Abstract
Sphingolipids, first described in the brain in 1884, are important structural components of biological membranes of all eukaryotic cells. In recent years, several lines of evidence support the critical role of sphingolipids such as sphingosine, sphingosine-1-phosphate (S1P), and ceramide as anti- or pro-inflammatory bioactive lipid mediators in a variety of human pathologies including pulmonary and vascular disorders. Among the sphingolipids, S1P is a naturally occurring agonist that exhibits potent barrier enhancing property in the endothelium by signaling via G protein-coupled S1P1 receptor. S1P, S1P analogs, and other barrier enhancing agents such as HGF, oxidized phospholipids, and statins also utilize the S1P/S1P1 signaling pathway to generate membrane protrusions or lamellipodia, which have been implicated in resealing of endothelial gaps and maintenance of barrier integrity. A better understanding of sphingolipids mediated regulation of lamellipodia formation and barrier enhancement of the endothelium will be critical for the development of sphingolipid-based therapies to alleviate pulmonary disorders such as sepsis-, radiation-, and mechanical ventilation-induced acute lung injury.
1. INTRODUCTION
Endothelial cell (EC) migration is important for a variety of physio logical and pathological conditions such as vasculogenesis, angiogenesis, wound healing, and atherogenesis (Lamalice, Le Boeuf, & Huot, 2007; Marcola & Rodrigues, 2015; Mostmans et al., 2017; Mudau, Genis, Lochner, & Strijdom, 2012; Tang & Conti, 2004). This complex multistep process involves protrusion of plasma membrane at the leading edges, formation of new adhesions at protrusion sites, and retraction of older adhesions at the rear and contraction. Generation of protruding membranes or lamellipodia at leading edge involves reorganization of actin microfilaments, microtubules, and intermediate filaments (Fletcher & Mullins, 2010). Current evidence suggests that the formation of lamellipodia, generated at the leading edge of migrating ECs in response to a variety of growth factors and extracellular stimuli, are primarily involved in motility (Krause & Gautreau, 2014), and the driving force for EC motility is fueled by continuous growth of actin filaments and rearrangement of the actin-binding proteins such as cortactin and filamin in the lamellipodia. In addition to motility, lamellipodia have been implicated in resealing of endothelial gaps and restoration of cell–cell junctions to maintain barrier integrity of the pulmonary endothelium (Adams & Nelson, 1998; Breslin, Zhang, Worthylake, & Souza-Smith, 2015; McNeill, Ryan, Smith, & Nelson, 1993).
Endothelial barrier integrity is a balance between the net effects of two competing forces that include intracellular contraction and adhesive cell–cell and cell–matrix tethering. The actin microfilament, through multiple linkage sites to intracellular and membrane proteins, is a critical determinant of EC adhesion and tight junctions. However, actin is also responsible for the generation of tensile intracellular forces via an actomyosin motor, which results in EC barrier disruption. The actin rearrangement is driven by the coordinated activation of calcium/calmodulin-dependent myosin light chain kinase (MLCK) and rho kinase and their activities together affect MLC phosphorylation and actin polymerization that regulate the contractile or relaxed phenotype of the ECs. On the other hand, agents such as sphingosine-1-phosphate (S1P), FTY-720 P, and it’s analogs, cAMP analogs and hepatocyte growth factor (HGF) that enhance integrity of endothelial barrier or reduce endothelial permeability stimulate activation of the small GTPase Rac1 that promotes redistribution of actin and cortactin at cell periphery and stabilizes cellular adherens junctions (Ephstein et al., 2013; Gonzalez, Kou, & Michel, 2006; Schlegel & Waschke, 2014; Wang, Bittman, Garcia, & Dudek, 2015). Lipopolysaccharide (LPS) or thrombin-induced endothelial hyper-permeability is associated with enhanced Rho/ROCK and decreased Rac1 activity in the endothelium, with concomitant decrease in localization of actin and cortactin at the periphery of ECs (Essler et al., 1998; Kwok & Clemens, 2014; Schlegel, Baumer, Drenckhahn, & Waschke, 2009; van Nieuw Amerongen, van Delft, Vermeer, Collard, & van Hinsbergh, 2000). The use of NSC23766 that blocks Rac1 activation by Tiam1, resulted in enhanced LPS- or thrombin-induced endothelial permeability, decreased formation of a stable cortical actin ring at the cell periphery that has been directly correlated with destabilized adherens junctions (Abbasi & Garcia, 2013; Natarajan et al., 2013).
In several lung inflammatory pathologies including sepsis, and acute respiratory distress syndrome (ARDS), endothelial barrier dysregulation results in pulmonary leakage and alveolar flooding. Immediate restoration of endothelial barrier is therefore critical to normalize lung function (Herrero, Sanchez, & Lorente, 2018; Lucas, Verin, Black, & Catravas, 2009). Recent studies suggest that altered sphingolipids metabolism and sphingolipid signaling play a critical role in the development, progression, and alleviation of pulmonary leak and lung inflammatory injury (Ebenezer, Fu, Suryadevara, Zhao, & Natarajan, 2017; Fu et al., 2018; Suryadevara et al., 2018). Among the sphingolipids, metabolism of sphingomyelin to ceramide by sphingomyelinases, conversion of ceramide to sphingosine by ceramidases, and generation of sphingosine-1-phosphate (S1P) from sphingosine by sphingosine kinases (SPHKs) 1 and 2 have been implicated in regulating endothelial barrier function and maintaining vascular integrity. While several reviews have dealt with regulation of endothelial barrier dys-function, the underlying mechanisms involved in endothelial barrier restoration after pulmonary leak and alveolar flooding have not been addressed. S1P and S1P analogs and hepatocyte growth factor (HGF) are known to promote EC barrier enhancement via formation of membrane protrusions at leading edges of ECs; however, molecular mechanism(s) of governing sealing of the endothelial gaps and tightening of the adherens junctions is yet to be fully defined. Here, we will summarize key aspects of S1P metabolism and signaling via G protein-coupled S1PRs in lamellipodia formation and restoration of endothelial barrier function and integrity during lung inflammatory injury.
2. DYNAMICS OF LAMELLIPODIA
Lamellipodia are thin and flat membrane structures formed at the cell periphery of the leading edge (Small, Stradal, Vignal, & Rottner, 2002) and are rich in branched actin filaments that polymerize against the membrane tension force to form cell protrusions (Ponti, Machacek, Gupton, Waterman-Storer, & Danuser, 2004). Typically, lamellipodia undergo cycles of protrusion and retraction, reflecting the dynamic nature of actin polymerization and depolymerization in these structures (Machacek et al., 2009; Tsai & Meyer, 2012). Branched actin filaments form on a pre-existing filament by Arp2/3 complex, a process known as actin nucleation (Abella et al., 2016; Rotty, Wu, & Bear, 2013). Arp2/3 complex is composed of seven evolutionarily conserved proteins (Abella et al., 2016; Rotty et al., 2013). Nucleation-promoting factors like SCAR/WAVE complex recruits Arp2/3 to a pre-existing actin filament and induce Arp2/3 conversion to its active conformation (Rotty et al., 2013). This initiates the F-actin nucleation process of a new actin filament from the side of a pre-existing actin filament. The newly formed actin filament can itself become a substrate for the formation of another actin branch and so on, thereby creating a network of actin filaments. Just as SCAR/WAVE regulate Arp2/3 complex positively, Arpin regulates the complex negatively by converting it to the inactive state (Dang et al., 2013, 2017). Cortactin binds to pre-existing actin junctions and stabilizes branched actin network in lamellipodia (Cai, Makhov, Schafer, & Bear, 2008; Sinha et al., 2016). Therefore, cortactin is used as a marker to label the lamellipodia width, in contrast to SCAR/WAVE complex that solely localizes at the lamellipodia periphery (Hahne, Sechi, Benesch, & Small, 2001).
Actin elongation is a process that follows actin nucleation. This process is managed by ENA/VASP proteins (Breitsprecher et al., 2011). These proteins bind to the barbed end of actin growing filaments and protect it from being capped by capping proteins, whose function is to prevent elongation of actin filament (Breitsprecher et al., 2008, 2011). ENA/VASP proteins also recruit ATP-G-actin bound to profilin to the barbed end, which are the building blocks of actin filaments. The equilibrium between the positive and negative regulators of nucleation, branching, and elongation of actin determines the life of a given lamellipodium as well as the directionality of cell migration.
To visualize lamellipodia under microscopes, it is common to fluorescently label actin and cortactin and identify lamellipodia as regions of co-localization of the two proteins (Fu et al., 2016). Rac1 and any of the seven subunits of the Arp2/3 complex for instance can also be labeled with fluorescent probes and used to visualize lamellipodia (Svitkina & Borisy, 1999). To measure the extent of lamellipodia protrusion, kymograph analysis is a common method. To perform kymograph analysis, cell images should be acquired every ~10s. This analysis could be done on unlabeled cells using differential interference contrast (DIC) microscopy imaging for instance (Bear et al., 2002) as well as fluorescently labeled cells. These kymo-graphs can track the distance of protrusion or retraction of single pixels at the cell edge as a function of time. On the other hand, global measurement of protrusive activity at the entire cell edge is also possible. This type of analysis measures the changes in total increase and decrease of cell surface area with time (Karginov et al., 2014; Klomp et al., 2016). Lamellipodia are indispensable for cell migration and are the site of new focal adhesions formation (Zaidel-Bar, Cohen, Addadi, & Geiger, 2004). These new focal adhesions connect the actomyosin cytoskeleton of the cell to the extracellular matrix (Gardel et al., 2008; Grashoff et al., 2010). The traction force generated by the actomyosin cytoskeleton on focal adhesions moves the cell body forward toward the lamellipodium (Gardel et al., 2008; Grashoff et al., 2010). While new focal adhesions are formed at the leading edge, focal adhesions at the lagging edge of the cell concomitantly disassemble that results in forward movement (Gupton, Eisenmann, Alberts, & Waterman-Storer, 2007).
To label nascent focal adhesions, protein such as VASP that predominantly localizes at nascent focal adhesions enables the exclusive visualization of nascent focal adhesions (Huttelmaier et al., 1998). On the other hand, Zyxin is believed to localize at mature focal adhesions, and therefore represents as a marker of mature focal adhesions in cells (Efimov et al., 2008).
Some proteins, like paxillin for instance, are present at both nascent and mature focal adhesions and thus used to label total focal adhesions. To quantify focal adhesions dynamics, one can measure the rate of increase or decrease in intensity of a focal adhesion markers and plot the changes as a function of time. From the slope of the graph, it is possible to determine the rate constant of appearance or disappearance of focal adhesions. Alternatively, Focal Adhesion Analysis Server (FAAS) is a great resource for studying focal adhesions in migrating cells (Berginski & Gomez, 2013). It is an open source and user-friendly website to analyze time lapse of fluorescently labeled focal adhesions. Using this server, Karginov et al. (2014) quantified changes in adhesion number and elongation in response to c-Src activation (Figs. 1 and 2). FAAS can measure focal adhesions assembly and disassembly rates as well as properties of static focal adhesions. It can also track single focal adhesion complex from the time it assembles to the moment it disassembles in addition to changes in focal adhesion orientation. FAAS also determines a focal adhesion alignment index (FAAI), which measures how total focal adhesions align across a cell.
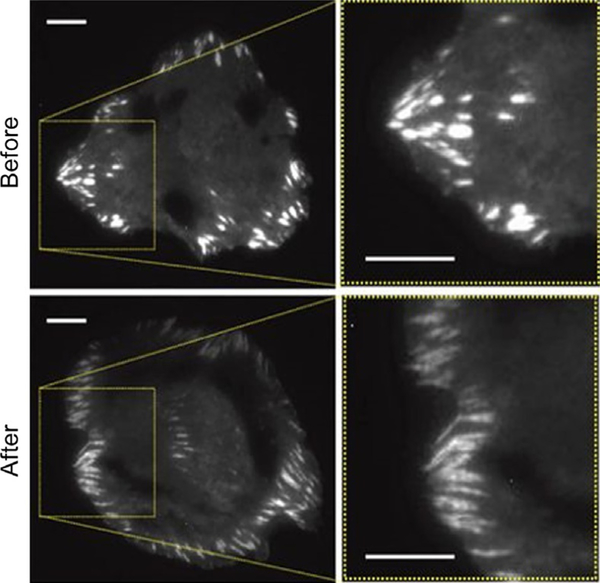
Fig. 1.
HeLa cells showing elongation and increase in focal adhesion number in response to c-Src stimulation. Cells were transiently overexpressing mCherry-paxillin and imaged using TIRF microscopy. Scale bars correspond to 10 μm (Karginov et al., 2014).
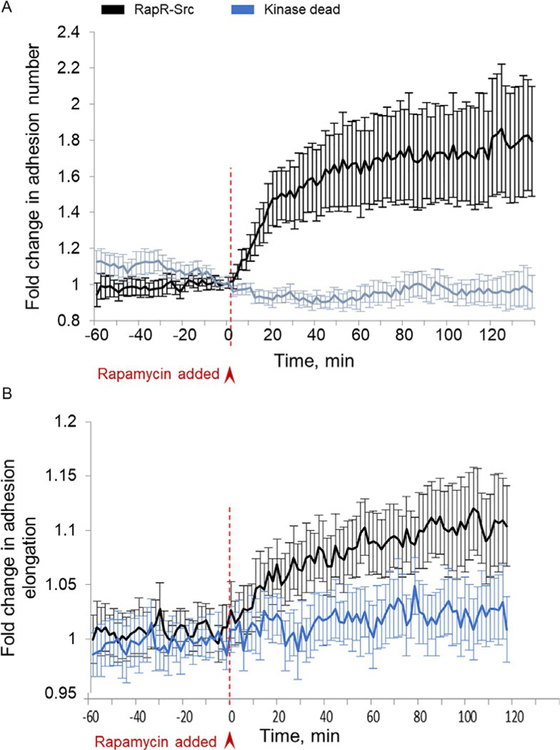
Fig. 2.
(A) Time-lapse quantification of focal adhesion numbers using metamorph and MATLAB softwares. Focal adhesion number at any time point was normalized to the average number of focal adhesions before c-Src activation. (B) Focal adhesion elongation was measured using the focal adhesion analysis server. Error bars represent 90% confidence intervals (n = 22 cells for RapR-Src; n= 15 cells for kinase dead) (Karginov et al., 2014).
3. MODULATION OF LUNG ENDOTHELIAL BARRIER FUNCTION BY INFLAMMATORY AND PROTECTIVE AGENTS
The loss of EC barrier integrity and alveolar flooding are hallmarks of inflammatory lung injury in ALI/ARDS and lung tumor metastases (Fanelli & Ranieri, 2015; Ferguson et al., 2012; Matthay & Ware, 2015). A number of inflammatory agonist such as vascular endothelial growth factor (VEGF) (Bates, 2010), thrombin (Bogatcheva, Garcia, & Verin, 2002; Garcia, Verin, & Schaphorst, 1996), histamine (Leach, Eaton, Westcott, & Firth, 1995), lipopolysaccharide (LPS) (Khakpour, Wilhelmsen, & Hellman, 2015; Vandenbroucke, Mehta, Minshall, & Malik, 2008), and particulate matter (Dai et al., 2016; Wang et al., 2012) disrupt EC barrier function. Sphingosine-1-phosphate (Abbasi & Garcia, 2013; Natarajan et al., 2013), high molecular weight hyaluronic acid (HMW HA) (Behling-Kelly, Vonderheid, Kim, Corbeil, & Czuprynski, 2006; Singleton et al., 2010), ATP (Gunduz et al., 2003; Kolosova et al., 2005), activated protein C (APC) (Okajima, 2004), oxidized phosphatidylcholine (Birukova, Chatchavalvanich, Oskolkova, Bochkov, & Birukov, 2007; Nonas et al., 2006), and hepatocyte growth factor (HGF) (Fu et al., 2015; Yamada et al., 2014) all promote EC migration and enhance barrier function. Increased blood levels of VEGF and HGF have been reported in sepsis. Plasma VEGF levels positively correlated with sepsis were elevated during sepsis and mortality (van der Flier et al., 2005). While elevated VEGF levels increase vascular endothelial permeability, HGF levels were also increased in early sepsis (Sekine, Fujishima, & Aikawa, 2004) suggesting initiation of tissue protection and regeneration after sepsis. Similarly, S1P levels were decreased in plasma of sepsis patients and animal models of ALI (Winkler et al., 2015; Zhao et al., 2011) and infusion of S1P offered protection against LPS- and CLP-mediated lung inflammation and injury (Szczepaniak et al., 2008). In other lung pathologies such as asthma, pulmonary hypertension, pulmonary fibrosis, bronchopulmonary dysplasia, and lung cancer, S1P levels are elevated in plasma and in lung tissues of patients and animal models (Gairhe et al., 2016; Harijith et al., 2013; Huang & Natarajan, 2015; Jolly, Rosenfeldt, Milstien, & Spiegel, 2002; Tabasinezhad et al., 2013). Blocking S1P production by inhibiting sphingosine kinase (SPHK) 1 and/or 2 seems to offer protection against lung inflammation and injury in several lung disorders (Ebenezer, Fu, & Natarajan, 2016; Huang & Natarajan, 2015; Natarajan et al., 2013). EC hyper-permeability induced by barrier disruptive agents such as VEGF or LPS involve signaling pathways of JNK mitogen-activated kinase, endothelial myosin light chain kinase (EC MLCK), and Rho GTPase that regulate actomyosin-dependent disruption of cell junctions and adherens junction proteins (Shimizu et al., 2015). On the contrary, barrier enhancement by HGF, S1P, simvastatin, ATP, and oxidized phospholipids (OxPLs) are associated with increased interaction between actin and cortactin, and α/β-catenin and VE-cadherin at leading edge of cells (Birukova, Malyukova, Mikaelyan, Fu, & Birukov, 2007; Dudek et al., 2004; Jacobson et al., 2004, 2006; Usatyuk et al., 2014). HGF and S1P promote cell migration, wound healing, and barrier enhancement by interacting with its cognate receptors c-Met (Fu et al., 2016; Usatyuk et al., 2014) and S1P 1–5 (McVerry & Garcia, 2005), respectively; however, the role of specific receptor(s) for OxPLs [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC) and 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphocholine (PGPC)] in barrier enhancement is yet to be clearly established.
4. MECHANISMS OF LAMELLIPODIA FORMATION AND ENDOTHELIAL BARRIER ENHANCEMENT
In lung endothelium, both S1P and HGF are known to stimulate lamellipodia formation and endothelial barrier enhancement (Fu et al., 2016, 2015). The driving force for cell motility results from a continuous growth of actin filaments and cortactin rearrangement in the lamellipodia at leading edge of the EC (Bugyi & Carlier, 2010; Gomez & Letourneau, 2014). Earlier studies have shown that lamellipodia formation is controlled by Rho family of GTPases, particularly Rac1 and Cdc42, which regulate the Arp2/3 complex via WASP (Hufner, Schell, Aepfelbacher, & Linder, 2002; Le Clainche et al., 2007; Ten Klooster et al., 2006), while Rho induces actomyosin contractility and regulates stress fiber formation and focal adhesions (Chrzanowska-Wodnicka & Burridge, 1996; Katoh, Kano, & Noda, 2011). HGF or scatter factor signals through its tyrosine kinase receptor, c-Met and promotes angiogenesis, tissue regeneration, and barrier tightening of the endothelium.
4.1. Sphingosine Kinase/Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptors Signaling and Focal Adhesions in Barrier Regulation
S1P, a bioactive lipid mediator, is a naturally occurring sphingolipid present in all mammalian tissues, erythrocytes, and circulating plasma (Hanel, Andreani, & Graler, 2007) and is a potent barrier enhancing sphingolipid that signals via both intracellular and extracellular mechanisms. Its extracellular effects are mediated through its five G protein-coupled receptors, S1P1–5 that are expressed on the plasma membrane of cells. Human lung ECs express higher levels of S1P1–3 compared to S1P4 and 5 (Natarajan et al., 2013). In vivo, S1P administration conferred protection against LPS-induced ALI. Intravenous administration of S1P (1μM) after 1h of intratracheal LPS challenge in mice attenuated lung inflammation and injury (Peng et al., 2004). Similarly, in a canine model of LPS-induced lung injury, infusion of S1P significantly reduced alveolar edema, and protein accumulation in BAL fluid (McVerry et al., 2004). These studies suggested that enhancing S1P levels in circulation and/or lung tissue has a beneficial effect against LPS-induced lung injury. Increasing S1P concentration in plasma and cells may have deleterious effects such as cardiac toxicity through activation of S1P3 in the heart (Forrest et al., 2004) or increase airway hyper-responsiveness in allergen-challenged mice (Roviezzo et al., 2007). Interestingly, inhibition of S1P lyase activity by S1P lyase inhibitor, 2-acetyl-4(5)-[1(R),2(S),3(R),4-tetrahydroxybutyl]-imidazole, in mice, increased S1P levels in lung tissue and BAL fluid and reduced LPS-induced lung injury and inflammation (Zhao et al., 2011). Further, down-regulation of S1P lyase with siRNA increased intracellular S1P concentration, and attenuated LPS-induced barrier disruption of HLMVECs by activation of Rac1 (Zhao et al., 2011). These results indicated a potential role for intracellularly generated S1P in barrier protection against ALI and suggest that S1P lyase could be a potential target.
Mechanisms of S1P-mediated regulation of endothelial barrier enhancement have been investigated in vitro using primary lung pulmonary artery and microvascular ECs isolated from human, mouse, and bovine species. Human lung ECs express high levels of S1P1 and S1P3 (Natarajan et al., 2013). While S1P1 signaling is coupled to Gi and Rac1 activation, S1P3 signaling is coupled to Gi, Gq/11, and G12/13 pathways that activate Rho A to a greater extent compared to Rac1 (Garcia et al., 2001; Lee et al., 2009; Natarajan et al., 2013; Sammani et al., 2010; Waeber, Blondeau, & Salomone, 2004). S1P, through activation of S1P1, stimulates Rac1-dependent translocation and co-localization of cortactin and non-muscle myosin light chain (MLC) kinase, MLC phosphorylation, and cortical actin stabilization to enhance barrier function (Dudek et al., 2004). Current evidence indicates that regulation of S1P-induced activation and recruitment of PI3K, Tiam1/Rac1 to caveolin-enriched microdomains (CEM) is necessary for α-actinin-mediated cortical actin rearrangement and endothelial barrier enhancement (Singleton, Dudek, Chiang, & Garcia, 2005). Additionally, integrin β4 (ITGB4) was also recruited to CEM and formed a complex with S1P1 after S1P challenge of human pulmonary artery ECs and down-regulation of ITGB4 expression with siRNA reduced S1P-induced barrier enhancement (Ephstein et al., 2013). These results support a role for S1P1 and ITGB4 complexes in CEM for enhanced barrier function and vascular integrity. S1P-induced lamellipodia formation and migration of ECs also require cofilin, a major actin-regulating protein involved in actin depolymerization (Pfaendtner, De La Cruz, & Voth, 2010). Another actin cross-linking protein, filamin A (FLNa), has been shown to link SPHK1 and S1P1 at lamellipodia to promote cell movement (Maceyka, Alvarez, Milstien, & Spiegel, 2008). Coronin, an actin-binding protein, has been identified as critical cofactor for cofilin-dependent signaling pathways. S1P stimulated phosphorylation of coronin 1B, its translocation to lamellipodia, co-localization with cortactin at lamellipodia and chemotaxis of lung ECs, which was PLD2, PKC, and Rac1 dependent (Usatyuk, Burns, et al., 2013). Taken together, these data suggest that S1P-mediated lamellipodia formation and enhancement of endothelial barrier require assembly/interaction of a number of actin and actin-binding proteins with S1P receptors, Rac1, Tiam1/IQGAP1, and integrins at the CEM.
Focal adhesion and cell–cell adherens junctional proteins have been implicated in endothelial barrier regulation (Belvitch & Dudek, 2012; Thennes & Mehta, 2012). S1P-induced EC barrier enhancement is associated with redistribution of focal adhesion proteins, paxillin, and focal adhesion kinase (FAK) to the cell periphery and co-localization with cortical actin (Shikata, Birukov, Birukova, Verin, & Garcia, 2003). S1P promotes tyrosine phosphorylation of FAK at Y576 leading to disruption of focal adhesions and redistribution to cell periphery and CEM and cell–matrix and cell–cell adherens junctional complex tightening to offset decrease in EC permeability (Shikata et al., 2003). On the contrary, thrombin induces FAK phosphorylation at multiple sites (Y397, Y576, Y925) that result in redistribution of focal adhesion proteins to stress fibers and barrier disruption (Shikata et al., 2003). S1P also stimulated paxillin tyrosine phosphorylation at Y31 and Y118 by c-Abl and down-regulation of c-Abl with siRNA attenuated S1P-mediated paxillin tyrosine phosphorylation and lamellipodia formation (Fu et al., 2015). These observations suggest a key role for FAK and paxillin tyrosine phosphorylation in S1P-mediated endothelial barrier enhancement.
4.2. S1P Analogs, FTY720, FTY720-P, and FTY-720 Phosphonate, and Endothelial Barrier Enhancement
Although S1P has been shown to be effective against pulmonary leak in animal ALI, its role as a therapeutic agent is limited due to potential side effects such as bradycardia and airway hyper-responsiveness in animal models of inflammation and injury. This has led to development and characterization of several S1P analogs to mimic the biological effects of S1P and its signaling via S1P receptors. FTY720, a synthetic derivative of a fungal metabolite myriocin, is an immunosuppressive agent, and approved by FDA for the treatment of multiple sclerosis in the United States (Sanchez et al., 2003). Both in vivo and in vitro FTY720 modulates vascular permeability. Lower doses of FTY720 attenuated LPS-induced pulmonary leak in mice (Muller et al., 2011). However, higher doses of FTY720 caused irreversible loss of vascular barrier integrity and apoptosis in mice subjected to ventilator-induced lung injury and an increase in permeability and loss of barrier function in ECs in vitro (McVerry et al., 2004). The mode of action of FTY720 in barrier regulation is not well understood; however, most likely involves phosphorylation of FTY720 to FTY720-P, an S1P analog, by sphingosine kinase 2 (Billich et al., 2003; Natarajan et al., 2013). The mechanism of FTY720-induced endothelial barrier enhancement seems to be distinct from S1P. While both S1P and FTY720 rapidly induced S1P1 accumulation in CEM, only S1P stimulated transient increase of [Ca2+]i and phosphorylation of S1P1 on threonine residue compared to FTY720 (Dudek et al., 2007). Further, phosphorylation of FTY720 was not essential for EC barrier enhancement and did not involve myosin light chain phosphorylation, Rac1 activation, cortactin tyrosine phosphorylation, and cytoskeletal rearrangement; however was coupled to Gi signaling through CEM (Dudek et al., 2007). Unlike S1P, FTY720 did not induce translocation of adherens junction or tight junction proteins to cell periphery and down-regulation of β-catenin, occludin, claudin-5, or zona occludins proteins with siRNA had no effect on FTY720-induced barrier enhancement of human pulmonary artery endothelial cells (Wang, Chiang, Simmons, Garcia, & Dudek, 2011). Interestingly, similar to S1P (Fu et al., 2015), FTY720-induced barrier enhancement was dependent on c-Abl kinase activation as c-Abl siRNA attenuated barrier enhancement by FTY720 (Wang et al., 2011). It is unclear if FTY720-induced endothelial barrier enhancement is paxillin mediated as S1P-induced lamellipodia formation was attenuated by paxillin siRNA (Fu et al., 2015). The usefulness of FTY720 or FTY720-P in treatment of ALI is also limited due to reported detrimental in vivo effects such as increased rates of dyspnea and decreased lung function in patients with multiple sclerosis (Kappos et al., 2006), most likely related to altered expression and degradation of S1P1.
Recently, a number of FTY720-P analogs including phosphonates, phosphothionates, and phenyimidazole derivatives have been synthesized and tested for their effects on endothelial barrier function. One such analog is FTY720-phosphonate, which is more stable and resistant to hydrolysis by lipid phosphate phosphatases (Lu et al., 2009). The (S)-isomer of FTY720-phosphonate exhibited a robust barrier protection compared to S1P and FTY720 in vitro and also showed a protective effect without immunosuppression in vivo (Camp et al., 2009). Further, FTY720-phosphonate had no effect on S1P1 expression compared to S1P or FTY720 both in vivo against bleomycin-induced ALI in mice and in vitro in human lung ECs (Wang et al., 2014). Unlike FTY720, FTY720-phosphonate activated Rac1, enhanced VE-cadherin redistribution to cell periphery, and induced focal adhesion and adherens junction rearrangement in ECs (Wang et al., 2015). Additionally, FTY720, FTY720-P, and FTY720-phosphonate stimulated redistribution of actin and cortactin in cell periphery of human lung ECs (Fig. 3) suggesting involvement of lamellipodia in barrier enhancement if the endothelium.
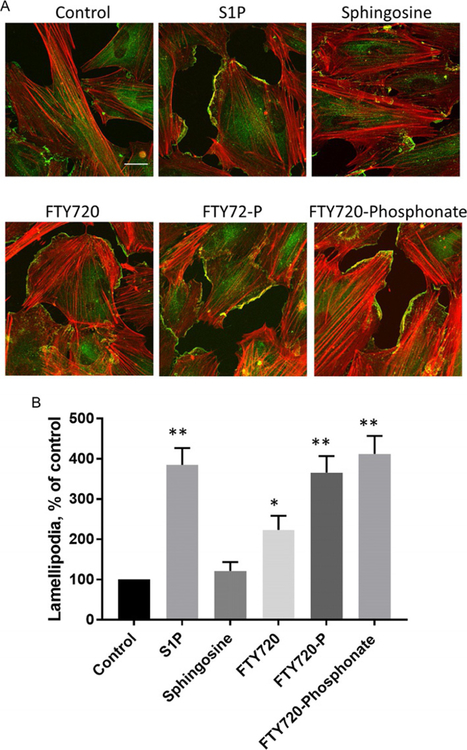
Fig. 3.
Effects of sphingolipids on endothelial lamellipodia formation. (A) Human lung microvascular endothelial (HLMVECs) cells were treated with vehicle, S1P (1μM), sphingosine (1μM), FTY720 (1μM), FTY720-P (1μM), or FTY720-phosphonate (1μM), for 30min. Cells were subjected to immunofluorescent staining for cortactin (green) and actin (red). Images were obtained with Zeiss LSM 880 confocal microscope, 63 × oil objective, scale bar=20μm. (B) Statistical analysis of lamellipodia formation presented as percentage of control, and at least 20 cells were analyzed for each condition. *P<0.05 vs control; **P<0.01 vs control.
4.3. HGF/c-Met/PI3K/Akt Signaling in Lamellipodia Formation and Endothelial Barrier Enhancement
HGF stimulated phosphorylation of C-Met at Tyr 1003, 1313, 1234, 1235, 1349, and Ser985 and potentiated lamellipodia formation via PI3K and Akt activation (Usatyuk et al., 2014). Inhibition of c-Met with SU11274 attenuated c-Met, PI3K and Akt phosphorylation, suppressed lamellipodia formation and EC migration implicating a significant role for c-Met in the process. Similarly, inhibition of PI3K with LY294002 or down-regulation of PI3K with siRNA attenuated Akt activation, and EC migration suggesting a role for PI3K/Akt signaling in HGF/c-Met-mediated lamellipodia formation and motility (Usatyuk et al., 2014). In addition to ECs, HGF also enhanced melanoma cell migration that was dependent on PI3K/Akt pathway and down-regulation of E-cadherin and β-catenin (Ye et al., 2008). In addition to PI3K/Akt pathway, HGF triggers other distinct mechanisms such as microtubule-independent Tiam1 activation and microtubule- and activated protein C (APC)-dependent stimulation of Asef (a novel Rac activator) that play a role in endothelial barrier enhancement (Birukova, Alekseeva, Mikaelyan, & Birukov, 2007; Higginbotham et al., 2014; Singleton et al., 2007). Additionally, IQGAP1, an effector of Rac1 and Cdc42, has been identified as a key regulator of actin cytoskeleton dynamics, thereby regulating lamellipodia formation, cell migration, and barrier protection (Watanabe, Wang, & Kaibuchi, 2015). In a recent study, increased Asef–IQGAP1 interaction has been implicated in HGF-induced regulation of cortical actin cytoskeleton remodeling via Arp3/cortactin and enhanced endothelial barrier (Tian et al., 2015). Thus Asef/IQGAP1/Tiam1 signaling may be involved in regulating local Rac activity and in response to barrier enhancing stimuli such as HGF, S1P, and OxPLs. The mechanism of HGF-induced activation of Asef/IQGAP1/Tiam1 signaling is not known; however, exposure of human pulmonary artery endothelial cells to hyperoxia caused activation and redistribution of Tiam1, Rac1, and IQGAP1 to cell periphery (Usatyuk et al., 2009). Further, down-regulation of Rac1 or IQGAP1 attenuated hyperoxia-induced cortactin tyrosine phosphorylation and ROS production, which was dependent on PLD2 (Usatyuk et al., 2009). HGF is known to activate PLD in endothelial cells (Natarajan, V., et al., unpublished data), and the role of PLD1 or PLD2 in HGF-mediated redistribution of Tiam1/IQGAP1 to lamellipodia and barrier enhancement needs further investigation.
4.4. Cross-Talk Between HGF/c-Met and SPHK1/S1P/S1PR Signaling in Lamellipodia Formation and Endothelial Barrier Enhancement
Recent evidence suggests a role for S1P signaling in HGF-mediated lamellipodia formation and barrier regulation. A critical role for S1PR1 and integrin β4 in HGF/c-Met-mediated enhanced barrier integrity has been reported. Reduced S1PR1 expression attenuated HGF/c-Met-mediated ITGB4 and Rac1 activation and decreased endothelial permeability (Ephstein et al., 2013). HGF stimulated S1P production in ECs by activation of SPHK1 (Fu et al., 2016), and HGF-mediated migration of ECs was dependent of SPHK1 activation (Duan et al., 2004). In HLMVECs, HGF enhanced co-localization of SPHK1/p-SPHK1 with actin/cortactin in lamellipodia and down-regulation SPHK1 or inhibition of SPHK1 activity with a SPHK1-specific inhibitor, PF-543 attenuated HGF-induced lamellipodia formation. In addition, down-regulation of SPNS2, a transporter of S1P in endothelial cells, also suppressed HGF-induced lamellipodia formation and EC migration suggesting a key role for “inside-out” S1P signaling (Fu et al., 2016). The interdependence between HGF-c-Met signaling and S1P was further evidenced when knockdown of S1P1, but not S1P2 or S1P3, abolished lamellipodia formation in HLMVECs (Fu et al., 2016). In addition to SPHK1, mammalian cells also express the second isoenzyme of SPHK1, namely SPHK2; however, it appears that blocking SPHK2 with specific inhibitor or down-regulation of Sphk2 with siRNA had no effect on HGF-induced lamellipodia formation (Fu et al., 2016). The cross-talk between HGF/c-Met and SPHK1/S1P/SPNS2/S1P1 signaling axis in HGF-mediated lamellipodia formation and endothelial barrier enhancement provides potential interaction between receptor tyrosine kinase(s) and its coupling to G protein-coupled receptors and their role in the regulation of EC barrier function (Fig. 4).
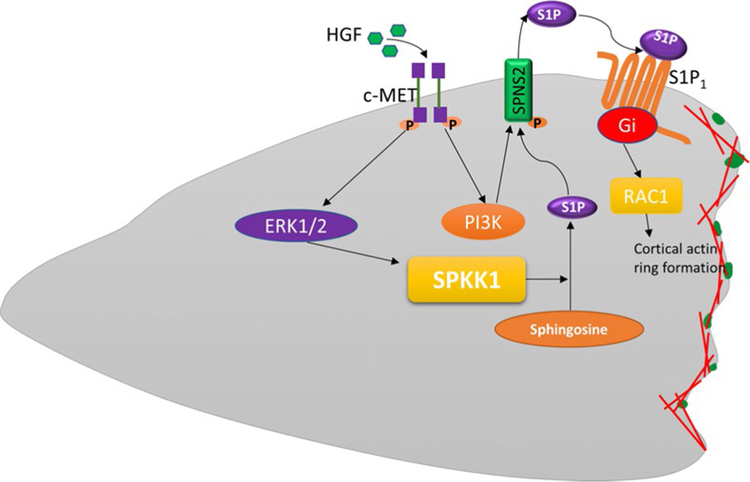
Fig. 4.
Proposed model for the cross-talk between HGF/c-Met and SphK1/S1P/Spns2/S1P1 signaling axis in HGF-mediated lamellipodia formation. HGF activates its receptor c-Met, which initiates phosphorylation of Erk and Akt kinase. Activation of Erk by HGF stimulates SPHK1 phosphorylation, which in turn converts sphingosine into S1P. HGF-mediated activation of PI3K/Akt pathway phosphorylates S1P transporter, Spns2, which facilitates efflux of intracellular S1P to extracellular milieu. The secreted S1P binds to its receptor S1P1 to initiate downstream pathways mediating lamellipodia formation of lung endothelial cells.
5. REACTIVE OXYGEN SPECIES, LAMELLIPODIA FORMATION, AND ENDOTHELIAL BARRIER ENHANCEMENT
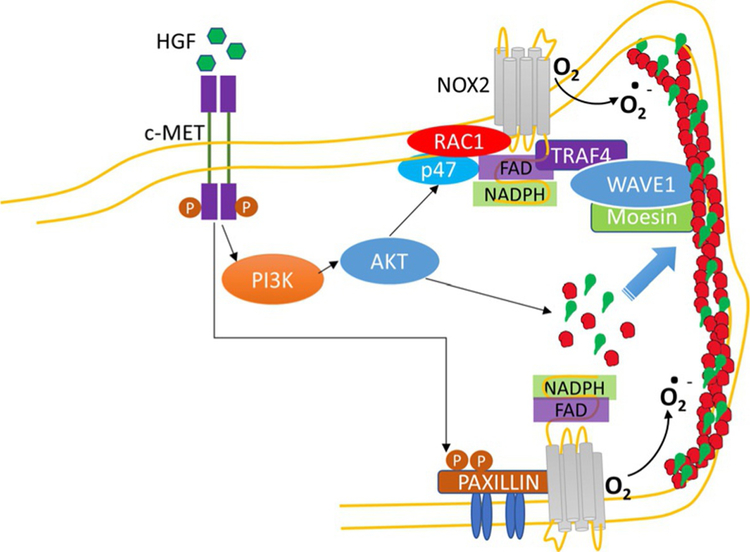
Low concentrations of reactive oxygen species (ROS) such as hydrogen peroxide, superoxide, NO, and hydroxyl radical are generated in cells under normal conditions; however, excess ROS are produced during bacterial infection and several human pathologies including ALI, pulmonary fibrosis, pulmonary hypertension, and radiation- and ventilator-induced lung injury. Although excess ROS is toxic, physiological concentrations of ROS generated within the various compartments in the cell are vital and function as signaling molecules to mediate responses such as proliferation, migration, differentiation, and gene expression (Schieber & Chandel, 2014; Ushio-Fukai & Urao, 2009; Wu et al., 2005; Zhang et al., 2016). There are conflicting reports regarding the role ROS in maintaining healthy vascular endothelium. ROS generated by NADPH oxidase (NOX) proteins at sites of injury disrupt endothelial barrier (Cai, 2005) while cell-specific increase of Nox2 expression in cardiomyocyte, but not endothelium, augmented cardiomyocyte hypertrophy, and interstitial fibrosis (Sirker et al., 2016). However, short-term conditional expression of Nox2 in vascular ECs increased ROS production and induced proliferation of ECs and angiogenic sprouting in the aorta (Shafique et al., 2017). These and other studies suggest different roles for spatio-temporally generated ROS in various cell functions both under normal and pathological conditions. Exogenous addition of hydrogen peroxide to ECs caused stress fiber formation, and disruption of endothelial barrier, which was mediated by PLD/PA signal transduction (Usatyuk, Kotha, Parinandi, & Natarajan, 2013). However, targeting Nox2-derived ROS to focal complexes in lamellipodia and membrane ruffles via interaction with p47phox, TRAF4 and WAVE1 generated ROS in the lamellipodia that were required for directed cell migration (Ushio-Fukai, 2006; Wu et al., 2005). Further, localizing the ROS signal in CEM is known to regulate endothelial dysfunction and vascular hyper-trophy (Ushio-Fukai et al., 2005). There is an evidence for ROS production is lamellipodia and a mechanism for targeting NOX2 to the lamellipodia. HGF stimulated hydrogen peroxide formation in lamellipodia of lung microvascular ECs and inhibition of c-Met or PI3K or Akt attenuated p47phox, cortactin, and Rac1 redistribution to lamellipodia (Usatyuk et al., 2014). Moreover, down-regulation of p47phox or cortactin or inhibition of NOX2 attenuated HGF-induced lamellipodia and hydrogen peroxide accumulation in lamellipodia (Usatyuk et al., 2014) (Fig. 5). In addition to p47phox, moesin and WAVE1, which catalyze the actin nucleation required for lamellar structure in co-operation with Rac1 targets NOX2 to the lamellipodia of ECs (Takac, Schroder, & Brandes, 2012; Wu et al., 2005). Cortactin tyrosine phosphorylation by Src is critical for HGF-induced lamellipodia formation and hydrogen peroxide generation, which might depend on IQGAP1 redistribution to lamellipodia as shown in ventral lamellipodia of ECs that were enriched in cortactin, IQGAP1, and p47phox (Martinelli et al., 2013). In addition to cortactin, Rac1, and IQGAP1, HGF-or S1P-induced tyrosine phosphorylation of paxillin is essential for ROS accumulation in lamellipodia. Down-regulation of paxillin with siRNA or ecto-expression of paxillin mutants Y118F in lung ECs attenuated HGF-mediated ROS accumulation in lamellipodia (Fu et al., 2015). Thus, the current evidence suggests Nox2-dependent ROS generation in lamellipodia of lung ECs and blocking p47phox or cortactin influenced not only the lamellipodia but also local ROS generation in lamellipodia. The role of other NOX family proteins in lamellipodia formation and lamellipodial ROS generation is to be determined.
Fig. 5.
c-Met/PI3K/Akt signaling in ROS generation, lamellipodia formation, and cell motility. c-Met/PI3K/Akt signaling cascade modulates actin and cortactin redistribution to cell periphery, translocation of p47phox and Rac1, components of NADPH oxidase (NOX) 2, resulting reactive oxygen species (ROS) generation at cell periphery, which is critical for lamellipodia formation. Phosphorylation of paxillin by HGF or S1P also regulates NOX2-generated ROS and lamellipodia formation.
6. OXIDIZED PHOSPHOLIPIDS, LAMELLIPODIA FORMATION, AND ENDOTHELIAL BARRIER ENHANCEMENT
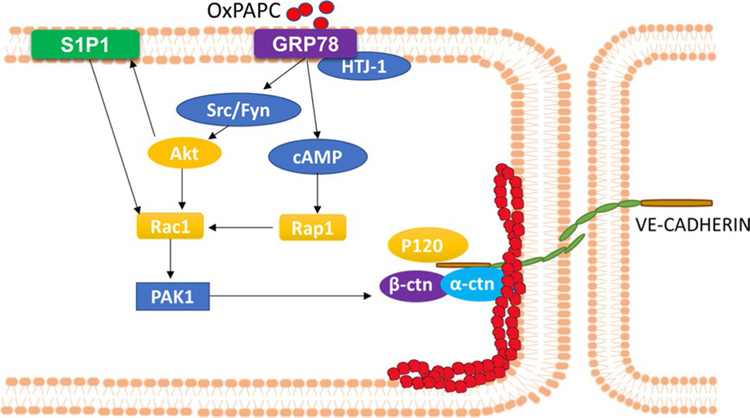
Oxidation of protein and phospholipid components of low-density lipoprotein (LDL) particles leads to generation of oxidized (Ox) LDL, which is a key initiating factor in plaque formation and atherogenesis (Kita et al., 2001). Oxidized phospholipids (OxPLs) in OxLDL are a mixture of oxidized phospholipid products primarily derived from 1-palmitoyl-2 glutaroyl-sn-glycero-3-phosphocholine (PGPC) and 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphocholine (POVPC) (Fu & Birukov, 2009). Accumulating evidence suggests that OxPLs are both pro- and anti-inflammatory and implicated in regulation of inflammation, angiogenesis, thrombosis, endothelial barrier function, and immune responses (Bochkov et al., 2017). OxPL triggers vascular inflammation in a murine carotid artery model using Pluronic gel (Furnkranz et al., 2005) and in atherosclerotic lesions induce monocyte–endothelial interactions and adhesion via MAPK activation (Birukov, Leitinger, Bochkov, & Garcia, 2004). While POVPC and PGPC, the fragmented OxPLs, were endothelial barrier disruptive, full-length OxPLs such as oxidized 1-palmtoyl-2-arachidonyl-sn-glycero-3-phosphocholine (OxPAPC) showed endothelial barrier protection both in vivo and in vitro. OxPAPC reversed barrier disruption mediated by thrombin, LPS, mechanical ventilation, and heat-inactivated Staphylococcus aureus lung infection (Birukov, Bochkov,etal.,2004;Melitonetal.,2015;Nonasetal.,2008,2006).Signaling pathways involved in OxPAPC-mediated barrier protection have been described in human lung ECs (Birukov & Karki, 2018). Barrier protective effects of OxPAPC were attenuated by inhibitors of small GTPases, PKA, PKC, and Src, while MAPKs and PI3K had no role to play in the barrier protection (Birukov, Bochkov, et al., 2004; Birukova, Chatchavalvanich, et al., 2007). The OxPAPC-mediated enhancement of barrier integrity required binding to a cell membrane-associated chaperone protein, GRP78, and its cofactor, HTJ-1 that facilitated GRP78 trafficking to CEM, activation of Src, Fyn, and Rac1 (Birukova et al., 2014), and Akt-dependent trans-activation action of S1P1 (Singleton et al., 2009) resulting in rearrangement and stabilization of cortical actin cytoskeleton to cell periphery (Fig. 6). Although these studies suggest a role for S1P1 in OxPAPC-mediated barrier protective responses, the role of S1P in S1P1 activation is unclear. Further study is necessary to determine if OxPAPC activation of S1P1 is mediated by intracellular S1P generation followed by “inside-out” signaling through S1P transporter Spns2. The contribution of S1P2 and S1P3 in OxPAPC-mediated enhancement of vascular integrity needs further clarification.
Fig. 6.
Signaling pathway activated by OxPAPC. OxPAPC binds to cell membrane chaperon GRP78 and its cofactor HTJ-1 to activate Src/Fyn kinase(s) and its downstream target Akt. Akt transactivates S1P1 and small GTPase Rac1. Also, OxPAPC up-regulates intracellular cAMP, which in turn activates Rap1 and further activates Rac1. Rac1 exerts its activity of cytoskeleton rearrangement through PAK1.
7. SIMVASTATIN ACTIVATION OF S1P1 IN ENDOTHELIAL BARRIER ENHANCEMENT
Simvastatin, a FDA approved drug that specifically inhibits HMGCoA reductase, is a widely prescribed drug to lower LDL cholesterol and triglyceride levels in hyperlipidemia subjects with proven beneficial effects in coronary artery disease. In addition to lipid-lowering property, simvastatin and other statins are known to regulate endothelial barrier function in animal models of lung injury and in vitro. In a murine model of radiation-induced lung injury (RILI), simvastatin administration reduced several indices of RILI including pulmonary leak, leukocyte infiltration, oxidative stress, and reversed RILI-associated dysregulated sphingolipid metabolic pathway genes expression (Pabst & Tschernig, 2010). In vitro, simvastatin attenuated thrombin-mediated stress fiber formation, and myosin light chain phosphorylation while increased Rac GTPase activity, and rearrangement of actin and cortactin to cell periphery (Jacobson et al., 2004). The endothelial barrier regulation by simvastatin was mediated by increased expression of S1P1 via the transcriptional factor, KLF2 (Sun, Mathew, Sammani, Jacobson, & Garcia, 2017). Simvastatin also up-regulated expression of SPHK1, but not SPHK2, in human lung ECs (Natarajan V, unpublished data), which might account for enhanced S1P production and inside-out signaling through S1P1 to enhance endothelial barrier function. The role of SPHK1/S1P/Spns2 signaling axis in simvastatin-mediated endothelial barrier enhancement needs to be investigated.
8. CONCLUSIONS AND FUTURE DIRECTIONS
The recent advances outlined here emphasize the crucial role of sphingolipid metabolism and signaling in regulating lamellipodia formation and endothelial barrier function. Endothelial barrier integrity is critical for normal vascular function that involves interaction between growth factors and its receptors with downstream targets in the cell that regulates reorganization of actin, cortactin, and other cytoskeletal proteins to membrane protrusions on the cell periphery. The finding that S1P receptors, especially S1P1, is central to barrier enhancement mediated by HGF, S1P, oxidized phospholipids, and statins underscores the importance of sphingolipid signaling in modulation of endothelial barrier function under normal and pathological conditions of several pulmonary disorders. The findings that HGF/c-Met signaling cross-talks with SPHK1/S1P/SPNS2/S1P1 and OxPAPC signaling is linked to S1P1 pathway highlights the importance and complexity involved in endothelial barrier regulation. Another salient aspect is the assembly of sphingolipid metabolites, metabolizing enzymes, and S1P receptors with cytoskeletal components such as cortactin and non-muscle MLCK in caveolin-enriched microdomains of the endothelium to facilitate lamellipodia formation and barrier protection. Spatio-temporal generation of ROS at caveolin-enriched microdomains mediated by recruitment of Rac1 and p47phox, and S1P signaling seems to be essential for lamellipodia formation and barrier enhancement. The current concept of generation of membrane protrusions that constitute lamellipodia is based on imaging of co-localized actin and cortactin at leading edges of the cell would be well served by developing reliable procedures to isolate lamellipodia, determine its sphingolipid composition, characterize the nature of cytoskeletal and other proteins recruited to lamellipodia and CEM, determine the expression and activity of enzymes involved in S1P and ROS generation and how modulation of these may affect the lamellipodia formation and function. Studies to define the role of other phospholipids such as inositol phospholipids and mitochondrial ROS and their signaling on lamellipodia formation and barrier enhancement should be investigated. Such future studies are likely to define key targets and result in novel therapies to combat various vascular diseases.
ACKNOWLEDGMENTS
We are grateful to Dr. Prasad Kanteti for the critical reading and editing of this manuscript. The research was supported by special research funds from the Dean’s Office of College of Medicine, UIC and National Institutes of Health grant P01 HL060678 (Project 4) to V.N.
REFERENCES
- Abbasi T, & Garcia JG (2013). Sphingolipids in lung endothelial biology and regulation of vascular integrity. Handbook of Experimental Pharmacology, 216, 201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abella JV, Galloni C, Pernier J, Barry DJ, Kjaer S, Carlier MF, et al. (2016). Isoform diversity in the Arp2/3 complex determines actin filament dynamics. Nature Cell Biology, 18, 76–86. [DOI] [PubMed] [Google Scholar]
- Adams CL, & Nelson WJ (1998). Cytomechanics of cadherin-mediated cell–cell adhesion. Current Opinion in Cell Biology, 10, 572–577. [DOI] [PubMed] [Google Scholar]
- Bates DO (2010). Vascular endothelial growth factors and vascular permeability. Cardiovascular Research, 87, 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, et al. (2002). Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell, 109, 509–521. [DOI] [PubMed] [Google Scholar]
- Behling-Kelly E, Vonderheid H, Kim KS, Corbeil LB, & Czuprynski CJ (2006) Roles of cellular activation and sulfated glycans in Haemophilus somnus adherence to bovine brain microvascular endothelial cells. Infection and Immunity, 74, 5311–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvitch P, & Dudek SM (2012). Role of FAK in S1P-regulated endothelial permeability. Microvascular Research, 83, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berginski ME, & Gomez SM (2013). The focal adhesion analysis server: A web tool for analyzing focal adhesion dynamics. F1000Research, 2, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, & Baumruker T (2003). Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. The Journal of Biological Chemistry, 278, 47408–47415. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, et al. (2004). Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circulation Research, 95, 892–901. [DOI] [PubMed] [Google Scholar]
- Birukov KG, & Karki P (2018). Injured lung endothelium: Mechanisms of self-repair and agonist-assisted recovery (2017 Grover conference series). Pulmonary Circulation, 8, 2045893217752660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukov KG, Leitinger N, Bochkov VN, & Garcia JG (2004). Signal transduction pathways activated in human pulmonary endothelial cells by OxPAPC, a bioactive component of oxidized lipoproteins. Microvascular Research, 67, 18–28. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Alekseeva E, Mikaelyan A, & Birukov KG (2007). HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. The FASEB Journal, 21, 2776–2786. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Chatchavalvanich S, Oskolkova O, Bochkov VN, & Birukov KG (2007). Signaling pathways involved in OxPAPC-induced pulmonary endothelial barrier protection. Microvascular Research, 73, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Malyukova I, Mikaelyan A, Fu P, & Birukov KG (2007). Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. Journal of Cellular Physiology, 211, 608–617. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Singleton PA, Gawlak G, Tian X, Mirzapoiazova T, Mambetsariev B, et al. (2014). GRP78 is a novel receptor initiating a vascular barrier protective response to oxidized phospholipids. Molecular Biology of the Cell, 25, 2006–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov V, Gesslbauer B, Mauerhofer C, Philippova M, Erne P, & Oskolkova OV (2017). Pleiotropic effects of oxidized phospholipids. Free Radical Biology & Medicine, 111, 6–24. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Garcia JG, & Verin AD (2002). Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry (Mosc), 67, 75–84. [DOI] [PubMed] [Google Scholar]
- Breitsprecher D, Kiesewetter AK, Linkner J, Urbanke C, Resch GP, Small JV, et al. (2008). Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. The EMBO Journal, 27, 2943–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Kiesewetter AK, Linkner J, Vinzenz M, Stradal TE, Small JV, et al. (2011). Molecular mechanism of Ena/VASP-mediated actin-filament elongation. The EMBO Journal, 30, 456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin JW, Zhang XE, Worthylake RA, & Souza-Smith FM (2015). Involvement of local lamellipodia in endothelial barrier function. PLoS One, 10, e0117970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugyi B, & Carlier MF (2010). Control of actin filament treadmilling in cell motility. Annual Review of Biophysics, 39, 449–470. [DOI] [PubMed] [Google Scholar]
- Cai H (2005). Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovascular Research, 68, 26–36. [DOI] [PubMed] [Google Scholar]
- Cai L, Makhov AM, Schafer DA, & Bear JE (2008). Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell, 134, 828–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp SM, Bittman R, Chiang ET, Moreno-Vinasco L, Mirzapoiazova T, Sammani S, et al. (2009). Synthetic analogs of FTY720 [2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol] differentially regulate pulmonary vascular permeability in vivo and in vitro. The Journal of Pharmacology and Experimental Therapeutics, 331, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, & Burridge K (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. The Journal of Cell Biology, 133, 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Sun C, Yao Z, Chen W, Yu L, & Long M (2016). Exposure to concentrated ambient fine particulate matter disrupts vascular endothelial cell barrier function via the IL-6/HIF-1alpha signaling pathway. FEBS Open Bio, 6, 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang I, Gorelik R, Sousa-Blin C, Derivery E, Guerin C, Linkner J, et al. (2013). Inhibitory signalling to the Arp2/3 complex steers cell migration. Nature, 503, 281–284. [DOI] [PubMed] [Google Scholar]
- Dang I, Linkner J, Yan J, Irimia D, Faix J, & Gautreau A (2017). The Arp2/3 inhibitory protein Arpin is dispensable for chemotaxis. Biology of the Cell, 109, 162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan HF, Wu CT, Lu Y, Wang H, Liu HJ, Zhang QW, et al. (2004). Sphingosine kinase activation regulates hepatocyte growth factor induced migration of endothelial cells. Experimental Cell Research, 298, 593–601. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, et al. (2007). Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cellular Signalling, 19, 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, et al. (2004). Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: Roles for cortactin and myosin light chain kinase. The Journal of Biological Chemistry, 279, 24692–24700. [DOI] [PubMed] [Google Scholar]
- Ebenezer DL, Fu P, & Natarajan V (2016). Targeting sphingosine-1-phosphate signaling in lung diseases. Pharmacology & Therapeutics, 168, 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer DL, Fu P, Suryadevara V, Zhao Y, & Natarajan V (2017). Epigenetic regulation of pro-inflammatory cytokine secretion by sphingosine 1-phosphate (S1P) in acute lung injury: Role of S1P lyase. Advances in Biological Regulation, 63, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov A, Schiefermeier N, Grigoriev I, Ohi R, Brown MC, Turner CE, et al. (2008). Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. Journal of Cell Science, 121, 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephstein Y, Singleton PA, Chen W, Wang L, Salgia R, Kanteti P, et al. (2013). Critical role of S1PR1 and integrin beta4 in HGF/c-Met-mediated increases in vascular integrity. The Journal of Biological Chemistry, 288, 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, & Aepfelbacher M (1998). Thrombin inactivates myosin light chain phosphatase via Rho and its target rho kinase in human endothelial cells. The Journal of Biological Chemistry, 273, 21867–21874. [DOI] [PubMed] [Google Scholar]
- Fanelli V, & Ranieri VM (2015). Mechanisms and clinical consequences of acute lung injury. Annals of the American Thoracic Society, 12(Suppl. 1), S3–S8. [DOI] [PubMed] [Google Scholar]
- Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. (2012). The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Medicine, 38, 1573–1582. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, & Mullins RD (2010). Cell mechanics and the cytoskeleton. Nature, 463, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, et al. (2004). Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. The Journal of Pharmacology and Experimental Therapeutics, 309, 758–768. [DOI] [PubMed] [Google Scholar]
- Fu P, & Birukov KG (2009). Oxidized phospholipids in control of inflammation and endothelial barrier. Translational Research, 153, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Ebenezer DL, Berdyshev EV, Bronova IA, Shaaya M, Harijith A, et al. (2016). Role of sphingosine kinase 1 and S1P transporter Spns2 in HGF-mediated lamellipodia formation in lung endothelium. The Journal of Biological Chemistry, 291, 27187–27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Ebenezer DL, Ha AW, Suryadevara V, Harijith A, & Natarajan V (2018). Nuclear lipid mediators: Role of nuclear sphingolipids and sphingosine-1-phosphate signaling in epigenetic regulation of inflammation and gene expression. Journal of Cellular Biochemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Usatyuk PV, Jacobson J, Cress AE, Garcia JG, Salgia R, et al. (2015). Role played by paxillin and paxillin tyrosine phosphorylation in hepatocyte growth factor/sphingosine-1-phosphate-mediated reactive oxygen species generation, lamellipodia formation, and endothelial barrier function. Pulmonary Circulation, 5, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, et al. (2005). Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 633–638. [DOI] [PubMed] [Google Scholar]
- Gairhe S, Joshi SR, Bastola MM, McLendon JM, Oka M, Fagan KA, et al. (2016). Sphingosine-1-phosphate is involved in the occlusive arteriopathy of pulmonary arterial hypertension. Pulmonary Circulation, 6, 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, et al. (2001). Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. The Journal of Clinical Investigation, 108, 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Verin AD, & Schaphorst KL (1996). Regulation of thrombin-mediated endothelial cell contraction and permeability. Seminars in Thrombosis and Hemostasis, 22, 309–315. [DOI] [PubMed] [Google Scholar]
- Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, & Waterman CM (2008). Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. The Journal of Cell Biology, 183, 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, & Letourneau PC (2014). Actin dynamics in growth cone motility and navigation. Journal of Neurochemistry, 129, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Kou R, & Michel T (2006). Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. The Journal of Biological Chemistry, 281, 3210–3216. [DOI] [PubMed] [Google Scholar]
- Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, et al. (2010). Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature, 466, 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz D, Hirche F, Hartel FV, Rodewald CW, Schafer M, Pfitzer G, et al. (2003). ATP antagonism of thrombin-induced endothelial barrier permeability. Cardiovascular Research, 59, 470–478. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Eisenmann K, Alberts AS, & Waterman-Storer CM (2007). mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. Journal of Cell Science, 120, 3475–3487. [DOI] [PubMed] [Google Scholar]
- Hahne P, Sechi A, Benesch S, & Small JV (2001). Scar/WAVE is localised at the tips of protruding lamellipodia in living cells. FEBS Letters, 492, 215–220. [DOI] [PubMed] [Google Scholar]
- Hanel P, Andreani P, & Graler MH (2007). Erythrocytes store and release sphingosine 1-phosphate in blood. The FASEB Journal, 21, 1202–1209. [DOI] [PubMed] [Google Scholar]
- Harijith A, Pendyala S, Reddy NM, Bai T, Usatyuk PV, Berdyshev E, et al. (2013). Sphingosine kinase 1 deficiency confers protection against hyperoxia-induced bronchopulmonary dysplasia in a murine model: Role of S1P signaling and Nox proteins. The American Journal of Pathology, 183, 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero R, Sanchez G, & Lorente JA (2018). New insights into the mechanisms of pulmonary edema in acute lung injury. Annals of Translational Medicine, 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham K, Tian Y, Gawlak G, Moldobaeva N, Shah A, & Birukova AA (2014). Hepatocyte growth factor triggers distinct mechanisms of Asef and Tiam1 activation to induce endothelial barrier enhancement. Cellular Signalling, 26, 2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, & Natarajan V (2015). Sphingolipids in pulmonary fibrosis. Advances in Biological Regulation, 57, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufner K, Schell B, Aepfelbacher M, & Linder S (2002). The acidic regions of WASp and N-WASP can synergize with CDC42Hs and Rac1 to induce filopodia and lamellipodia. FEBS Letters, 514, 168–174. [DOI] [PubMed] [Google Scholar]
- Huttelmaier S, Mayboroda O, Harbeck B, Jarchau T, Jockusch BM, & Rudiger M (1998). The interaction of the cell-contact proteins VASP and vinculin is regulated by phosphatidylinositol-4,5-bisphosphate. Current Biology, 8, 479–488. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, et al. (2004). Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. American Journal of Respiratory Cell and Molecular Biology, 30, 662–670. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, & Garcia JG (2006). Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. American Journal of Physiology. Lung Cellular and Molecular Physiology, 291, L289–L295. [DOI] [PubMed] [Google Scholar]
- Jolly PS, Rosenfeldt HM, Milstien S, & Spiegel S (2002). The roles of sphingosine-1-phosphate in asthma. Molecular Immunology, 38, 1239–1245. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. (2006). Oral fingolimod (FTY720) for relapsing multiple sclerosis. The New England Journal of Medicine, 355, 1124–1140. [DOI] [PubMed] [Google Scholar]
- Karginov AV, Tsygankov D, Berginski M, Chu PH, Trudeau ED, Yi JJ, et al. (2014). Dissecting motility signaling through activation of specific Src-effector complexes. Nature Chemical Biology, 10, 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kano Y, & Noda Y (2011). Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. Journal of the Royal Society Interface, 8, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpour S, Wilhelmsen K, & Hellman J (2015). Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immunity, 21, 827–846. [DOI] [PubMed] [Google Scholar]
- Kita T, Kume N, Minami M, Hayashida K, Murayama T, Sano H, et al. (2001). Role of oxidized LDL in atherosclerosis. Annals of the New York Academy of Sciences, 947, 199–205 [discussion 205–196]. [DOI] [PubMed] [Google Scholar]
- Klomp JE, Huyot V, Ray AM, Collins KB, Malik AB, & Karginov AV (2016). Mimicking transient activation of protein kinases in living cells. Proceedings of the National Academy of Sciences of the United States of America, 113, 14976–14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova IA, Mirzapoiazova T, Adyshev D, Usatyuk P, Romer LH, Jacobson JR, et al. (2005). Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circulation Research, 97, 115–124. [DOI] [PubMed] [Google Scholar]
- Krause M, & Gautreau A (2014). Steering cell migration: Lamellipodium dynamics and the regulation of directional persistence. Nature Reviews. Molecular Cell Biology, 15, 577–590. [DOI] [PubMed] [Google Scholar]
- Kwok W, & Clemens MG (2014). Rho-kinase activation contributes to Lps-induced impairment of endothelial nitric oxide synthase activation by endothelin-1 in cultured hepatic sinusoidal endothelial cells. Shock, 42, 554–561. [DOI] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, & Huot J (2007). Endothelial cell migration during angiogenesis. Circulation Research, 100, 782–794. [DOI] [PubMed] [Google Scholar]
- Le Clainche C, Schlaepfer D, Ferrari A, Klingauf M, Grohmanova K, Veligodskiy A, et al. (2007). IQGAP1 stimulates actin assembly through the N-WASP-Arp2/3 pathway. The Journal of Biological Chemistry, 282, 426–435. [DOI] [PubMed] [Google Scholar]
- Leach L, Eaton BM, Westcott ED, & Firth JA (1995). Effect of histamine on endothelial permeability and structure and adhesion molecules of the paracellular junctions of perfused human placental microvessels. Microvascular Research, 50, 323–337. [DOI] [PubMed] [Google Scholar]
- Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, et al. (2009). Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. American Journal of Physiology. Heart and Circulatory Physiology, 296, H33–H42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Sun C, Valentine WJ, Shuyu E, Liu J, Tigyi G, et al. (2009). Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. The Journal of Organic Chemistry, 74, 3192–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R, Verin AD, Black SM, & Catravas JD (2009). Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochemical Pharmacology, 77, 1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Alvarez SE, Milstien S, & Spiegel S (2008). Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Molecular and Cellular Biology, 28, 5687–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, et al. (2009). Coordination of Rho GTPase activities during cell protrusion. Nature, 461, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcola M, & Rodrigues CE (2015). Endothelial progenitor cells in tumor angiogenesis: Another brick in the wall. Stem Cells International, 2015, 832649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli R, Kamei M, Sage PT, Massol R, Varghese L, Sciuto T, et al. (2013). Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier micro-wounds. The Journal of Cell Biology, 201, 449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, & Ware LB (2015). Resolution of alveolar edema in acute respiratory distress syndrome. Physiology and biology. American Journal of Respiratory and Critical Care Medicine, 192, 124–125. [DOI] [PubMed] [Google Scholar]
- McNeill H, Ryan TA, Smith SJ, & Nelson WJ (1993). Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion. The Journal of Cell Biology, 120, 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVerry BJ, & Garcia JG (2005). In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: Mechanistic insights. Cellular Signalling, 17, 131–139. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, & Garcia JG (2004). Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. American Journal of Respiratory and Critical Care Medicine, 170, 987–993. [DOI] [PubMed] [Google Scholar]
- Meliton AY, Meng F, Tian Y, Sarich N, Mutlu GM, Birukova AA, et al. (2015). Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. American Journal of Physiology. Lung Cellular and Molecular Physiology, 308, L550–L562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostmans Y, Cutolo M, Giddelo C, Decuman S, Melsens K, Declercq H, et al. (2017). The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmunity Reviews, 16, 774–786. [DOI] [PubMed] [Google Scholar]
- Mudau M, Genis A, Lochner A, & Strijdom H (2012). Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovascular Journal of Africa, 23, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HC, Hocke AC, Hellwig K, Gutbier B, Peters H, Schonrock SM, et al. (2011). The sphingosine-1 phosphate receptor agonist FTY720 dose dependently affected endothelial integrity in vitro and aggravated ventilator-induced lung injury in mice. Pulmonary Pharmacology & Therapeutics, 24, 377–385. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, et al. (2013). Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. American Journal of Respiratory Cell and Molecular Biology, 49, 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, et al. (2008). Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Critical Care, 12, R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonas S, Miller I, Kawkitinarong K, Chatchavalvanich S, Gorshkova I, Bochkov VN, et al. (2006). Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. American Journal of Respiratory and Critical Care Medicine, 173, 1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima K (2004). Prevention of endothelial cell injury by activated protein C: The molecular mechanism(s) and therapeutic implications. Current Vascular Pharmacology, 2, 125–133. [DOI] [PubMed] [Google Scholar]
- Pabst R, & Tschernig T (2010). Bronchus-associated lymphoid tissue: An entry site for antigens for successful mucosal vaccinations? American Journal of Respiratory Cell and Molecular Biology, 43, 137–141. [DOI] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, et al. (2004). Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. American Journal of Respiratory and Critical Care Medicine, 169, 1245–1251. [DOI] [PubMed] [Google Scholar]
- Pfaendtner J, De La Cruz EM, & Voth GA (2010). Actin filament remodeling by actin depolymerization factor/cofilin. Proceedings of the National Academy of Sciences of the United States of America, 107, 7299–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, & Danuser G (2004). Two distinct actin networks drive the protrusion of migrating cells. Science (New York, N.Y.), 305, 1782–1786. [DOI] [PubMed] [Google Scholar]
- Rotty JD, Wu C, & Bear JE (2013). New insights into the regulation and cellular functions of the ARP2/3 complex. Nature Reviews. Molecular Cell Biology, 14, 7–12. [DOI] [PubMed] [Google Scholar]
- Roviezzo F, Di Lorenzo A, Bucci M, Brancaleone V, Vellecco V, De Nardo M, et al. (2007). Sphingosine-1-phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. American Journal of Respiratory Cell and Molecular Biology, 36, 757–762. [DOI] [PubMed] [Google Scholar]
- Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, et al. (2010). Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. American Journal of Respiratory Cell and Molecular Biology, 43, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, et al. (2003). Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. The Journal of Biological Chemistry, 278, 47281–47290. [DOI] [PubMed] [Google Scholar]
- Schieber M, & Chandel NS (2014). ROS function in redox signaling and oxidative stress. Current Biology, 24, R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel N, Baumer Y, Drenckhahn D, & Waschke J (2009). Lipopolysaccharide-induced endothelial barrier breakdown is cyclic adenosine monophosphate dependent in vivo and in vitro. Critical Care Medicine, 37, 1735–1743. [DOI] [PubMed] [Google Scholar]
- Schlegel N, & Waschke J (2014). cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier—A signaling pathway compromised in inflammation. Cell and Tissue Research, 355, 587–596. [DOI] [PubMed] [Google Scholar]
- Sekine K, Fujishima S, & Aikawa N (2004). Plasma hepatocyte growth factor is increased in early-phase sepsis. Journal of Infection and Chemotherapy, 10, 110–114. [DOI] [PubMed] [Google Scholar]
- Shafique E, Torina A, Reichert K, Colantuono B, Nur N, Zeeshan K, et al. (2017). Mitochondrial redox plays a critical role in the paradoxical effects of NAPDH oxidase-derived ROS on coronary endothelium. Cardiovascular Research, 113, 234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata Y, Birukov KG, Birukova AA, Verin A, & Garcia JG (2003). Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: Role of Src and GIT. The FASEB Journal, 17, 2240–2249. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Camp SM, Sun X, Zhou T, Wang T, & Garcia JG (2015). Sp1-mediated nonmuscle myosin light chain kinase expression and enhanced activity in vascular endothelial growth factor-induced vascular permeability. Pulmonary Circulation, 5, 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, et al. (2009). Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched micro-domains regulates endothelial barrier enhancement by oxidized phospholipids. Circulation Research, 104, 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton PA, Dudek SM, Chiang ET, & Garcia JG (2005). Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. The FASEB Journal, 19, 1646–1656. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, et al. (2010). High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. American Journal of Physiology. Lung Cellular and Molecular Physiology, 299, L639–L651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, et al. (2007). CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. The Journal of Biological Chemistry, 282, 30643–30657. [DOI] [PubMed] [Google Scholar]
- Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M, et al. (2016). Cortactin promotes exosome secretion by controlling branched actin dynamics. The Journal of Cell Biology, 214, 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirker A, Murdoch CE, Protti A, Sawyer GJ, Santos CX, Martin D, et al. (2016). Cell-specific effects of Nox2 on the acute and chronic response to myocardial infarction. Journal of Molecular and Cellular Cardiology, 98, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Stradal T, Vignal E, & Rottner K (2002). The lamellipodium: Where motility begins. Trends in Cell Biology, 12, 112–120. [DOI] [PubMed] [Google Scholar]
- Sun X, Mathew B, Sammani S, Jacobson JR, & Garcia JGN (2017). Simvastatin-induced sphingosine 1-phosphate receptor 1 expression is KLF2-dependent in human lung endothelial cells. Pulmonary Circulation, 7, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadevara V, Fu P, Ebenezer DL, Berdyshev E, Bronova IA, Huang LS, et al. (2018). Sphingolipids in ventilator induced lung injury: Role of sphingosine-1-phosphate lyase. International Journal of Molecular Sciences, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, & Borisy GG (1999). Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. The Journal of Cell Biology, 145, 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepaniak WS, Zhang Y, Hagerty S, Crow MT, Kesari P, Garcia JG, et al. (2008). Sphingosine 1-phosphate rescues canine LPS-induced acute lung injury and alters systemic inflammatory cytokine production in vivo. Translational Research, 152, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabasinezhad M, Samadi N, Ghanbari P, Mohseni M, Saei AA, Sharifi S, et al. (2013). Sphingosin 1-phosphate contributes in tumor progression. Journal of Cancer Research and Therapeutics, 9, 556–563. [DOI] [PubMed] [Google Scholar]
- Takac I, Schroder K, & Brandes RP (2012). The Nox family of NADPH oxidases: Friend or foe of the vascular system? Current Hypertension Reports, 14, 70–78. [DOI] [PubMed] [Google Scholar]
- Tang DG, & Conti CJ (2004). Endothelial cell development, vasculogenesis, angiogenesis, and tumor neovascularization: An update. Seminars in Thrombosis and Hemostasis, 30, 109–117. [DOI] [PubMed] [Google Scholar]
- Ten Klooster JP, Evers EE, Janssen L, Machesky LM, Michiels F, Hordijk P, et al. (2006). Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. The Biochemical Journal, 397, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thennes T, & Mehta D (2012). Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function. Microvascular Research, 83, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Gawlak G, Shah AS, Higginbotham K, Tian X, Kawasaki Y, et al. (2015). Hepatocyte growth factor-induced Asef-IQGAP1 complex controls cytoskeletal remodeling and endothelial barrier. The Journal of Biological Chemistry, 290, 4097–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai FC, & Meyer T (2012). Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Current Biology, 22, 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usatyuk PV, Burns M, Mohan V, Pendyala S, He D, Ebenezer DL, et al. (2013). Coronin 1B regulates S1P-induced human lung endothelial cell chemotaxis: Role of PLD2, protein kinase C and Rac1 signal transduction. PLoS One, 8, e63007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usatyuk PV, Fu P, Mohan V, Epshtein Y, Jacobson JR, Gomez-Cambronero J, et al. (2014). Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in hepatocyte growth factor (HGF)-mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. The Journal of Biological Chemistry, 289, 13476–13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usatyuk PV, Gorshkova IA, He D, Zhao Y, Kalari SK, Garcia JG, et al. (2009). Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. The Journal of Biological Chemistry, 284, 15339–15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usatyuk PV, Kotha SR, Parinandi NL, & Natarajan V (2013). Phospholipase D signaling mediates reactive oxygen species-induced lung endothelial barrier dysfunction. Pulmonary Circulation, 3, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M (2006). Localizing NADPH oxidase-derived ROS. Science’s STKE: Signal Transduction Knowledge Environment, 2006, re8. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, & Urao N (2009). Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxidants & Redox Signaling, 11, 2517–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, & Alexander RW (2005). cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: Role in vascular hypertrophy. Circulation Research, 97, 829–836. [DOI] [PubMed] [Google Scholar]
- van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, & Geelen SP (2005). Plasma vascular endothelial growth factor in severe sepsis. Shock, 23, 35–38. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, & van Hinsbergh VW (2000). Activation of RhoA by thrombin in endothelial hyper-permeability: Role of rho kinase and protein tyrosine kinases. Circulation Research, 87, 335–340. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke E, Mehta D, Minshall R, & Malik AB (2008). Regulation of endothelial junctional permeability. Annals of the New York Academy of Sciences, 1123, 134–145. [DOI] [PubMed] [Google Scholar]
- Waeber C, Blondeau N, & Salomone S (2004). Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors. Drug News & Perspectives, 17, 365–382. [DOI] [PubMed] [Google Scholar]
- Wang L, Bittman R, Garcia JG, & Dudek SM (2015). Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate. Microvascular Research, 99, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chiang ET, Simmons JT, Garcia JG, & Dudek SM (2011). FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. The European Respiratory Journal, 38, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sammani S, Moreno-Vinasco L, Letsiou E, Wang T, Camp SM, et al. (2014). FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury. Critical Care Medicine, 42, e189–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang L, Moreno-Vinasco L, Lang GD, Siegler JH, Mathew B, et al. (2012). Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Particle and Fibre Toxicology, 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Wang S, & Kaibuchi K (2015). IQGAPs as key regulators of actincytoskeleton dynamics. Cell Structure and Function, 40, 69–77. [DOI] [PubMed] [Google Scholar]
- Winkler MS, Nierhaus A, Holzmann M, Mudersbach E, Bauer A, Robbe L, et al. (2015). Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Critical Care, 19, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA Jr., & Terada LS (2005). Subcellular targeting of oxidants during endothelial cell migration. The Journal of Cell Biology, 171, 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Nakagawa S, Horai S, Tanaka K, Deli MA, Yatsuhashi H, et al. (2014). Hepatocyte growth factor enhances the barrier function in primary cultures of rat brain microvascular endothelial cells. Microvascular Research, 92, 41–49. [DOI] [PubMed] [Google Scholar]
- Ye M, Hu D, Tu L, Zhou X, Lu F, Wen B, et al. (2008). Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Investigative Ophthalmology & Visual Science, 49, 497–504. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Cohen M, Addadi L, & Geiger B (2004). Hierarchical assembly of cell–matrix adhesion complexes. Biochemical Society Transactions, 32, 416–420. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. (2016). ROS and ROS-mediated cellular signaling. Oxidative Medicine and Cellular Longevity, 2016, 4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Gorshkova IA, Berdyshev E, He D, Fu P, Ma W, et al. (2011). Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. American Journal of Respiratory Cell and Molecular Biology, 45, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]