Abstract
How do humans learn to adapt their motor actions to achieve task success? Recent behavioral and patient studies have challenged the classic notion that motor learning arises solely from the errors produced during a task, suggesting instead that explicit cognitive strategies can act in concert with the implicit, error-based, motor learning component. In this study, we show that the earliest wave of directionally tuned neuromuscular activity that begins within ~100 ms of peripheral visual stimulus onset is selectively influenced by the implicit component of motor learning. In contrast, the voluntary neuromuscular activity associated with reach initiation, which evolves ~100–200 ms later, is influenced by both the implicit and explicit components of motor learning. The selective influence of the implicit, but not explicit, component of motor learning on the directional tuning of the earliest cascade of neuromuscular activity supports the notion that these components of motor learning can differentially influence descending motor pathways.
NEW & NOTEWORTHY Motor learning can be driven both by an implicit error-based component and an explicit strategic component, but the influence of these components on the descending pathways that contribute to motor control is unknown. In this study, we show that the implicit component selectively influences a reflexive circuit that rapidly generates a visuomotor response on the human upper limb. Our results show that the substrates mediating implicit and explicit motor learning exert distinct influences on descending motor pathways.
Keywords: EMG, human, motor learning, reaching
INTRODUCTION
Motor learning occurs throughout the human lifespan, from children learning to walk to the aged adjusting to a new set of reading glasses. Motor learning involves establishing and constantly recalibrating the mapping of a desired goal onto the required motor commands (Shadmehr et al. 2010). A predominant theory of motor learning posits that learning arises from an implicit error-based process, in which the brain learns by computing an error between actual and predicted sensory consequences of the generated motor command (Thoroughman and Shadmehr 2000; Wolpert et al. 1998). Recent behavioral work using a visuomotor rotation task (Krakauer 2009), which systematically rotates the visual cursor denoting hand position around the center of the workspace, has suggested that a second explicit process also contributes during motor learning (Mazzoni and Krakauer 2006; Taylor and Ivry 2011; Taylor et al. 2014). The explicit process is driven by awareness of task errors, which participants exploit to achieve task success. The implicit and explicit components of motor learning appear largely independent, because research with individuals who have brain lesions shows that the implicit and explicit components of motor learning have distinctive neural substrates, relying on the integrity of cerebellar (Morehead et al. 2017; Taylor et al. 2010) and frontal circuits (Slachevsky et al. 2001, 2003), respectively (but see Butcher et al. 2017 for evidence showing that an explicit aiming process is also impaired following cerebellar damage). However, multiple descending pathways originating from the cortex and brain stem contribute to motor control in healthy individuals (Alstermark and Isa 2012; Kuypers 1981; Lemon 2008), and the comparative influence of the implicit and explicit components of motor learning on these pathways is not known.
Our interest in this report is to examine the comparative effects of implicit and explicit motor learning on the first wave of directionally tuned upper limb muscle activity that occurs time-locked ~100 ms after visual stimulus onset (termed stimulus-locked responses, or SLRs) (Pruszynski et al. 2010). We compared these learning effects against the changes in muscle activity associated with reach initiation, occurring roughly 200–300 ms after stimulus onset (Welford 1980). Previous work has shown that the largest SLRs occur when stimuli are presented at locations associated with the largest reach-related responses (Gu et al. 2018; Pruszynski et al. 2010), and SLRs persist even if the ensuing reach movement is withheld (Atsma et al. 2018; Wood et al. 2015) or proceeds in the opposite direction (Gu et al. 2016). These response properties, as well as the fact that SLRs evolve at latencies that preclude extensive cortical processing, have led us to propose that SLRs and later reach-related activity arise from distinct descending motor pathways (Gu et al. 2016; Pruszynski et al. 2010).
In this study, we examine how the implicit and explicit components of motor learning influence these two waves of electromyographic (EMG) activity during the visuomotor rotation task. Success in this task requires that participants learn a new mapping between the location of the visual stimulus and the direction of the reach movement. We quantify the change in directional tuning of the SLR and reach-related activity across three different variants of the visuomotor rotation task that either combine or isolate the implicit and explicit components of motor learning. We show that changes in SLR tuning only occur during tasks that involve implicit motor learning and that the partial shifts in SLR tuning observed during these experiments (~10°–15° for different rotation sizes) are consistent with previous estimates of implicit learning based on measures of participants’ gaze behavior (de Brouwer et al. 2018) or verbal reports of aiming direction (Bond and Taylor 2015; Taylor et al. 2014).
In contrast, the tuning of reach-related activity shifts completely in all tasks, consistent with influences of both implicit and explicit motor learning. Taken together, our results show that the earliest wave of muscle activity following a visual stimulus is selectively influenced by implicit motor learning, whereas the later voluntary waves of muscle activity are influenced by both implicit and explicit motor learning.
MATERIALS AND METHODS
Participants and Procedures
In total, we had 32 participants (21 men and 11 women, age: 25 ± 5 yr old, mean ± SD) perform at least one of the three experiments. All participants were self-declared right-handed except for one left-handed man and four left-handed women, all had normal or corrected-to-normal vision, and all reported no current visual, neurological, and/or musculoskeletal disorders. Participants provided written consent, were paid for their participation, and were free to withdraw from any experiment at any time. All procedures were approved by the Health Science Research Ethics Board at the University of Western Ontario.
Method Details
The apparatus, EMG recording setup, and parts of the data analyses have been previously described (Gu et al. 2016, 2018; Wood et al. 2015).
Apparatus and kinematic acquisition.
Briefly, in all three experiments, participants sat at a desk with their right elbow supported by a custom-built air sled. They performed right-handed horizontal planar reaches while holding the handle of a planar robotic manipulandum (InMotion Technologies, Watertown, MA). The x- and y-positions of the manipulandum were sampled and recorded at 600 Hz. A constant rightward load force of 5 N was applied throughout experiments 2 and 3 to increase the baseline activity of the muscle of interest, due to the use of surface electrodes. No load was applied in experiment 1, because we used both surface and intramuscular electrodes. Note that even though we applied a constant load in experiments 2 and 3, Franklin et al. (2012) found that rapid visuomotor responses are not modulated with changes in constant background load. Thus we assumed that the background load also did not affect any of our results. All visual stimuli were presented onto an upward-facing horizontal mirror, located just below the participant’s chin level, which reflected the display of a downward-facing LCD monitor with a refresh rate of 75 Hz. The precise timing of the peripheral visual stimulus onset on the LCD screen was determined by a photodiode. The mirror occluded view of the participant’s right arm throughout the experiment, and real-time visual feedback of the handle of the manipulandum was given by a small red cursor on a white background.
EMG acquisition.
EMG activity from the clavicular head of the right pectoralis major muscle was recorded using either intramuscular (experiment 1) and/or surface recordings (experiments 1–3). Intramuscular EMG activity was recorded using fine-wire (A-M Systems, Sequim, WA) electrodes inserted into the pectoralis muscle (see Wood et al. 2015 for insertion procedure). Briefly, for each recording, we inserted two monopolar electrodes ~2.5 cm into the belly of the pectoralis muscle. Insertions were aimed ~1 cm inferior to the inflection point of the clavicle and staggered by 1 cm along the muscle’s fiber direction. All intramuscular EMG activity was recorded with a Myopac Junior System (Run Technologies, Mission Viejo, CA). Surface recordings were made with doubled-differential electrodes (Delsys, Natick, MA) placed at the same location as the intramuscular recordings. EMG activity and the photodiode signal were digitized and recorded at 4 kHz.
Experimental Tasks
Experiment 1: Abrupt visuomotor rotation task.
Each trial began with the appearance of a central start position. Participants (N = 7/8 with a detectable SLR, SLR+; see detection criterion in SLR detection) moved the cursor into the start position, and after a randomized delay in the start position (1–1.25 s), a peripheral black circle appeared (10 cm away from the start position at 1 of 8 equidistant locations). The onset of the peripheral visual stimulus coincided with the offset of the start position. Participants were instructed to perform an out-and-back reach movement toward the peripheral stimulus. Additionally, they were instructed to reach as accurately as possible with the cursor to the peripheral stimulus during the outward phase of the reach movement. A small yellow circle also appeared at the position where the cursor crossed the 10-cm radius of the start position until the start of the next trial (1 s); this provided additional visual feedback on the accuracy of the outward reach movement.
Each participant performed 11 subblocks during the experiment, each subblock consisted of 20 cycles (see Fig. 2A; 1 cycle consists of 8 trials, 1 trial for each of the 8 different stimulus locations). In the first three subblocks (prerotation block), the cursor veridically represented handle position. During the next four subblocks (perirotation block), the cursor representing handle position was rotated by 60° clockwise (CW) around the start position. In the final four subblocks (postrotation block), the cursor once again represented handle position.
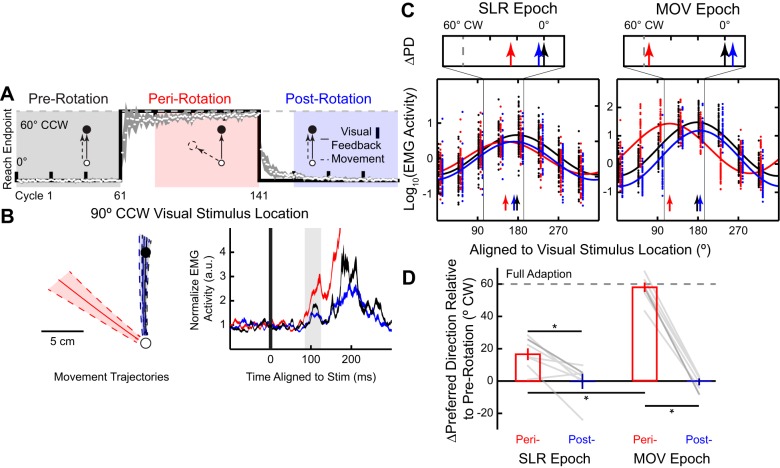
Fig. 2.
Partial adaptation of the stimulus-locked response (SLR) tuning during the abrupt visuomotor rotation task. A: timeline and behavioral performance during a 60° clockwise (CW) abrupt visuomotor rotation. Group mean (±SE; white circles and shaded line) reach end point per cycle relative to the stimulus location is plotted against perfect task performance (black line). Veridical visual feedback was provided during pre- (black) and postrotation (blue) blocks. During the perirotation (red) block, the virtual cursor feedback was rotated around the start position by 60°CW. B and C: electromyography (EMG) data from the representative participant. B: normalized mean (±SD) movement trajectories and mean (±SE) right pectoralis major EMG activity (a.u., arbitrary units) for the outward visual stimulus location of a representative participant. The EMG activity is aligned to stimulus onset, and the SLR epoch (85–125 ms after stimulus onset) is highlighted. C: sinusoidal tuning curve fits (Eq. 1) between visual stimulus location and the normalized mean EMG activity during the SLR (left) and reach-related movement (MOV) epochs (right). Each dot indicates data from a single trial, and solid lines show the best fit for each block; vertical arrows indicate the preferred directions (PDs) for each fit. Note for illustration purposes only, we have staggered the individual trial data. Top insets show the shifts in PD (∆PD) during the peri- and postrotation blocks relative to the prerotation block. Vertical dashed gray line represents full adaptation to the 60°CW visuomotor rotation. D: group mean (±SE) ∆PD for both perirotation (red bars) and postrotation blocks (blue bars) during both the SLR and MOV epochs across all participants. ∆PD = 0° or ∆PD = 60°CW would indicate either no adaptation or a complete adaptation to the imposed rotation, respectively. Each gray line represents data from an individual participant, with the darker gray line indicating data from the participant in C. *P < 0.05.
Experiment 2: Gradual visuomotor rotation task.
As in experiment 1, participants (N = 14/14 SLR+) moved the cursor into the start position, and after a randomized delay in the start position (1–1.25 s), a peripheral black circle appeared at one of eight equidistant locations around the start position. Participants were instructed to perform an out-and-back reach movement toward the peripheral stimulus and to reach as accurately as possible with the cursor to the peripheral stimulus during the outward movement. We did not present the yellow cursor feedback after each outward reach movement, because we were concerned that subjects would notice persistent shifts in the feedback relative to the target and adjust their strategy accordingly.
Each participant performed 9 subblocks, each consisting of 20 cycles (see Fig. 3A). In the first two subblocks (test block 1), the cursor veridically represented handle position. Afterward, participants performed reaches during either a 20°CW or 20° counterclockwise (CCW) visuomotor rotation, with a gradual imposition of this rotation. A gradual rotation was imposed during the third subblock, in which the cursor representing handle position was rotated by 1° around the start position after each cycle; over the entire block, the total rotation was 19°. Participants were counterbalanced between experiencing either a CW or CCW rotation first (N = 7 per group; see Fig. 3A). During test block 2 (subblocks 4 and 5), participants performed reaches while the cursor was constantly rotated by 20°. In the next two subblocks (subblocks 6 and 7), a gradual rotation was imposed 1° per cycle in the opposite direction as in subblock 3; thus, by the end of subblock 7, the total rotation imposed during the two subblocks was 39°. During test block 3 (subblocks 8 and 9), participants reached with a constant 20° rotation, which was in the opposite direction as test block 2. Thus all participants performed visually guided reaches with veridical feedback (prerotation) and reaches with both 20°CW and 20° CCW rotations (see Fig. 3A).
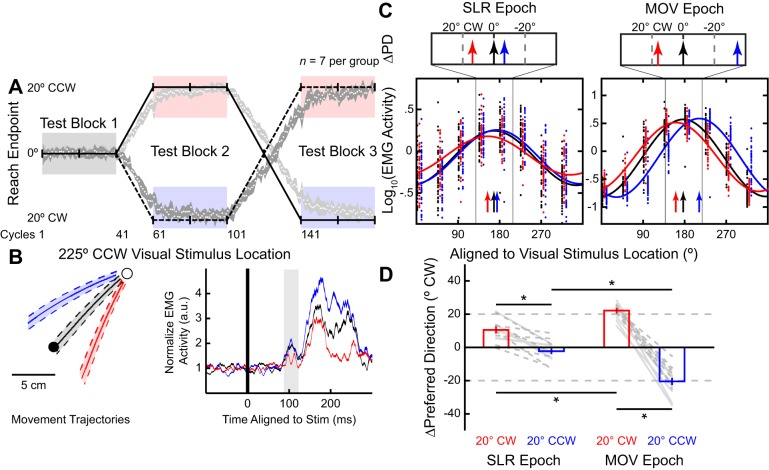
Fig. 3.
Partial adaptation of the stimulus-locked response (SLR) tuning during the gradual visuomotor rotation task. Same layout as Fig. 2. A: timeline and behavioral performance during a gradual visuomotor rotation task. After the 40 cycles of reaches (test block 1) with veridical cursor feedback, the cursor was gradually rotated 1° per cycle to 20° clockwise (CW; solid line) or counterclockwise (CCW; dashed line). After participants performed 40 cycles with the cursor constantly rotated 20°CW or 20°CCW (test block 2), the cursor was rotated in the opposite direction for 40 cycles. Finally, participants performed 40 cycles with the cursor constantly rotated 20°CCW or CW (test block 3). Both groups performed reaches with veridical (prerotation; black), 20°CW (red), and 20°CCW (blue) visual feedback blocks. B and C: electromyography (EMG) data from the representative participant. B: mean(±SD) movement trajectories and mean (±SE) EMG activity (a.u., arbitrary units) for the left inward visual stimulus location during the 3 blocks from a participant who experienced the CW rotation first. C: preferred direction (PD) for each of the test blocks during both the SLR and reach-related movement (MOV) epochs (vertical arrows). D: group mean (±SE) ∆PD for CW and CCW blocks compared with prerotation block for both the SLR and MOV epochs across all participants. Dashed or solid lines indicate participants who first experienced CW or CCW rotation, respectively. *P < 0.05.
Experiment 3: Mental visuomotor rotation task.
Each trial began with the appearance of a start position and black outlines of the eight equidistant locations 10 cm from the start position. Participants (N = 13/18 SLR+) moved the cursor into the start position, and after a randomized delay in the start position (1–1.25 s), one of the peripheral stimulus location was filled. Each participant performed 6 subblocks of 20 cycles (Fig. 4A). In three of the subblocks (VIS block), participants performed out-and-back reach movements to the peripheral stimulus, whereas in the other three rotation subblocks (ROT block), participants were instructed to reach toward the open stimulus location 90°CCW to the filled in peripheral stimulus location. Unlike in experiments 1 and 2, the cursor always veridically represented handle position throughout the experiment. The order of the blocks was counterbalanced between participants (N = 9 per group).
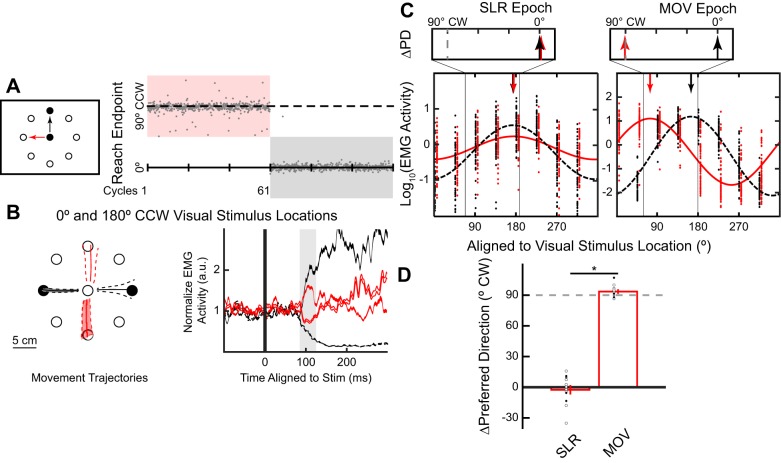
Fig. 4.
Stimulus-locked response (SLR) tuning did not adapt during a mental visuomotor rotation task. Same layout as Fig. 2. A: task schematic, timeline, and behavioral performance for a representative participant during the mental visuomotor rotation task. Veridical visual feedback was given throughout the whole experiment. Participants were instructed to reach directly (VIS block, black) or 90° counterclockwise (CCW; ROT block, red) to the stimulus location, with the order counterbalanced across participants. B and C: electromyography (EMG) data from the representative participant. B: mean (±SD) movement trajectories and mean (±SE) EMG activity for both the leftward and rightward stimulus locations. C: preferred directions (PD) for both the VIS and ROT blocks during both the SLR and reach-related movement (MOV) epochs (vertical arrows). D: group mean (±SE) ∆PD between VIS and ROT blocks across all participants. Open and closed circles indicate participants who first performed the VIS and ROT block, respectively. *P < 0.05.
Experimental Design and Statistical Analysis
Data preprocessing.
All analyses were performed with custom-written scripts in MATLAB (version R2014b; The MathWorks, Natick, MA). To achieve sample matching between the kinematics and EMG data, all kinematic data were up-sampled from 600 to 1,000 Hz with a low-pass interpolation algorithm and then low-pass filtered with a second-order Butterworth filter with a cutoff at 150 Hz. Reach reaction times (RTs) were calculated as the time from the onset of the peripheral visual stimulus (measured by the photodiode) to the initiation of the reach movement. Reach initiation was identified by first finding the peak tangential movement velocity after stimulus onset and then moving backward to the closest time at which the tangential velocity profile surpassed 8% of the peak velocity. All EMG data were rectified and then either integrated into 1-ms bins (intramuscular) or down-sampled (surface) to 1,000 Hz. EMG activity was then normalized relative to each block’s mean baseline EMG activity (defined as the mean EMG activity 40 ms before the onset of the peripheral visual stimulus). We defined the SLR epoch as 85–125 ms after stimulus onset and the SLR magnitude as the mean EMG activity during the SLR epoch. We also defined the reach-related movement (MOV) epoch as 20 ms before to 20 ms after reach RT. All trials with RTs <185 ms were excluded to prevent contamination of the SLR epoch by shorter latency reach-related responses (Gu et al. 2016; Wood et al. 2015).
To determine the normalized movement trajectories, we first determined the movement duration for each trial individually. The movement duration was defined as the time when the handle position surpassed 2 cm from the center of the start position to 50 ms after the time when the handle position surpassed 8 cm from the center of the start position. We then interpolated the movement duration into 101 equally spaced time samples and calculated the x- and y-positions at each given time sample.
SLR detection.
On the basis of previous studies detecting the presence of the SLR (Corneil et al. 2004; Pruszynski et al. 2010), we also used a receiver operating characteristic (ROC) analysis to quantitatively detect the presence of a SLR. In all experiments, we examined EMG activity for leftward and rightward reaches during veridical visual feedback, and we performed the following ROC analysis. For every time sample (1-ms bin) between 100 ms before to 300 ms after visual stimulus onset, we calculated the area under the ROC curve between the leftward and rightward trials. This metric indicates the probability that an ideal observer could discriminate the side of the stimulus location based solely on EMG activity. A ROC value of 0.5 indicates chance discrimination, whereas a value of 1 or 0 indicates perfectly correct or incorrect discrimination, respectively. We set the thresholds for discrimination at 0.6; these criteria exceed the 95% confidence intervals of data randomly shuffled with a bootstrap procedure (Chapman and Corneil 2011). The earliest discrimination time was defined as the time after stimulus onset at which the ROC was above 0.6 and remained above that threshold for at least 5 of the next 10 samples. Previous studies have also reported decreased SLR magnitude during an anti-reach task (Gu et al. 2016); thus we lowered our threshold to 0.55 for the ROT block in experiment 3. On the basis of the ROC analyses, we defined the SLR epoch as from 85 to 125 ms after visual stimulus onset and categorized any participant with a discrimination time <125 ms as having a SLR (SLR+ participant). Across the three experiments, we could reliably detect a SLR in 29 of 32 participants.
Tuning curve fit.
To determine the tuning curve of EMG activity during both the SLR and MOV epochs, we assumed that the relationship between EMG activity and the peripheral visual stimulus location took the form of a sinusoidal function (Eq. 1):
| (1) |
in which x is the angular location of the peripheral visual stimulus in degrees, EMG(x) is the logarithm of the normalized EMG activity for the given stimulus location, A is the amplitude of the sinusoidal fit, θ is the preferred direction (PD) of the sinusoidal fit, and γ is the offset of the sinusoidal fit. We used MATLAB’s curve fitting toolbox, in which we constricted our parameters so that A < 0 and 0° ≤ θ < 360°, and the starting points of the parameters were A = 1, θ = 180°, and γ = 0.
Statistical analyses.
For statistical analyses done on the EMG data from the representative participants of experiments 1 and 2, we performed a 1-way ANOVA (visuomotor rotation blocks) for both the SLR and MOV epochs separately. For experiment 3, we performed a 2-way ANOVA (direction × visuomotor rotation block) for the SLR epoch. For the group RT data, we performed either a repeated-measures 1-way ANOVA (visuomotor rotation blocks; experiments 1 and 2) or paired t-test (experiment 3). For the group ∆PD data, we performed either a repeated-measures 2-way ANOVA (epochs × visuomotor rotation blocks) or a one-sample t-test to compare against zero. For ANOVA post hoc testing, we performed a Tukey’s honestly significant difference (HSD) correction. The statistical significance was set as P < 0.05.
Data and Software Availability
All data were analyzed using MATLAB R2014b.
RESULTS
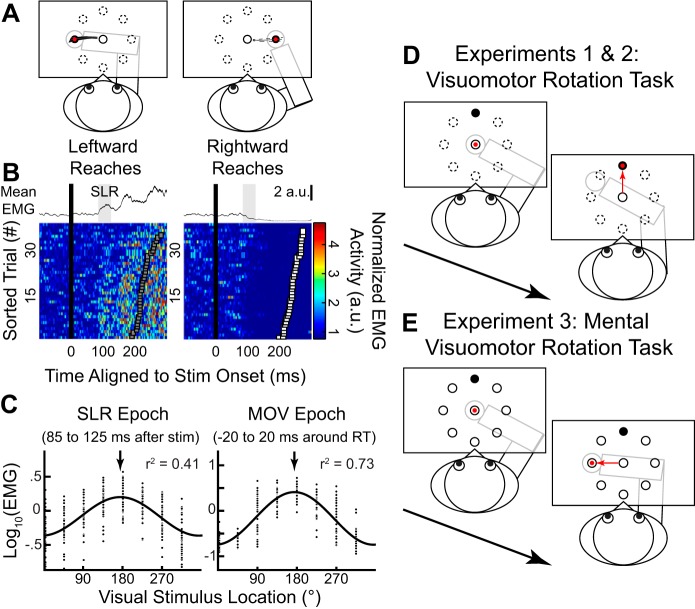
Figure 1A shows the normalized (mean ± SD) movement trajectories for both the leftward (180°CCW from straight right) and rightward (0°) stimulus locations from a representative participant when he or she had veridical visual feedback of hand position (i.e., the cursor moved in register with the participant’s hand). Figure 1B shows the corresponding normalized (mean ± SE; top) and individual (bottom) pectoralis EMG activity from leftward and rightward trials. EMG activity was aligned to the onset of the peripheral visual stimulus onset, and individual trials were sorted on the basis of RT (squares, fastest to slowest from bottom to top). We observed a reliable SLR, which consisted of a brief increase or decrease in EMG activity ~100 ms after the presentation of leftward or rightward stimulus locations, respectively (Gu et al. 2016; Pruszynski et al. 2010; Wood et al. 2015). We defined the SLR magnitude for each trial as the mean EMG activity during the SLR epoch (85–125 ms after stimulus onset; Fig. 1B, top). Our previous work has shown that the latency of the SLR on neck muscles is basically the same for movements with shorter or longer than average RTs (Goonetilleke et al. 2015).
Fig. 1.
Experimental paradigm and spatial tuning of the stimulus-locked response (SLR) on human limb muscle during visually guided reaches. A: normalized mean (±SD) movement trajectories for leftward and rightward visually guided reach for a participant performing reaches with veridical feedback in experiment 2. B: the corresponding normalized mean (±SE; top) and individual trials (bottom) of electromyographic (EMG) activity (a.u., arbitrary units) from the right pectoralis major muscle aligned to visual stimulus onset (black line). In the color panels, each row represents EMG activity from a single trial, with trials sorted on the basis of reach reaction time (RT; squares). EMG activity diverged during the SLR epoch (shaded regions, 85–125 ms after stimulus onset), regardless of the ensuing RT. C: sinusoidal relationship between the normalized mean EMG activity and visual stimulus location during the SLR (left) and reach-related movement (MOV) epochs (right) for this participant. Arrows indicate the preferred direction of each fit. D: experiments 1 and 2, the visuomotor rotation task. Participants generate reach movements to move the cursor (red circle) to the visual stimulus location (black circle). To induce motor learning, the cursor was systematically rotated (60° clockwise in this case) around the start position. E: experiment 3, the mental rotation task. During the task, the cursor always gave veridical feedback of the robotic handle, but participants were explicitly instructed to reach to the stimulus location 90° counterclockwise to the visual stimulus location.
To determine the directional tuning of the EMG activity during both the SLR and the later reach-related response (MOV; −20 to 20 ms around RT) epochs, we derived the PD of each epoch, assuming a sinusoidal fit (Eq. 1). Figure 1C shows the log-normalized EMG activity as a function of visual stimulus location (arrows indicate the PDs of each fit). With veridical feedback, a reliable SLR was detected in 29 of 32 participants (see materials and methods, SLR detection for detection criteria). Consistent with a previous study (Pruszynski et al. 2010), we also found a small but reliable difference in PD of EMG activity between the SLR and MOV epochs [172.5 ± 1.6° and 180.0 ± 1.2° (means ± SE), respectively; paired t-test: t(36) = −4.0, P = 0.001]. Data from participants who did not exhibit an SLR were excluded from all subsequent analyses (see materials and methods for exact numbers for each experiment). Having established the tuning of EMG activity during the SLR and MOV epochs with veridical hand position feedback, we next examined how the PDs changed during two different visuomotor rotation tasks (Fig. 1D) and a mental visuomotor rotation task (Fig. 1E).
Partial Adaptation of the SLR During an Abrupt 60°CW Visuomotor Rotation
In experiment 1, we used an abrupt visuomotor rotation task that has been previously shown to engage both implicit and explicit motor learning components (Mazzoni and Krakauer 2006; Taylor et al. 2014). During both the pre- and postrotation blocks (Fig. 2A), participants (N = 7) performed 60 and 80 cycles (a cycle consists of 8 reaches, 1 reach per direction) of visually guided reaches under veridical visual feedback, respectively. During the perirotation block (80 cycles), we imposed a 60°CW rotation on the visual cursor around the start position. Figure 2A also shows the group mean (±SE) reach end point plotted relative to the stimulus location, where the solid line indicates perfect task performance. Consistent with previous experiments (Krakauer et al. 2005; Pine et al. 1996), participants rapidly adapted their end-point reach direction during the beginning of the perirotation block and exhibited signs of motor learning as shown by the aftereffect during the beginning of the postrotation block (Mazzoni and Krakauer 2006). We excluded the first 20 cycles of both the peri- and postrotation blocks to ensure that participants’ behavioral performance had plateaued. We observed an increase in median RTs during the perirotation block (see Fig. 5A; 301 ± 17 ms, group mean ± SE) compared with both pre- and postrotation blocks [246 ± 14 and 254 ± 13 ms, respectively, repeated-measures 1-way ANOVA, F(2,12) = 11.99, P = 0.001, post hoc Tukey’s HSD, both P < 0.01]. Prolonged RTs during the visuomotor rotation task have been associated with explicit motor learning as participants employ an aiming strategy (Fernandez-Ruiz et al. 2011; Haith et al. 2015). Thus participants’ behavior provided evidence for the engagement of both implicit and explicit motor learning components during this task.
Fig. 5.
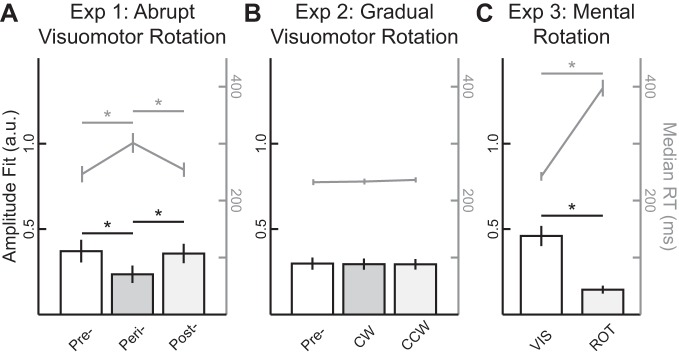
An explicit aiming strategy attenuated stimulus-locked response (SLR) magnitude and increased reaction time (RT). A–C: group means (±SE) of both the amplitude parameter (a.u., arbitrary units) for the sinusoidal fits during the SLR epoch (bars, left axis) and median RTs (gray lines, right axis) across the 3 different experiments. Pre, prerotation; peri, perirotation; post, postrotation; CW, clockwise; CCW, counterclockwise; VIS, direct reach to stimulus; ROT, 90°CCW rotation. *P < 0.05.
Figure 2B shows mean movement trajectories and pectoralis EMG activity for the outward visual stimulus location (90°CCW) across the three different blocks for one participant. As shown by the mean movement trajectories, during perirotation the participants learned that the imposed 60°CW visuomotor rotation required them to generate a left outward reach movement ~60°CCW to the stimulus location. These left-outward movements during the perirotation block required more pectoralis recruitment compared with straight outward movements during both pre- and postrotation blocks. As expected, during the MOV epoch we observed reliable modulation in pectoralis EMG activity across blocks [1-way ANOVA, main effect, F(2,176) = 486.4, P < 10−71], with greater EMG activity during peri- compared with both pre- and postrotation blocks (post hoc Tukey’s HSD, both P < 10−9).
For the outward stimulus location, we also observed a similar pattern of modulation during the SLR epoch [1-way ANOVA, main effect, F(2,176) = 7.97, P = 0.001], with greater EMG activity during the SLR epoch for peri- compared with both pre- and postrotation blocks (post hoc Tukey’s HSD, P = 0.006 and P = 0.001, respectively). Thus, even though the same visual stimulus location was presented across all three blocks, the magnitude of the SLR changed during motor learning.
To quantify the influence of motor learning on directional tuning, we derived the PDs of EMG activity during the two different epochs for all three blocks (colored arrows in Fig. 2C). We normalized the results across participants by using each participant’s PD during the prerotation block as a baseline and quantified the shifts in PD (∆PD) for both peri- and postrotation blocks (Fig. 2C, top). Across participants (Fig. 2D), we found that ∆PD for the MOV epoch adapted almost completely during the perirotation block [∆PD = 57.7 ± 2.9°CW (mean ± SE); one-sample t-test, t(6) = 19.61, P < 10−5] to the imposed 60°CW visuomotor rotation. Note this is expected because we aligned the tuning curves relative to visual stimulus location rather than the reach direction. We also found that ∆PD returned to baseline during the postrotation bock [∆PD = 0.7 ± 1.6°CW; one sample t-test, t(6) = 0.46, P = 0.66], and there was a reliable difference in ∆PD between the peri- and postrotation blocks [repeated-measures 2-way ANOVA, epoch and rotation blocks, interaction effect, F(1,6) = 74.15, P < 10−6, post hoc Tukey’s HSD, P = 0.0001]. Thus we observed nearly complete adaptation (∆PD ≈60°CW) and deadaptation (∆PD ≈0°CW) during the MOV epoch for the peri- and postrotation blocks, respectively.
We next examined the change in the directional tuning of EMG activity during the SLR epoch. Like the later MOV epoch, we also observed reliable adaptation during the perirotation block [∆PD = 16.7 ± 3.6°CW; one-sample t-test, t(6) = 4.6, P = 0.004] and deadaptation during the postrotation block [∆PD = 0.0 ± 4.2°CW; one-sample t-test, t(6) = 0.01, P = 0.99]. However, the extent of adaptation during perirotation for the SLR epoch was reliably smaller than that during the later MOV epoch [repeated-measures 2-way ANOVA, post hoc Tukey’s HSD, perirotation, SLR vs. MOV epoch, P = 0.0001].
To summarize the results from experiment 1, motor learning induced via an abrupt 60°CW visuomotor rotation systematically altered the tuning of the SLR, despite its short latency. However, unlike the full adaptation of EMG in the later MOV epoch, we observed only partial adaptation of EMG during the SLR interval. The abrupt visuomotor rotation task is thought to engage both implicit and explicit motor learning components. In experiment 2, we tested whether the shift in SLR tuning is still present when the explicit component of motor learning is minimized.
SLR Adaptation Occurs Despite a Lack of Explicit Awareness of a Visuomotor Rotation
In experiment 2, participants (N = 14) performed a gradual visuomotor rotation task (Fig. 3A). A previous imaging study has suggested that abrupt and gradual visuomotor rotation tasks engage different neural substrates (Werner et al. 2014), and behavioral studies have shown that gradual visuomotor rotations produced larger aftereffects (Kagerer et al. 1997) and longer lasting retention (Klassen et al. 2005) compared with abrupt visuomotor rotations. In the present study, we imposed a visuomotor rotation gradually (1° per cycle). Once again, participants initially performed visually guided reaches to one of eight equidistant visual stimuli with veridical feedback (Fig. 3A, test block 1, prerotation) for 40 cycles; and then, for the next 20 cycles, the visual feedback of the cursor was rotated either 1°CW or CCW per cycle, counterbalanced between participants. Over the next 40 cycles, the visual feedback remained rotated at 20°CW or CCW (test block 2). Afterward, the feedback was rotated 1° per cycle in the opposite direction to the initial imposed rotation for 40 cycles. Finally, the feedback remained constantly rotated at 20°CCW or CW (test block 3). We found no reliable differences in end-point reach direction between the three test blocks based on the order of imposed rotation [2-way ANOVA, test blocks and group, interaction effect, F(1,24) = 7.14, P = 0.01, post hoc Tukey’s HSD, both P > 0.21]. Thus we pooled data from all participants together for the subsequent analyses.
The size of the imposed visuomotor rotation, 1° per cycle, during experiment 2 is less than the trial-by-trial variance of the participants’ reach end point during the prerotation block (Gaussian fit, means ± SD: µ = 0.4 ± 0.1, σ2 = 5.0 ± 0.2, adjusted r2 = 0.94 ± 0.01). Consistent with previous studies (Galea et al. 2010; Honda et al. 2012), participants reported no explicit awareness of changes in the underlying sensorimotor mapping at any point during the experiment. Furthermore, unlike in experiment 1, we found no difference in median RTs between veridical feedback (see Fig. 5B, prerotation, 232 ± 5 ms, mean ± SE) and the two rotation blocks [CW and CCW, 233 ± 5 and 236 ± 5 ms, respectively; repeated-measures 1-way ANOVA, F(2,26) = 1.79, P = 0.19]. This lack of RT increase during the gradual visuomotor rotation is also consistent with a minimal influence of explicit aiming during the experiment.
Figure 3B shows mean movement trajectories and pectoralis EMG activity from one participant for the left inward stimulus location (225°CCW) across the three test blocks: prerotation, 20°CW, and 20°CCW. As in experiment 1, we found reliable differences in normalized EMG activity across the three blocks for both the SLR and MOV epochs for this stimulus location [1-way ANOVA, main effect, F(2,109) = 5.74 and 57.6, P = 0.004 and P < 10−17, respectively]. For example, during the 20°CW rotation block, the participant generated reaches away from the PD of the pectoralis muscle; hence, there was a decrease in mean EMG activity both during the MOV epoch (red trace in Fig. 3B, starting ~150 ms after stimulus onset, post hoc Tukey’s HSD, P < 10−5) and during the SLR epoch (shaded region, post hoc Tukey’s HSD, P = 0.01). Figure 3C shows the tuning curve fits during both the SLR and MOV epochs across the three different blocks for this participant, demonstrating the changes in the PD in both the SLR and MOV epochs.
When we examined the shifts in PD across our sample, as expected we observed full ∆PD adaptations of 22.2 ± 1.1°CW and 20.4 ± 2.1°CCW during the MOV epoch for the 20°CW and 20°CCW rotation blocks relative to the prerotation block, respectively [Fig. 3D, right; repeated-measures 2-way ANOVA, epoch and rotation, interaction effect, F(1,13) = 122.08, P < 10−10, post hoc Tukey’s HSD, P < 10−8]. When we performed the same analysis during the SLR epoch (Fig. 3D, left), we found that the SLR ∆PD rotated 10.5 ± 1.7°CW and 2.3 ± 1.6°CCW for the 20°CW and 20°CCW rotations, respectively (post hoc Tukey’s HSD, P < 10−4). Similar to the reach direction error, we found no difference between the ∆PD of the SLR based on the order of visuomotor rotation for both the 20°CW and 20°CCW blocks [2-way ANOVA, order and block, main effect of order, F(1,24) = 0.31, P = 0.59]. Although there is an asymmetry in how much the tuning of the SLR changed for CW and CCW rotations, the main contrast that the experiment was designed to examine was the difference in PDs between the 20°CW and 20°CCW blocks. As in experiment 1, we observed a reliable smaller overall change in ∆PD during the SLR vs. MOV epochs when collapsing these changes across the 20°CW and 20°CCW rotation blocks [12.8 ± 1.9° and 42.6 ± 2.1°; paired t-test, t(13) = 11.0, P < 10−7].
Thus, as with an abrupt visuomotor rotation, motor learning induced by a gradual visuomotor rotation systematically altered the tuning of the SLR. Experiment 2 also demonstrated that explicit awareness of changes in the underlying visuomotor mapping is not required for the tuning of the SLR to change. However, the extent of adaptation during the SLR epoch was still reliably less than that observed in the later MOV epoch. This finding is consistent with literature suggesting that another cognitive strategy, such as reward-based learning, could still be engaged in the gradual visuomotor rotation task, despite the lack of explicit awareness (Galea et al. 2010).
Changes in the Explicit Aiming Strategy Do Not Alter the PD of the SLR
In experiment 3, participants (N = 13) performed a mental visuomotor rotation task (Georgopoulos and Massey 1987; Mazzoni and Krakauer 2006; Taylor and Ivry 2011). Unlike in the first two experiments, participants received veridical visual feedback of their hand position throughout the experiment. It has been proposed that this eliminates implicit motor learning, because such learning is thought to occur only when there is a mismatch between the visual location of the virtual cursor and the participant’s hand position (Mazzoni and Krakauer 2006; Morehead et al. 2017). Instead, participants were explicitly instructed to reach either directly to the stimulus location (VIS block; Fig. 4A, black arrow) or 90°CCW relative to the stimulus location (ROT block, red arrow). The order of the blocks was counterbalanced between participants. To assist participants, all eight stimulus locations were presented as open circles throughout the whole experiment, and the peripheral stimulus onset occurred when one of the open circles filled in. As in experiment 1, we found an increase in median RTs during the ROT (see Fig. 5C, 398 ± 15 ms, mean ± SE) compared with VIS block [243 ± 7 ms; paired t-test, t(12) = –17.8, P < 10−9], supporting the idea that participants used an aiming strategy during the ROT block.
Figure 4A shows the end-point reach direction from a participant who performed the ROT block first. There was no aftereffect during the initial few cycles after the end of the ROT block, which is consistent with the absence of implicit motor learning. Figure 4B shows a participant’s mean movement trajectories and pectoralis EMG activity for leftward and rightward stimulus locations (180° and 0° locations). Note that regardless of the voluntary movement direction, we observed greater EMG activity after leftward compared with rightward stimulus presentation during the SLR epoch in both the VIS [Fig. 4B, black lines; 2-way ANOVA, direction and block, interaction effect, F(1,225) = 12.57, P = 0.0005, post hoc Tukey’s HSD, P < 10−8] and ROT blocks (red lines; post hoc Tukey’s HSD, P < 10−7). Like the previous two experiments, we derived the PD of EMG activity during both the SLR and MOV epochs (Fig. 4C).
Across our sample, we observed a reliable shift in PD between the VIS and ROT blocks during the MOV epoch [Fig. 4D, ∆PD = 93.6 ± 1.5°CW; one-sample t-test, t(12) = 63.0, P < 10−15]. In contrast, the SLR tuning did not reliably differ between the two blocks [∆PD = −2.5 ± 3.8°CW; one-sample t-test, t(12) = –0.7, P = 0.52]. Although there was a significant attenuation in the amplitude of the SLR tuning curve between the VIS and ROT blocks [paired t-test, t(12) = 5.96, P < 10−4], this attenuation could be related to the corresponding increase in RT during the ROT block, because SLR magnitude is known to decrease when preceding movements with longer RTs (Gu et al. 2016; Pruszynski et al. 2010). This decrease in amplitude was also observed during the perirotation block in experiment 1, when there was also an increase in median RTs, but a decrease in amplitude was not seen in experiment 2, when there was no reliable increase in median RTs (see Fig. 5 for the relationship between SLR amplitudes and median RTs in all 3 experiments). Thus, in experiment 3, learning induced during a mental visuomotor rotation task did not systematically alter the tuning of the SLR.
DISCUSSION
Recent studies have suggested that motor learning can be driven by multiple learning components: an implicit learning component related to the mismatch between the actual and predicted sensory consequences of a generated motor command (Mazzoni and Krakauer 2006; Morehead et al. 2017) and an explicit learning component that involves changes to aiming strategy (Taylor and Ivry 2011; Taylor et al. 2014). What has not been clear from this literature is how such components engage various descending motor pathways. In this study, we measured the changes in the directional tuning of EMG activity on the human pectoralis muscle during three variations of the visuomotor rotation task. We found both the implicit and explicit components of motor learning modulated the tuning of voluntary reach-related EMG activity. In contrast, we found that only the implicit motor learning component modulated the tuning of the earliest wave of muscle activity that is time-locked to the onset of a peripheral visual stimulus.
Implicit Motor Learning Drives the Partial Adaptation of SLR Tuning During Visuomotor Rotations
Our central result is that implicit motor learning altered the directional tuning during the SLR epoch (85–125 ms after stimulus onset), whereas both implicit and explicit motor learning altered the tuning of reach-related MOV activity (−20 to 20 ms around RT, ~200–300 ms after stimulus onset). Thus implicit motor learning can induce adaptation in the fastest, essentially reflexive, visuomotor pathway. The amount of adaptation was considerably less than either of our imposed visuomotor rotations: SLR tuning changed by 16.7 ± 3.6° for a 60° visuomotor rotation in experiment 1, and by 12.8 ± 1.9° for the overall 40° visuomotor rotation in experiment 2 (when the PDs for the 20°CW vs. 20°CCW blocks are compared). These observations match well with previous behavioral estimates of implicit learning component of ~10°–15° from both the initial aftereffect (Taylor and Ivry 2011) and during the visuomotor rotation regardless of the magnitude of the imposed visuomotor rotation (Bond and Taylor 2015; Taylor et al. 2014). The latter estimates are based on a subtraction logic, wherein the implicit component is estimated as the difference between the actual reach direction and the verbal reporting of the participant’s aiming direction. Recent work has also shown that gaze behavior in a subset of subjects correlates with their explicit aiming strategy (de Brouwer et al. 2018).
The gradual visuomotor rotation used in experiment 2 attempted to minimize the explicit aiming component of motor learning. Evidence that participants learned the new visuomotor mapping without using an explicit aiming strategy is found in the lack of difference in RTs between the veridical and rotation blocks (Fig. 5) and postexperiment confirmation that our participants were unaware of any changes in the visuomotor mapping during the experiment (Galea et al. 2010; Honda et al. 2012). However, a previous study has reported impaired learning rates during a similar gradual visuomotor task when participants concurrently performed a cognitively demanding task (Galea et al. 2010), suggesting a distinction between explicit awareness and contribution of other forms of learning. This may explain why we only observed a partial adaptation of SLR tuning (~13°) compared with a full adaptation during the MOV epoch (~40°). Our paradigm was designed to test the influence of error-based learning but also may have engaged reinforcement-based learning (Lee et al. 2012). Reinforcement-based learning was likely engaged in all three experiments, as participants gauged their success in hitting the target. Previous studies have shown that changes in sensorimotor mapping can be driven purely by reinforcement learning (Izawa and Shadmehr 2011; Shmuelof et al. 2012; Therrien et al. 2016), which can occur without awareness (Alamia et al. 2016). Furthermore, recent studies have shown that reward signals can modulate the extent of implicit motor learning (Kim et al. 2018; Leow et al. 2018; Reichenthal et al. 2016). At the current time, whether modulation of reward can alter the tuning of the SLR is not known.
Distinct Neural Substrates for the Implicit and Explicit Components of Motor Learning
To our knowledge, no previous animal neurophysiological or human imaging studies have described a neural correlate for partial adaptation during either a gradual or an abrupt visuomotor rotation task. Previous functional magnetic resonance imaging (fMRI) studies have shown that blood oxygen level-dependent (BOLD) activity within the posterior parietal cortex (PPC) faithfully encodes visual stimulus location during the visuomotor rotation task, regardless of the ensuing reach direction (Fernandez-Ruiz et al. 2007; Haar et al. 2015). Similarly, during saccadic adaptation, neurons within the lateral intraparietal cortex also encode visual stimulus location rather than saccadic end point (Steenrod et al. 2013). Conversely, both fMRI and neurophysiological studies have shown that both premotor and primary motor cortices encode the final movement direction, regardless of the visual stimulus location (Haar et al. 2015; Paz et al. 2003; Perich et al. 2017; Shen and Alexander 1997a, 1997b). Thus the pattern of the modulation of SLR tuning is distinct from signals observed in either the PPC or motor cortices, which would presumably be relayed via corticospinal projections.
Previous clinical studies suggested that implicit and explicit components of motor learning have distinct underlying neural substrates. For example, even though patients with prefrontal lesions lacked any explicit awareness of changes during an abrupt visuomotor rotation task, they still partially adapted their reaching movements (Slachevsky et al. 2001, 2003). This result suggested that although the explicit aiming component is impaired, the implicit motor learning component is spared in such patients. Conversely, patients with cerebellar damage showed impairment when adapting to novel environments (Morton and Bastian 2004; Rabe et al. 2009; Tseng et al. 2007), regardless of the size or how the perturbation was imposed (Gibo et al. 2013; Schlerf et al. 2013). Although these patients could still compensate for the sensorimotor perturbations through either reinforcement learning (Izawa and Shadmehr 2011; Therrien et al. 2016) or the use of an explicit aiming strategy (Taylor et al. 2010), they still had impaired implicit error-based learning (Morehead et al. 2017; Taylor et al. 2010; Therrien et al. 2016) and displayed much smaller aftereffects after motor learning (Werner et al. 2010).
A Cerebellar Influence on the Tectoreticulospinal Pathway
Given that the cerebellum has been strongly implicated in implicit motor learning, we surmise that the changes in SLR tuning observed in experiments 1 and 2 are modulated via the cerebellum. How then could the cerebellum be altering this visuomotor mapping? We have speculated that the SLR is mediated by a tectoreticulospinal pathway (Gu et al. 2016; Pruszynski et al. 2010; Wood et al. 2015), and there is substantial evidence for interaction between the cerebellum and the reticular formation. Consistent with cerebellar projections to the reticular formation (Bantli and Bloedel 1975a; Cohen et al. 1958; Gonzalo-Ruiz et al. 1988), electrical stimulation to both human (Mottolese et al. 2013) and nonhuman primate (Bantli and Bloedel 1975b; Soteropoulos and Baker 2008) cerebellum evokes short-latency EMG response on upper limb muscles. These responses are still intact even after the inactivation of the contralateral primary motor cortex (Bantli and Bloedel 1975b). Furthermore, the cerebellum receives an internal copy of the descending reticulospinal command from propriospinal neurons via the lateral reticular nucleus (Azim et al. 2014).
The (tecto)reticulospinal pathway has also been implicated in other rapid motor responses such as the startReact effect (Carlsen et al. 2004; Honeycutt et al. 2013; Oude Nijhuis et al. 2007; Valls-Solé et al. 1995), forced-RT paradigms (Haith et al. 2015, 2016), or corrective reach movements (Carlton 1981; Day and Brown 2001; Reynolds and Day 2012). Our results, which demonstrate a selective influence of implicit motor learning on this descending pathway, may also explain the adaptation of these responses during various motor learning paradigms. For example, both startReact and corrective reach movements are modulated during motor learning induced by a force field (Franklin et al. 2012; Wright et al. 2015) or, as studied here, a visuomotor rotation (Hayashi et al. 2016; Telgen et al. 2014). However, the contribution of implicit vs. explicit components of motor learning was not considered in these paradigms. By isolating EMG activity attributable to the tectoreticulospinal pathway and segregating the implicit and explicit components of motor learning, we can directly quantify the influence of different components of motor learning via the changes in the tuning of the SLR. Such an approach may be particularly useful for future work on motor learning in animal models to directly quantify both the implicit and the explicit components via the SLR and eye tracking (de Brouwer et al. 2018), because these objective measures could serve as benchmarks for comparison with simultaneously recorded neural activity.
GRANTS
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Grants RGPIN-311680 (to B. D. Corneil), RGPIN-238338 (to P. L. Gribble), and RGPIN-2015-06714 (to J. A. Pruszynski), Canadian Institutes of Health Research Grant MOP-93796 (to B. D. Corneil), an NSERC Canada Graduate Doctoral Scholarship (to C. Gu), and a salary award from the Canada Research Chairs Program (to J. A. Pruszynski).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.G., J.A.P., P.L.G., and B.D.C. conceived and designed research; C.G. performed experiments; C.G. and B.D.C. analyzed data; C.G., J.A.P., P.L.G., and B.D.C. interpreted results of experiments; C.G. prepared figures; C.G. drafted manuscript; C.G., J.A.P., P.L.G., and B.D.C. edited and revised manuscript; C.G., J.A.P., P.L.G., and B.D.C. approved final version of manuscript.
REFERENCES
- Alamia A, Orban de Xivry JJ, San Anton E, Olivier E, Cleeremans A, Zenon A. Unconscious associative learning with conscious cues. Neurosci Conscious 2016: niw016, 2016. doi: 10.1093/nc/niw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci 35: 559–578, 2012. doi: 10.1146/annurev-neuro-062111-150527. [DOI] [PubMed] [Google Scholar]
- Atsma J, Maij F, Gu C, Medendorp WP, Corneil BD. Active braking of whole-arm reaching movements provides single-trial neuromuscular measures of movement cancellation. J Neurosci 38: 4367–4382, 2018. doi: 10.1523/JNEUROSCI.1745-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508: 357–363, 2014. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantli H, Bloedel JR. Monosynaptic activation of a direct reticulo-spinal pathway by the dentate nucleus. Pflugers Arch 357: 237–242, 1975a. doi: 10.1007/BF00585978. [DOI] [PubMed] [Google Scholar]
- Bantli H, Bloedel JR. The action of the dentate nucleus on the excitability of spinal motoneurons via pathways which do not involve the primary sensorimotor cortex. Brain Res 88: 86–90, 1975b. doi: 10.1016/0006-8993(75)90952-X. [DOI] [PubMed] [Google Scholar]
- Bond KM, Taylor JA. Flexible explicit but rigid implicit learning in a visuomotor adaptation task. J Neurophysiol 113: 3836–3849, 2015. doi: 10.1152/jn.00009.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher PA, Ivry RB, Kuo SH, Rydz D, Krakauer JW, Taylor JA. The cerebellum does more than sensory prediction error-based learning in sensorimotor adaptation tasks. J Neurophysiol 118: 1622–1636, 2017. doi: 10.1152/jn.00451.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen A, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Mot Behav 36: 253–264, 2004. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carlton LG. Processing visual feedback information for movement control. J Exp Psychol Hum Percept Perform 7: 1019–1030, 1981. doi: 10.1037/0096-1523.7.5.1019. [DOI] [PubMed] [Google Scholar]
- Chapman BB, Corneil BD. Neuromuscular recruitment related to stimulus presentation and task instruction during the anti-saccade task. Eur J Neurosci 33: 349–360, 2011. doi: 10.1111/j.1460-9568.2010.07496.x. [DOI] [PubMed] [Google Scholar]
- Cohen D, Chambers WW, Sprague JM. Experimental study of the efferent projections from the cerebellar nuclei to the brainstem of the cat. J Comp Neurol 109: 233–259, 1958. doi: 10.1002/cne.901090207. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Visual responses on neck muscles reveal selective gating that prevents express saccades. Neuron 42: 831–841, 2004. doi: 10.1016/S0896-6273(04)00267-3. [DOI] [PubMed] [Google Scholar]
- Day BL, Brown P. Evidence for subcortical involvement in the visual control of human reaching. Brain 124: 1832–1840, 2001. doi: 10.1093/brain/124.9.1832. [DOI] [PubMed] [Google Scholar]
- de Brouwer AJ, Albaghdadi M, Flanagan JR, Gallivan JP. Using gaze behavior to parcellate the explicit and implicit contributions to visuomotor learning. J Neurophysiol 120: 1602–1615, 2018. doi: 10.1152/jn.00113.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Goltz HC, DeSouza JF, Vilis T, Crawford JD. Human parietal “reach region” primarily encodes intrinsic visual direction, not extrinsic movement direction, in a visual motor dissociation task. Cereb Cortex 17: 2283–2292, 2007. doi: 10.1093/cercor/bhl137. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wong W, Armstrong IT, Flanagan JR. Relation between reaction time and reach errors during visuomotor adaptation. Behav Brain Res 219: 8–14, 2011. doi: 10.1016/j.bbr.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Franklin S, Wolpert DM, Franklin DW. Visuomotor feedback gains upregulate during the learning of novel dynamics. J Neurophysiol 108: 467–478, 2012. doi: 10.1152/jn.01123.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Sami SA, Albert NB, Miall RC. Secondary tasks impair adaptation to step- and gradual-visual displacements. Exp Brain Res 202: 473–484, 2010. doi: 10.1007/s00221-010-2158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Massey JT. Cognitive spatial-motor processes. 1. The making of movements at various angles from a stimulus direction. Exp Brain Res 65: 361–370, 1987. [DOI] [PubMed] [Google Scholar]
- Gibo TL, Criscimagna-Hemminger SE, Okamura AM, Bastian AJ. Cerebellar motor learning: are environment dynamics more important than error size? J Neurophysiol 110: 322–333, 2013. doi: 10.1152/jn.00745.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Leichnetz GR, Smith DJ. Origin of cerebellar projections to the region of the oculomotor complex, medial pontine reticular formation, and superior colliculus in New World monkeys: a retrograde horseradish peroxidase study. J Comp Neurol 268: 508–526, 1988. doi: 10.1002/cne.902680404. [DOI] [PubMed] [Google Scholar]
- Goonetilleke SC, Katz L, Wood DK, Gu C, Huk AC, Corneil BD. Cross-species comparison of anticipatory and stimulus-driven neck muscle activity well before saccadic gaze shifts in humans and nonhuman primates. J Neurophysiol 114: 902–913, 2015. doi: 10.1152/jn.00230.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Pruszynski JA, Gribble PL, Corneil BD. Done in 100 ms: path-dependent visuomotor transformation in the human upper limb. J Neurophysiol 119: 1319–1328, 2018. doi: 10.1152/jn.00839.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Wood DK, Gribble PL, Corneil BD. A trial-by-trial window into sensorimotor transformations in the human motor periphery. J Neurosci 36: 8273–8282, 2016. doi: 10.1523/JNEUROSCI.0899-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar S, Donchin O, Dinstein I. Dissociating visual and motor directional selectivity using visuomotor adaptation. J Neurosci 35: 6813–6821, 2015. doi: 10.1523/JNEUROSCI.0182-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. The influence of movement preparation time on the expression of visuomotor learning and savings. J Neurosci 35: 5109–5117, 2015. doi: 10.1523/JNEUROSCI.3869-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith AM, Pakpoor J, Krakauer JW. Independence of movement preparation and movement initiation. J Neurosci 36: 3007–3015, 2016. doi: 10.1523/JNEUROSCI.3245-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yokoi A, Hirashima M, Nozaki D. Visuomotor map determines how visually guided reaching movements are corrected within and across trials. eNeuro 3: ENEURO.0032-16.2016, 2016. doi: 10.1523/ENEURO.0032-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Hirashima M, Nozaki D. Adaptation to visual feedback delay influences visuomotor learning. PLoS One 7: e37900, 2012. doi: 10.1371/journal.pone.0037900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Kharouta M, Perreault EJ. Evidence for reticulospinal contributions to coordinated finger movements in humans. J Neurophysiol 110: 1476–1483, 2013. doi: 10.1152/jn.00866.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol 7: e1002012, 2011. doi: 10.1371/journal.pcbi.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115: 557–561, 1997. doi: 10.1007/PL00005727. [DOI] [PubMed] [Google Scholar]
- Kim HE, Parvin DE, Ivry RB. Intrinsic rewards modulate sensorimotor adaptation (Preprint). bioRxiv 363606, 2018. doi: 10.1101/363606. [DOI]
- Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164: 250–259, 2005. doi: 10.1007/s00221-005-2247-4. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol 629: 405–421, 2009. doi: 10.1007/978-0-387-77064-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci 25: 473–478, 2005. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers HG. Anatomy of the descending pathways. In: Handbook of Physiology. The Nervous System. Motor and Control, edited by Brookhart JM, Mountcastle VB. Bethesda, MD: American Physiological Society, 1981, p. 597–666. [Google Scholar]
- Lee D, Seo H, Jung MW. Neural basis of reinforcement learning and decision making. Annu Rev Neurosci 35: 287–308, 2012. doi: 10.1146/annurev-neuro-062111-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Leow LA, Marinovic W, de Rugy A, Carroll T. Task errors contribute to implicit remapping in sensorimotor adaptation (Preprint). bioRxiv 263988, 2018. doi: 10.1101/263988. [DOI] [PubMed]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead JR, Taylor JA, Parvin DE, Ivry RB. Characteristics of implicit sensorimotor adaptation revealed by task-irrelevant clamped feedback. J Cogn Neurosci 29: 1061–1074, 2017. doi: 10.1162/jocn_a_01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. J Neurophysiol 92: 2497–2509, 2004. doi: 10.1152/jn.00129.2004. [DOI] [PubMed] [Google Scholar]
- Mottolese C, Richard N, Harquel S, Szathmari A, Sirigu A, Desmurget M. Mapping motor representations in the human cerebellum. Brain 136: 330–342, 2013. doi: 10.1093/brain/aws186. [DOI] [PubMed] [Google Scholar]
- Oude Nijhuis LB, Janssen L, Bloem BR, van Dijk JG, Gielen SC, Borm GF, Overeem S. Choice reaction times for human head rotations are shortened by startling acoustic stimuli, irrespective of stimulus direction. J Physiol 584: 97–109, 2007. doi: 10.1113/jphysiol.2007.136291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Boraud T, Natan C, Bergman H, Vaadia E. Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nat Neurosci 6: 882–890, 2003. doi: 10.1038/nn1097. [DOI] [PubMed] [Google Scholar]
- Perich MG, Gallego JA, Miller LE. A neural population mechanism for rapid learning (Preprint). bioRxiv 138743, 2017. doi: 10.1101/138743. [DOI] [PMC free article] [PubMed]
- Pine ZM, Krakauer JW, Gordon J, Ghez C. Learning of scaling factors and reference axes for reaching movements. Neuroreport 7: 2357–2361, 1996. doi: 10.1097/00001756-199610020-00016. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, King GL, Boisse L, Scott SH, Flanagan JR, Munoz DP. Stimulus-locked responses on human arm muscles reveal a rapid neural pathway linking visual input to arm motor output. Eur J Neurosci 32: 1049–1057, 2010. doi: 10.1111/j.1460-9568.2010.07380.x. [DOI] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101: 1961–1971, 2009. doi: 10.1152/jn.91069.2008. [DOI] [PubMed] [Google Scholar]
- Reichenthal M, Avraham G, Karniel A, Shmuelof L. Target size matters: target errors contribute to the generalization of implicit visuomotor learning. J Neurophysiol 116: 411–424, 2016. doi: 10.1152/jn.00830.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RF, Day BL. Direct visuomotor mapping for fast visually-evoked arm movements. Neuropsychologia 50: 3169–3173, 2012. doi: 10.1016/j.neuropsychologia.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Xu J, Klemfuss NM, Griffiths TL, Ivry RB. Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. J Neurophysiol 109: 1164–1173, 2013. doi: 10.1152/jn.00654.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Shen L, Alexander GE. Neural correlates of a spatial sensory-to-motor transformation in primary motor cortex. J Neurophysiol 77: 1171–1194, 1997a. doi: 10.1152/jn.1997.77.3.1171. [DOI] [PubMed] [Google Scholar]
- Shen L, Alexander GE. Preferential representation of instructed target location versus limb trajectory in dorsal premotor area. J Neurophysiol 77: 1195–1212, 1997b. doi: 10.1152/jn.1997.77.3.1195. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Huang VS, Haith AM, Delnicki RJ, Mazzoni P, Krakauer JW. Overcoming motor “forgetting” through reinforcement of learned actions. J Neurosci 32: 14617–14621, 2012. doi: 10.1523/JNEUROSCI.2184-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slachevsky A, Pillon B, Fourneret P, Pradat-Diehl P, Jeannerod M, Dubois B. Preserved adjustment but impaired awareness in a sensory-motor conflict following prefrontal lesions. J Cogn Neurosci 13: 332–340, 2001. doi: 10.1162/08989290151137386. [DOI] [PubMed] [Google Scholar]
- Slachevsky A, Pillon B, Fourneret P, Renié L, Levy R, Jeannerod M, Dubois B. The prefrontal cortex and conscious monitoring of action: an experimental study. Neuropsychologia 41: 655–665, 2003. doi: 10.1016/S0028-3932(02)00225-7. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Baker SN. Bilateral representation in the deep cerebellar nuclei. J Physiol 586: 1117–1136, 2008. doi: 10.1113/jphysiol.2007.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenrod SC, Phillips MH, Goldberg ME. The lateral intraparietal area codes the location of saccade targets and not the dimension of the saccades that will be made to acquire them. J Neurophysiol 109: 2596–2605, 2013. doi: 10.1152/jn.00349.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. PLoS Comput Biol 7: e1001096, 2011. doi: 10.1371/journal.pcbi.1001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Klemfuss NM, Ivry RB. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum 9: 580–586, 2010. doi: 10.1007/s12311-010-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34: 3023–3032, 2014. doi: 10.1523/JNEUROSCI.3619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgen S, Parvin D, Diedrichsen J. Mirror reversal and visual rotation are learned and consolidated via separate mechanisms: recalibrating or learning de novo? J Neurosci 34: 13768–13779, 2014. doi: 10.1523/JNEUROSCI.5306-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien AS, Wolpert DM, Bastian AJ. Effective reinforcement learning following cerebellar damage requires a balance between exploration and motor noise. Brain 139: 101–114, 2016. doi: 10.1093/brain/awv329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature 407: 742–747, 2000. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett 195: 97–100, 1995. doi: 10.1016/0304-3940(94)11790-P. [DOI] [PubMed] [Google Scholar]
- Welford AT. Choice reaction time: basis concepts. In: Reaction Times, edited by Welford AT. New York: Academic, 1980, p. 73–128. [Google Scholar]
- Werner S, Bock O, Gizewski ER, Schoch B, Timmann D. Visuomotor adaptive improvement and aftereffects are impaired differentially following cerebellar lesions in SCA and PICA territory. Exp Brain Res 201: 429–439, 2010. doi: 10.1007/s00221-009-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Schorn CF, Bock O, Theysohn N, Timmann D. Neural correlates of adaptation to gradual and to sudden visuomotor distortions in humans. Exp Brain Res 232: 1145–1156, 2014. doi: 10.1007/s00221-014-3824-1. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998. doi: 10.1016/S1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Wood DK, Gu C, Corneil BD, Gribble PL, Goodale MA. Transient visual responses reset the phase of low-frequency oscillations in the skeletomotor periphery. Eur J Neurosci 42: 1919–1932, 2015. doi: 10.1111/ejn.12976. [DOI] [PubMed] [Google Scholar]
- Wright ZA, Carlsen AN, MacKinnon CD, Patton JL. Degraded expression of learned feedforward control in movements released by startle. Exp Brain Res 233: 2291–2300, 2015. doi: 10.1007/s00221-015-4298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were analyzed using MATLAB R2014b.