Abstract
Osteoarthritis (OA) is a degenerative disease of synovial joints characterized by progressive loss of articular cartilage, subchondral bone remodeling, and intra-articular inflammation with synovitis that results in chronic pain and motor impairment. Despite the economic and health impacts, current medical therapies are targeted at symptomatic relief of OA and fail to alter its progression. Given the complexity of OA pathogenesis, we hypothesized that a combinatorial gene therapy approach, designed to inhibit inflammation with interleukin-1 receptor antagonist (IL-1Ra) while promoting chondroprotection using lubricin (PRG4), would improve preservation of the joint compared to monotherapy alone. Employing two surgical techniques to model mild, moderate and severe posttraumatic OA, we found that combined delivery of helper-dependent adenoviruses (HDVs), expressing IL-1Ra and PRG4, preserved articular cartilage better than either monotherapy in both models as demonstrated by preservation of articular cartilage volume and surface area. This improved protection was associated with increased expression of proanabolic and cartilage matrix genes together with decreased expression of catabolic genes and inflammatory mediators. In addition to improvements in joint tissues, this combinatorial gene therapy prolonged protection against thermal hyperalgesia compared to either monotherapy. Taken together, our results show that a combinatorial strategy is superior to monotherapeutic approaches for treatment of posttraumatic OA.
Keywords: helper-dependent adenovirus, lubricin, proteoglycan 4, interleukin-1 receptor antagonist, osteoarthritis, gene therapy
Introduction
Osteoarthritis (OA) is a degenerative disease of synovial joints characterized by loss of articular cartilage, subchondral bone remodeling with osteophytes, and intra-articular inflammation with synovitis that lead to the development of chronic pain, joint dysfunction, and motor impairment.1 It is one of the most common forms of disability in adults worldwide, and risk factors associated with disease onset include age, weight, genetic predisposition, and joint injury.2,3 Despite the health and socioeconomic impacts, current treatment paradigms are limited to symptomatic relief of pain, physical therapy, and, in end-stage disease, total joint arthroplasty.4 Intra-articular treatment for OA is favored, given the potential for increased local concentrations of therapeutic agents and reduced potential for adverse events; however, because small molecules and recombinant proteins are quickly filtered from the joint cavity by synovial capillaries and lymphatics, it is difficult to achieve effective and persistent therapeutic concentrations.5–8 In this regard, intra-articular gene therapy holds great promise given that a single intra-articular injection can lead to long-term, robust expression of a therapeutic protein within joint tissues and bypasses difficulties associated with systemic delivery.
OA gene therapy has classically focused on delivery of a single therapeutic gene that either reduces inflammation or modulates anabolic and catabolic pathways important for tissue homeostasis and repair.9 Interleukin-1 (IL-1) is one of the key players in OA pathogenesis, and its expression in cartilage and synovial tissue correlates with increasing OA severity.10 Mechanistically, IL-1 amplifies inflammation within arthritic joints by inducing expression of other inflammatory mediators, including IL-6 and IL-8, which leads to upregulation of extracellular proteolytic enzymes, such as matrix metalloproteinases (MMP).11 Elevations in matrix catabolism and markers of chondrocyte hypertrophy are accompanied by suppression of type II collagen and proteoglycan synthesis.12 From a therapeutic perspective, one of the most efficient inhibitors of the IL-1 pathway is the endogenous interleukin 1 receptor antagonist (IL-1Ra). Recombinant IL-1Ra is available as the drug anakinra and has been approved for treatment of rheumatoid arthritis.13 Anakinra was also evaluated for OA in a clinical safety and tolerability trial where slight, but significant, improvements in pain were observed at an early time point following single intra-articular injection compared with placebo; however, given its limited half-life of only 4 hours, this effect was not sustained.14 Gene therapy with IL-1Ra allows for sustained and/or regulated production within joint tissues and synovial fluid and has been shown to reduce cartilage degradation and to delay disease progression in animal models of posttraumatic OA.15–19
In addition to anti-inflammatory strategies, another means of altering OA progression is to target positive regulators of tissue homeostasis. Proteoglycan 4 (PRG4; also known as lubricin) is present within articular cartilage, synovium, and synovial fluid and functions as a boundary lubricant within the joint. Human patients with PRG4 loss-of-function mutations develop Camptodactyly-Arthropathy-Coxa Vara-pericarditis Syndrome—a broad spectrum disorder characterized by early-onset OA with synovial hyperplasia. An OA-like phenotype is also observed in Prg4 knockout mice early in their lives.20 Intra-articular injection of recombinant PRG4 has been shown to reduce cartilage damage in animal models, yet also requires multiple injections to maintain efficacy as the protein is cleared from the synovial fluid.21 At the same time, genetic and helper-dependent adenovirus (HDV)-mediated constitutive overexpression of PRG4 within the joint has no detrimental effects on joint structure or function, maintains stable protein expression over the long-term, and protects mice from development of posttraumatic OA in the cruciate ligament transection (CLT) model of severe disease.22,23
Given that OA is a disease with a complex pathogenesis, it is unlikely that a single therapeutic approach will be capable of mediating long-term and disease-modifying efficacy. Therefore we hypothesized that, by combining the chondroprotective properties of PRG4 and the anti-inflammatory actions of IL-1Ra, we could extend the duration of therapeutic efficacy beyond that of either gene therapy alone. Using surgical mouse models of moderate and severe posttraumatic OA, we show that protective effects associated with intra-articular delivery of HDV, encoding either inflammation-inducible IL-1Ra (HDV-NFκB-Il-1ra) or constitutive Prg4 (HDV-EF1-Prg4), are lost over time as evidenced primarily by a decrease in cartilage volume and covered surface area as well as the development of thermal hyperalgesia; in contrast, combinatorial gene therapy protected from cartilage loss in more advanced disease, induced a chondroprotective and anti-inflammatory gene expression pattern, and maintained protection from the development of thermal hyperalgesia.
Materials and Methods
Animals
FVB mice were obtained from the Baylor College of Medicine Center for Comparative Medicine (Houston, TX) mouse colony. All studies were performed with approval from the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). Mice were housed 4 to 5 mice to a cage in a pathogen-free environment with ad libitum access to food and water and under a 14h light/10h dark cycle. Male mice were used to minimize variability between weight and activity levels and subchondral bone differences.24 Mice from different experimental groups were housed together, with each experimental group being represented in each surgical batch of five mice.
Helper-dependent adenoviral vector generation
Wild-type serotype 5 HDV vectors were generated as previously described.22 The Prg4 vector is driven by the constitutively active elongation factor 1 (Ef1) promoter element whereas the Il-1ra vector is driven by an inflammation-sensitive Nfkb promoter element. As a control, an HDV vector containing only stuffer sequence (termed HDV-Empty) was used.
Posttraumatic surgery models
Two surgical models of posttraumatic OA were utilized to represent both mild-to-moderate and severe end-stage disease. Each surgical batch consisted of five mice, in which four underwent the surgical procedure and one underwent the sham procedure. Surgeries were performed on both left and right legs of 8-week-old male mice because both knees were required for microCT and histological analysis and unilateral surgery results in altered loading of the contralateral limb. For this reason, we utilize a sham-surgery mouse as the control. The destabilization of the medial meniscus (DMM) surgery was performed as previously described, with the sham surgery being identical in all steps with the exception of the medial meniscotibial ligament transection. The CLT surgery was performed as previously described, with the sham surgery consisting of an incision to the skin above the patella followed by closure with skin glue.20,25
Intra-articular HDV injections
Injections were performed two weeks postsurgery as previously described.23 A total of 1 × 108 viral particles (VPs)/joint in 5 μl of sterile phosphate-buffered saline (PBS) were injected into each knee. Monotherapy groups included HDV-NFκB-Il1ra, HDV-EF1-Prg4. Controls for inflammation due to the innate immune response to the virus and/or the injection process were accounted for by the inclusion of a group injected with HDV-empty (in the DMM model) or PBS (in the CLT model). In the combinatorial therapy group, mice received injections of both 1 × 108 each of HDV- NFκB-Il1ra and HDV-EF1-Prg4, while sham mice received no injections.
Histology
Mice were euthanized at 2.5 months (CLT and early DMM) and 3.5 months (late DMM) postsurgery, and the left hind limb was dissected and fixed overnight in 10% neutral-buffered formalin on a shaker at room temperature. Samples were decalcified at room temperature in 10% EDTA with 1 × PBS for one week prior to paraffin embedding using a standard protocol. Six-μm samples were sectioned and stained with Safranin O/Fast Green, according to a standard protocol, to visualize proteoglycan content. Samples were scored by an independent pathologist who was blinded to treatment and procedure.
Phase-contrast microCT imaging and analysis
The dissected right hind limb of each mouse were fixed, scanned by phase-contrast μCT, and then analyzed using TriBON software (RATOC, Tokyo, Japan) as previously described.26 Subchondral bone was also analyzed by isolating the medial tibial plateau within the software and measuring the bone volume compared to the total volume of the tibial plateau from the lower boundary of the uncalcified cartilage to the upper boundary of the growth plate in the region of articulation. This region consisted of 75 slices from the femoral attachment of the posterior cruciate ligament, which served as the medial landmark.
Hotplate nociception
Mice were individually placed on a hotplate set to 55°C and enclosed within plexiglass walls. A video camera (set to record 60 fps) was also placed approximately 12 inches from the front of the apparatus to record all responses. The latency to a response (hind paw shake, hind paw lick, or jump) was measured up to 45 seconds, after which mice were removed from the apparatus.
Whole-joint RNA isolation, purification, and quantitative real-time PCR analysis
Three weeks after injection, mice were euthanized, and hind limbs were immediately removed and placed in an ice-cold PBS bath. Working quickly, we removed all skin and muscle tissue from each leg using forceps, scissors, and KimWipes, leaving the joint intact. Using forceps to apply gentle pressure at the growth plate, the bone was removed on each side of the joint, which was then quick frozen in liquid nitrogen before being transferred to storage at −80°C for further processing.
To isolate RNA, the tissue was transferred to Safe-Lock microcentrifuge tubes (Eppendorf, Hamburg, Germany) containing 500 μl of chilled TRIzol (Invitrogen, Carlsbad, CA) and one 5-mm stainless steel bead, and then placed into the adapters on a TissueLyzer II (Qiagen, Hilden, Germany) kept in a 4°C cold room. Tissue was completely homogenized using seven cycles of 120 s at 23 Hz shaking followed by 120 s of rest (rechilling) between each cycle. After homogenization, an additional 500 μl of TRIzol was added to each micro tube, and an isopropanol extraction (TRIzol protocol MAN0001271) was used to isolate the RNA. To remove any potential TRIzol contaminants, a LiCl cleanup step was immediately performed. Briefly, 12.5 μl of LiCl Solution (Ambion, AM9480) and 200 μl of 100% ethanol were added to 50 μl of RNA resuspended in nuclease-free water and incubated overnight at −80°C. The next day, samples were centrifuged at 13,500 RPM for 20 minutes at 4°C after which the supernatant was removed. The pellet was then washed with 70% ethanol and resuspended in 50 μl of nuclease-free water. RNA quantity was assessed using the DS-11 microvolume spectrophotometer (Denovix Inc., Wilmington, DE).
To prepare samples for quantitative real-time PCR, RNA was converted to cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen 18080-051) following the manufacturer's protocol. TaqMan Universal PCR Mix (Applied Biosystems) and PerfeCTa SYBR Green SuperMix (Quanta Biosciences) were used for the following genes using these primers: mPrg4, ACTTCAGCTAAAGAGACACGGAGT (forward) and GTTCAGGTGGTTCCTTGGTTGTAGTAA (reverse); mTgfb1, TCCAAACTAAGGCTCGCCAGTC (forward) and GGTTCAGCCACTGCCGTACAACTC (reverse); mAcan, CCTGCTACTTCATCGACCCC (forward) and AGATGCTGTTGACTCGAACCT (reverse); mCol2a1, GCTCATCCAGGGCTCCAATGATGTAG (forward) and CGGGAGGTCTTCTGTGATCGGTA (reverse); mCol10a1, AAAGCTTACCCAGCAGTAGG (forward) and ACGTACTCAGAGGAGTAGAG (reverse); mIl1b, GACCTGTTCTTTGAAGTTGACGGA (forward) and TGATGTGCTGCTGCGAGATTT (reverse); mIl6, GGAAATCGTGGAAATGAGAAA (forward) and GAATTGGATGGTCTTGGTCCTTAG (reverse); mPtgs2, ACATGGAGTAAACAGCTATCATAAACGTA (forward) and TTTGCTGGCTACCACTCATCAACGA (reverse); mB2mg, GGTCTTTCTGGTGCTTGTC (forward) and CGTATGTATCAGTCTCAGT (reverse).
Statistics
Statistical analyses comparing multiple groups with parametric data were performed by one-way ANOVA followed by Tukey's or Fisher's Least Significant Difference (LSD) post hoc. All analyses were performed using Prism 7. A p-value of <0.05 was considered statistically significant.
Results
Combinatorial PRG4 and IL-1Ra gene therapy better preserves articular cartilage compared to monotherapy in a severe disease model
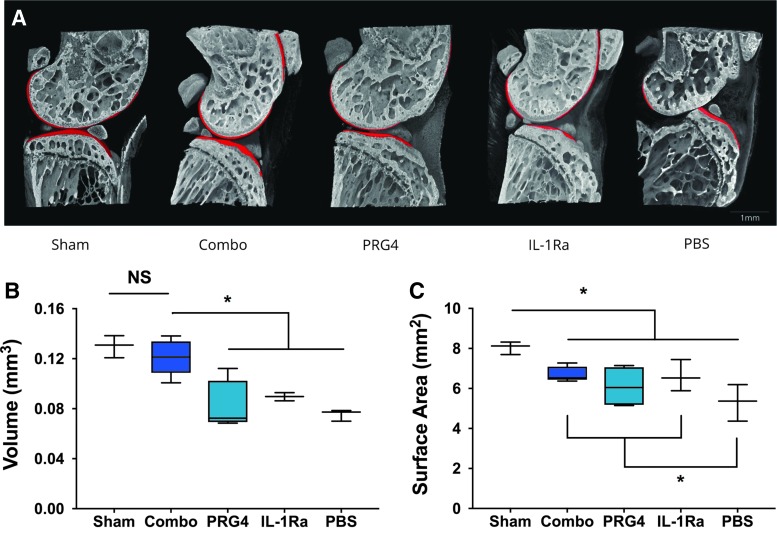
We have shown previously in the CLT model of severe posttraumatic OA that intra-articular delivery of HDV expressing murine Il-1ra on an inflammation-inducible Nfκb promoter (HDV-NFκB-Il-1ra) or murine Prg4 (lubricin) driven by the constitutive Ef1 promoter (HDV-EF1-Prg4) was protective at eight weeks postsurgery.22,27 To test whether combinatorial gene therapy improves this protection, we injected joints with HDV-NFκB-Il-1ra and HDV-EF1-Prg4 alone or in combination at two weeks following surgery. At 2.5 months post-CLT, we collected joints from each group and assessed for OA development by phase-contrast microcomputed tomography (μCT) (Fig. 1). Compared to sham controls, mice receiving HDV-NFκB-Il-1ra or HDV-EF1-Prg4 alone showed significant cartilage volume loss similar to that observed for empty HDV (Fig. 1a,b). In support of our hypothesis, cartilage volume in the combinatorial group was comparable to the sham controls and significantly greater than that observed for the groups receiving either HDV-NFκB-Il-1ra or HDV-EF1-Prg4 monotherapy (Fig. 1a-b).
Figure 1.
Combinatorial gene therapy preserves articular cartilage in a severe model of posttraumatic OA. Joints were collected 2.5 months after CLT surgery and analyzed by phase-contrast microCT. (A) Representative images of the medial compartment of the joint from each experimental group. Red volumes represent articular cartilage. (B) Medial cartilage volume is preserved only in joints treated with combinatorial therapy, whereas joints treated with HDV-EF1-Prg4 and HDV-NFκB-IL-1ra monotherapies have significantly lost cartilage volume. (C) Surface area of bone covered by cartilage is decreased in all surgical groups; however, joints of groups treated with combinatorial therapy and IL-1ra monotherapy retain significantly more surface area than did the untreated joints. Data are represented by min-to-max box and whisker plots. n = 3 (PBS, sham), 4 (IL-1Ra, PRG4), 5 (combinatorial). *p < 0.05 ANOVA. Scale bar, 1mm. OA, osteoarthritis; PBS, phosphate-buffered saline.
Though all surgical groups exhibited less bone surface area covered by cartilage compared to sham controls, the combinatorial and HDV-NFκB-Il-1ra alone treated groups retained significantly more covered surface area compared to mice injected with empty HDV (Fig. 1c). Bones of animals treated with HDV-EF1-Prg4 trended toward greater surface area but did not reach statistical significance. The greatest surface area loss observed with this model was predominately at sites of articulation between the femur and posterior tibia in animals that experienced posterior dislocation. These data support that combined PRG4 and IL-1Ra expression are able to better preserve cartilage volume and covered surface area compared with monotherapy in a severe model of OA.
Gene therapy induces protective gene expression changes in anabolic and catabolic genes
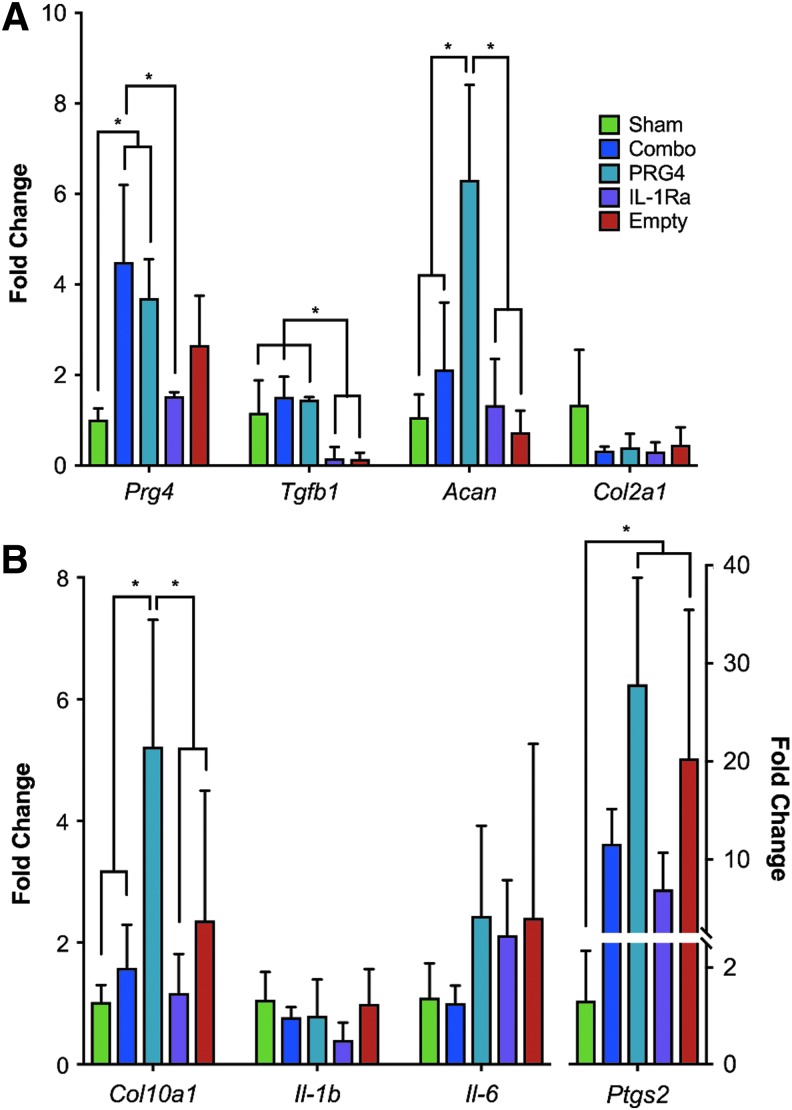
OA pathogenesis is associated with increased inflammatory signaling and a shift from anabolic to catabolic processes as disease progresses.3 To further elucidate a potential molecular mechanism contributing to the preservation seen in the phase-contrast microCT results, we examined the effects of our gene therapy strategies on molecular markers of disease progression. To do so, we injected joints with HDV-NFκB-Il-1ra and HDV-EF1-Prg4 alone or in combination at two weeks post-CLT and collected whole joints three weeks later for real-time PCR analysis (Fig. 2). As expected, HDV-EF1-Prg4 and combinatorial therapy significantly increased Prg4 expression when compared to other experimental groups. Aggrecan (Acan), a proteoglycan present within the cartilage extracellular matrix, was also significantly elevated in the HDV-EF1-Prg4 group compared to sham and empty-virus groups, while both combinatorial and HDV-NFκB-Il-1ra treated groups trended toward a moderate increase in its expression. In contrast, no significant changes were observed among surgical groups in expression of type II collagen (Col2a1)—the most abundant extracellular matrix protein in articular cartilage (Fig. 2a). Interestingly, expression of transforming growth factor beta-1 (Tgfb1)—a growth factor essential for cartilage development and maturation—was maintained in the HDV-EF1-Prg4 and combinatorial groups compared to sham, whereas its expression was significantly reduced in HDV-NFκB-Il-1ra and empty HDV treated mice supporting differential effects on catabolic versus anabolic markers.
Figure 2.
Combinatorial PRG4 and IL-1Ra gene therapy induces protective expression changes in anabolic and catabolic genes. Whole joints were collected from CLT mice, and RNA expression was analyzed by Real Time PCR three weeks postinjection. (A) Expression of anabolic and matrix genes, including Prg4, Tgfb1, and Acan, were elevated in combinatorial and HDV-EF1-Prg4-treated animals, whereas there was no effect on Col2a1 expression. (B) Expression of hypertrophy marker Col10a1 was maintained in combinatorial and HDV- NFκB-Il-1ra-treated animals, whereas HDV-EF1-Prg4 and empty HDV-treated mice exhibited elevated expression. Inflammatory genes Il-1b, Il-6, and Ptgs2 showed a protective expression pattern in combinatorially treated animals. Data are represented as mean + SD. n = 3 *p < 0.05 ANOVA.
In addition to expression changes in anabolic and cartilage matrix genes, inflammatory and catabolic genes were inhibited in the combinatorial group when compared to the monotherapies. In terms of inflammatory markers, Il-1b expression across the entire joint showed a mild decrease in all three treatment groups, whereas the combinatorial group alone inhibited upregulation of interleukin 6 (Il-6) expression comparable to sham treatment. Both HDV-NFκB-Il-1ra and combinatorial gene therapy but not HDV-EF1-Prg4 also partially inhibited the upregulation of cyclooxygenase-2 (Ptgs2) observed in the empty HDV group compared to sham (Fig. 2b). In terms of chondrocyte hypertrophy, the hypertrophic marker type X collagen (Col10a1) was significantly elevated only in the HDV-EF1-Prg4 and empty HDV groups compared to sham, which again indicated that HDV-NFκB-IL-1ra and combinatorial gene therapy provides greater protection from inflammation and chondrocyte hypertrophy compared to HDV-EF1-Prg4 alone (Fig. 2b). Taken together, these data indicate that treatment with HDV-EF1-Prg4 alone prevents decreases in anabolic and cartilage matrix gene expression whereas HDV-NFκB-IL-1ra blocks the upregulation of inflammatory and catabolic pathways associated with OA pathogenesis. Interestingly, combinatorial therapy mediates protective expression in all three aspects of disease pathogenesis, supporting that Prg4 and Il-1Ra are complementary therapies and together they may more effectively inhibit disease progression.
Combinatorial therapy and PRG4 monotherapy maintain cartilage volume and covered surface area of underlying bone in a milder model of posttraumatic OA
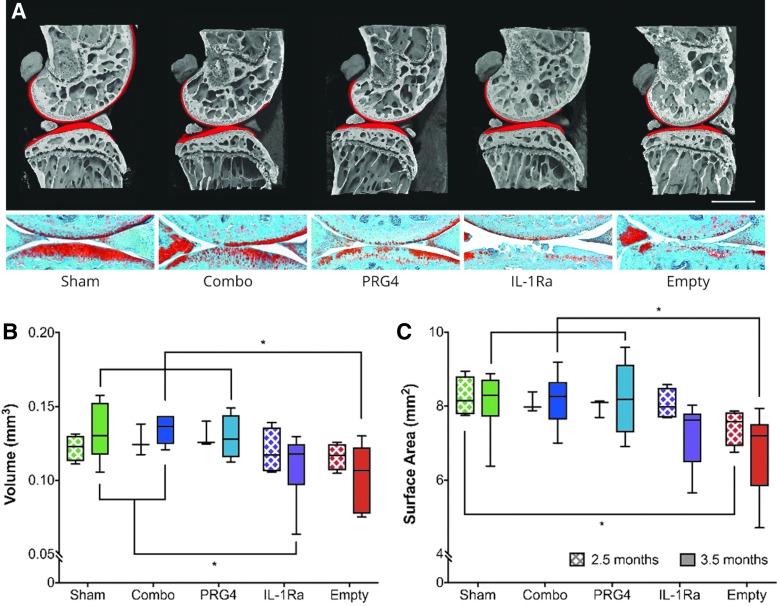
To better characterize temporal differences between our mono- and combinatorial gene therapy approaches, we next examined the efficacy of our combinatorial strategy again via phase-contrast microCT imaging using the milder DMM model of post-traumatic OA. Compared to CLT, DMM generates a milder joint instability that leads to progressive but moderate articular cartilage destruction, subchondral bone thickening, and osteocyte formation more reminiscent of human disease pathogenesis.28 In these experiments, we injected joints with HDV-NFκB-Il-1ra and HDV-EF1-Prg4 alone or in combination at two weeks following DMM. Joints were then collected at 2.5 or 3.5 months postsurgery and assessed for differences in the degree of OA progression via phase-contrast μCT and histology (Fig. 3). At 2.5 months postsurgery, there was a mild but statistically insignificant decline in cartilage volume in the HDV-NFκB-Il-1ra and empty HDV treated groups; however, by 3.5 months postinjury, HDV-NFκB-Il-1ra lost its protective effect, and both it and empty HDV-treated groups showed a significant loss of cartilage volume when compared to sham controls. In contrast, both combinatorial and HDV-EF1-Prg4 alone retained near normal cartilage volumes at this timepoint (Fig. 3a-b).
Figure 3.
Combinatorial therapy and PRG4 monotherapy preserve articular cartilage at early and late time points in a mild-to-moderate model of posttraumatic OA. Joints were collected and analyzed at 2.5- and 3.5-months after DMM surgery by phase-contrast microCT. (A) Representative images of joints at 3.5-months post-surgery that show the medial compartment (femur + tibia) from each experimental group. Red volumes represent articular cartilage. (B) Cartilage volume is maintained in all groups at the 2.5-month time point; however, significant volume loss in cartilage has occurred in the IL-1Ra and untreated groups by 3.5 months. (C) At 2.5 months the untreated group has begun to lose covered surface area when compared to sham; however, by 3.5 months the untreated group has lost significantly more than the sham-treated group, combinatorial-treated, and PRG4-treated groups, with IL-1Ra-treated animals trending toward a reduction in surface area. Data are represented by min-to-max box and whisker plots. n = 6 (3.5 months; all groups); n = 3 (2.5 months; combinatorial, PRG4) and 4 (2.5 months; IL-1Ra, empty, and sham). *p < 0.05 ANOVA. Scale bar, 1mm. DMM, destabilization of the medial meniscus.
Empty HDV-treated mice displayed a reduction in covered surface area even at our earliest timepoint of 2.5 months postsurgery, while HDV-NFκB-Il-1ra retained surface area measures comparable to sham controls at this timepoint. Similar to what was seen for cartilage volume measures, HDV-NFκB-Il-1ra also lost its protective effect on surface area by 3.5 months postsurgery, while both combinatorial and HDV-EF1-Prg4 demonstrated significantly greater preservation comparable to sham controls (Fig. 3c). These results suggest HDV-NFκB-Il-1ra provides protection at early stages of moderate, posttraumatic disease. While we cannot rule out that differences between therapeutic groups in this model would have been seen in earlier timepoints if the sample size were increased, as a post-hoc power analysis did indicate that this model was slightly underpowered, it is still clear that constitutive overexpression of PRG4 mediated by HDV maintains protection for a longer period, and protection can be further enhanced using our combinatorial approach.
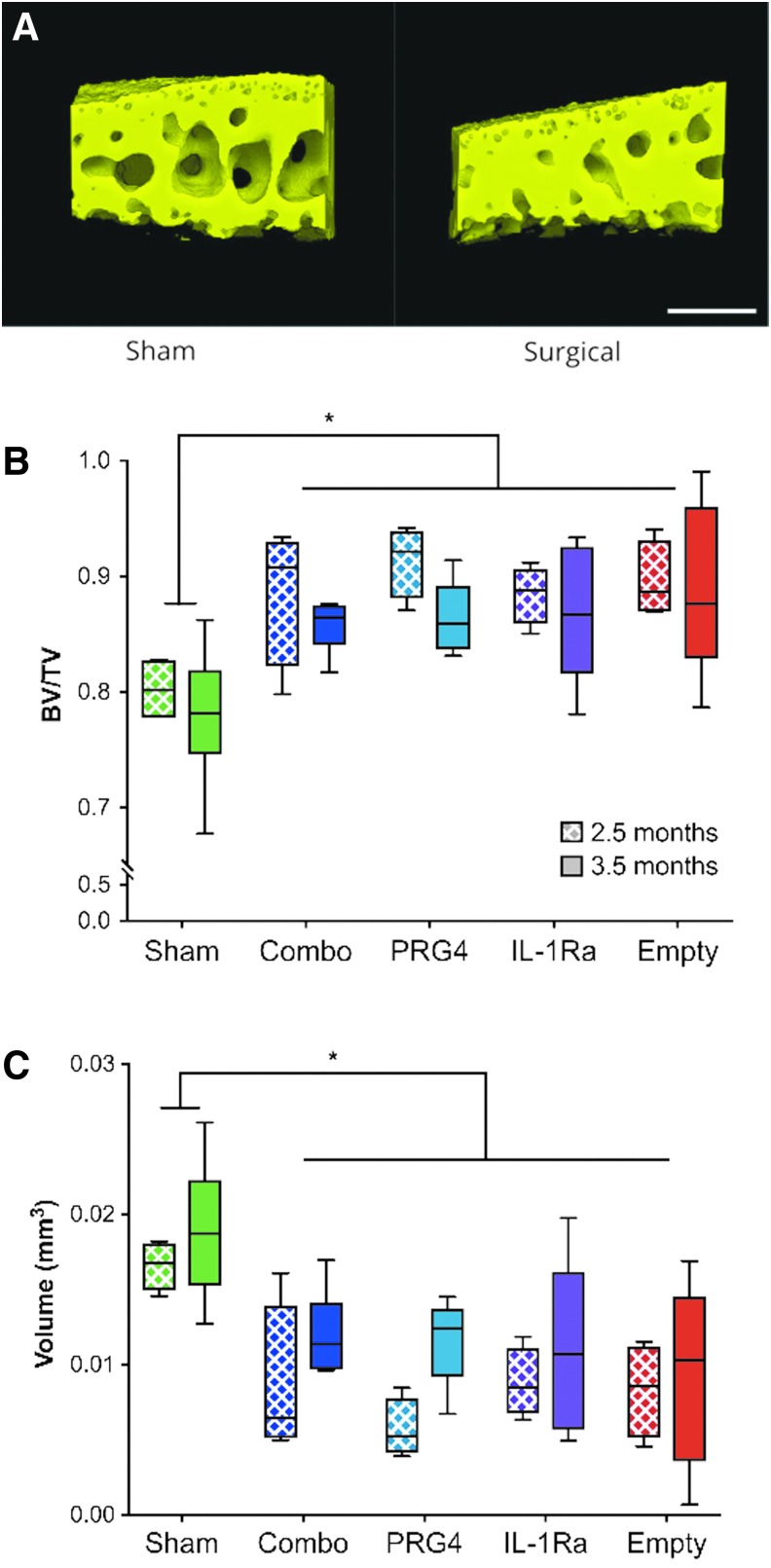
OA-induced subchondral bone changes are unaffected by either mono- or combinatorial gene therapy
In addition to cartilage degeneration, alterations to subchondral bone have been associated with OA progression in humans.1 To assess whether HDV-NFκB-Il-1ra and HDV-EF1-Prg4 alone or in combination alters OA-induced changes in subchondral bone, we used phase-contrast microCT to measure subchondral bone volume and trabecular spacing of the tibia in the DMM model. All surgical groups, irrespective of treatment, displayed a significant level of subchondral thickening (i.e., “sclerosis”) at both 2.5- and 3.5-months postsurgery. Specifically, all surgical groups at both timepoints had significant decreases in trabecular bone spacing within the region of interest and concurrent increases in bone volume (BV)/total volume (TV) (Fig. 4). These subchondral bone alterations likely occur as a result of altered loading in the DMM model, which did not seem to be prevented by any of the gene therapies assessed. The physiological importance of this finding will require additional investigation.
Figure 4.
Treatment had no effect on subchondral bone changes in the mild-to-moderate posttraumatic OA model. (A) Representative images of tibial subchondral bone from the zone of articulation for both a sham and surgical animal. Scale bar represents 0.25 mm. (B) A significant elevation in bone volume/total volume (BV/TV) was seen at both 2.5- and 3.5-months post-surgery in all groups except sham. (C) Consistent with BV/TV, trabecular spacing (depicted as volume of trabecular space) was significantly decreased in all experimental groups compared to sham controls at both 2.5- and 3.5-months post-surgery indicating bone sclerosis. Data are represented by min-to-max box and whisker plots. n = 4 (2.5 months; all groups) and 6 (3.5 months; all groups). *p < 0.05 ANOVA.
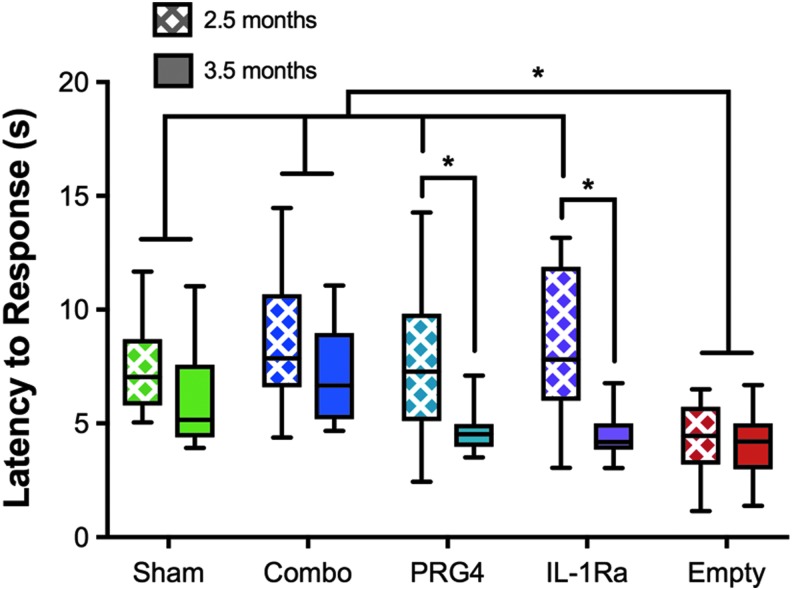
Combinatorial gene therapy prevents thermal hyperalgesia
During OA pathogenesis, alterations to synovial joint structure lead to chronic pain and secondary functional impairments.29 To measure hypersensitivity to a noxious stimulus, we examined the latency to hind limb response using the hot plate nociception assay one week prior to collection.27 When tested at 2.5-months post-DMM surgery where cartilage damage was minimal, mice treated with empty HDV responded significantly faster than sham-treated mice to the noxious heat stimulus, indicating that the empty HDV-treated animals are already experiencing a chronic nociceptive state due to disease (Fig. 5). Interestingly, the response time for mice treated with HDV-EF1-Prg4 and HDV-NFκB-Il-1ra alone or in combination was comparable to sham-treated mice and significantly less than that observed for the empty-HDV group, indicating that both mono- and combinatorial therapy were protective at this time point. In contrast, while combinatorial gene therapy maintained protection from thermal hyperalgesia at 3.5 months postDMM, HDV-EF1-Prg4 or HDV-NFκB-Il-1ra monotherapy treated mice lost protection (Fig. 5). This suggests that, despite obvious differences in cartilage protection between combinatorial therapy and HDV-EF1-Prg4, the combination with HDV-NFκB-Il-1ra provides a superior functional benefit in the inhibition of central sensitization over monotherapeutic approaches for reducing OA-associated pain.
Figure 5.
Combinatorial therapy protects test animals from developing hyperalgesia in the mild-to-moderate posttraumatic OA model at both early and late time points. Behavior tests were conducted one week prior to collection. The untreated group responded significantly faster than sham controls at both early and late time points. Both monotherapy groups responded to the noxious thermal stimuli similarly to sham controls at 2.5 months after surgery; however, they develop a hyperalgesic response like the untreated animals at 3.5-months. Combinatorial therapy-treated animals responded like sham controls at both early and late time points, indicating a protection from the development of hyperalgesia. Data are represented by min-to-max box and whisker plots. N = 11 (2.5 months; Empty, 3.5 months; Sham) N = 12 (2.5 months; IL-1Ra), n = 13 (all other groups). *p < 0.05 ANOVA.
Discussion
Despite significant investment, disease-modifying therapeutics for OA have been elusive. Unlike most efforts to date that have focused on different categories of monotherapy, our work suggests that a combinatorial approach may provide improved outcomes. Specifically, we demonstrated that by combining anti-inflammatory and chondroprotective therapeutics we were able to significantly delay cartilage degradation and thermal hyperalgesia in moderate and severe models of posttraumatic OA. This delayed degradation represents both a physiological and a functional improvement in outcomes when compared to monotherapies. Furthermore, this delay was achieved using an inducible promoter to drive the expression of the anti-inflammatory agent, ensuring a precise delivery of IL-1Ra when inflammation occured as opposed to constitutively as seen in other gene therapies. We have demonstrated previously that this vector is capable of driving expression of IL-1Ra in response to an inflammatory challenge in the context of the synovial joint, which then subsides until subsequent rechallenge.27 This approach better models normal tissue repair and homeostatic pathways as recent work has shown that a complete suppression of inflammatory signaling can worsen OA through the inhibition of anti-apoptotic gene expression; however, moderate inhibition of inflammation can suppress expression of disease-related catabolic genes.30
OA is better understood as the clinical endpoint of multiple converging disorders, each with their own contributing pathophysiological determinants. In posttraumatic cases, such as those modeled in our study, the primary contributing factor is instability of the joint organ with a secondary contribution from inflammation. The majority of cases of OA are not trauma-related and therefore have a variety of potential primary causes including genetic predispositions, aging, altered biomechanics, obesity, or systemic inflammatory conditions. The individual contributions of inflammation or catabolism differ at early stages of disease, and therefore an individual treatment targeting only one pathological mechanism would be susceptible to being overcome by other pathological drivers.
We chose HDV for its improved safety profile over other adenoviral vectors, its larger packaging capacity (necessary for both large transgenes, such as Prg4,as well as for future downstream multitransgene vector development), its demonstrated ability to transduce synoviocytes and chondrocytes, as well as its ability to mediate more robust, stable long-term expression of gene targets within the joint (more than 1 year in mouse models).22,31 Despite improvements in safety profiles of viral gene therapy vectors over the years, the potential for an immune response to the viral capsid still exists; however, this potential is mitigated in our approach via the use of an intra-articular, rather than systemic, injection. It is also important to note that the dose used in this study is 10-fold lower than that used in prior preclinical studies, yet a comparable level of joint preservation was seen in the combinatorial group to previously published high-dose monotherapies, which is also more favorable for clinical applications.22 This suggests that combinatorial therapy could provide an additional value through reduced toxicity and viral response, but with synergistic efficacy. Similar benefits, including reductions in toxicity, have also been seen in combination pharmacotherapies used for hypertension and cancer.32 Logistical and regulatory obstacles to the use of combinatorial therapy in humans include the need to validate that a single combinatorial vector expressing both transgenes is capable of driving a similar level of expression of target genes as the coinjected monotherapies in addition to the potential requirement for regulatory approval of the separate monotherapies prior to approval of a combinatorial treatment.
It has been shown previously that IL-1 and PRG4 expression are inversely related to one another, with increases in IL-1 leading to decreases in PRG4 expression and vice versa. For instance, treatment with recombinant IL-1Ra improved PRG4 expression in a murine ACLT model, and rhPRG4 treatment reduced IL-1β in serum and synovial fluids in the Yucatan minipig DMM model.21,33 Furthermore, PRG4 has been shown to interact with CD44 and TLR2/4 which leads to an overall reduction in inflammation and catabolic gene expression in both in vitro and in vivo models.25,34,35 Interestingly our RNA expression experiment, even though evaluated at the whole joint level rather than strictly at the articular cartilage, appears to recapitulate these phenomena as shown by the increase in Prg4 expression in combinatorial therapy animals and a decrease in inflammatory signaling molecules, including Il-6. Furthermore, we found that treatment with HDV-EF1-Prg4 was capable of inducing expression of proanabolic and cartilage matrix genes but had no effect on catabolic and inflammatory mediators; on the other hand, HDV-NFκB-Il-1ra inhibited expression of genes associated with inflammation and catabolic genes but did not induce anabolic expression patterns. Only when applied in combination did all three protective patterns of gene expression occur, further illustrating the synergistic effect of combinatorial therapy. Future studies could evaluate more immediate gene and protein expression changes via RNA sequencing and/or immunohistochemistry at an even earlier timepoint to further identify immediate effects of combinatorial therapy that is divorced from disease-mediated changes.
The contribution of subchondral bone alterations to disease pathogenesis has only recently begun to receive attention in the field because osteoarthritis was long assumed to be a disease solely of the articular cartilage. It is known that subchondral bone sclerosis is associated with late-stage OA; however, whether these changes are a driver of disease or a consequence of progression is controversial.36 While subchondral bone abnormalities have also been linked to increased pain in both animal models and human patients, our results suggest that subchondral bone sclerosis may not correlate with the development of a hypersensitive response to noxious thermal stimuli as the sclerotic phenotype occurred independently of hyperalgesia in all treatment groups.37,38
Development of chronic pain in OA is now believed to be driven by continuous nociceptive input from the joint leading to central nervous system sensitization; however, no strong correlate has been found in humans between degree of damage and perceived pain level or central sensitivity.29 Consistent with this, other groups have shown how nerve growth factor (NGF) is capable of inducing central sensitization and hyperalgesia and that blocking it via intra-articular injection of anti-NGF antibody prevents the development of hyperalgesia even in the presence of joint destruction.39 Similarly, we have demonstrated that we can block development of central sensitivity through intra-articular combinatorial HDV-EF1-Prg4 and HDV- NFκB-Il-1ra treatment while also simultaneously providing preservation of articular cartilage. The importance of this finding is further underscored by the fact that clinical trials with anti-NGF were placed on hold in 2010 when results from long-term safety and efficacy trials revealed that a significantly greater number of patients in the treatment group developed a more aggressive form of OA and required one or more joint replacements when compared to the placebo group.40
In summary, we report that combinatorial gene therapy using IL-1Ra and PRG4 extends both physiological and functional protection in posttraumatic models of OA. These findings are clinically important given that PRG4 therapies are in preclinical development with an eye toward human trials and Sc-rAAV2.5IL-1Ra is beginning a Phase I trial (NCT02790723) while an HDV-IL-1Ra trial is slated to begin in 2019.41 Based on our results, combination of these monotherapies, together with a inflammation-triggered target-gene induction strategy, may be necessary to achieve the standard primary endpoint of clinically relevant pain relief as well as achieving secondary endpoints of joint structure preservation.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number 1F31AR067609. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by the Cell and Gene Therapy T32 program award number T32HL092332, Translational Biology and Molecular Medicine T32 program award number T32GM088129, and the Howard Hughes Medical Institute Med-into-Grad Program, BCM Intellectual and Developmental Disabilities Research Center (HD024064) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the BCM Advanced Technology Cores with funding from the NIH (AI036211, CA125123, and RR024574), the Rolanette and Berdon Lawrence Bone Disease Program of Texas, and the BCM Center for Skeletal Medicine and Biology and the Pamela and David Ott Center for Heritable Disorders of Connective Tissue. M.W.G. was also partially supported by a postdoctoral fellowship from the Canadian Institutes of Health Research.
Author Contributions
Conceptualization, A.S, M.W.G, M.R., and B.L.; Methodology, A.S., M.W.G., and M.R.; Investigation, A.S., M.W.G., M.R., B.D., Y.C., M.J., I.S., and P.J.; Formal Analysis, A.S., M.W.G., and F.G.; Visualization, A.S.; Writing – Original Draft, A.S.; Writing – Review & Editing, A.S., M.W.G. and B.L.; Resources, R.C., B.D.; Supervision, B.L.
Author Disclosure
No competing financial interests exist.
References
- 1. Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol 2016;12:632–644 [DOI] [PubMed] [Google Scholar]
- 2. Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage 2018;26:319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med 2016;59:333–339 [DOI] [PubMed] [Google Scholar]
- 4. Hochberg MC, Altman RD, April KT, et al. . American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care & Research 2012;64:465–474 [DOI] [PubMed] [Google Scholar]
- 5. Simkin PA, Bassett JE. Pathways of microvascular permeability in the synovium of normal and diseased human knees. J Rheumatol 2011;38:2635–2642 [DOI] [PubMed] [Google Scholar]
- 6. Simkin PA. Synovial perfusion and synovial fluid solutes. Ann Rheum Dis 1995;54:424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol 2014;10:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans CH, Ghivizzani SC, Robbins PD. Gene delivery to joints by intra-articular injection. Hum Gene Ther 2018;29:2–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans CH, Gouze JN, Gouze E, et al. . Osteoarthritis gene therapy. Gene Ther 2004;11:379–389 [DOI] [PubMed] [Google Scholar]
- 10. Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop 2015;6:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen D, Shen J, Zhao W, et al. . Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res 2017;5:16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dayer J-M, Oliviero F, Punzi L. A brief history of IL-1 and IL-1 Ra in rheumatology. Front Pharmacol 2017;8:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grol MW, Stone A, Ruan MZC, et al. . Prospects of gene therapy for skeletal diseases. In: Genetics of Bone Biology and Skeletal Disease. Elsevier; 2018; pp. 119–137 [Google Scholar]
- 14. Chevalier X, Goupille P, Beaulieu AD, et al. . Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double‐blind, placebo‐controlled study. Arthritis & Rheumatology 2009;61:344–352 [DOI] [PubMed] [Google Scholar]
- 15. Elsaid KA, Zhang L, Shaman Z, et al. . The impact of early intra-articular administration of interleukin-1 receptor antagonist on lubricin metabolism and cartilage degeneration in an anterior cruciate ligament transection model. Osteoarthritis Cartilage 2015;23:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H-J, Yu C-L, Kishi H, et al. . Suppression of experimental osteoarthritis by adenovirus-mediated double gene transfer. Chin Med J 2006;119:1365–1373 [PubMed] [Google Scholar]
- 17. Zhang P, Zhong Z-H, Yu H-T, et al. . Exogenous expression of IL-1Ra and TGF-β1 promotes in vivo repair in experimental rabbit osteoarthritis. Scand J Rheumatol 2015;44:404–411 [DOI] [PubMed] [Google Scholar]
- 18. Nixon AJ, Grol MW, Lang HM, et al. . Disease-modifying osteoarthritis treatment with interleukin-1 receptor antagonist gene therapy in small and large animal models. Arthritis Rheumatol 2018. [Epub ahead of print]; DOI: 10.1002/art.40668 [DOI] [PubMed] [Google Scholar]
- 19. Grol MW, Lee BH. Gene therapy for repair and regeneration of bone and cartilage. Current Opinion in Pharmacology 2018;40:59–66 [DOI] [PubMed] [Google Scholar]
- 20. Coles JM, Zhang L, Blum JJ, et al. . Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis & Rheumatology 2010;62:1666–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waller KA, Chin KE, Jay GD, et al. . Intra-articular recombinant human proteoglycan 4 mitigates cartilage damage after destabilization of the medial meniscus in the Yucatan minipig. Am J Sports Med 2017;45:1512–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruan MZC, Erez A, Guse K, et al. . Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med 2013;5:176ra34–176ra34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruan MZ, Cerullo V, Cela R, et al. . Treatment of osteoarthritis using a helper-dependent adenoviral vector retargeted to chondrocytes. Mol Ther Methods Clin Dev 2016;3:16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma H-L, Blanchet TJ, Peluso D, et al. . Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage 2007;15:695–700 [DOI] [PubMed] [Google Scholar]
- 25. Iqbal SM, Leonard C, Regmi SC, et al. . Lubricin/proteoglycan 4 binds to and regulates the activity of toll-like receptors in vitro. Sci Rep 2016;6:18910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruan MZC, Dawson B, Jiang M-M, et al. . Quantitative imaging of murine osteoarthritic cartilage by phase-contrast micro-computed tomography. Arthritis & Rheumatology 2013;65:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nixon AJ, Grol MW, Lang H, et al. . Disease-modifying ssteoarthritis treatment with interleukin-1 receptor antagonist gene therapy in small and large animal models. Arthritis & Rheumatology 2018 [DOI] [PubMed] [Google Scholar]
- 28. Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 2007;15:1061–1069 [DOI] [PubMed] [Google Scholar]
- 29. Arendt-Nielsen L. Pain sensitisation in osteoarthritis. Clin Exp Rheumatol 2017;35 Suppl 107:68–74 [PubMed] [Google Scholar]
- 30. Kobayashi H, Chang SH, Mori D, et al. . Biphasic regulation of chondrocytes by Rela through induction of anti-apoptotic and catabolic target genes. Nat Commun 2016;7:13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmer DJ, NG DP. Helper-dependent adenoviral vectors for gene therapy. https://homeliebertpubcom/hum 2005;16:1–16 [DOI] [PubMed]
- 32. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. . Combination therapy in combating cancer. Oncotarget 2017;8:38022–38043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elsaid KA, Zhang L, Shaman Z, et al. . The impact of early intra-articular administration of interleukin-1 receptor antagonist on lubricin metabolism and cartilage degeneration in an anterior cruciate ligament transection model. Osteoarthritis Cartilage 2015;23:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alquraini A, Jamal M, Zhang L, et al. . The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res Ther 2017;19:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alquraini A, Garguilo S, D'Souza G, et al. . The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther 2015;17:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol 2012;8:665–673 [DOI] [PubMed] [Google Scholar]
- 37. Sowers MF, Hayes C, Jamadar D, et al. . Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthritis Cartilage 2003;11:387–393 [DOI] [PubMed] [Google Scholar]
- 38. Fang H, Huang L, Welch I, et al. . Early changes of articular cartilage and subchondral bone in the DMM mouse model of osteoarthritis. Sci Rep 2018;8:2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu L, Nwosu LN, Burston JJ, et al. . The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthritis Cartilage 2016;24:1587–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanga P, Katz N, Polverejan E, et al. . Long-term safety and efficacy of fulranumab in patients with moderate-to-severe osteoarthritis pain: phase II randomized, double-blind, placebo-controlled extension study. Arthritis Rheumatol 2017;69:763–773 [DOI] [PubMed] [Google Scholar]
- 41. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000. February 29 Identifier NCT02790723, Safety of intra-articular Sc-rAAV2.5IL-1Ra in subjects with moderate knee OA (AAVIL-1Ra); 2016. June 6 [cited 2018. May 12]; [about 7 screens]. Available from: https://clinicaltrials.gov/ct2/show/NCT02790723 [Google Scholar]