Abstract
Transient receptor potential vanilloid type 1 (TRPV1) is a ligand-gated ion channel expressed in the peripheral and central nervous systems. TRPV1-dependent mechanisms take part in a wide range of physiological and pathophysiological pathways including the regulation of homeostatic functions. TRPV1 expression in the hypothalamus has been described as well as evidence that TRPV1-dependent excitatory inputs to hypothalamic preautonomic neurons are diminished in diabetic conditions. Here we aimed to determine the functional expression of TRPV1 in two hypothalamic nuclei known to be involved in the central control of metabolism and to test the hypothesis that TRPV1-expressing neurons receive TRPV1-expressing inputs. A mouse model (TRPV1Cre/tdTom) was generated to identify TRPV1-expressing cells and determine the cellular properties of TRPV1-expressing neurons in adult mice. Our study demonstrated the functional expression of TRPV1 in the dorsomedial hypothalamic nucleus and paraventricular nucleus in adult mice. Our findings revealed that a subset of TRPV1Cre/tdTom neurons receive TRPV1-expressing excitatory inputs, indicating direct interaction between TRPV1-expressing neurons. In addition, astrocytes likely play a role in the modulation of TRPV1-expressing neurons. In summary, this study identified specific hypothalamic regions where TRPV1 is expressed and functional in adult mice and the existence of direct connections between TRPV1Cre/tdTom neurons.
NEW & NOTEWORTHY Transient receptor potential vanilloid type 1 (TRPV1) is expressed in the hypothalamus, and TRPV1-dependent regulation of preautonomic neurons is decreased in hyperglycemic conditions. Our study demonstrated functional expression of TRPV1 in two hypothalamic nuclei involved in the control of energy homeostasis. Our results also revealed that a subset of TRPV1-expressing neurons receive TRPV1-expressing excitatory inputs. These findings suggest direct interaction between TRPV1-expressing neurons.
Keywords: capsaicin, dorsomedial hypothalamic nucleus, paraventricular nucleus of the hypothalamus, patch-clamp, TRPV1
INTRODUCTION
Transient receptor potential vanilloid type 1 (TRPV1), a member of the transient receptor potential superfamily and the first identified member of the vanilloid subfamily, is a ligand-gated nonselective cation channel (Caterina et al. 1997; Szallasi et al. 2007; Tominaga and Tominaga 2005). TRPV1 plays important roles in inflammation, weight control, energy homeostasis, and the development and progression of diabetes mellitus (Derbenev and Zsombok 2016; Kawada et al. 1986; Razavi et al. 2006; Suri and Szallasi 2008; Tsui et al. 2007, 2011; Wang et al. 2012; Zhang et al. 2007; Zsombok and Derbenev 2016). TRPV1 expression has been shown in multiple tissues and organs including those involved in metabolism (Akiba et al. 2004; Baboota et al. 2014; Gram et al. 2007; Razavi et al. 2006).
Initially, widespread TRPV1 expression was demonstrated in the hypothalamus and brain stem (Cristino et al. 2006; Mezey et al. 2000), brain areas known to govern homeostatic functions. Electrophysiological studies demonstrated that TRPV1 modulates neurotransmission by increasing excitatory neurotransmitter release in the hypothalamus (Boychuk et al. 2013; Li et al. 2004) or enhancing the frequency of both excitatory (Peters et al. 2010) and inhibitory (Derbenev et al. 2006) postsynaptic currents in the brain stem. TRPV1-dependent control of preautonomic neurons was also demonstrated in the brain stem and hypothalamus (Gao et al. 2012; Zsombok et al. 2011, 2014). Furthermore, the TRPV1-dependent regulation of excitatory neurotransmission was diminished in hyperglycemic conditions (Gao et al. 2012, 2017; Zsombok et al. 2011), which suggests altered autonomic circuits in diabetes mellitus. These initial studies established TRPV1 expression in specific brain regions and revealed a functional role for TRPV1 in the central nervous system. On the other hand, recent reports by Cavanaugh and colleagues demonstrated highly restricted distribution of TRPV1 in the adult central nervous system and suggested developmental downregulation of TRPV1 (Cavanaugh et al. 2011a, 2011b).
In this study, a mouse model with an in vitro reporter assay system (TRPV1Cre/tdTom) was generated to identify TRPV1-expressing neurons, and we tested the hypothesis that TRPV1-expressing neurons receive TRPV1-expressing inputs in adult mice. TRPV1Cre/tdTom neurons were identified in the hypothalamus including the paraventricular nucleus of the hypothalamus (PVN), dorsomedial hypothalamic nucleus (DMH), lateral hypothalamus, and posterior hypothalamus. Whole cell patch-clamp recordings were conducted in TRPV1Cre/tdTom neurons in the DMH and PVN to establish their cellular properties and determine their synaptic response to a potent exogenous agonist. Our study demonstrated functional expression of TRPV1 in the PVN and DMH in adult mice and revealed functional interaction between TRPV1-expressing neurons.
MATERIALS AND METHODS
Animals.
TRPV1Cre [B6.129-Trpv1tm1(cre)Bbm/J; Jackson Laboratories no. 017769] and tdTomato [B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; Jackson Laboratories no. 007914] mice were crossed to generate reporter mice, which express red fluorescence (tdTomato) in cells expressing TRPV1 (TRPV1Cre/tdTom). Adult male and female TRPV1Cre/tdTom mice (10–30 wk old) were used as specified in the experimental settings. Experiments were performed according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by Tulane University’s Institutional Animal Care and Use Committee.
Genotyping.
Genotyping was performed by PCR on DNA extracted from tail snips from breeding parents (TRPV1Cre homozygous, which expresses Cre recombinase only in TRPV1-expressing cells, and tdTomato homozygous) with the following primers: trpv1: forward primer: GCG GTC TGG CAG TAA AAA CTA TC; reverse primer: GTG AAA CAG CAT TGC TGT CAC TT and tdTomato: forward primer: CTG TTC CTG TAC GGC ATG G; reverse primer: GGC ATT AAA GCA GCG TAT CC. The first-generation mice expressed red fluorescence in cells expressing TRPV1 receptors and were used for experiments. Thus all mice used in the experiments had both TRPV1Cre (heterozygous) and tdTomato (heterozygous) genes.
Brain injections.
Male and female TRPV1Cre and tdTomato mice were injected with an adeno-associated virus (AAV) that expresses a red fluorescent protein (mCherry) under the control of human synapsin 1 promoter in a Cre-dependent manner (AAV8-hSyn-DIO-mCherry, a gift from Bryan Roth, Addgene viral prep no. 50459-AAV8) to determine the distribution of TRPV1-expressing neurons in adult mice. Each mouse was anesthetized with isoflurane and placed into a stereotaxic apparatus (Stoelting). Coordinates were chosen to cover the area where neurons were recorded during the study. Glass micropipettes connected to a nanoinjector (Nanojet III; Drummond) were used to bilaterally inject the virus (3 × 200 nl, 1 nl/s) 1.1 ± 0.5 mm posterior, 0.25 ± 0.05 mm lateral, and 5 mm ventral compared with bregma. After injection, the micropipette remained in place for an additional 7 min to prevent backflow. Three weeks after the viral injections, mice were anesthetized and transcardially perfused with 0.01 M phosphate-buffered saline (PBS) and then with 4% paraformaldehyde. Brains were processed, cryostat sections (50 µm) were prepared, and fluorescent images were taken (Olympus BX51, Nikon Eclipse Ti2).
Gene expression with digital droplet PCR.
To determine that tdTomato-positive neurons in the TRPV1Cre/tdTom mice express TRPV1, droplet digital PCR (ddPCR) was used to detect target gene expression in microdissected tissue and in single cells (Faragó et al. 2013). To detect TRPV1 mRNA, the hypothalamus and cortex were microdissected from male TRPV1Cre/tdTom mice (n = 2; 18 and 22 wk old) and stored in RNAlater (Qiagen). Total RNA was extracted with an RNeasy Mini Kit (Qiagen). After quantification of the isolated RNA, 10 ng of total RNA was used in each ddPCR reaction. The ddPCR reactions were prepared with the One-Step RT-ddPCR Advanced Kit for Probes (Taqman PCR system; Bio-Rad) and specific primers and a probe for mouse TRPV1 mRNA as follows: forward primer, 5′-TGT CTT CAT CAT CCT GTT AC-3′; reverse primer, 5′-GGA AAC TCT TCT CTG TAT CC-3′; probe, 5′/6-FAM/CTG CTC AAC ATG CTC ATT GC/BHQ1/3′. Subsequently, ddPCR was performed with the QX200 ddPCR system (Bio-Rad). The thermal profile used in the ddPCR reaction consisted of 60 min at 45°C, 10 min at 95°C, 40 cycles of 30 s at 95°C and 1 min at 54°C, 10 min at 98°C, and holding at 4°C.
In addition, TRPV1 mRNA expression was evaluated in single cells with ddPCR. Single-cell cytoplasm samples were collected from six tdTomato-positive and two non-tdTomato neurons located in the PVN and DMH of TRPV1Cre/tdTom mice. Collection of cytoplasm was performed with the same setup and methods described for whole cell patch-clamp recordings (see below). Neurons were patched with glass pipettes filled with Cs-gluconate solution. Whole cell configuration was maintained for 5 min before the cytoplasm was collected, and then the electrode was rapidly removed, the tip of the glass pipette was broken at the bottom of an Eppendorf tube containing 100 µl of lysis buffer containing 0.7% β-mercaptoethanol (Absolutely RNA Nanoprep Kit; Agilent), and the solution containing the cytoplasm was pushed out from the recording pipette. Samples were stored at −80°C until processing. Total RNA in these collected single-cell cytoplasm samples were purified according to RNA extracting kit instructions (Agilent). In addition to these RNA samples from single cells, diluted total RNA isolated from hypothalamus was used as positive control and water as a no-template control. The SuperScript III First Strand kit (Invitrogen) was used to synthetize cDNAs (total 20 µl). In the reactions to create cDNAs both 2.5 nM oligo(dT) primer and 2.5 ng/µl random hexamer primer were used. TRPV1 cDNA in 12 µl of cDNA library was preamplified with PreAmp Supermix (SsoAdvanced) containing the TRPV1 primers described above. The thermal profile used in the preamplification of TRPV1 cDNA consisted of 3 min at 95°C, 15 cycles of 15 s at 95°C and 4 min at 54°C, and holding at 4°C. The PCR products containing preamplified TRPV1 cDNA were diluted with water to 1:100, and 5 µl of the diluted PCR products was used in ddPCR as described above.
Brain slice preparation.
Acute brain slices were made as described previously (Gao et al. 2012; Jiang et al. 2013). After anesthesia with isoflurane, the brain was removed and immersed in ice-cold oxygenated artificial cerebrospinal fluid containing the following (in mM): 124 NaCl, 26 NaHCO3, 1.4 NaH2PO4, 11 d-glucose, 3 KCl, 1.3 MgCl2, and 1.5 CaCl2 (pH 7.3–7.4). Transverse hypothalamic slices containing the PVN or DMH (300 μm) were made with a vibrating microtome. The slices were stored in a holding chamber at 34–36°C and then transferred to a recording chamber mounted on a fixed stage under an upright microscope (Nikon FN1).
Whole cell patch-clamp recordings.
Whole cell patch-clamp recordings from TRPV1Cre/tdTom neurons in the DMH and PVN were performed at 34–36°C under ×40 water-immersion objective (NA = 0.8). Neurons were identified by their red fluorescence, and infrared-differential interference contrast optics was used to target specific cells. For whole cell patch-clamp recordings, electrodes (4–6 MΩ) were filled with a solution containing the following (in mM): 135 K+ or Cs+ gluconate, 10 HEPES, 5 EGTA, 1 NaCl, 1 MgCl2, 1 CaCl2, 3 KOH or CsOH, and 2–3 Mg-ATP, with 0.1% biocytin or 0.1% Neurobiotin 350 (pH 7.3–7.4). Electrophysiological signals were recorded with an Axoclamp 700B amplifier (Molecular Devices) and acquired by pCLAMP (Molecular Devices). Inhibitory postsynaptic currents (IPSCs) were recorded at 0 mV and excitatory postsynaptic currents (EPSCs) at −60 mV. Bath application of tetrodotoxin (TTX, 1 μM; Tocris Bioscience) was used to block action potentials and monitor miniature IPSCs (mIPSCs) and EPSCs (mEPSCs). Capsaicin (1 µM; Tocris Bioscience) was used to activate TRPV1. Brain slices were perfused with oxygenated artificial cerebrospinal fluid with or without capsaicin, which was bath applied for 10 min, and continuous recordings were conducted. The effect of capsaicin was analyzed in a 3-min period (between 4 and 7 min) after the drug reached the chamber. This time frame was based on our previous studies demonstrating that the peak response of capsaicin is observed within this time period (Gao et al. 2012). A TRPV1 antagonist, 5′-iodoresiniferatoxin (5′-IRTX, 1 µM; Tocris Bioscience) was used to prevent the effect of capsaicin. Activity of astrocytes was blocked with the glia-specific Krebs cycle inhibitor fluorocitrate (FC, 100 µM; Sigma-Aldrich). FC was prepared as described previously (Paulsen et al. 1987), and slices were incubated with FC at least 2 h before the recording. Synaptic currents and action potentials were analyzed off-line with pCLAMP or Mini Analysis (Synaptosoft). Amplitude and area detection threshold were set as three times the root mean square noise with Mini Analysis. After the software-based automatic detection, the events were carefully reviewed and manually selected. Input resistance was calculated from current steps. The threshold was computed with Clampfit by averaging 10 action potentials. The derivative of the average trace was used to measure the maximum speed of depolarization (mV/s). The threshold time point was set to 5% of maximum speed of depolarization, and the threshold of action potential was determined based on this time.
Visualization of recorded cells.
After recordings, neurons filled with Neurobiotin 350 (Vector Laboratories) or biocytin were fixed in paraformaldehyde (4%) and stored at 4°C. After extensive rinse in PBS (0.01 M), slices with biocytin-filled neurons were incubated for 4 h in a solution containing PBS, AMCA Avidin D (1:200), and Triton X-100 (1%) at room temperature. Rinsed slices were mounted on glass slides and coverslipped with Vectashield (Vector Laboratories). Pictures were taken with a fluorescence microscope (Olympus BX51).
Statistical analysis.
Continuous recordings were conducted, and 3-min periods were analyzed with Mini Analysis to measure peak amplitude, frequency, and baseline. Effects of capsaicin on the frequency of mEPSCs and mIPSCs in individual neurons were determined by Kolmogorov-Smirnov test on interevent interval values. Numbers are reported as means ± SE. Statistical analysis was performed with GraphPad Prism 7 software. Kolmogorov-Smirnov test with Dallal-Wilkinson-Lillie for P value was used to test normality of value distribution. Paired and unpaired t-tests were used to compare two groups with Gaussian-distributed values, whereas non-Gaussian values were tested with a Mann-Whitney test or a Wilcoxon test. Comparison of more than two groups with non-Gaussian distribution was conducted by Friedman test with post hoc Dunn’s multiple comparisons test, whereas groups with Gaussian distribution were analyzed by multiple comparisons with post hoc Tukey’s test based on one-way ANOVA. A two-tailed P < 0.05 was considered significant.
RESULTS
Localization of TRPV1-expressing neurons in hypothalamus.
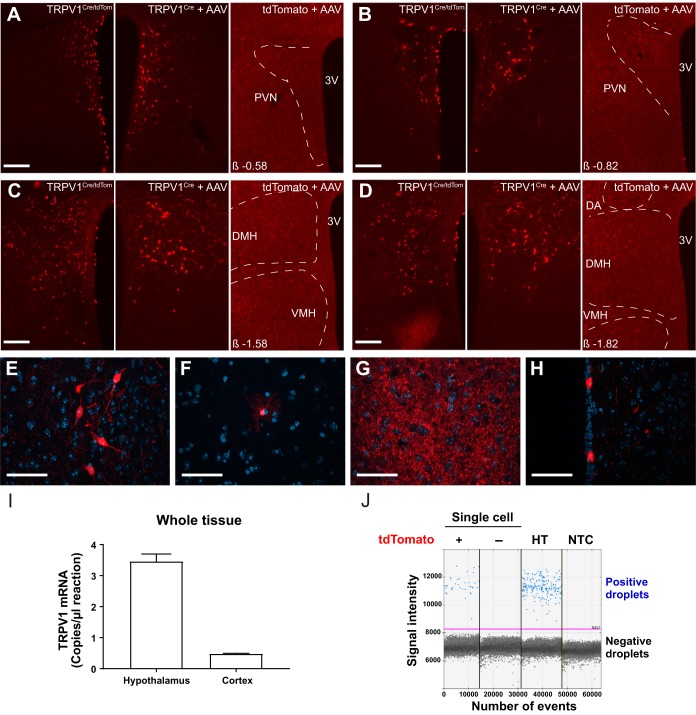
Representative examples of the distribution of TRPV1-expressing neurons within the PVN and DMH are shown in Fig. 1. Strong red fluorescence signal by tdTomato expression reporting TRPV1 expression was observed in the anterior hypothalamic nuclei, DMH (Fig. 1, C and D), and posterior hypothalamus. Moderate signal was identified in the PVN (Fig. 1, A and B) and lateral hypothalamus, whereas sparse expression was noted in the arcuate nucleus. Several cell types known to express TRPV1 were identified according to their morphology, including neurons (Fig. 1E), astrocyte-like cells (Fig. 1F), and ependymal-like cells lining the third ventricle (Fig. 1H) (Jo et al. 2013; Mannari et al. 2013). In addition, prominent red fluorescence labeling indicating a dense network of TRPV1-expressing processes was observed in the ventromedial hypothalamic nucleus (Fig. 1G), with few identifiable cell bodies.
Fig. 1.
Expression of transient receptor potential vanilloid type 1 (TRPV1) in the dorsomedial hypothalamic nucleus (DMH) and the paraventricular nucleus of the hypothalamus (PVN). A–D: representative images demonstrate TRPV1-expressing neurons in PVN (A and B) and DMH (C and D) in TRPV1Cre/tdTom mice (left), in TRPV1Cre mice injected with a Cre-dependent adeno-associated virus (AAV) construct (center), and in control tdTomato mice injected with the same viral construct (right). E–H: representative images show TRPV1 expression in neurons (E) and astrocyte-like cells (F) in the DMH, dense processes in the ventromedial hypothalamus (G), and ependymal-like cells lining the third ventricle (H). I: TRPV1 mRNA in the hypothalamus and cortex in adult mice. J: expression of TRPV1 in single cells. Droplet digital PCR was used to reveal TRPV1 expression in single cells. Positive droplets containing a single copy of TRPV1 cDNA (blue dots) were identified in tdTomato-expressing neurons and in diluted hypothalamic mRNA (HT; positive control), which demonstrates TRPV1 expression in tdTomato fluorescent neurons. There was no positive droplet in tdTomato-negative neurons or in the no-template control (NTC; negative control). Negative droplets (black dots) are droplets that do not contain TRPV1 cDNA. DA, dorsal hypothalamic area; VMH, ventromedial hypothalamic nucleus; 3V, 3rd ventricle. β, distance from bregma; tdTomato +, tdTomato-positive neuron; tdTomato, nonfluorescent neuron. Scale bars: 100 µm (A–D), 50 µm (E–H).
To confirm that tdTomato is a reliable marker for TRPV1 expression, TRPV1Cre mice at the age when the electrophysiological recordings were performed were injected with a Cre-dependent viral construct in the DMH and PVN (n = 6; 20- to 23-wk-old male and female mice). Strong fluorescent labeling was identified in TRPV1Cre mice, whereas there was no labeling in the control tdTomato mice (n = 2; 32-wk-old male mice), which received injection with the same Cre-dependent viral construct (Fig. 1, A–D). The distribution and amount of TRPV1-expressing hypothalamic cells were comparable between the virally injected TRPV1Cre mice and TRPV1Cre/tdTom-expressing mice (Fig. 1, A–D), suggesting that tdTomato-positive cells express TRPV1.
Next, TRPV1 mRNA was detected in hypothalamic tissue. Higher expression of TRPV1 mRNA was observed in the hypothalamus compared with the cortex (Fig. 1I). In addition, single-cell ddPCR analysis of TRPV1Cre/tdTom neurons in the DMH and PVN showed positive droplets in tdTomato-positive neurons as well as in diluted hypothalamic tissues (positive control), which indicates that TRPV1 is expressed in TRPV1Cre/tdTom neurons (Fig. 1J). In the case of tdTomato-positive neurons, TRPV1 mRNA expression was detected in five of six TRPV1Cre/tdTom neurons. There were only negative droplets in tdTomato-negative neurons and in the no-template control (negative control). Together, these findings indicate that TRPV1 is expressed in tdTomato-positive neurons in the hypothalamus of adult mice.
Membrane properties of TRPV1-expressing neurons in DMH and PVN.
Previous studies suggested that TRPV1 in the central nervous system is involved in the control of excitatory neurotransmission in nuclei involved in the regulation of metabolism (Boychuk et al. 2013; Gao et al. 2012). Therefore, we revealed the cellular properties of TRPV1Cre/tdTom neurons in the DMH and PVN, two nuclei that are well known to contribute to the regulation of homeostatic functions. Whole cell patch-clamp recordings were conducted to determine the membrane properties of TRPV1Cre/tdTom neurons in the DMH and PVN of male mice. TRPV1-expressing neurons were identified by their red fluorescence. Recordings were performed throughout the DMH and PVN, including a variety of subnuclei (Fig. 2B), and post hoc visualization of the recorded neurons confirmed that the recordings were made from TRPV1-expressing neurons (Fig. 2A).
Fig. 2.
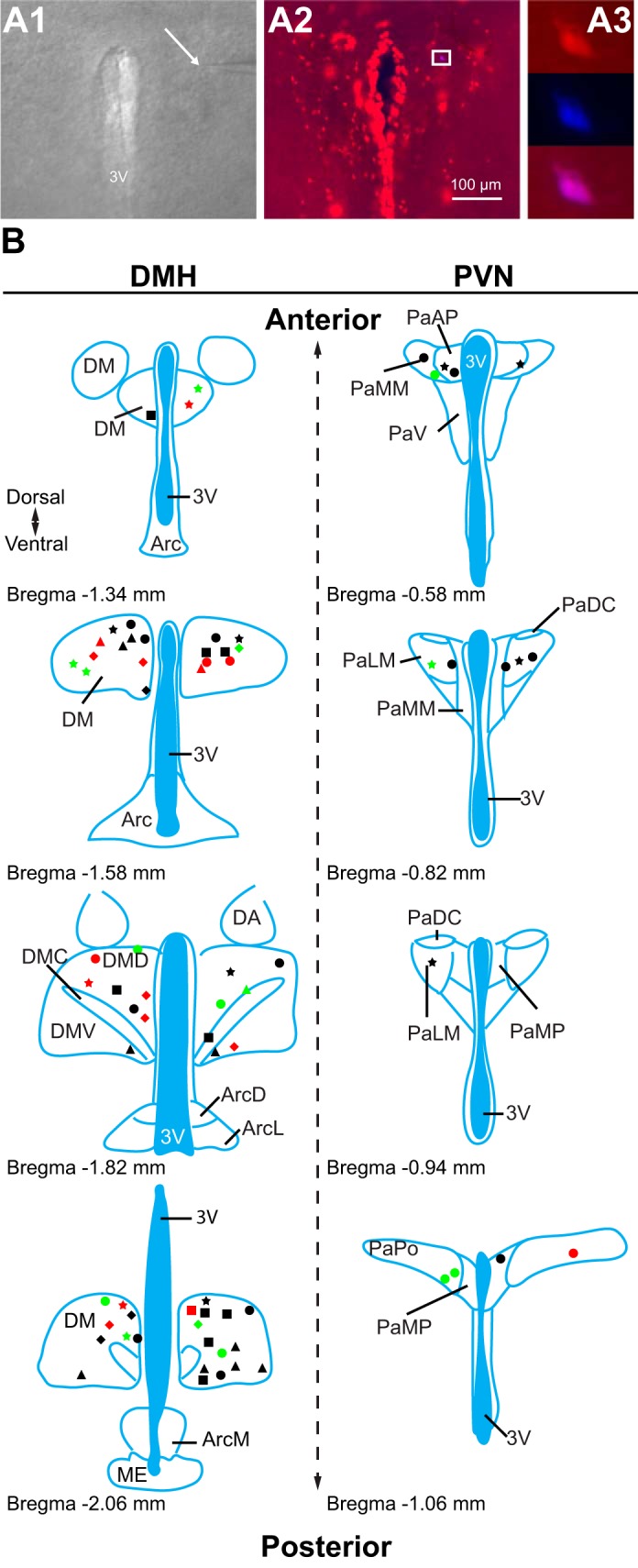
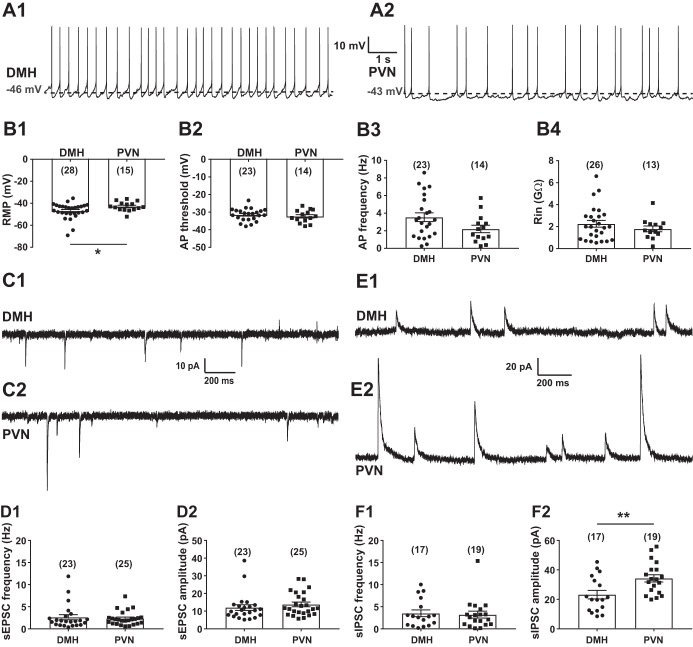
Location of recorded transient receptor potential vanilloid type 1 (TRPV1)-expressing neurons. A: patch-clamp recordings were conducted from TRPV1-expressing neurons. A1: differential interference contrast image during patch-clamp recording (×4). Arrow points to the tip of a recording pipette. A2: TRPV1-expressing neurons in the paraventricular nucleus of the hypothalamus (PVN; red, ×10). A3: enlarged view of boxed area shown in A2 (×40) illustrates a recorded TRPV1-expressing neuron (red) filled with Neurobiotin 350 (blue). B: schematic illustrations of the recorded TRPV1Cre/tdTom neurons in the dorsomedial hypothalamic nucleus (DMH) and the PVN. Each shape represents a different experimental setting as follows. In male mice: circle, miniature excitatory postsynaptic currents (mEPSCs) + capsaicin (Cap); square, mEPSCs +5′-iodoresiniferatoxin (5′-IRTX) + Cap; triangle, mEPSC + fluorocitrate (FC) + Cap; star, mIPSCs + Cap; in female mice: diamond, mEPSCs + Cap. Red indicates the location of neurons that had increased frequency after Cap application, green indicates the location of neurons that had decreased frequency, and black indicates the location of neurons not responding to Cap. 3V, 3rd ventricle; DM, DMH; DMD, dorsal part of DMH; DMC, compact part of DMH; DMV, ventral part of DMH; Arc, arcuate nucleus; ArcD, dorsal part of Arc; ArcL, lateral part of Arc; ArcM, medial part of Arc; ME, median eminence; DA, dorsal hypothalamic area; Pa, PVN; PaAP, anterior PVN; PaMM, medial magnocellular PVN; PaV, ventral PVN; PaDC, dorsal cap of PVN; PaLM, lateral PVN; PaMP, medial PVN; PaPO, posterior PVN. Schematics are based on Franklin and Paxinos (2007).
In general, the majority of recorded TRPV1-expressing neurons had spontaneous firing in both the DMH (23 of 28; n = 14 mice) and the PVN (14 of 15; n = 11 mice) (Fig. 3A). The resting membrane potential of TRPV1-expressing neurons in the DMH was significantly different from the resting membrane potential of TRPV1Cre/tdTom neurons in the PVN (−47.00 ± 1.35 mV vs. −42.88 ± 1.09 mV; Mann-Whitney, P = 0.048; Fig. 3B1). Although TRPV1-expressing neurons in the DMH were more hyperpolarized, their threshold for action potentials and firing rates were not significantly different from TRPV1Cre/tdTom neurons in the PVN [−31.49 ± 0.74 mV vs. −32.41 ± 0.96 mV (unpaired t-test, P = 0.45) and 3.54 ± 0.50 Hz vs. 2.21 ± 0.43 Hz (unpaired t-test, P = 0.072); Fig. 3, B2 and B3). The input resistance of TRPV1-expressing neurons was also similar in these brain areas (2.26 ± 0.31 GΩ vs. 1.80 ± 0.26 GΩ; unpaired t-test, P = 0.33; Fig. 3B4).
Fig. 3.
Basic cellular properties of transient receptor potential vanilloid type 1 (TRPV1)-expressing neurons in the dorsomedial hypothalamic nucleus (DMH) and the paraventricular nucleus of the hypothalamus (PVN). A: representative recordings from TRPV1-expressing neurons in the DMH (A1) and the PVN (A2). B: cellular properties of TRPV1-expressing neurons in the DMH and the PVN. B1: resting membrane potential (RMP). B2: threshold of action potentials (APs). B3: frequency of APs. B4: input resistance (Rin). C: representative recordings of spontaneous excitatory postsynaptic currents (sEPSCs) at holding potential of −60 mV in the DMH (C1) and the PVN (C2). D: frequency (D1) and amplitude (D2) of sEPSCs in TRPV1Cre/tdTom neurons in the DMH and the PVN. E: representative recordings of spontaneous inhibitory postsynaptic currents (sIPSCs) at holding potential of 0 mV in the DMH (E1) and the PVN (E2). F: frequency (F1) and amplitude (F2) of sIPSCs in TRPV1Cre/tdTom neurons in the DMH and the PVN. Numbers of recorded cells are shown in parentheses. *Significance (P < 0.05); **significance (P < 0.01).
Synaptic currents of TRPV1-expressing neurons in DMH and PVN.
EPSCs and IPSCs to TRPV1-expressing neurons in male mice were revealed in voltage-clamp mode (Fig. 3, C and E). The spontaneous (s)EPSC frequency was similar in TRPV1Cre/tdTom neurons of the DMH and PVN [2.63 ± 0.58 Hz (n = 23 from 12 mice) vs. 2.34 ± 0.32 Hz (n = 25 from 15 mice); Mann-Whitney, P = 0.55; Fig. 3D1]. The amplitude of sEPSCs was also comparable in the DMH and the PVN (12.05 ± 1.61 pA vs. 13.84 ± 1.33 pA; Mann-Whitney, P = 0.22; Fig. 3D2). Similarly to sEPSC frequency, the frequency of sIPSCs was not significantly different between TRPV1-expressing neurons in the DMH and PVN [3.51 ± 0.76 Hz (n = 17 from 10 mice) vs. 3.20 ± 0.78 Hz (n = 19 from 13 mice); Mann-Whitney, P = 0.97; Fig. 3F1]. In contrast, the amplitude of sIPSCs was smaller in the DMH compared with the PVN (23.2 ± 2.89 pA vs. 34.46 ± 2.48 pA; unpaired t-test, P = 0.006; Fig. 3F2).
Effect of capsaicin on excitatory neurotransmission to TRPV1-expressing neurons in DMH.
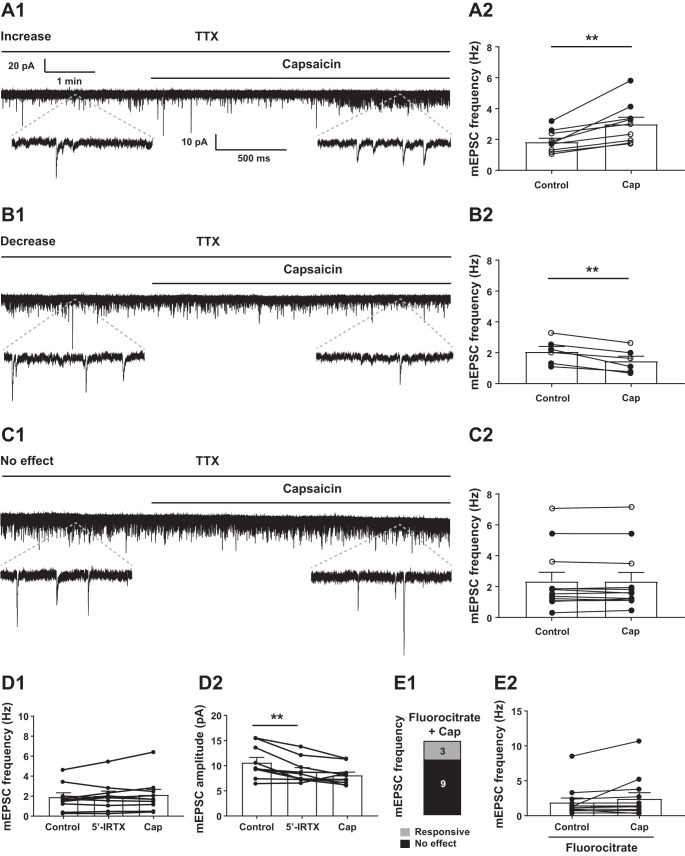
Capsaicin, the potent exogenous TRPV1 agonist, was bath applied to determine its effect on excitatory neurotransmission to TRPV1Cre/tdTom neurons of male and female mice. Voltage-clamp recordings were performed at −60 mV to determine the effect of TRPV1 activation on excitatory neurotransmission (Fig. 4, A–C). In females, the frequency of sEPSCs in TRPV1Cre/tdTom neurons was 5.47 ± 1.46 Hz (n = 12 from 7 female mice), whereas in males the average frequency was 3.00 ± 0.81 Hz (n = 15 from 8 male mice) (Wilcoxon, P = 0.077) (Table 1). Our data did not reveal significant difference or trend in the frequency (Mann-Whitney, P = 0.68) and amplitude (unpaired t-test, P = 0.28) of mEPSCs of male and female mice (Table 1). The proportion of capsaicin-responding neurons was also similar (7 of 15 neurons in males; 8 of 12 in females); therefore, we combined the data from male and female mice and grouped them on the basis of their response to capsaicin.
Fig. 4.
Capsaicin (Cap) modulates excitatory neurotransmission of transient receptor potential vanilloid type 1 (TRPV1)-expressing neurons. A–C: Cap increased (A), decreased (B), or did not affect (C) the frequency of miniature excitatory postsynaptic currents (mEPSCs) in TRPV1Cre/tdTom neurons in the dorsomedial hypothalamic nucleus (DMH). Representative recordings of mEPSCs from TRPV1Cre/tdTom neurons in the DMH show increase (A1), decrease (B1), or no response (C1) to Cap application. TTX, tetrodotoxin. Bar graphs demonstrate increase (A2), decrease (B2), and no response (C2). D: in the presence of a TRPV1 antagonist, 5′-iodoresiniferatoxin (5′-IRTX), Cap did not increase mEPSC frequency (D1) and amplitude (D2); however, 5′-IRTX alone decreased mEPSC amplitude. E: in the presence of fluorocitrate, Cap increased mEPSC frequency in a small subset of TRPV1-expressing DMH neurons (E1), without altering the overall mEPSC frequency (E2). Numbers indicate number of neurons. ○, recording was conducted in female mice; ●, recording was conducted in male mice. **Significance (P < 0.01).
Table 1.
Excitatory neurotransmission in TRPV1-expressing DMH neurons in male and female mice
| sEPSCs |
mEPSCs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Frequency, Hz |
Amplitude, pA |
Frequency, Hz |
Amplitude, pA |
|||||
| Average | Range | Average | Range | Average | Range | Average | Range | |
| Male (n = 15) | 3.00 ± 0.81 | 0.37–11.87 | 10.98 ± 1.53 | 5.47–29.73 | 1.94 ± 0.31 | 0.29–5.43 | 8.47 ± 0.67 | 5.32–14.00 |
| Female (n = 12) | 5.47 ± 1.46 | 1.40–18.93 | 14.35 ± 1.84 | 6.91–26.88 | 2.34 ± 0.49 | 1.00–7.06 | 9.63 ± 0.82 | 5.81–14.46 |
Values are means ± SE. DMH, dorsomedial hypothalamic nucleus; mEPSC, miniature excitatory postsynaptic current; sEPSC, spontaneous excitatory postsynaptic current; TRPV1, transient receptor potential vanilloid type 1.
Overall, pooled data from TRPV1Cre/tdTom neurons in the DMH of male and female mice showed that capsaicin application resulted in a small baseline shift from −30.34 ± 2.09 pA to −31.92 ± 2.1 pA (paired t-test, P = 0.03; n = 27 from 15 mice). This baseline shift likely indicates tonic activation of TRPV1 that may produce sustained shift in the baseline. Analysis of the individual neurons by Kolmogorov-Smirnov test revealed that approximately half of the recorded DMH TRPV1Cre/tdTom neurons (15 of 27) receive TRPV1-expressing inputs. Capsaicin increased the frequency of mEPSCs in nine recorded neurons (1.85 ± 0.24 vs. 3.00 ± 0.44 Hz; paired t-test, P = 0.0029; Fig. 4A) and decreased the frequency of mEPSCs in six (2.08 ± 0.33 Hz vs. 1.47 ± 0.31 Hz; paired t-test, P = 0.0032; Fig. 4B). The frequency of mEPSCs was not altered in the remaining neurons (2.343 ± 0.581 Hz vs. 2.336 ± 0.583 Hz, n = 12; Wilcoxon, P = 0.95; Fig. 4C). The amplitude of mEPSCs was unaltered in the increasing and no-response groups but showed a decrease in the decreasing group.
Application of 5′-IRTX, a TRPV1 antagonist, alone did not alter the frequency of mEPSCs in DMH TRPV1Cre/tdTom neurons (Fig. 4D1); however, it decreased mEPSC amplitude (10.65 ± 1.00 pA vs. 8.86 ± 0.75 pA, n = 10; Tukey’s multiple comparisons test, P = 0.007; Fig. 4D2). In the presence of 5′-IRTX, capsaicin did not alter mEPSC frequency in TRPV1Cre/tdTom neurons in the DMH (2.05 ± 0.46 Hz vs. 2.15 ± 0.54 Hz, n = 10 from 4 male mice; 1-way ANOVA, P = 0.41, F = 0.78) or amplitude (Tukey’s multiple comparisons test, P = 0.21) (Fig. 4D) and also failed to cause an overall baseline shift (−29.80 ± 3.31 pA vs. −30.48 ± 3.35 pA; 1-way ANOVA, P = 0.16, F = 2.32).
Since glial cells in the hypothalamus express TRPV1 (Huda et al. 2018; Mannari et al. 2013), we determined the contribution of astrocytes to the observed capsaicin effect. Astrocytes were neutralized with FC, and the effect of capsaicin on mEPSCs was determined. Analysis of individual cells by Kolmogorov-Smirnov test revealed that 3 of 12 TRPV1Cre/tdTom neurons responded to capsaicin application in the presence of FC (Fig. 4E1); however, capsaicin application did not cause a significant change in the overall frequency (1.91 ± 0.64 Hz vs. 2.44 ± 0.86 Hz, n = 12 from 4 male mice; P = 0.204; Fig. 4E2) or amplitude (8.95 ± 0.45 pA vs. 8.81 ± 0.57 pA, n = 12; Wilcoxon, P = 0.204) of mEPSCs in the presence of FC. These data suggest the existence of direct interaction between TRPV1-expressing neurons and that astrocytes likely contribute to the observed effect of capsaicin.
Effect of capsaicin on excitatory neurotransmission of TRPV1-expressing neurons in PVN.
In the PVN, capsaicin application resulted in a baseline shift from −26.78 ± 3.80 pA to −29.91 ± 4.37 pA (paired t-test, P = 0.009). Similarly to the DMH, application of capsaicin caused an increase (n = 2), decrease (n = 2), or no change (n = 6) in mEPSC frequency in TRPV1Cre/tdTom neurons in the PVN analyzed by Kolmogorov-Smirnov test. Capsaicin application did not have a significant effect on the amplitude of mEPSCs in TRPV1-expressing neurons of PVN (11.52 ± 1.45 pA vs. 11.57 ± 1.64 pA; paired t-test, P = 0.90).
Effect of capsaicin on inhibitory neurotransmission to TRPV1-expressing neurons in DMH and PVN.
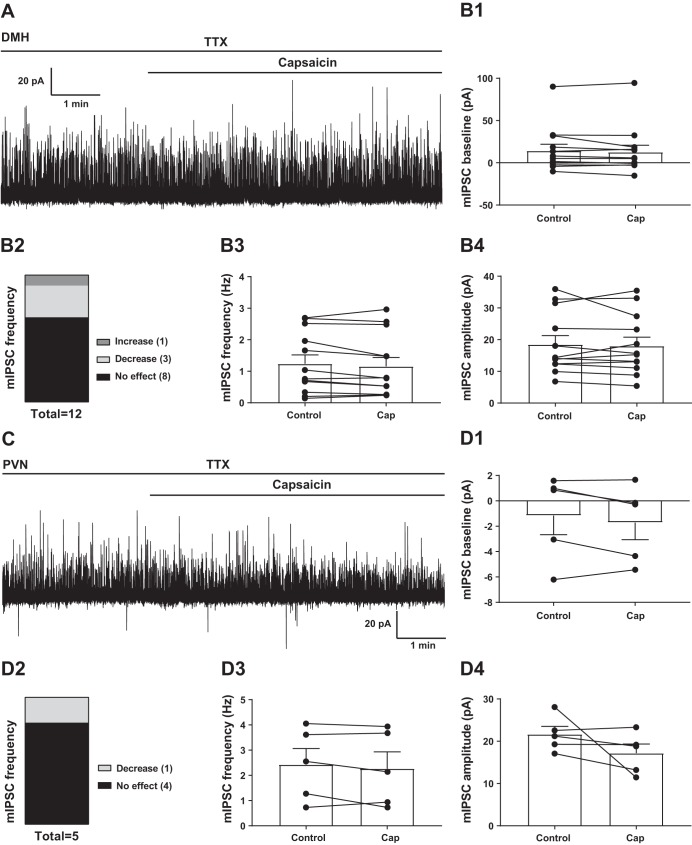
Voltage-clamp recordings were conducted at 0 mV to determine the effect of TRPV1 activation on mIPSCs of TRPV1Cre/tdTom neurons in the DMH and PVN (Fig. 5). Capsaicin application did not alter the baseline either in the DMH [14.15 ± 7.85 pA vs. 12.64 ± 8.16 pA, n = 12 from 8 mice (Wilcoxon, P = 0.38); Fig. 5B1] or in the PVN [−1.16 ± 1.50 pA vs. −1.71 ± 1.35 pA (paired t-test, P = 0.25); Fig. 5D1]. Capsaicin did not have a significant effect on the overall frequency of mIPSCs in the DMH [1.24 ± 0.28 Hz vs. 1.15 ± 0.28 Hz (paired t-test, P = 0.16); Fig. 5B3] or in the PVN [2.43 ± 0.64 Hz vs. 2.27 ± 0.66 Hz (paired t-test, P = 0.32); Fig. 5D3]. Similarly, capsaicin did not alter mIPSC amplitude in the DMH [18.47 ± 2.81 pA vs. 18.02 ± 2.75 pA (Wilcoxon, P = 0.68); Fig. 5B4] or in the PVN [21.65 ± 1.85 pA vs. 17.19 ± 2.15 pA (paired t-test, P = 0.23); Fig. 5D4].
Fig. 5.
Capsaicin (Cap) does not modulate the overall inhibitory neurotransmission of TRPV1Cre/tdTom neurons. A: representative recording of miniature inhibitory postsynaptic currents (mIPSCs) from a transient receptor potential vanilloid type 1 (TRPV1)-expressing dorsomedial hypothalamic nucleus (DMH) neuron. TTX, tetrodotoxin. B: Cap had no significant effect on the baseline (B1), overall frequency (B3), and amplitude (B4) of mIPSCs in TRPV1Cre/tdTom DMH neurons. C: representative recording of mIPSCs from a TRPV1-expressing paraventricular nucleus of the hypothalamus (PVN) neuron. D: Cap had no significant effect on the baseline (D1), overall frequency (D3), and amplitude (D4) of mIPSCs in TRPV1Cre/tdTom PVN neurons. Numbers in B2 and D2 indicate number of neurons.
DISCUSSION
In this study, an in vivo reporter assay model that displays red fluorescence where TRPV1 is expressed was used to reveal the expression of TRPV1 in the hypothalamus, establish the basic cellular properties of TRPV1Cre/tdTom neurons in the DMH and the PVN, and determine interaction between TRPV1-expressing neurons. Together, these findings demonstrate functional expression of TRPV1 in the hypothalamus of adult mice and interaction between TRPV1-expressing neurons.
Previous studies revealed abundant TRPV1 expression in multiple brain nuclei (Cristino et al. 2006; Mezey et al. 2000; Vennekens et al. 2012). By immunohistochemistry TRPV1 expression was shown in the suprachiasmatic nucleus, anterior hypothalamic nucleus, PVN, DMH, and arcuate nucleus in addition to many other brain nuclei (Mezey et al. 2000). The same study also confirmed TRPV1 mRNA throughout the central nervous system of the rat. In a subset of mouse PVN neurons, TRPV1 was shown to be expressed in the same neurons where cannabinoid type 1 receptors are expressed (Cristino et al. 2006), and TRPV1-expressing neurons were identified in hypothalamic nuclei as described above. In our study, TRPV1Cre/tdTom cells were abundantly expressed in the anterior hypothalamic nuclei, DMH, and posterior hypothalamus, whereas moderate red fluorescence was detected in the PVN and lateral hypothalamus. TRPV1Cre/tdTom cells, based on their morphology, were recognized as neurons, astrocyte-like cells, and ependymal-like cells lining the third ventricle (Jo et al. 2013; Mannari et al. 2013). We also noted that the ventromedial hypothalamic nucleus contains a dense network of TRPV1-expressing processes. These observations are consistent with the previous immunostaining studies describing expression of TRPV1 in the hypothalamus. In addition, our data are consistent with a study by Cavanaugh and colleagues that revealed that TRPV1 expression was not as widespread as suggested earlier and is limited to a few distinct brain areas in the adult rodent (Cavanaugh et al. 2011b).
TRPV1 undergoes downregulation in the brain of adult mice; thus the presence of tdTomato in TRPV1Cre/tdTom mice does not necessarily confirm that TRPV1 is expressed at that time (Cavanaugh et al. 2011a, 2011b). To address this issue, Cavanaugh and colleagues used a mouse model expressing a LacZ reporter under the control of the same regulatory elements as TRPV1, showing a limited but robust expression of TRPV1 in the hypothalamus (Cavanaugh et al. 2011b). Similarly, we used a viral approach to label TRPV1-expressing neurons in TRPV1Cre mice, where Cre recombinase expression is regulated by TRPV1 promoter, thus relating the actual presence of TRPV1 in these cells. Unexpected recombination can occur when transmitting Cre recombinase and floxed alleles through the germ line, leading to nonspecific expression of tdTomato fluorescence (Song and Palmiter 2018). Here, by showing that parental mouse line TRPV1Cre mice expressed Cre recombinase with a distribution similar to that observed in TRPV1Cre/tdTom mice, we revealed that recombination between Cre and tdTomato expression occurs as expected. In addition, ddPCR single-cell analysis of tdTomato-positive neurons and analysis of hypothalamic tissue revealed that TRPV1 is expressed in TRPV1Cre/tdTom neurons and in the hypothalamus of adult mice. Together, the viral approach and the ddPCR analysis of individual neurons support that TRPV1 is expressed in the hypothalamus of adult mice.
In this study, we established the cellular properties of TRPV1Cre/tdTom neurons in the DMH and the PVN, including the basic characteristics of TRPV1-expressing neurons. The majority of TRPV1Cre/tdTom neurons fired spontaneously in both the DMH and the PVN. TRPV1Cre/tdTom neurons in the PVN were more depolarized, and the amplitudes of sIPSCs were larger compared with TRPV1Cre/tdTom neurons in the DMH. These differences are likely nucleus-specific differences.
Hypothalamic nuclei are heterogeneous and contain functionally different neuronal populations. Regarding TRPV1 expression, vasopressin-producing magnocellular neurosecretory neurons in the supraoptic nucleus and PVN have been shown to express an NH2-terminal variant of TRPV1 (Sharif Naeini et al. 2006). That study demonstrated that TRPV1 plays a role in osmosensory transduction, and abnormal osmotic control of vasopressin release was observed in TRPV1−/− mice. In addition, trpv1 gene was shown to be necessary for thermosensory transduction in vasopressin-producing neurons and for the thermal control of vasopressin release (Sharif-Naeini et al. 2008; Sudbury et al. 2010). In circumventricular organs such as the vascular organ of the lamina terminalis trpv1 gene expression was required for hypertonicity sensing (Ciura and Bourque 2006), and recently arcuate proopiomelanocortin neurons were reported to express TRPV1-like channels (Jeong et al. 2018). Our study did not identify specific subsets of neurons expressing TRPV1 but determined functional interaction between TRPV1-expressing neurons.
Our data revealed that in the DMH ~55% (15 of 27) of the recorded TRPV1Cre/tdTom neurons received TRPV1-expressing inputs and that in the PVN ~ 40% of the neurons responded with a change in frequency of postsynaptic currents. In the case of the DMH, recordings were conducted in male and female mice. Our data did not reveal a sex difference in the frequency and amplitude of mEPSCs of TRPV1Cre/tdTom. Furthermore, the percentage of neurons receiving capsaicin-sensitive inputs was similar. These findings suggest sex-independent miniature excitatory neurotransmission in TRPV1Cre/tdTom neurons of the DMH. On the other hand, the frequency of sEPSCs in DMH TRPV1Cre/tdTom showed an increasing trend in females compared with males (Wilcoxon, P = 0.077). This observation may suggest potential sex differences in the action potential-dependent excitatory neurotransmission; however, further detailed studies are required to determine synaptic control in males and females. Our data revealing capsaicin-sensitive inputs are aligned with previous studies. In presympathetic PVN neurons, capsaicin increased the frequency of mEPSCs and action potential firing (Li et al. 2004), and the authors suggested that TRPV1 is located on presynaptic terminals. Our previous data also demonstrated that stomach- and liver-related neurons receive TRPV1-expressing inputs (Boychuk et al. 2013; Gao et al. 2012, 2017). A recent report demonstrated that arcuate proopiomelanocortin neurons express TRPV1 and are involved in temperature-induced inhibition of feeding (Jeong et al. 2018). Taken together, the increasing body of evidence suggests that activity of subsets of hypothalamic neurons is modulated by TRPV1.
Astrocytes express TRPV1 (Mannari et al. 2013), which was confirmed in our TRPV1Cre/tdTom mouse, and activation of astrocytes via TRPV1 may indirectly contribute to the observed increase of mEPSC frequency (Bari et al. 2011). Our findings revealed that in the presence of an astrocyte blocker a subset of TRPV1Cre/tdTom neurons responded to capsaicin, suggesting direct interaction between TRPV1-expressing neurons. On the other hand, we have to note that the subset of responding neurons in the presence of FC was smaller (25%) compared with the absence of astrocyte neutralizer (55%). Accordingly, astrocytes likely play a role in modulating TRPV1-expressing neurons; however, further detailed studies are required to delineate the exact contribution of astrocytes (Huda et al. 2018).
In contrast to the excitatory neurotransmission, with the exception of very few recorded cells, the overall inhibitory neurotransmission is not modulated by TRPV1 receptors in the DMH and the PVN. These findings are consistent with previous observations, demonstrating that capsaicin increases excitatory neurotransmission to preautonomic PVN neurons (Gao et al. 2012, 2017). In light of our present data and previous observations from others (Li et al. 2004) demonstrating that excitatory rather than inhibitory neurotransmission is regulated by TRPV1, we can speculate that in the hypothalamus TRPV1 provides a unique segregation. In contrast to the brain stem, where neurons receive capsaicin-sensitive excitatory and inhibitory inputs, in the hypothalamus a functional separation of neurons might exist. The segregation could be based on neurochemical phenotype or in a circuit-dependent manner; however, further studies would be required to delineate this scenario.
In a small subset of neurons we observed a decrease of mEPSC frequency after capsaicin application, which is consistent with a recent observation made in hippocampal neurons (Lu et al. 2017). The decrease of mEPSC frequency in DMH neurons after capsaicin application was accompanied by decreased amplitude, which may be due to TRPV1-dependent “kiss and run” release as recently described in dorsal root ganglia, which showed that TRPV1 activation leads to a transient opening and closure of a restricted fusion pore that might limit the release of neurotransmitters and may result in decreased amplitude of mEPSCs (Wang et al. 2017). Although we revealed direct interaction between TRPV1-expressing neurons, the origin of inputs is not known. We can speculate that excitatory inputs arrive from other hypothalamic nuclei, for example, DMH-PVN interactions; but this requires further detailed investigation.
In this study TRPV1Cre/tdTom mice were generated to test the hypothesis that TRPV1-expressing neurons in the DMH and the PVN receive TRPV1-expressing inputs. TRPV1-expressing neurons were identified in the hypothalamus, and our electrophysiological investigations revealed interaction between TRPV1-expressing neurons and a potential role of astrocytes in modulating TRPV1-expressing neurons.
GRANTS
This work was supported by research grants from the National Institutes of Health (NIH) (DK-099598 to A. Zsombok; HL-122829 to A. V. Derbenev) and core grant support [NIH CoBRE in Hypertension (P30 GM-103337) and Louisiana Clinical and Translational Science Center (U54 GM-104940)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.M., L.D.D., B.V.H., S.M.B., I.J.A., K.M., C.L.E., C.M.D., and R.S. performed experiments; A.J.M., S.M.B., and I.J.A. analyzed data; A.J.M., L.D.D., I.J.A., R.S., A.V.D., and A.Z. interpreted results of experiments; A.J.M. prepared figures; A.Z. drafted manuscript; A.J.M., L.D.D., B.V.H., S.M.B., I.J.A., K.M., C.L.E., R.S., A.V.D., and A.Z. edited and revised manuscript; A.J.M., L.D.D., B.V.H., and A.Z. approved final version of manuscript.
REFERENCES
- Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun 321: 219–225, 2004. doi: 10.1016/j.bbrc.2004.06.149. [DOI] [PubMed] [Google Scholar]
- Baboota RK, Singh DP, Sarma SM, Kaur J, Sandhir R, Boparai RK, Kondepudi KK, Bishnoi M. Capsaicin induces “brite” phenotype in differentiating 3T3-L1 preadipocytes. PLoS One 9: e103093, 2014. doi: 10.1371/journal.pone.0103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari M, Bonifacino T, Milanese M, Spagnuolo P, Zappettini S, Battista N, Giribaldi F, Usai C, Bonanno G, Maccarrone M. The endocannabinoid system in rat gliosomes and its role in the modulation of glutamate release. Cell Mol Life Sci 68: 833–845, 2011. doi: 10.1007/s00018-010-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk CR, Zsombok A, Tasker JG, Smith BN. Rapid glucocorticoid-induced activation of TRP and CB1 receptors causes biphasic modulation of glutamate release in gastric-related hypothalamic preautonomic neurons. Front Neurosci 7: 3, 2013. doi: 10.3389/fnins.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Bráz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci 31: 10119–10127, 2011a. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31: 5067–5077, 2011b. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26: 9069–9075, 2006. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 139: 1405–1415, 2006. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Monroe MJ, Glatzer NR, Smith BN. Vanilloid-mediated heterosynaptic facilitation of inhibitory synaptic input to neurons of the rat dorsal motor nucleus of the vagus. J Neurosci 26: 9666–9672, 2006. doi: 10.1523/JNEUROSCI.1591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbenev AV, Zsombok A. Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin Immunopathol 38: 397–406, 2016. doi: 10.1007/s00281-015-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragó N, Kocsis AK, Lovas S, Molnár G, Boldog E, Rózsa M, Szemenyei V, Vámos E, Nagy LI, Tamás G, Puskás LG. Digital PCR to determine the number of transcripts from single neurons after patch-clamp recording. Biotechniques 54: 327–336, 2013. doi: 10.2144/000114029. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates (3rd ed). San Diego, CA: Academic, 2007. [Google Scholar]
- Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 61: 1381–1390, 2012. doi: 10.2337/db11-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Molinas AJ, Miyata K, Qiao X, Zsombok A. Overactivity of liver-related neurons in the paraventricular nucleus of the hypothalamus: electrophysiological findings in db/db mice. J Neurosci 37: 11140–11150, 2017. doi: 10.1523/JNEUROSCI.1706-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram DX, Ahrén B, Nagy I, Olsen UB, Brand CL, Sundler F, Tabanera R, Svendsen O, Carr RD, Santha P, Wierup N, Hansen AJ. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci 25: 213–223, 2007. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- Huda R, Chang Z, Do J, McCrimmon DR, Martina M. Activation of astrocytic PAR1 receptors in the rat nucleus of the solitary tract regulates breathing through modulation of presynaptic TRPV1. J Physiol 596: 497–513, 2018. doi: 10.1113/JP275127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Lee DK, Liu SM, Chua SC Jr, Schwartz GJ, Jo YH. Activation of temperature-sensitive TRPV1-like receptors in ARC POMC neurons reduces food intake. PLoS Biol 16: e2004399, 2018. doi: 10.1371/journal.pbio.2004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Gao H, Krantz AM, Derbenev AV, Zsombok A. Reduced GABAergic inhibition of kidney-related PVN neurons in streptozotocin-treated type 1 diabetic mouse. J Neurophysiol 110: 2192–2202, 2013. doi: 10.1152/jn.00013.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo KD, Lee KS, Lee WT, Hur MS, Kim HJ. Expression of transient receptor potential channels in the ependymal cells of the developing rat brain. Anat Cell Biol 46: 68–78, 2013. doi: 10.5115/acb.2013.46.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada T, Hagihara K, Iwai K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr 116: 1272–1278, 1986. doi: 10.1093/jn/116.7.1272. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol 92: 1807–1816, 2004. doi: 10.1152/jn.00171.2004. [DOI] [PubMed] [Google Scholar]
- Lu CW, Lin TY, Hsie TY, Huang SK, Wang SJ. Capsaicin presynaptically inhibits glutamate release through the activation of TRPV1 and calcineurin in the hippocampus of rats. Food Funct 8: 1859–1868, 2017. doi: 10.1039/C7FO00011A. [DOI] [PubMed] [Google Scholar]
- Mannari T, Morita S, Furube E, Tominaga M, Miyata S. Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia 61: 957–971, 2013. doi: 10.1002/glia.22488. [DOI] [PubMed] [Google Scholar]
- Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA 97: 3655–3660, 2000. doi: 10.1073/pnas.97.7.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem 48: 1377–1385, 1987. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65: 657–669, 2010. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 127: 1123–1135, 2006. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini R, Ciura S, Bourque CW. TRPV1 gene required for thermosensory transduction and anticipatory secretion from vasopressin neurons during hyperthermia. Neuron 58: 179–185, 2008. doi: 10.1016/j.neuron.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Sharif Naeini R, Witty MF, Séguéla P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 9: 93–98, 2006. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- Song AJ, Palmiter RD. Detecting and avoiding problems when using the Cre-lox system. Trends Genet 34: 333–340, 2018. doi: 10.1016/j.tig.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbury JR, Ciura S, Sharif-Naeini R, Bourque CW. Osmotic and thermal control of magnocellular neurosecretory neurons—role of an N-terminal variant of trpv1. Eur J Neurosci 32: 2022–2030, 2010. doi: 10.1111/j.1460-9568.2010.07512.x. [DOI] [PubMed] [Google Scholar]
- Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci 29: 29–36, 2008. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 6: 357–372, 2007. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch 451: 143–150, 2005. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- Tsui H, Paltser G, Chan Y, Dorfman R, Dosch HM. “Sensing” the link between type 1 and type 2 diabetes. Diabetes Metab Res Rev 27: 913–918, 2011. doi: 10.1002/dmrr.1279. [DOI] [PubMed] [Google Scholar]
- Tsui H, Razavi R, Chan Y, Yantha J, Dosch HM. “Sensing” autoimmunity in type 1 diabetes. Trends Mol Med 13: 405–413, 2007. doi: 10.1016/j.molmed.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Menigoz A, Nilius B. TRPs in the brain. Rev Physiol Biochem Pharmacol 163: 27–64, 2012. doi: 10.1007/112_2012_8. [DOI] [PubMed] [Google Scholar]
- Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, Ma L, Zhao Z, Liu D, Zhu Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 61: 2155–2165, 2012. doi: 10.2337/db11-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu Q, Hu M, Liu B, Chai Z, Huang R, Wang Y, Xu H, Zhou L, Zheng L, Wang C, Zhou Z. Ligand- and voltage-gated Ca2+ channels differentially regulate the mode of vesicular neuropeptide release in mammalian sensory neurons. Sci Signal 10: eaal1683, 2017. doi: 10.1126/scisignal.aal1683. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res 100: 1063–1070, 2007. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci 31: 14024–14031, 2011. doi: 10.1523/JNEUROSCI.2081-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Derbenev AV. TRP channels as therapeutic targets in diabetes and obesity. Pharmaceuticals (Basel) 9: E50, 2016. doi: 10.3390/ph9030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Jiang Y, Gao H, Anwar IJ, Rezai-Zadeh K, Enix CL, Münzberg H, Derbenev AV. Regulation of leptin receptor-expressing neurons in the brainstem by TRPV1. Physiol Rep 2: e12160, 2014. doi: 10.14814/phy2.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]