Abstract
Osteoarthritis (OA) is a debilitating conditioning with pain as the major clinical symptom. Understanding the mechanisms that drive OA-associated chronic pain is crucial for developing the most effective analgesics. Although the degradation of the joint is the initial trigger for the development of chronic pain, the discordance between radiographic joint damage and the reported pain experience in patients, coupled with clinical features that cannot be explained by purely peripheral mechanisms, suggest there are often other factors at play. Therefore, this study considers the central contributions of chronic pain, using a monoiodoacetate (MIA) model of OA. Particularly, this study explores the functionality of descending controls over the course of the model by assessing diffuse noxious inhibitory controls (DNIC). Early-phase MIA animals have a functional DNIC system, whereas DNIC are abolished in late-phase MIA animals, indicating a dysregulation in descending modulation over the course of the model. In early-phase animals, blocking the actions of spinal α2-adrenergic receptors completely abolishes DNIC, whereas blocking the actions of spinal 5-HT7 receptors only partially decreases the magnitude of DNIC. However, activating the spinal α2-adrenergic or 5-HT7 receptors in late-phase MIA animals restored DNIC-induced neuronal inhibition. This study confirms that descending noradrenergic signaling is crucial for DNIC expression. Furthermore, we suggest a compensatory increase in descending serotonergic inhibition acting at 5-HT7 receptors as the model progresses such that receptor activation is sufficient to override the imbalance in descending controls and mediate neuronal inhibition.
NEW & NOTEWORTHY This study showed that there are both noradrenergic and serotonergic components contributing to the expression of diffuse noxious inhibitory controls (DNIC). Furthermore, although a tonic descending noradrenergic tone is always crucial for the expression of DNIC, variations in descending serotonergic signaling over the course of the model mean this component plays a more vital role in states of sensitization.
Keywords: descending modulation, diffuse noxious inhibitory controls, norepinephrine, serotonin
INTRODUCTION
Osteoarthritis (OA) is the most common rheumatic condition caused by degradation of the synovial joints. Despite pain being the major clinical symptom, current analgesics remain relatively ineffective. This may be due to analgesics principally concentrating on the peripherally driven aspects of joint pain, yet centrally driven aspects also contribute to the pain experience (Wieland et al. 2005). A subset of OA patients develop referred pain at sites distant to initial joint damage or suffer with chronic pain following total knee replacement surgery, indicating that the pain associated with OA cannot be considered purely peripheral and may be attributed to a long-term centrally dysfunctional amplification of the pain response (Malfait and Schnitzer 2013; Wylde et al. 2011).
The continuous barrage of peripheral nociceptive signaling due to joint damage means spinal neurons with joint input can become hyperexcitable, which can subsequently lead to an altered function of supraspinal structures (Schaible 2012). Indeed, in patients with hip OA, an increased activity within the periaqueductal gray (PAG) was found when areas of referred pain were stimulated (Gwilym et al. 2009). Monoaminergic descending controls are coordinated in the brain stem and modulate spinal nociceptive processing. An enhanced descending serotonergic facilitatory drive has been reported in animal models of OA as contributing to the hyperexcitability of spinal neurons (Rahman et al. 2009). Therefore, adaptive changes in the brain stem and descending modulatory pathways are important features to be considered when treating OA-associated chronic pain.
One method for assessing the functionality of descending controls is by measuring diffuse noxious inhibitory controls (DNIC) (Yarnitsky 2015). DNIC are a unique form of endogenous inhibitory control whereby evoked activity of convergent neurons is strongly inhibited by a concurrent noxious stimulus outside of the receptive field (Bannister et al. 2015; Cadden 1993; Le Bars et al. 1979a). Conditioned pain modulation (CPM) is the human counterpart of DNIC and can be assessed in the clinic. DNIC cannot be observed in anesthetized animals with spinal cord transection, and CPM is lost in tetraplegics, indicating that both rely on the activation of supraspinal structures and functional descending controls (Le Bars et al. 1979b; Roby-Brami et al. 1987). Furthermore, CPM measurements in the clinic provide valuable insights into a patient’s physiology, such as their likelihood of developing chronic pain or responding to drugs that restore descending inhibition (Yarnitsky et al. 2008, 2012).
DNIC rely on the conditioning stimulus activating descending noradrenergic inhibitory controls, which activate α2-adrenergic receptors in the spinal cord to mediate neuronal inhibition, because blocking these receptors abolishes DNIC in naive animals (Bannister et al. 2015). There is also a serotonergic component to DNIC, but its role is more difficult to unravel because descending serotonergic signaling mediates both facilitatory and inhibitory actions on nociceptive processing (Bannister et al. 2017). Serotonergic actions at spinal 5-HT3 receptors mediate facilitatory actions, and an enhancement of this pathway contributes to the loss of DNIC in models of nerve injury (Bannister et al. 2015). Yet, increasing the synaptic content of serotonin in the spinal cord with serotonin reuptake inhibitors (SSRIs) in a rat model of neuropathy restored neuronal inhibition induced by DNIC (Bannister et al. 2017). Interestingly, spinal 5-HT7 receptors mediate antinociception, and it was demonstrated to be the activation of this receptor via SSRIs that restored DNIC (Brenchat et al. 2012; Dogrul et al. 2009). Furthermore, an increase in the number of 5-HT7 receptors has been reported in the ipsilateral dorsal horn of neuropathic rats, indicating that sensitizing conditions may regulate serotonergic receptor expression (Brenchat et al. 2010).
We used a monoiodoacetate (MIA) model of OA, because variations in descending monoaminergic controls have been reported as the model progresses (Burnham and Dickenson 2013). We investigated the expression of DNIC in both early and late phases of the MIA model and pharmacologically manipulated the α2-adrenergic and 5-HT7 receptors to better understand their roles in DNIC expression in sensitized states.
METHODS
Animals.
In all experiments, male Sprague-Dawley rats were used. Food and water were provided ab libitum, with cages kept in a 12:12-h light-dark cycle. All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986.
The MIA model.
Male Sprague-Dawley rats (190–210 g for early phase and 120–140 g for late phase) were anesthetized with isoflurane, and arthritis was induced in the left knee with an intra-articular injection of 2 mg of MIA (Sigma) in 25 μl of 0.9% saline. Sham animals received an intra-articular injection of 25 μl of 0.9% saline only.
Electrophysiology.
Electrophysiological experiments were carried out 2–6 days post-MIA injection for early-phase animals and 14–20 days post-MIA injection for late-phase animals as previously described (Urch and Dickenson 2003). Briefly, animals were anesthetized for the duration of the experiment with isoflurane (1.5%) delivered in a gaseous mix of O2 (33%) and N2O (66%). A laminectomy was performed to expose the L4–L5 segments of the spinal cord. Extracellular single unit recordings were made from deep dorsal horn wide dynamic range (WDR) neurons (lamina V–VI) using parylene-coated tungsten electrodes (A-M Systems). All neurons were polymodal WDRs, because they responded to both mechanical and thermal innocuous and noxious stimulations in a graded manner, coding intensity. Data were captured and analyzed by a CED 1401 interface coupled to a computer running Spike2 software (Cambridge Electronic Design; rate functions).
DNIC study design.
First, the preconditioned mechanically evoked neuronal firing rates were quantified in response to 8-, 26-, and 60-g von Frey filament stimulation applied to the hind paw. This was repeated three times to obtain a stable preconditioned response (where all neurons met the inclusion criteria of <10% variation in action potential firing). For the DNIC response, the same von Frey filaments were applied to the hind paw receptive field with a concurrent noxious ear pinch (15.75 × 2.3-mm Bulldog Serrefine; InterFocus, Linton, UK). This trial was repeated, and preconditioned and DNIC responses were calculated as the mean from the two trials. A DNIC response was quantified as an inhibition on mechanically evoked neuronal firing in the presence of the conditioning noxious ear pinch. A 1-min nonstimulation recovery period was allowed between each test, and a 10-min nonstimulation recovery period was allowed between each trial to ensure neuronal responses had returned to baseline.
Drug administration.
First, two DNIC trials were carried out to collect predrug baseline controls. Each individual drug dose was then administered, and the neuronal response was followed for 1 h, with tests carried out at 20 and 40 min (1 neuron per animal). For each time point, another DNIC trial was conducted, which consisted of preconditioned responses to 8-, 26-, and 60-g mechanical stimulations repeated three times to obtain stable responses, followed by a DNIC response with a concurrent noxious ear pinch. For postdrug effects, the maximal changes for preconditioned and DNIC responses are presented in the graphs for Fig. 1–4.
Fig. 1.
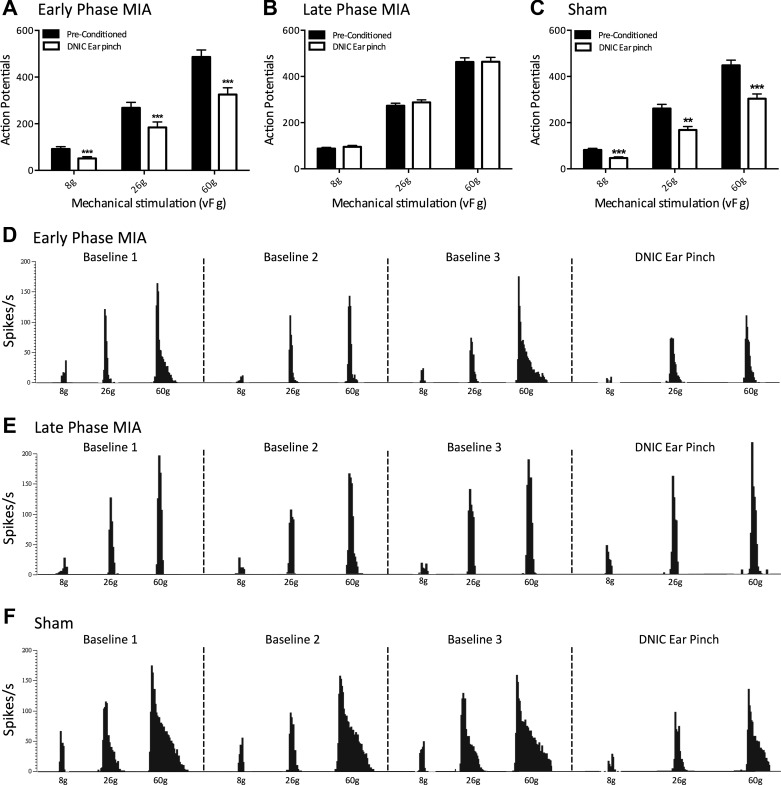
Expression of diffuse noxious inhibitory controls (DNIC) in early- and late-phase monoiodoacetate (MIA) animals and sham controls. A: a conditioning noxious ear pinch produced a significant reduction in mechanically evoked neuronal firing in ipsilateral wide dynamic range (WDR) neurons in early-phase MIA animals (n = 19). B: in late-phase MIA animals, a conditioning noxious ear pinch no longer produces a reduction in mechanically evoked neuronal firing in ipsilateral WDR neurons (n = 42). C: a conditioning noxious ear pinch produced a significant reduction in mechanically evoked neuronal firing in ipsilateral WDR neurons in sham controls (n = 27). D–F: representative tracts from ipsilateral WDR neurons in early-phase MIA (D), late-phase MIA (E), and sham animals (F) showing 3 baseline preconditioned responses and a DNIC response with a concurrent noxious ear pinch. **P < 0.01; ***P < 0.001; two-way ANOVA with Bonferroni correction.
Fig. 4.
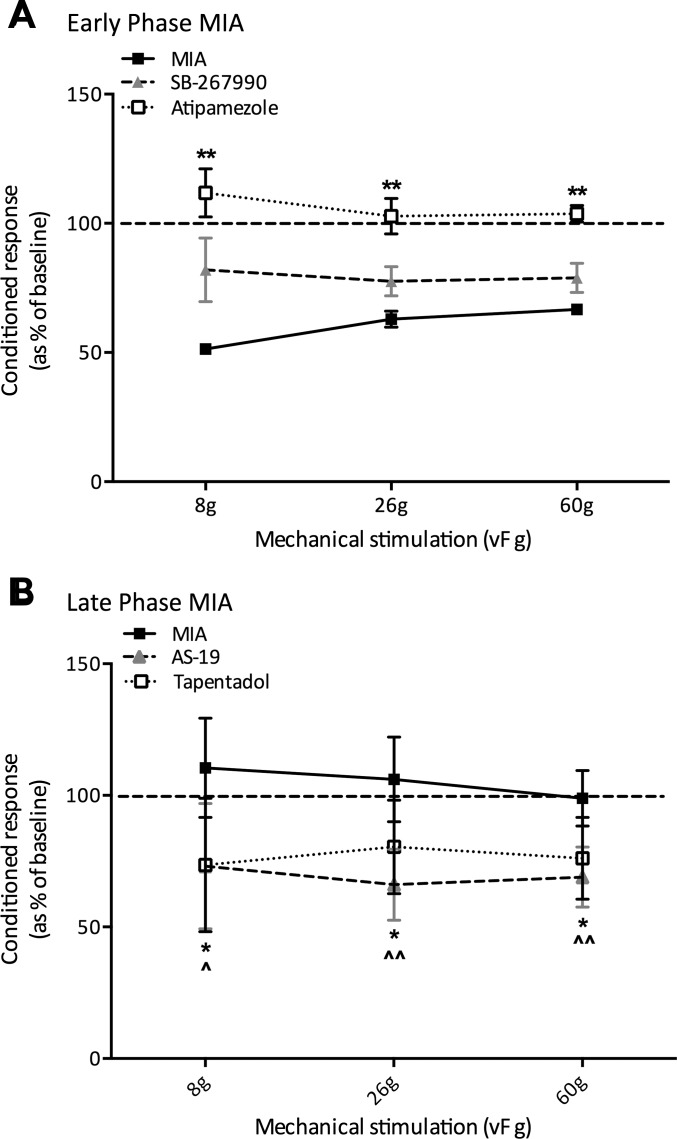
Conditioned responses (as a percentage of the baseline) in early- and late-phase monoiodoacetate (MIA) animals. A: in early-phase MIA animals, the spinal application of atipamezole completely abolished DNIC-induced neuronal inhibition, whereas the spinal application of SB-269970 only partially reversed it (atipamezole, n = 5; SB-269970, n = 6). B: in late-phase MIA animals, both the subcutaneous injection of tapentadol and the spinal application of AS-19 significantly restored neuronal inhibition to levels comparable to that observed in sham controls (tapentadol, n = 6; AS-19, n = 6). *P < 0.05; **P < 0.01; ^P < 0.05; ^^P < 0.01; Kruskal-Wallis.
The 5-HT7 receptor antagonist SB-269970 (Tocris) was dissolved in saline and applied topically to the spinal cord (100 μg/50 μl) of early-phase MIA animals. The α2-adrenoceptor antagonist atipamezole (Sigma) was dissolved in vehicle (97% saline, 2% cremaphor, and 1% DMSO) and applied topically to the spinal cord (100 μg/50 μl) of early-phase MIA animals. Tapentadol, a norepinephrine reuptake inhibitor (NRI) and μ-opioid receptor (MOR) agonist (Grünenthal) was dissolved in saline and delivered via a subcutaneous injection at a dose of 2 mg/kg in late-phase MIA animals. The 5-HT7 receptor agonist AS-19 (Tocris) was dissolved in vehicle (97% saline, 2% cremaphor, and 1% DMSO) and applied topically to the spinal cord (100 μg/50 μl) of late-phase MIA animals.
Quantitative PCR.
Animals were terminally anesthetized with an overdose of isoflurane, and the ipsilateral lumbar dorsal horn and L3–L5 dorsal root ganglia (DRG) were dissected, snap-frozen in liquid nitrogen, and stored at −80°C. RNA was extracted from homogenized tissue using a RNase micro kit (Qiagen). First-strand cDNA synthesis was performed on 500 ng of RNA using a Superscript III reverse transcriptase kit (Invitrogen) according to manufacturer’s instructions with deoxynucleotide triphosphates (Promega) and random primers (Promega). mRNA levels of the α2-adrenergic and 5-HT7 receptors were measured with quantitative PCR using specific primers (Table 1) and LightCycler 480 SYBR Green I master mix (Roche, Welwyn Garden City, UK). The mRNA levels were normalized to GAPDH and expressed relative to sham controls.
Table 1.
Primer sequences
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Source |
|---|---|---|---|
| HTR7 | ACCTGAGGACCACCTATCGT | GGTCAGAGTTTTGTCTTACAGCA | Invitrogen |
| ADRA2 | ACACGGACCTGCTTTGACAT | TATGCTGTTAGGCACAGGGG | Invitrogen |
| GAPDH | CTGCACCACCAACTGCTTAG | TGATGGCATGGACTGTGG | Sigma |
Statistical analysis.
Statistical analyses were performed using SPSS v22 (IBM, Armonk, NY). All data plotted are means ± SE. For electrophysiology, statistical differences in neuronal responses with noxious conditioning ear pinch or following drug application were determined using a two-way repeated-measures ANOVA with Bonferroni post hoc test. For analysis of percentage change for conditioned responses before and after drug application, a Kruskal-Wallis independent samples one-way ANOVA was used. For quantitative PCR, statistical differences in mRNA expression levels between MIA and sham controls were determined with a Kruskal-Wallis independent samples one-way ANOVA. Asterisks in figures denote statistically significantly differences (*P < 0.05, **P < 0.01, ***P < 0.001).
RESULTS
DNIC expression in early- and late-phase MIA animals.
Throughout this study, DNIC was induced by a concurrent noxious ear pinch ipsilateral to the WDR neurons being recorded. The noxious conditioning ear pinch produced a consistent and substantial reduction in mechanically evoked neuronal firing in both early-phase MIA (n = 19) and sham animals (n = 27) (Fig. 1, A and C). The degree of neuronal inhibition induced by the conditioning noxious ear pinch in early-phase animals was 44%, 31%, and 33% for 8-, 26-, and 60-g mechanical stimulations, respectively (Fig. 1A), whereas the degree of neuronal inhibition in sham controls was 45%, 38%, and 34% for 8-, 26-, and 60-g stimulation, respectively (Fig. 1C), indicating that the magnitude of DNIC induced inhibition is comparable between these groups. The significant difference between preconditioned neuronal firing and neuronal firing with a concurrent conditioning ear pinch in early-phase MIA and sham animals confirms the presence of DNIC and functional descending controls in these groups (early-phase MIA: P < 0.001 for all mechanical forces; sham: P < 0.001 for 8 g, P < 0.01 for 26 g, and P < 0.001 for 60 g). On the other hand, the conditioning ear pinch produced no reduction in mechanically evoked neuronal firing in late-phase MIA animals (n = 42), indicating a loss of DNIC and a dysfunctional endogenous inhibitory system (P > 0.05 for all mechanical forces).
Descending noradrenergic controls and the expression of DNIC in MIA animals.
Because it has been demonstrated that DNIC are reliant on descending inhibitory noradrenergic controls acting at α2-adrenergic receptors in the spinal cord, we assessed the consequences of blocking this receptor on DNIC expression in early-phase MIA animals (Bannister et al. 2015, 2017). Following the spinal application of the selective α2-adrenergic receptor antagonist atipamezole, there was no longer a reduction in neuronal firing with a concurrent noxious ear pinch, indicating that blocking spinal noradrenergic actions resulted in a complete loss of DNIC (Fig. 2A; P > 0.05 for all mechanical forces, n = 5). The spinal application of atipamezole also resulted in a facilitated preconditioned response, with a significant increase in neuronal firing in response to mechanical stimulation at 8 (P < 0.01), 26 (P < 0.05), and 60 g (P < 0.05).
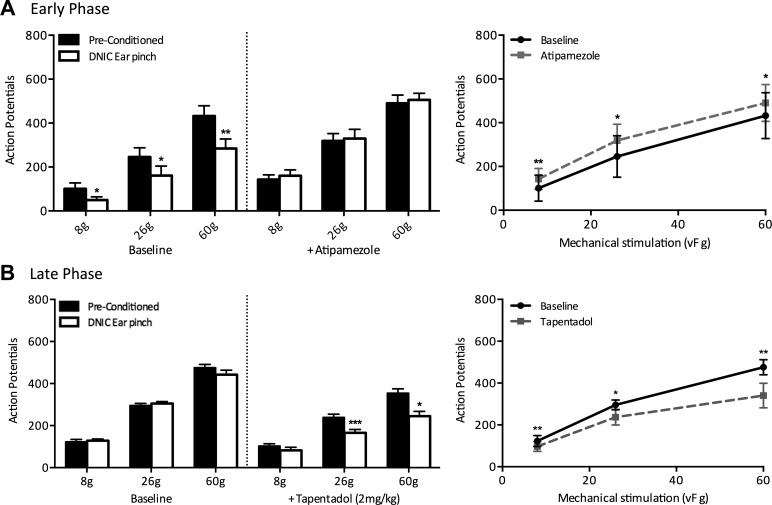
Fig. 2.
The preconditioned and diffuse noxious inhibitory controls (DNIC) response profiles of ipsilateral wide dynamic range neurons before and after spinal application of atipamezole (100 μg/50 μl) or subcutaneous tapentadol (2 mg/kg) in early- and late-phase monoiodoacetate (MIA) animals, respectively. A: in early-phase MIA animals, spinal application of the α2-adrenergic receptor antagonist atipamezole stopped the noxious conditioning ear pinch from producing a reduction in mechanically evoked neuronal firing and also facilitated preconditioned neuronal responses (n = 5). B: in late-phase MIA animals, a subcutaneous injection of tapentadol restored DNIC-induced neuronal inhibition such that there was a significant reduction in mechanically evoked neuronal firing with a concurrent noxious ear pinch (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001; two-way ANOVA with Bonferroni correction.
Having shown that blocking the actions of α2-adrenergic receptors caused a loss of DNIC in early phase animals, we investigated if enhancing this descending inhibitory noradrenergic system could restore DNIC in late phase MIA animals. Tapentadol, which acts as both an NRI and MOR agonist, restored neuronal inhibition induced by a noxious conditioning ear pinch (n = 6; Fig. 2B). Following systemic tapentadol, a concurrent noxious ear pinch resulted in a significant reduction in mechanically evoked neuronal firing, with the magnitude of neuronal inhibition of 21%, 31%, and 31% for mechanical stimulation at 8 (P < 0.01), 26 (P < 0.05), and 60 g (P < 0.01), respectively. Additionally, tapentadol significantly inhibited preconditioned mechanically evoked neuronal firing (8 g, P < 0.01; 26 g, P < 0.05; 60 g, P < 0.01).
The role played by spinal 5-HT7 receptors in DNIC expression in MIA animals.
In early-phase MIA animals, the 5-HT7 receptor antagonist SB-269970 reduced the magnitude of DNIC induced neuronal inhibition in the presence of a concurrent noxious ear pinch but did not abolish it. Indeed, there was no significant difference in neuronal inhibition before and after drug administration (Fig. 4A). Following spinal application of SB-269970, the degree of neuronal inhibition induced by a conditioning ear pinch fell from 45%, 33%, and 32% to 15%, 21%, and 19% for 8-, 26-, and 60-g mechanical stimulations, respectively (n = 6; Fig. 3A). Therefore, the neuronal inhibition was reduced by 12–30% when the actions of serotonin at 5-HT7 receptors in the spinal cord were blocked, yet DNIC remained, because there was still a significant reduction in neuronal firing with a conditioning ear pinch compared with preconditioned responses (26 g, P < 0.05; 60 g, P < 0.05).
Fig. 3.
The preconditioned and diffuse noxious inhibitory controls (DNIC) response profiles of ipsilateral wide dynamic range neurons before and after spinal application of SB-269970 (100 μg/50 μl) or AS-19 (100 μg/50 μl) in early- and late-phase monoiodoacetate (MIA) animals, respectively. A: in early-phase MIA animals, spinal application of the 5-HT7 receptor antagonist SB-269970 reduced the magnitude of neuronal inhibition induced by a conditioning noxious ear pinch, but both before and after application there remained a significant reduction in mechanically evoked neuronal firing with concurrent ear pinch (n = 6). B: in late-phase MIA animals, the spinal application of the 5-HT7 receptor agonist AS-19 restored DNIC-induced neuronal inhibition such that a noxious conditioning ear pinch produced a significant reduction in mechanically evoked neuronal firing (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001; two-way ANOVA with Bonferroni correction.
In late-phase MIA animals, the spinal application of the 5-HT7 agonist AS-19 restored DNIC such that a significant reduction in mechanically evoked neuronal firing was achieved with a concurrent noxious ear pinch in response to 26- and 60-g stimulations (P < 0.05, n = 6; Fig. 3B). Following the spinal application of AS-19, the degree of DNIC induced neuronal inhibition was 28%, 34%, and 32% in response to 8-, 26-, and 60-g stimulations, which is comparable to that we would expect to see in naive animals and sham controls (Bannister et al. 2015). Furthermore, preconditioned mechanically evoked neuronal firing was significantly inhibited by AS-19 (8 g, P < 0.01; 26 g, P < 0.01; 60 g, P < 0.001).
The function of norepinephrine and serotonin in DNIC in the MIA model.
In agreement with previous studies, blocking the actions of spinal α2-adrenergic receptors completely abolished DNIC, whereas enhancing the descending noradrenergic inhibitory system restored DNIC to levels similar to those observed in naive animals, which suggests that a tonic noradrenergic tone is crucial for the expression of DNIC (Bannister et al. 2015, 2017) (Fig. 4). Meanwhile, blocking the actions of spinal 5-HT7 receptors did not abolish DNIC in early-phase MIA animals, where descending controls are functional, likely because the conditioning stimulus still activates descending noradrenergic signaling to mediate neuronal inhibition. However, when DNIC were absent in late-phase MIA animals, activating spinal 5-HT7 receptors was sufficient to restore neuronal inhibition to levels similar to shoe observed in naive animals (Fig. 4). Interestingly, spinal application of AS-19 had limited inhibitory effects on either preconditioned or DNIC responses in sham controls (data not shown), which indicates the function or activation properties of spinal 5-HT7 receptors may be modulated in the sensitized state.
The expression of α2-adrenergic and 5-HT7 receptors in MIA animals.
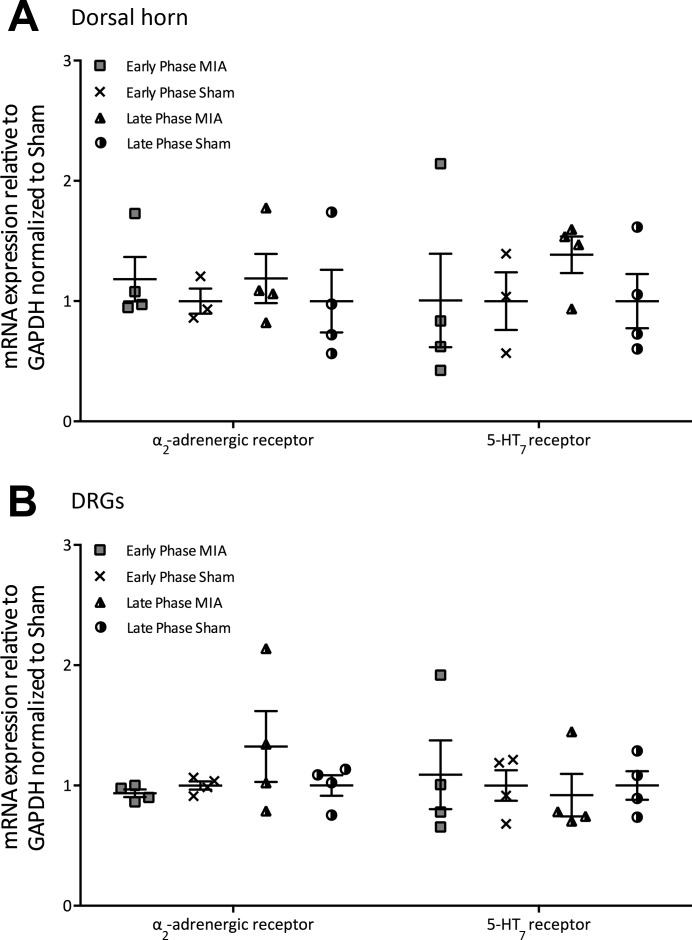
The absence of DNIC in late-phase MIA animals strongly suggests an imbalance in descending inhibitory and facilitatory controls exists, specifically a reduction in noradrenergic controls acting at α2-adrenergic receptors and enhanced serotonergic controls acting at 5-HT3 receptors in the spinal cord. One mechanism through which this imbalance may be mediated could be an increase or decrease in the expression of receptors in the dorsal horn such that the release of monoaminergic neurotransmitters into the spinal cord will subsequently produce larger or smaller effects. However, we found no significant difference in the mRNA expression of α2-adrenergic and 5-HT7 receptors between MIA and sham groups in either the lumbar DRG or dorsal horn, suggesting the imbalance in descending controls is not a due to an up- or downregulation of these receptors (Fig. 5).
Fig. 5.
mRNA expression of the α2-adrenergic and 5-HT7 receptors in the ipsilateral lumbar dorsal horn and dorsal root ganglia (DRG) from early- and late-phase monoiodoacetate (MIA) animals (early phase, n = 4; early phase sham; n = 4; late phase, n = 4; late phase sham, n = 4). A: in the dorsal horn, there was no significant change in mRNA expression levels of the α2-adrenergic or 5-HT7 receptors between groups (Kruskal-Wallis). B: in the L3–L5 DRG, there was no significant change in mRNA expression levels of the α2-adrenergic or 5-HT7 receptors between groups (Kruskal-Wallis).
DISCUSSION
DNIC rely on supraspinal signals, because they are absent following spinal transection, and this descending inhibitory system acts on all activities of spinal and trigeminal WDR neurons (Dickenson and Le Bars 1983; Le Bars et al. 1979a, 1979b). Therefore, this study confirms previous findings that assessing DNIC responses provides valuable insights into the functionality of descending controls. Previous studies demonstrate that the 2-mg MIA model causes mechanical hypersensitivity at the hind paw; therefore, this site of secondary hyperalgesia was stimulated during electrophysiological experiments. The presence of DNIC was confirmed in early-phase MIA animals by a reduction in mechanically evoked neuronal firing in the presence of a noxious conditioning ear pinch, indicating that descending controls remained functional. On the other hand, DNIC were absent in late-phase MIA animals, which is in agreement with preexisting evidence that central changes and variations in descending controls develop as the model progresses (Burnham and Dickenson 2013). First, although NSAIDs prove effective at relieving pain in early-phase MIA animals, as the model progresses animals develop NSAID resistant pain, which reflects the clinical situation, because tackling joint inflammation provides relief in only a portion of patients (Fernihough et al. 2004; Havelin et al. 2016). Furthermore, a reduction in descending noradrenergic inhibition and an enhanced descending serotonergic facilitation acting at spinal 5-HT3 receptors have been demonstrated in the late-phase MIA model (Burnham and Dickenson 2013; Rahman et al. 2009). This dysregulation in descending controls has been proposed to cause a loss of DNIC in an animal model of neuropathy (Bannister et al. 2015). Specifically, the authors revealed DNIC by blocking spinal 5-HT3 receptors with the antagonist ondansetron, and they suggested that an enhancement of descending facilitatory serotonergic actions coupled with a reduced noradrenergic control compromised the ability to induce DNIC (Bannister et al. 2015). A clinical study found patients with severe knee OA pain had significantly less CPM than healthy controls; therefore, it is important to understand the pharmacological basis of DNIC/CPM and how this may be manipulated to restore effective endogenous analgesia in OA patients that do not respond to traditional analgesics (Arendt-Nielsen et al. 2010).
Blocking the actions of spinal α2-adrenergic receptors completely blocked neuronal inhibition induced by a noxious conditioning ear pinch in early-phase MIA animals, indicating DNIC expression is reliant on inhibitory actions of spinal norepinephrine. An enhanced descending noradrenergic inhibitory drive has been reported in early-phase MIA animals, which agrees with our finding that spinal atipamezole significantly facilitated preconditioned mechanically evoked neuronal responses (Burnham and Dickenson 2013). We found that tapentadol restored DNIC in late-phase MIA animals. Tapentadol has a dual mode of action, acting as both an NRI and MOR agonist, but previous studies have demonstrated that the NRI contributions of tapentadol’s actions are predominant in sensitized states (Bee et al. 2011; Schröder et al. 2010; Tzschentke et al. 2007). Therefore, although DNIC are thought to function through a partially opioidergic mechanism, we propose that tapentadol can restore DNIC predominantly through its ability to increase the synaptic content of norepinephrine, which subsequently activates spinal α2-adrenergic receptors to mediate neuronal inhibition (Le Bars et al. 1981). Overall, our study confirms that variations in descending noradrenergic inhibitory tone over the course of the MIA model play a principal role in the resultant expression of DNIC.
DNIC is thought to have a serotonergic component because serotonin transporter gene polymorphisms affect the magnitude of CPM in healthy subjects (Lindstedt et al. 2011). However, the predominant inhibitory or faciliatory actions of serotonin are more complex to unravel because of the myriad of spinal receptors that can be activated. The 5-HT7 receptor has been demonstrated to play an inhibitory role in uninjured animals, because blocking the actions of spinal 5-HT7 receptors blocked the antinociceptive effects observed following both a systemic or RVM microinjection of morphine (Dogrul and Seyrek 2006; Dogrul et al. 2009). We found that although the selective 5-HT7 receptor antagonist SB-269970 reduced the degree of neuronal inhibition induced by a noxious conditioning ear pinch, it did not abolish DNIC expression in early-phase MIA animals. However, in late-phase MIA animals, where DNIC are absent, the spinal application of the selective 5-HT7 receptor agonist AS-19 restored DNIC-induced neuronal inhibition to a degree similar to that observed in sham controls. Interestingly, a previous study demonstrated that spinal application of SSRIs restored DNIC in an animal model of neuropathy through inhibitory actions at spinal 5-HT7 receptors (Bannister et al. 2017). Yet, when the spinal actions of norepinephrine acting at α2-adrenergic receptors were completely blocked with atipamezole, SSRIs could no longer reveal neuronal inhibition (Bannister et al. 2017). These findings agree with our study: in early-phase MIA animals, which are thought to have functional descending noradrenergic inhibitory controls, blocking the actions of 5-HT7 receptors cannot abolish DNIC because the noxious conditioning stimulus still activates descending noradrenergic signaling to activate spinal α2-adrenergic receptors and mediate neuronal inhibition. However, in late-phase MIA animals, where the descending noradrenergic inhibitory system is reported to be reduced, the activation of spinal 5-HT7 receptors is sufficient to override the imbalance in descending controls and produce DNIC-induced neuronal inhibition.
Spinal 5-HT7 receptors are localized to opioidergic and GABAergic interneurons, suggesting the release of serotonin in the spinal cord may mediate neuronal inhibition through the activation of inhibitory interneurons (Brenchat et al. 2010; Viguier et al. 2012). A previous study with the MIA model found that the antinociceptive effects of the serotonin-norepinephrine reuptake inhibitor (SNRI) milnacipran could be preferentially mediated by either noradrenergic or serotonergic signaling depending on the stage of the MIA model, with the late phase acting through an increased descending serotonergic inhibitory drive acting at 5-HT7 receptors (Burnham and Dickenson 2013). The fact that blocking the actions of 5-HT7 receptors only has a small effect in early-phase MIA animals yet can completely restore DNIC in late-phase animals suggests that a compensatory upregulation of 5-HT7 receptor-mediated inhibition may occur when noradrenergic inhibitory controls are reduced in states of sensitization.
One mechanism through which an imbalance in descending controls may be mediated is through alterations in the expression levels of noradrenergic or serotonergic receptors in the dorsal horn such that the release of monoaminergic neurotransmitters into the spinal cord subsequently produces larger or smaller effects. Indeed, an increase in 5-HT7 receptor density was demonstrated in the ipsilateral dorsal horn of mice with partial sciatic nerve ligation, suggesting a compensatory mechanism whereby receptor expression was increased to mediate inhibition (Brenchat et al. 2010). However, we found no significant differences in the mRNA expression of α2-adrenergic or 5-HT7 receptors in the ipsilateral lumbar dorsal horn or DRG in either early- or late-phase MIA animals. The lack of changes in mRNA expression suggests that the imbalance in descending inhibitory and facilitatory controls resulting in a loss of DNIC are not necessarily a result of an altered number of receptors in the spinal cord. Because the monoaminergic descending controls modulate nociceptive transmission at the level of the spinal cord mainly through volume transmission, the most likely mechanism for the imbalance in descending controls is alterations in supraspinal structures and the subsequent decreased release of norepinephrine and an increased release of serotonin from the brain stem (Todd 2010). However, although this study indicates there are no changes in the mRNA receptor expression, other posttranslational changes may occur and influence the binding affinity or downstream signaling of receptors at the level of the spinal cord. It should also be noted that changes in mRNA expression may occur in specific areas of the dorsal horn and that neuronal cells only constitute a portion of the cellular composition of the dorsal horn and DRG, and therefore subtle changes in receptor expression may be missed with this technique (Thakur et al. 2014).
Importantly, our study confirms that DNIC and CPM share similar pharmacology. Clinical studies in patients with diabetic neuropathy have reiterated that CPM rely on noradrenergic and serotonergic signaling. First, similarly to our study, tapentadol activated abolished CPM responses while providing significant pain relief in patients (Niesters et al. 2014). Second, patient’s CPM responses were found to predict the likelihood of them responding to the SNRI duloxetine such that duloxetine was most effective in patients with an inefficient CPM (Yarnitsky et al. 2012). These findings are in agreement with our study, showing that analgesics that activate reduced descending inhibitory pathways may prove the most effective in patients with central changes. Interestingly, SSRIs have proved relatively ineffective in the clinic, which may be due to serotonin mediating both inhibitory and facilitatory actions. Because our study indicates that norepinephrine signaling is critical for DNIC expression, selective NRIs or SNRIs may prove the most effective at restoring endogenous inhibition. Furthermore, these clinical studies highlight how valuable insights into a patient’s physiology can be obtained from assessing CPM responses, and could lead the way toward patient segmentation and a personalized medicine approach such that each patient receives the most appropriate analgesic.
GRANTS
This study was funded by the Wellcome Trust Pain Consortium (102645—Defining pain circuitry in health and disease).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.L., K.B., and A.H.D. conceived and designed research; S.M.L. performed experiments; S.M.L. analyzed data; S.M.L. interpreted results of experiments; S.M.L. prepared figures; S.M.L. drafted manuscript; K.B. edited and revised manuscript; A.H.D. approved final version of manuscript.
REFERENCES
- Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain 149: 573–581, 2010. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Bannister K, Lockwood S, Goncalves L, Patel R, Dickenson AH. An investigation into the inhibitory function of serotonin in diffuse noxious inhibitory controls in the neuropathic rat. Eur J Pain 21: 750–760, 2017. doi: 10.1002/ejp.979. [DOI] [PubMed] [Google Scholar]
- Bannister K, Patel R, Goncalves L, Townson L, Dickenson AH. Diffuse noxious inhibitory controls and nerve injury: restoring an imbalance between descending monoamine inhibitions and facilitations. Pain 156: 1803–1811, 2015. doi: 10.1097/j.pain.0000000000000240. [DOI] [PubMed] [Google Scholar]
- Bee LA, Bannister K, Rahman W, Dickenson AH. Mu-opioid and noradrenergic α2-adrenoceptor contributions to the effects of tapentadol on spinal electrophysiological measures of nociception in nerve-injured rats. Pain 152: 131–139, 2011. doi: 10.1016/j.pain.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Nadal X, Romero L, Ovalle S, Muro A, Sánchez-Arroyos R, Portillo-Salido E, Pujol M, Montero A, Codony X, Burgueño J, Zamanillo D, Hamon M, Maldonado R, Vela JM. Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity. Pain 149: 483–494, 2010. doi: 10.1016/j.pain.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Zamanillo D, Hamon M, Romero L, Vela JM. Role of peripheral versus spinal 5-HT7 receptors in the modulation of pain undersensitizing conditions. Eur J Pain 16: 72–81, 2012. doi: 10.1016/j.ejpain.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Burnham LJ, Dickenson AH. The antinociceptive effect of milnacipran in the monosodium iodoacetate model of osteoarthritis pain and its relation to changes in descending inhibition. J Pharmacol Exp Ther 344: 696–707, 2013. doi: 10.1124/jpet.112.199489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadden SW. The ability of inhibitory controls to ‘switch-off’ activity in dorsal horn convergent neurones in the rat. Brain Res 628: 65–71, 1993. doi: 10.1016/0006-8993(93)90938-J. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Le Bars D. Diffuse noxious inhibitory controls (DNIC) involve trigeminothalamic and spinothalamic neurones in the rat. Exp Brain Res 49: 174–180, 1983. doi: 10.1007/BF00238577. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Ossipov MH, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res 1280: 52–59, 2009. doi: 10.1016/j.brainres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Seyrek M. Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br J Pharmacol 149: 498–505, 2006. doi: 10.1038/sj.bjp.0706854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain 112: 83–93, 2004. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum 61: 1226–1234, 2009. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- Havelin J, Imbert I, Cormier J, Allen J, Porreca F, King T. Central sensitisation and neuropathic features on ongoing pain in a rat model of advanced Osteoarthritis. J Pain 17: 374–382, 2016. doi: 10.1016/j.jpain.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D, Chitour D, Kraus E, Dickenson AH, Besson JM. Effect of naloxone upon diffuse noxious inhibitory controls (DNIC) in the rat. Brain Res 204: 387–402, 1981. doi: 10.1016/0006-8993(81)90597-7. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain 6: 305–327, 1979b. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickeonson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 6: 283–304, 1979a. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- Lindstedt F, Berrebi J, Greayer E, Lonsdorf TB, Schalling M, Ingvar M, Kosek E. Conditioned pain modulation is associated with common polymorphisms in the serotonin transporter gene. PLoS One 6: e18252, 2011. doi: 10.1371/journal.pone.0018252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol 9: 654–664, 2013. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth 113: 148–156, 2014. doi: 10.1093/bja/aeu056. [DOI] [PubMed] [Google Scholar]
- Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain 5: 45, 2009. doi: 10.1186/1744-8069-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B, Willer JC, Le Bars D. An electrophysiological investigation into the pain-relieving effects of heterotopic nociceptive stimuli. Probable involvement of a supraspinal loop. Brain 110: 1497–1508, 1987. doi: 10.1093/brain/110.6.1497. [DOI] [PubMed] [Google Scholar]
- Schaible HG. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep 14: 549–555, 2012. doi: 10.1007/s11926-012-0279-x. [DOI] [PubMed] [Google Scholar]
- Schröder W, Vry JD, Tzschentke TM, Jahnel U, Christoph T. Differential contribution of opioid and noradrenergic mechanisms of tapentadol in rat models of nociceptive and neuropathic pain. Eur J Pain 14: 814–821, 2010. doi: 10.1016/j.ejpain.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Thakur M, Crow M, Richards N, Davey GIJ, Levine E, Kelleher JH, Agley CC, Denk F, Harridge SD, McMahon SB. Defining the nociceptor transcriptome. Front Mol Neurosci 7: 1–11, 2014. doi: 10.3389/fnmol.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11: 823–836, 2010. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Christoph T, Kögel B, Schiene K, Hennies HH, Englberger W, Haurand M, Jahnel U, Cremers TI, Friderichs E, De Vry J. (−)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel μ-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 323: 265–276, 2007. doi: 10.1124/jpet.107.126052. [DOI] [PubMed] [Google Scholar]
- Urch CE, Dickenson AH. In vivo single unit extracellular recordings from spinal cord neurones of rats. Brain Res Brain Res Protoc 12: 26–34, 2003. doi: 10.1016/S1385-299X(03)00068-0. [DOI] [PubMed] [Google Scholar]
- Viguier F, Michot B, Kayser V, Bernard JF, Vela JM, Hamon M, Bourgoin S. GABA, but not opioids, mediates the anti-hyperalgesic effects of 5-HT7 receptor activation in rats suffering from neuropathic pain. Neuropharmacology 63: 1093–1106, 2012. doi: 10.1016/j.neuropharm.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis—an untreatable disease? Nat Rev Drug Discov 4: 331–344, 2005. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 152: 566–572, 2011. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 156, Suppl 1: S24–S31, 2015. doi: 10.1097/01.j.pain.0000460343.46847.58. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 138: 22–28, 2008. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 153: 1193–1198, 2012. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]