Abstract
Flavonoid supplementation improves brachial artery flow-mediated dilation (FMD), but it is not known whether flavonoids protect against vascular dysfunction induced by ischemia-reperfusion (IR) injury and associated respiratory burst. In a randomized, double-blind, placebo-controlled, crossover study, we investigated whether 4 wk supplementation with freeze-dried Montmorency cherry (MC) attenuated suppression of FMD after IR induced by prolonged forearm occlusion. Twelve physically inactive overweight, middle-aged men (52.8 ± 5.8 yr, BMI: 28.1 ± 5.3 kg/m2) consumed MC (235 mg/day anthocyanins) or placebo capsules for 4 wk, with supplementation blocks separated by 4 wk washout. Before and after each supplementation block, FMD responses and plasma nitrate and nitrite ([]) concentrations were measured at baseline and 15, 30, and 45 min after prolonged (20 min) forearm occlusion. FMD response was significantly depressed by the prolonged occlusion (P < 0.001). After a 45-min reperfusion, FMD was restored to baseline levels after MC (ΔFMD presupplementation: −30.5 ± 8.4%, postsupplementation: −0.6 ± 9.5%) but not placebo supplementation (ΔFMD presupplementation: −11.6 ± 10.6, postsupplementation: −25.4 ± 4.0%; condition × supplement interaction: P = 0.038). Plasma [] decreased after prolonged occlusion but recovered faster after MC compared with placebo (Δ45 min to baseline; MC: presupplementation: −15.3 ± 9.6, postsupplementation: −6.2 ± 8.1; Placebo: presupplementation: −16.3 ± 5.9, postsupplementation: −27.7 ± 11.1 nmol/l; condition × supplement × time interaction: P = 0.033). Plasma peroxiredoxin concentration ([Prx2]) was significantly higher after MC (presupplementation: 22.8 ± 1.4, postsupplementation: 28.0 ± 2.4 ng/ml, P = 0.029) but not after placebo supplementation (presupplementation: 22.1 ± 2.2, postsupplementation: 23.7 ± 1.5 ng/ml). In conclusion, 4 wk MC supplementation enhanced recovery of endothelium-dependent vasodilatation after IR, in parallel with faster recovery of plasma [], suggesting NO dependency. These protective effects seem to be related to increased plasma [Prx2], presumably conferring protection against the respiratory burst during reperfusion.
NEW & NOTEWORTHY This is the first study to demonstrate that 4 wk of Montmorency cherry powder supplementation exerted protective effects on endothelium-dependent vasodilation after transient ischemia-reperfusion injury in overweight, physically inactive, nonmedicated, hypertensive middle-aged men. These effects seem to be due to increased nitric oxide availability, as evidenced by higher plasma nitrite concentration and peak arterial diameter during the flow-mediated dilation measurement. This may be a consequence of increased concentration of peroxiredoxin and other antioxidant systems and, hence, reduced reactive oxygen species exposure.
Keywords: endothelial function, forearm occlusion, Montmorency cherry, nitrite/nitrate, peroxiredoxin-2
INTRODUCTION
Cardiovascular diseases (CVD) remain the leading worldwide cause of death with an estimated 17.3 million deaths in 2008, which is projected to increase to 23 million by 2030 (27). Higher consumption of fruit and vegetables is associated with a lower risk of all-cause mortality, particularly cardiovascular disease mortality (43). Epidemiological data suggest that polyphenols within fruits and vegetables may contribute to this observed reduction in CVD risk (4, 13). Specifically, a systematic review of prospective cohort studies found that the dietary intake of different classes of flavonoids, including flavonols, flavones, flavanones, flavan-3-ols, anthocyanidins, and proanthocyanidins significantly decreased the risk of CVD (44). Risk factors for CVD, including hypertension, hypercholesterolemia, and diabetes mellitus are associated with enhanced production of reactive oxygen species (ROS), particularly superoxide, generated by enzymes such as xanthine oxidase and NADPH oxidase (NOX) and via the electron transport chain, which induce endothelial dysfunction and oxidative stress in the vascular wall (29). Ischemia-reperfusion injuries are relatively common vascular events during general surgical procedures, and they present a significant problem for organ transplant. For instance, myocardial injury was experienced by 19% of individuals aged over 45 yr after noncardiac surgery in vascular surgical patients, with the majority of these events clinically silent (7). Despite this incidence level, and the significant tissue damage resulting from ischemia-reperfusion leading to irreversible pathophysiology, such as myocardial infarction and stroke, the availability and efficacy of therapeutic strategies are still poor.
Rodriguez-Mateos et al. (38) found that acute blueberry supplementation (766 mg of total polyphenols) increased flow-mediated dilation (FMD) in healthy young men, peaking 1 and 6 h postconsumption, which paralleled the reduction in neutrophil NOX activity. Acute Montmorency cherry (MC) consumption (74 mg anthocyanin, 179 mg total polyphenols) significantly lowered systolic blood pressure in tandem with increases in circulating phenolic acids (22). A number of studies have also found improvements in endothelium-dependent vasodilation after chronic supplementation with fruit polyphenols, especially among study populations with impaired cardiovascular function. Grape juice supplements have been shown to improve FMD in patients with coronary artery disease (56 days) (14) and patients with hypercholesterolemia (14 days) (16). Red grapes (72 g/day for 21 days) reduced the suppression of FMD induced by a high-fat meal (13), grape polyphenols improved FMD in patients with metabolic syndrome (267 mg polyphenols for 30 days) (6) and grape seed extract (2 g/day for 4 wk) improved FMD in patients with increased CVD risk (15). Chokeberry juice supplementation (1 g polyphenols/day) for 6 wk also increased FMD in patients with hypercholesterolemia (36), and Khan et al. (24) found that black currant polyphenol supplementation for 6 wk (815 mg polyphenols, including 143 mg anthocyanins per day) improved FMD in healthy adults consuming two or fewer portions of fruit and vegetables per day. Consumption of blueberry, cherry, cacao, and black currant polyphenols appear to enhance vascular function (19, 22, 24, 38), most likely through reduced ROS exposure and, hence, increased NO bioavailability (40, 47). Although, not all studies have shown favorable effects, for example, chronic supplementation (2–6 wk) with grapeseed extract (0.6–1.0 g/day) had no effect on FMD in patients with Type 2 diabetes (23), participants with elevated CVD risk (although baseline brachial artery diameter was increased) (36), and healthy young men (37).
To date, only one study has investigated whether polyphenol supplementation preserved endothelium-dependent vasodilatation after exposure to prolonged forearm occlusion in healthy adults (39). Schreuder et al. (39) found that after a 7-day period consuming three cups of black tea per day (~900 mg total polyphenols) FMD was enhanced 90 min after consumption of ~600 mg black tea polyphenols, but FMD was not preserved 20 min after prolonged forearm occlusion. However, prior chronic supplementation with both blueberry polyphenols (2) and cherry polyphenols (5) reduced ischemia-reperfusion (IR)-induced infarct size, better preserved cardiac function, and protected against postischemic cardiac remodeling in isolated rat hearts. Collectively, these data suggest that a longer period of cherry or berry polyphenol supplementation may be necessary to provide vasoprotection against IR injury or that effects may be more likely to be observed in a study population with elevated cardiovascular risk factors. Therefore, we hypothesized that 4 wk of Montmorency cherry supplementation would increase endogenous antioxidant capacity and, thus, improve FMD and protect against the impairment in endothelial-dependent vasodilation induced by ischemia reperfusion. We adopted an experimental model of a prolonged (20-min) occlusion of the forearm that induces a short-lasting impairment to FMD (14, 31) and provides an in vivo model of transient IR injury. We recruited a nonmedicated, physically inactive, overweight, hypertensive, middle-aged male population to take part in the study.
MATERIALS AND METHODS
Participants.
Twelve physically inactive, middle-aged male participants (age: 52.8 ± 5.8 yr, BMI: 28.1 ± 5.3 kg/m2; Table 1) completed the trials, from a total of 14 participants recruited by Z. Aboo-Bakkar from local residents in Devon, UK. All participants were nonsmokers, with no known history of diabetes, cardiovascular diseases, or bone or joint problems, and they were not taking any drugs or nutritional supplements, as assessed by questionnaires. Habitual physical activity was assessed using the International Physical Activity Questionnaire, and if participants reported <150 min/week moderate or vigorous activity, they were deemed to have met the physically inactive inclusion criterion. The study was approved by the University of Exeter Research Ethics Committee (2013/409) and conformed with the Declaration of Helsinki. All participants provided their written informed consent before taking part. Two participants withdrew from the study after baseline measurements were completed, but before commencing supplementation.
Table 1.
Participant characteristics
| Parameter | Value |
|---|---|
| Age, yr | 54 ± 1 |
| Height, cm | 179 ± 1 |
| Weight, kg | 91.5 ± 4.0 |
| Body mass index, kg/m2 | 28.2 ± 1.6 |
| Resting heart rate, beats/min | 66 ± 0 |
| Systolic blood pressure, mmHg | 142 ± 6 |
| Diastolic blood pressure, mmHg | 88 ± 3 |
Values are expressed as means ± SE.
Study design.
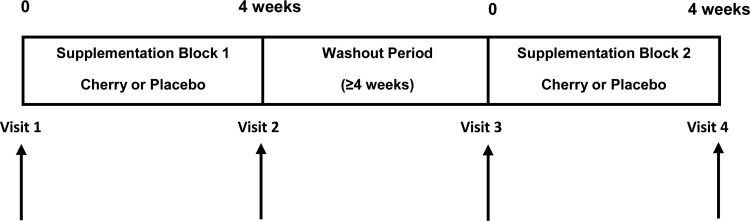
The study used a double-blind, randomized crossover design with two experimental arms and a washout period of ≥4 wk (Fig. 1). Each of the participants was randomly assigned by J. L. Bowtell using a sealed opaque envelope system to receive either MC capsules in the first supplementation block followed by placebo or vice versa and were scheduled to attend the laboratory for four trial visits. Participants and the research team involved in performing the data analysis remained blind to treatment until all analyses were completed. Baseline measurements before each supplementation block were made during visits 1 and 3, followed by the 4-wk supplementation blocks, and postsupplementation measurements were made in visits 2 and 4. The participants consumed either 1.7 g MC powder per day for 28 days in the form of six capsules (226 mg anthocyanins and 456 mg total phenolics measures as gallic acid equivalents, CherryActive, Sunbury, UK) or placebo capsules (glucose). Three capsules were consumed in the morning and three in the evening, with the last dose taken on the evening before the postsupplementation measures. In line with previous studies (10, 21, 42), there was at least a 4-wk washout period between trials.
Fig. 1.
Schematic diagram of study design.
Experimental protocol.
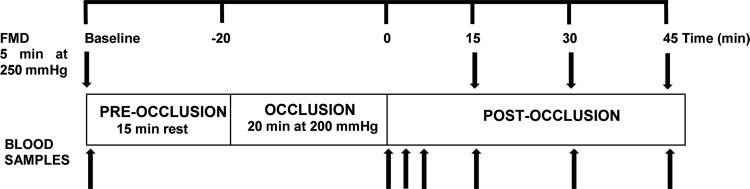
Participants reported to the laboratory at 8:00 AM on each visit, preceded by an overnight fast (≥10 h) and having abstained from alcohol and caffeine for more than 24 h. Participants were asked to avoid food and beverages containing high phenolic compounds and record their diet for two consecutive days immediately before their first laboratory visit and to replicate this for 2 days before subsequent visits. Diet diaries were analyzed using Nutritics software, and data are presented in Table 2. Upon arrival, a cannula was placed in the antecubital vein of the left arm, the same arm upon which FMD measurements were made. Trials were conducted in a quiet room with dim light and room temperature set at 24°C. Participants rested in a supine position for 10 min, before baseline FMD was assessed in the left arm with the cuff placed just below the elbow. Fifteen minutes after the baseline FMD measurement, the cuff was inflated to 200 mmHg (Hokanson, Bellevue, WA) for 20 min to induce transient ischemia, and then FMD measures were repeated after 15, 30, and 45 min of reperfusion. Venous blood samples were obtained at baseline, immediately, 2, 4, 15, 30, and 45 min after the 20-min occlusion (Fig. 2). Blood samples at 15, 30, and 45 min were collected immediately after cuff release for FMD measurement. Samples were centrifuged at 4,000 rpm and 4°C for 10 min, within 1 min of collection. Plasma was subsequently extracted and immediately frozen at −80°C for later analysis for nitrite (), nitrate (), IL-6, C-reactive protein (CRP), protein carbonyl (PC), peroxiredoxin 2 (Prx2) content, and catalase (CAT) activity.
Table 2.
Average dietary composition
| Dietary Composition | Value |
|---|---|
| Energy, kcal/day | 1883 ± 115 |
| Protein, g/day | 74.6 ± 4.1 |
| Protein, g·kg−1·day−1 | 0.9 ± 0.1 |
| Carbohydrate, g/day | 237.8 ± 29.9 |
| Fat, g/day | 66.9 ± 5.5 |
| Total cholesterol, mg/day | 328.5 ± 48.0 |
| Saturated fat, g/day | 23.2 ± 3.1 |
| Dietary fiber, g/day | 16.3 ± 2.2 |
| Sugars, g/day | 77.5 ± 22.4 |
Values are expressed as means ± SE. Average dietary composition for the 48-h period before each trial.
Fig. 2.
Brachial artery flow-mediated dilation (FMD) and venous blood collection before and after prolonged occlusion protocol to induce transient ischemia-reperfusion injury. Arrows represent FMD measurements and blood samples for plasma analysis.
Brachial artery FMD.
Brachial artery FMD was examined in the left arm, with the arm extended and positioned at an angle of ~80° from the torso. Three ECG electrodes were positioned on the left and right intraclavicular fossa and left iliac crest region, respectively. A high-resolution ultrasonography system (Sequoia 512, Acuson; Siemens, Munich, Germany) with a 13-MHz linear array transducer was used to image the brachial artery in the distal one-third of the upper arm in accordance with the established protocol in our laboratory (8, 9) and recent guidelines (41). To ensure arm stability and transducer placement, a customized arm rest and transducer holder device cradled the arm and locked the transducer in the position, providing the optimal image of the brachial artery. Ultrasound parameters were set to optimize the longitudinal B-mode images of the lumen–arterial wall interface and were then held stable for a 1-min baseline recording of the image and Doppler velocity. Continuous Doppler velocity assessment was collected using a 60° insonation angle, which did not vary during each study. The occlusion cuffs were inflated to 250 mmHg to completely block the arterial inflow for 5 min. Diameter and flow recordings resumed 30 s before cuff deflation and continued for 3 min thereafter. All FMD analyses were performed by the same investigator who was blind to the condition. Ultrasound images triggered at the beginning of the R-wave, which marks the end of the diastolic phase, were captured and then analyzed off-line using a validated ECG-gating software (Brachial Analyzer, five vascular devices; Medical Imaging Applications, Coralville, IA). The region of interest was carefully defined and frame-by-frame analysis of artery diameter (mm), and blood flow velocity (m/s) was performed by the same analyst. Baseline diameter was calculated as the mean of the artery diameter from a 1-min baseline recording before each cuff inflation. Peak diameter was determined as the highest artery diameter measurements after each cuff deflation. Endothelium-dependent vasodilation was calculated as the percentage increase in arterial diameter after a 5-min ischemic stimulus. The total shear rate under the curve (SR AUC) was calculated as the area under the shear curve versus time immediately after cuff deflation until peak arterial diameter (41).
Plasma nitrite and nitrate concentration.
All glassware, utensils, and surfaces were rinsed with deionized water to remove residual [] and [] before blood analyses. The undiluted (nondeproteinized) and deproteinized plasma was used to analyze [] and [], respectively, using a Sievers gas-phase chemiluminescence NO analyzer (NOA; Sievers NOA 280i; Analytix, Durham, UK) as described in previous studies (45, 46).
Plasma Prx2 concentration and CAT activity.
Plasma was diluted 16-fold and analyzed for [Prx2] using a commercial ELISA kit, according to the manufacturer’s instructions (Cloud-Clone, Katy, TX). For CAT activity, plasma was diluted five-fold and analyzed using a commercial ELISA kit, according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI).
Plasma CRP and IL-6 concentration.
Plasma [CRP] was determined using a turbidometric assay on a Siemens Advia 2400 autoanalyzer and using commercially available reagents (PZ Cormay, Lublin, Poland). Plasma [IL-6] was assessed using a commercially available ELISA kit (IBL International, Hamburg, Germany).
Plasma PC concentration.
Protein content in all plasma samples were measured using NanoDrop Lite (Thermo Fisher Scientific, Wilmington, DE). Each plasma sample was diluted to 10 µg/ml of protein in 1× PBS. The OxiSelect protein carbonyl ELISA kit (STA-310; Cell Biolabs, San Diego, CA) was used to measure protein carbonyls. Briefly, samples were allowed to adsorb to wells of a 96-well plate and then reacted with dinitrophenol hydrazine. The protein carbonyls derivatized to dinitrophenyl hydrazone (DNP) were then probed with an anti-DNP antibody. The standard curve was prepared from commercially prepared reduced and oxidized BSA standards as provided.
Statistical analysis.
Sample size was based on the power curves for the use of FMD in crossover designs reported by Donald et al. (16) and the effect size reported by Khan et al. (24) after polyphenol supplementation. With 80% power, 5% significance, and an effect size of 0.8 for increase in FMD (24), 12 participants are required in each group. All data are presented as means ± SE. FMD, baseline and peak brachial artery diameter, SR AUC, and plasma [] and [] were analyzed by three-way, repeated-measures ANOVA: condition (MC × placebo) by supplement (presupplementation × postsupplementation) by time (FMD measures: baseline, 15, 30, and 45 min postocclusion; plasma nitrate and nitrite measures: baseline, 0, 2, 4, 15, 30, and 45 min postocclusion). Targeted post hoc two-way repeated-measures ANOVA were completed where significant three-way interaction effects were identified. Baseline plasma [CRP], [IL-6], [PC], [Prx2], and CAT activity before and after each supplementation block were analyzed by two-way repeated-measures ANOVA. Mauchly’s sphericity test was used to check the homogeneity of variance for all ANOVA analyses; when necessary, violations of the assumption were corrected with the use of the Greenhouse-Geisser adjustment. Significant main effects were followed up with the use of least significant difference post hoc analysis. Data were analyzed using statistical software (SPSS version 19; IBM; New York), with significance accepted as P ≤ 0.05.
RESULTS
Brachial artery FMD.
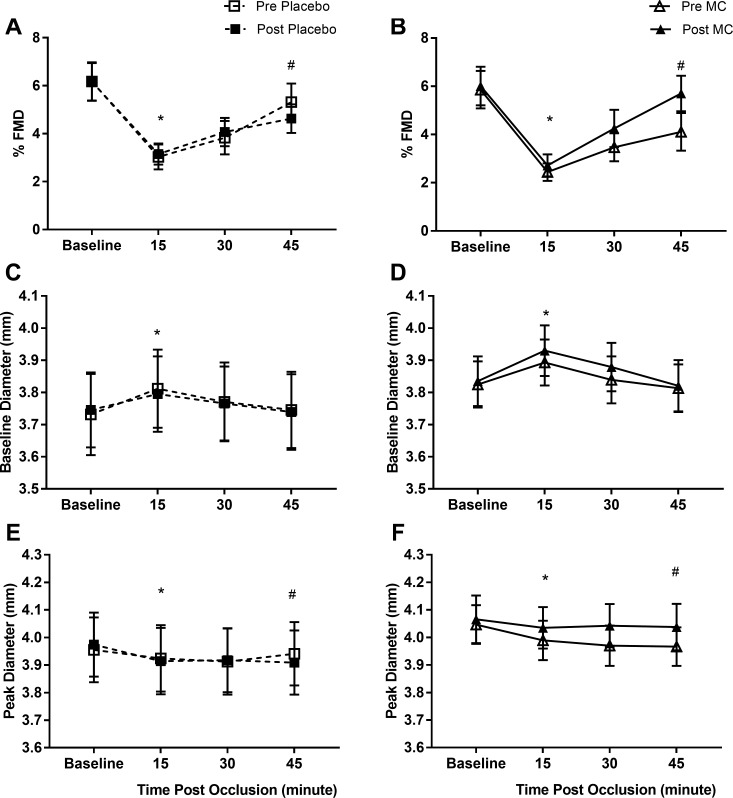
There were no significant differences in baseline FMD values for the four measurement visits. The FMD response was significantly depressed by the prolonged occlusion in all visits (main effect of time: P < 0.001; Fig. 3A) with a significant reduction in FMD 15 and 30 min postocclusion, but FMD was not significantly different to baseline 45 min after prolonged occlusion. There was a significant condition × supplementation × time interaction (P = 0.022), whereby 45 min after prolonged occlusion, the FMD response was reduced after placebo supplementation but better preserved after MC supplementation (Fig. 3B). Specifically, the magnitude of FMD suppression 45 min after the prolonged occlusion (change from baseline FMD to 45 min postocclusion) was significantly attenuated after MC (pre: −30.5 ± 8.4% vs. post: −0.6 ± 9.5%) compared with placebo (pre: −11.6 ± 10.6 vs. post: −25.4 ± 4.0) supplementation (condition × supplementation interaction: P = 0.038). Similarly, there was a reduction in SR AUC (main time effect: P < 0.001; Table 3) after prolonged occlusion, but there was no condition or supplementation effect. Normalization of the FMD data to SR AUC (FMD:SR AUC) did not affect the outcome of the analysis (Table 3). Peak brachial artery diameter was significantly suppressed after prolonged occlusion (main time effect, P < 0.001). There was a condition × supplementation × time interaction (P = 0.011; Fig. 3D), whereby peak diameter was higher following prolonged occlusion post- versus pre-MC supplementation but not post- versus pre-placebo supplementation. In contrast, basal diameter of the brachial artery (immediately before occlusion for FMD measurement) was increased after the prolonged occlusion (main time effect, P < 0.001; Fig. 3C), but there was no condition or supplementation effect.
Fig. 3.
Flow-mediated dilation (FMD; A and B), baseline diameter (C and D), and peak diameter (E and F) before and after prolonged occlusion for placebo (A, C, and E) and Montmorency cherry (MC; B, D, and F) conditions. Values are expressed as means ± SE; n = 12. *Significant difference from baseline (main effect for time, P < 0.05). #Condition vs. supplementation vs. time interaction, P < 0.05.
Table 3.
Shear rate area under the curve and FMD normalized to SR AUC
| Time |
||||
|---|---|---|---|---|
| Baseline | 15 min Post | 30 min Post | 45 min Post | |
| SR AUC* | ||||
| Placebo | ||||
| Pre | 663 ± 91 | 623 ± 63 | 594 ± 75 | 629 ± 68 |
| Post | 683 ± 101 | 533 ± 60 | 550 ± 52 | 579 ± 85 |
| Montmorency cherry | ||||
| Pre | 669 ± 85 | 525 ± 58 | 524 ± 47 | 577 ± 64 |
| Post | 632 ± 63 | 456 ± 54 | 444 ± 57 | 544 ± 63 |
| FMD:SR AUC* | ||||
| Placebo | ||||
| Pre | 0.99 ± 0.16 | 0.46 ± 0.06 | 0.66 ± 0.12 | 0.85 ± 0.12 |
| Post | 1.00 ± 0.13 | 0.67 ± 0.13 | 0.75 ± 0.10 | 0.92 ± 0.15 |
| Montmorency cherry | ||||
| Pre | 0.90 ± 0.09 | 0.49 ± 0.07 | 0.65 ± 0.10 | 0.70 ± 0.11 |
| Post | 1.06 ± 0.22 | 0.61 ± 0.09 | 1.11 ± 0.29 | 1.15 ± 0.20 |
Values are expressed as means ± SE. Shear rate area under the curve (SR AUC) and flow-mediated dilation (FMD) were normalized to SR AUC (FMD:SR AUC) measured 15 min before 20-min occlusion (baseline), 15, 30, and 45 min after reperfusion and before and after each supplementation block.
Main effect of time, P < 0.001.
Plasma analytes.
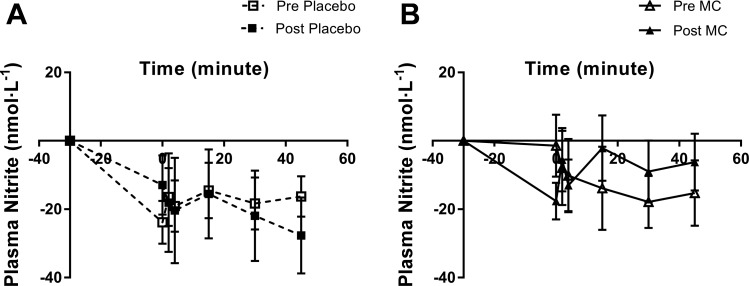
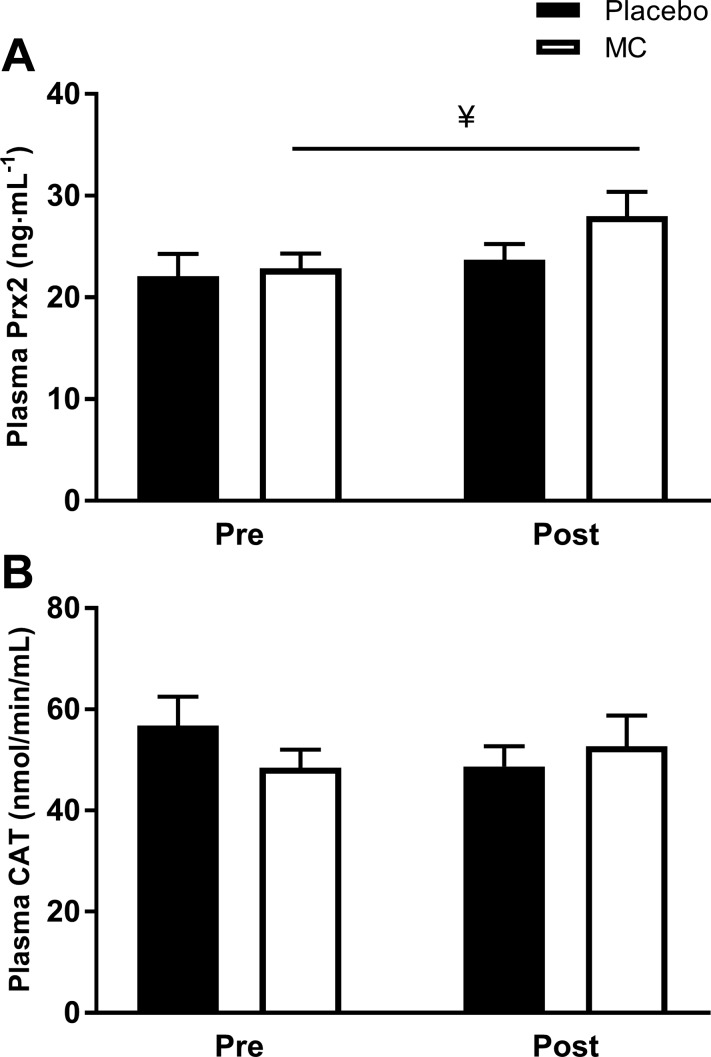
Plasma [] and [] were significantly depressed by the prolonged occlusion in all visits (main effects of time, P < 0.001; Table 4). Plasma [] returned toward baseline during the 45-min recovery after prolonged occlusion more quickly after MC supplementation compared with placebo (condition × supplement × time interaction, P = 0.033; Fig. 4, A and B). There was no significant effect of supplementation on plasma []. Basal plasma [Prx2] was higher after MC (P = 0.029) but not after placebo supplementation (Fig. 5A). However, there was no effect of supplementation on basal plasma CAT activity (P > 0.05; Fig. 5B), or plasma [CRP], [IL-6], and [PC] (P > 0.05, Table 5).
Table 4.
Plasma nitrite [] and nitrate [] concentrations
| Baseline | Time 0, min Post | Time 2, min Post | Time 4, min Post | Time 15, min Post | Time 30, min Post | Time 45, min Post | |
|---|---|---|---|---|---|---|---|
| [], nmol/l*# | |||||||
| Placebo | |||||||
| Pre | 87.3 ± 7.6 | 63.5 ± 4.2 | 70.9 ± 3.4 | 68.3 ± 4.8 | 72.8 ± 10.8 | 69.0 ± 5.7 | 71.0 ± 7.4 |
| Post | 104.2 ± 13.5 | 91.3 ± 9.7 | 86.2 ± 9.1 | 83.8 ± 11.3 | 88.7 ± 7.6 | 82.3 ± 6.5 | 76.5 ± 6.9 |
| MC | |||||||
| Pre | 82.1 ± 10.0 | 80.7 ± 11.1 | 74.2 ± 11.3 | 71.9 ± 10.1 | 68.2 ± 14.9 | 64.2 ± 11.8 | 66.8 ± 10.1 |
| Post | 83.2 ± 10.5 | 65.6 ± 7.3 | 77.7 ± 8.6 | 70.2 ± 6.4 | 81.1 ± 7.3 | 74.2 ± 9.4 | 77.0 ± 10.7 |
| [], µmol/l* | |||||||
| Placebo | |||||||
| Pre | 32.8 ± 3.9 | 29.7 ± 3.6 | 28.6 ± 3.5 | 28.7 ± 3.3 | 27.7 ± 2.9 | 27.9 ± 2.9 | 27.2 ± 3.0 |
| Post | 27.3 ± 2.6 | 25.7 ± 3.0 | 26.8 ± 3.3 | 25.9 ± 2.4 | 25.7 ± 2.7 | 24.4 ± 2.8 | 25.8 ± 2.7 |
| MC | |||||||
| Pre | 27.6 ± 4.9 | 24.8 ± 4.4 | 26.2 ± 4.7 | 25.8 ± 4.8 | 25.6 ± 4.6 | 25.0 ± 4.5 | 24.7 ± 4.1 |
| Post | 29.5 ± 5.2 | 28.2 ± 4.9 | 29.3 ± 4.9 | 27.9 ± 4.9 | 26.8 ± 4.7 | 26.6 ± 4.4 | 26.3 ± 4.4 |
Values are expressed as means ± SE. Plasma [] and [] measured 15 min before 20-min occlusion (baseline), and immediately, 2, 4, 15, 30, and 45 min after reperfusion, before, and after placebo or Montmorency cherry (MC) supplementation.
Main effect of time P < 0.001.
Three-way interaction effect.
Fig. 4.
Change from baseline in plasma nitrite concentrations after prolonged occlusion performed pre- and post- 4 wk of placebo (A) and Montmorency cherry (MC) (B) supplementation. Values are expressed as means ± SE; n = 9. #Condition × supplement × time interaction, P < 0.05.
Fig. 5.
Baseline plasma peroxiredoxin 2 [Prx2] (A) and catalase (CAT) activity (B) before and after prolonged occlusion performed pre and post 4 wk of placebo and Montmorency cherry (MC) supplementation. Values are expressed as means ± SE; n = 12. ¥ denotes supplemental effect, P < 0.05.
Table 5.
Plasma biomarkers
| Placebo |
Montmorency Cherry |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Interleukin-6, pg/ml | 0.06 ± 0.04 | 0.06 ± 0.06 | 0.06 ± 0.03 | 0.07 ± 0.09 |
| C-reactive protein, mg/l | 1.01 ± 0.33 | 3.90 ± 2.69 | 1.90 ± 0.40 | 3.40 ± 1.27 |
| Protein carbonyl, nmol/mg | 0.86 ± 0.11 | 0.69 ± 0.18 | 0.93 ± 0.17 | 0.71 ± 0.10 |
Values are expressed as means ± SE. Baseline plasma biomarkers before and after 4 wk of Montmorency cherry and placebo supplementation.
DISCUSSION
Four weeks of MC supplementation enhanced recovery of endothelium-dependent vasodilation in overweight, physically inactive, nonmedicated hypertensive, middle-aged men after the transient ischemia-reperfusion injury induced by prolonged forearm occlusion. Plasma nitrite concentration was higher after MC supplementation during the recovery period after ischemia. Moreover, plasma peroxiredoxin 2 concentration was increased after 4 wk MC supplementation, indicating increased capacity for reduction of hydrogen peroxide and, therefore, superoxide quenching. This supports the notion that MC supplementation enhanced NO bioavailability and vascular function under conditions of increased ROS generation after prolonged blood flow occlusion via increased endogenous antioxidant capacity.
Prolonged forearm occlusion induced transient IR injury as evidenced by attenuated endothelium-dependent vasodilation. DeVan et al. (14) reported that FMD in middle-aged sedentary subjects was decreased by 68% by the same occlusion protocol employed in the present study, while Schreuder et al. (39) and Rakobowchuk et al. (37) reported 33% and 36% reductions in FMD 15 min after prolonged occlusion in 8 healthy women and 20 healthy older adults, respectively. In the present study, the reduction in FMD after prolonged occlusion appears to be reproducible across trials with FMD ~53% lower, 15 min after prolonged occlusion when compared with baseline (52 ± 19%, pre-MC; 58 ± 13%, pre-placebo; 49 ± 21%, post-placebo; and 55 ± 17%, post-MC, respectively). In the present study, the reduction in FMD after prolonged occlusion appears to be reproducible across trials with FMD ~53% lower, 15 min after prolonged occlusion when compared to baseline (52 ± 19 %, pre-MC; 58 ± 13 %, pre-placebo; 49±21 %, post-placebo; and 55±17 %, post-MC, respectively). The FMD reduction after prolonged occlusion seems to be a consequence of both increased baseline artery diameter (12, 37, 39) and reduced peak artery diameter (25, 37). It has been suggested that this impaired endothelium-dependent vasodilation is due to reduced NO bioavailability as a consequence of increased ROS production during reperfusion (17, 35). Our data are consistent with this hypothesis, since plasma nitrate and nitrite concentrations were lower in the venous blood exiting the forearm subjected to prolonged occlusion. Under normal resting conditions, the rate of superoxide production is lower than the flux of NO, and this allows effective scavenging of the low intracellular levels of hydrogen peroxide and superoxide by antioxidant systems, including PRX2, as well as by NO, resulting in low levels of the cytotoxic derivative, peroxynitrite (18). However, during reperfusion immediately after prolonged occlusion, superoxide production is increased (35, 42), resulting in greater peroxynitrite formation (30) and reduction in NO availability (34, 35). We cannot exclude the possibility that the reduced pH and Po2 induced by ischemia enhanced degradation of nitrite to NO (32, 33), which may also contribute to the reduction in plasma nitrite concentration observed in the present study.
Fruit-derived polyphenols improve endothelium-dependent vasodilatation; for instance, FMD response peaked between 1 and 2 h, and 6 h after consuming 766 mg of blueberry polyphenols [310 mg anthocyanins (38)]. Keane et al. (22) showed that systolic blood pressure (SBP) was significantly lowered in hypertensive men (SBP ≥ 130 mmHg) 2 h after ingestion of 60 ml of MC concentrate (74 mg cyanidin-3-glucoside/l, 179 mean gallic acid equivalent/l). Chronic supplementation interventions of 4 to 6 wk with fruit juice or powder (32–143 mg anthocyanins/day) increased FMD response in men with mild hypercholesterolemia (36) and metabolic syndrome (6), as well as men and women with low fruit and vegetable intake (<2 portions/day) (24). However, in the present study, 4 wk of MC powder supplementation providing 256 mg/day anthocyanins (split into two doses, morning and evening) did not improve FMD response in overnight fasted, overweight, physically inactive, nonmedicated hypertensive middle-aged men. Collectively, our findings and previous literature suggest that resting vascular function is only improved after chronic fruit-based supplement consumption in those with clinical conditions or low habitual polyphenol intake, but not in those with elevated CVD risk factors. However, MC supplementation was able to protect the vasculature from the decline in endothelium-dependent vasodilation induced by ischemia reperfusion in the present study. Polyphenols are suggested to increase calcium-dependent endothelium nitric oxide synthase (eNOS) phosphorylation through the phosphatidylinositol 3-kinase-Akt pathway (3), thus enhancing NO production. Phenolic metabolites such as vanillic acid have also been shown in vivo to inhibit NOX activity in neutrophils (38). The resulting reduction in superoxide production and, hence, reduced NO degradation to peroxynitrite will also improve NO bioavailability. Anthocyanins and their metabolites have been shown to interact with cysteine residues present in Kelch-like ECH-associated protein 1 (Keap1), resulting in nuclear translocation and phosphorylation of Nrf2 (20, 26, 28), with subsequent signaling through the antioxidant response element pathway to increase production of downstream phase II antioxidant enzymes, such as the peroxiredoxins (1). The increased plasma PRX2 concentration observed after MC supplementation is consistent with this mechanism and suggests that enhanced capability to quench ROS may contribute to the improved NO bioavailability evident in the present study.
Prolonged forearm occlusion resulted in elevated baseline diameter in all trials in this and previous studies (12, 37, 39). This basal dilation might be related to increased endothelium-derived hyperpolarizing factor production since this pathway is upregulated during eNOS dysfunction and results in arterial vasodilation via vascular smooth muscle hyperpolarization (11). In contrast to the favorable effects of MC supplementation on the NO-dependent measures (peak brachial artery diameter and plasma nitrite concentration), MC supplementation did not influence this NO-independent effect on basal brachial artery diameter.
In conclusion, the results of this randomized placebo-controlled, crossover study demonstrate that 4 wk of MC powder supplementation exerted protective effects on endothelium-dependent vasodilation after transient IR injury in overweight, physically inactive, nonmedicated hypertensive middle-aged men. These effects seem to be due to increased NO availability, as evidenced by higher plasma nitrite concentration and peak arterial diameter during the FMD measurement. This may be a consequence of increased concentration of peroxiredoxin and other antioxidant systems, and, hence, reduced ROS exposure.
GRANTS
This study was partially funded by a grant from the Cherry Research Committee, Aboo-Bakkar was supported by a Ph.D. studentship from the Universiti Kuala Lumpur, and Fulford’s salary was provided by National Institute for Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.F., P.E.G., S.R., A.M.J., and J.B. conceived and designed research; Z.A.-B., P.E.G., S.R.J., B.B., and J.L.B. performed experiments; Z.A.-B., J.F., and B.B. analyzed data; Z.A.-B., J.F., P.E.G., A.M.J., B.B., and J.L.B. interpreted results of experiments; Z.A.-B. prepared figures; Z.A.-B. drafted manuscript; Z.A.-B., J.F., P.E.G., S.R.J., A.M.J., B.B., and J.L.B. approved final version of manuscript; J.F., P.E.G., S.R.J., A.M.J., B.B., and J.L.B. edited and revised manuscript. All authors read and approved the final manuscript.
REFERENCES
- 1.Aboonabi A, Singh I. Chemopreventive role of anthocyanins in atherosclerosis via activation of Nrf2-ARE as an indicator and modulator of redox. Biomed Pharmacother 72: 30–36, 2015. doi: 10.1016/j.biopha.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Ahmet I, Spangler E, Shukitt-Hale B, Joseph JA, Ingram DK, Talan M. Survival and cardioprotective benefits of long-term blueberry-enriched diet in dilated cardiomyopathy following myocardial infarction in rats. PLoS One 4: e7975, 2009. doi: 10.1371/journal.pone.0007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anter E, Thomas SR, Schulz E, Shapira OM, Vita JA, Keaney JF Jr. Activation of endothelial nitric-oxide synthase by the p38 MAPK in response to black tea polyphenols. J Biol Chem 279: 46637–46643, 2004. doi: 10.1074/jbc.M405547200. [DOI] [PubMed] [Google Scholar]
- 4.Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81, Suppl: 317S–325S, 2005. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 5.Bak I, Lekli I, Juhasz B, Nagy N, Varga E, Varadi J, Gesztelyi R, Szabo G, Szendrei L, Bacskay I, Vecsernyes M, Antal M, Fesus L, Boucher F, de Leiris J, Tosaki A. Cardioprotective mechanisms of Prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am J Physiol Heart Circ Physiol 291: H1329–H1336, 2006. doi: 10.1152/ajpheart.01243.2005. [DOI] [PubMed] [Google Scholar]
- 6.Barona J, Aristizabal JC, Blesso CN, Volek JS, Fernandez ML. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J Nutr 142: 1626–1632, 2012. doi: 10.3945/jn.112.162743. [DOI] [PubMed] [Google Scholar]
- 7.Biccard BM, Scott DJA, Chan MTV, Archbold A, Wang C-Y, Sigamani A, Urrútia G, Cruz P, Srinathan SK, Szalay D, Harlock J, Tittley JG, Rapanos T, Elias F, Jacka MJ, Malaga G, Abraham V, Berwanger O, Montes FR, Heels-Ansdell DM, Hutcherson MT, Chow CK, Polanczyk CA, Szczeklik W, Ackland GL, Dubois L, Sapsford RJ, Williams C, Cortés OL, Le Mananch Y, Devereaux PJ. Myocardial injury after noncardiac surgery (MINS) in vascular surgical patients. Ann Surg 268: 357–363. 2018. doi: 10.1097/SLA.0000000000002290. [DOI] [PubMed] [Google Scholar]
- 8.Bond B, Cockcroft EJ, Williams CA, Harris S, Gates PE, Jackman SR, Armstrong N, Barker AR. Two weeks of high-intensity interval training improves novel but not traditional cardiovascular disease risk factors in adolescents. Am J Physiol Heart Circ Physiol 309: H1039–H1047, 2015. doi: 10.1152/ajpheart.00360.2015. [DOI] [PubMed] [Google Scholar]
- 9.Bond B, Gates PE, Jackman SR, Corless LM, Williams CA, Barker AR. Exercise intensity and the protection from postprandial vascular dysfunction in adolescents. Am J Physiol Heart Circ Physiol 308: H1443–H1450, 2015. doi: 10.1152/ajpheart.00074.2015. [DOI] [PubMed] [Google Scholar]
- 10.Bowtell JL, Sumners DP, Dyer A, Fox P, Mileva KN. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med Sci Sports Exerc 43: 1544–1551, 2011. doi: 10.1249/MSS.0b013e31820e5adc. [DOI] [PubMed] [Google Scholar]
- 11.Bryan RM Jr, You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology 102: 1261–1277, 2005. doi: 10.1097/00000542-200506000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Carter SE, Faulkner A, Rakobowchuk M. The role of prostaglandin and antioxidant availability in recovery from forearm ischemia-reperfusion injury in humans. J Hypertens 32: 339–351, 2014. doi: 10.1097/HJH.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr 136: 2588–2593, 2006. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 14.DeVan AE, Umpierre D, Harrison ML, Lin H-F, Tarumi T, Renzi CP, Dhindsa M, Hunter SD, Tanaka H. Endothelial ischemia-reperfusion injury in humans: association with age and habitual exercise. Am J Physiol Heart Circ Physiol 300: H813–H819, 2011. doi: 10.1152/ajpheart.00845.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, Kluge MA, Wang N, Palmisano J, Milbury PE, Blumberg JB, Vita JA. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr 93: 934–940, 2011. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SML, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol 51: 1959–1964, 2008. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 17.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull 70: 71–86, 2004. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 18.Grisham MB, Granger DN, Lefer DJ. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic Biol Med 25: 404–433, 1998. doi: 10.1016/S0891-5849(98)00094-X. [DOI] [PubMed] [Google Scholar]
- 19.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 88: 38–50, 2008. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 20.Hwang YP, Choi JH, Yun HJ, Han EH, Kim HG, Kim JY, Park BH, Khanal T, Choi JM, Chung YC, Jeong HG. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem Toxicol 49: 93–99, 2011. doi: 10.1016/j.fct.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Keane KM, Bell PG, Lodge JK, Constantinou CL, Jenkinson SE, Bass R, Howatson G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur J Nutr 55: 1695–1705, 2016. doi: 10.1007/s00394-015-0988-9. [DOI] [PubMed] [Google Scholar]
- 22.Keane KM, George TW, Constantinou CL, Brown MA, Clifford T, Howatson G. Effects of Montmorency tart cherry (Prunus Cerasus L.) consumption on vascular function in men with early hypertension. Am J Clin Nutr 103: 1531–1539, 2016. doi: 10.3945/ajcn.115.123869. [DOI] [PubMed] [Google Scholar]
- 23.Kelishadi R, Gidding SS, Hashemi M, Hashemipour M, Zakerameli A, Poursafa P. Acute and long term effects of grape and pomegranate juice consumption on endothelial dysfunction in pediatric metabolic syndrome. J Res Med Sci 16: 245–253, 2011. [PMC free article] [PubMed] [Google Scholar]
- 24.Khan F, Ray S, Craigie AM, Kennedy G, Hill A, Barton KL, Broughton J, Belch JJF. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: a randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic Biol Med 72: 232–237, 2014. doi: 10.1016/j.freeradbiomed.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation 103: 1624–1630, 2001. doi: 10.1161/01.CIR.103.12.1624. [DOI] [PubMed] [Google Scholar]
- 26.Kropat C, Mueller D, Boettler U, Zimmermann K, Heiss EH, Dirsch VM, Rogoll D, Melcher R, Richling E, Marko D. Modulation of Nrf2-dependent gene transcription by bilberry anthocyanins in vivo. Mol Nutr Food Res 57: 545–550, 2013. doi: 10.1002/mnfr.201200504. [DOI] [PubMed] [Google Scholar]
- 27.Laslett LJ, Alagona P Jr, Clark BA III, Drozda JP Jr, Saldivar F, Wilson SR, Poe C, Hart M. Clark BA, Drozda JP, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol 60: S1–S49, 2012. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Lee SG, Kim B, Yang Y, Pham TX, Park Y-K, Manatou J, Koo SI, Chun OK, Lee J-Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J Nutr Biochem 25: 404–411, 2014. doi: 10.1016/j.jnutbio.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci 34: 313–319, 2013. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Hock CE, Nagele R, Wong PY. Formation of nitric oxide, superoxide, and peroxynitrite in myocardial ischemia-reperfusion injury in rats. Am J Physiol Heart Circ Physiol 272: H2327–H2336, 1997. doi: 10.1152/ajpheart.1997.272.5.H2327. [DOI] [PubMed] [Google Scholar]
- 31.Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ, Kuijpers TW, Deanfield JE. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation 121: 2310–2316, 2010. doi: 10.1161/CIRCULATIONAHA.108.814731. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14: 623–641, 2015. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 34.Pernow J, Böhm F, Beltran E, Gonon A. L-arginine protects from ischemia-reperfusion-induced endothelial dysfunction in humans in vivo. J Appl Physiol (1985) 95: 2218–2222, 2003. doi: 10.1152/japplphysiol.00515.2003. [DOI] [PubMed] [Google Scholar]
- 35.Pleiner J, Schaller G, Mittermayer F, Marsik C, MacAllister RJ, Kapiotis S, Ziegler S, Ferlitsch A, Wolzt M. Intra-arterial vitamin C prevents endothelial dysfunction caused by ischemia-reperfusion. Atherosclerosis 197: 383–391, 2008. doi: 10.1016/j.atherosclerosis.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Poreba R, Skoczynska A, Gac P, Poreba M, Jedrychowska I, Affelska-Jercha A, Turczyn B, Wojakowska A, Oszmianski J, Andrzejak R. Drinking of chokeberry juice from the ecological farm Dzieciolowo and distensibility of brachial artery in men with mild hypercholesterolemia. Ann Agric Environ Med 16: 305–308, 2009. [PubMed] [Google Scholar]
- 37.Rakobowchuk M, Parsloe ER, Gibbins SE, Harris E, Birch KM. Prolonged low flow reduces reactive hyperemia and augments low flow mediated constriction in the brachial artery independent of the menstrual cycle. PLoS One 8: e55385, 2013. doi: 10.1371/journal.pone.0055385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, Tabatabaee S, George TW, Heiss C, Spencer JP. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am J Clin Nutr 98: 1179–1191, 2013. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- 39.Schreuder THA, Eijsvogels TMH, Greyling A, Draijer R, Hopman MTE, Thijssen DHJ. Effect of black tea consumption on brachial artery flow-mediated dilation and ischaemia-reperfusion in humans. Appl Physiol Nutr Metab 39: 145–151, 2014. doi: 10.1139/apnm-2012-0450. [DOI] [PubMed] [Google Scholar]
- 40.Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E, Dugo G, Caputi AP, Cuzzocrea S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci 73: 1097–1114, 2003. doi: 10.1016/S0024-3205(03)00356-4. [DOI] [PubMed] [Google Scholar]
- 41.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traustadóttir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ II, Harman SM. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr 139: 1896–1900, 2009. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349, jul29 3: g4490, 2014. [Erratum in Brit Med J 349: 5472, 2014]. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr 111: 1–11, 2014. doi: 10.1017/S000711451300278X. [DOI] [PubMed] [Google Scholar]
- 45.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 46.Wylie LJ, Mohr M, Krustrup P, Jackman SR, Ermιdis G, Kelly J, Black MI, Bailey SJ, Vanhatalo A, Jones AM. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol 113: 1673–1684, 2013. doi: 10.1007/s00421-013-2589-8. [DOI] [PubMed] [Google Scholar]
- 47.Xu J-W, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 44: 217–222, 2004. doi: 10.1161/01.HYP.0000135868.38343.c6. [DOI] [PubMed] [Google Scholar]