Abstract
BIOMEX (BIOlogy and Mars EXperiment) is an ESA/Roscosmos space exposure experiment housed within the exposure facility EXPOSE-R2 outside the Zvezda module on the International Space Station (ISS). The design of the multiuser facility supports—among others—the BIOMEX investigations into the stability and level of degradation of space-exposed biosignatures such as pigments, secondary metabolites, and cell surfaces in contact with a terrestrial and Mars analog mineral environment. In parallel, analysis on the viability of the investigated organisms has provided relevant data for evaluation of the habitability of Mars, for the limits of life, and for the likelihood of an interplanetary transfer of life (theory of lithopanspermia). In this project, lichens, archaea, bacteria, cyanobacteria, snow/permafrost algae, meristematic black fungi, and bryophytes from alpine and polar habitats were embedded, grown, and cultured on a mixture of martian and lunar regolith analogs or other terrestrial minerals. The organisms and regolith analogs and terrestrial mineral mixtures were then exposed to space and to simulated Mars-like conditions by way of the EXPOSE-R2 facility. In this special issue, we present the first set of data obtained in reference to our investigation into the habitability of Mars and limits of life. This project was initiated and implemented by the BIOMEX group, an international and interdisciplinary consortium of 30 institutes in 12 countries on 3 continents. Preflight tests for sample selection, results from ground-based simulation experiments, and the space experiments themselves are presented and include a complete overview of the scientific processes required for this space experiment and postflight analysis. The presented BIOMEX concept could be scaled up to future exposure experiments on the Moon and will serve as a pretest in low Earth orbit.
Key Words: EXPOSE-R2, BIOMEX, Habitability, Limits of life, Extremophiles, Mars
1. Results from Previous Spaceflight and Ground-Based Experiments
Previous experiments in spaceflight and ground-based studies, which were performed before the BIOMEX (BIOlogy and Mars EXperiment) proposal submission to ESA, showed that, in particular, microcolonies of bacteria, meristematic black fungi, and symbiotic associations of microorganisms such as lichens are able to survive and be reactivated after simulated and direct space experiments (Tarasenko et al., 1990; Horneck et al., 1994; de Vera et al., 2003, 2004a, 2004b, 2007, 2008, 2010; de la Torre Noetzel et al., 2007; Sancho et al., 2007; Onofri et al., 2008, 2010; Olsson-Francis et al., 2009; de la Torre et al., 2010; de Vera and Ott, 2010). Bacteria strains such as Bacillus subtilis and Deinococcus radiodurans have shown a certain radiation and vacuum tolerance (Horneck, 1993; Horneck et al., 1994, 2001; Rettberg et al., 2002, 2004; Möller et al., 2007a, 2007b, 2007c; Pogoda de la Vega et al., 2007; Wassmann et al., 2012; Panitz et al., 2014). Gram-negative endophytic bacteria and cyanobacteria survived a 14-day shuttle flight (within the shuttle interior) and exhibited enhanced plant colonizing activity in microgravity (Tarasenko et al., 1990). During the BIOPAN 5 and 6 experiments, the lichens Rhizocarpon geographicum and Xanthoria elegans were analyzed after exposure to space conditions of about 11–14 days coupled with parallel tests in ground-based facilities. These results have led to the conclusion that the tested symbiotic eukaryotic associations of alga and fungi in the lichen were not seriously damaged, and nearly 70–100% of the tested lichens survived. The lichens were physiologically active and able to germinate and grow. Furthermore, investigations on the mutation rate of photoproducts on the DNA have shown that the mycobiont (the fungal symbiont) is practically unaffected by UV radiation and that the algal symbiont is more sensitive (de Vera et al., 2003, 2004a, 2004b, 2007, 2008, 2010; de Vera, 2005; de la Torre Noetzel et al., 2007; Sancho et al., 2007; de la Torre et al., 2010; de Vera and Ott, 2010). Cyanobacteria, as has been shown by analysis on akinetes (resting-state cells of cyanobacteria), were also able to survive the low Earth orbit and simulated extraterrestrial conditions (Olsson-Francis et al., 2009), while vegetative cells of Chroococcidiopsis sp. CCMEE 029 survived prolonged desiccation periods (Billi, 2009; Fagliarone et al., 2017) and a few minutes of exposure to an attenuated Mars-like UV flux and 4 h of exposure to a Mars-like UV flux (Cockell et al., 2005). Numerous species mentioned in this study were even able to survive simulated catastrophes as induced by asteroid impact simulations (Horneck et al., 2001, 2008; Stöffler et al., 2007; Meyer et al., 2011). Mars simulation tests with methanogenic archaea have also shown a remarkable level of survival and demonstrated physiological activity during exposure to Mars-like environmental conditions (Morozova and Wagner, 2007; Morozova et al., 2007, 2015; Schirmack et al., 2014). The same has been observed for meristematic black fungi during a ground-based experiment in the facilities at the German Aerospace Center (DLR) Cologne named EVT (Experiment Verification Test), which was performed for the Lichens and Fungi Experiment (LIFE) on EXPOSE-E (Onofri et al., 2008) and after the final space experiment (Onofri et al., 2012, 2015). In other ground-based experiments, we were able to show that Paenibacillus sp. caused biocorrosion of anorthosite rock (Lytvynenko et al., 2006). In total, we can presume that a wide variety of different microorganisms, even from higher evolutionary advanced levels than those of archaea or bacteria, are able to resist and survive space and Mars-like conditions for a period of time (at least for 1.5 years). However, because of the limited capacity of the space exposure facilities, further work with replicates and other samples is still needed to finally answer questions on the degree of Mars' habitability or the kind of space and Mars-like environmental conditions that are limiting factors in reference to the most important vital functions of life (de Vera et al., 2014; Schulze-Makuch et al., 2015).
The BIOMEX results presented here further advance our knowledge and address pressing questions as mentioned above to an extended degree in comparison to previously executed space experiments, which, for the most part, were more restricted and focused on investigating the likelihood of an interplanetary transfer of life as is formulated in the lithopanspermia hypothesis (Richter, 1865; Thomson, 1894; Arrhenius, 1903; see also Lee et al., 2017). Accordingly, these new BIOMEX experiments in space were intended to address new questions in planetary research and improve future space exploration goals. Nevertheless, it is clear that the results obtained by BIOMEX could also be used to evaluate previously performed space experiments in reference to lithopanspermia.
2. Sample Selection
As a consequence of results obtained in previous space experiments that engendered a significant number of still-open questions, a proposal named BIOMEX (ILSRA-2009-0834) was submitted in 2009. This was in response to the ESA international research announcement for research in space life sciences at the International Space Station (ISS)—ILSRA-2009—and BIOMEX was successfully selected. The proposal included replicate exposure of known species used in previous space experiments such as the reassessed Antarctic fungus Cryomyces antarcticus, the cyanobacterium Chroococcidiopsis sp., the lichen Circinaria gyrosa (formerly known as Aspicilia fruticulosa before reclassification; Sohrabi et al., 2013), and a set of new, preselected organisms for further preflight experiments (see Table 1). After survival of these organisms was shown, the samples were incorporated into the BIOMEX experiment, integrated into the final EXPOSE-R2 hardware, and sent to space on the ISS.
Table 1.
Selected Samples for BIOMEX
| BIOMEX selected samples for spaceflight | |
|---|---|
| Archaea | Methanosarcina sp. strain SMA-21 (terrestrial permafrost) (GFZ/AWI Potsdam) |
| Bacteria | Deinococcus radiodurans wild type and crtI or crtB (nonpigmented) (DLR Cologne) |
| Biofilm containing Leptothrix, Pedomicrobium, Pseudomonas, Hyphomonas, Tetrasphaera (TU Berlin) | |
| Cyanobacterium Nostoc sp. strain CCCryo 231-06 (Fraunhofer IZI-BB) | |
| Cyanobacterium Gloeocapsa OU-20 (Astrobiology Center Edinburgh) | |
| Cyanobacterium Chroococcidiopsis sp. CCMEE 029 (Uni Roma) | |
| Alga | Green alga Sphaerocystis sp. CCCryo 101-99 (Fraunhofer IZI-BB) |
| Lichens | Circinaria gyrosa (INTA) |
| Buellia frigida (Antarctic lichen) (H-H-Uni Düsseldorf) | |
| Fungi | Cryptoendolithic Antarctic black fungus Cryomyces antarcticus CCFEE 515 (Uni Viterbo) |
| Bryophytes | Grimmia sessitana (alpine samples) (Uni Potsdam) |
| Marchantia polymorpha L. (Uni Potsdam) | |
| Biomolecules | Pigment Chlorophyll (H-H-Uni Düsseldorf) |
| Pigment beta-Carotene (H-H-Uni Düsseldorf) | |
| Pigment Naringenin (H-H-Uni Düsseldorf) | |
| Pigment Quercitin (H-H-Uni Düsseldorf) | |
| Pigment Parietin (H-H-Uni Düsseldorf) | |
| Pigment Melanin (H-H-Uni Düsseldorf) | |
| Cellulose (H-H-Uni Düsseldorf) | |
| Chitin (H-H-Uni Düsseldorf) | |
| Biofilm | Kombucha biofilm containing: Yeasts:Saccharomyces ludwigii, Schizosaccharomyces pombe, Zygosaccharomyces rouxii, Zygosaccharomyces bailii, Brettanomyces bruxellensis;Bacteria:Paenibacillus sp. IMBG221, Acetobacter nitrogenifigens, Gluconacetobacter kombuchae sp. nov., Gluconacetobacter xylinum (NAS Ukraine) |
| Substrates/Minerals | Agar (as a substitute for Murein) (H-H-Uni Düsseldorf) |
| Minerals lunar analog mixture (MfN Berlin) | |
| Minerals P-MRS: Early acidic Mars analog (Mixture of Fe2O3, montmorillonite, chamosite, kaolinite, siderite, hydromagnesite, quartz, gabbro, and dunite) (MfN Berlin) | |
| Minerals S-MRS: Late basic Mars analog (Mixture of hematite, goethite, gypsum, quartz, gabbro, dunite) (MfN Berlin) | |
| Silica discs (glass) (Astrobiology Center Edinburgh) | |
Gray shaded cells indicate the samples for which results are available and included in this special collection.
This new sample set was chosen systematically and comprised a selection from archaea, bacteria, and eukaryotes, which represent the three main domains of the tree of life. Most of these organisms were collected from Mars analog habitats distributed on different continents, which include the Alps (climatic and geomorphologic Mars analogy: gullies, polygons, temperatures below 0°C, dryness, and elevated UV irradiation), the steppe highlands of Central Spain (characterized by extreme insolation, high temperature contrasts, and arid summers Crespo and Barreno, 1978]), and regions in the Arctic and Antarctica. The aim of selecting a wide variety of species was to identify which are able to demonstrate the limits of life with regard to the applied space and Mars-like conditions in low Earth orbit, as well as further our understanding of the kind of species for which Mars could be habitable.
2.1. Biological samples
A set of organisms was tested, which were embedded in, or grown on, Mars (optionally lunar) analogs and other terrestrial minerals. Bacteria, biofilms of bacteria and yeast species, cyanobacteria, archaea, lichens, snow/permafrost algae, meristematic black fungi, and bryophytes of mostly alpine and polar habitats of desiccation- and radiation-resistant strains were chosen because some of these organisms are thought to be among the oldest on Earth (Wang et al., 1999; Schidlowski, 2001; Campbell et al., 2003; Yuan et al., 2005) and, over time, have evolutionarily adapted to different environmental conditions. Some of these organisms could even be Mars-relevant because they use Mars-atmosphere resources such as CO2 to form methane, a trace gas found remotely in the martian atmosphere (Formisano et al., 2004; Mumma et al., 2009) and in situ at Gale Crater by way of the rover Curiosity (Webster et al., 2015). The processes that lead to extreme variations in the methane concentration of the martian atmosphere, in particular the potential for abiogenic origin (Lefèvre and Forget, 2009), have recently been reviewed (Yung et al., 2018). In previous studies, some of the organisms studied in the BIOMEX experiments have also exhibited a high resistance under simulated and real space conditions or simulated martian conditions. Others, like the newly selected bryophytes, provide insights on the resistance capabilities of an evolutionarily younger life-form. Details on the selected species are listed in Table 1.
2.2. Mars and lunar analog mineral mixtures
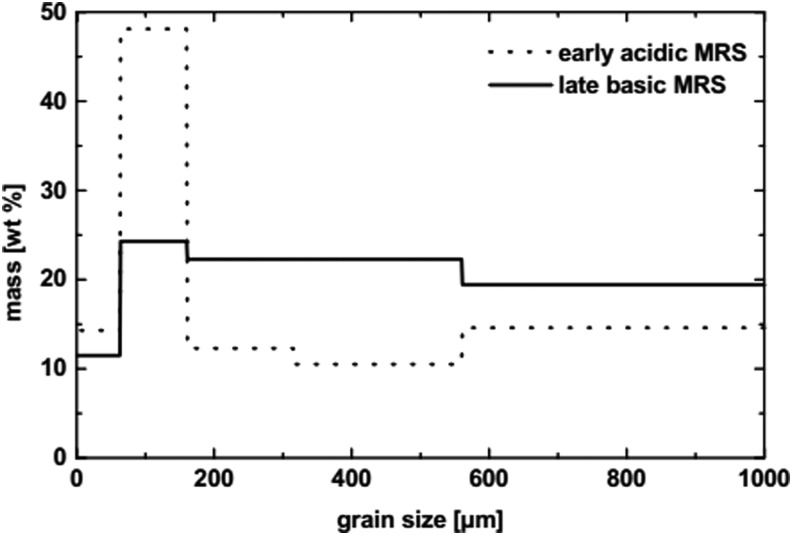
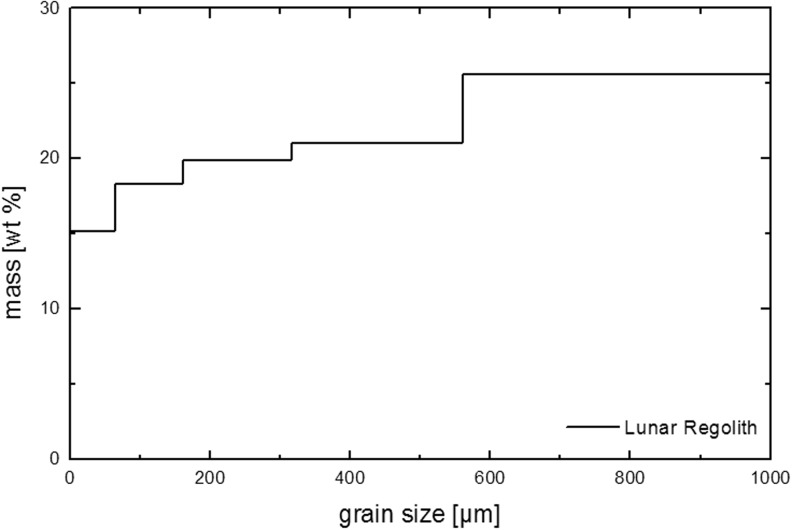
With the BIOMEX experiment, our goal was to analyze the effects of a space environment that approaches as closely as possible Mars-like environmental conditions and includes the use of Mars analog mixtures that could serve as a substrate or an embedding matrix for biological samples (see Table 2 and Figs. 1 and 2). These Mars analog mineral mixtures mimic the regolith cover from early and late evolutionary stages of Mars (Böttger et al., 2012). The components of the mixtures were developed in the Museum für Naturkunde (MfN) Berlin (Germany) in the context of the Helmholtz-Alliance “Planetary Evolution and Life” proposal and based on several observational studies (Bibring et al., 2005, 2006; Poulet et al., 2005; Chevrier and Mathé, 2007). It is important to test the effects of space and the martian environment on minerals in parallel biological investigations. A welcome consequence of this space experiment would be that the investigated samples would also be tested for viability and space-resistance capacity and provide valuable data if used in a replicate space experiment with regard to the probability of lithopanspermia in the Earth-Mars system. The lithopanspermia hypothesis has also been investigated in previous space experiments on FOTON/BIOPAN some years ago and on the EXPOSE-E mission on the ISS. However, replicates are still needed. Besides the Mars analog mineral mixtures, the MfN also provided a lunar regolith analog (see Table 2 and Figs. 1 and 3) for investigation into the influence of the lunar surface material on organisms, which could be relevant for life-support systems such as the selected and tested cyanobacteria (not part of this special collection of articles).
Table 2.
Mars and Lunar Analog Mineral Mixtures
| Component | P-MRS (wt %) | S-MRS (wt %) | LRA (wt %) |
|---|---|---|---|
| Gabbro (Groß-Bieberau, Germany) | 3 | 32 | - |
| Dunite—Olivine Fo96 (Åheim, Norway) | 2 | 15 | 5.7 |
| CPx—Diopside (Kragerö, Norway) | - | - | 8.9 |
| OPx—Hyperstehn (Egersund, Norway) | - | - | 5.7 |
| Anorthosite—Plagioclase (Larvik, Norway) | - | - | 66.8 |
| Quarzite (Bayerischen Wald, Germany) | 10 | 3 | - |
| Apatite (Minas Gerais, Brasil) | - | - | 1.1 |
| Hematite (Cerro Bolivar, Venezuela) | 5 | 13 | - |
| Illmenite (Flekkefiord, Norway) | - | - | 1.1 |
| Iron (Fe) | - | - | 1.3 |
| Montmorillonite (Hallertau, Germany) | 45 | - | - |
| Chamosite (Nucic, Czech Republic) | 20 | - | - |
| Kaolinite (Hirschau, Germany) | 5 | - | - |
| Siderite (Hüttenberg, Austria) | 5 | - | - |
| Hydromagnesite (Albaner Berge, Italy) | 5 | - | - |
| Goethite (Salchendorf, Germany) | - | 7 | - |
| Gypsum (Nüttermoor, Germany) | - | 30 | - |
| Volcanic slag (Aeolian islands, Italy) | - | - | 9.4 |
P-MRS: phyllosilicatic martian regolith = early acidic. S-MRS: sulfatic martian regolith = late basic. LRA: lunar regolith analog.
FIG. 1.
Mars analog pellets integrated in the EXPOSE-R2 hardware.
FIG. 2.
Grain size distribution of martian regolith analog P-MRS (early acidic MRS) and S-MRS (late basic MRS).
FIG. 3.
Grain size distribution of lunar regolith analog material LRA.
2.3. Ground-based environmental and space simulations
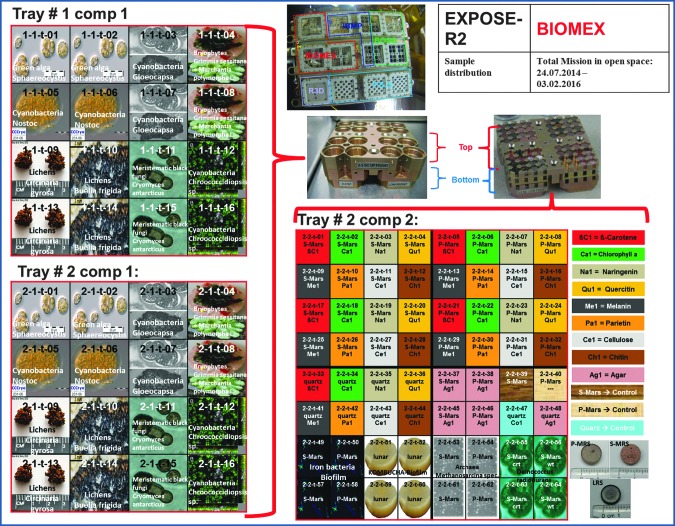
Many preflight tests were performed to discern whether the chosen samples (Fig. 4) are able to resist extreme conditions with reference to space and martian environments. After an array of experiments on Mars-like regolith and desiccation tests, the organisms were exposed to Experiment Verification Tests (EVTs) and Scientific Verification Tests (SVTs) (Rabbow et al., 2017). In the EVTs, a number of parameters were tested individually on the selected samples. Among these tests, vacuum, a low-pressure Mars-like CO2 atmosphere, extreme temperature cycles from far below zero to more than 40°C, and UVC irradiation were applied (see Table 3). The SVT experiments were conducted inside hardware with conditions that approached those of the space environment at the ISS (see Table 4).
FIG. 4.
Visual table of the sample distribution within the EXPOSE-R2 hardware.
Table 3.
Experiment Verification Tests (EVTs)
| EXPOSE-R2 EVT part 1 | BIOMEX Experiment |
|---|---|
| Test parameter | performed |
| Vacuum | 7 d, pressure: 3.5 × 10−2 ± 0.12 Pa |
| 10−5 Pa | |
| Mars atmosphere | 7 d, pressure: 6.5 × 102 ± 0.12 Pa |
| (CO2 gas composition) | |
| 103 Pa | |
| Temperature | 48 cycles |
| −10°C to +45°C | 8 h each |
| Temperature max and min | −25°C ± 0.5°C, 1 h |
| −25°C and +60°C | +60°C ± 0.5°C, 1 h |
| Irradiation | 0 s → 0 J/m2 |
| 254 nm | 18 s → 10.1 J/m2 |
| Hg low-pressure lamp | 2 min 59 s → 100.2 J/m2 |
| @ 56 μW/cm2 | 29 min 46 s → 1000.2 J/m2 |
| 4 h 57 min 37 s → 9999.9 J/m2 | |
| EXPOSE-R2 EVT part 2 (run 1 + 2) | BIOMEX Experiment |
| Run 1 | 0 s → dark |
| Irradiation | 18 min →1.4 × 103 kJ/m2 |
| 200–400 nm | 3 h → 1.4 × 104 kJ/m2 |
| SOL2000 | 30 h → 1.4 × 105 kJ/m2 |
| @ 1,271.2 W/m2200–400nm | 99 h → 4.5 × 105 kJ/m2 |
| 148 h → 6.8 × 105 kJ/m2 | |
| Run 2 | 0 s → dark |
| Irradiation | 432 s → 5.5 × 102 kJ/m2 (0.1% ND filter) |
| 200–400 nm | 1 h 12 min →5.5 × 103 kJ/m2 (1.0% ND filter) |
| SOL2000 | 30 h → 1.4 × 105 kJ/m2 |
| @ 1,271.2 W/m2200–400nm | 60 h → 2.7 × 105 kJ/m2 |
| (as for a 12-month mission duration) | 120 h → 5.5 × 105 kJ/m2 |
| Gluing test | >24 h vulcanization, glue: Wacker-silicone |
ND: neutral density.
Table 4.
Scientific Verification Tests (SVTs)
| SVT | Duration | Pressure | Atmosphere | Temperature (T) | T extremes | Irradiation |
|---|---|---|---|---|---|---|
| Tray 1 | December 2013–January 2014, 38 d | vacuum pressure at 4.1 × 10−5 Pa | T cycles between −25°C (16 h in the dark) and +10°C (8 h during irradiation) | The upper layers of each tray: UVR200–400nm with 1271 Wm−2 (5.7 × 105 kJ m−2) for 5924 min | ||
| The lower layers of the trays were kept in the dark | ||||||
| Tray 2 | Mars atmosphere (95.55% CO2, 2.7% N2, 1.6% Ar, 0.15% O2, and ∼370 ppm H2O at 1 kPa) | −23°C |
2.4. Radiation conditions in space
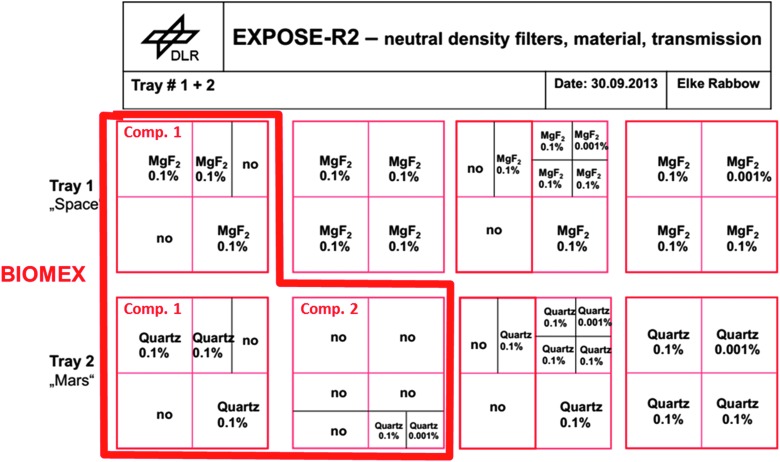
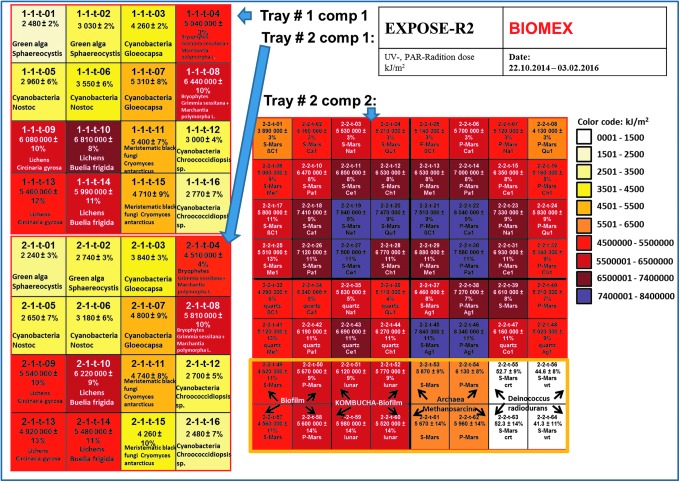
For several of the BIOMEX experiments, we used different neutral density filters (Fig. 5) as covers below the sample window or as cutoff filters, which allowed for a radiation spectral range such as that present at the surface of Mars. For the more protected samples, doses varied between 4.13 × 101 kJ/m2 and 6.5 × 103 kJ/m2 (see Fig. 5 for details). Samples such as the cyanobacteria of the genera Nostoc, Gloeocapsa, and Chroococcidiopsis; the green snow/permafrost alga Sphaerocystis; and the fungus Cryomyces antarcticus were partly shielded by special MgF2 neutral density filters with transmission of 0.1% for space conditions and quartz filters with transmission of 0.1% for Mars-like conditions (for detailed description of the EXPOSE-R2 facility and the space mission, see Rabbow et al. [2017]). These allowed for an approximation of martian subsurface radiation conditions, representing a thin soil cover with a significantly reduced amount of transmission. The reason for these filters, which covered select samples, was also to mimic those conditions that occur in natural habitats where organisms primarily colonize endolithic niches or fissures and cracks in rocks or soils or are embedded by shielding snow or ice. Some of these organisms occur naturally as endoliths, such as Chroococcidiopsis sp. and Cryomyces antarcticus. Furthermore, the neutral density filters with 0.1% transmission were additionally applied to the samples of the methanogen archaeon Methanosarcina soligelidi SMA 21. In this case, these filters were used because of the specific nature of this organism's original habitat, which is situated within permafrost-affected soils and protected by soil particles with different grain sizes. Deinococcus radiodurans was covered by neutral density filters with a transmission of 0.01%. The measured and calculated data with regard to the final doses the samples experienced, which included UVA, UVB, UVC, PAR (photosynthetically active radiation), and Lyman alpha, are represented in Fig. 6 and were kindly provided by ESA via computations completed by the company RedShift Design and Engineering BVBA.
FIG. 5.
The distribution of neutral density filters and the values of transmission depending on the used material.
FIG. 6.
Final UV/PAR radiation dose distribution on the BIOMEX compartments within EXPOSE-R2 after exposure on the ISS (data provided by RedShift).
Significant variation was observed in the dose of UV among samples that were placed within sample sites not protected by filters. The observed variations could be explained by the dependence of the sample position in the hardware, which would have been exposed to a variety of shadowing effects during the orbit of the ISS. When filters were not used in the BIOMEX experiments, the final doses varied between 4.5 × 106 and 8.4 × 106 kJ/m2. Specific organisms exposed without any neutral density filters include the epilithic lichens Circinaria gyrosa and Buellia frigida, the epilithically living bryophytes Grimmia sp. and Marchantia polymorpha, the iron bacteria biofilm, and the kombucha biofilm. Biomolecules exposed on the surface and embedded in the Mars analog mineral pellets also endured the same direct space conditions without any neutral density filters.
3. Overview of Results within This Special Collection
The results presented in this special collection are arranged to provide an overview of each step of the processes involved in the BIOMEX experiment, that is, from selecting samples for simulation experiments to the final exposure experiments in space. Results from pretests on the chosen methanogenic archaeon of the genus Methanosarcina at the preselection level are reported in Serrano et al. (2019). This archaeon was chosen because of its relevance with regard to its potential for being metabolically active on Mars. Therefore, the first tests were designed to use different substrates that contained magnesium perchlorate to establish a Mars-relevant perchlorate environment and mineral mixture before attempting the next selection step of applying atmospheric and radiation-related environmental conditions within the preflight experiments EVTs and SVTs. With regard to the EVTs and SVTs, we present the results obtained by analysis of the fungus Cryomyces antarcticus (Pacelli et al., 2019) and the moss Grimmia sp. (Huwe et al., 2019). Results obtained after space exposure are shown from a series of analyses on the biofilm kombucha (Podolich et al., 2019), the cyanobacterium Chroococcidiopsis (Billi et al., 2019), the cryptoendolithic Antarctic fungus Cryomyces antarcticus (Onofri et al., 2019), and the lichens Buellia frigida (Backhaus et al., this issue) and Circinaria gyrosa (de la Torre et al., not part of this issue). A rough summary of the different studies is given in Table 5, and more details are shown in Table 6. Survival, physiological activity, and growth capacity were detected in all organisms tested. However, life's vital functions decreased from slight to significant, and the reader is directed to specific articles in this collection for detailed discussion of these findings. Several of the selected archaea, bacteria, and heterogenic multilayered biofilms formed by a multitude of species were found to be the most resistant to simulated or direct space and Mars-like conditions. Less resistance and a significant decrease in cell numbers and vitality with regard to the Mars-like environment were shown for multicellular life-forms such as the tested fungus Cryomyces antarcticus (Onofri et al., 2019) and the lichens Buellia frigida (Backhaus et al., 2019) and Circinaria gyrosa (de la Torre Noetzel et al., 2019). The bryophyte Grimmia sp. was an exception (Huwe et al., 2019), but further analysis after space exposure might show whether this specific moss is also resistant to the conditions in space. Actual results in reference to the bryophytes were shown only for the preflight selection mode of EVT and SVT. Our results so far indicate that present Mars seems to be habitable for archaea and bacteria over longer timescales. However, a clearer understanding of the limits of life would be achievable with the implementation of extended space exposure experiments on the Moon, for example, with similar space exposure facilities as those used in the present study (see de Vera et al., 2012).
Table 5.
Results Listed According to the Topics “Limits of Life” and Habitability of Life
 |
( ) Survival / metabolically active / growth capacity, (
) Survival / metabolically active / growth capacity, ( ) partly survival, more damaged
) partly survival, more damaged
Table 6.
Detailed Result List Explaining the Classification Shown in Table 5
 |
S-MRS: sulfatic martian regolith. P-MRS: phyllosilicatic martian regolith. LRA: lunar regolith analog.
Acknowledgments
This research was supported by the Italian Space Agency (ASI grant BIOMEX Cyano 051-R.0 to D.B., ASI grant BIOMEX MicroColonial Fungi 063-R.0 to S.O.); the German Aerospace Center (DLR-grants: Department of Infrastructure and Management, Astrobiology Laboratories through a grant DLR-FuW-Project BIOMEX (2474128)/Department of Radiation Biology supported by the grant DLR-FuE-Projekt ISS LIFE, Programm RF-FuW, Teilprogramm 475); the German Helmholtz Association through the Helmholtz-Alliance “Planetary Evolution and Life”; the Spanish Ministry of Economy, Industry and Competitiveness (MINECO, project SUBLIMAS “SUrvival of Bacteria and LIchens on Mars Analogs and Space,” ESP2015-69810-R, 2015, to R. de la Torre, and project “CTM2015-64728-C2-1-R” to L.G. Sancho); and the National Academy of Sciences of Ukraine (grant 47/2017). We also kindly acknowledge support from the Alexander von Humboldt Foundation, the German Federal Ministry of Economics and Technology (BMWi: grant to D.W. (50WB1152) and S.O., T.B. (50WB1153) in the frame of the BIOMEX project). L.S. acknowledges The Italian Antarctic National Museum (MNA) for financial support to the Culture Collection of Fungi From Extreme Environments (CCFEE). Also, Dirk Schulze-Makuch acknowledges the support of the ERC Advanced Grant HOME (# 339231). We would like to thank personally Antje Hermelink and the Robert Koch Institute for the SEM images, and Victor Parro (Centro de Astrobiología) for his opinion and suggestions concerning microbial survival to perchlorate exposure. Thank you also to Ralf Liebermann as well as Dorit Siebert and Sandra Jönsson, University of Potsdam, for supporting sample handling. The authors express appreciation to Mr. V'yacheslav Moskaluyk for excellent service regarding the examination of extracellular membrane vesicles, using a scanning electron microscope. We would like to express a special thank you to the BGR for the logistics for the necessary field work in Antarctica during the GANOVEX 10 expedition so that the collection of samples within Mars-analog field sites for BIOMEX was possible, and we are very thankful to Andreas Läufer, the expedition leader.
We thank ESA for supporting the EXPOSE experiments and in particular the BIOMEX project (ESA-ILSRA 2009-0834, PI: J.-P. de Vera), and we thank the cosmonauts for their excellent EVA work. Thank you also for the final dose calculation and providence of data by RedShift. Moreover, we would like to thank the anonymous reviewers for constructive feedback.
Abbreviations Used
- BIOMEX
BIOlogy and Mars EXperiment
- DLR
German Aerospace Center
- EVTs
Experiment Verification Tests
- ISS
International Space Station
- MfN
Museum für Naturkunde
- PAR
photosynthetically active radiation
- SVTs
Scientific Verification Tests
Author Disclosure Statement
No competing financial interests exist.
References
- Arrhenius S. (1903) Die Verbreitung des Lebens im Weltenraum. Umschau 7:481–485 [Google Scholar]
- Backhaus T., Meeßen J., Demets R., de Vera J.-P., and Ott S. (2019) Characterization of viability of the lichen Buellia frigida after 1.5 years in space on the International Space Station. Astrobiology, 19:233–241; doi: 10.1089/ast.2018.1894 [DOI] [PubMed] [Google Scholar]
- Bibring J.-P., Langevin Y., Gendrin A., Gondet B., Poulet F., Berthé M., Soufflot A., Arvidson R., Mangold N., Mustard J., Drossart P., OMEGA Team, Erard S., Forni O., Combes M., Encrenaz T., Fouchet T., Merchiorri R., Belluci G., Altieri F., Formisano V., Bonello G., Capaccioni F., Cerroni P., Coradini A., Fonti S., Kottsov V., Ignatiev N., Moroz V., Titov D., Zasova L., Mangold M., Pinet P., Douté S., Schmitt B., Sotin C., Hauber E., Hoffmann H., Jaumann R., Keller U., Duxbury T., and Forget F. (2005) Mars surface diversity as revealed by the OMEGA/Mars express observations. Science 307:1576–1581 [DOI] [PubMed] [Google Scholar]
- Bibring J.-P., Squyres S.W., and Arvidson R.E. (2006) Planetary science. Merging views on Mars. Science 313:1899–1901 [DOI] [PubMed] [Google Scholar]
- Billi D. (2009) Subcellular integrities in Chroococcidiopsis sp. CCMEE 029 survivors after prolonged desiccation revealed by molecular probes and genome stability assays. Extremophiles 13:49–57 [DOI] [PubMed] [Google Scholar]
- Billi D., Verseux C., Fagliarone C., Napoli A., Baqué M., and de Vera J.-P. (2019) A desert cyanobacterium under simulated Mars-like conditions in low Earth orbit: implications for the habitability of Mars. Astrobiology 19:158–169; doi: 10.1089/ast.2017.1807 [DOI] [PubMed] [Google Scholar]
- Böttger U., de Vera J.-P., Fritz J., Weber I., Hübers H.-W., and Schulze-Makuch D. (2012) Optimizing the detection of carotene in cyanobacteria in a martian regolith analogue with a Raman spectrometer for the ExoMars mission. Planet Space Sci 60:356–362 [Google Scholar]
- Campbell N.A., Reece J.B., and Markl J. (2003) Biologie. Spektrum Akademischer Verlag, 533–639 [Google Scholar]
- Chevrier V. and Mathé P.E. (2007) Mineralogy and evolution of the surface of Mars: a review. Planet Space Sci 55:289–314 [Google Scholar]
- Cockell C.S., Schuerger A.C., Billi D., Friedmann E.I., and Panitz C. (2005) Effects of a simulated martian UV flux on the cyanobacterium, Chroococcidiopsis sp. 029. Astrobiology 5:127–140 [DOI] [PubMed] [Google Scholar]
- Crespo A. and Barreno E. (1978) Sobre las comunidades terrícolas de liquenes vagantes Sphaerothalio, Xanthoparmelion vagantis al. Nova). Acta Botànica Malacitana 4:55–62 [Google Scholar]
- de la Torre R., Sancho L.G., Horneck G., Ascaso C., de los Ríos A., Olsson-Francis K., Cockell C.S., Rettberg P., Berger T., de Vera J.P.P., Ott S., Frías J.M., Wierzchos J., Reina M., Pintado A., and Demets R. (2010) Survival of lichens and bacteria exposed to outer space conditions—results of the Lithopanspermia experiments. Icarus 208:735–748 [Google Scholar]
- de la Torre Noetzel R., Sancho L.G., Pintado A., Rettberg P., Rabbow E., Panitz C., Deutschmann U., Reina M., and Horneck G. (2007) BIOPAN experiment LICHENS on the Foton M2 mission: preflight verification tests of the Rhizocarpon geographicum-granite ecosystem. Adv Space Res 40:1665–1671 [Google Scholar]
- de la Torre Noetzel R., Ortega García M.V., Miller A.Z., Bassy O., Granja C., Cubero B., Jordão L., Martínez Frías J., Rabbow E., Backhaus T., Ott S., Sancho L.G., and de Vera J.P. (2019) Lichens survive on board the EXPOSE-R2 facility outside the ISS: results of the BIOMEX experiment. Astrobiology, in press; doi: 10.1089/ast.2018.1959 [DOI] [PubMed] [Google Scholar]
- de Vera J.-P. (2005) Grenzen des Überlebens: Flechten als Modellsystem für das Potential von Adaptationsmechanismen eines Symbioseorganismus unter Extrembedingungen. Inaugural Dissertation at the Heinrich-Heine-University, ULB Düsseldorf, Düsseldorf, Germany [Google Scholar]
- de Vera J.P. and Ott S. (2010) Resistance of symbiotic eukaryotes to simulated space conditions and asteroid impact catastrophes. In Symbioses and Stress, edited by Seckbach J. and Grube M., Springer Science Series Volume 17: Cellular Origins, Life in Extreme Habitats and Astrobiology, Springer Science+Business Media, Dordrecht, the Netherlands, pp 595–611 [Google Scholar]
- de Vera J.P., Horneck G., Rettberg P., and Ott S. (2003) The potential of lichen symbiosis to cope with extreme conditions of outer space—I. Influence of UV radiation and space vacuum on the vitality of lichen symbiosis and germination capacity. Int J Astrobiol 1:285–293 [Google Scholar]
- de Vera J.P., Horneck G., Rettberg P., and Ott S. (2004a) The potential of lichen symbiosis to cope with the extreme conditions of outer space II: germination capacity of lichen ascospores in response to simulated space conditions. Adv Space Res 33:1236–1243 [DOI] [PubMed] [Google Scholar]
- de Vera J.-P., Horneck G., Rettberg P., and Ott S. (2004b) In the context of panspermia: may lichens serve as shuttles for their bionts in space? In Proceedings of the III European Workshop on Exo-Astrobiology. Mars: The Search for Life, ESA SP-545, edited by Harris R.A. and Ouwehand L., European Space Agency, Noordwijk, the Netherlands, pp 197–198 [Google Scholar]
- de Vera J.P., Tilmes F., Heydenreich T., Meyer C., Horneck G., and Ott S. (2007) Potential of prokaryotic and eukaryotic organisms in a Mars like environment and as reference system for the search of life on other planets. In Proceedings of the DGLR International Symposium “To the Moon and Beyond,” DGLR, Bremen [Google Scholar]
- de Vera J.-P., Rettberg P., and Ott S. (2008) Life at the limits: capacities of isolated and cultured lichen symbionts to resist extreme environmental stresses. Orig Life Evol Biosph 38:457–468 [DOI] [PubMed] [Google Scholar]
- de Vera J.-P., Möhlmann D., Butina F., Lorek A., Wernecke R., and Ott S. (2010) Survival potential and photosynthetic activity of lichens under Mars-like conditions: a laboratory study. Astrobiology 10:215–227 [DOI] [PubMed] [Google Scholar]
- de Vera J.-P., Boettger U., Noetzel R.D.L.T., Sánchez F.J., Grunow D., Schmitz N., Lange C., Hübers H.-W., Billi D., Baqué M., Rettberg P., Rabbow E., Reitz G., Berger T., Möller R., Bohmeier M., Horneck G., Westall F., Jänchen J., Fritz J., Meyer C., Onofri S., Selbmann L., Zucconi L., Kozyrovska N., Leya T., Foing B., Demets R., Cockell C.S., Bryce C., Wagner D., Serrano P., Edwards H.G.M., Joshi J., Huwe B., Ehrenfreund P., Elsaesser A., Ott S., Meessen J., Feyh N., Szewzyk U., Jaumann R., and Spohn T. (2012) Supporting Mars exploration: BIOMEX in low Earth orbit and further astrobiological studies on the Moon using Raman and PanCam technology. Planet Space Sci 74:103–110 [Google Scholar]
- de Vera J.-P., Schulze-Makuch D., Khan A., Lorek A., Koncz A., Möhlmann D., and Spohn T. (2014) Adaptation of an Antarctic lichen to martian niche conditions can occur within 34 days. Planet Space Sci 98:182–190 [Google Scholar]
- Fagliarone C., Mosca C., Ubaldi I., Verseux C., Baqué M., Wilmotte A., and Billi D. (2017) Avoidance of protein oxidation correlates with the desiccation and radiation resistance of hot and cold desert strains of the cyanobacterium Chroococcidiopsis. Extremophiles 21:981–991 [DOI] [PubMed] [Google Scholar]
- Formisano V., Atreya S., Encrenaz T., Ignatiev N., and Giurann M. (2004) Detection of methane in the atmosphere of Mars. Science 306:1758–1761 [DOI] [PubMed] [Google Scholar]
- Horneck G. (1993) Responses of Bacillus subtilis spores to space environment: results from experiments in space. Orig Life Evol Biosph 23:37–52 [DOI] [PubMed] [Google Scholar]
- Horneck G., Bücker H., and Reitz G. (1994) Long term survival of bacterial spores in space. Adv Space Res 14:41–45 [DOI] [PubMed] [Google Scholar]
- Horneck G., Stöffler D., Eschweiler U., and Hornemann U. (2001) Bacterial spores survive simulated meteorite impact. Icarus 149:285–290 [Google Scholar]
- Horneck G., Stöffler D., Ott S., Hornemann U., Cockell C.S., Möller R., Meyer C., de Vera J.P., Fritz J., Schade S., and Artemieva N. (2008) Microbial rock inhabitants survive hypervelocity impacts on Mars-like host planets: first phase of lithopanspermia experimentally tested. Astrobiology 8:17–44 [DOI] [PubMed] [Google Scholar]
- Huwe B., Fiedler A., Moritz S., Rabbow E., de Vera J.-P., and Joshi J. (2019) Mosses in low Earth orbit—implications for the limits of life and the habitability of Mars. Astrobiology 19:221–232. doi: 10.1089/ast.2018.1889 [DOI] [PubMed] [Google Scholar]
- Lee N.N., Fritz J., Fries M.D., Gil J.F., Beck A., Pellinen-Wannberg A., Schmitz B., Steele A., and Hofmann B.A. (2017) The extreme biology of meteorites: their role in understanding the origin and distribution of life on Earth and in the Universe. In Adaption of Microbial Life to Environmental Extremes: Novel Research Results and Application, edited by Stan-Lotter H. and Fendrihan S., Springer, Cham, Switzerland, pp 283–325 [Google Scholar]
- Lefèvre F. and Forget F. (2009) Observed variations of methane on Mars unexplained by known atmospheric chemistry and physics. Nature 460:720–723 [DOI] [PubMed] [Google Scholar]
- Lytvynenko T., Zaetz I., Voznyuk T., Kovalchuk M., Rogutskyy I., Mytrokhyn O., Estrela-Lopis V., Borodinova T., Mashkovska S., Foing B., and Kozyrovska N. (2006) A rationally assembled microbial community for growing Tagetes patula L. in a lunar greenhouse. Res Microbiol 157:87–92 [DOI] [PubMed] [Google Scholar]
- Meyer C., Fritz J., Misgaiski M., Stöffler D., Artemieva N.A., Hornemann U., Moeller R., de Vera J.-P., Cockell C., Horneck G., Ott S., and Rabbow E. (2011) Shock experiments in support of the lithopanspermia theory: the influence of host rock composition, temperature, and shock pressure on the survival rate of endolithic and epilithic microorganisms. Meteorit Planet Sci 46:701–718 [Google Scholar]
- Möller R., Stackebrandt E., Reitz G., Berger T., Rettberg P., Doherty A.J., Horneck G., and Nicholson W.L. (2007a) Role of DNA repair by non-homologous end joining (NHEJ) in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV and ionizing radiation. J Bacteriol 189:3306–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller R., Douki T., Cadet J., Stackebrandt E., Nicholson W.L., Rettberg P., Reitz G., and Horneck G. (2007b) UV-radiation-induced formation of DNA bipyrimidine photoproducts in Bacillus subtilis endospores and their repair during germination. Int Microbiol 10:39–46 [PubMed] [Google Scholar]
- Möller R., Stackebrandt E., Douki T., Cadet J., Rettberg P., Mollenkopf H.J., Reitz G., and Horneck G. (2007c) DNA bipyrimidine photoproduct repair and transcriptional response of UV-C irradiated Bacillus subtilis. Arch Microbiol 188:421–431 [DOI] [PubMed] [Google Scholar]
- Morozova D. and Wagner D. (2007) Stress response of methanogenic archaea from Siberian permafrost compared with methanogens from non permafrost habitats. FEMS Microbiol Ecol 61:16–25 [DOI] [PubMed] [Google Scholar]
- Morozova D., Möhlmann D., and Wagner D. (2007) Survival of methanogenic archaea from Siberian permafrost under simulated martian thermal conditions. Orig Life Evol Biosph 37:189–200 [DOI] [PubMed] [Google Scholar]
- Morozova D., Moeller R., Rettberg P., Wagner D. (2015) Enhanced Radiation Resistance of Methanosarcina soligelidi SMA21, a new Methanogenic Archaeon isolated from a Siberian Permafrost-Affected Soil in direct comparison to Methanosarcina barkeri. Astrobiology 15:951–960 [DOI] [PubMed] [Google Scholar]
- Mumma M.J., Villanueva G.L., Novak R.E., Hewagama T., Bonev B.P., DiSanti M.A., Mandell A.M., and Smith M.D. (2009) Strong release of methane on Mars in northern summer 2003. Science 323:1041–1045 [DOI] [PubMed] [Google Scholar]
- Olsson-Francis K., de la Torre R., Towner M.C., and Cockell C.S. (2009) Survival of akinetes (resting-state cells of cyanobacteria) in low Earth orbit and simulated extraterrestrial conditions. Orig Life Evol Biosph 39:565–579 [DOI] [PubMed] [Google Scholar]
- Onofri S., Barreca D., Agnoletti A., Rabbow E., Horneck G., de Vera J.P.P., Selbmann L., Zucconi L., and Hatton J. (2008) Resistance of Antarctic black fungi and cryptoendolithic communities to simulated space and Mars conditions. Stud Mycol 61:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofri S., Selbmann L., Barreca D., Isola D., and Zucconi L. (2010) Do fungi survive under actual space conditions? Searching for evidence in favour of lithopanspermia. Plant Biosyst 143:S85–S87 [Google Scholar]
- Onofri S., de la Torre R., de Vera J.P., Ott S., Zucconi L., Selbmann L., Scalzi G., Venkateswaran K., Rabbow E., and Horneck G. (2012) Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiology 12:508–516 [DOI] [PubMed] [Google Scholar]
- Onofri S., de Vera J.-P., Zucconi L., Selbmann L., Scalzi G., Venkateswaran K.J., Rabbow E., de la Torre R., and Horneck G. (2015) Survival of Antarctic cryptoendolithic fungi in simulated martian conditions on board the International Space Station. Astrobiology 15:1052–1059 [DOI] [PubMed] [Google Scholar]
- Onofri S., Selbmann L., Pacelli C., Zucconi L., Rabbow E., and de Vera J.-P. (2019) Survival, DNA, and ultrastructural integrity of a cryptoendolithic Antarctic fungus in Mars and lunar rock analogs exposed on the ISS. Astrobiology 19:170–182. doi: 10.1089/ast.2017.1728 [DOI] [PubMed] [Google Scholar]
- Pacelli C., Selbmann L., Zucconi L., Coleine C., de Vera J.-P., Rabbow E., Böttger U., Dadachova E., and Onofri S. (2019) Responses of the black fungus Cryomyces antarcticus to simulated martian and space conditions on rock analogs. Astrobiology 19:209–220. doi: 10.1089/ast.2016.1631 [DOI] [PubMed] [Google Scholar]
- Panitz C., Horneck G., Rabbow E., Rettberg P., Moeller R., Cadet J., Douki Th., and Reitz G. (2014) The SPORES experiment of the EXPOSE-R mission: Bacillus subtilis spores in artificial meteorites. Int J Astrobiol 14:105–114 [Google Scholar]
- Podolich O., Kukharenko O., Haidak A., Zaets I., Zaika L., Storozhuk O., Palchikovska L., Orlovska I., Reva O., Borisova T., Khirunenko L., Sosnin M., Rabbow E., Kravchenko V., Skoryk M., Kremenskoy M., Demets R., Olsson-Francis K., Kozyrovska N., and de Vera J.-P. (2019) Multimicrobial kombucha culture tolerates Mars-like conditions simulated on low Earth orbit. Astrobiology 19:183–196. doi: 10.1089/ast.2017.1746 [DOI] [PubMed] [Google Scholar]
- Pogoda de la Vega U., Rettberg P., and Reit G. (2007) Simulation of the environmental climate conditions on martian surface and its effect on Deinococcus radiodurans. Adv Space Res 40:1672–1677 [Google Scholar]
- Poulet F., Bibring J.-P., Mustard J.F., Gendrin A., Mangold N., Langevin Y., Arvidson R.E., Gondet B., Gomez C., and The Omega Team. (2005) Phyllosilicates on Mars and implications for early martian climate. Nature 438:623–627 [DOI] [PubMed] [Google Scholar]
- Rabbow E., Rettberg P., Parpart A., Panitz C., Schulte W., Molter F., Jaramillo R., Demets R., Weiß P., and Wilnecker R. (2017) EXPOSE-R2: the astrobiological ESA mission on board of the International Space Station. Front Microbiol 8, doi: 10.3389/fmicb.2017.01533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettberg P., Eschweiler U., Strauch K., Reitz G., Horneck G., Wänke H., Brack A., and Barbier B. (2002) Survival of microorganisms in space protected by meteorite material: results of the experiment EXOBIOLOGIE of the PERSEUS mission. Adv Space Res 30:1539–1545 [DOI] [PubMed] [Google Scholar]
- Rettberg P., Rabbow E., Panitz C., and Horneck G. (2004) Biological space experiments for the simulation of martian conditions: UV radiation and martian soil analogues. Adv Space Res 33:1294–1301 [DOI] [PubMed] [Google Scholar]
- Richter H. (1865) Zur Darwinschen Lehre. Schmidts Jahrb. Ges Med 126:243–249 [Google Scholar]
- Sancho L.G., de la Torre R., Horneck G., Ascaso C., de los Rios A., Pintado A., Wierzchos J., and Schuster M. (2007) Lichens survive in space: results from the 2005 LICHENS experiment. Astrobiology 7:443–454 [DOI] [PubMed] [Google Scholar]
- Schidlowski M. (2001) Carbon isotopes as biogeochemical recorders of life over 3.8 Ga of Earth history: evolution of a concept. Precambrian Res 106:117–134 [Google Scholar]
- Schirmack J., Böhm M., Brauer C., Löhmannsröben H.-G., de Vera J.-P., Möhlmann D., and Wagner D. (2014) Laser spectroscopic real time measurements of methanogenic activity under simulated martian subsurface analogue conditions. Planet Space Sci 98:198–204 [Google Scholar]
- Schulze-Makuch D., Schulze-Makuch A., and Houtkooper J.M. (2015) The physical, chemical, and physiological limits of life. Life 5:1472–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P., Alawi M., de Vera J.-P., and Wagner D. (2019) Response of methanogenic archaea from Siberian permafrost and nonpermafrost environments to simulated Mars-like desiccation and the presence of perchlorate. Astrobiology 19:197–208; doi: 10.1089/ast.2018.1877 [DOI] [PubMed] [Google Scholar]
- Sohrabi M., Stenroos S., Myllys L., Søchting U., Ahti T., and Hyvönen J. (2013) Phylogeny and taxonomy of the ‘manna lichens’. Mycol Progress 12:231–269 [Google Scholar]
- Stöffler D., Horneck G., Ott S., Hornemann U., Cockell C.S., Möller R., Meyer C., de Vera J.P., Fritz J., and Artemieva N.A. (2007) Experimental evidence for the impact ejection of viable microorganisms from Mars-like planets. Icarus 186:585–588 [Google Scholar]
- Tarasenko V.A., Kozyrovska N., Nechitailo G.P., Ngo Ke S., and Tarnavskaja E.B. (1990) Cytological aspects of relationships of eucaryotes and nitrogen-fixing eu- and cyanobacteria in artificial association under microgravity. In Abstracts of the XXIII COSPAR 1990, The Hague, the Netherlands, p 55 [Google Scholar]
- Thomson W. (1894) 1871 presidential address to the British Association. In Popular Lectures and Addresses, MacMillan and Co., New York, pp 132–205 [Google Scholar]
- Wang D.Y.C., Kumar S., and Hedges S.B. (1999) Divergence time estimated for the early history of animal phyla and the origin of plants, animals and fungi. Proc Biol Sci 266:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmann M., Moeller R., Rabbow E., Panitz C., Horneck G., Reitz G., Douki Th., Cadet J., Stan-Lotter H., Cockell Ch.S., and Rettberg P. (2012) Survival of spores of the UV-resistant Bacillus subtilis strain MW01 after exposure to low Earth orbit and simulated martian conditions: data from the space experiment ADAPT on EXPOSE-E. Astrobiology 12:498–507 [DOI] [PubMed] [Google Scholar]
- Webster C.R., Mahaffy P.R., Atreya S.K., Flesch G.J., Mischna M.A., Meslin P.-Y., Farley K.A., Conrad P.G., Christensen L.E., Pavlov A.A., Martín-Torres J., Zorzano M.-P., McConnochie T.H., Owen T., Eigenbrode J.L., Glavin D.P., Steele A., Malespin C.A., Archer P.D., Jr, Sutter B., Coll P., Freissinet C., McKay C.P., Moores J.E., Schwenzer S.P., Bridges J.C., Navarro-Gonzalez R., Gellert R., Lemmon M.T., the MSL Science Team. (2015) Mars methane detection and variability at Gale Crater. Science 347:415–417 [DOI] [PubMed] [Google Scholar]
- Yuan X., Xiao S., and Taylor T.N. (2005) Lichen like symbiosis 600 million years ago. Science 308:1017–1020 [DOI] [PubMed] [Google Scholar]
- Yung Y.L., Chen P., Nealson K., Atreya S., Beckett P., Blank J.G., Ehlmann B., Eiler J., Etiope G., Ferry J.G., Forget F., Gao P., Hu R., Kleinböhl A., Klusman R., Lefèvre F., Miller C., Mischna M., Mumma M., Newman S., Oehler D., Okumura M., Oremland R., Orphan V., Popa R., Russell M., Shen L., Sherwood Lollar B., Staehle R., Stamenković V., Stolper D., Templeton A., Vandaele A.C., Viscardy S., Webster C.R., Wennberg P.O., Wong M.L., and Worden J. (2018) Methane on Mars and habitability: challenges and responses. Astrobiology 18:1221–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]