hispanic, 68% NHW), (2) “My doctor recommended testing” (60% Hispanic, 54% NHW), and (3) “Minimal cost to me” (59% Hispanic, 72% NHW). Only 27% of tested and 15% of untested women were aware of GINA; misuse of test results was reported as a barrier for only 6.5%.
Conclusions: Rates of genetic testing recommendation are lower among Hispanics, but both groups reported additional barriers. Most are unaware of GINA, yet misuse is not a highly cited barrier. Findings suggest the need to educate providers on the importance of recommending testing to all who meet criteria; increase awareness of newer options for more affordable testing; and bolster facilitators that may increase testing uptake.
Keywords: hereditary breast and ovarian cancer, access, genetic testing, barriers and facilitators, GINA awareness
Introduction
In the United States, breast cancer (BC) has the highest cancer incidence among women regardless of race or ethnicity, with an estimated 266,120 new cases and 40,920 deaths during 2018 (American Cancer Society, 2018). Furthermore, it is the most common cause of death from cancer in Hispanic women and the second most common among non-Hispanic white (NHW) women (Centers for Disease Control and Prevention, 2018). Approximately 5–10% of BC is due to highly penetrant inherited pathogenic variants (Claus et al., 1996), with BRCA1/2 (BRCA) being the most commonly found by current genetic tests (Buys et al., 2017). The high risks of breast (∼70%) and ovarian (up to 44%) (Kuchenbaecker et al., 2017) cancers among women with a BRCA pathogenic variant can be reduced to below that of the general population through proven cancer prevention and early detection options (Pal and Vadaparampil, 2012). Yet <10% of U.S. women with BRCA pathogenic variants are aware they are carriers (Drohan et al., 2012), with lower rates of BRCA testing in Hispanics (Hall et al., 2009; Jagsi et al., 2015). Thus, there is an urgent need to better understand factors associated with access and uptake of cancer genetic testing across populations.
All women diagnosed with BC of age ≤50 years should be offered cancer genetic risk assessment (CGRA) services according to national practice guidelines (National Comprehensive Cancer Network, 2018), yet few (50% or less) are referred by their health care provider (Bellcross et al., 2011; Trivers et al., 2011; Wood et al., 2014), and <1 in 5 who meet national criteria receive genetic testing for hereditary breast and ovarian cancer (Childers et al., 2017). Most BRCA testing has occurred in NHW populations compared with underserved minority populations, contributing to health care disparities in clinical cancer genetics (Hall et al., 2009; Anderson et al., 2012; Lynce et al., 2016; McCarthy et al., 2016). Racial, ethnic, and socioeconomic disparities have been well documented with regard to awareness, access, and uptake of CGRA and genetic testing services (Huang et al., 2013; Butrick et al., 2015; Cragun et al., 2015, 2017b; Hann et al., 2017).
Although many articles have reported low awareness of genetic testing among Hispanics (Vadaparampil, 2006; Pagán et al., 2009; Gammon et al., 2011; Huang et al., 2013), few have included information about barriers and facilitators. Across studies, the most common barriers to CGRA included lack of awareness, lack of physician referral, competing life concerns, out-of-pocket expenses, concerns about emotional reactions to the results, anticipated worry about family, concerns about reactions of family members, fears of genetic discrimination or misuse of information, and concerns about losing insurance (Thompson et al., 2003; Ramirez et al., 2006; Sussner et al., 2009, 2010, 2013; Kinney et al., 2010; Gammon et al., 2011; Vadaparampil et al., 2011; Hann et al., 2017). Key motivators or factors that facilitated testing included benefit to relatives, desire to know future cancer risks, provider recommendation/referral, desire to reduce cancer concerns or uncertainty, and opportunity to facilitate cancer screening and prevention (Gammon et al., 2011; Vadaparampil et al., 2011; Anderson et al., 2012). Only two large population-based studies have evaluated self-reported barriers and facilitators to CGRA among high-risk women with BC; one had low representation of Hispanics (<2%) (Anderson et al., 2012), whereas the other included 149 high-risk Hispanic women, but made no comparisons across race/ethnicity (Kurian et al., 2017).
The passing of the Genetic Information Nondiscrimination Act (GINA) in 2008 represents an effort to implement national protections against discrimination based on genetic information by health insurers and employers (Clifton et al., 2010), yet the impact of this law on testing access remains unclear. Findings from a limited number of studies among the general U.S. population suggest that ∼9–20% of adults are aware of GINA (Huang et al., 2013; Parkman et al., 2015). However, awareness was substantially higher among individuals who had genetic testing and were in contact with a hereditary breast and ovarian cancer advocacy group, with 46% being aware of GINA before survey completion between 2009 and 2010 (Allain et al., 2012). Most prior studies have been conducted among primarily Caucasians; thus, there remains a paucity of data about GINA awareness and impact among non-Caucasians and untested high-risk populations.
Existing research gaps in knowledge about differences in genetic services utilization, including awareness of GINA and facilitators or barriers to receipt of services in an ethnically diverse group of patients, have been identified by the U.S. Preventive Services Task Force (USPSTF) (Nelson et al., 2013). This information is needed to develop interventions to increase access to and utilization of genetic services across populations, and is particularly important given the potential for genomics to widen the gap in cancer health disparities (Levy et al., 2011; McClellan et al., 2013). Through a population-based sample of young NHW and Hispanic BC survivors, we sought to compare self-reported barriers and facilitators to genetic testing as well as awareness of GINA.
Materials and Methods
Participant recruitment
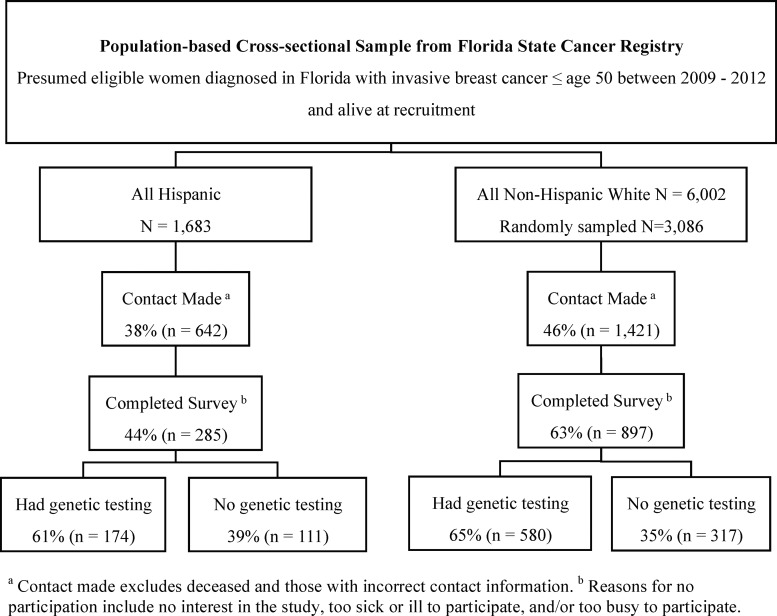
Eligible participants were NHW and Hispanic women diagnosed with invasive BC ≤50 between the years 2009 and 2012 living in Florida at the time of diagnosis and alive at the time of recruitment. Through protocols approved by the Institutional Review Boards (IRB) at the University of South Florida and the Florida Department of Health, recruitment to the study was initiated in 2014. After IRB approval, the Florida Cancer Data System (FCDS) released names and contact information on all eligible participants. Verification of contact information and vital status was performed using LexisNexis. Contact was attempted among all Hispanic women in the sampling frame and a random sample of white women (Fig. 1).
FIG. 1.
Recruitment from the Florida State Cancer Registry, participation rates, and prior genetic testing utilization.
Participants were recruited using state-mandated recruitment efforts as previously described (Bonner et al., 2016; Cragun et al., 2017b), which consisted of two recruitment packet mailings, 2 weeks apart. Recruitment packets in both English and Spanish (which included an introductory letter, consent form, questionnaire, and opt out response card) were mailed out in batches to a randomly selected sample of eligible NHW women and to all presumably eligible Hispanic women. If a response was not received within 3 weeks of the second mailing, a study team member attempted to contact the recruit by phone to explain the study and determine their interest in participating. Informed consent was obtained on those willing to participate. All participants were asked to complete a questionnaire and those who reported having genetic testing for hereditary breast cancer (HBC) were asked to complete a medical records release for verification of genetic test results.
Data collection and measures
Data collected from FCDS for all eligible women included year of birth, date and clinical details of diagnosis, race and ethnicity, primary payer at diagnosis, marital status, and employment status. Questionnaires were completed by participants either online or on paper and consisted of questions from several prior studies conducted by us and others, including basic demographic questions, reasons for not having genetic testing (19-item checklist with an open-ended response option to write in other barriers) (Anderson et al., 2012), and reasons for having genetic testing or what made it easier to get testing (motivators/facilitators) (6- and 8-item checklists with open-ended response options) (Anderson et al., 2012). We also asked whether they had previously heard of a federal law, GINA, which protects people from genetic discrimination by health insurers and employers. Ethnicity was reported by participants on the survey, and Hispanic participants were classified into two subgroups based on whether or not they reported speaking primarily Spanish at home. Individuals reported on the survey whether they previously had genetic testing for HBC and were asked to sign a release to obtain verification of results.
Data analysis
The primary objective of the parent study was to compare access to genetic testing between 400 Hispanic and 800 NHW women with 89% power to detect a difference of 10% between the proportions of individuals who accessed genetic testing across groups. Current analyses were not the primary aim of the parent study; thus, power was not calculated for this secondary objective.
Among participants, clinical and demographic characteristics from the survey were summarized and compared using Pearson chi-square tests for categorical variables and Mann–Whitney tests for continuous variables. The number of barriers and facilitators to genetic testing was calculated for Hispanic and NHW by calculating and comparing the proportions of women who endorsed each barrier and facilitator among untested and tested women, respectively. Specifically, barriers and facilitators endorsed by >5% of respondents were then ranked and compared across the two ethnic groups using Pearson chi-square tests. The Holm step-down procedure with an alpha of 0.05 was used to control the type 1 error rate when reporting significant differences for each of the three main sets of analyses comparing barriers, facilitators, and awareness of GINA.
Results
Participants included a total of 1182 BC survivors, consisting of 897 NHW and 285 Hispanics (168 English-speaking Hispanics and 117 Spanish-speaking Hispanics), which represents 63% and 44% of NHW and Hispanics, respectively, in the sampling frame with whom contact was established (Fig. 1). Comparisons between participants and all other presumed eligible women within each of the two respective ethnic strata revealed no statistically significant differences with regard to median age, histologic subtype, marital status, or employment at diagnosis (results not shown).
Of the 1182 participants, 754 reported prior testing at study enrollment (“tested participants”), including 61% (174/285) of Hispanics versus 65% (580/897) of NHW (p = 0.270) (Fig. 1). Of the 754 tested participants, 516 germline genetic test reports were obtained, including 68% through signed medical release and/or the participant providing the test result to the study team. Among Hispanics, those who primarily spoke Spanish at home were significantly less likely to have had testing than other Hispanics (50% Spanish-speaking Hispanic, 69% English-speaking Hispanic; p = 0.0009). Hispanic participants were significantly less likely to report annual household income ≥$25,000 (65% Hispanic, 84% NHW; p < 0.0001), to have private health insurance (66% Hispanic, 84% NHW; p < 0.0001), and to have been referred for genetic testing (79% Hispanic, 86% NHW; p = 0.02) (Table 1).
Table 1.
Clinical and Demographic Comparisons of Hispanic and Non-Hispanic White
| Hispanic | NHW | ||||
|---|---|---|---|---|---|
| N = 285 | N = 897 | ||||
| Characteristics | n | % | n | % | p |
| Had previous genetic testing | 174 | 61.1 | 580 | 64.7 | 0.27 |
| Referred for genetic testing | 226 | 79.3 | 769 | 85.7 | 0.02 |
| Diagnosed of age ≤45 yearsa | 144 | 50.5 | 432 | 48.2 | 0.49 |
| Triple negative (ER-/PR-/HER2-)b | 30 | 10.5 | 119 | 13.3 | 0.06 |
| Family history of breast cancer | 139 | 48.8 | 501 | 55.9 | 0.04 |
| Family history of ovarian cancer | 28 | 9.8 | 102 | 11.4 | 0.47 |
| Married or cohabiting | 192 | 67.4 | 644 | 71.8 | 0.15 |
| Has childrenc | 174 | 61.1 | 611 | 68.1 | 0.07 |
| Income ≥25,000d | 186 | 65.3 | 754 | 84.1 | <0.0001 |
| College education or higher | 147 | 51.6 | 504 | 56.2 | 0.38 |
| Private insurance | 188 | 66.0 | 754 | 84.1 | <0.0001 |
All women diagnosed at age 45 and below meet National Comprehensive Cancer Center criteria for genetic testing, whereas those aged 46–50 years should have a genetic risk evaluation, but must meet additional personal or family history criteria for testing.
Unknown for 13.7% of Hispanics (n = 39) and 9.1% of NHW (n = 82).
Unknown for 2.4% of Hispanics (n = 7) and 2.6% of NHW (n = 23).
Unknown for 6.3% of Hispanics (n = 18) and 7% of NHW (n = 63).
NHW, non-Hispanic white.
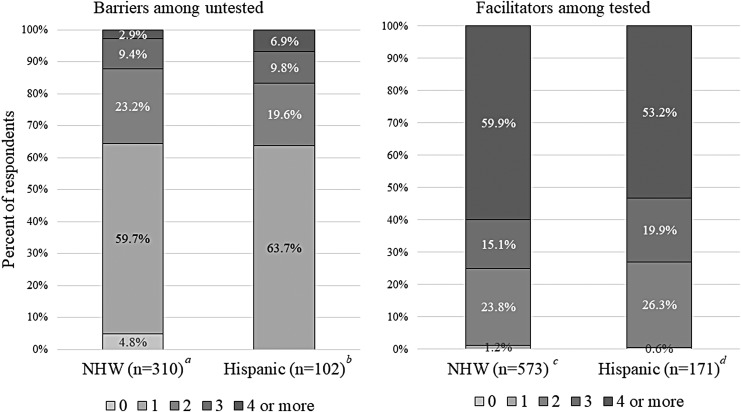
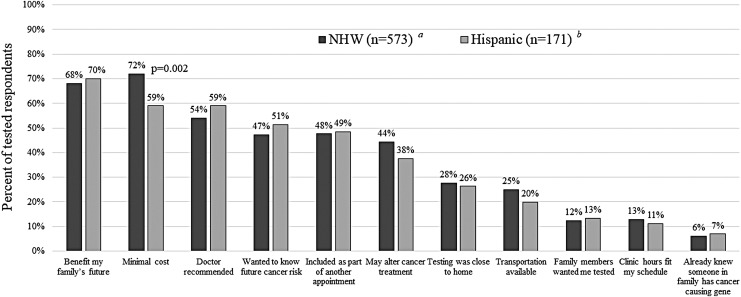
Among 754 tested participants, the numbers (Fig. 2) and types (Fig. 3) of facilitators were similar for Hispanics and NHW. The top three facilitators were as follows: (1) “To benefit my family's future” (70% Hispanic, 68% NHW), (2) “My doctor recommended I have testing” (60% Hispanic, 54% NHW), and (3) “Minimal cost to me” (59% Hispanic, 72% NHW). Other motivators or facilitators, endorsed by nearly half of both ethnic groups, included wanting to know about future risk for recurrent cancer, having genetic counseling as part of or in conjunction with a cancer treatment/follow-up appointment, and the potential to alter cancer treatment. Although less common, similar proportions of both ethnic groups cited proximity to home, availability of transportation, and ease of scheduling as additional facilitators of testing.
FIG. 2.
Number of barriers and facilitators reported among participant groups. Does not reflect the type of barrier or facilitator reported; therefore, multiple barriers or facilitators may comprise one category. Additional participants in each respective group who did not answer these questions include: a7 NHW; b9 Hispanic; c7 NHW; d3 Hispanic. NHW, Non-Hispanic White.
FIG. 3.
Most commonly reported facilitators among those who had genetic testing. P-values are listed for those that were statistically significant between the two groups. Additional participants in each respective group who did not answer these questions include: a7 NHW; b3 Hispanic. NHW, Non-Hispanic White.
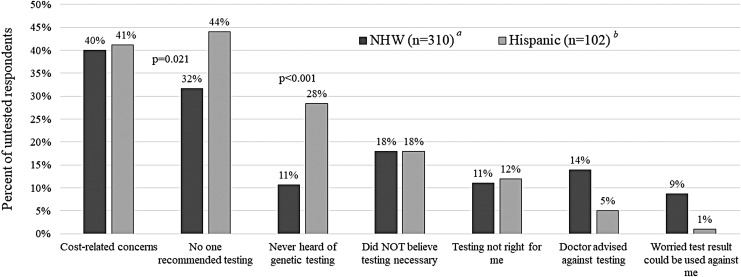
Among 412 untested participants, the number of barriers was similar across Hispanics and NHW (Fig. 2), with ∼36% reporting multiple barriers. Hispanics were significantly more likely to report having never heard of genetic testing (28% Hispanic, 11% NHW; p < 0.0001). Lack of awareness about the availability of genetic testing was the third most common barrier for Hispanics, but this was only the sixth most highly endorsed barrier among NHW participants. The two most commonly cited barriers to testing were lack of testing recommendation (44% Hispanic, 32% NHW; p = 0.021) and cost-related concerns (41% Hispanic, 40% NHW; p = 0.834). The vast majority of untested participants (75% NHW, 70% Hispanic) reported one or more additional barriers to testing besides or in addition to being unaware of testing or lacking a testing recommendation (Fig. 4).
FIG. 4.
Most commonly reported barriers among those who had not yet undergone genetic testing. P-values are listed for those that were statistically significant between the two groups. Additional participants in each respective group who did not answer these questions include: a7 NHW; b9 Hispanic. NHW, Non-Hispanic White.
Other barriers cited by >5% included the misconception that genetic testing was not necessary because they already had a cancer diagnosis, the feeling that testing was not right for them, and having a health care provider who advised against testing. Among the 48 participants (5 Hispanic, 43 NHW) who indicated a provider advised against testing, at least 15 clearly met National Comprehensive Cancer Center personal history criteria for genetic testing [either being diagnosed at or below age 45 (n = 11) or diagnosed with triple negative cancer at or below age 60 (n = 4)]. Among those who had not accessed genetic testing, 77% NHW and 84% Hispanic were interested in genetic testing resources or additional information (p = 0.148)
Of note, only 7% of all respondents reported concerns that their test result could be used against them as a reason for not having testing. Among those who reported this as a barrier, 19% were aware of GINA, which is similar to 14% awareness among the untested individuals for whom such concerns were not a barrier to testing. The proportion reporting awareness of GINA was the same across tested patients for whom we obtained results (27%) and those for whom results were not obtained (27%). The proportions who were aware of GINA differed significantly based on testing status (27% tested vs. 15% untested; p < 0.0001). After stratifying by testing status, differences in GINA awareness were not statistically significant across Hispanic and NHW populations (29% tested NHW, 22% tested Hispanic, 16% untested NHW, 12% untested Hispanic; p = 0.12).
Discussion
To our knowledge, this is the first study to systematically compare self-reported barriers and facilitators to hereditary cancer genetic testing and awareness of GINA across a large cancer registry-based sample of young Hispanics and NHW BC survivors. Our findings suggest similar numbers of facilitators and barriers across the two populations. Furthermore, among tested women, there were no substantial differences in types of reported facilitators among Hispanics compared with NHW. In contrast, among the untested group, Hispanics were significantly less aware of genetic testing and less likely to have received a testing recommendation from their provider compared with NHW. Yet, the vast majority of both groups were interested in testing resources or additional information. Most participants were unaware of GINA; awareness was lower among those who were untested, but was similar across ethnic groups.
Prior studies have reported that compared with NHW, Hispanic women have lower awareness about genetic testing and are less likely to receive a provider referral (Vadaparampil, 2006; Heck et al., 2008; Sussner et al., 2013; Hann et al., 2017), which is consistent with our findings. Testing rates in our study differ from a national sample of newly diagnosed BC patients <40 years of age, which showed that Hispanic women were half as likely to undergo genetic testing compared with NHW women (Levy et al., 2011). This difference in testing rates may be the result of population variation at the national and state levels, the impact of direct-to-consumer advertising that has previously occurred in Florida (Centers for Disease Control and Prevention (CDC), 2004; Mai et al., 2014), or increased awareness due to our more recent sampling frame. Nevertheless, access to genetic services may be improving among Hispanics, given that a more recent population-based study found no significant differences in receipt of genetic counseling between NHW and Hispanic, although the proportion of Hispanics accessing testing remained lower (Kurian et al., 2017).
Cost-related concern was the most frequently cited barrier for NHW participants in our study and the second most frequently cited barrier for Hispanic participants, consistent with prior reports that cost or out-of-pocket expenses are a common barrier to genetic testing among Hispanics (Kinney et al., 2010; Sussner et al., 2010; Vadaparampil et al., 2011). Despite the lower annual income and lower rates of private health insurance among Hispanics compared with NHW, similar proportions of untested participants across both ethnic groups reported financial concerns as a barrier to genetic testing. This finding highlights the ongoing need to continue to advocate for insurance coverage and policies that will encourage insurance companies to pay for genetic services.
Among our respondents, “Worry a genetic test result could be used against me (by an employer or future health insurance)” was the seventh most commonly endorsed barrier, cited by 38 NHW (9% of NHW) and only a single Hispanic participant (<1%). Young Latina women have previously reported less worry about misuse of genetic information and lower levels of medical mistrust compared with older Latina women (Sussner et al., 2009), perhaps explaining results among our young population. In addition, the frequency of concern about discrimination among NHW is consistent with the 9.5% of young, primarily NHW BC survivors who reported this barrier in a registry-based study of young BC survivors conducted in Michigan before the U.S. law (GINA) was passed to protect against discrimination by health insurers and employers (Anderson et al., 2012). The frequency of reported concerns varies across studies and may depend on the population, when they were sampled, and variation in question wording (Thompson et al., 2003; Ramirez et al., 2006; Sussner et al., 2009, 2010; Suther and Kiros, 2009). Furthermore, prior studies are inconsistent, revealing both lower rates of concerns among Hispanics (17.2% NHW vs. 7.9% Hispanic) (Gammon et al., 2011) and higher rates among Hispanics (20% NHW vs. 28% Hispanic) (Suther and Kiros, 2009). Notably, concerns may not always prevent genetic services access or uptake, as suggested by findings from a study of 120 Latina women, where concerns about genetic discrimination and medical mistrust failed to predict intention to have genetic testing in their multivariate model (Sussner et al., 2013).
Other potential barriers to testing that have been reported in the literature, but were endorsed by <5% of participants in our study, include concerns about family reactions, lack of interest in learning future cancer risks, fears of testing, adverse psychological consequences (anxiety, stress, etc.), and competing life concerns (e.g., taking care of family, too busy) (Kinney et al., 2010; Sussner et al., 2010, 2013; Anderson et al., 2012). Our results were similar to the Michigan study of primarily NHW BC survivors where <5% reported these as reasons for not accessing genetic services (Anderson et al., 2012). In contrast, studies that have worded questions differently found these concerns to be more prevalent among both Hispanic and NHW (Ramirez et al., 2006; Sussner et al., 2010, 2013; Gammon et al., 2011).
The majority of untested NHW and Hispanic women in our study reported a single barrier to genetic testing, consistent with the Michigan study of young BC survivors in which 1.2 barriers to uptake of cancer genetic services was reported (Anderson et al., 2012). Interestingly, the number of facilitators in that study was higher than barriers, with an average of 2.7 reasons for attending genetic counseling and 1.8 reasons enabling them to attend (Anderson et al., 2012). This is also consistent with our findings where the majority of tested NHW and Hispanic participants reported four or more facilitators and/or motivators combined. Taken together, these findings suggest that in addition to focusing on overcoming barriers, interventions should help individuals recognize motivations for testing and identify facilitators, including a recommendation from their health care provider.
The three most common motivators and facilitators identified in our study (i.e., benefit to family, minimal cost, and physician recommendation for testing) were consistent with prior reports (Ramirez et al., 2006; Sussner et al., 2010; Gammon et al., 2011; Vadaparampil et al., 2011; Anderson et al., 2012; Hann et al., 2017; Kurian et al., 2017). Given that strong family values have been shown to influence genetic testing uptake in Latinas, family members' support of genetic testing may further motivate some Hispanic women (Hann et al., 2017); however, our results found that only 12% of Hispanics and 13% of NHW reported having a family member who wanted them tested to be a motivator. Accessibility, which was identified in our study through several reported facilitators (i.e., coordination of appointments, convenience of locations, and/or ease of scheduling), has also been previously associated with uptake of cancer genetic services (Hann et al., 2017).
Many of the prior studies evaluating barriers were conducted before GINA was passed in 2008 to provide protection against health insurance and employment discrimination (Clifton et al., 2010). Despite our study population being diagnosed after GINA, only 20% of participants were aware of GINA. Awareness of GINA was higher among tested participants (27%) compared with untested participants (15%); however, this remains a concerning finding given that all tested participants should have been informed about GINA as part of the informed consent process. In addition, although GINA may not necessarily be pertinent to the study participants due to their previous BC diagnosis, it remains important for BC survivors to be aware of GINA as it would apply to their unaffected relatives.
This study has several strengths, including that being the first population-based study to systematically compare facilitators and barriers to genetic testing across NHW and Hispanic BC survivors. In addition, the statewide sampling frame and use of the cancer registry enabled comparisons between participants and nonparticipants to confirm the recruitment of a generalizable sample based on data available from the registry. Furthermore, medical record documentation of testing was obtained on the majority of tested participants, rather than the reliance on self-reported data, increasing the validity of our findings. Finally, the study is among the first to explore whether discrimination concerns and GINA awareness differ across populations.
Despite these strengths there remain limitations, including the survey design. For instance, certain questions were formatted as check-all-that-apply with multiple column arrangement of answer options, which has been shown to bias responses (Dillman and Smyth, 2007). There is also possible response bias due to lower response rates among Hispanics compared with NHW. Although this may have been due to the language barrier that would have differentially impacted Hispanics, study materials were provided in both English and Spanish to all women who were identified as Hispanic in the cancer registry data set. In addition, there is heterogeneity in Hispanics residing in Florida, yet we did not collect subethnicity data and cannot comment on attitudes and barriers across those groups. Furthermore, this study was not designed to identify whether differences in barriers or facilitators exist across Hispanics who are primarily Spanish-speaking versus primarily English-speaking Hispanics. Given that our study is cross-sectional, longitudinal follow-up would be critical to determine whether awareness and testing practices change over time. Ongoing studies are particularly important given that our participants were all diagnosed before a number of practice changing events that occurred after 2012, including the fall of the BRCA patent, plummeting genetic testing costs, and celebrity disclosures (Caplan, 2013; Cragun et al., 2017a). Finally, our sample was confined to Florida, and thus may not be generalizable to other parts of the country where awareness and testing practices may vary.
Ultimately, knowledge of hereditary predisposition is valuable as it provides options for future cancer prevention and can impact treatment options. Therefore, it is critical to understand barriers and facilitators to cancer genetic services to inform the development of appropriate and effective interventions to assure access and uptake. Our study highlights that there are more similarities than differences in facilitators and barriers to genetic testing across NHW and Hispanic BC survivors. Importantly, top barriers (i.e., low awareness, lack of provider recommendation, and cost-related concerns) are consistent across other ethnic groups as well, including African American and Asian minorities (Suther and Kiros, 2009; Cragun et al., 2017b; Hann et al., 2017). Despite the lower rates of cancer genetic testing awareness and provider recommendation among Hispanics, the vast majority of untested Hispanic and NHW participants were interested in testing resources or additional information. Regardless of whether participants were tested, the majority across both ethnic groups were unaware of GINA; although few indicated that discrimination was a barrier to testing. Although medical mistrust and concern for misuse of genetic information tends to be higher among minorities, it may be a more salient barrier for African Americans than for Hispanics (Suther and Kiros, 2009).
Our findings suggest that a multipronged approach may be needed to increase provider testing recommendations, to educate women and providers about insurance coverage for those who meet certain criteria and to raise awareness of the decreases in costs of testing that have occurred. Successfully reducing these common barriers is critical, but strategies to bolster motivators or facilitate testing may also be important. Such strategies may include raising awareness of how testing can benefit women and their family members even if they have already had cancer and making testing more accessible by including it as part of another appointment or ensuring available testing close to their homes or through telemedicine. Future research should explore interventions that incorporate several of these strategies and go beyond raising awareness of genetic testing availability and GINA among patients and health care providers.
Acknowledgments
This work was supported by grants through the Bankhead Coley Granting agency (4BB15 and IBG10-34199). Support for Deborah Cragun's time was provided in part by an NCI R25T training grant awarded to Moffitt Cancer Center (5R25CA147832-04).
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review boards and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Author Disclosure Statement
No competing financial interests exist.
References
- Allain DC, Friedman S, Senter L. (2012) Consumer awareness and attitudes about insurance discrimination post enactment of the Genetic Information Nondiscrimination Act. Fam Cancer 11:637–644 [DOI] [PubMed] [Google Scholar]
- American Cancer Society (2018) Cancer facts & figures 2018. Available at: www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html (Last accessed July29, 2018)
- Anderson B, McLosky J, Wasilevich E, et al. (2012) Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol 2012:298745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellcross CA, Kolor K, Goddard KAB, et al. (2011) Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med 40:61–66 [DOI] [PubMed] [Google Scholar]
- Bonner D, Cragun D, Reynolds M, et al. (2016) Recruitment of a population-based sample of young Black women with breast cancer through a state cancer registry. Breast J 22:166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butrick M, Kelly S, Peshkin BN, et al. (2015) Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genet Med 17:467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys SS, Sandbach JF, Gammon A, et al. (2017) A study of over 35,000 women with breast cancer tested with a 25‐gene panel of hereditary cancer genes. Cancer 123:1721–1730 [DOI] [PubMed] [Google Scholar]
- Caplan AL. (2013) The actress, the court, and what needs to be done to guarantee the future of clinical genomics. PLoS Biology 11 Available at: 10.1371/journal.pbio.1001663 (last accessed July30, 2018) [DOI] [PMC free article] [PubMed]
- Centers for Disease Control and Prevention (2018, June 12) Breast cancer statistics. Available at: www.cdc.gov/cancer/breast/statistics/index.htm (Last accessed July28. 2018)
- Centers for Disease Control and Prevention (CDC) (2004) Genetic testing for breast and ovarian cancer susceptibility: evaluating direct-to-consumer marketing—Atlanta, Denver, Raleigh-Durham, and Seattle, 2003. MMWR Morb Mortal Wkly Rep 53:603–606 [PubMed] [Google Scholar]
- Childers CP, Childers KK, Maggard-Gibbons M, et al. (2017) National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol 35:3800–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus EB, Schildkraut JM, Thompson WD, et al. (1996) The genetic attributable risk of breast and ovarian cancer. Cancer 77:2318–2324 [DOI] [PubMed] [Google Scholar]
- Clifton JM, VanBeuge SS, Mladenka C, et al. (2010) The Genetic Information Nondiscrimination Act 2008: what clinicians should understand. J Am Acad Nurse Pract 22:246–249 [DOI] [PubMed] [Google Scholar]
- Cragun D, Bonner D, Kim J, et al. (2015) Factors associated with genetic counseling and BRCA testing in a population-based sample of young Black women with breast cancer. Breast Cancer Res Treat 151:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragun D, Kinney AY, Pal T. (2017a) Care delivery considerations for widespread and equitable implementation of inherited cancer predisposition testing. Expert Rev Mol Diagn 17:57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragun D, Weidner A, Lewis C, et al. (2017b) Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 123:2497–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman DA, Smyth JD. (2007) Design effects in the transition to web-based surveys. Am J Prev Med 32(5 Suppl):S90–S96 [DOI] [PubMed] [Google Scholar]
- Drohan B, Roche CA, Cusack JC, et al. (2012) Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol 19:1732–1737 [DOI] [PubMed] [Google Scholar]
- Gammon AD, Rothwell E, Simmons R, et al. (2011) Awareness and preferences regarding BRCA1/2 genetic counseling and testing among Latinas and non-Latina white women at increased risk for hereditary breast and ovarian cancer. J Genet Couns 20:625–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MJ, Reid JE, Burbidge LA, et al. (2009) BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer 115:2222–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann KEJ, Freeman M, Fraser L, et al. (2017) Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Public Health 17:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Franco R, Jurkowski JM, et al. (2008) Awareness of genetic testing for cancer among United States Hispanics: the role of acculturation. Community Genet 11:36–42 [DOI] [PubMed] [Google Scholar]
- Huang MY, Huston SA, Perri M. (2013) Awareness of the US Genetic Information Nondiscrimination Act of 2008: an online survey. J Pharm Health Serv Res 4:235–238 [Google Scholar]
- Jagsi R, Griffith KA, Kurian AW, et al. (2015) Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol 33:1584–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney AY, Gammon A, Coxworth J, et al. (2010) Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 mutation testing and preventive strategies. Genet Med 12:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317:2402–2416 [DOI] [PubMed] [Google Scholar]
- Kurian AW, Griffith KA, Hamilton AS, et al. (2017) Genetic testing and counseling among patients with newly diagnosed breast cancer. JAMA 317:531–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Byfield SD, Comstock CB, et al. (2011) Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genet Med 13:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynce F, Graves KD, Jandorf L, et al. (2016) Genomic disparities in breast cancer among Latinas. Cancer Control 23:359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai PL, Vadaparampil ST, Breen N, et al. (2014) Awareness of cancer susceptibility genetic testing. Am J Prev Med 46:440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, Bristol M, Domchek SM, et al. (2016) Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. J Clin Oncol 34:2610–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KA, Avard D, Simard J, et al. (2013) Personalized medicine and access to health care: potential for inequitable access? Eur J Hum Genet 21:143–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2018, July 30) Genetic/familial high-risk assessment: breast and ovarian (Version 2.2019). Available at: www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf (Last accessed November2, 2018)
- Nelson HD, Fu R, Goddard K, et al. (2013) Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: systematic review to update the U.S. Preventive Services Task Force recommendation. Rockville (MD): Agency for Healthcare Research and Quality (US) Available at: www.ncbi.nlm.nih.gov/books/NBK179201 (last accessed July28, 2018) [PubMed] [Google Scholar]
- Pagán JA, Su D, Li L, et al. (2009) Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am J Prev Med 37:524–530 [DOI] [PubMed] [Google Scholar]
- Pal T, Vadaparampil ST. (2012) Genetic risk assessments in individuals at high risk for inherited breast cancer in the breast oncology care setting. Cancer Control 19:255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman AA, Foland J, Anderson B, et al. (2015) Public awareness of genetic nondiscrimination laws in four states and perceived importance of life insurance protections. J Genet Couns 24:512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AG, Aparicio-Ting FE, de Majors SSM, et al. (2006) Interest, awareness, and perceptions of genetic testing among Hispanic family members of breast cancer survivors. Ethn Dis 16:398–403 [PubMed] [Google Scholar]
- Sussner KM, Jandorf L, Thompson HS, et al. (2010) Interest and beliefs about BRCA genetic counseling among at-risk Latinas in New York City. J Genet Couns 19:255–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner KM, Jandorf L, Thompson HS, et al. (2013) Barriers and facilitators to BRCA genetic counseling among at-risk Latinas in New York City. Psychooncology 22:1594–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner KM, Thompson HS, Valdimarsdottir HB, et al. (2009) Acculturation and familiarity with, attitudes towards and beliefs about genetic testing for cancer risk within Latinas in East Harlem, New York City. J Genet Couns 18:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suther S, Kiros GE. (2009) Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med 11:655–662 [DOI] [PubMed] [Google Scholar]
- Thompson HS, Valdimarsdottir HB, Jandorf L, et al. (2003) Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Educ Couns 51:217–227 [DOI] [PubMed] [Google Scholar]
- Trivers KF, Baldwin LM, Miller JW, et al. (2011) Reported referral for genetic counseling or BRCA ½ testing among United States physicians: a vignette-based study. Cancer 117:5334–5343 [DOI] [PubMed] [Google Scholar]
- Vadaparampil ST. (2006) The impact of acculturation on awareness of genetic testing for increased cancer risk among Hispanics in the year 2000 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 15:618–623 [DOI] [PubMed] [Google Scholar]
- Vadaparampil ST, Quinn GP, Dutil J, et al. (2011) A pilot study of knowledge and interest of genetic counseling and testing for hereditary breast and ovarian cancer syndrome among Puerto Rican women. J Community Genet 2:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ME, Kadlubek P, Pham TH, et al. (2014) Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol 32:824–829 [DOI] [PMC free article] [PubMed] [Google Scholar]