Abstract
Hydrogen sulfide (H2S) is a highly neurotoxic gas. It is the second most common cause of gas-induced deaths. Beyond mortality, surviving victims of acute exposure may suffer long-term neurological sequelae. There is a need to develop countermeasures against H2S poisoning. However, no translational animal model of H2S-induced neurological sequelae exists. Here, we describe a novel mouse model of H2S-induced neurotoxicity for translational research. In paradigm I, C57/BL6 mice were exposed to 765 ppm H2S for 40 min on day 1, followed by 15-min daily exposures for periods ranging from 1 to 6 days. In paradigm II, mice were exposed once to 1000 ppm H2S for 60 minutes. Mice were assessed for behavioral, neurochemical, biochemical, and histopathological changes. H2S intoxication caused seizures, dyspnea, respiratory depression, knockdowns, and death. H2S-exposed mice showed significant impairment in locomotor and coordinated motor movement activity compared with controls. Histopathology revealed neurodegenerative lesions in the collicular, thalamic, and cortical brain regions. H2S significantly increased dopamine and serotonin concentration in several brain regions and caused time-dependent decreases in GABA and glutamate concentrations. Furthermore, H2S significantly suppressed cytochrome c oxidase activity and caused significant loss in body weight. Overall, male mice were more sensitive than females. This novel translational mouse model of H2S-induced neurotoxicity is reliable, reproducible, and recapitulates acute H2S poisoning in humans.
Keywords: inhalation exposure, hydrogen sulfide, neurotoxicity, neurodegeneration, acute toxicity, translational model
Introduction
Hydrogen sulfide (H2S) is typical of compounds that are beneficial at normal physiological concentrations but toxic at high doses. Endogenously produced H2S functions as a signaling molecule in the brain.1,2 However, H2S in high acute-dose exposures is a severely toxic xenobiotic.3,4 H2S is a by-product of many industries, including the oil and gas industry, intensive animal farming operations, and sewer and waste treatment plants, and is present in gas-storage facilities.5–8 It is also used as a raw material for several industrial applications.8 It is classified as a highly toxic industrial compound and is the second leading cause of gas-induced death, after carbon monoxide.9,10 H2S is a colorless gas and has a distinctive rotten-egg odor. It is sometimes referred to as sewer gas. It is heavier than air, and therefore collects in low-lying, enclosed spaces, such as manholes, sewer lines, and manure pits, where it is a hazard.11,12 It can be made easily and inexpensively at home from raw materials commonly found in local stores. For this reason, it is increasingly being used as suicidal agent.13,14 H2S was used during World War I as a chemical weapon.15 For this reason, there is significant concern by the Department of Homeland Security about its potential misuse as a weapon of mass destruction, particularly in confined spaces, such as underground transit facilities.16 Mass civilian exposure to H2S could also occur if terrorists targeted H2S storage facilities or industrial plants producing or using this gas in or near highly populated areas.17 Industrial plant accidents can also lead to mass civilian population exposures and deaths, as occurred in the Poza Rica Mexico recycling incident18 or in sour-gas well blow-outs, as occurred in the Kaixian district in China, resulting in 243 civilian deaths.19
Acute toxicity and lingering long-term consequences of acute H2S exposure in humans have been reported for many years.5,20,21,25 Acute exposure to high levels of H2S has been reported to cause acute neurotoxic, cardiovascular, and respiratory effects, as well as long-term neurological sequelae, including neurodegeneration, memory and motor impairment, neuropsychiatric disturbances, moribund state, and even death.6,12,22–25 It is a systemic toxicant, but the central nervous system, respiratory, and cardiovascular systems are the most sensitive.6,26 Clinical signs of acute H2S intoxication include convulsions, respiratory distress, and acute death.6,26,27 H2S is also known as the “knockdown” gas. A few breaths at a high concentration can cause immediate collapse without warning.24 Some survivors of acute H2S exposure suffer lingering neurotoxic effects, including movement disorders, persistent headache, seizures, neurobehavioral and cognitive deficits, blindness, hearing impairment, and, sometimes, permanent vegetative states.6,12,22,23,25,28,29 The toxic mechanisms of H2S poisoning are not well known. However, ischemia–hypoxia, cytochrome c oxidase enzyme inhibition, changes in brain amino acid profiles, changes in neurotransmitters, and oxidative stress have been proposed as some of the neurotoxic mechanisms.30,31 Regardless of the trigger of H2S-induced neurotoxicity, the downstream cellular molecular mechanisms leading to neurodegeneration have yet to be defined.6,23,32
Currently, there is no antidote for the treatment of H2S poisoning, particularly for field treatment of mass civilian casualties in the event of terrorism or industrial accidents. Our laboratory is interested in the treatment of acute neurotoxicity and neurological sequelae of H2S-induced poisoning. The objective of this study was to develop a novel translational mouse model of H2S neurotoxicity to recapitulate human inhalation exposure and subsequent health effects. This model will be used to study H2S-induced neurotoxic mechanisms and as a benchmark to evaluate the efficacy of potential countermeasures for the treatment of acute H2S poisoning. Before commencement of this study, an extensive review of the literature revealed that existing animal models do not faithfully recapitulate the human exposure routes and or/focus on modeling H2S-induced mortality. For example, Jiang et al. used anesthetized mice in their H2S inhalation exposure model.33 In rat models, rats were injected with sodium hydrosulfide (NaHS) intraperitoneally, which does not faithfully reflect the common route of exposure in humans, which is inhalation.34,35 Other mouse models of inhalation exposure only reported metabolic effects and did not report behavioral changes or neurodegeneration.36,37 Our mouse model is unique because it is comprehensive and uses freely moving mice exposed to H2S by whole-body inhalation exposure, recapitulating the typical human exposure route. Most importantly, this mouse model reproduces significant neurological lesions in different parts of the brain and allows evaluation of drug candidates easily.
Material and methods
Chemicals and reagents
Methanol (high-performance liquid chromatography (HPLC) grade), acetonitrile (HPLC grade), MD-TM mobile phase, sodium dodecyl sulfate (SDS), Tris-Cl, phosphate buffered saline (PBS), Alexa Fluor 680 goat anti-mouse secondary antibody, and formic acid were obtained from Thermo Fisher Scientific (Waltham, MA). (d- and l-)Glutamic acid, γ-aminobutyric acid (GABA), dopamine (DA), 3,4 dihydroxyphenlyacetic acid (DOPAC), homovanillic acid (HVA), norepinephrine (NE), 5-hydroxtryptaamine (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), paraformaldehyde (PFA), ethylenediaminetetraacetic acid (EDTA), Triton X-100, sodium chloride (NaCl), ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), sodium fluoride (NaF), sodium orthovanadate (Na3VO4), sodium pyrophosphate tetrabasic (Na4P2O7), sodium deoxycholate, and 60% perchloric acid (HClO4) were purchased from Sigma–Aldrich (St. Louis, MO). l-Glutamic-2,3,3,4,4-d5 acid (Glu-d5) and 4-aminobutyric-2,2,3,3,4,4-d6 acid (GABA-d6) were purchased from C/D/N Isotopes (Pointe-Claire, Quebec, Canada). IRDye 800 donkey anti-rabbit secondary antibody was purchased from Rockland Antibodies and Assays (Limerick, PA). Li-COR Blocking buffer was purchased from LI-COR (Lincoln, Nebraska). Tris-buffered saline (TBS) and Tween-20 were purchased from Bio-Rad Laboratories (Hercules, CA). Fluoro-jade C was purchased from Histo-Chem, Inc. (Jefferson, AR). Vectastain Elite ABC HRP kit and diaminobenzidine were purchased from Vectastain Laboratories (Burlingame, CA). NeuN, Iba-1, and GFAP antibody were purchased from Abcam (Cambridge, UK). Aqueous solutions were prepared using 18.2 MΩ·cm water (Aries Filter work, Berlin Township, NJ).
Animals
All animal studies were approved by the Iowa State University Institutional Animal Care and Use Committee. The 7- to 8-week-old C57/BL6 mice used in these studies were purchased from the Jackson Laboratories(Bar Harbor, ME). In paradigm I experiments, both male and female mice were included in the experiment. In paradigm II experiments, only male mice were used. All mice weighed 20–25 g at the beginning of the experiment. Mice were housed five per cage, for each sex, in the Laboratory Animal Resource Facility of the Iowa State University College of Veterinary Medicine (ISU CVM, Ames, IA). They were housed at a room temperature of 68–70 °F, relative humidity of 35–50%, and a 12-h light/dark cycle and were provided 14% Protein Rodent maintenance diet (Teklad HSD Inc., WI) and drinking water ad libitum. Mice were acclimated for 1 week before the start of the studies.
H2S inhalation exposure
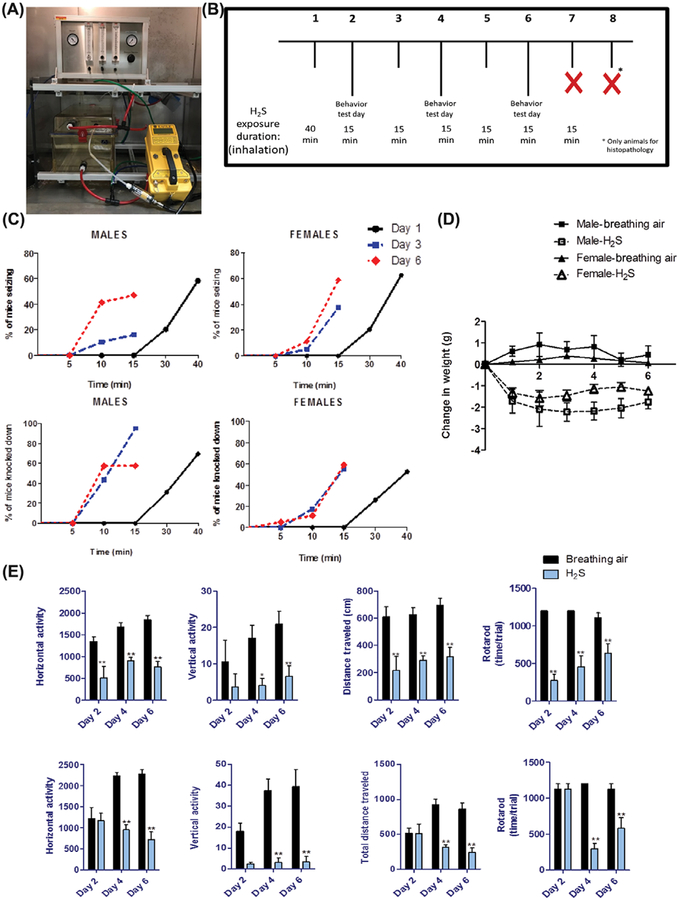
Fully conscious and freely moving mice were placed in a whole-body exposure chamber (Fig. 1A). The chamber is designed to hold up to 10 mice at a time. H2S is a highly toxic gas, and all experiments were conducted under a chemical fume hood certified by the Environmental Health & Safety at ISU. Gas access to the chamber was from two lines; one for a breathing air tank under pressure and the other from a tank containing H2S under pressure. The two lines connect to a control panel that allowed separate regulation of breathing air and gas in flow (l/min). Breathing air was introduced first, followed by H2S 2 min later. The concentration of H2S in the exposure chamber was constantly monitored using a H2S monitor (Environmental Equipment and Supply, Harrisburg, PA) that was custom designed to measure concentrations of up to 1000 ppm of H2S.
Figure 1.
(A) Whole-body inhalation chamber and system used in the present studies. (B) Treatment paradigm I of H2S-induced neurodegeneration in mice. (C) Seizures and knockdown induced by H2S in male and female mice (n = 8–10/group). (D) Weight loss in mice exposed to H2S (765 ppm) (n = 8–10/group). (E) Behavior deficits induced by H2S (765 ppm) in male and female mice (n = 8–10/group). *, P < 0.05; **, P < 0.01 compared with breathing-air controls of each sex.
Exposure paradigm I
Exposure paradigm I is summarized in Figure 1B. Mice were exposed to H2S by inhalation, either once or once followed by 2–7 short additional acute exposures. On the first day of H2S exposure, mice in groups of 10 were exposed to 765 ppm H2S for 40 minutes. On subsequent days, groups of mice were exposed to 765 ppm H2S for 15 min each day. Following each exposure, the chamber was flushed out with breathing air for 2 min before mice could be safely removed. Separate groups of mice were euthanized after one, three, or seven exposures 2–10 min following removal from the H2S exposure chamber. Mice in the negative control breathing air group were exposed following a similar paradigm but were euthanized after the seventh exposure. This experimental approach allowed progressive evaluation of clinical signs, behavioral deficits, and histo-logical lesions in brains of mice following a single or repeated short-term exposure to H2S over a 1-week period.
Exposure paradigm II
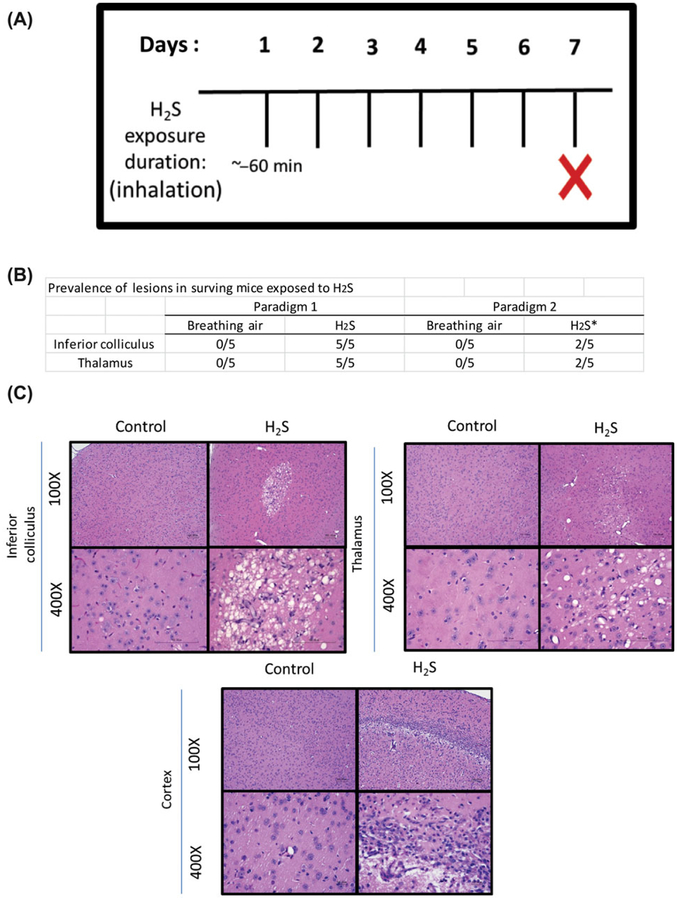
Exposure paradigm II is summarized in Figure 7A. This paradigm reflects the typical human exposure scenario of a single acute exposure to high H2S concentrations, followed by rescue. This experiment used only male mice, because results of previous experiments indicated that males are more sensitive to H2S than females. Separate groups of mice were exposed either to breathing air or 1000 ppm H2S by whole-body inhalation for 60 minutes. Following exposure, the chamber was flushed out with breathing air for 2 min before mice were retrieved from the chamber. Mice were euthanized 7 days after H2S exposure for histopathological evaluation of brain lesions using the same protocol as in paradigm I above.
Figure 7.
(A) Treatment paradigm II of H2S-induced neurodegeneration in mice. (B) Prevalence of lesions in paradigm I versus paradigm II in the inferior colliculus and thalamus. (C) Vacuolization of the neuropil and neuronal death in the inferior colliculus, thalamus, and cortex of mice exposed to H2S (1000 ppm) (n = 5/group).*, 30% died in chamber during H2S exposure. 100×.
Clinical assessment
To obtain baseline data, animals were clinically evaluated and weighed starting 3 days before H2S exposure. Mice were weighed daily until euthanasia. Toxicity signs monitored during H2S exposure were a modification of the McDaniel and Moser functional observational battery.38 Adopted end points included dyspnea, seizure activity, and latency to seizure activity. Knockdown, a clinical effect of H2S poisoning presenting in mice as lateral recumbency following seizure activity, was an end point that we added. The time latency to knockdown and number of mice in knockdown were recorded. Mice were evaluated for these clinical signs during exposure in the chamber and again 2 h postexposure. For post-exposure assessment, mice were removed from their home cages and placed individually in an empty chamber for 2 min to assess gait, posture, tremors, or convulsions. The same trained observer assessed the mice throughout the entirety of the experiment.
Behavior testing
Behavior assessments for open field activity were performed 3 h after mice were exposed to H2S. This was performed after two, four, or six H2S exposures using a protocol adapted from Ghosh et al.39 Briefly, an automated computer-controlled device (Model RXYZCM-16; Accuscan, Columbus, OH) was used to measure the spontaneous activity of mice in this open-field test. The dimensions of the activity chamber were 40 × 40 × 30.5 cm; it was made of clear Plexiglass and covered with a Plexiglass lid with holes for ventilation. Data were collected and analyzed by a VersaMax Analyzer (Model CDA-8; AccuScan). Mice were acclimated to the chamber 2 days before H2S exposure. On test days, mice were placed inside the infrared monitor for 2 min to acclimate to the chamber. Open-field activities were recorded for 10-min test sessions assessing multiple parameters, including vertical activity, horizontal activity, and distance traveled. To test coordination and balance, mice were subjected to the AccuRotor 4-Channel RotaRod test (rod diameter = 30 mm, height = 38 cm). Mice were also trained on the rotating rod for 2 consecutive days before H2S exposure at 24 rpm for 20 minutes. Following H2S exposure, mice were given five trials for 20 min at 24 rpm on test days after H2S exposure.
Thiosulfate analysis
Thiosulfate is a good biomarker of H2S exposure. Blood was allowed to clot at room temperature for 15 min and then spun down at 2900 × g for 10 minutes. The serum was analyzed for thiosulfate concentration according to Togawa et al.40 Briefly, a 50-μL serum sample was added to a 7-dram glass vial, along with 50 μL water and 100 μL 5 mM monobromobimane. This was vortexed and incubated for 1 h at room temperature in the dark. A standard curve was prepared by adding 100 μL water and 100 μL 5 mM monobromobimane as a blank along with three standards of 55, 110, and 220 ng of thiosulfate adjusted with water to a volume of 100 μL. A 100 μL volume of 5 mM monobromobimane was added to all samples. After 1 h, 100 μL of stopping reagent 0.05 M KCl–HCl (pH 2.0) was added to all samples. All samples were analyzed by HPLC with fluorescence detection. The mobile phase consisted of 25 mM sodium perchlo-rate (pH 3.0) and acetic acid:acetonitrile ratio of 70:30 V:V, with a flow rate of 1 mL/min on a Waters separation module 2695 HPLC system. A Waters 2475 fluorescent detector was used with a 396 nm excitation and 476 nm emission. The thiosulfate was separated on a Dionex WAX-1 150 × 4.6 mm column; retention time was approximately 15 minutes.
Histopathology and immunohistochemistry
Mice were euthanized 24 h after the last H2S exposure. First, mice were anesthetized deeply with a cocktail of 100 mg/kg body weight ketamine and 10 mg/kg body weight xylazine given intramuscularly. Once in a surgical plane of anesthesia, the thoracic cavity was surgically opened to expose the heart. Fresh 4% PFA solution (PFA, pH 7.4) was then injected through the left ventricle to perfuse the animal. After perfusion, brains were postfixed in 4% PFA for 24 h, paraffin embedded, sectioned at 5 μm, and stained with hematoxylin and eosin for routine histopathology. Additional brain sections were stained using fluoro-jade C or stained using an indirect immunostaining protocol that employed primary antibodies directed against NeuN, GFAP, Iba-1, or 4-HNE. Diaminobenzidine was the chromogen used. Stained sections were examined microscopically using a Nikon Eclipse Ci-L microscope with DS-Fi2 camera or EVOS FL fluorescence microscope. Routine histopathology, as well as immunohistopathology, was conducted by a board-certified pathologist blinded to the study design. The semiquantitative scale used for scoring the severity of lesions is summarized in Table S1.
Cytochrome c oxidase enzyme activity
Mice for all biochemical assays were euthanized by decapitation. Brains were immediately removed from the skull and microdissected into different brain regions on ice. Brain tissue samples were stored at −80 °C until analysis. Cytochrome c oxidase enzyme was extracted from microdissected brain regions, and enzyme activity determined using an assay kit (ab109909) from Abcam (Cambridge, MA) according to the manufacturer’s protocol.
Neurochemical analysis
Monoamines.
To determine H2S-induced neurochemical changes, different brain regions were analyzed for changes in DA and its metabolites, DOPAC and HVA. Samples from these brain regions were also analyzed for serotonin (5-HT), its metabolite 5-HIAA, and NE. Samples were prepared and quantified as described previously.41 Briefly, neurotransmitters were extracted from different brain regions in 0.2 M perchloric acid solution containing 0.05% Na2EDTA, 0.1% Na2S2O5, and isoproterenol (internal standard).
DA, 5-HT, NE, and their respective metabolites were separated isocratically by a reverse-phase column with a flow rate of 0.6 mL/min mobile phase (10% acetonitrile, 1% sodium phosphate, and 89% water) using a Dionex Ultimate 3000 HPLC system (pump ISO-3100SD, Thermo Scientific, Bannockburn, IL) equipped with a refrigerated automatic sampler (model WPS-3000TSL). The electrochemical detection system included a CoulArray model 5600A coupled with an analytical cell (microdialysis cell 5014B) and a guard cell (model 5020). Data acquisition and analysis were performed using Chromeleon 7 and ESA Coularray 3.10 HPLC Software.
GABA/glutamate.
Microdissected mouse brain tissues were weighed and transferred to 1.5-mL Eppendorf tubes. All samples were spiked with 10 μL Glu-d5 (1400 μg/mL) and GABA-d6 (780 μg/mL) as internal standards and mixed thoroughly. This procedure was performed on ice. Subsequently, samples were extracted with 900 μL methanol/water 85/15 (v/v)42 and vortex-mixed for 5 min at 2500 rpm on a multitube vortexer for thorough mixing. The mixtures were then filtered through 0.45-μm filter disks and subjected to liquid chromatography‒mass spectroscopy (LC–MS/MS) analysis.
GABA and glutamate levels were measured by LC–MS/MS analysis on a Quattro Premier XE triple quadruple mass spectrometer (Milford, MA), equipped with a Waters e2695 HPLC separation module. The separation of GABA and glutamic acid was performed using a reversed phase C18 HPLC column (Luna, 150 × 4.6 mm, 5 μm) obtained from Phenomenex (Torrance, CA). The mobile phase consisted of 0.1% formic acid aqueous solution (A) and acetonitrile (B). The analysis was performed using an isocratic elution of 75%:25% mobile phase A:mobile phase B at a rate of 0.6 mL/min. Column temperature was 30 °C, and autosampler temperature was 4 °C. Analytes were detected in the positive electrospray ionization mode with a capillary voltage of 3.1 kV. The source temperature was 350 °C. The desolvation gas flow rate was 800 L/h; the cone gas flow rate was 20 L/h. Data were acquired and analyzed using MassLynx version 4.1 (Waters, Mil-ford, MA).
Western blot
Microdissected brain tissues were lysed in modified RIPA lysis buffer (1% Triton X-100, 1 mM EDTA, 100 mM NaCl, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 50 mM Tris-HCl, and pH 7.4) via sonication. Brain homogenates were prepared as described previously.43 Protein concentration of samples was measured using the Bradford assay. Western blotting was performed as described previously.43 Briefly, samples containing equal amounts of proteins were loaded and fractionated in a 10–12% SDS-PAGE gel and transferred onto a nitrocellulose membrane. Membranes were blocked with 5% milk in TBS supplemented with 0.1 % Tween-20 or LI-COR blocking buffer. Primary antibodies against specific proteins were incubated with the membrane overnight at 4 °C. After rinsing thoroughly in PBS supplemented with 0.1% Tween-20, the membrane was incubated with Alexa Fluor 680 goat anti-mouse or IRDye 800 donkey anti-rabbit secondary antibodies. For the loading control, β-actin antibody was used. Immunoblot imaging was performed with an Odyssey Infrared Imaging system (LI-COR, Lincoln, NE). ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantify western blot bands.
Data analyses
Data are presented as mean and standard error of the mean. Biochemical and neurochemical end points were analyzed using a Student’s t-test comparing the H2S-exposed mice to the breathing air mice. Behavioral data were analyzed using two-way analysis of variance (ANOVA) with a post hoc test comparing the H2S groups with the breathing air group. End point data from male and female mice were compared using ANOVA models with treatment, gender and their interaction, and fixed effects. Behavioral data were analyzed using repeated-measures ANOVA models, with treatment, day, gender and their interactions, and fixed effects, and mouse as the subject of repeated measures. Statistical tests were performed on Prism 6 (GraphPad Prism Software) or SAS. A P value of less than 0.05 was accepted as statistically significant.
Results
Paradigm I
H2S-induced seizures and knockdowns in mice.
Both male and female mice exposed to H2S showed similar clinical toxic effects. The main clinical effects of H2S exposure are summarized in Table S2. Mice exposed to H2S exhibited hunched posture, pilo-erection, and dyspnea during exposure. Neurologic effects observed during exposure included tonic–clonic seizures, knockdown, and ataxia (Table S2). Before seizure activity, mice exhibited characteristic running fits followed by tonic–clonic seizures with paddling of limbs and urination. Salivation was frequently observed in mice in the knockdown state. Upon termination of gas exposure, mice regained consciousness and activity within 5–10 min. For mice that received more than one exposure, a notable observation was that H2S-exposed mice showed increased sensitivity with each subsequent exposure. For example, during the first exposure, the seizures were not observed until after 20 min of H2S exposure, whereas seizures were observed as early 5 min after subsequent exposures (Fig. 1C). The average time until mice experienced the first seizures and knockdown was shorter in males than in females, indicating that males are more sensitive than females. In males, the average time at first seizure was 21±6 min, while in females it was observed at 29±4 minutes. The first knockdown was observed at 25 ± 9 min in males, compared with 27±4 min in ±females. Cumulative mortality at the end of the study was relatively low: 15% for male mice and 13% for female mice. H2S also caused a statistically significant loss in body weight in both male and female mice compared with controls. Negative control mice exposed to breathing air continued to gain weight during the course of the study (Fig. 1D).
Evaluation of behavioral deficits caused by H2S exposure was tested by performance on the RotaRod and VersaMax open-field tests. Mice exposed to H2S had a statistically significant reduction in locomotor activity in the open-field test on the basis of vertical activity, horizontal activity, and total distance traveled compared with negative controls. On the RotaRod, mice exposed to H2S fell off the RotaRod at a significantly shorter time compared with negative controls. Statistically significant changes were observed on all days of behavioral assessment for males, including following a single exposure. In female mice, however, changes were observed on days 4 and 6, again suggesting higher susceptibility of males than females (Fig. 1E).
Thiosulfate is an oxidation product of H2S and is used as a biomarker of H2S exposure.14,44 In this model, thiosulfate levels were significantly increased in H2S-exposed mice in both sexes compared with control mice(Fig. S1). Our data provide further confirmation over the validity of our model to assess H2S-induced neurodegenerative changes. Collectively, these results indicate that H2S caused significant motor impairment in mice exposed to H2S relative to breathing-air negative control, with the males being more sensitive than females.
H2S-induced lesions in the brain.
H2S exposure induced morphological changes in select brain regions. Brain regions affected and the severity of neurological lesions evaluated are summarized in Figure 2. Neurodegeneration was observed in different brain regions but especially in the inferior colliculus (IC) and the thalamus. Severity of the lesion increased with additional exposures and varied from activated glial cells and oxidative stress with one or two exposures to frank bilateral or, much less commonly, unilateral, well-circumscribed necrosis in the IC and or thalamus on day 7 (Fig. 2). Bilateral neurodegeneration and necrosis of the IC were consistently present following 7 days of H2S exposure.
Figure 2.
Vacuolization of the neuropil and neuronal death in the inferior colliculus and thalamus of mice brains after one, three, or seven exposures to H2S (765 ppm). (A) Severe and widespread necrosis and glial response in the IC (arrows) after 7 days of exposure to H2S. Size bars = 1000 μm for 10× and 100 μm for 400×. The attached graph summarizes changes in histopathology over time. (B) Histopathology score determined from semiquantitative grading scheme in Table S2 (n = 5/group). ***, P < 0.001 compared with breathing-air controls.
This was the first region to be affected, and lesions were noticeable as early as 3 days after H2S exposure. The thalamus was the second most frequently affected region after the IC. As in the IC, thalamic lesions were also well demarcated from adjacent, nonnecrotic tissue. In both regions, lesions began as fine vacuolation of the neuropil, followed by gliosis and degeneration and necrosis of neurons, ultimately leading to loss of all cellular components. Mild-to-moderate hemorrhage was also frequently observed, especially in the IC region. Late-stage morphological changes were characterized by gliosis and Gitter cell phagocytosis of cellular debris, as well as proliferation of capillaries in necrotic areas.
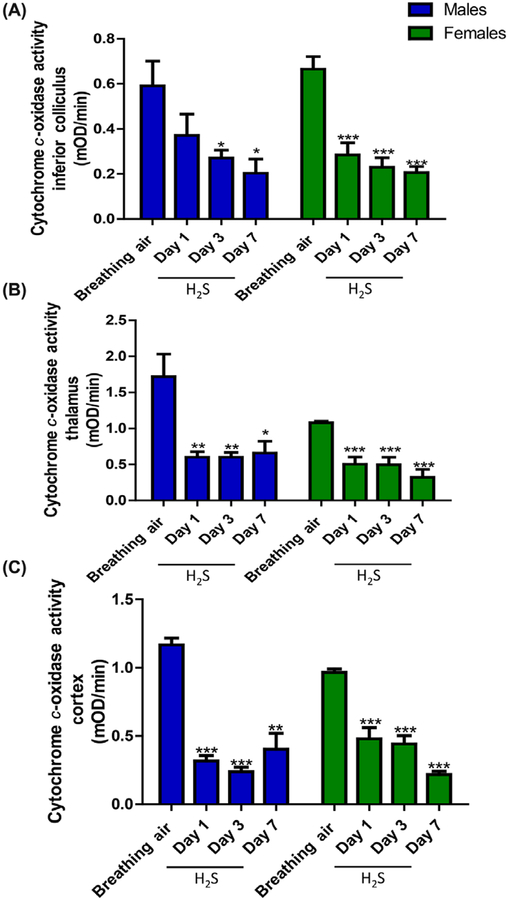
H2S-inhibited cytochrome c oxidase activity in mice.
We characterized the effects of H2S in different brain regions over time. Results are summarized in Figure 3. H2S inhibited cytochrome c oxidase activity in all brain regions examined in this study, compared to negative control mice (P < 0.05). By day 7, H2S exposure resulted in 70% inhibition of cytochrome c oxidase enzyme activity in the IC of both male and female mice, indicating that the effects of H2S on this enzyme are cumulative. There were no statistically significant differences between male and female mice with respect to enzyme inhibition by H2S. Statistically significant inhibition of cytochrome c enzyme activity was also observed in the thalamus and in other brain regions where histologic lesions were not prominent, such as the cortex (Fig. 3C).
Figure 3.
Decrease in cytochrome c oxidase activity in (A) inferior colliculus, (B) thalamus, and (C) cortex of male and female mice following exposure to H2S (765 ppm) (n = 3–5/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with breathing-air controls.
H2S-induced changes in neurotransmitter levels in various brain regions.
Because of the observed clinical, behavioral, and morphologic changes in H2S-exposed mice, we measured DA, 5-HT, NE, and their metabolites in different brain regions. Results are summarized in Table 1. There were statistically significant differences among neurotransmitters, which were dependent on anatomical location. Interestingly, DA concentration, with a few exceptions, was significantly increased across all brain regions examined (Table 1). These results indicate that H2S exposure induced statistically significant changes in monoamine neurotransmitter content across the brain regions studied, including those where neurodegeneration was not manifested (Table S3).
Table 1.
Dopamine and metabolite changes in various brain regions of mice following exposure to 765 ppm of H2S
| H2S | ||||||
|---|---|---|---|---|---|---|
| Breathing air | Day 1 | Day 3 | Day 7 | |||
| Inferior colliculus | Dopamine | |||||
| Male | 100.0 ± 3.33 | 162.4 ± 20.29** | 146.3 ± 9.87** | 173.5 ± 26.45* | ||
| Female | 100.0 ± 7.13 | 195.3 ± 23.00* | 166 ± 15.97** | 164 ± 11.99** | ||
| DOPAC | ||||||
| Male | 100.0 ± 7.41 | 272.6 ± 44.19** | 148.9 ± 9.56* | 186.3 ± 32.42* | ||
| Female | 100.0 ± 3.31 | 210.3 ± 25.85* | 135 ± 20.43** | 157.4 ± 11.75** | ||
| HVA | ||||||
| Male | 100.0 ± 3.26 | 129.1 ± 7.04** | 139.1 ± 8.14** | 150 ± 20.53* | ||
| Female | 100.0 ± 13.05 | 155.1 ± 12.59* | 74.07 ± 25.58 | 112.5 ± 8.82 | ||
| Thalamus | Dopamine | |||||
| Male | 100.0 ± 31.89 | 214.7 ± 89.37 | 75.39 ± 58.87 | 138.5 ± 62.97 | ||
| Female | 100.0 ± 16.82 | 80.58 ± 15.67 | 62.35 ± 6.99 | 51.86 ± 20.70 | ||
| DOPAC | ||||||
| Male | 100.0 ± 16.82 | 80.58 ± 15.67 | 62.35 ± 6.99 | 51.86 ± 20.70 | ||
| Female | 100.00 ± 20.42 | 103.5 ± 15.74 | 38.57 ± 2.26 | 56.81 ± 18.02 | ||
| HVA | ||||||
| Male | 100.0 ± 20.99 | 180.3 ± 26.67 | 79.74 ± 48.29 | 218.7 ± 49.09 | ||
| Female | 100.0 ± 14.33 | 77.47 ± 13.55 | 64.54 ± 7.55 | 65.33 ± 24.21 | ||
| Cortex | Dopamine | |||||
| Male | 100.00 ± 19.07 | 192.9 ± 75.87 | 241.9 ± 35.64** | 191.1 ± 42.53 | ||
| Female | 100.0 ± 9.05 | 127.1 ± 21.2 | 159.9 ± 41.83 | 104.6 ± 7.36 | ||
| DOPAC | ||||||
| Male | 95.6 ± 14.73 | 99.94 ± 32.47 | 217.2 ± 34.50* | 126.8 ± 9.04 | ||
| Female | 95.6 ± 8.54 | 118.7 ± 18.99 | 186.9 ± 43.53 | 108 ± 7.35 | ||
| HVA | ||||||
| Male | 95.6 ± 10.97 | 116.1 ± 32.05 | 230.4 ± 30.91** | 128.5 ± 11.12 | ||
| Female | 95.6 ± 8.58 | 113.3 ± 17.8 | 186.6 ± 42.63 | 114.1 ± 11.4 | ||
| Striatum | Dopamine | |||||
| Male | 95.6 ± 11.84 | 171 ± 28.32** | 163.6 ± 52.26 | 104.2 ± 18.66 | ||
| Female | 100.0 ± 17.74 | 187.2 ± 44.11 | 100.1 ± 8.93 | 83.67 ± 6.43 | ||
| DOPAC | ||||||
| Male | 100.0 ± 10.5 | 131.9 ± 15.84 | 132 ± 10.79 | 152.7 ± 34.4 | ||
| Female | 100.0 ± 19.77 | 59.1 ± 41.65 | 15.17 ± 13.52** | 2.16 ± 0.52** | ||
| HVA | ||||||
| Male | 100.0 ± 8.19 | 142.8 ± 22.03 | 125.1 ± 6.84 | 131.2 ± 20.09 | ||
| Female | 100 ± 21.92 | 166.3 ± 36.74 | 97.2 ± 7.49 | 94.36 ± 6.11 | ||
Note: n = 4–5/group.
P < 0.05;
P < 0.01 compared with breathing-air controls.
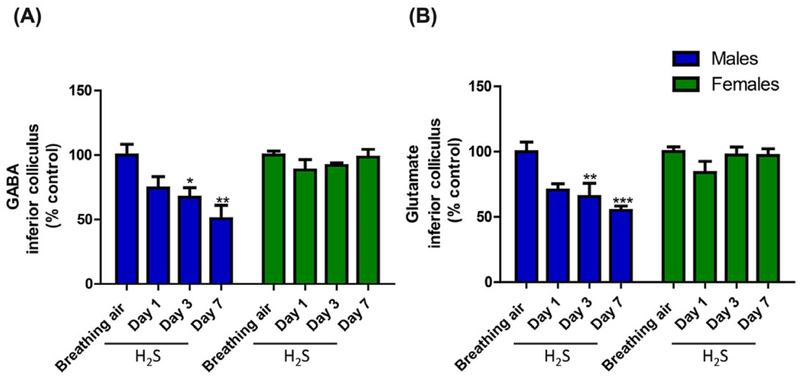
GABA and glutamate play roles in seizure activity, so we investigated the effect of H2S on the levels of these neurotransmitters. The results are summarized in Figure 6. In male mice only, H2S caused a significant decrease in GABA and glutamate following three or seven exposures compared with breathing-air negative controls (Fig. 4A and B). These changes were not observed in female mice.
Figure 6.
Oxidative stress in the inferior colliculus of male mice following exposure to H2S (765 ppm) (n = 3/group). *, P < 0.05 compared with breathing-air controls.
Figure 4.
Changes in (A) GABA and (B) glutamate levels in the inferior colliculus of mice following exposure to H2S (765 ppm) (n = 5/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with breathing-air controls.
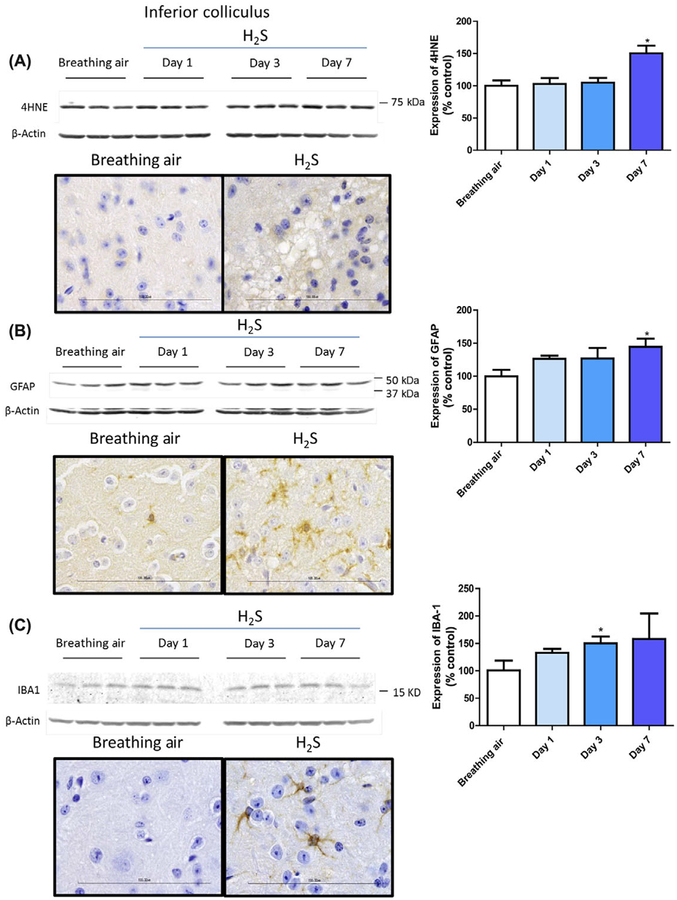
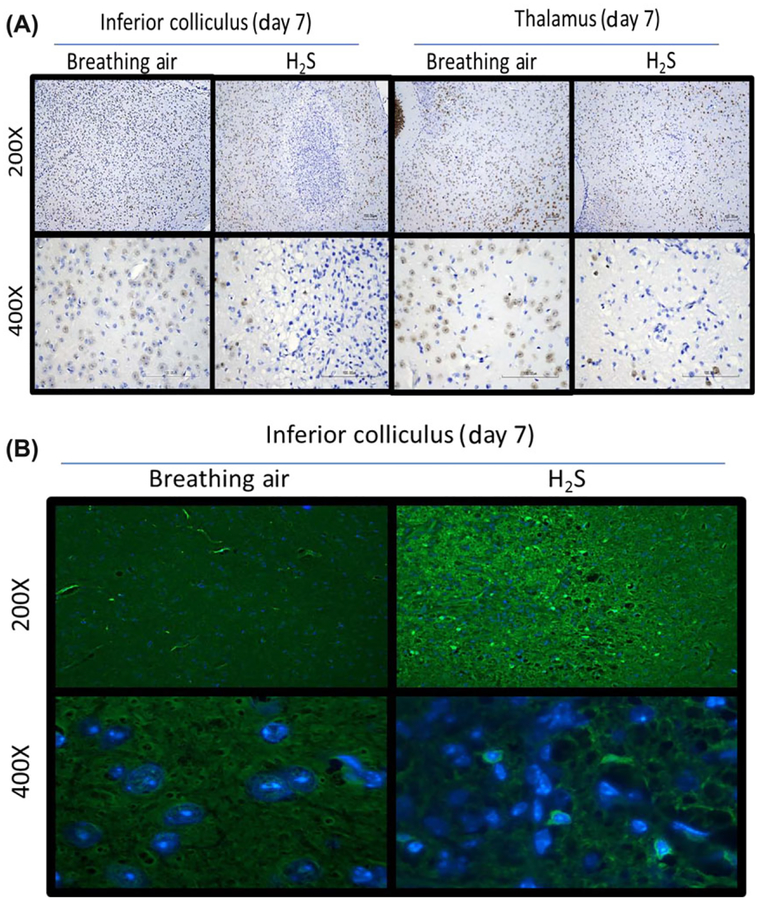
H2S-induced neuronal loss and oxidative stress.
Immunohistochemical studies focused on the IC and thalamus. Immunohistochemical staining with antibody directed at NeuN, a marker of neurons, revealed loss of neurons in these regions (Fig. 5A). Immunostaining with fluoro jade C revealed degenerating neurons in the IC of H2S-exposed mice (Fig. 5B). Therefore, neuronal cell loss in these brain regions is a major effect of H2S-induced neurotoxicity. We also examined the level of 4-hydroxynonenol (4-HNE), a by-product of lipid peroxidation, via western blot analysis and immunohistochemistry. H2S exposure produced 50% more 4-HNE in the IC on day 7 of H2S exposure compared with controls (Fig. 6A). Ionized calcium-binding adaptor molecule 1 (Iba-1), a biomarker of microglial activation, was significantly increased by more than 50% on day 3 (Fig. 6B). Expression of GFAP was also induced by H2S exposure, reflecting activated astrocytes (Fig. 6C). These results indicate the roles of oxidative stress and neuroinflammation in H2S-induced neurotoxicity.
Figure 5.
(A) Loss of neurons (NeuN) in the inferior colliculus and thalamus of mice following exposure to H2S (765 ppm). (B) Degenerating neurons, stained with fluoro jade c, (white arrows) within a representative inferior colliculus of mice following exposure of H2S (n = 6/group).
Exposure paradigm II
H2S-induced seizures and knockdowns in mice.
A single acute exposure to a higher concentration of H2S (1000 ppm for 60 min) resulted in mice exhibiting similar clinical signs as in paradigm I but with high mortality. Mice started seizing within 6–9 min of H2S exposure, and manifested knockdown by 10–16 min of exposure. Seizure activities were similar to those described in paradigm I. Cumulative mortality at the end of the 7-day study was 40%.
H2S-induced lesions in the brain.
Approximately 40% of surviving mice manifested neurological lesions, compared with 100% of the mice in paradigm I (Fig. 7B). Control animals had no lesions. A single exposure to a high concentration of H2S also resulted in vacuolar lesions with degeneration and loss of neurons and formation of a glial response, similar to those observed in animals in exposure paradigm I. Brain regions affected included the IC, thalamus, and additional foci of degeneration, necrosis, and scarring in the cortex (one of five mice) and pons (two of five mice) in the single, acute exposure (Fig. 7C). The animal that had a cortical lesion had a large, unilateral area of necrosis in the frontal and parietal region with marked vacuolization and neuron loss in layer 4 of the cortex, neuronal degeneration and necrosis in more superficial layers, and moderate gliosis and endothelial hypertrophy and hyperplasia in all layers of the affected cortical regions.
Discussion
The goal of this study was to develop a rodent model of H2S-induced neurotoxicity, with a relevant route to human exposure, for characterizing mechanisms of H2S-induced neurotoxicity and for use in translational studies aimed at evaluating the efficacy of countermeasures against H2S neurotoxicity.2 This study has led to the development of a novel inhalation mouse model of H2S-induced neurotoxicity and neurodegeneration that recapitulates many of the features of the human condition,2 including clinical signs, behavioral responses, and neurodegeneration.5,45–48 There have been some recent attempts at developing such models, but the routes of H2S exposure employed were not relevant to chemical terrorism or farm/industrial H2S exposure. These animal models also failed to express reproducible brain lesions in an appreciable number of test subjects. For example, a rat model demonstrated neuronal necrotic lesions in the superficial and middle layers of the cerebral cortex and posterior thalamus, but the route of H2S exposure was by intraperitoneal (IP) injection of NaHS.35 This study was also limited in scope, focusing on histopathological and behavioral end points, without evaluating neurochemical and biochemical changes. This model was characterized by high mortality, with only a small number of rats surviving for morphological evaluation of neuropathology. Another excellent study using a similar rat model of IP NaHS injection highlighted the difficulty of inducing brain lesions in this model.34 Only one in three surviving rats that received a higher dose demonstrated frank lesions 1 week after exposure.34 In an inhalation mouse model using anesthetized mice, the focus of the study was biochemical changes, and neurodegeneration was not evaluated.33 None of these alternative models examined sex differences in rats or mice. An additional advantage of our mouse model, besides using the appropriate route of exposure in conscious mice, is that multiple end points were evaluated, and neurotoxicity and neurodegeneration were assessed using various exposure paradigms to assess the progression and severity of H2S-induced toxicity. Additionally, gender-specific differences in susceptibility to H2S-induced neuropathology were also evaluated, which were lacking in other models.
The mechanisms underlying acute toxicity and continued neurodegeneration of victims of H2S toxicity are not well understood, but cytochrome c oxidase inhibition, ATP depletion, and ischemia/hypoxia are often cited as causal to H2S-induced neurodegeneration.30,49–51 H2S exposure has been known to cause a wide array of neurological clinical effects, including persistent headaches, nausea, lethargy, seizures, dizziness, abnormal reflexes, sleeping disorders, hearing impairment, movement disorders, and loss of consciousness, sometimes leading to permanent vegetative states.21 All mice in this study exhibited seizures and knockdown effects during H2S exposure, replicating what is commonly reported in humans. Humans have also been reported to have a lower threshold to H2S-induced neurotoxicity following additional H2S exposure, as was observed in this mouse model.50 Mice receiving repeated H2S exposure were more vulnerable to H2S poisoning. In a pilot dose range–finding study preceding this detailed study, upon a second exposure to 765 ppm H2S for 40 min, there was almost 100% mortality; yet, on first exposure, only about 5%mortality was observed at the end of a 40-min exposure. The earlier manifestations of seizures and knockdown in mice in this study upon subsequent second and third exposures, compared with a single naive exposure, further confirm the validity of our model and its relevance to human exposure. In a study conducted on female mice exposed four times to 100 ppm at 4-day intervals, Savolainen et al. reported that biochemical effects of repeated H2S exposure are cumulative.37 Collectively, these results suggest that the acute effects of H2S-induced neurotoxicity manifested in mice in paradigm I reflect cumulative effects of H2S, as was observed by Savolainen et al.37 This study showed cumulative inhibition of cytochrome c oxidase activity, neuroinflammatory response, and reduced GABA and glutamate with repeated short-term exposures. The mechanisms of these cumulative effects are beyond the scope of this study and should be the subject of future investigations. Cumulative effects are likely one of the factors contributing to enhanced sensitivity of mice to subsequent acute H2S exposures.
The brain is a primary target organ of H2S intoxication, and many victims of acute H2S poisoning report persistent neurological problems after H2S exposure, including movement disorders, such as spastic gait.22,23,25,50 Consistent with this observation, both open-field tests and RotaRod tests revealed impairments in locomotor activity and motor coordination in mice exposed to H2S. Interestingly, male mice were more sensitive to these motor deficits than females, and males displayed these symptoms earlier than females. The notion that male mice are more sensitive than female mice is further supported by the observation that time to seizure induction was shorter in males than females and that only male mice manifested significant inhibition of GABA. The reasons for these qualitative sex differences in response to H2S poisoning are not currently clear. Future studies to examine the role of hormones in these qualitative differences are recommended.
It is interesting that H2S induced brain region–specific neurodegeneration and necrosis. Necrosis in the IC and thalamus of H2S-exposed mice suggests that these regions are the most vulnerable to H2S-induced neurotoxicity. Severe neuronal damage and an intense glial response induced by H2S bear close resemblance to that induced by NaHS and carbonyl sulfide (COS) gas, a precursor of H2S.35,52 Unlike NaHS and COS, which cause severe cortical necrosis, H2S exposure by inhalation did not result in severe cortical necrosis in this mouse model, although the cortex was also affected. The mechanisms underlying the selective sensitivity of the IC and thalamus to H2S-induced neurodegeneration are not clear. However, the IC is a brain region known to receive the highest blood supply and to have a very high metabolic rate.53 The IC is part of the auditory pathway; it is interesting that hearing impairment is a frequently reported lingering effect of H2S intoxication in humans.50 The thalamus is a key brain region that is at the intersection of key neural tracts.54 Neurodegeneration in this region can virtually manifest in many of the clinical signs reported in human victims of H2S poisoning, including migraines.55 It will be of interest to determine why other regions are not vulnerable to the same extent as the IC and thalamus. Considering the cytochrome c oxidase enzyme activity was globally affected in the brain, inhibition of this enzyme by H2S is unlikely to be the explanation for regional differential susceptibilities. Previous studies suggest that ischemia–hypoxia could be contributing factors to the neurodegeneration induced by H2S;30,34,36 further studies would need to be conducted to elucidate the exact role that hypoxia plays in H2S-induced neurodegeneration.
A key observation in this study is that histopathological and immunohistopathologic findings were readily reproducible and served as reliable end points to assess the severity of H2S-induced neurotoxicity in this model. We therefore recommend histopathology and immunohistopathology as key end points for translational studies evaluating the efficacy of medical countermeasures for treatment of H2S-induced neurodegeneration.
In human victims of acute H2S exposure, lesions are reported in various parts of the brain, with the cortex, thalamus, and the basal ganglia being most commonly involved.20,22,23 Lesions in similar brain regions, including the collicular regions, have also been reported in animals.56 Faithfully reproducing lesions in brains of laboratory animals following a single H2S exposure has been a challenge.34,35,57 This may be a result of physiological differences between rodents and nonhuman primates and humans. Baldelli and Sonobe using injected NaHS showed that lesions were manifested in only a small number of surviving rats. Besides the brain, the lungs and the cardiovascular system are secondary target organs of H2S exposure. H2S-induced lung edema in particular may contribute to hypoxia and is best replicated in an inhalation model. A good inhalation model of H2S-induced neurotoxicity was shown in an elegant study in rhesus monkeys exposed either once or at least twice to H2S. Using this primate model, Lund et al. reproduced lesions in the cortex and basal ganglia of monkeys exposed twice to 500 ppm with 3 days between exposures for 25 min initially and 17 min on second exposure.57 Monkeys that died acutely lacked brain lesions. Those with at least two exposures that lived at least 3 days after exposure developed lesions. The results of this primate study are consistent with observations in the mouse model presented here. Although the nonhuman primate model is attractive because it yields lesions in similar brain regions as humans, facilities to support this kind of research are not widely available.
The mouse model reported here followed two exposure paradigms. Though exposure paradigm I is atypical of exposure scenarios in humans, it has the advantage that it is associated with low mortality and a high frequency of mice exhibiting brain lesions. This exposure paradigm is ideal for assessment of countermeasures against H2S-induced neurodegeneration with minimal numbers of mice to achieve meaningful statistical significance. Because the molecular mechanisms leading to cell death have yet to be defined, the window of opportunity for efficacy of such drugs is not known.30 From our observations in this study, neurodegeneration becomes morphologically manifested at least 3 days after H2S exposure, suggesting a wider intervention time for the treatment of neurodegeneration. Paradigm II, on the other hand, faithfully replicates the typical human single exposure, but it is associated with high mortality and is ideal for evaluating the efficacy for countermeasures that increase survival. In our models, most deaths occurred during H2S exposure, as is reported in human case reports of H2S poisoning18 and other rodent models.33–35 Therefore, rescue countermeasures to maximize survival must be given during exposure.
Neurochemical alterations in the brain have been correlated with many movement disorders,58,59 particularly lesions in the basal ganglia.60 In order to understand the origin of the motor deficits observed in this mouse model, the striatum was analyzed for monoamine neurotransmitter changes after H2S exposure. A single exposure or repeated short-term exposures to H2S resulted in increased DA concentration (Table 1). This is consistent with the literature demonstrating H2S to be a monoamine oxidase enzyme inhibitor.61 Interestingly, mice exposed to H2S also had lower NE levels compared with negative controls (Table S3). A positive correlation in the levels of the aforementioned neurotransmitters was observed in the thalamus, cortex, and striatum of mice exposed to H2S (Table 1). It is notable that these neurochemical changes were observed in brain regions that did not manifest morphological lesions, suggesting that neurodegeneration may not be an essential prerequisite for the manifestation of neurochemical changes.
The mechanisms of H2S-induced seizures have not been characterized. However, neurotransmitter imbalances can contribute to seizures,62,63 and in this study we demonstrated significant changes in neurotransmitter levels in the brain. GABA and glutamate are important regulators of inhibitory and excitatory signals in the brain. H2S exposure time-dependently decreased GABA and glutamate in the IC of the brain. Dysregulation of glutamate and GABA may partially explain the seizurogenic and neurotoxic effects of H2S.
A consistent hallmark of H2S exposure that has been well characterized is the inhibition of cytochrome c oxidase (complex IV) of the electron transport chain.33,64,65 Our model recapitulates this biochemical effect: cytochrome c oxidase activity was significantly decreased on all days of H2S exposure in both male and female mice. Specifically, cytochrome c oxidase activity was reduced in the IC, thalamus, and cortex. These data suggest that inhibition of cytochrome c oxidase may not be the sole cause of H2S-induced neurotoxicity and neurodegeneration.
A biomarker of H2S exposure that has been reported in the literature is increased thiosulfate.14,44
In this study, we also observed that H2S exposure increases serum thiosulfate levels. It is anticipated that serum thiosulfate concentration will be a valuable biomarker for evaluation of efficacy of countermeasures, such as cobinamide, which bind H2S to prevent it from interacting with critical sensitive molecules, such as cytochrome c oxidase.
We also evaluated gender differences in susceptibility to H2S-induced neurotoxicity in paradigm I. Some differences were found, and overall males were found to be more sensitive to H2S-induced acute toxicity than females. This is supported by data examining body weight loss, average latency time to exhibit seizures and time taken to demonstrate knockdown, severity of brain injury, and performance in behavioral tests. It is for this reason that only male mice were used in paradigm II of the study. The reasons for this apparent increased susceptibility of male mice to H2S are unknown, but they could be hormonal, or magnitude of locomotor activity could be a critical determinant. For this reason, we recommend using male mice with inclusion of a smaller cohort of female mice in future translational efficacy studies evaluating potential drug candidates for the treatment of acute and lingering effects of H2S-induced neurotoxicity.
In conclusion, we have developed a mouse model that recapitulates many of the hallmarks of H2S-induced neurotoxicity and neurodegeneration using two exposure paradigms. In paradigm I, all animals exposed to H2S had a reduction in body weight and motor deficits and exhibited clinical signs characteristic of H2S poisoning, including seizure activity and the knockdown response. Male mice were more sensitive than females, as determined using multiple end points. The animals exposed to H2S also showed a cumulative toxicity to H2S, a phenomenon also observed in humans. These animals also had neurochemical imbalances in almost all of the brain regions investigated, which could be a contributing factor to the seizure activity and persistent neurological sequelae observed in this model. Mortality was low in paradigm I, and consistently reproducible necrotic lesions were present in brain regions previously shown to be sensitive to H2S toxicity, such as the IC and thalamus. We recommend this approach for testing the efficacy of countermeasures against neurodegeneration, because it uses a small number of mice to achieve statistically significant and meaningful results. Paradigm II, on the other hand, is associated with high mortality, with a small percentage of surviving mice manifesting similar lesions in same brain regions, albeit less severe. We recommend this approach for testing the efficacy of rescue countermeasures to increase survival rate. There is no perfect animal model of H2S-induced neurotoxicity and neurodegeneration. The mouse model presented here is novel and for the first time provides a tool for mechanistic and translational studies. Currently, no other animal model uses whole-body H2S inhalation exposure on fully awake animals. A wide variety of end points to be evaluated in this model have been established, including mortality and clinical, biochemical, and neurochemical changes, which parallel those observed in H2S toxicity in humans and will provide metrics suitable for the evaluation and screening of antidotes of H2S-induced neurotoxicity.
Supplementary Material
Figure S1. Thiosulfate levels in serum of male and female mice following exposure to H2S (n=5/group *, P < 0.05; **, P <0.01; ***, P < 0.001) compared with breathing-air controls.
Table S1. Semiquantitative grading scheme to assess histological lesions caused by H2S in mouse brains.
Table S2. Summary of functional observation of battery evaluation during H2S exposure (765 ppm) of male and female mice, including seizure activity, knockdowns, and other clinical signs.
Table S3. Serotonin, 5-HIAA, and norepinephrine levels in various brain regions of mice following exposure to 765 ppm of H2S.
Acknowledgments
The experiments were conceived and designed by P.A., W.K.R, and A.K. The experiments were performed by P.A., B.M., and M.R.L. Analysis was done by P.A., D.S.K., D.S., P.M.I., and E.M.W. Data were analyzed by P.A, D.S.K, P.M.I, and E.M.W, and they take responsibility for the integrity of the data analyzed. The manuscript was prepared by P.A., W.K.R., A.K., E.M.W., and D.S.K. We thank Dr. Jacek Koziel for setting up the inhalation chamber, Moriah Jenkins for aiding with the animal work, and Dr. Chong Wong for his assistance with statistical analysis. This research was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number NS089487.
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article.
Competing interests
The authors declare no competing interests.
References
- 1.Tan BH, Wong PT & Bian JS. 2010. Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochem. Int 56: 3–10. [DOI] [PubMed] [Google Scholar]
- 2.Moustafa A & Habara Y. 2015. Hydrogen sulfide: a novel gaseous signaling molecule and intracellular Ca2+ regulator in rat parotid acinar cells. Am. J. Physiol. Cell Physiol 309: C480–C490. [DOI] [PubMed] [Google Scholar]
- 3.Olas B 2017. Hydrogen sulfide as a “double-faced” compound: one with pro- and antioxidant effect. Adv. Clin. Chem 78: 187–196. [DOI] [PubMed] [Google Scholar]
- 4.Gadalla MM & Snyder SH. 2010. Hydrogen sulfide as a gasotransmitter. J. Neurochem 113: 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauchamp RO Jr. et al. 1984. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol 13: 25–97. [DOI] [PubMed] [Google Scholar]
- 6.Woodall GM, Smith RL & Granville GC. 2005. Proceedings of the hydrogen sulfide health research and risk assessment symposium October 31–November 2, 2000. Inhal. Toxicol 17: 593–639. [DOI] [PubMed] [Google Scholar]
- 7.National Research Council. 1979. Hydrogen sulfide In Medical and Biological Effects of Environmental Pollutants. Baltimore, MD: University Park Press; pp. 1–183. [Google Scholar]
- 8.Rischitelli GD & Schaumburg HH. 2000. Hydrogen sulfide In Experimental and Clinical Neurotoxicology. Spencer P & Schaumburg HH, Eds.: 655–658. New York: Oxford University Press. [Google Scholar]
- 9.OSHA. 2016. Safety and health topics: hydrogen sulfide. Accessed June 16, 2017 https://www.osha.gov/SLTC/hydrogensulfide/index.html.
- 10.OSHA. 2016. Toxic industrial chemical guide. Vol. 2017 United States Department of Labor, Washington, DC. [Google Scholar]
- 11.OSHA. Hydrogen sulfide In OSHA Fact Sheet. Vol. 2015 Accessed June 16, 2017 https://www.osha.gov/SLTC/hydrogensulfide/hazards.html. [Google Scholar]
- 12.EPA. 2003. Toxicological review of hydrogen sulfide. Washington, DC: EPA. [Google Scholar]
- 13.Anderson AR 2016. Characterization of chemical suicides in the United States and its adverse impact on responders and bystanders. West. J. Emerg. Med 17: 680–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maebashi K et al. 2011. Toxicological analysis of 17 autopsy cases of hydrogen sulfide poisoning resulting from the inhalation of intentionally generated hydrogen sulfide gas. Forensic Sci. Int 207: 91–95. [DOI] [PubMed] [Google Scholar]
- 15.Foulkes CH 2009. “Gas!” The Story of the Special Brigade. Naval and Military Press. [Google Scholar]
- 16.Department of Homeland Security. 2007. Appendix A: chemicals of interest list. Fed. Reg.2017. Accessed June 22, 2017 https://www.dhs.gov/appendix-a-chemicals-interest-list. [Google Scholar]
- 17.Department of Homeland Security. 2007. 6 CFR Part 27. Appendix to Chemical Facility anti-terrorism Standards; Final Rule. Fed. Reg 72(223): 65423. [Google Scholar]
- 18.McCabe LC & Clayton GD. 1952. Air pollution by hydrogen sulfide in Poza Rica, Mexico; an evaluation of the incident of Nov. 24, 1950. AMA Arch. Ind. Hyg. Occup. Med 6: 199–213. [PubMed] [Google Scholar]
- 19.Yang D, Chen G & Zhang R. 2006. Estimated public health exposure to H2S emissions from a sour gas well blowout in Kaixian County, China. Aerosol Air Qual. Res 6: 430–443. [Google Scholar]
- 20.Schneider JS et al. 1998. Persistent cognitive and motor deficits following acute hydrogen sulphide poisoning. Occup. Med 48: 255–260. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti TL 1996. Hydrogen sulphide. Occup. Med 46: 367–371. [DOI] [PubMed] [Google Scholar]
- 22.Snyder JW et al. 1995. Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am. J. Emerg. Med 13: 199–203. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo M, Cummins JW & Anderson RE. 1979. Neurological sequelae of massive hydrogen sulfide inhalation. Arch. Neurol 36: 451–452. [DOI] [PubMed] [Google Scholar]
- 24.Doujaiji B & Al-Tawfiq JA. 2010. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med 30: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tvedt B et al. 1991. Delayed neuropsychiatric sequelae after acute hydrogen sulfide poisoning: affection of motor function, memory, vision and hearing. Acta Neurol. Scand 84: 348–351. [DOI] [PubMed] [Google Scholar]
- 26.Reiffenstein RJ, Hulbert WC & Roth SH. 1992. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol 32: 109–134. [DOI] [PubMed] [Google Scholar]
- 27.Fujita Y et al. A fatal case of acute hydrogen sulfide poisoning caused by hydrogen sulfide: hydroxocobalamin therapy for acute hydrogen sulfide poisoning. J. Anal. Toxicol 35: 119–123. [DOI] [PubMed] [Google Scholar]
- 28.Parra O et al. 1991. Inhalation of hydrogen sulphide: a case of subacute manifestations and long term sequelae. Br. J. Ind. Med 48: 286–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilburn KH 2003. Effects of hydrogen sulfide on neurobehavioral function. South Med. J 96: 639–646. [DOI] [PubMed] [Google Scholar]
- 30.Rumbeiha W et al. 2016. Acute hydrogen sulfide-induced neuropathology and neurological sequelae: challenges for translational neuroprotective research. Ann. N.Y. Acad. Sci 1378: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truong DH et al. 2006. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab. Rev 38: 733–744. [DOI] [PubMed] [Google Scholar]
- 32.Smith RP 1997. Sulfide poisoning. J. Toxicol. Clin. Toxicol 35: 305–306. [DOI] [PubMed] [Google Scholar]
- 33.Jiang J et al. 2016. Hydrogen sulfide—mechanisms of toxi-city and development of an antidote. Sci. Rep 6: 20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldelli RJ, Green FH & Auer RN. 1993. Sulfide toxicity: mechanical ventilation and hypotension determine survival rate and brain necrosis. J. Appl. Physiol (1985) 75: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 35.Sonobe T et al. 2015. Immediate and long-term outcome of acute H2S intoxication induced coma in unanesthetized rats: effects of methylene blue. PLoS One 10: e0131340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haouzi P, Chenuel B & Sonobe T. 2015. High-dose hydroxocobalamin administered after H2S exposure counteracts sulfide-poisoning-induced cardiac depression in sheep. Clin. Toxicol. (Phila.) 53: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savolainen H et al. 1980. Cumulative biochemical effects of repeated subclinical hydrogen sulfide intoxication in mouse brain. Int. Arch. Occup. Environ. Health 46: 87–92. [DOI] [PubMed] [Google Scholar]
- 38.McDaniel KL & Moser VC. 1993. Utility of a neurobehavioral screening battery for differentiating the effects of two pyrethroids, permethrin and cypermethrin. Neurotoxicol. Teratol 15: 71–83. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh A et al. Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free Radic. Biol. Med 49: 1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Togawa T et al. 1992. High performance liquid chromatographic determination of bound sulfide and sulfite and thiosulfate at their low levels in human serum by pre-column fluorescence derivatization with monobromobimane. Chem. Pharm. Bull 40: 3000–3004. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh A et al. 2016. Mitoapocynin treatment protects against neuroinflammation and dopaminergic neurodegeneration in a preclinical animal model of Parkinson’s disease. J. Neuroimmune Pharmacol 11: 259–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Freitas Silva DM, Ferraz VP & Ribeiro AM. 2009. Improved high-performance liquid chromatographic method for GABA and glutamate determination in regions of the rodent brain. J. Neurosci. Methods 177: 289–293. [DOI] [PubMed] [Google Scholar]
- 43.Kim DS et al. 2016. p73 gene in dopaminergic neurons is highly susceptible to manganese neurotoxicity. Neurotoxicology 59: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daldal H et al. 2010. Hydrogen sulfide toxicity in a thermal spring: a fatal outcome. Clin. Toxicol 48: 755–756. [DOI] [PubMed] [Google Scholar]
- 45.Guidotti TL 2015. Hydrogen sulfide intoxication. Handb. Clin. Neurol 131: 111–133. [DOI] [PubMed] [Google Scholar]
- 46.Guidotti TL 1994. Occupational exposure to hydrogen sulfide in the sour gas industry: some unresolved issues. Int. Arch. Occup. Environ. Health 66: 153–160. [DOI] [PubMed] [Google Scholar]
- 47.Guidotti TL 2010. Hydrogen sulfide: advances in understanding human toxicity. Int. J. Toxicol 29: 569–581. [DOI] [PubMed] [Google Scholar]
- 48.Arnold IM et al. 1985. Health implication of occupational exposures to hydrogen sulfide. J. Occup. Med 27: 373–376. [DOI] [PubMed] [Google Scholar]
- 49.Dorman DC et al. 2002. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol. Sci 65: 18–25. [DOI] [PubMed] [Google Scholar]
- 50.Tvedt B et al. 1991. Brain damage caused by hydrogen sulfide: a follow-up study of six patients. Am. J. Ind. Med 20: 91–101. [DOI] [PubMed] [Google Scholar]
- 51.Gerasimon G et al. 2007. Acute hydrogen sulfide poisoning in a dairy farmer. Clin. Toxicol 45: 420–423. [DOI] [PubMed] [Google Scholar]
- 52.Bartholomaeus AR & Haritos VS. 2005. Review of the toxicology of carbonyl sulfide, a new grain fumigant. Food Chem. Toxicol 43: 1687–1701. [DOI] [PubMed] [Google Scholar]
- 53.Sokoloff L 1977. Relation between physiological function and energy metabolism in the central nervous system. J. Neurochem 29: 13–26. [DOI] [PubMed] [Google Scholar]
- 54.Moustafa AA et al. 2017. The thalamus as a relay station and gatekeeper: relevance to brain disorders. Rev. Neurosci 28: 203–218. [DOI] [PubMed] [Google Scholar]
- 55.Noseda R et al. 2014. Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One 9: e103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jubb KVF & Palmer KP. 1992. Pathology of Domestic Animals. San Diego, CA: Academic Press Inc. (Harcourt Brace Jovanovich). [Google Scholar]
- 57.Lund OE & Wieland H. 1966. Pathologic–anatomic findings in experimental hydrogen sulfide poisoning (H2S). A study on rhesus monkeys. Int. Arch. Arbeitsmed 22: 46–54. [PubMed] [Google Scholar]
- 58.Contreras-Vidal JL & Stelmach GE. 1995. A neural model of basal ganglia–thalamocortical relations in normal and Parkinsonian movement. Biol. Cybern 73: 467–476. [DOI] [PubMed] [Google Scholar]
- 59.Jankovic J 2016. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin. Pharmacother 17: 2461–2470. [DOI] [PubMed] [Google Scholar]
- 60.Groenewegen HJ 2003. The basal ganglia and motor control. Neural Plast. 10: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warenycia MW et al. 1989. Monoamine oxidase inhibition as a sequel of hydrogen sulfide intoxication: increases in brain catecholamine and 5-hydroxytryptamine levels. Arch. Toxicol 63: 131–136. [DOI] [PubMed] [Google Scholar]
- 62.Obata K 2013. Synaptic inhibition and γ-aminobutyric acid in the mammalian central nervous system. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 89: 139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiang J & Jiang Y. 2013. Antiepileptic potential of matrine via regulation the levels of gamma-aminobutyric acid and glutamic acid in the brain. Int. J. Mol. Sci 14: 23751–23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall AH & Rumack BH. 1997. Hydrogen sulfide poisoning: an antidotal role for sodium nitrite? Vet. Hum. Toxicol 39: 152–154. [PubMed] [Google Scholar]
- 65.Veeranki S & Tyagi SC. 2015. Role of hydrogen sulfide in skeletal muscle biology and metabolism. Nitric Oxide 46: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Thiosulfate levels in serum of male and female mice following exposure to H2S (n=5/group *, P < 0.05; **, P <0.01; ***, P < 0.001) compared with breathing-air controls.
Table S1. Semiquantitative grading scheme to assess histological lesions caused by H2S in mouse brains.
Table S2. Summary of functional observation of battery evaluation during H2S exposure (765 ppm) of male and female mice, including seizure activity, knockdowns, and other clinical signs.
Table S3. Serotonin, 5-HIAA, and norepinephrine levels in various brain regions of mice following exposure to 765 ppm of H2S.