Abstract
Cholesterol has been shown to modulate the activity of multiple G Protein-coupled receptors (GPCRs), yet whether cholesterol acts through specific interactions, indirectly via modifications to the membrane, or via both mechanisms is not well understood. High-resolution crystal structures of GPCRs have identified bound cholesterols; based on a β2-adrenergic receptor (β2AR) structure bound to cholesterol and the presence of conserved amino acids in class A receptors, the cholesterol consensus motif (CCM) was identified. Here in mammalian cells expressing A2aR, ligand dependent production of cAMP is reduced following membrane cholesterol depletion with methyl-beta-cyclodextrin (MβCD), indicating that adenosine A2a receptor (A2aR) signaling is dependent on cholesterol. In contrast, ligand binding is not dependent on cholesterol depletion. All-atom molecular simulations suggest that cholesterol interacts specifically with the CCM when the receptor is in an active state, but not when in an inactive state. Taken together, the data support a model of receptor state-dependent binding between cholesterol and the CCM, which could facilitate both G-protein coupling and downstream signaling of A2aR.
Introduction
G-protein coupled receptors (GPCRs) represent the largest family of receptors in the human genome, having approximately 800 human genes predicted; however, the crystal structures of only approximately 40 GPCRs have been reported (1,2). GPCRs are ideal drug targets because of their location at the plasma membrane and their ability to initiate many intracellular responses from varying extracellular stimuli(3). Approximately 35% of all drugs currently on the market target GPCRs, but these drugs only target approximately 10% of known GPCRs(4, 5). An increased understanding of the structure-function relationship, as well as protein-membrane interactions of these receptors could lead to superior drug specificity and efficacy.
Cholesterol is an essential component of the plasma membrane in eukaryotic cells(6), and has been shown to modulate the activity of many GPCRs both positively and negatively including rhodopsin(7, 8), the CXCR4 chemokine receptor(9, 10), and the serotonin1A receptor (5-HT1a)(11–14). The most common method used to study the role of cholesterol in signaling of GPCRs is to deplete cholesterol from live cells or isolated membranes by treatment with methyl-β-cyclodextrin (MβCD), Cholesterol depletion with MβCD can give insight into cholesterol dependent signaling of GPCRs but can also be used as a model for disease states where the concentration of cholesterol at the plasma membrane is altered.
While membrane cholesterol concentrations can vary greatly between different cell types, in a single cell type, cholesterol homeostasis is well regulated and maintained(15, 16). However, changes in membrane cholesterol homeostasis have been implicated in a number of disease pathologies. One example of this dysregulation is Niemann-Pick disease type C, which is caused by mutations in the NPC1 or NPC2 gene. NPC1 and NPC2 are necessary for proper movement of lipids within cells; mutations to these proteins lead to an accumulation of intracellular cholesterol in lysosomal compartments, resulting in a reduction in cholesterol at the plasma membrane(17). Interestingly, treatment with an adenosine A2a receptor (A2aR) agonist has been suggested as a therapeutic for Niemann-Pick disease type C, and successfully tested in both cell and mouse models of the disease(18–20). Another example of a disease with abnormal cholesterol homeostasis is Alzheimer’s disease (AD), where membrane cholesterol can be increased by 30% in cerebral cortex brain samples from diseased patients (21–23). Additionally, the greatest genetic risk factor for AD is the presence of an ε4 allele of apolipoprotein E, which regulates cholesterol metabolism(24, 25). Transcriptome analysis of embryonic rat cortical neurons loaded with 30% additional cholesterol (using MβCD: Cholesterol complexes) revealed the expression of many genes was significantly altered, including increased expression of adenylate cyclase II and reduced expression of the Adenosine A1 receptor (A1R) (26). This result is consistent with reported findings of reduced A1R expression in postmortem hippocampal brain slices from AD patients(27). Although A2aR is not as prevalent in the healthy brain as A1R, reports of upregulation of A2aR expression in glial cells in the hippocampus and cerebral cortex of AD patients has been reported(28).
Cholesterol is predicted to have specific interactions with A2aR (a class A GPCR) due to the presence of cholesterol binding motifs in the sequence of A2aR. Both the cholesterol consensus motif (CCM) and cholesterol recognition amino acid consensus (CRAC) motif are found in the A2aR sequence. Additionally, simulations predict two specific cholesterol-binding sites, one on the inner leaflets of helix five and six (h5/6i) and the other on the outer leaflet of helix six (h6o)(29). The h6o site is consistent with bound cholesterols seen in crystal structures of A2aR in complex with the antagonist ZM241385(30–32). In previous experimental studies, addition of cholesteryl hemi-succinate (CHS), a cholesterol derivative, was required to retain activity of the receptor when purified A2aR was reconstituted into protein-detergent complexes (PDCs)(33). Further in vitro studies utilizing radioligand binding and small angle neutron scattering (SANS) demonstrated that an optimal CHS level gave maximal activity of A2aR, likely as a result of both physical and chemical changes to the PDC environment(34).
In this work, bulk membrane cholesterol depletion in live mammalian cells is shown to inhibit the activity of the adenosine A2a receptor, as indicated by the reduction of cyclic adenosine monophosphate (cAMP) production upon ligand binding. Extensive, all-atom simulations totaling 46 μsec confirm the cholesterol interaction at the CCM observed for A2aR and suggest that cholesterol preferentially interacts with the CCM in the active-state receptor.
Materials and methods
Materials.
Cholesterol and Methyl-β-cyclodextrin (MβCD) were obtained from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS), Lipofectamine 2000 transfection reagent and Opti-MEM reduced serum media were from Invitrogen Life Technologies (Carlsbad, CA). CGS21680 and ZM241385 were obtained from Tocris (Bristol, UK), FITC-APEC was obtained from the NIMH synthesis program, http://nimh-repository.rti.org, NIMH Code: D-906, and [3H]NECA and [3ZM241385] were obtained from American Radiolabeled Chemicals (St. Louis, MO).
Cell culture.
Human embryonic kidney (HEK293) cells were maintained in growth media containing Dulbecco’s modified eagle medium (DMEM; Cellgro, Manassas, VA) with 10% FBS at 37°C in a 5% C02 incubator.
Lipofectamine transfection.
Cells were seeded on day 0 in a T-25 flask to be approximately 70% confluent. On day 1, cells were transfected using 10 μl Lipofectamine 2000 reagent, and 1 μg DNA in 2 ml Opti-MEM reduced serum media. On day 2 cells were placed back in growth media and used for experimentation on day 3.
Cholesterol depletion by MβCD) of cells in culture.
HEK-A2aR cells were depleted of cholesterol using 1.25 mM to 5 mM methyl-β-cyclodextrin (MβCD) for 30 minutes at 37°C following a 3 hour incubation in serum free media (DMEM) as previously described(14). Cells were also treated with cholesterol: MβCD complexes to account for off-target effects of MβCD, while retaining native membrane cholesterol concentrations. 5 mM MβCD and 0.1 mM cholesterol were added to serum free media (DMEM) and vortexed constantly for 1 hour at room temperature as previously described(35). The cholesterol: MβCD complexes were then added to cells previously incubated for 3 hours in serum free media for a 30-minute incubation at 37°C. Cells were rinsed with PBS prior to experimentation.
Cell membrane isolation and cholesterol content analysis.
Cell membranes were isolated via ultracentrifugation as previously described(33). BCA assay (Pierce; Rockford, IL) was performed to determine the total protein concentration of isolated membrane, using BSA as a standard, and membrane preparations isolated that day. The cholesterol content in 5 μg of total protein from isolated cell membranes was determined using the Amplex Red cholesterol assay kit (Invitrogen Molecular Probes; Eugene, OR) according to the manufacturer’s protocol.
Giant Plasma Membrane Vesicle Preparation and Lipidomics Analysis.
On day 0, flasks were incubated with 0.1 mg/ml Poly-D-Lysine (PDL) (A-003-E; Sigma Aldrich, St. Louis, MO) for 1 hr at 37°C then washed 3 times with PBS. HEK293 were seeded into PDL coated flasks to be approximately 80% confluent on day 1. On day 1, cells were treated with MβCD, washed with PBS and then incubated in GPMV buffer (10 mM HEPES, 150 mM NaCl, 2 mM CaCl2, pH 7.4) supplemented with 50 mM paraformaldehyde (PFA) and 4 mM Dithiothreitol (DTT) for 3 hrs at 37°C, as previously described(36, 37). Following the incubation, supernatant containing the giant plasma membrane vesicles (GPMVs) was gently removed from flasks and centrifuged at 20,000 xg for 1 hr at 4°C to pellet GPMVs. The GPMVs were resuspended in 100 μl of ABC buffer (150 mM ammonium bicarbonate) and stored at −20°C. GPMVs were thawed on ice and spun through a 5 μm centrifugal filter (UFC30SV00, EMD Millipore) at 10,000 xg for 3 min at 4°C. BCA assay (Pierce; Rockford, IL) was performed (using low volume protocol) to determine the total protein concentration of isolated GPMVs. 10 or 20 μg of protein in 150 μl ABC buffer was sent for lipidomics analysis by Lipotype GmbH (Dresden Germany).
Radioligand binding assay.
Isolated cell membranes were resuspended in ligand binding buffer (50 mM Tris-HCl, 10 mM MgCl2 and 1 mM EDTA, pH, 7.4) and 5 μg membrane protein / well were loaded onto poly(ethyleneimine) (0.1% v/v) treated 96-well glass fiber filter plates (MultiScreen-FC filter type B, Millipore, Billerica, MA) as previously described(38). Cells were incubated with 1.25-40 nM [3H]ZM241385 in the presence or absence of unlabeled competitor ligand (50 μ NECA) for 1.5 hours. Once binding equilibrium was reached, membranes were washed 3 times with ice-cold binding buffer and then 30 μl of scintillation solution (ULTIMA gold, Perkin Elmer) was added to each well. Radioactive counts (CPMs) using a Perkin-Elmer 1450 Microbeta liquid scintillation counter were measured to determine ligand binding approximately 24 hours after the addition of the scintillation solution. Non-specific binding was determined from binding to membranes in the presence of an unlabeled competitor and CPMs were subtracted from total binding to calculate specific binding.
cAMP activity assay.
Transiently transfected and parental HEK cells were incubated for 30 minutes in the presence or absence of 1 μM CGS21680 at a cell density of 1,000 cells/well in a white 384 well plate (Grenier bio-one #784075, Kremsmiinster, Austria). The concentration of cAMP per well was measured using the cAMP dynamic 2 kit (cisbio, Bedford, MA) using a BioTek Synergy H1 Plate Reader according to manufacturer’s protocol.
Statistical Analysis.
Graphpad Prism 7 (Graphpad software) was used for student’s t-test analysis. Values were considered statistically significant if P<.05.
All-atom simulation details.
Initial coordinates for the inactive and apo states were based on the same high resolution, ZM241385 structure as the Martini simulations (31). Initial coordinates for the active state were based on a structure of the NECA-bound receptor (39). Initial coordinates for residues 208 to 219 were obtained by aligning to the final frame of a previously published 1 μsec simulation(40). The apo structure was created by simply deleting the ZM241385 ligand. The protein was embedded in a symmetric lipid bilayer containing 504 dipalmitoyl phosphatidyl choline (DPPC), 132 dioleoyl phosphatidyl choline, and 273 cholesterol and solvated with approximately 55,000 tip3p(41) waters and one sodium ion as observed in the crystal structure, and 10 chloride ions to neutralize the system. The protein and lipids were modeled with the CHARMM36 force field(42, 43) and the ligand with the CHARMM general force(44) with atoms typed by the ParamChem server(45).
Several equilibration steps were run with NAMD v2.9(46). The initial configuration was relaxed by 4000 steps of steepest descent. The protein backbone was then restrained using a force constant of 2 kcal/mol/Å2 while the system was heated to 295 K, reassigning velocities from a Maxwell-Boltzmann distribution every 4 timesteps, with the simulation cell volume allowed to change semi-isotropically via a Langevin piston with a damping timescale of 0.1 psec and period 0.2 psec(47). An additional 20,000 equilibration steps were performed, rescaling velocities every 100 steps to enforce a temperature of 295 K. Finally the Langevin equation was integrated for 1 nsec with a 1.0 fsec timestep, followed by 10 nsec with a 2.0 fsec timestep and all covalently bonded hydrogens constrained by SHAKE(48). During all relaxation steps electrostatics were computed with the particle-mesh Ewald method(49) on a 1 Å grid, with a tolerance of 10−6 and 4th order interpolation. Lennard-Jones interactions were cutoff at 10 Å and shifted to zero at 12.5 Å ensuring both continuous potential and force.
The equilibrated binary restart files were converted to dms format for production simulation on Anton2. Forcefield information was added using Viparr v4.5.34. Integration was performed under constant pressure (1 atm), temperature (295 K), and particle number with the multigrator(50) method, with temperature (295 K) controlled by a Nose-Hoover(51) chain coupled every 24 timesteps and pressure by the Martyna-Tobias-Klein barostat (pressure 1 atm, semi-isotropic) coupled every 480 timesteps(52). Electrostatics were computed using the k-space Gaussian split Ewald method(53), with long-range interactions computed every third timestep. Lennard-Jones interactions were cutoff at at least 11 Å. (Full details are available in the simulation configuration file in the Supporting Material.) Both ZM241385 bound and apo simulations were run for 6 μsec with a 2.5 fsec timestep (hydrogens constrained by M-SHAKE(54)) and simulation configurations stored every 240 psec.
Data availability.
All data generated or analyzed during this study are included in this published article (and its supporting information file). Any additional information will be provided by the authors upon request.
Results
Bulk cholesterol depletion effects on HEK293 cells.
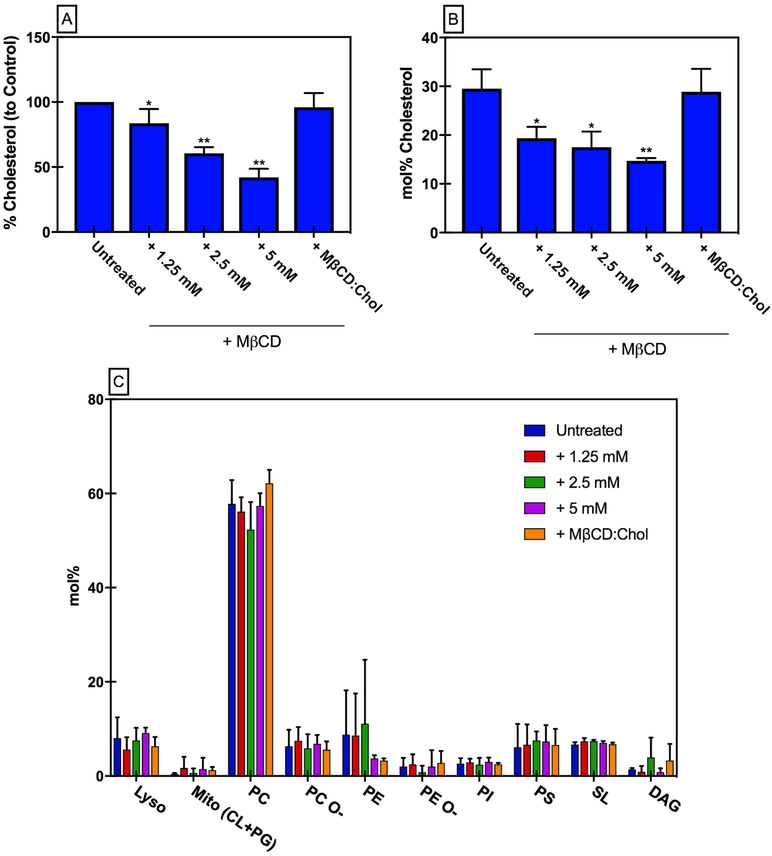
HEK293-A2aR cells were depleted of cholesterol with increasing concentrations of methyl-β-cyclodextrin (MβCD), which preferentially extracts cholesterol from the membrane by capturing it in its hydrophobic cavity (55). Cholesterol levels were measured in cells following this protocol to confirm the decrease in membrane cholesterol (Fig. 1A). To account for off-target effects of MβCD, cells were treated with MβCD:cholesterol complexes to provide a control in which cells are treated with MβCD, but native membrane cholesterol concentrations are maintained — A2aR in this control should behave as in untreated cells. Cells treated with 5 mM MβCD contained approximately 42% of the membrane cholesterol of the untreated control, while cells treated with MβCD:cholesterol complexes (5 mM MβCD + 0.1 mM Cholesterol) contained approximately 95% of the untreated control membrane cholesterol levels. Cell viability of HEK293 cells was measured following MβCD treatment and no statistically significant differences in viability were observed (Fig. S1), consistent with previously published results(56).
Figure 1. Effect of MβCD on HEK293 cells.
(A) Cholesterol content from membrane preparations following MβCD treatment compared to untreated control. Mean ± S.D. values from n≥4 experiments done in triplicate. (B) Cholesterol content in GPMV preps following MβCD treatment determined by lipidomics analysis. Data are mean±S.D. values from n=3 independent samples. (C) Headgroup distribution of lipids in GPMV preps following MβCD treatment determined by lipidomics analysis. Data are mean±S.D. values from n=3 samples.
To verify that MβCD was predominantly impacting cholesterol levels, and not depleting other membrane lipids, giant plasma membrane vesicles (GPMVs) were prepared to enrich and isolate the plasma membrane (57, 58). Lipidomics analysis of GPMVs was performed following MβCD cholesterol depletion and replenishment. Consistent with the cholesterol assay results, lipidomics analysis confirmed that cholesterol was depleted from the plasma membrane following MβCD treatment, and that treatment with MβCD: Cholesterol complexes results in near native cholesterol concentrations (Fig. 1B). Lipidomics analysis also gave insight into changes in the lipid profile of GPMV preps from cells treated with MβCD. Overall, it appeared that short-term MβCD treatment had little effect on the lipidomes of GPMVs from HEK293 cells, with the exception of cholesterol (Fig. 1C). Similarly, treatment with MβCD did not lead to any significant changes in the unsaturation or length of plasma membrane lipids (Fig. S2).
GPMV lipidomes may not represent the actual composition of the living cell plasma membrane because of the effects of isolation chemicals and conditions. However, such effects would be constant across treatments; therefore, we conclude that MβCD has no notable off-target effects on lipidomes.
Bulk cholesterol depletion effects on A2aR.
To examine the effect of MβCD treatment on A2aR levels, HEK293 cells were transiently transfected with pCep4-A2aR plasmid DNA, and membrane preparations were obtained for analysis. Non-transfected HEK293 cells had no detectable levels of native A2aR, as determined by western blot analysis (Fig. S3). A2aR was quantified by densitometry in all transiently transfected cells and no significant changes in protein levels were detected; thus, MβCD treatment had no effect on A2aR levels in membrane preparations (Fig. S3).
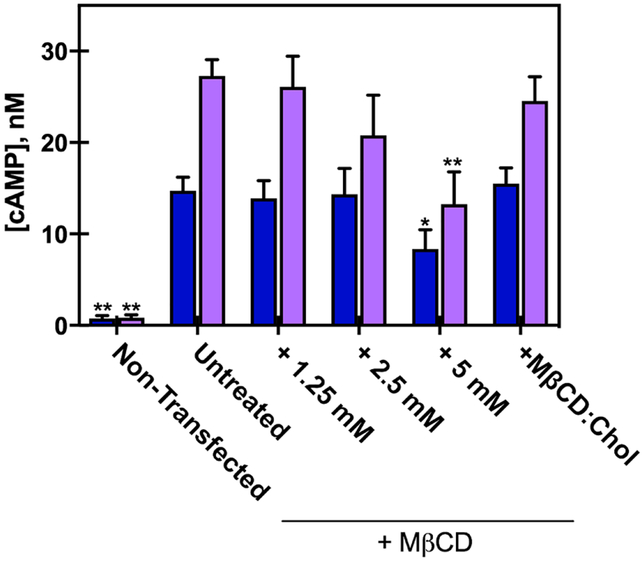
A2aR couples to Gs±, which activates adenylyl cyclase (a membrane-embedded protein) to upregulate synthesis of cyclic AMP, and thus ligand binding of A2aR should result in the production of cAMP. Although MβCD treatment did not affect total A2aR levels, bulk changes to the membrane due to MβCD cholesterol depletion caused significant changes in the downstream activation of A2aR, as determined by cAMP measurements (Fig. 2). Decreases in cell membrane cholesterol led to an overall reduction in the accumulation of the downstream signaling molecule cAMP following incubation with an A2aR selective agonist (1 μM CGS21680). As expected, non-transfected control cells showed negligible cAMP accumulation upon ligand addition. Basal values of cAMP (constitutive A2aR activation) were decreased only at the highest concentration of MβCD (Fig 2), however forskolin-stimulated cAMP production (forskolin directly activates adenylyl cyclase, independent of GPCR activity) was not affected by 5 mM MβCD treatment (Fig S4). This suggests that the reduction in membrane cholesterol reduced activity of A2aR but not adenylyl cyclase, which is also a membrane embedded protein. The measured cAMP concentration following agonist incubation was most significantly reduced in cells treated with 5mM MβCD compared to untreated cells (14 nM vs. 27 nM cAMP, respectively) (Fig. 2). Cells treated with MβCD:cholesterol complexes (50:1 molar ratio) showed unchanged basal activity relative to the control, and showed increased cAMP synthesis (compared to 5 mM MβCD treatment alone) following agonist addition, yielding results similar to untreated cells. These data suggest that the decrease in downstream signaling of A2aR is caused directly by changes in membrane cholesterol concentration, and not off-target effects of MβCD treatment.
Figure 2. Effect of MβCD on downstream signaling of HEK293-A2aR cells.
cAMP concentration measured from HEK293-A2aR cells following MβCD treatment, in cells treated with either no ligand (blue) or 1 μM CGS21860 (a selective agonist for A2aR) (purple). The data are mean ± S.E.M values from n≥4 independent experiments done in triplicate. *P<.05 and **P< 005 are significantly different from the untreated A2aR control for each ligand treatment.
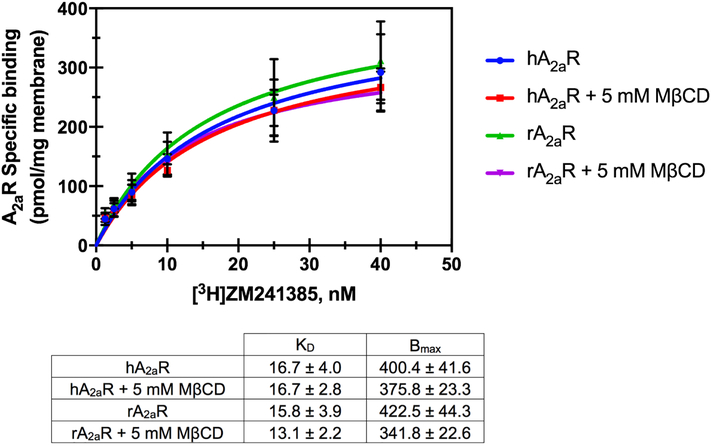
In order to determine whether reduction in cAMP synthesis was due to reduced binding of agonist, ligand binding was measured following cholesterol depletion. Surprisingly, equilibrium ligand binding of the fluorescent agonist APEC-FITC was unchanged following cholesterol depletion when measured via flow cytometry (Fig. S5). In a competition equilibrium binding experiment, we found that the binding of the selective antagonist for A2aR, ZM241385, was also unchanged following cholesterol depletion (Fig. S5). Similar results were observed when specific binding of a saturating concentration of the radioligand [3H]NECA (a non-selective agonist for adenosine receptors) was measured (Fig. S6). Additional ligand binding experiments comparing the effect of 5mM MβCD treatment on both human A2aR and rat A2aR revealed similar results. 5 mM MβCD treatment had no effect on the specific binding affinity of [3H]ZM241385 to A2aR in membrane preparations from cells expressing either receptor (Fig. 3). These data suggest that membrane cholesterol levels did not affect ligand binding, but downstream activation (via cAMP) requires sufficient membrane cholesterol for full potentiation of the G-protein coupled response for A2aR.
Figure 3. Effect of MβCD on hA2aR and rA2aR specific binding.
Specific binding of increasing concentrations of [3H]ZM241385 to membrane preparations from cells expressing hA2aR or rA2aR following treatment with 5 mM MβCD compared to untreated control. Mean ± S.D values from n=3 experiments done in duplicate.
Cholesterol Interactions at the CCM of A2R
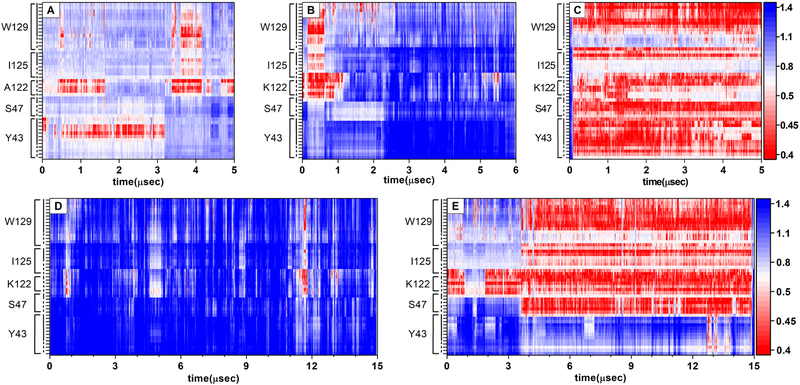
Following previous work(29, 59, 60), unbiased, all-atom simulations were performed on the receptor in five different ligation states: Bound to two agonists (NECA and UK432097), one inverse agonist and one antagonist (caffeine and ZM241385, respectively), and apo. The apo structure was obtained by deleting the ligand from the ZM241385 structure, and therefore resembles the antagonist bound structures. (The overall protein structure does not relax significantly on the simulation timescale.) Because cholesterol binding events at the surface of integral membrane proteins are slow on conventional all-atom MD time-scales, long contiguous trajectories were obtained of at least 5 μsec in each case, with the UK432097 and ZM241385 trajectories each extended to 15 μsec; in total, the five trajectories represent 46 μsec of simulation time.
The distance between cholesterol and the heavy atoms of the CCM as observed in each of these trajectories are shown as heatmaps, where close proximity is shown in red and farther distances in blue (Fig.4). When the receptor is in active state (panels C and E), a cholesterol is bound in the CCM for the majority of the trajectory, indicated by contact (heavy atom to heavy atom distance of ca. 4 nm) between atoms of a single cholesterol and the side chains of the CCM residues. (Note that a bound cholesterol is indicated in this analysis by simultaneous contact between cholesterol and several residues of the CCM, indicated by vertically oriented swaths of red in Fig. 4.) In contrast, few such interactions are observed in the inverse agonist or antagonist bound states (Panels A and D) or the antagonist-like “apo” state (Panel C; see also Fig. 5).
Figure 4. Heavy atom distance between nearest cholesterol and the CCM.
. Cholesterol binds the CCM in all-atom simulations of the receptor when bound to the agonists NECA (Panel C) or UK432097 (Panel E), as indicated by contact between heavy atoms of a single cholesterol and the sidechains of the CCM. In simulations of the receptor in inactive states (caffeine bound, Panel A; ZM241385 bound, Panel D; an apo simulation derived from the ZM241385 state, Panel B), no such interactions are observed. The residues of the CCM are labeled on the vertical axis, dashed line indicates the protein backbone, solid lines are the sidechains. The distance in nm is shown in the scale bar at right. A binding event appears as a close approach (red) of all residues of the CCM simultaneously.
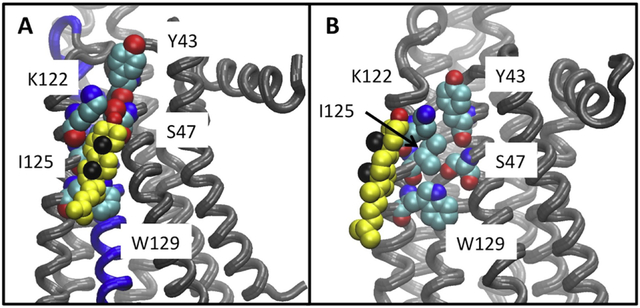
Figure 5. Cholesterol bound to the CCM during simulations.
Panel A: A snapshot from the UK432097 bound simulation, showing the disposition of cholesterol (yellow, black spheres show the methyls of the beta face and red the hydroxyl) relative to the CCM (shown in space filling representation) in a tightly bound configuration. Panel B: The closest approach obtained between cholesterol and the CCM in any of the inactive receptor simulations (snapshot from ca. 0.8 μsec of the ZM241385 bound simulation.)
The lysine at residue 122 is positioned to hydrogen bond with the hydroxyl of the cholesterol (See Fig. 5) and seems likely critical to binding — other reported cholesterol binding sites on GPCRs have a charged residue positioned similarly(61, 62). Note that this residue is mutated to alanine in the sequence that is thermostabilized for binding antagonist. Tyrosine does not appear to be critical for a tight interaction with cholesterol at the CCM, as it hardly interacts at all in Panel E (Fig. 4).
Discussion
In the present work we have studied the effect that MβCD treatment has on HEK293 cells and the effect that membrane cholesterol has on the modulation of A2aR activity through bulk changes to the membrane via MβCD cholesterol depletion. MβCD depletion experiments demonstrated that cholesterol reduction from HEK293 cells expressing A2aR led to a diminished downstream signaling (via cAMP), which is a measure of the protein’s activity. This reduction in downstream signaling was observed even though membrane receptor levels as well as ligand binding of the fluorescent agonist, FITC-APEC, radiolabeled agonist [3H]NECA, radiolabeled antagonist [3H]ZM241385 and competition binding of the unlabeled selective antagonist, ZM241385, were unchanged. Taken together, this data suggests that cholesterol depletion has a greater impact on the protein’s ability to activate the associated G-protein following ligand binding than it does on ligand binding. This model is supported by the all-atom simulation data, in which cholesterol is observed to bind the CCM of the active receptor, but not the inactive form.
Our results are consistent with prior studies of A2aR activity dependence on cholesteryl-hemisuccinate (CHS) in micelles(34). However our data contradict recently published claims that cholesterol negatively modulates A2aR(56). Guixà-González et al(56) reported an increase in the specific binding of A2aR to the selective antagonist [3H]ZM241385 in rat C6 glioma cells as the concentration of MβCD was increased and cholesterol levels decreased. It seemed unlikely that differences in ligand binding following MβCD cholesterol depletion between that observed by Guixà-González, et al. and the present work could be due to receptor differences between species. Rat A2aR (uniprot entry P30543) and human A2aR (uniprot entry P29274) are 80% identical, and rat A2aR contains all the amino acids within the CCM found in human A2aR with the exception of Y43. In addition, we expressed rat A2aR in HEK293 cells and performed ligand binding experiments to detect specific binding between [3H]ZM241385 and rat A2aR in membrane preparations from both untreated and 5 mM MβCD treated cells (Fig. 2). In our system we could not replicate the results from Guixà-González, et al. However, cellular differences in membrane lipidomes could potentially explain the observed discrepancies. It has been shown recently that lipidomes are highly diverse and flexible, with even closely related cell types having significantly diverging membrane lipid compositions. Although detailed data on the lipids of C6 cells is unavailable, Quintero and colleagues (2007) show that these cells are high in storage lipids like TAGs and CEs(63), which were not detected in our lipidomics analysis of HEK293 cells (Fig. 1C). These differences in the lipid compositions may explain the differences we observe.
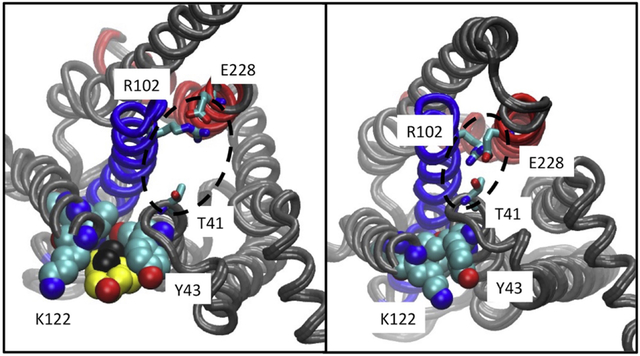
It is clear from our data that the activity of A2aR is dependent on membrane cholesterol levels; however, MβCD cholesterol depletion experiments alone do not give any insight into the mechanism by which cholesterol modulates the receptor. It has long been debated whether cholesterol indirectly modulates GPCR activity through biophysical changes to the membrane environment, or if cholesterol modulates GPCR function through specific interactions with the receptor. The crystal structure of A2a-T4L-ΔC purified in complex with the antagonist ZM241385 revealed a phospholipid, not cholesterol, bound in the CCM pocket(64). Additionally several high-resolution structures of A2aR in complex with ZM241385 all show cholesterol bound at a different site(30, 31, 65). All-atom simulation of A2aR in complex with ZM241385 reveal cholesterol bound at two sites, in the inner and outer leaflets at helix 6(29); the site at the outer leaf being consistent with the cholesterol binding sites reported from crystal structures of A2aR in complex with ZM241385 (29–31, 65). Comparisons of the extracellular or ligand binding pocket of the inactive receptor and that bound to agonist show no significant changes, consistent with our ligand binding data. The CCM is located on the intracellular side of the receptor and is positioned to influence the conformation of helix 3, the intracellular end of which contains El02 of the D/ERY ionic lock motif. In order to couple to G-protein, the ionic lock must be “broken,” opening the intracellular face of the receptor and freeing El02 to interact with the α5 helix of the G-protein(66). In inactive structures of the A2A receptor, T41 — located one-half turn of a helix away from Y43 of the CCM — participates in the ionic lock interaction network(64). It is therefore noteworthy that when cholesterol binds the CCM, it favors conformations that break the T41:ionic lock interaction, opening the intracellular face of the receptor, offering a possible mechanism whereby cholesterol binding at the CCM favors G-protein coupling (Fig. 6).
Figure 6. Ionic lock interactions with and without cholesterol at the CCM.
The left panel shows a typical state of the ionic lock when cholesterol is bound at the CCM, in which T41 (1/2 turn of helix removed from the Y43 of the CCM) does not participate in the ionic lock interaction network. The right panel shows the tight interaction between the residues of the ionic lock, including T41 from helix 2 and R102 and E228 from the conserved ionic lock motif.
Taken together, the crystal structure and simulation data suggest that cholesterol binds to A2aR at the CCM in a receptor state-dependent manner (Fig. 5). Two possible models would explain these data: Either the receptor samples active and inactive-like conformations when apo, and cholesterol binding at the CCM shifts the population toward the active state, or the conformation of the receptor in the vicinity of the CCM is changed upon binding agonist, and cholesterol subsequently binds at the CCM, stabilizing the active state. The observation that ligand binding is unaffected by cholesterol depletion is inconsistent with the former model, suggesting a two-step mechanism as described in the latter model. An alternative hypothesis that cannot be ruled out by the present data is that cholesterol depletion dissolves raft-like, ordered domains, which may enhance A2aR-Gs coupling by colocalization. However, it should be noted that enhanced G-protein coupling via raft partitioning is not mutually exclusive with a structure-based allosteric mechanism, in which partitioning to ordered domains also favors a receptor state that is ready to couple to G-protein, mediated by structural changes that occur upon cholesterol binding to the CCM.
In conclusion, our work gives important insight into the complicated mechanism by which cholesterol modulates A2aR, and other class A GPCRs. Our results suggest that sufficient quantities of cholesterol are necessary to fully activate the downstream signaling pathway of A2aR measured via cAMP accumulation. Overall, this work substantiates the importance of cholesterol in GPCR activation and opens the door to potential therapies to modulate GPCR disease related pathways. Future experiments will further investigate the specific interaction between cholesterol and A2aR at the CCM, and its role in the modulation of the receptor.
Supplementary Material
Highlights.
Treatment with methyl-β-cyclodextrin (MβCD) reduced membrane cholesterol without depleting other membrane lipids.
Cholesterol depletion reduced downstream signaling activity of the Adenosine A2A receptor measured via cAMP accumulation.
Membrane cholesterol depletion did not affect A2aR ligand binding.
Cholesterol interacts specifically with the cholesterol consensus motif of A2AR when the receptor is in an active state.
Acknowledgements
M. was supported by LA Board of Regents Fellowship LEQSF(2012-17)-GF-16 and NIH RO1GM120351; E.L., and L.Y. were supported by NIH P20GM104316. Anton2 computer time was provided by the National Resource for Biomedical Supercomputing (NRBSC), the Pittsburgh Supercomputing Center (PSC), and the Biomedical Technology Research Center for Multiscale Modeling of Biological Systems through grant P41GM103712-S1 from the National Institutes of Health. The Anton2 machine at NRBSC/PSC was generously made available by D.E. Shaw Research. A special thanks to Kirsten Swonger for her help handling the radioligands.
Footnotes
Conflict of Interest statment
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghosh E, Kumari P, Jaiman D, and Shukla AK. 2015. Methodological advances: the unsung heroes of the GPCR structural revolution. Nat. Rev. 16: 69–81. [DOI] [PubMed] [Google Scholar]
- 2.White S 2017. Membrane Proteins of Known 3D Structure. . [Google Scholar]
- 3.Katritch V, Cherezov V, and Stevens RC. 2013. Structure-Function of the G-protein-Coupled Receptor Superfamily. Annu. Rev. Pharmacol. Toxicol 53: 531–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rask-Andersen M, Almén MS, and Schiöth HB. 2011. Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov 10: 579–590. [DOI] [PubMed] [Google Scholar]
- 5.Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, Karlsson A, Al-Lazikani B, Hersey A, Oprea TI, and Overington JP. 2017. A comprehensive map of molecular drug targets. Nat. Rev. ∣ DRUG Discov 16: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeagle PL 1985. Cholesterol and the cell membrane. Biochim. Biophys. Acta 822: 267–87. [DOI] [PubMed] [Google Scholar]
- 7.Albert AD, and Boesze-Battaglia K. 2005. The role of cholesterol in rod outer segment membranes. Prog Lipid Res 44: 99–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niu S-L, Mitchell DC, and Litman BJ. 2002. Manipulation of cholesterol levels in rod disk membranes by methyl-beta-cyclodextrin: effects on receptor activation. J. Biol. Chem 277: 20139–45. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DH, and Taub D. 2002. CXCR4 Function Requires Membrane Cholesterol: Implications for HIV Infection. J. Immunol 168: 4121–26. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen DH, and Taub DD. 2003. Inhibition of chemokine receptor function by membrane cholesterol oxidation. Exp. Cell Res 291: 36–45. [DOI] [PubMed] [Google Scholar]
- 11.Pucadyil TJ, and Chattopadhyay A. 2004. Cholesterol modulates ligand binding and G-protein coupling to serotonin1A receptors from bovine hippocampus. Biochim. Biophys. Acta - Biomembr 1663: 188–200. [DOI] [PubMed] [Google Scholar]
- 12.Pucadyil TJ, and Chattopadhyay A. 2005. Cholesterol modulates the antagonist-binding function of hippocampal serotonin1A receptors. Biochim. Biophys. Acta - Biomembr 1714: 35–42. [DOI] [PubMed] [Google Scholar]
- 13.Saxena R, and Chattopadhyay A. 2012. Membrane cholesterol stabilizes the human serotonin1A receptor. Biochim. Biophys. Acta - Biomembr 1818: 2936–2942. [DOI] [PubMed] [Google Scholar]
- 14.Prasad R, Devi Paila Y, Jafurulla M, and Chattopadhyay A. 2009. Membrane cholesterol depletion from live cells enhances the function of human serotonin 1A receptors. Biochem. Biophys. Res. Commun 389: 333–337. [DOI] [PubMed] [Google Scholar]
- 15.Brown MS, and Goldstein JL. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47. [DOI] [PubMed] [Google Scholar]
- 16.Lange Y, Ye J, and Steck TL. 2004. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc. Natl. Acad. Sci. U. S. A. 101: 11664–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wojtanik KM, and Liscum L. 2003. The Transport of Low Density Lipoprotein-derived Cholesterol to the Plasma Membrane Is Defective in NPC1 Cells. J. Biol. Chem 278: 14850–14856. [DOI] [PubMed] [Google Scholar]
- 18.Ferrante A, Pezzola A, Matteucci A, Di Biase A, Attorri L, Armida M, Martire A, Chern Y, and Popoli P. 2018. The adenosine A2A receptor agonist T1–11 ameliorates neurovisceral symptoms and extends the lifespan of a mouse model of Niemann-Pick type C disease. Neurobiol. Dis 110: 1–11. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante A, De Nuccio C, Pepponi R, Visentin S, Martire A, Bernardo A, Minghetti L, and Popoli P. 2016. Stimulation of adenosine A2A receptors reduces intracellular cholesterol accumulation and rescues mitochondrial abnormalities in human neural cell models of Niemann-Pick C1. Neuropharmacology. 103: 155–162. [DOI] [PubMed] [Google Scholar]
- 20.Visentin S, De Nuccio C, Bernardo A, Pepponi R, Ferrante A, Minghetti L, and Popoli P. 2013. The Stimulation of Adenosine A2A Receptors Ameliorates the Pathological Phenotype of Fibroblasts from Niemann-Pick Type C Patients. J. Neurosci 33: 15388–15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazar AN, Bich C, Panchal M, Desbenoit N, Petit VW, Touboul D, Dauphinot L, Marquer C, Laprévote O, Brunelle A, and Duyckaerts C. 2013. Time-of-flight secondary ion mass spectrometry (TOF-SIMS) imaging reveals cholesterol overload in the cerebral cortex of Alzheimer disease patients. Acta Neuropathol 125: 133–144. [DOI] [PubMed] [Google Scholar]
- 22.Xiong FL, Callaghan D, Jones A, Walker DG, Lue L-F, Beach TG, Sue LI, Woulfe J, Xu H, Stanimirovic DB, and Zhang W. 2008. Cholesterol retention in Alzheimer’s brain is responsible for high β- and γ-secretase activities and Aβ production. Neurobiol. Dis 29: 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, and Mattson MP. 2004. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 101: 2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, and Pericak-Vance MA. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 261: 921–3. [DOI] [PubMed] [Google Scholar]
- 25.Di Paolo G, and Kim T-W. 2011. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat. Rev. Neurosci 12: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquer C, Laine J, Dauphinot L, Hanbouch L, Lemercier-Neuillet C, Pierrot N, Bossers K, Le M, Corlier F, Benstaali C, Saudou F, Thinakaran G, Cartier N, Octave J-N, Duyckaerts C, and Potier M-C. 2014. Increasing membrane cholesterol of neurons in culture recapitulates Alzheimer’s disease early phenotypes. Mol. Neurodegener 9: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalaria RN, Sromek S, Wilcox BJ, and Unnerstall JR. 1990. Hippocampal adenosine A1 receptors are decreased in Alzheimer’s disease. Neurosci. Lett 118: 257–60. [DOI] [PubMed] [Google Scholar]
- 28.Angulo E, Casadó V, Mallol J, Canela EI, Viñals F, Ferrer I, Lluis C, and Franco R. 2003. A1 adenosine receptors accumulate in neurodegenerative structures in Alzheimer disease and mediate both amyloid precursor protein processing and tau phosphorylation and translocation. Brain Pathol 13: 440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouviere E, Arnarez C, Yang L, and Lyman E. 2017. Identification of Two New Cholesterol Interaction Sites on the A2AAdenosine Receptor. Biophys. J 113: 2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batyuk A, Galli L, Ishchenko A, Han GW, Gati C, Popov PA, Lee M-Y, Stauch B, White TA, Barty A, Aquila A, Hunter MS, Liang M, Boutet S, Pu M, Liu Z-J, Nelson G, James D, Li C, Zhao Y, Spence JCH, Liu W, Fromme P, Katritch V, Weierstall U, Stevens RC, and Cherezov V. 2016. Native phasing of x-ray free-electron laser data for a G protein-coupled receptor. Sci. Adv 2: el600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, Dzerman AP, Cherezov V, and Stevens RC. 2012. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 337: 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segala E, Guo D, Cheng RKY, Bortolato A, Deflorian F, Doré AS, Errey JC, Heitman LH, IJzerman AP, Marshall FH, and Cooke RM. 2016. Controlling the Dissociation of Ligands from the Adenosine A2A Receptor through Modulation of Salt Bridge Strength. J. Med. Chem 59: 6470–6479. [DOI] [PubMed] [Google Scholar]
- 33.O’Malley MA, Lazarova T, Britton ZT, and Robinson AS. 2007. High-level expression in Saccharomyces cerevisiae enables isolation and spectroscopic characterization of functional human adenosine A2a receptor. J. Struct. Biol 159: 166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Malley MA, Helgeson ME, Wagner NJ, and Robinson AS. 2011. The morphology and composition of cholesterol-rich micellar nanostructures determine transmembrane protein (GPCR) activity. Biophys. J 100: LI 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jafurulla M, Rao BD, Sreedevi S, Ruysschaert J-M, Covey DF, and Chattopadhyay A. 2014. Stereospecific requirement of cholesterol in the function of the serotonin1A receptor. Biochim. Biophys. Acta 1838: 158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sezgin E, Kaiser H-J, Baumgart T, Schwille P, Simons K, and Levental I. 2012. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc 7: 1042–1051. [DOI] [PubMed] [Google Scholar]
- 37.Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, and Webb WW. 2007. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. U. S. A. 104: 3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naranjo AN, McNeely PM, Katsaras J, and Robinson AS. 2016. Impact of purification conditions and history on A 2A adenosine receptor activity: The role of CHAPS and lipids. Protein Expr. Purif 124: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AGW, and Tate CG. 2011. Agonist-bound adenosine A 2A receptor structures reveal common features of GPCR activation. Nature. 474: 521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JY, Patel R, and Lyman E. 2013. Ligand-dependent cholesterol interactions with the human A 2A adenosine receptor. Chem. Phys. Lipids 169: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, and Klein ML. 1983. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys 79: 926–935. [Google Scholar]
- 42.Best RB, Zhu X, Shim J, Lopes PEM, Mittal J, Feig M, Mackerell AD Jr. 2012. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput 8: 3257–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klauda JB, Venable RM, Freites JA, O’Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD, Pastor RW, and Pastor RW. 2010. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114: 7830–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, and Mackerell AD. 2010. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem 31: 671–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanommeslaeghe K, and MacKerell AD. 2012. Automation of the CHARMM General Force Field (CGenFF) I: Bond Perception and Atom Typing. J. Chem. Inf. Model. 52:3144–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, and Schulten K. 2005. Scalable molecular dynamics with NAMD. J. Comput. Chem 26: 1781–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feller SE, Zhang Y, Pastor RW, and Brooks BR. 1995. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys 103: 4613–4621. [Google Scholar]
- 48.Ryckaert J-P, Ciccotti G, and Berendsen HJC 1977. Numerical integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys 23: 321–341. [Google Scholar]
- 49.Darden T, York D, and Pedersen L. 1993. Particle mesh Ewald: An N ·log(N) method for Ewald sums in large systems. J. Chem. Phys 98: 10089–10092. [Google Scholar]
- 50.Lippert RA, Predescu C, Ierardi DJ, Mackenzie KM, Eastwood MP, Dror RO, and Shaw DE. 2013. Accurate and efficient integration for molecular dynamics simulations at constant temperature and pressure. J. Chem. Phys 139: 164106. [DOI] [PubMed] [Google Scholar]
- 51.Nosé S 1984. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys 81: 511–519. [Google Scholar]
- 52.Martyna GJ, Tobias DJ, and Klein ML. 1994. Constant pressure molecular dynamics algorithms. J. Chem. Phys 101: 4177–89. [Google Scholar]
- 53.Shan Y, Klepeis JL, Eastwood MP, Dror RO, and Shaw DE. 2005. Gaussian split Ewald: A fast Ewald mesh method for molecular simulation. J. Chem. Phys 122: 054101. [DOI] [PubMed] [Google Scholar]
- 54.Kräutler V, van Gunsteren WF, and Hiinenberger PH. 2001. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem 22: 501–508. [Google Scholar]
- 55.Zidovetzki R, and Levitan I. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta 1768: 1311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guixà-González R, Albasanz JL, Rodriguez-Espigares I, Pastor M, Sanz F, Martí-Solano M, Manna M, Martinez-Seara H, Hildebrand PW, Martin M, and Selent J. 2017. ARTICLE Membrane cholesterol access into a G-protein-coupled receptor. Nat. Commun 8: 14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scott RE, and Maercklein PB. 1979. PLASMA MEMBRANE VESICULATION IN 3T3 AND SV3T3 CELLS II. FACTORS AFFECTING THE PROCESS OF VESICULATION. J. Cell Set 35: 245–252. [DOI] [PubMed] [Google Scholar]
- 58.Scott RE 1976. Plasma membrane vesiculation: a new technique for isolation of plasma membranes. Science. 194: 743–5. [DOI] [PubMed] [Google Scholar]
- 59.Lee JY, and Lyman E. 2012. Predictions for Cholesterol Interaction Sites on the A 2A Adenosine Receptor. J. Am. Chem. Soc 134: 16512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cang X, Du Y, Mao Y, Wang Y, Yang H, and Jiang H. 2013. Mapping the Functional Binding Sites of Cholesterol in β 2-Adrenergic Receptor by Long-Time Molecular Dynamics Simulations. [DOI] [PubMed] [Google Scholar]
- 61.Jafurulla M, Tiwari S, and Chattopadhyay A. 2011. Identification of cholesterol recognition amino acid consensus (CRAC) motif in G-protein coupled receptors. Biochem. Biophys. Res. Commun 404: 569–573. [DOI] [PubMed] [Google Scholar]
- 62.Fantini J, and Barrantes FJ. 2013. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quintero ñ., Cabanas ME, and Arús C. 2007. A possible cellular explanation for the NMR-visible mobile lipid (ML) changes in cultured C6 glioma cells with growth. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1771: 31–44. [DOI] [PubMed] [Google Scholar]
- 64.Jaakola V-P, Griffith MT, Hanson MA, Cherezov V, Chien EYT, Lane JR, Ijzerman AP, and Stevens RC. 2008. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 322: 1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doré AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F, Tate CG, Weir M, and Marshall FH. 2011. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure. 19: 1283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carpenter B, Nehme R, Warne T, Leslie AGW, and Tate CG. 2016. Structure of the adenosine A2A receptor bound to an engineered G protein. Nature. 536: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supporting information file). Any additional information will be provided by the authors upon request.