Abstract
There is growing evidence that aerobic exercise protects against age-related cognitive decline and that cardiorespiratory fitness is an important factor for these benefits. Studies also suggest that combining physical activity with cognitive enrichment is beneficial. We further examine these predictions by comparing effects of a nutritional supplement promoting exercise capacity to a lower-intensity activity with cognitive enrichment on functional network and cognitive outcomes that otherwise decline with aging. Inactive healthy older adults were randomized to one of four groups including a low-intensity activity with complex cognitive demands (dancing), walking, walking+supplement, or an active control. Results showed that walking+supplement increased salience network functional connectivity (FC), with less training benefit for default mode network FC. Although cognitive performance did not increase for any training group, participants in the walking+supplement group who were on medication that boosted key neurotransmitters (e.g., selective serotonin reuptake inhibitors) showed improved processing speed. Overall, this study provides new insight into how to boost the protective effects of exercise on brain systems that otherwise deteriorate with aging.
NEW & NOTEWORTHY Aerobic exercise effects on brain networks that otherwise decline with aging can be boosted with a nutritional supplement including beta-alanine. Beta-alanine supplementation could enhance the extent to which aerobic adaptations benefit the brain. In contrast, cognitive enrichment with low-intensity physical activity through dance did not affect functional networks. Medications that modulate neurotransmitters affected by aging (e.g., selective serotonin reuptake inhibitors) may modify effects of exercise on cognition.
Keywords: aging, cardiorespiratory fitness, default mode network, functional connectivity, physical activity, randomized controlled trial, salience network
INTRODUCTION
Given the rising proportion of older adults and progressive cognitive decline with aging, we need more effective methods to improve brain and cognitive function in late life (42). Studies across species support that aerobic exercise protects brain systems negatively affected by aging (32, 71) and that aerobic exercise capacity as measured by cardiorespiratory fitness (CRF) is an important protective factor (51, 67, 69, 72). This evidence leads to the prediction that CRF gains are a critical factor in whether aerobic exercise benefits the brain and cognition. On the other hand, studies also suggest that cognitive enrichment could increase benefits from physical activity (PA; 6, 66). The present study aimed to test these predictions in a randomized controlled trial with healthy older adults.
Studies have used resting-state functional connectivity (FC) to identify networks of brain regions affected negatively by aging that appear modifiable through PA (65, 70). The default mode network (DMN), which is anchored by the posterior cingulate, the medial and lateral temporal cortices, and the medial prefrontal cortex, has been linked broadly to cognitive health in aging and is thought to manage internal representations such as episodic memory (4, 17). Results suggest that protective effects of CRF on brain aging are strongest for frontal-temporal connections in the DMN (18, 36, 68–70). For instance, in a 12-mo training study that randomized previously inactive older adults to receive 150 min of weekly walking or an active control, only those in the walking condition improved CRF by 4.5% and improved temporal-frontal FC of the DMN to the level of young adults (70). Another network known as the salience network (SAL) is anchored by the dorsal anterior cingulate, anterior insula, and anterior-lateral prefrontal cortex, and is thought to process salient information from interoceptive and environmental contexts to expectations in sensory and cognitive systems (61, 62). In addition to the DMN, the study also found increased SAL FC between anterior prefrontal cortex (aPFC) regions. Yet, changes in DMN FC were most strongly linked with changes in fluid abilities such as cognitive control and working memory. It should be noted, though, that the battery of cognitive tasks was targeted most to fluid abilities. We extend this work by evaluating how PA-related changes in network FC correspond to changes in cognitive performance using a broader assessment of abilities affected by aging.
Some evidence suggests that CRF is an important factor in whether and how PA benefits resting-state FC. In a cross-sectional study we compared the relationships of PA and CRF to brain network FC. Of the network interactions where CRF was related to higher FC, almost half (46%) were from DMN regions, 23% were from a frontoparietal dorsal attention network (DAN), 4% were from SAL, 0% were from a frontoparietal executive control network (ECN), and the remaining 27% of connections were between networks (72). Critically, the relation of CRF with FC was strongest after covarying for daily PA. These results suggest that either genetic aspects of CRF or the physiological response to PA, or both, are important factors over and above PA alone.
Here we further test the importance of CRF by determining whether a nutritional supplement that enhances exercise capacity would augment changes in resting FC and cognition. Beta-alanine has been shown to increase lean muscle mass and intramuscular levels of carnosine, which enhance pH buffering of lactic acid buildup during moderate-to-vigorous PA (MVPA), acts as an antioxidant to clear cell damage, and improves Ca2+ and protein regulation (29). Although intramuscular carnosine has been shown to decline with aging, beta-alanine supplementation appears to increase muscle carnosine in young (39, 41) and older adults (30), and chronic supplementation increases exercise capacity for untrained individuals across a range of ages and even without an exercise stimulus (60). Together, these data support the prediction that beta-alanine supplementation could enhance gains in exercise capacity during aerobic training by decreasing muscular fatigue and facilitating recovery after workouts.
In addition to boosting improvements in exercise capacity from training, cognitive enrichment is another factor that could be combined with PA for enhanced brain and cognitive outcomes. Studies with animal models have compared benefits of running with benefits of running plus novel arrangements of toys to explore (66). These studies have found that running is sufficient to increase learning and hippocampal neurogenesis and that enrichment does not significantly enhance effects (14, 53). On the other hand, there is evidence from intervention trials with aging populations that combining physical and mental exercise may bring greater benefit than either intervention alone (6, 75). Yet a general pattern of cognitive enrichment studies is that changes in the brain and cognition are directly related to practiced skills (21, 40). This is consistent with a recent report showing that the dance intervention applied here led to increased white matter integrity of the fornix, which may be involved in spatial and sequential learning involved in the training (19). Thus, if a dance intervention is of insufficient intensity or duration to produce improvements in cognition associated with MVPA, we may expect FC changes in networks involved in learning dance. This could include DAN, associated with orienting visuospatial attention, SAL, associated with updating while learning skills that involve overriding habits (27, 31), or DMN, which includes pathways through the fornix important for spatial memory (15).
Therefore, the present study was designed to compare effects of interventions that differentially enhance CRF gains and cognitive enrichment on brain networks and cognitive abilities that decline with aging. In the aerobic group (Walk), participants were assigned to a walking program designed to gradually improve CRF. Another group received the same program with an added daily liquid supplement containing beta-alanine (Walk+). A third group (Dance) participated in a combination of English country and contra dance with increasingly complex choreographies. Finally, the strength, stretching, and stability control group (SSS) participated in exercises focusing on strength, stretching, and stability not designed to improve CRF and thus served as an active control condition. We used a linear mixed-effects (LME) approach to test the predictions that 1) the Walk+ group would show both increased CRF and increased FC of DMN and SAL relative to the SSS control group, coupled with weaker benefits for the Walk group, and 2) PA with added cognitive enrichment in the Dance group would enhance resting FC in DAN, SAL, and DMN related to visuospatial attention, learning, and memory.
MATERIALS AND METHODS
The study was preregistered (https://clinicaltrials.gov/ct2/show/NCT01472744) with a focus on outcomes reported in this paper. We focus on resting-state FC, which is a subset of measures to assess brain function. Results for secondary outcomes such as quality of life, self-efficacy, physical function, and psychosocial outcomes are reported elsewhere (see https://clinicaltrials.gov). The University of Illinois institutional review board approved this study, and written informed consent was obtained from all participants.
Participants.
Neuroimaging, cognitive, and PA- and CRF-related data were initially collected from 247 community-dwelling healthy older adults (average age of 65 yr, 68% female, average of 15.6 yr of education). A full consort diagram is available in another recent report of the study (19), which focuses on the effects of the interventions on white matter integrity. Eligible participants met the following criteria: 1) were between the ages of 60 and 80 yr old, 2) were free from psychiatric and neurological illness and had no history of stroke, transient ischemic attack, or head trauma, 3) scored ≥23 on the Mini-Mental State Exam (MMSE) and >21 on a Telephone Interview of Cognitive Status (TICS-M) questionnaire, 4) scored <10 on the geriatric depression scale (GDS-15), 5) scored ≥75% right-handedness on the Edinburgh Handedness Questionnaire, 6) demonstrated normal or corrected-to-normal vision of at least 20/40 and no color blindness, 7) were screened for safe participation in an MRI environment (e.g., no metallic implants that could interfere with the magnetic field or cause injury and no claustrophobia), and 8) were active at a self-reported moderate intensity for <30 min two times per week for the last 6 mo. We further excluded participants with MMSE <27 to exclude those with possible mild cognitive impairment. Participants were also excluded from our analysis if they did not have resting functional MRI (fMRI) data or there was significant and consistent motion, signal dropout, or distortion that could not be corrected in the baseline resting function scan (n = 58). There was no absolute cutoff for motion. Scans were excluded on the basis of an evaluation of consistency and direction of motion, temporal signal-to-noise ratio, and dropout and on the basis of agreement from coauthors M. W. Voss and T. B. Weng on scan usability. Scan quality evaluation was done while blinded to intervention group membership. Median and interquartile range for framewise displacement (FD) are provided in Table 1, and a full listing of quality control metrics for all participants is provided in the supporting materials (see endnote). All participants provided a full health history and self-reported medications at enrollment.
Table 1.
Demographics for each intervention group
|
n |
FD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | Pre | Post | Age | %Female | Education, yr | MMSE | Pre | Post |
| SSS | 52 | 43 | 65.85 (4.29) | 65.38 | 16.49 (3.09) | 29.00 (1.10) | 0.31 (0.18) | 0.31 (0.17) |
| Dance | 53 | 46 | 65.66 (4.62) | 67.92 | 15.64 (3.28) | 28.87 (0.96) | 0.32 (0.21) | 0.34 (0.26) |
| Walk | 39 | 35 | 65.49 (4.67) | 71.79 | 15.73 (2.94) | 28.85 (1.09) | 0.32 (0.17) | 0.33 (0.18) |
| Walk+ | 45 | 39 | 64.62 (4.10) | 68.89 | 15.73 (2.21) | 28.78 (0.95) | 0.31 (0.20) | 0.28 (0.19) |
Age, education, and Mini-Mental Status Exam (MMSE) values are means (SD); frame-to-frame displacement (FD) values are medians (interquartile range) because they are positively skewed; n = no. of participants. Intervention groups were not significantly different in age, sex distribution, education, MMSE score, or baseline FD in the scanner. No group showed differential change in FD from preintervention (Pre) to postintervention (Post), suggesting that changes in functional connectivity could not be driven by changes in FD. Dance, dance group; SSS, strength, stretching, and stability active control group; Walk, walking group; Walk+, walking+supplement group.
The final sample of older adults included 189 community-dwelling healthy older adults (68% female) with an average age of 65.4 (4.4) yr [mean (SD)] and average education of 15.9 (2.9) yr. This is the sample described in our cross-sectional paper (n = 189; 72), which represents the baseline measurement before randomization. Participant demographics broken down by intervention group are shown in Table 1. Twenty-six participants did not complete the postintervention fMRI (n = 9 SSS, n = 7 Dance, n = 4 Walk, and n = 6 Walk+), and overall their age [65.5 (4.2) yr], sex (70% female), and education level [16 (2.8) yr] were representative of the baseline sample. Because our analysis approach with longitudinal LME models can accept missing data, all participants with baseline data were included in the longitudinal analyses.
Intervention groups.
All participants completing baseline assessments were randomized to one of four intervention groups (SSS, Dance, Walk, and Walk+; 19). Participants in all intervention groups attended supervised sessions three times per week for 60 min each for 6 mo, and groups did not differ in program adherence or enjoyment (33). The intervention was conducted in four waves from October 2011 to November 2014.
The Walk intervention was designed to mimic our previous aerobic exercise interventions showing benefits for brain structure and function in older adults (25, 69, 70). The program was designed to improve CRF through a gradual increase in heart rate relative to individualized maximum heart rate measures during the graded maximal exercise test. Participants were instructed to walk within a target heart rate of 50–60% of their maximal heart rate for the first 6 wk and 60–75% for the last 18 wk. Frequent assessment of heart rate, using either palpation or Polar heart rate monitors, and rating of perceived exertion ensured that participants’ exercise intensity was performed at the prescribed level. Participants in the Walk+ group participated in the same walking program as the Walk group and additionally received a daily Ensure shake provided by Abbott Nutrition that contained their standard multivitamin formula as well as beta-alanine.
The Dance intervention was designed to provide simultaneous cognitive and social enrichment combined with PA. Participants were instructed to learn complex social dance sequences, and choreographed dance combinations became progressively more challenging over the course of the 6-mo program. The dance styles were selected (i.e., contra and English country dancing) to minimize lead-follow roles to require participants to move between partners during each dance. In each session, participants learned ~4 dances and recorded their heart rate and perceived exertion after each dance. Each participant learned and alternated between two roles for each dance, increasing the cognitive challenge.
Finally, the SSS group served as the active control group to account for social engagement and provided nonadaptive cognitive enrichment. Similar to previous studies from our group, a trained exercise specialist instructed participants in a program designed to improve mobility and balance. The program includes nonaerobic stretches, simple strength exercises, and basic stability activities for all the large muscle groups. Each stretch was gently held to a point of slight tension but not pain for ~20–30 s. Each stretching and toning session included a 10–15-min warm-up and cooldown and 30–45 min of the above-described stretching and toning exercises. The number of sets for each activity varied depending on the type of activity being performed. Typically, strength-related activities comprised 1 or 2 sets of 8–12 repetitions. Stretching and stability exercise comprised both repetitions and sets as well as activities that were held (e.g., poses) for varying lengths of time. A new series of activities was introduced each month to maintain interest. Note that the toning exercises were not designed to build muscle mass based on an individual’s one-repetition maximum, which is typically done in studies designed to test whether resistance training would increase cognitive and brain health (47, 48). Rather, the SSS group was more similar to the balance-and-toning active control groups from these studies.
CRF and PA assessment.
Descriptive statistics for PA, resting heart rate, and CRF before and after the intervention are provided in Table 2. CRF was defined as peak oxygen consumption (V̇o2peak, in ml·kg−1·min−1), measured with indirect calorimetry during a modified Balke graded maximal exercise test on a motor-driven treadmill test. The exercise test was completed when the participant terminated the test volitionally or the physician stopped the test because of medical concerns. A Parvo Medics metabolic system and software were used to measure expired oxygen, expired carbon dioxide, ventilation, and respiratory exchange ratio. Blood pressure was monitored continuously, and heart rate was measured via direct 12-lead electrocardiographic monitoring. V̇o2peak was assessed via continuous sampling of expired gases sampled at 30-s intervals and was indicated as the highest value achieved during the test. Supine resting heart rate was recorded using the same system preceding the maximal fitness test.
Table 2.
Descriptive statistics for PA and CRF data preintervention and postintervention
| Light PA, min/day |
MVPA, min/day |
CRF, ml·kg−1·min−1 |
RHR, beats/min |
|||||
|---|---|---|---|---|---|---|---|---|
| Group | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| SSS | 270.09 (64.82) | 286.59 (57.26) | 40.56 (39.25) | 46.43 (30.19) | 19.19 (4.92) | 20.23 (4.38) | 74.48 (11.20) | 72.02 (9.53) |
| Dance | 274.65 (64.30) | 274.43 (64.38) | 43.57 (31.93) | 47.43 (30.29) | 20.34 (4.76) | 20.25 (5.15) | 75.11 (10.69) | 74.02 (9.42) |
| Walk | 281.34 (63.86) | 269.89 (79.41) | 40.67 (55.06) | 64.29 (47.43) | 19.91 (5.20) | 21.40 (5.74) | 73.54 (9.47) | 71.71 (9.73) |
| Walk+ | 281.44 (74.49) | 263.82 (70.46) | 39.17 (34.57) | 57.62 (30.17) | 19.88 (4.24) | 21.42 (3.70) | 70.76 (10.69) | 70.78 (10.63) |
Light-intensity physical activity (Light PA), cardiorespiratory fitness (CRF), and resting heart rate (RHR) values are means (SD); moderate-intensity physical activity (MVPA) values are medians (interquartile range) because they are positively skewed. Table shows PA and physiological outcomes related to CRF. Note that participants were assessed in the last week of the program, so the postassessment includes intervention sessions. Dance, dance group; Post, postintervention; Pre, preintervention; SSS, strength, stretching, and stability active control group; Walk, walking group; Walk+, walking+supplement group.
To assess PA, participants were instructed to wear the ActiGraph GT3X accelerometer (ActiGraph, Pensacola, Florida) for 7 consecutive days on an elastic belt on the left hip during all waking hours, except for when bathing or swimming. The participants completed a daily log to record the time that the accelerometer was worn, and this log was used to verify the accelerometer data for processing with the ActiLife v5.6.0 software. Each valid measurement epoch (1 min) was classified using activity intensity cutoff ranges appropriate for older adults (26): sedentary activity [0–50 counts per minute (cpm)], light PA (51–1,040 cpm), or MVPA (>1,040 cpm). The total minutes of each intensity were divided by total valid days to yield average daily minutes of sedentary activity, light PA, and MVPA. MVPA was positively skewed; therefore, we used a natural log transformation to improve normality of the distribution.
Cognitive assessment and preprocessing.
All participants completed a comprehensive cognitive assessment including the Virginia Cognitive Aging Project task battery (59), as well as two cognitive experimental tasks used in our previous exercise-training studies (task switching and spatial working memory; 34, 68, 70). The full task battery (7, 19) and modifying effects of baseline brain modularity on intervention-related change in cognition (7) are described in our recent publications and in our links with supporting material. Cognitive outcomes are examined as secondary outcomes in this report, based on composite measures of fluid abilities (matrix reasoning, Shipley abstraction, letter sets, spatial relations, paper folding, form boards, spatial working memory overall accuracy, and task-switching reaction time measure of local cost computed as reaction time on the switch trials relative to repeat trials within the task-switching block), perceptual speed (digit symbol, letter comparison, and pattern comparison), memory (logical memory, free recall, and paired associates), and vocabulary (Wechsler Adult Intelligence Scale vocabulary, picture vocabulary, and synonym/antonym). Composite measures were created by first winsorizing each raw cognitive variable with the psych R package, by trimming scores outside of 0.3% (i.e., 3 SDs) of the full sample distribution for each session. Winsorized scores were standardized across all participants at pretest and posttest separately and then averaged according to construct groupings.
MRI acquisition.
All images were acquired during a single session on a 3-T Siemens Trio Tim system (Siemens, Erlangen, Germany). T2*-weighted resting-state images were acquired with a fast echo-planar imaging (EPI) sequence with blood oxygen level-dependent (BOLD) contrast [6 min, repetition time (TR) = 2 s, echo time (TE) = 25 ms, flip angle = 80°, 3.4 × 3.4-mm2 in-plane resolution, 35 four-millimeter-thick slices acquired in ascending order, generalized autocalibrating partial parallel acquisition (GRAPPA) acceleration factor = 2, 64 × 64 matrix] while the participants were asked to lie still with their eyes closed. Additionally, dual-echo gradient field maps were acquired to account for geometric distortions caused by magnetic field inhomogeneity (44). The gradient field maps were collected as 35 four-millimeter-thick slices, 3.4 × 3.4-mm2 in-plane resolution, TR = 700 ms, TE = 10/12.46 ms, and flip angle = 35°. Resting-state and field map images were obtained parallel to the anterior-posterior commissure plane with no interslice gap. High-resolution structural MR scans were acquired using a three-dimensional magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted sequence (TR = 1,900 ms, TE = 2.32 ms, inversion time = 900 ms, flip angle = 9°, 256 × 256 matrix, field of view = 230 mm, 192 slices, resolution = 0.9 × 0.9 × 0.9 mm, GRAPPA acceleration factor = 2) and used as an intermediate step in registration of functional images to standard Montreal Neurological Institute (MNI) space.
MRI data processing.
Resting-state image processing and analyses were performed with an in-house script library using tools from the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL 5.0.4, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki), Analysis of Functional NeuroImages (AFNI, https://afni.nimh.nih.gov), FreeSurfer (http://surfer.nmr.mgh.harvard.edu), and MATLAB (The MathWorks, Natick, MA). All procedures for our primary FC analyses are the same as previously described in our study that focuses on the relationships between PA, CRF, and functional brain networks (72). Briefly, we applied standard preprocessing including motion correction using AFNI’s “3dvolreg” function, distortion correction with gradient field maps, brain extraction with FSL’s Brain Extraction Tool (BET), spatial smoothing with FSL’s Smallest Univalue Segment Assimilating Nucleus (SUSAN; 6-mm full width at half maximum), temporal filtering (0.008 < f < 0.08 Hz, where f is frequency), and nuisance regression (6 motion parameters, white matter, cerebrospinal fluid, and global signal). Therefore, the processing stream used for all primary analyses included global signal regression (GSR). Although the use of GSR is an active area of research, its use has been shown to account for a large proportion of noise due to motion and physiological noise and to improve specificity of FC measurement within specific networks (52, 56). However, for source of comparison in the Supplemental Material we report results based on a processing stream that does not include GSR, which is consistent with the CompCor processing stream (9).
For all analyses, volumes above an FD threshold of 0.5 mm that coincided with BOLD signal changes in the residual time series were removed from subsequent FC analyses (38). Medians and interquartile ranges for preintervention and postintervention FD are shown in Table 2. Longitudinal models indicated that motion variables such as FD did not differ between intervention groups at baseline or from preintervention to postintervention (all P > 0.59). In addition, FD showed strong stability between pretest and posttest [r(161) = 0.71, P < 0.001, 95% confidence interval = 0.63–0.78], which is consistent with this variable reflecting a trait-level variable rather than only day-to-day fluctuations in scanner movement (74).
FC analysis of resting-state networks.
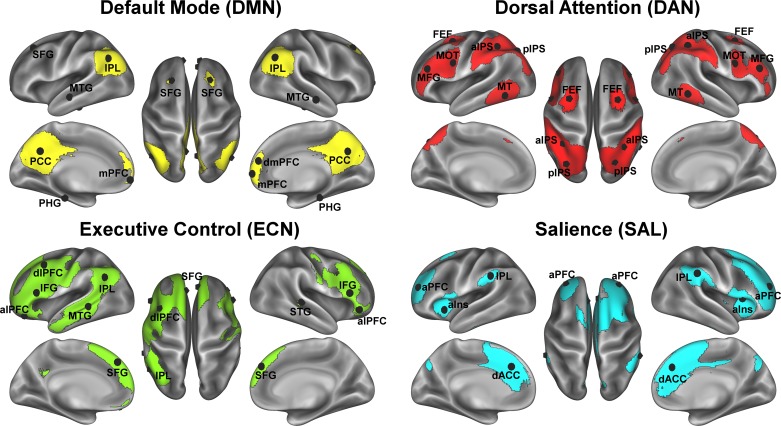
As described in our cross-sectional paper with the baseline sample (72), we identified well-replicated cortical association networks in our sample using group-level independent-component analysis with FSL’s Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) tool. Briefly, residual fMRI signal following the nuisance regression as described above was decomposed into 13 independent spatiotemporal components, from which we identified 4 networks of interest for the present study: 1) DMN, 2) DAN, 3) ECN, and 4) SAL (see Fig. 1). For each of these four networks we constructed 14-mm-diameter spheres centered on the peak coordinates in standard MNI space within the independent component statistical map (listed in the Supplemental Material; illustrated in Fig. 1). A seed of this size in standard MNI space ensured a sufficient sampling of functional voxels (~13) in native space for a reliable mean time series for FC analyses. Network regions of interest (ROIs) were registered to native (functional) space with the following steps: 1) each participant’s EPI was registered to their high-resolution structural T1 image using the Boundary-Based Registration (BBR) algorithm (37), 2) each participant’s high-resolution structural image was registered to standard MNI space with FMRIB’s Nonlinear Image Registration Tool (FNIRT) nonlinear registration using a default 10-mm warp resolution (2, 3), 3) these two transformations were then concatenated and applied to the participant’s functional image to register their functional EPI to standard MNI space, and 4) a reverse transform was used to register the network ROIs from standard MNI space to each participant’s native EPI space.
Fig. 1.
Association networks examined for intervention effects. Association networks and their region-of-interest node peaks derived from independent-component analysis of the baseline resting-state scans. Region-of-interest acronyms correspond to the labels in Supplemental Table S1; lateralized regions of interest are visualized on the appropriate hemisphere (in each group, the left hemisphere is shown at left, and the right hemisphere is shown at right). aIns, anterior insula; aIPS, anterior intraparietal sulcus; alPFC, anterior lateral prefrontal cortex (PFC); aPFC, anterior PFC; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral PFC; dmPFC, dorsomedial PFC; FEF, frontal eye fields; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; mPFC, medial PFC; MFG, middle frontal gyrus; MOT, somatomotor cortex; MT, middle temporal; MTG, middle temporal gyrus; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; pIPS, posterior intraparietal sulcus; SFG, superior frontal gyrus; STG, superior temporal gyrus.
For FC analyses, the mean (preprocessed) time series was extracted from all ROIs in native EPI space. Cross-correlation between ROIs was estimated with Pearson correlation coefficients and transformed to Fisher’s Z-estimates Z(r) using Fisher’s r-to-z transformation. Functional network integrity for each network of interest (DMN, SAL, DAN, and ECN) was estimated as the average of all within-network pairwise Z(r) estimates. We also examined the DMN core subsystem, including the posterior cingulate and ventral medial prefrontal cortex, and the DMN core regions with the bilateral parahippocampus (BPHG), because our previous studies have highlighted relationships between CRF and exercise training with these specific aspects of the DMN (68, 70, 72) and the fornix has a known role in medial temporal lobe connectivity (15).
Statistical analysis with longitudinal mixed-effects modeling.
Because our hypotheses target effects of each intervention relative to SSS, intervention effects were evaluated using a contrast matrix with three dummy codes for four groups such that SSS was the reference. With respect to group × session interactions, we report the specific results for each experimental group compared with the reference group. Contrasts were evaluated within a longitudinal LME model performed with the lme4 and lmerTest packages in R. For all models, continuous predictor and dependent variables were z-score scaled, so fixed-effect coefficients reflect standardized β-coefficients.
Although randomization equalizes the effect of different medications on baseline measures, some types of medication have known interactions with aerobic exercise training. First, 70% of participants were taking medications that affect the cardiovascular system to control cholesterol, blood pressure, and a mixture of other conditions. Because these medications could affect the measurement of CRF, cardiovascular medication use was entered as a covariate (0 = no, 1 = yes) when testing whether training enhanced CRF. It is important to note that here we collapsed across drugs that can have different mechanistic effects on exercise tolerance and CRF (i.e., statins and antihypertensives). The purpose of the covariate was to account for individual differences in a factor generally affecting measurement of CRF. We did not have sufficient sample size for thorough coding of unique effects while also accounting for comorbidities. Second, 26% of participants reported taking either antianxiety medication or antidepressants such as selective serotonin reuptake inhibitors or other drugs known to block reuptake of norepinephrine and dopamine (e.g., bupropion), most often for mild anxiety or depressive symptoms. Because these drugs have been shown to interact with exercise-training effects on the brain and cognition (50, 58), we included use of antianxiety medication and antidepressants as a covariate (referred to as “AA medication,” 0 = no, 1 = yes) in all longitudinal models with brain and behavioral measures as outcomes to account for their main effect, interaction with session, and the three-way interaction with session and group.
In principle, the intercepts should be equivalent in manipulated variables and covariates such as age and gender because of randomization. Indeed, on the basis of a one-way ANOVA with group as a factor, groups did not differ in baseline CRF [F(3,185) = 0.52, P = 0.67], MVPA [F(3,185) = 1.04, P = 0.38], age [F(3,185) = 0.70, P = 0.55], sex [F(3,185) = 0.14, P = 0.94], years of education [F(3,185) = 0.93, P = 0.43], or cognitive status assessed with the MMSE [F(3,185) = 0.40, P = 0.75]. The critical null hypotheses are that the experimental-versus-reference group pairs have statistically equivalent slopes. Support for the null hypothesis would be indicated by a nonsignificant coefficient for the group × session and group × session × medication interaction. In contrast, positive statistically significant coefficients for these interaction terms for each group reflect the extent to which a greater change was observed for the experimental group compared with SSS and whether medication use interacted with this effect. Inference of statistical significance was performed with lmerTest at alpha P < 0.05. We report model-estimated effects and unadjusted two-tailed P values with an emphasis on patterns of effect sizes across outcome measures.
RESULTS
Intervention-related changes in CRF and PA.
Baseline and postintervention CRF and PA variables for all four groups are shown in Table 2. The longitudinal LME for CRF showed a significant session × group interaction for the Walk [β = 0.58 ± 0.26 (mean ± SE), t(156.32) = 2.20, P = 0.03] and Walk+ groups [β = 0.44 ± 0.22, t(159.27) = 2.00, P = 0.04]. A post hoc comparison of walking groups showed no difference in their CRF gains [β = −0.15 ± 0.25, t(70.4) = −0.59, P = 0.56]. Similarly, the model for MVPA showed a statistically significant session × group interaction for the Walk [β = 0.36 ± 0.18, t(170.31) = 2.03, P = 0.04] and Walk+ groups [β = 0.50 ± 0.17, t(172.08) = 2.88, P = 0.005]. A post hoc comparison of walking groups showed no difference in their MVPA gains [β = 0.14 ± 0.17, t(78.1) = 0.81, P = 0.42]. Thus, the supervised walking interventions led to greater gains in CRF and MVPA relative to the SSS active control group, whereas this was not observed for the Dance group. However, Walk and Walk+ showed similar gains, so the supplement did not appear to increase CRF gains from aerobic training.
Intervention-related changes in FC of cortical association networks that degrade with aging.
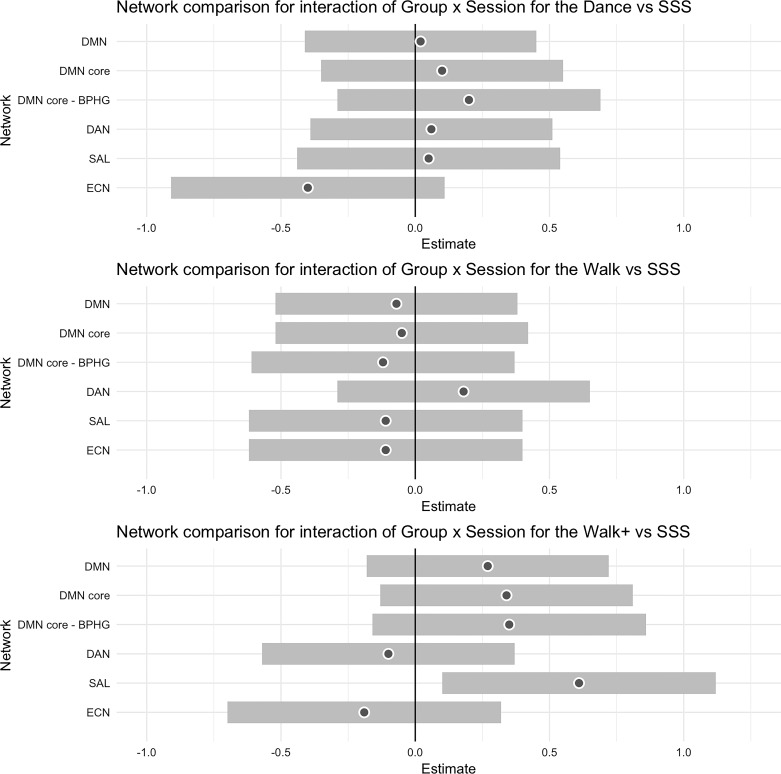
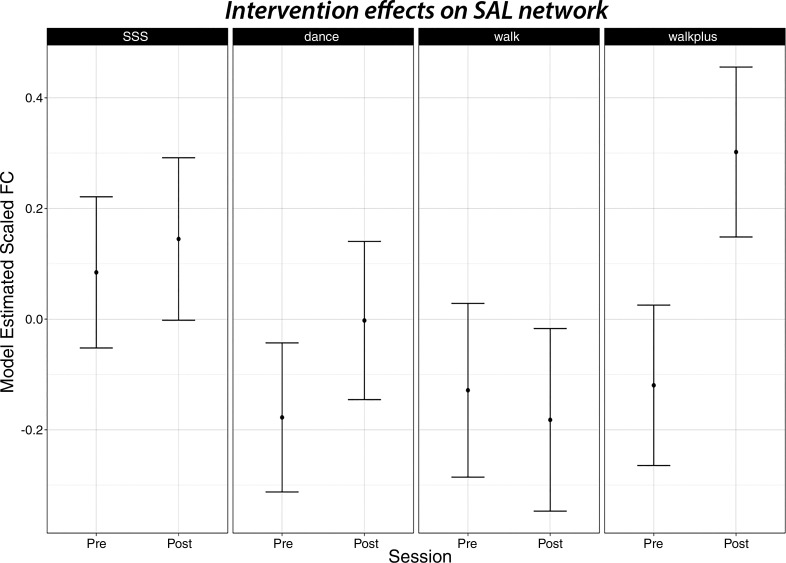
The standardized β-coefficients for all network outcomes with 95% confidence interval for each group relative to the reference are shown in Fig. 2. Contrary to our prediction, no groups showed statistically significant increases in DMN FC relative to the reference. The strongest effect size for the DMN was the DMN core FC with the BPHG [β = 0.35 ± 0.26, t(172.35) = 1.36, P = 0.17]. Consistent with our prediction, there was a statistically significant session × group interaction in favor of the Walk+ group for SAL [β = 0.61 ± 0.26, t(176.72) = 2.34, P = 0.02]. Figure 3 shows the model-estimated FC at preintervention and postintervention for each group. Note that this effect was also statistically significant when using the CompCor processing stream [β = 0.58 ± 0.24, t(182.90) = 2.41, P = 0.02]. We further examined whether the bilateral aPFC regions of SAL showed enhanced FC, as they did from 1 yr of walking in our previous study (70). Indeed, of all bilateral pairs, only the aPFC showed greater increases for the Walk+ group relative to the active control group [β = 0.73 ± 0.28, t(175.40) = 2.65, P = 0.009]. Additionally, there was a three-way interaction between group, session, and AA medication use for aPFC [β = −1.17 ± 0.53, t(178.48) = −2.22, P = 0.03], which reflected increased SAL aPFC FC for Walk+ participants not on AA medication (n = 32) [β = 0.73 ± 0.28, t(129.07) = 2.61, P = 0.01], whereas there was no change in Walk+ on AA medication (n = 13). Neither DAN nor ECN showed significant session × group or session × group × medication interactions for any intervention group (all P ≥ 0.05).
Fig. 2.
Summary of standardized β-coefficients for intervention effects on functional brain networks. Coefficients for each experimental group [dance group (Dance), walking group (Walk), and walking+supplement group (Walk+)] are shown with reference to the strength, stretching, and stability active control group (SSS). For each estimate, the gray dot reflects the standardized β-coefficient, and the surrounding band is the 95% confidence interval. BPHG, bilateral parahippocampus; DAN, dorsal attention network; DMN, default mode network; ECN, executive control network; SAL, salience network.
Fig. 3.
The walking+supplement group (Walk+) showed selective increases in salience network (SAL) functional connectivity (FC). Model-adjusted SAL FC estimates are shown preintervention (Pre) and postintervention (Post) for each intervention group; no groups differed in baseline FC from the strength, stretching, and stability active control group (SSS). Point estimates are shown along with their 95% confidence intervals. Dance, dance group; Walk, walking group.
Cognitive change and relation with change in network FC.
We used the same predictor model as above to determine whether there were changes in cognitive constructs as a function of the intervention and, if so, whether change in DMN core-BPHG or SAL FC interacted with change in cognition.
There were no statistically significant intervention effects for memory or vocabulary constructs for any group. The model for fluid processing showed only a two-way interaction of session × group such that the Dance group showed greater decline than the SSS group [β = −0.31 ± 0.12, t(170.79) = −2.62, P = 0.01]. Only processing speed showed a positive benefit for an active intervention. The model for processing speed showed a three-way interaction between session, group, and AA medication use for the Dance [β = 0.50 ± 0.22, t(168.59) = 2.26, P = 0.03] and Walk+ groups [β = 0.63 ± 0.23, t(169.26) = 2.67, P = 0.008]. Both interactions reflected a greater improvement in perceptual speed for those on AA medication. The Dance participants not on AA medication (n = 38) showed decline relative to SSS [β = −0.27 ± 0.12, t(125.58) = −2.29, P = 0.02], whereas the Dance participants on AA medication (n = 15) showed a positive but nonsignificant effect [β = 0.24 ± 0.18, t(43.21) = 1.32, P = 0.19]. Similarly, the Walk+ participants not on AA medication (n = 32) showed a negative trend [β = −0.21 ± 0.12, t(125.10) = −1.67, P = 0.10], whereas the Walk+ group on AA medication (n = 13) showed a statistically significant positive effect [β = 0.44 ± 0.19, t(43.48) = 2.30, P = 0.03].
With respect to correspondence of functional network changes with perceptual speed, those in the Walk+ group on AA medication showed a significant interaction with DMN Core-BPHG FC [β = 0.54 ± 0.24, t(43.31) = 2.24, P = 0.03], such that greater increases in DMN core-BPHG FC for this group were related to greater improvement in perceptual speed. SAL did not show this interaction.
DISCUSSION
Here we used functional neuroimaging of brain systems known to deteriorate with aging to gain mechanistic insight into well-replicated behavioral observations that physical exercise and higher CRF reduce risk of cognitive decline and dementia (16, 46, 73). In this randomized controlled trial, both the Walk and Walk+ groups increased CRF from 6 mo of aerobic (walking) training. Although the supplement did not increase CRF gains from aerobic training, it did increase gains in functional coupling of SAL. Results do not support our prediction that DMN FC would increase from an intervention that improved exercise capacity. These results are consistent with other recent studies showing that on average, training-induced change in DMN FC is not evident after 6 mo of training (35, 70) but do not rule out that longer periods of PA that increase exercise capacity are beneficial for DMN FC (11, 70). Although we did not observe reliable training-related changes in cognitive performance, we found that participants in the Dance and Walk+ groups on AA medication improved perceptual speed relative to the control group. For the Walk+ group, improved perceptual speed was related to increased FC of the DMN core with the BPHG. Thus, our results partially support our predictions and provide some mechanistic clues as to how aerobic exercise and CRF are protective for the aging brain.
First, the pattern of results suggests that there are different mechanisms for the relationship between aerobic exercise and functional integrity of the SAL and DMN systems.
For instance, only the Walk+ (and not Walk) group increased SAL FC as assessed with and without GSR. We did not observe training-related changes in DMN FC in any group. However, as we previously reported (72), DMN core FC was related to CRF independent of MVPA with and without GSR after adjusting for medication usage [see Voss et al. (72) and links below with supporting material]. Together, this pattern suggests that increases in SAL FC are most strongly linked to training-related changes in MVPA and CRF and that the nutritional supplement amplified the effects of aerobic training on SAL FC. In contrast, DMN core FC may be more related to genetic aspects of CRF or the response to PA, such as inherited mitochondrial function (13), and these pathways may be distinct from or slower to respond to aerobic training than pathways modifying SAL FC (12).
Because the Walk+ group did not have greater improvements in CRF than the Walk group, the mechanism by which the supplement with added beta-alanine increased SAL FC presumably worked downstream from adaptions related to CRF. In other words, the supplement may have increased the interaction between training-related CRF adaptations and the brain. Beta-alanine is thought to improve aerobic exercise capacity by buffering accumulation of lactic acid in the muscle during MVPA, and there are some reports of an antioxidant effect (10, 29, 60). However, without measures of muscle content or circulating factors secreted from the muscle, we cannot directly link or rule out any putative downstream effects to changes in resting FC or cognition.
With respect to cognitive enrichment, the Dance intervention did not increase or decrease FC for any of the association networks examined here. In contrast to our functional network results, we have recently shown that the Dance group showed increased white matter integrity in the fornix (19). However, we note that no training-related changes in white matter integrity were detected at a broader scale for larger tracts in the frontal and temporal lobes as we have observed in correlational studies (23, 55, 63) and in relation to changes in CRF following 1 yr of aerobic training (69). This may suggest that the Dance intervention had insufficient intensity or duration to enhance benefits of cognitive enrichment. Indeed, participants in the Dance group rated their perceived exertion as lower than that of the other groups (33). There is theoretical and empirical support for the benefits of aerobic exercise coupled with cognitive enrichment to be maximized when training is at a moderate-to-vigorous intensity (57). The dance program implemented here incorporated a large volume of low-intensity instruction time during each class. Future work that prioritizes time spent dancing while reducing time spent in instruction would help to elucidate whether greater intensity coupled with cognitive enrichment would be needed for changes in the fornix to appear in widespread functional networks. Exergaming would also provide a good medium for simultaneous and adaptive cognitive and physical training (6). Our null results for the Dance group may also suggest that more specific systems need to be probed through higher-resolution fMRI or task-evoked FC. In sum, our results do not support the prediction that cognitive enrichment added to PA through dance improves large-scale functional network integrity. However, there remain several viable explanations for these null results that could be examined in follow-up analyses and future studies.
We also extend our and others’ previous studies by including a comprehensive assessment of cognitive constructs as a parallel outcome with functional networks. On average, the group × session interaction results did not show training-related changes in cognitive constructs of perceptual speed, fluid abilities, episodic memory, or vocabulary. This null effect may have been partly due to the use of neuropsychological tasks that were less sensitive to subtle change over 6 mo in cognitively healthy adults. The literature is also quite mixed with respect to benefits of relatively short aerobic training interventions for cognitive performance in unimpaired older adults (5, 24, 45, 49, 54). This may suggest that there are important modifiers or boundary conditions that are not typically accounted for (e.g., diet, sleep, and medications).
Our results suggest that medication may be an important, albeit complex, modifier. Participants in the Dance and Walk+ groups on medications that increase availability of one or more of the neurotransmitters serotonin, norepinephrine, and dopamine showed a statistically significant improvement in perceptual speed. This result is post hoc because participants were not randomized on the basis of medication status. Yet it is worth noting that the result is consistent with basic exercise neuroscience showing that these are neurotransmitters involved in exercise benefits for cognition (8, 28) and that antidepressants that increase serotonin availability can facilitate exercise-induced production of brain-derived neurotrophic factor in the hippocampus (50, 58). The pattern of FC changes also suggests that the hippocampus may play a role in this effect. For those in the Walk+ group, AA medication suppressed detection of training-related changes in SAL aPFC FC, whereas increased DMN core FC with the BPHG was related to increased perceptual speed on configural discrimination tasks. Still other complicating issues are that previous studies have found that selective serotonin reuptake inhibitors that increase availability of serotonin can have a vasoconstrictive effect (22, 43) and that elderly adults with depressive symptoms tend to have lower resting FC in the aPFC SAL regions (1). We also have no explanation for why those in the Dance and Walk+ groups not on AA medications tended to show declines in perceptual speed relative to the SSS control group. Therefore, although our results highlight a potential factor for boosting exercise effects on the brain and cognition with nutritional and pharmacological supplements, well-powered experiments with healthy individuals randomized to short-term pharmacological treatments combined with exercise and imaging modalities less dependent on neurovascular coupling would provide a starting point for strengthening conclusions from these results. Additionally, with more precise sample description with respect to medications in published studies, future meta-analyses could examine whether patterns similar to ours are consistent across samples.
Several limitations of our study should be noted. We did not collect follow-up measures of FC or cognition to measure the duration of training-related effects. However, the goal of the study was rather an initial proof of concept to test the potential for treatments in combination with physical exercise. Along these lines, we present results without correction for multiple comparisons across network and cognitive outcomes. On the basis of these results, future studies as described above may focus on synergistic treatments for optimizing CRF gains, complemented with tighter control on multiple comparisons and long-term follow-up of brain and behavioral outcomes. Similarly, because of the proof-of-concept nature of the randomized controlled trial, there was no placebo-controlled condition for the nutritional supplement. However, given the positive results in this report, future studies may consider focusing comparison on a factorial combination of both active control and aerobic training with and without nutritional supplementation, while also including measures of overall nutrient intake and absorption. A final consideration is that we did not measure potential physiological confounds such as heart rate and respiration during scanning. Studies have found that these variables can affect observed resting-state FC in the DMN and SAL (20, 64). These concerns are somewhat minimized by the fact that there were no statistically significant changes in resting heart rate for any intervention group (all P > 0.05, descriptive data in Table 2). However, it is important for future research to include such variables during scanning to more fully characterize their role in exercise-related changes in resting FC.
Despite these limitations, our results provide new insight into how to boost the protective effects of exercise on brain systems that otherwise are known to deteriorate with aging, which in turn may help boost the ability for exercise to counteract age-related neurodegeneration.
GRANTS
This work was supported by the National Institute on Aging at the National Institutes of Health (Grant R37-AG-025667) and funding from Abbott Nutrition through the Center for Nutrition, Learning, and Memory at the University of Illinois.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.W.V., A.Z.B., E.M., and A.F.K. conceived and designed research; M.W.V., A.Z.B., J.F., E.S., and N.P.G. performed experiments; M.W.V., M.S., T.B.W., J.F., E.S., and D.K.E. analyzed data; M.W.V., M.S., E.M., and A.F.K. interpreted results of experiments; M.W.V. and T.B.W. prepared figures; M.W.V. drafted manuscript; M.W.V., M.S., A.Z.B., J.F., E.S., D.K.E., E.M., and A.F.K. edited and revised manuscript; M.W.V., M.S., T.B.W., A.Z.B., J.F., E.S., N.P.G., D.K.E., E.M., and A.F.K. approved final version of manuscript.
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at the institutional website of one of the authors, which at the time of publication they indicate is: https://github.com/mwvoss/ProjectFAST. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
ACKNOWLEDGMENTS
We thank Anya Knecht, Susan Houseworth, Nancy Dodge, Holly Tracy, and all the Lifelong Brain and Cognition Laboratory and Exercise Psychology Laboratory graduate students and staff for help in participant recruitment and data collection.
REFERENCES
- 1.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord 139: 56–65, 2012. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson JL, Jenkinson M, Smith S. Non-linear Optimisation. Oxford, UK: FMRIB, 2007. (FMRIB Analysis Group Tech. Rep. TR07JA1). [Google Scholar]
- 3.Andersson JL, Jenkinson M, Smith S. Non-linear Registration, aka Spatial Normalisation. Oxford, UK: FMRIB, 2007. (FMRIB Analysis Group Tech. Rep. TR07JA2). [Google Scholar]
- 4.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18: 251–270, 2012. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angevaren M, Aufdemkampe G, Verhaar H, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 3: CD005381, 2008. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Bamidis PD, Vivas AB, Styliadis C, Frantzidis C, Klados M, Schlee W, Siountas A, Papageorgiou SG. A review of physical and cognitive interventions in aging. Neurosci Biobehav Rev 44: 206–220, 2014. doi: 10.1016/j.neubiorev.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Baniqued PL, Gallen CL, Voss MW, Burzynska AZ, Wong CN, Cooke GE, Duffy K, Fanning J, Ehlers DK, Salerno EA, Aguiñaga S, McAuley E, Kramer AF, D’Esposito M. Brain network modularity predicts exercise-related executive function gains in older adults. Front Aging Neurosci 9: 426, 2018. doi: 10.3389/fnagi.2017.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basso JC, Shang A, Elman M, Karmouta R, Suzuki WA. Acute exercise improves prefrontal cortex but not hippocampal function in healthy adults. J Int Neuropsychol Soc 21: 791–801, 2015. doi: 10.1017/S135561771500106X. [DOI] [PubMed] [Google Scholar]
- 9.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37: 90–101, 2007. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev 93: 1803–1845, 2013. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 11.Boraxbekk C, Salami A, Wahlin A, Nyberg L. Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network: a multimodal approach. Neuroimage 131: 133–141, 2016. doi: 10.1016/j.neuroimage.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Pérusse L, Leon AS, Rao DC. Familial aggregation of V̇o2max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985) 87: 1003–1008, 1999. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard C, Daw EW, Rice T, Pérusse L, Gagnon J, Province MA, Leon AS, Rao DC, Skinner JS, Wilmore JH. Familial resemblance for V̇o2max in the sedentary state: the HERITAGE Family Study. Med Sci Sports Exerc 30: 252–258, 1998. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 17: 2042–2046, 2003. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 15.Bubb EJ, Kinnavane L, Aggleton JP. Hippocampal-diencephalic-cingulate networks for memory and emotion: an anatomical guide. Brain Neurosci Adv 1: 2398212817723443, 2017. doi: 10.1177/2398212817723443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78: 1323–1329, 2012. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38, 2008. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 18.Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci 2: 23, 2010. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burzynska AZ, Jiao Y, Knecht AM, Fanning J, Awick EA, Chen T, Gothe N, Voss MW, McAuley E, Kramer AF. White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Front Aging Neurosci 9: 59, 2017. doi: 10.3389/fnagi.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage 47: 1448–1459, 2009. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark R, Wendel C, Voss MW. Physical activity and cognitive training: impact on hippocampal structure and function. In: The Hippocampus from Cells to Systems: Structure, Connectivity, and Functional Contributions to Memory and Flexible Cognition, edited by Hannula DE and Duff MC. Cham, Switzerland: Springer, 2017, p. 209–243. doi: 10.1007/978-3-319-50406-3_8. [DOI] [Google Scholar]
- 22.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol 50: 335–362, 1996. doi: 10.1016/S0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 23.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 58: 176–180, 2003. doi: 10.1093/gerona/58.2.M176. [DOI] [PubMed] [Google Scholar]
- 24.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14: 125–130, 2003. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 25.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA 101: 3316–3321, 2004. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act 17: 17–30, 2009. doi: 10.1123/japa.17.1.17. [DOI] [PubMed] [Google Scholar]
- 27.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 28.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30: 464–472, 2007. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Culbertson JY, Kreider RB, Greenwood M, Cooke M. Effects of beta-alanine on muscle carnosine and exercise performance: a review of the current literature. Nutrients 2: 75–98, 2010. doi: 10.3390/nu2010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Favero S, Roschel H, Solis MY, Hayashi AP, Artioli GG, Otaduy MC, Benatti FB, Harris RC, Wise JA, Leite CC, Pereira RM, de Sá-Pinto AL, Lancha-Junior AH, Gualano B. Beta-alanine (Carnosyn™) supplementation in elderly subjects (60–80 years): effects on muscle carnosine content and physical capacity. Amino Acids 43: 49–56, 2012. doi: 10.1007/s00726-011-1190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci 12: 99–105, 2008. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function? Brain 139: 662–673, 2016. doi: 10.1093/brain/awv407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehlers DK, Fanning J, Awick EA, Kramer AF, McAuley E. Contamination by an active control condition in a randomized exercise trial. PLoS One 11: e0164246, 2016. doi: 10.1371/journal.pone.0164246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108: 3017–3022, 2011. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flodin P, Jonasson LS, Riklund K, Nyberg L, Boraxbekk CJ. Does aerobic exercise influence intrinsic brain activity? An aerobic exercise intervention among healthy old adults. Front Aging Neurosci 9: 267, 2017. doi: 10.3389/fnagi.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauthier C, Lefort M, Mekary S, Desjardins-Crepeau L, Skimminge A, Iversen P, Madjar C, Desjardins M, Lesage F, Garde E, Frouin F, Bherer L, Hoge R. Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiol Aging 36: 304–314, 2015. doi: 10.1016/j.neurobiolaging.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48: 63–72, 2009. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 82: 208–225, 2013. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30: 279–289, 2006. doi: 10.1007/s00726-006-0299-9. [DOI] [PubMed] [Google Scholar]
- 40.Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced? Psychol Sci Public Interest 9: 1–65, 2008. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 41.Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32: 225–233, 2007. doi: 10.1007/s00726-006-0364-4. [DOI] [PubMed] [Google Scholar]
- 42.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med 368: 1326–1334, 2013. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins BG. Pharmacologic magnetic resonance imaging (phMRI): imaging drug action in the brain. Neuroimage 62: 1072–1085, 2012. doi: 10.1016/j.neuroimage.2012.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med 34: 65–73, 1995. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- 45.Kane RL, Butler M, Fink HA, Brasure M, Davila H, Desai P, Jutkowitz E, McCreedy E, Nelson VA, McCarten JR, Calvert C, Ratner E, Hemmy LS, Barclay T. Comparative Effectiveness Reviews, Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia. Rockville, MD: Agency for Healthcare Research and Quality, 2017, vol. 188. doi: 10.23970/AHRQEPCCER188. [DOI] [PubMed] [Google Scholar]
- 46.Liu R, Sui X, Laditka JN, Church TS, Colabianchi N, Hussey J, Blair SN. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med Sci Sports Exerc 44: 253–259, 2012. doi: 10.1249/MSS.0b013e31822cf717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging 33: 1690–1698, 2012. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med 170: 170–178, 2010. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care. Lancet 390: 2673–2734, 2017. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 50.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27: 589–594, 2004. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, Wilkins HM, Brooks WM, Billinger SA, Swerdlow RH, Burns JM. Aerobic exercise for Alzheimer’s disease: a randomized controlled pilot trial. PLoS One 12: e0170547, 2017. doi: 10.1371/journal.pone.0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154: 169–173, 2017. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience 219: 62–71, 2012. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 52: 154–160, 2018. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 55.Oberlin LE, Verstynen TD, Burzynska AZ, Voss MW, Prakash RS, Chaddock-Heyman L, Wong C, Fanning J, Awick E, Gothe N, Phillips SM, Mailey E, Ehlers D, Olson E, Wojcicki T, McAuley E, Kramer AF, Erickson KI. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. Neuroimage 131: 91–101, 2016. doi: 10.1016/j.neuroimage.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Power JD, Plitt M, Laumann TO, Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage 146: 609–625, 2017. doi: 10.1016/j.neuroimage.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raichlen DA, Alexander GE. Adaptive capacity: an evolutionary neuroscience model linking exercise, cognition, and brain health. Trends Neurosci 40: 408–421, 2017. doi: 10.1016/j.tins.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology 29: 2189–2199, 2004. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- 59.Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychol Aging 18: 91–110, 2003. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- 60.Saunders B, Elliott-Sale K, Artioli GG, Swinton PA, Dolan E, Roschel H, Sale C, Gualano B. β-Alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Br J Sports Med 51: 658–669, 2017. doi: 10.1136/bjsports-2016-096396. [DOI] [PubMed] [Google Scholar]
- 61.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 62: 42–52, 2009. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356, 2007. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sexton C, Betts J, Demnitz N, Dawes H, Ebmeier K, Johansen-Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage 131: 81–90, 2016. doi: 10.1016/j.neuroimage.2015.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage 38: 306–320, 2007. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suo C, Singh M, Gates N, Wen W, Sachdev P, Brodaty H, Saigal N, Wilson G, Meiklejohn J, Singh N, Baune B, Baker M, Foroughi N, Wang Y, Mavros Y, Lampit A, Leung I, Valenzuela M. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry 21: 1633–1642, 2016. doi: 10.1038/mp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci 1: 191–198, 2000. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 67.Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol Aging 33: 1624–1632, 2012. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, Hu L, Szabo A, Klamm E, McAuley E, Kramer AF. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 48: 1394–1406, 2010. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wójcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp 34: 2972–2985, 2013. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wójcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2: 32, 2010. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 17: 525–544, 2013. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, Fanning J, Awick E, Gothe NP, Olson EA, McAuley E, Kramer AF. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage 131: 113–125, 2016. doi: 10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wendell CR, Gunstad J, Waldstein SR, Wright JG, Ferrucci L, Zonderman AB. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci 69: 455–462, 2014. doi: 10.1093/gerona/glt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng LL, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, Buckner RL, Liu H. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci USA 111: 6058–6062, 2014. doi: 10.1073/pnas.1317424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu X, Yin S, Lang M, He R, Li J. The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res Rev 31: 67–79, 2016. doi: 10.1016/j.arr.2016.07.003. [DOI] [PubMed] [Google Scholar]