Abstract
BACKGROUND:
Deficiency of lysosomal acid lipase (LAL) causes Wolman and cholesterol ester storage diseases. With the recent introduction of enzyme replacement therapy to treat LAL deficiency comes the need for a reliable assay of LAL enzymatic activity that can be applied to dried blood spots (DBS).
METHODS:
We prepared and tested a library of analogs of palmitoyl 4-methylumbelifferyl esters to find a highly active and specific substrate for LAL in DBS. The LAL assay was optimized leading to both a liquid chromatography-tandem mass spectrometry (LC-MS/MS) and fluorimetric assay of LAL. We tested the new assay on DBS from healthy and LAL-deficient patients.
RESULTS:
The ester formed between palmitic acid and 4-propyl-8-methyl-7-hydroxycoumarin (P-PMHC) was found to be >98% selective for LAL in DBS based on the sensitivity of its activity to the LAL-specific inactivator Lalistat-2 and the fact that the activity was close to zero using DBS from patients previously shown to be LAL deficient. Use of P-PMHC and heavy isotope-labeled internal standard with optimized assay conditions led to a ~2-fold increase in the specific activity of LAL compared to the previously reported LAL assay. Patients deficient in LAL were readily distinguished from normal persons with the new LAL assay using UPLC-MS/MS or fluorimetric assay platforms.
CONCLUSIONS:
The new assay can measure LAL in DBS with a single measurement compared to the previous method involving two assays done in parallel.
Keywords: lysosomal storage disease, newborn screening, tandem mass spectrometry, Wolman disease, cholesterol ester storage disease, inborn errors of metabolism, biochemical genetics
Introduction
Lysosomal acid lipase (LAL) deficiency is an autosomal recessive lysosomal storage diseases with two distinct phenotypes: a severe infantile form known as Wolman disease, and a milder, later-onset form referred to as cholesterol ester storage disease (1). Both forms are due to mutations in the LIPA gene that encodes for LAL, a low pH-active, serine lipase in the same family as gastric lipase (2). Patients with Wolman disease at birth or within the first few weeks of life with signs of hepatosplenomegaly, adrenal calcification and failure to thrive. Without treatment, this lysosomal storage disease is fatal within the first year. The presentation of cholesterol ester storage disease is more variable but typically presents in early adolescence with hypercholesterolemia and early development of atherosclerosis in major arteries. These affected persons typically succumb from fatal cardiac disease within the third decade of life. There are reports of affected individuals living into their sixties or seventies. These clinical differences are thought to be due to the degree of residual LAL activity, with patients having cholesterol ester storage disease typically displaying 1–5% residual LAL activity and patients with Wolman disease patients displaying no detectable activity (3). Over 40 mutations in the LIPA gene have been reported (3).
LAL is detected in leukocytes or fibroblasts using radiometric, immunologic, or fluorescence assays (4). Hamilton and colleagues recently developed a fluorimetric assay to detect LAL in dried blood spots (DBS) (5). The assay is based on the release of 4-methylumbelliferone from its ester with palmitic acid. However, the substrate is not specific for LAL, and the method involves two assays performed in parallel, one in the presence of an LAL-specific covalent inactivator Lalistat-2 (5). LAL activity is calculated from the difference in these two assays.
Recently, enzyme replacement therapy with recombinant LAL (Sebelipase alpha) has been approved by the FDA for treatment of LAL deficiency (6). As with many lysosomal storage diseases, initiation of treatment prior to the onset of irreversible symptoms may be advantageous. Thus, newborn screening for LAL deficiency may be warranted. However, given the rarity of LAL deficiency, newborn screening for this enzyme might be practical if it could be added in a multiplex fashion to an existing lysosomal storage disease newborn screening panel, if it could be done inexpensively, and if the number of follow-up samples was minimal. Here we describe the discovery of a substrate that is selective for LAL in DBS, and thereby allowing the activity of this enzyme to be measured in a single incubation.
Materials and Methods
MATERIALS
All patient samples were obtained with Institutional Review Board approval from the University of Washington. DBS from anonymized samples from LAL-deficient patients were obtained from Dr. Rhona Jack (Seattle Children’s Hospital). All patient samples were stored at 4˚C in sealed plastic bags. Other DBS samples were stored in plastic bags at −20˚C in sealed containers with desiccant. Recombinant LAL was obtained as a generous gift from Alexion Corp. The synthesis of all new reagents is detailed in online Supplemental Material. Lalistat-2 was provided as a gift from Dr. J. Hamilton (Yorkhill Hospital, UK) or synthesized as described (7).
LC-MS/MS LAL ASSAY
Assay cocktail was prepared by combining 9 volumes of 0.1 M sodium acetate buffer (made by adding reagent grade NaOH to 0.1 M reagent grade acetic acid in water), pH 4.5, containing 2.5 mM sodium taurodeoxycholate hydrate (Sigma, 287245) with 1 volume of 0.5% (w/v) bovine heart cardiolipin in ethanol (Sigma, C1649) containing 3 mM substrate 4-propyl-8-methyl-7-hydroxycoumarin (P-PMHC) and 50 μM carbon-13-labeled 4-propyl-8-methyl-7-hydroxycoumarin (internal standard). The latter was prepared by mixing solid substrate P-PMHC with internal standard solution in absolute ethanol, removing solvent in a centrifugal concentrator under vacuum (or with a jet of nitrogen) and then mixing with commercial cardiolipin/ethanol solution. This ethanol stock could be stored at −20°C for several months (avoid more than 2–3 freeze/thaw cycles), and the aqueous buffer can be stored at 4°C for several months. This is based on no change in assay results when new and stored solutions are used (not shown). Assay cocktail was made fresh before each use.
To a 3 mm punch from a DBS in a 1.5 mL polypropylene Eppendorf tube was added 200 μL of purified water (Milli-Q, EMD Millipore). The tubes were shaken on an orbital platform shaker for 1 h at ambient temperature. The tube of DBS extract was mixed briefly on a vortex mixer, and a 10 μL aliquot was added to the well of a polypropylene, deep-well, 96-well plate (Costar, 3959). Assay cocktail (30 μL, ambient temperature) was added to each well. The plate was centrifuged at 3000 × g for 5 min at ambient temperature to ensure that all liquid was at the well bottom. The plate was sealed with a film cover (Advantage 96 well round clear Silicone/PTFE cap mat #962611). The plate was shaken at 37°C in an incubator with orbital mixing at 400 rpm for 3 h. The reactions were quenched by addition of 80 μL of purified water (Milli-Q, EMD Millipore) followed by 400 μL of HPLC-grade ethyl acetate. The well contents were mixed by pipetting up and down ~10 times. The plate was centrifuged at 3000 × g for 5 min at ambient temperature, then 120 μL of the upper ethyl acetate layer was transferred to a shallow well, 96-well, polypropylene plate (Greigner, 651201). Solvent was removed at ambient temperature with a jet of N2. To each well was added 200 μL water/methanol (1/1, Fisher Optima Grade), and samples were mixed by pipetting up and down a few times. The plate was wrapped with aluminum foil and placed in the autosampler chamber at 8°C in preparation for LC-MS/MS analysis.
LC was carried out using a Waters Acquity binary solvent pump system with a CSH, C18, 1.7 μm, 2.1× 50 mm column (Waters, 186005296) and a CSH, C18, 1.7 μ guard column (Vanguard, 186005303). The solvent program was: 99% A (70/30, water/acetonitrile, 0.1% formic acid, Fisher Optima grade)/1% B (50/50, acetonitrile/isopropanol, 0.1% formic acid) to 30% A/70 % B over 1 min, then jump to 100% B and hold for 0.5 min, then to 99% A over 0.5 min (flow rate 0.8 mL/min). The total run time was 2.5 min. Mass spectrometry was carried out with a Waters Xevo TQ tandem-quadrupole instrument. Additional LC and MS/MS parameters are provided in online Supplemental Table 1.
FLUORIMETRIC LAL ASSAY
The fluorimetric assay up to the end of the incubation was identical to the LC-MS/MS assay. After incubation, the fluorimetric assay was continued as follows: Reactions were quenched by addition of 200 μL of water/methanol (1:1, Fisher Optima Grade). The well contents were mixed by pipetting up and down ~10 times. A portion (150 μL) of the well contents were transferred to a black, flat-bottomed 96-well microtiter plate (NUNC 437112). Samples were immediately read on a fluorimeter (Perkin Elmer Victor3V 1420) with an excitation wavelength of 355 nm, an emission wavelength of 460 nm, and an excitation time of 0.1 s. The fluorimeter reading was converted to μmole of product by generating a standard curve. DBS extract (60 μL) and assay cocktail (450 μL) were incubated separately. To a well was added 200 μL of water/methanol solution (1:1), 10 μL of DBS extract and 30 μL of assay cocktail. To each well was added 2 μL of LAL product (4-propyl-8-methyl-7-hydroxycoumarin) (0, 0.2, 1, or 2 nmole) from an aqueous stock solution. After mixing by pipetting up and down ~10 times, a 150 μL aliquot was transferred to the fluorimeter plate and submitted to fluorimetry as above. The well in which no product 2 was added serves as the blank. Note that since DBS extract and assay cocktail were incubated separately, this blank reflected i) the fluorescence of the blood extract and substrate; ii) any fluorescence due to non-enzymatic breakdown of substrate; and iii) any quenching of the fluorescence by components of the blood.
Results
DEVELOPMENT OF A NEW LAL ASSAY
Our goal was to find a new substrate that was acted upon only by LAL in DBS, thus avoiding the need to carry out two assays in parallel in the presence and absence of the LAL-specific inactivator Lalistat-2 (5). The most obvious choice was to consider the natural LAL substrates, cholesterol esters and triglycerides. By carrying out the published assay (5) with palmitoyl-4MU on a DBS extract and also with known amounts of recombinant LAL, we determined that there was no more than 50–100 pg of LAL in the DBS extract. Using this number and the published specific activities for purified LAL acting on the natural substrates cholesterol oleate and triolein (8), we concluded that the amount of products produced with a 3 mm DB punch and the natural substrates would be well below the detection limits for mass spectrometry. Thus, assays using these natural substrates were not attempted.
The next stage was to test a variety of new fatty acid esters as possible substrates for LAL (online Supplemental Material). We first tested variants of palmitoyl-4MU containing replacements for the 4MU group that were structurally similar to the leaving groups we used in our previously designed substrates for other lysosomal enzymes (9) (1, Supplemental Material). We also tested the ester formed between cholesterol and a fatty acyl group containing a bis-amide at the omega end of the acyl chain (2, Supplemental Material). Next we tested analogs containing of palmitoyl-4MU containing alkyl-amide chains attached to the methyl of the 4MU moiety (10) (3, 5 Supplemental Material). Finally, we tested analogs of palmitoyl-4MU containing bis-amides at the omega terminus of the fatty acyl group of palmitoyl-4MU (4, 6, Supplemental Material). None of these compounds displayed detectable LAL activity when tested with DBS under the conditions given in Supplemental Material. These studies show that addition of polar groups to the palmitoyl chain abrogated activity toward LAL. Also, extension of the 4MU moiety with polar chains was also not tolerated.
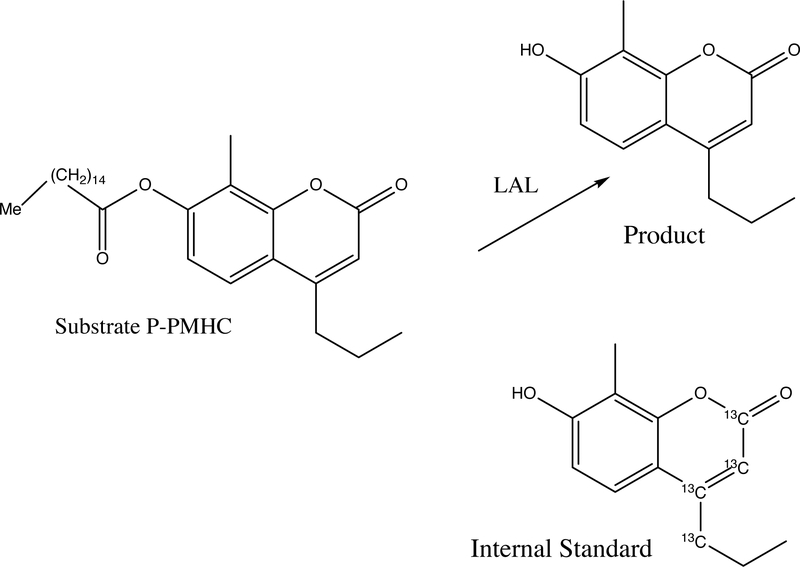
At this stage, the only substrate that displayed measurable LAL activity using DBS was palmitoyl-4MU. This is presumably because of the highly reactive nature of the ester linkage to the strong leaving group 4MU and the fact that LAL prefers highly hydrophobic substrates. Thus, we turned to the preparation of a library of compounds that contained relatively small structural variations of the palmitoyl-4MU substrate. The structures of all library components are shown in Supplemental Table 3. From this library of 25 compounds, P-PMHC (Figure 1) emerged as the substrate with the best combination of high specific activity on LAL and high specificity for LAL. Thus, we carried out additional studies with P-PMHC.
Figure 1.
Structure of LAL-specific substrate P-PMHC, LAL product, and heavy atom substituted internal standard.
We studied a variety of phospholipids as replacements for cardiolipin, which was used in the original LAL assay (5). The following phospholipids were tested individually at 0.1 mM: 1,2-dioleoyl-phosphatidylethanolamine, 1,2-dioleoyl-phosphatidylmethanol, 1,2-dioleoyl-phosphatidylcholine, 1,2-dioleoyl-phosphatidylserine, brain phosphatidylethanolamine, 1,2-heptadecyl-phosphatidylcholine, and 1,2-lauryl-phosphatidylcholine. None of these phospholipids led to any substantial increase in LAL over that measured with cardiolipin (data not shown), and thus we continued our studies with the latter.
In the original assay (5), dimethylsulfoxide (DMSO) was used as a solvent to prepare the palmitoyl-4MU and Lalistat-2 stock solutions. The final concentration of DMSO in the LAL assay was 2.4%. Over the course of our studies we noticed that the quality of the DMSO was critical to being able to detect LAL activity in DBS. Previously opened bottles of DMSO stored for ~1 month or longer led to essentially complete loss of LAL activity measured in DBS. Based on this result we decided to replace DMSO initially with dimethylformamide for preparation of the substrate stock solution, and to use ethanol to prepare the Lalistat-2 solution (however, with the use of LAL-specific P-PMHC, Lalistat-2 was no longer used). In our final assay conditions, we used ethanol as the only solvent other than water.
The previous LAL assay used Triton X-100 as the detergent (5). We replaced this with sodium taurodeoxycholate because this detergent largely remains in the aqueous phase following liquid-liquid extraction with ethyl acetate (thus minimizing injection of large amounts of detergent onto the UPLC column). Replacement of Triton X-100 with 2.5 mM sodium taurodeoxycholate resulted in a 20% drop of LAL activity, and increasing the detergent concentration further led to an additional loss of activity. Thus, 2.5 mM was chosen for all subsequent studies.
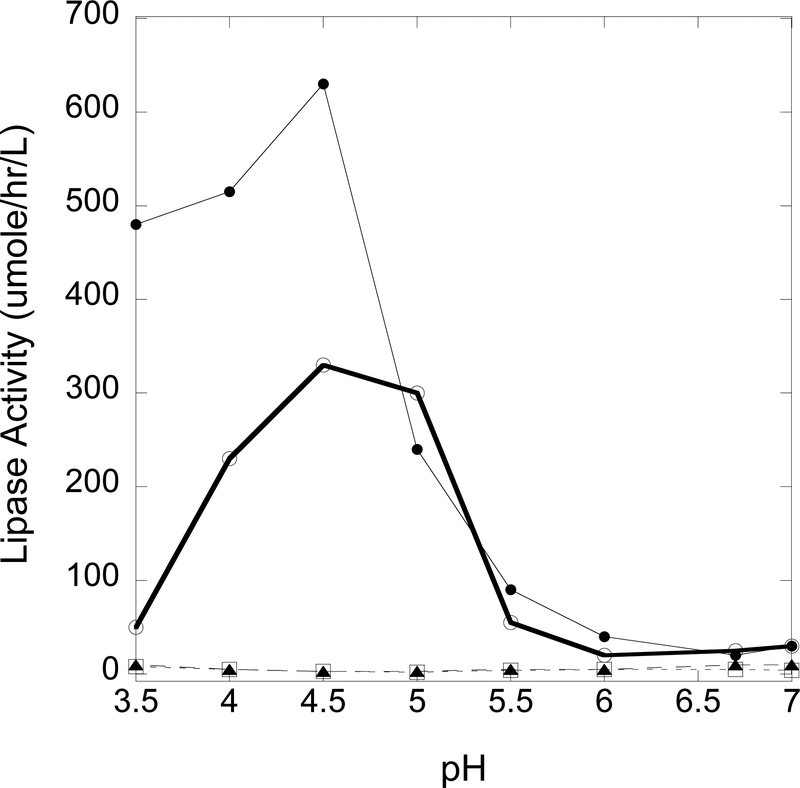
The LAL pH-rate profile was studied from 3.5 to 7.0 in 0.1 M succinate or from 3.5 to 5.5 in 0.1 M sodium acetate (Fig. 2). Based on this study, we chose 0.1 M sodium acetate, pH 4.5 in order to maximize LAL activity.
Figure 2.
pH-Rate profile for LAL activity using substrate P-PMHC from pH 3.5 to 7.0 in 0.1 M succinate (SA, ) or from pH 3.5 to 5.5 in 0.1 M sodium acetate (AA, ). Buffer (9 volumes) was combined with 2.5 mM sodium taurodeoxycholate, 3.7 mM substrate P-PMHC, and 1 volume of 0.5% (w/v) cardiolipin in ethanol. Full assays were carried out with DBS extract from a healthy adult and in the absence of Lalistat-2. Blank assays were carried out using water and 300 μM Lalistat-2. Inhibited assays were carried out as for the full assays but in the presence of 300 μM Lalistat-2.
We settled on a concentration of P-PMHC in the cocktail of 3 mM based on a good combination of high LAL activity and minimal background (no-DBS control, data not shown). The Hamilton assay (5) used 40 μL of an aqueous extract of a 3 mm DBS added to 160 μL of assay cocktail. To keep the solvent volumes for the liquid-liquid extraction to a minimum, thus avoiding long times for solvent removal, we added 10 μL of an aqueous extract of a 3 mm DBS punch to 30 μL of assay cocktail.
With the final assay conditions chosen, we obtained a specific activity of 874 μmole/hr/L using a 3 mm punch of a DBS from a healthy adult. The activity was measured by UPLC-MS/MS after extraction of the reaction mixture with ethyl acetate. The MS/MS multiple reaction monitoring response was converted to μmoles of product with the use of a chemically identical, but isotopically differentiated internal standard (carbon 13-labeled 4-propyl-8-methyl-7-hydrdoxycoumarin) (Fig. 1). The use of the internal standard accounts for all product losses due to sample processing and analysis. Using a 3 mm punch from an identical DBS and the previous fluorimetric LAL assay with and without Lalistat-2 (5), we obtained a specific activity of 466 μmole/hr/L (within the range of values reported previously) for the LAL component, which is 1.9-fold lower than the activity measured with the new UPLC-MS/MS assay.
The following studies established that LAL substrate P-PMHC was highly specific for LAL in DBS (Table 1). When extracts from two healthy persons DBS were pre-incubated with 10 μM Lalistat-2, 98% of the activity toward P-PMHC was inhibited. This increases to 99% inhibition if 100 μM Lalistat-2 was used (Table 1). In contrast, when the same DBS extract was submitted to the previously reported assay with palmitoyl-4MU (5), 78% of the total lipase activity was blocked by 10 μM Lalistat-2 (not shown). Under the assumption that Lalistat-2 was completely selective for LAL, the data showed that P-PMHC, but not palmitoyl-4MU, was highly specific for LAL in DBS.
Table 1.
Effect of Lalistat-2 on the activity (MS/MS method) of LAL toward substrate P-PMHC.1
| DBS | Activity (μmole/hr/L) without Lalistat-2 | Activity (μmole/hr/L) with 10 μM Lalistat-2 | Activity (μmole/hr/L) with 100 μM Lalistat-2 |
|---|---|---|---|
| Adult 1 (triplicate) | 554, 582, 582 | 10, 9, 10 | 6, 4, 5 |
| Adult 2 (triplicate) | 519, 539, 473 | 5, 2, 3 | 2, 0, 1 |
| Blank (15 measurements) | 16.8–26.2 |
Activity values for the adult DBS were blank corrected. The blank was obtained by using an equal volume of water instead of water-extracted DBS.
FLUORIMETRIC LAL ASSAY
Since hydrolysis of P-PMHC leads to the fluorescent product 4-propyl-8-methyl-7-hydroxycoumarin, we used used a standard plate reader fluorimeter to measure LAL activity in DBS. Table 2 shows LAL activities measured with by UPLC-MS/MS and by fluorimetry on an identical set of DBS from 10 adults. Agreement between the two assays general showed <30% difference in activity values for all but one pair where the difference approached 50%.
Table 2.
LAL activity in DBS measured by UPLC-MS/MS and fluorimetric assays.
| Type | Samples | LAL Activity (μmol/hr/L blood)1 |
Mean | %CV |
|---|---|---|---|---|
| Fluorescence | ||||
| Adult | 1 | 746,707,596,599 | 662 | 9.94 |
| 2 | 541,751,675,519,803 | 658 | 17.08 | |
| 3 | 618,592,583,628 | 605 | 3.05 | |
| 4 | 933,1683,1073,938,1261,1348 | 1206 | 21.81 | |
| 5 | 809,917,901,768,815 | 842 | 6.83 | |
| 6 | 1198,1635,1361,1382,1284 | 1372 | 10.67 | |
| 7 | 758,854,841,645,781 | 794 | 9.95 | |
| 9 | 427,100,376,239 | 286 | 44.54 | |
| 10 | 767,857,1150,1158,900 | 980 | 15.89 | |
| 12 | 989,843,792,918,604,703 | 808 | 15.89 | |
| Blank2 | 48–78 | 58 | 19.03 | |
| UPLC-MS/MS | ||||
| Adults | 1 | 598, 555, 653, 578, 608 | 598.5 | 5.5 |
| 2 | 712, 701, 674, 632, 588, 636 | 657.1 | 6.5 | |
| 3 | 492, 499, 506, 480, 453, 465 | 482.2 | 3.9 | |
| 4 | 962, 842, 862, 848, 1016, 849 | 896.3 | 7.5 | |
| 5 | 846, 852, 852, 864, 923, 907 | 874.0 | 3.4 | |
| 6 | 990, 979, 1129, 1152, 929 | 1035.8 | 8.5 | |
| 7 | 613, 555, 488, 536, 514, 470 | 529.0 | 8.9 | |
| 9 | 316, 330, 220, 247, 292, 236 | 273.4 | 15.2 | |
| 10 | 938, 796, 818, 627, 1112, 740 | 838.3 | 18.3 | |
| 12 | 424, 534, 376, 496, 368, 385 | 430.4 | 14.7 | |
| Blank2 | 6.3 – 24.9 | 15 | 36.4 | |
Activity values are blank corrected.
Range of blank values given for 12 and 56 repeats of the fluorimetric and MS/MS assays, respectively.
The analytical range is an important assay parameter that is defined as the ratio of assay response measured with the quality control HIGH sample (typical of a healthy person) divided by the assay response for all elements independent of LAL (11). The larger the analytical range, the greater the activity values will be spread out, leading to greater accuracy especially when activity is low. The mean analytical range for the UPLC-MS/MS assay was 44 compared to a value of 14 for the fluorimetric assay.
STUDIES WITH PATIENT SAMPLES
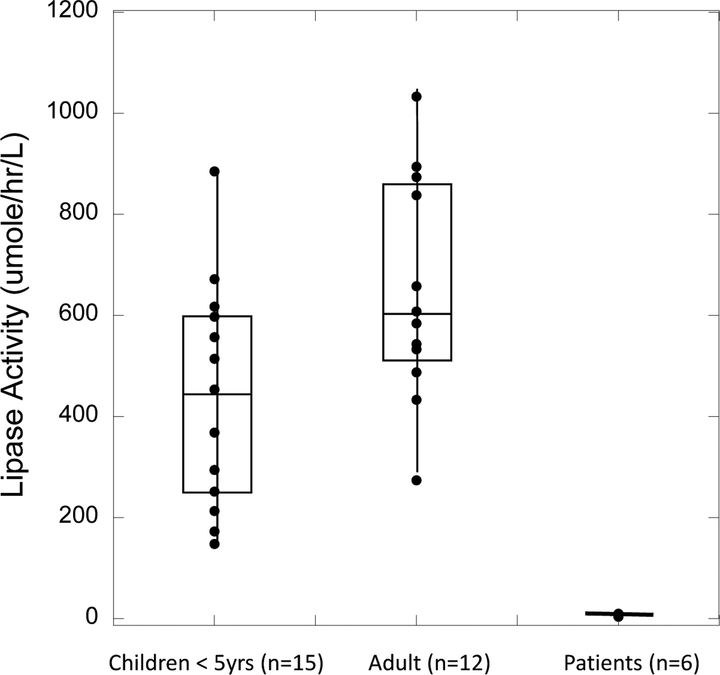
Figure 3 shows the LAL activity data in DBS from 15 healthy children (<5y old), 12 healthy adults, and 6 patients previously shown to be LAL deficient. All patients had symptoms consistent with LAL deficiency, and these 6 patients were shown to be LAL deficient by the original LAL assay method on DBS (5). The patients are de-identified, and we have no additional information.
Figure 3.
LAL activity in DBS measured with substrate P-PMHC and UPLC-MS/MS. The bottom of each box is the value of the first quartile, the middle line is the medium value, and the top line is the third quartile. All values are blank corrected. Individual patient values are given in Supplemental Table 4.
All assays were carried out with UPLC-MS/MS using the optimized conditions with substrate P-PMHC in the absence of Lalistat-2 (thus only a single assay per patient was needed). Individual values are shown in online Supplemental Table 4. All healthy persons showed LAL activity well separated from that measured with LAL-deficient patients. The fact that LAL activity was close to zero in all 6 LAL-deficient patients was further evidence that substrate P-PMHC was LAL specific.
DISCUSSION
The new LAL assay makes use of a substrate that is highly specific for LAL, thus allowing its activity to be measured in DBS using a single incubation. In contrast, about 1/3 of the standard substrate used to assay LAL, palmitoyl-4MU, is hydrolyzed by one or more esterases/lipases in DBS other than LAL. In the original assay, the LAL activity is obtained as the fraction of palmitoyl-4MU hydrolase activity that is sensitive to Lalistat-2 (5). Since the LAL activity component is obtained as the difference in two substantial activity values, the error in LAL activity must include the propagation of errors in two separate measurements. Thus, it would be required to raise the cutoff value in a newborn screening program based on the Lalistat-2 method. This would result in an increase in the false positive rate. With the new LAL specific substrate P-PMHC, not only can the effort to perform the assay be reduced, but the screen cutoff could be lowered as well.
It has been shown by comparison of large pilot newborn screening studies of lysosomal storage diseases that the MS/MS enzymatic activity method gives a substantially lower number of screen positives than the fluorimetric method when compared at equivalent cutoff values (12). This may be due to the fact that the analytical range of the MS/MS assays are > 3-fold greater than that of the corresponding fluorimetric assays (11). Thus, the use of UPLC-MS/MS may be the method of choice for newborn screening of LAL deficiency, but this remains to be determined. Finally, it should be possible to multiplex the new UPLC-MS/MS LAL assay with other MS/MS assays for lysosomal storage diseases simply by addition of substrate P-PMHC to a single assay cocktail containing a collection of additional substrates and internal standards and performing a single UPLC-MS/MS in which all products and internal standards are detected by multiple reaction monitoring mode.
Supplementary Material
ACKNOWLEDGEMENTS.
This work was supported by grants from the National Institutes of Health (R01 DK067859 to M.H.G. and F30 DH081853 to S.M.). We also thank Dr. Rhona Jack (Seattle Children’s Hospital) for DBS from LAL-deficient patients, J. Hamilton (Yorkhill Hospital, UK) for Lalistat-2, and Alexion Corp. for recombinant LAL.
Abbreviations:
- DBS
dried blood spot on a newborn screening card
- LAL
lysosomal acid lipase
- MS/MS
tandem mass spectrometry
- UPLC
ultra-high pressure liquid chromatography.
REFERENCES
- 1.Aguisanda F, Thorne N, Zheng W. Targeting Wolman disease and Cholesterol Ester Storage DIsease: Disease Pthogenesis and Therapeutic Development. Curr Chem Genom Transl Med 2017;11:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canaan S, Roussel A, Verger R, Cambillau C. Gastric lipase: crystal structure and activity. Biochim Biophysi Acta 1999;1441:197–204. [DOI] [PubMed] [Google Scholar]
- 3.Rajamohan F, Reyes AR, Ruangsiriluk W, Hoth LR, Han S, Caspers N, et al. Expression and functional characterization of human lysosomal acid lipase gene (LIPA) mutation responsible for cholesteryl ester storage disease (CESD) phenotype. Prot Expr Purif 2015;110:22–9. [DOI] [PubMed] [Google Scholar]
- 4.Gravel RA, Kaback MM, Proia RL, Sandhoff K, Suzuki K. The Metabolic & Molecular Bases of Inherited Disease In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler B, et al. , editors. The Metabolic and Molecular Bases of Inherited Disease. 8 ed. New York: McGraw-Hill; 2001. pages 3827–3387. [Google Scholar]
- 5.Hamilton J, Jones I, Srivastava R, Galloway P. A new method for the measurement of lysosomal acid lipase in dried blood spots using the inhibitor Lalistat 2. Clin Chim Acta 2012;413:1207–10. [DOI] [PubMed] [Google Scholar]
- 6.Jones SA, Rojas-Caro S, Quinn AG, Friedman M, Marulkar S, Ezgu F, et al. Survival in infants treated with sebelipase Alfa for lysosomal acid lipase deficiency: an open-label, multicenter, dose-escalation study. Orphanet J Rare Disl; 2017;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebdrup S, Refsgaard HHF, Fledelius C, Jacobsen P. Synthesis and structure-activity relationship for a novel class of potent and selective carbamate-based inhibitors of hormone selective lipase with acute in vivo antilipolytic effects. J Med Chem; 2007;50:5449–56. [DOI] [PubMed] [Google Scholar]
- 8.Negre A, Salvayre R, Dagan A, Borrone C, Gatt S. New spectrophotometric assays of acid lipase and their use in the diagnosis of Wolman and cholesteryl ester storage diseases. Anal Biochem 1985;145:398–405. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, et al. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clinical Chemistry. Clin Chem 2004;50:1785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe BJ, Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis II (Hunter Syndrome). Anal Chem. 2011;83:1152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar AB, Spacil Z, Masi S, Ghomashchi F, Ito M, Scott CR, et al. Tandem Mass Spectrometry Has a Larger Analytical Range than Fluorescence Assays of Lysosomal Enzymes: Application to Newborn Screening and Diagnosis of Mucopolysaccharidoses Types II, IVA, and VI. Clin Chem 2015;61:1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliot S, Buroker N, Cournoyer J, Potier A, Trometer J, Elbin C, et al. Pilot study of newborn screening for six lysosomal storage diseases using Tandem Mass Spectrometry. Molec Genet and Metab 2016;118:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.