Abstract
Retest learning impacts estimates of cognitive aging, but its bases are uncertain. Here we test the hypothesis that dementia related neurodegeneration impairs retest learning. Older persons without cognitive impairment at enrollment (n=567) had annual cognitive testing for a mean of 11 years, died, and had a neuropathologic examination to quantify 5 neurodegenerative pathologies. Change point models were used to divide cognitive trajectories into an early retest sensitive component and a later component less sensitive to retest. Performance on a global cognitive measure (baseline mean = 0.227, SD=0.382) increased an estimated mean of 0.142-unit per year for a mean of 1.5 years and declined an estimated mean of 0.123-unit per year thereafter. No pathologic marker was related to cognitive change before the change point; each was related to cognitive decline after the change point. Results were comparable in analyses that used specific cognitive outcomes, included 220 individuals with mild cognitive impairment at enrollment, or allowed a longer retest learning period. The findings suggest that neurodegeneration does not impact cognitive retest learning.
Keywords: retest learning, cognitive decline, longitudinal study, clinical-pathologic study, Alzheimer’s disease, TDP-43 pathology
1. INTRODUCTION
Gradually accelerating decline in cognitive function over many years is the primary clinical manifestation of dementia and its precursor, mild cognitive impairment. Assessing these trajectories in individuals requires repeated administration of cognitive tests. However, repeated cognitive testing has long been known to enhance performance (Schaie, 1965; Baltes, 1968; Rabbitt et al., 2004), and some studies have reported that this retest learning is reduced in persons with dementia (Cooper et al., 2001; Hassenstab et al., 2015) and mild cognitive impairment (Cooper et al., 2004; Duff et al., 2011). These observations suggest that estimates of cognitive decline represent an uncertain mix of actual cognitive loss plus ability to benefit from prior test experience.
However, quantifying retest effects in cognitive aging studies poses substantial challenges. The most basic problem is that because “most studies use widely-spaced measurement occasions (i.e., of sufficient duration in which systematic change over time is expected to occur) that are relatively constant across individuals, the effects of aging-related change and retest gains within a given individual in such designs are inherently confounded” (Hoffman et al., 2011). Even in data sets with some variation between time and number of measurement occasions, making separation of retest and aging effects possible, it is uncertain whether to specify retest effects as a boost after the initial measurement occasion, as constant or diminishing boosts after multiple measurement occasions, or in some other way (Vivot et al., 2016), and misspecification of retest effects is likely to impact estimates of cognitive aging (Hoffman et al., 2011). In addition to these obstacles to direct assessment of person-specific variation in cognitive retest effects, much prior research is based on relatively short retest intervals (e.g., ≤ 1 month), but most cognitive aging research uses longer retest intervals (e.g., ≥ 1 year). Further, with few exceptions (Galvin et al., 2005; Duff et al., 2014) previous research has used cognitive data to characterize the exposure (i.e., mild cognitive impairment, dementia) and the outcome, possibly biasing estimates of the association between them.
In the present analyses, we use data from two longitudinal cohort studies to test the hypothesis that dementia reduces the ability to benefit from prior cognitive test experience. A battery of 17 cognitive tests was administered at annual intervals for a mean of more than a decade. Two study features are noteworthy. First, because of the constant interval between testing occasions, we assessed cognitive retest effects indirectly based on prior observations that the rate of retest learning diminishes with subsequent re-exposures (Thorndike, 1922; Schaie, 1965; Baltes, 1968; Rapport et al., 1997; Theisen et al., 1998; Ivnik et al., 2000; Collie et al., 2003; Hausknecht et al., 2007; Bartels et al., 2010). Specifically, we statistically decomposed each individual cognitive trajectory into an early component assumed to be highly affected by retest learning and a later component assumed to be less affected. Second, at death all participants underwent a brain autopsy and uniform neuropathologic examination in which we quantified common dementia related pathologies. Analyses tested the relation of each postmortem pathologic marker to cognitive trajectory components.
2. METHODS
2.1. Participants
Analyses are based on participants in two longitudinal clinical-pathologic cohort studies. The Religious Orders Study (ROS) began in 1994 and involves older Catholic nuns, priests, and monks from across the United States (Wilson et al., 2004; Bennett et al., 2012a). The Rush Memory and Aging Project (MAP) began in 1997 and involves older lay persons from the Chicago metropolitan area (Bennett et al., 2005; Bennett et al., 2012b). Eligibility for both studies requires agreement to annual clinical evaluations and brain autopsy and neuropathologic examination at death. The clinical and neuropathologic evaluations in the two studies are identical in essential details. After a thorough discussion with study personnel, participants signed informed consent forms and an Anatomical Gift Act. Each study was approved by the institutional review board of Rush University Medical Center.
Inclusion in the present analyses required that participants in the parent studies meet 3 criteria. First, we required a minimum of 5 cognitive assessments to adequately capture nonlinear cognitive change. Second, we required a completed postmortem neuropathologic examination, to test the hypothesized association of dementia related pathologies with cognitive trajectory components. Third, because mild cognitive impairment is a precursor of dementia, we excluded those with the condition at baseline from primary analyses, but we included them in sensitivity analyses to determine whether there was enough pathology in the primary analytic group to support hypothesis testing.
At the time of these analyses, 3072 individuals without dementia had completed a baseline clinical evaluation. We excluded 822 persons with mild cognitive impairment. Of the remaining 2250 individuals without cognitive impairment, 242 died before the fourth annual follow-up evaluation and 474 had enrolled less than 3 years earlier, leaving 1534 persons with sufficient follow-up data. Of these, 729 had died and 670 (92%) had a brain autopsy. The neuropathologic examination was pending in 20 cases and 83 had some missing data. This left 567 persons in the primary analytic group. They had a mean age of 78.7 (SD=6.6; range: 64.5-96.9) at baseline, a mean age of 89.7 (SD=6.3; range: 71.3-104.3) at death, and a mean of 11.0 years of follow-up (SD=4.2; range: 3.7-21.8). They had completed a mean of 16.5 years of education (SD=3.7) and 393 (69.3%) were women.
In sensitivity analyses, we included 220 individuals who had mild cognitive impairment at baseline but otherwise met eligibility criteria. Compared to the 567 individuals in the primary analytic group, the additional 220 individuals were older (81.0 vs 79.0, t [785] = 4.5, p<0.001) and had fewer years of follow-up (9.9 vs. 11.0, x2 [1] =11.1, p<0.001); they had a similar level of education (16.3 vs 16.5, x2 [1] = 0.1, p = 0.776) and percent of women (69.1 vs 69.3, x2 [1] = 0.0, p=0.952); and aside from Lewy bodies (24.8% vs. 22.2%, x2 [1] = 0.5, p = 0.486), they had higher postmortem levels of pathology, including tangles (8.89 vs 5.33, x2 [1] = 36.5, p < 0.001), amyloid (1.90 vs 1.64, x2 [1] = 7.4, p=0.007), TDP-43 pathology (x2 [3] = 8.1, p = 0.044), and hippocampal sclerosis (15.9% vs 8.3%, x2 [1] = 9.9, p = 0.002).
2.2. Clinical evaluation
At annual intervals, participants had a uniform clinical evaluation that included a medical history, neurological examination, and assessment of cognitive function, as previously described (Bennett et al., 2006a; Bennett, et al., 2012a; Bennett et al., 2012b). On the basis of this evaluation, diagnoses of dementia, mild cognitive impairment, and other common conditions were rendered. The diagnosis of dementia followed the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984) which require a history of cognitive decline and impairment in at least two domains of cognition. Those who had impairment in at least one cognitive domain but did not meet dementia criteria were classified as mild cognitive impairment. Further information on these diagnostic criteria and their relation to clinical and pathologic outcomes is published elsewhere (Bennett et al., 2006b).
2.3. Assessment of cognitive function
A battery of cognitive performance tests was administered each year in an approximately one hour session. There were 7 episodic memory measures: Word List Memory, Word List Recall, and Word List Recognition (Welsh et al., 1994; Wilson et al., 2002) and immediate and delayed recall of Logical Memory Story A (Wechsler, 1987) and the East Boston Story (Albert et al., 1991; Wilson et al., 2002). Semantic memory was assessed with a category fluency test (Welsh et al., 1994; Wilson et al., 2002), 15-item version (Welsh et al., 1994) of the Boston Naming Test (Kaplan et al., 1983), and a brief word reading test (Wilson et al., 2002). There were 3 working memory tests: Digit Span Forward and Digit Span Backward (Wechsler, 1987) plus a modified form (Wilson et al., 2002) of Digit Ordering (Cooper et al., 1991). Modified versions (Wilson et al., 2002) of the Symbol Digit Modalities Test (Smith, 1982) and Number Comparison (Ekstrom et al., 1976) were used to assess perceptual speed, and short forms of Judgment of Line Orientation (Benton et al., 1994) and Standard Progressive Matrices (Raven et al., 1992) assessed visuospatial ability. In analyses, these individual measures were used to create composite measures of global cognition (based on all 17 tests), episodic memory (7 tests), and perceptual speed (2 tests). In each case, raw scores on individual tests were converted to z scores using the baseline mean and SD from the pooled parent cohorts, and z scores on component tests were averaged to yield the composite scores. Further information on the individual tests and development of the composite measures is published elsewhere (Wilson et al., 2002; Wilson et al., 2003; Wilson et al., 2005).
2.4. Neuropathologic examination
A standard protocol was used for removal of the brain and sectioning and preservation of the tissue (Bennett et al. 2006b; Schneider, Arvanitakis, Leurgans, & Bennett, 2009). Density of tau-immunoreactive neurofibrillary tangles was assessed in 8 brain regions (CA1/subiculum, entorhinal cortex, anterior cingulate cortex, dorsolateral prefrontal cortex, superior frontal cortex, inferior parietal cortex, inferior temporal cortex, primary visual cortex) with an antipaired helical filaments-tau antibody clone AT8 (ThermoScientific;1:2000). Beta-amyloid-immunoreactive plaques in the same 8 brain regions were assessed with a monoclonal antibody (1:50; Beta-amyloid, Clone 6F/3D, Dako). Raw regional data were averaged to provide separate composite measures of tangle density and beta-amyloid burden (Bennett et al., 2004). Monoclonal antibodies to phosphorylated TDP-43 (p5409/410; 1:100) (Neumann et al., 2009) were used to identify TDP-43 cytoplasmic inclusions in 6 brain regions (amygdala, CA1/subiculm, dentate gyrus, entorhinal cortex, midfrontal cortex, middle temporal cortex). The density of inclusions was rated on a 4-point scale and averaged across regions to yield a composite measure of TDP-43 pathology (Wilson et al., 2013; Nag et al., 2017). Severe neuronal loss and gliosis in any cornu ammonis subfield or the subiculum was classified as hippocampal sclerosis (Nag et al., 2015). A monoclonal antibody to alpha-synuclein (Zymed LB 509; 1: 50) was used to identify Lewy bodies in 6 brain regions (substantia nigra, anterior cingulate cortex, entorhinal cortex, midfrontal cortex, superior or middle temporal cortex, inferior parietal cortex) as previously described (Schneider et al., 2006). Lewy bodies and hippocampal sclerosis were treated as present or absent in analyses.
2.5. Statistical analysis
To assess trajectories of change in cognitive function over time, we constructed linear mixed-effects models (Laird and Ware, 1982). To capture retest learning we included a change point that allowed the rate of cognitive change to shift (Hall et al., 2001). We constrained the change point to occur within the first 3 years of observation because retest learning is thought to be strongest following initial test experiences and to diminish thereafter and because we did not want acceleration in cognitive decline proximate to death (i.e., terminal cognitive decline [Thorraldsson et al., 2008; Wilson, Segawa, Hizel, et al. 2012]) to impact placement of the retest learning change point. We did not add another change point (to separate the trajectory after the retest learning segment into preterminal and terminal components) because we needed time since baseline to capture retest effects but we would need time before death to capture terminal decline. Even if time were not misaligned, convergence can be a problem in models with two change points unless data are restricted to those with incident dementia (Wilson, Segawa, Boyle, et al., 2012). The model was implemented with OpenBugs software (Lunn et al., 2009) using a Bayesian Monte Carlo Markov Chain approach (Gelman et al., 2004) and had 4 components: intercept, initial slope, change point, and slope after change point. The first model included terms for age (at baseline), sex, education, and time from baseline to death. The second model added terms for each of the 5 postmortem pathologic markers. The primary outcome was the composite measure of global cognition. We repeated the second model separately with composite measures of episodic memory and perceptual speed.
To form subgroups based on combinations of pathologies to represent differences in overall pathologic burden in figures, we investigated the underlying structure of the pathologic data with multiple correspondence analysis (Benzecri, 1973; Benzecri, 1992) with binary versions of continuous variables (i.e. above or below the median).
We added individuals with baseline mild cognitive impairment to the analytic group and conducted 2 sensitivity analyses. First, we repeated the second model (which had terms for demographic and neuropathologic variables) with the global cognitive outcome. Second, we used the same model terms and outcome but constrained the change point to occur in the first 4 years of observation (rather than the first 3 years).
3. RESULTS
At baseline, the composite measure of global cognition ranged from −0.902 to 1.412 (mean=0.227, SD=0.382) with higher scores indicating better functioning. Younger age (r =−0.32, p<0.001) and higher educational attainment (r=0.39, p<0.001) were associated with higher baseline level of global cognition; there were no sex differences (t [565]=1.9, p=0.059).
We constructed a mixed-effects change point model to decompose cognitive trajectories into components differentially affected by retest learning. Because retest learning tends to be strongest following initial test experiences, the change point was constrained to occur in the first 3 study years. This also reduced the likelihood of impending death influencing placement of the change point. The initial model included terms for age at baseline, sex, and education (Table 1). In this analysis, there was an initial increase of 0.142-unit per year (95% CI: 0.120, 0.167) for a mean of 1.478 years (95% CI: 1.345, 1.615) after which performance declined at a mean rate of 0.123-unit per year (95% CI: −0.141, −0.107). Age was not related to the initial rate of cognitive decline or the timing of the change point, but older age was associated with lower initial cognitive level and more rapid cognitive decline after the change point. Neither sex nor education was related to global cognitive trajectories except for an association of higher level of education with higher initial level of cognition.
Table 1.
Relation of demographic measures to trajectories of global cognition
| Mean | Estimate | 95% credible interval |
|---|---|---|
| Intercept | 0.126 | 0.076, 0.176 |
| Initial slope | 0.142 | 0.120, 0.167 |
| Change point | 1.478 | 1.345, 1.615 |
| Slope after change point | −0.123 | −0.141, −0.107 |
| Age at baseline | ||
| Intercept | −0.017 | −0.023, −0.002 |
| Initial slope | −0.000 | −0.003, 0.002 |
| Change point | 0.007 | −0.024, 0.011 |
| Slope after change point | −0.001 | −0.004, −0.0001 |
| Male gender | ||
| Intercept | −0.045 | −0.124, 0.033 |
| Initial slope | −0.018 | −0.047, 0.012 |
| Change point | 0.191 | −0.028, 0.400 |
| Slope after change point | 0.023 | −0.005, 0.050 |
| Education | ||
| Intercept | 0.039 | 0.028, 0.049 |
| Initial slope | −0.003 | −0.007,0.001 |
| Change point | 0.022 | −0.010, 0.053 |
| Slope after change point | −0.003 | −0.007, 0.000 |
Note. From a mixed-effects change point model adjusted for duration of follow-up.
On postmortem neuropathologic examination, the mean tangle density was 5.33 (SD=5.86, skewness=2.42, range: 0.00 – 44.59). Because of the skewed distribution of beta-amyloid, scores were subjected to a square root transformation, resulting in a mean of 1.64 (SD=1.18, skewness = 0.08, range: 0.00 – 4.73). The mean TDP-43 pathologic stage was 0.97 (SD=1.10, skewness = 0.64, range: 0-3). There were 47 individuals with hippocampal sclerosis (8.3%) and 126 individuals with Lewy bodies (22.2%).
To test the hypothesis that neurodegenerative disease is associated with reduced retest learning, we repeated the initial change point model with terms added for the 5 postmortem markers of neurodegeneration (Table 2). In this analysis, age was no longer related to cognitive decline. More importantly, none of the pathologic markers was related to the portion of the cognitive trajectory preceding the change point, but each marker was related to more rapid decline after the change point.
Table 2.
Relation of postmortem neuropathologic markers to trajectories of global cognition
| Fixed effects | Estimate | 95% credible interval |
|---|---|---|
| Mean | ||
| Intercept | 0.161 | 0.098, 0.223 |
| Initial slope | 0.138 | 0.122, 0.167 |
| Change point | 1.444 | 1.254, 1.629 |
| Slope after change point | −0.093 | −0.112, −0.075 |
| Amyloid (square roof) | ||
| Intercept | 0.015 | −0.020, 0.051 |
| Initial slope | −0.001 | −0.013, 0.015 |
| Change point | −0.078 | −0.022, −0.179 |
| Slope after change point | −0.010 | −0.020, 0.000 |
| Tangles | ||
| Intercept | −0.005 | −0.013, 0.002 |
| Initial slope | 0.001 | −0.002, 0.004 |
| Change point | 0.007 | −0.009, 0.024 |
| Slope after change point | −0.008 | −0.009,−0.005 |
| Lewy bodies | ||
| Intercept | −0.017 | −0.070,0.103 |
| Initial slope | 0.025 | −0.011, 0.063 |
| Change point | −0.013 | −0.229, 0.206 |
| Slope after change point | −0.049 | −0.075, −0.024 |
| TDP-43 pathology | ||
| Intercept | −0.036 | −0.074, 0.002 |
| Initial slope | −0.004 | −0.015, 0.015 |
| Change point | 0.047 | −0.056, 0.151 |
| Slope after change point | −0.020 | −0.031, −0.009 |
| Hippocampal sclerosis | ||
| Intercept | 0.009 | −0.136, 0.155 |
| Initial slope | 0.016 | −0.040, 0.079 |
| Change point | 0.154 | −0.178, 0.488 |
| Slope after change point | −0.045 | −0.086, −0.006 |
Note. From a mixed-effects change point model adjusted for age, sex, education and duration of follow-up.
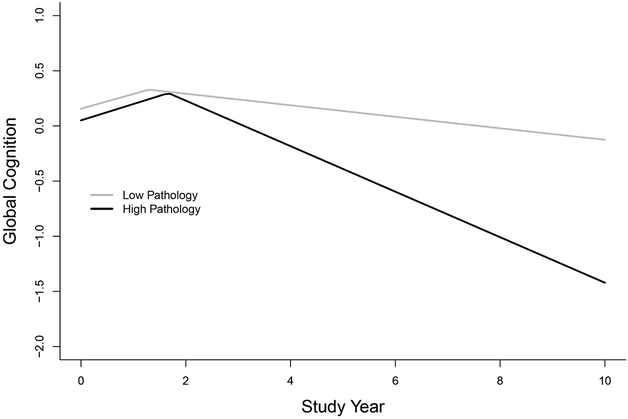
To illustrate the results, we used two subgroups differing in overall pathologic burden based on multiple correspondence analysis of the structures underlying the pathologic data. We identified subgroups with a low pathologic burden (n=174: tangles and beta-amyloid below median, no other pathologic condition present) and high pathologic burden (n=82:tangles and beta-amyloid above median, stage 2 TDP-43 pathology [present in hippocampus], 34.2% with hippocampal sclerosis, 30.5% with Lewy bodies). We then plotted in Figure 1 the global cognitive trajectories predicted by the model for a typical participant from the low pathologic burden subgroup (gray line: subgroup mean level of tangles [1.42] and beta-amyloid [0.46] with no other pathology) and a typical participant from the high pathologic burden subgroup (black line: subgroup mean level of tangles [14.28] and beta-amyloid [2.74], stage 2 TDP-43 pathology, and no other pathology]. The trajectories in the figure are parallel before the change point and diverge after it suggesting that dementia related pathologies have little impact on retest learning but substantial association with loss of cognitive ability.
Figure 1.
Predicted trajectories of change in global cognition for typical participants from the primary analytic group with a high (black line) versus low (gray line) burden of pathology from a mixed-effects change point model adjusted for age, sex, education, and duration of follow-up.
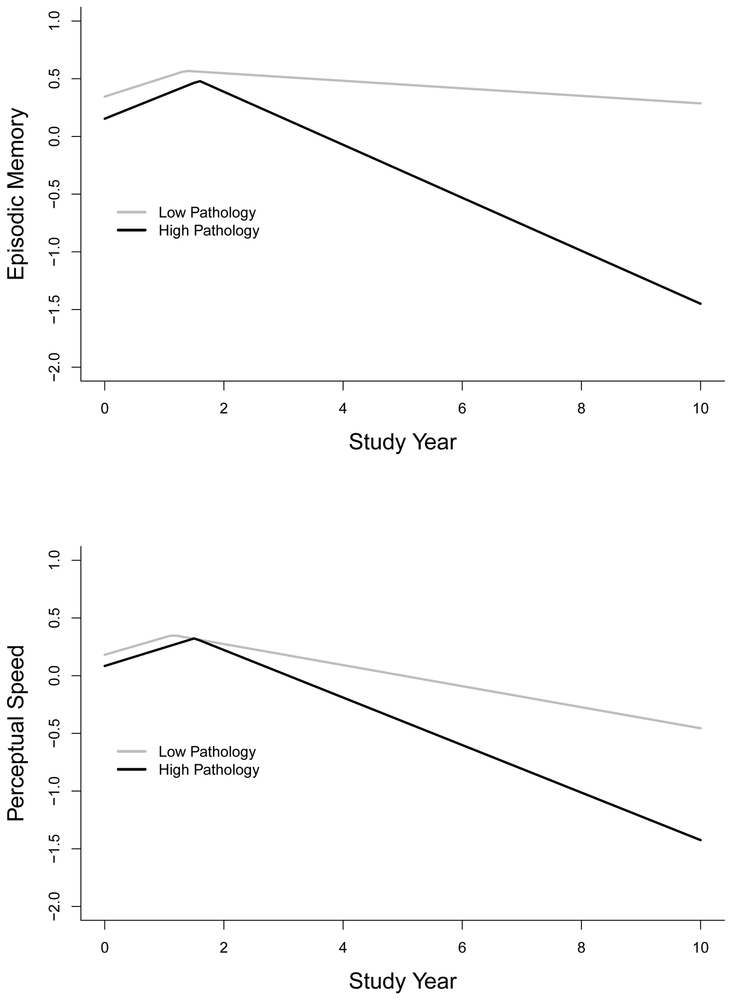
To determine whether results varied in specific cognitive domains, we repeated the analysis separately with composite measures of episodic memory and perceptual speed (Table 3). Results, shown in Figure 2 for typical individuals with a low (gray line) versus a high (black line) pathologic burden, were comparable to the analysis of global cognition. Thus, no pathologic marker was related to the initial part of either cognitive trajectory. By contrast, each pathologic marker was related to more rapid decline after each change point with the exception of hippocampal sclerosis which was related to decline in episodic memory but not perceptual speed.
Table 3.
Relation of postmortem neuropathologic markers to trajectories of episodic memory and perceptual speed
| Fixed effects | Episodic Memory Estimate (95% CI) |
Perceptual Speed Estimate (95% CI) |
|---|---|---|
| Mean | ||
| Intercept | 0.298 (0.240, 0.355) | 0.163 ((0.070, 0.257) |
| Initial slope | 0.181, (0.147, 0.217) | 0.151 (0.115, 0.192) |
| Change point | 1.446 (1.269, 1.622) | 1.326 (1.095, 1.550) |
| Slope after change point | −0.086 (−0.103, −0.068) | −0.131 (−0.147, −0.115) |
| Amyloid (square root) | ||
| Intercept | 0.006 (−0.026, 0.038) | 0.002 (−0.050, 0.055) |
| Initial slope | 0.000 (−0.017, 0.018) | −0.000 (−0.018, 0.018) |
| Change point | −0.003 (−0.019, 0.014) | −0.003 (−0.012, 0.030) |
| Slope after change point | −0.010 (−0.012, −0.008) | −0.010 (−0.005, −0.001) |
| Tangles | ||
| Intercept | −0.016(−0.023, −0.009) | −0.006 (−0.017, 0.005) |
| Initial slope | 0.004 (−0.000, 0.009) | 0.000 (−0.003, 0.004) |
| Change point | −0.003 (−0.019, 0.014) | −0.003(−0.012, 0.030) |
| Slope after change point | −0.010(−0.012, −0.008) | −0.010 (−0.005, −0.001) |
| Lewy Bodies | ||
| Intercept | −0.058 (−0.143, 0.026) | 0.057 (−0.079, 0.195) |
| Initial slope | 0.016 (−0.030, 0.070) | 0.030 (−0.020, 0.088) |
| Change point | 0.065 (−0.287, 0.147) | 0.098 (−0.344, 0.178) |
| Slope after change point | −0.043 (−0.068, −0.018) | −0.048 (−0.071, −0.026) |
| TDP-43 pathology | ||
| Intercept | −0.002 (−0.037, 0.033) | −0.014 (−0.071, 0.045) |
| Initial slope | −0.007, (−0.026, 0.014) | 0.151 (0.115, 0.192) |
| Change point | 0.058 (−0.041, 0.154) | −0.010 (−0.106, 0.126) |
| Slope after change point | −0.022 (−0.033, −0.012) | −0.016 (−0.025, −0.006) |
| Hippocampal sclerosis | ||
| Intercept | −0.055 (−0.195, 0.086) | −0.059 ((−0.281, 0.166) |
| Initial slope | 0.054, (−0.026, 0.143) | −0.007 (−0.076, 0.078) |
| Change point | 1.446 (1.269, 1.622) | 0.339 (−1.188, 0.815) |
| Slope after change point | −0.086 (−0.103, −0.068) | −0.021 (−0.059, 0.017) |
Note. From 2 mixed-effects change point models adjusted for age, sex, education and duration of follow-up. CI, credible interval.
Figure 2.
Predicted trajectories of change in episodic memory (upper panel) and perceptual speed (lower panel) for typical participants from the primary analytic group with a high (black line) versus low (gray line) burden of pathology from mixed-effects change point models adjusted for age, sex, education, and duration of follow-up.
3.1. Sensitivity analyses
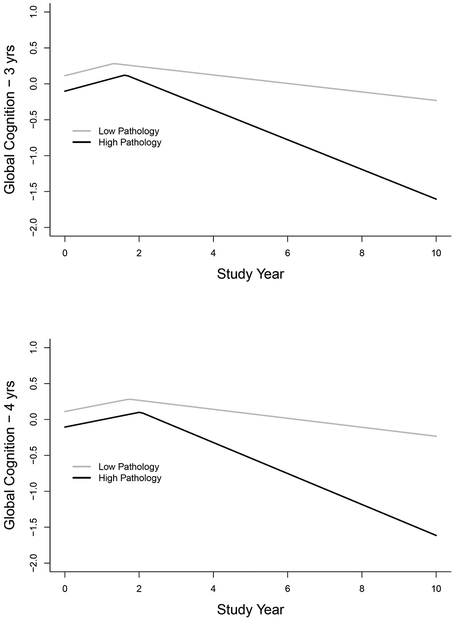
We considered the possibility that excluding those with mild cognitive impairment at study baseline may have reduced the overall neuropathologic burden in the analytic group and thereby limited our ability to detect an association of pathology with initial cognitive performance. Therefore, we repeated the core model (Table 2) including 220 persons who had mild cognitive impairment at baseline and higher neuropathologlic levels at autopsy. As shown in the upper part of Figure 3 and model A of Table 4, results were comparable to the original analysis, with no neuropathologic markers related to the original part of the global cognitive trajectory and each neuropathologic marker related to more rapid decline after the change point.
Figure 3.
Predicted trajectories of change in global cognition for typical participants from the expanded analytic group with a high (black line) versus low (gray line) burden of pathology and a 3-year (upper panel) or a 4-year (lower panel) limit on the maximum duration of the retest learning period, from mixed-effects change point models adjusted for age, sex, education, and duration of follow-up.
Table 4.
Relation of postmortem neuropathologic markers to trajectories of global cognition in the expanded analytic group (n=787)
| Fixed effects | Model A (maximal retest period = 3 years) Estimate (95% CI) |
Model B (maximal retest period = 4 years) Estimate (95% CI) |
|---|---|---|
| Mean | ||
| Intercept | 0.051(−0.000, 0.102) | 0.045 (−0.005, 0.095) |
| Initial slope | 0.132(0.110, 0.156) | 0.101(0.085, 0.118) |
| Change point | 1.436(1.287,1.581) | 1.884(1.717,2.054) |
| Slope after change point | −0.105(−0.117,−0.093) | −0.111(−0.124,−0.098) |
| Amyloid | ||
| Intercept | 0.002(−0.027,0.030) | −0.001(−0.029,0.027) |
| Initial slope | −0.001(−0.013,0.011) | −0.001(−0.010,0.007) |
| Change point | 0.084(0.012,0.158) | 0.122(0.040,0.204) |
| Slope after change point | −0.013(−0.020,−0.006) | −0.015(−0.022,−0.008) |
| Tangles | ||
| Intercept | −0.018(−0.023,−0.013) | −0.018(−0.023,−0.013) |
| Initial slope | 0.001(−0.001,0.004) | 0.001(−0.001,0.003) |
| Change point | −0.003(−0.014,0.008) | −0.009(−0.021,0.004) |
| Slope after change point | −0.007(−0.008,−0.006) | −0.051(−0.009,−0.006) |
| Lewy bodies | ||
| Intercept | −0.040(−0.115,0.035) | −0.040(−0.112,0.032) |
| Initial slope | 0.020(−0.011,0.055) | 0.012(−0.011,0.037) |
| Change point | −0.012(−0.186,0.167) | −0.025(−0.231,0.177) |
| Slope after change point | −0.041,(−0.059,−0.024) | −0.043(−0.061,−0.024) |
| TDP-43 pathology | ||
| Intercept | 0.007(−0.024,0.037) | 0.008(−0.023,0.037) |
| Initial slope | −0.003(−0.015,0.010) | −0.002(−0.011,0.007) |
| Change point | 0.079(−0.003),0.161) | 0.071(−0.022,0.162) |
| Slope after change point | −0.013(−0.020,−0.005) | −0.013(−0.021,−0.005) |
| Hippocampal sclerosis | ||
| Intercept | −0.191(−0.300,−0.082) | −0.193(−0.302,−0.086) |
| Initial slope | 0.021(−0.025,0.076) | 0.009(−0.023,0.046) |
| Change point | −0.072(−0.337,0.193) | −0.074(−390,0.234) |
| Slope after change point | −0.013(−0.080,−0.027) | −0.056(−0.084,−0.027) |
Note. From 2 mixed-effects change point models adjusted for age, sex, education, and duration of follow-up. CI, credible interval.
To determine whether constraining the maximum duration of the retest learning period to 3 years affected results, we repeated the previous analysis allowing a maximum retest learning period of 4 years. As shown in the lower part of Figure 3 and model B of Table 4, the mean change point increased to 1.88 years, but the results were otherwise consistent with previous analyses: neuropathological markers were unrelated to the initial slope and consistently related to the slope after the retest period.
4. DISCUSSION
The present analyses are based on more than 500 older persons who had annual cognitive testing for a mean of 11 years, died, and underwent a brain autopsy and neuropathologic examination to quantify 5 postmortem markers of neurodegenerative conditions associated with late-life cognitive decline and dementia. We estimated individual trajectories of change in cognitive function with a person-specific change point that allowed for retest learning in initial follow-up visits. On average, cognitive performance improved during the first 1.5 to 2.0 years of observation and declined thereafter. None of the postmortem neurodegenerative markers was related to cognitive change in the initial part of the trajectory; each was associated with more rapid decline in the latter part of the trajectory. The results suggest that common age-related neurodegeneration does not substantially influence long-term cognitive retest learning.
Previous research on dementia and retest learning has had mixed results. When retest learning is assessed over a period of a few hours or days, lower level of learning has been associated with lower current (Duff et al., 2011) and subsequent (Duff et al., 2012) levels of cognitive function. With retest intervals of at least one year, measures of retest learning appear to be associated with adverse cognitive outcomes assessed during the same observation period (Machulda et al., 2013; Hassenstab et al., 2015), but higher initial level of cognitive function has been associated with higher (Rabbitt et al., 2008) and lower (Gross et al., 2015) subsequent levels of retest learning. This inconsistency likely reflects several factors, but a fundamental problem is that it is uncertain how best to quantify long term cognitive retest effects while accounting for possible associations between retest learning and actual change in cognitive ability. The present study confronted this challenge in two ways. First, because the rate of retest learning tends to diminish with subsequent test experience, we statistically decomposed each cognitive trajectory into components more or less influenced by retest, as in previous research (Wilson et al., 2015; Wilson et al., 2016). Second, we defined dementia pathologically rather than clinically avoiding the potential bias involved in using cognitive data to ascertain the exposure (i.e., dementia) and the outcome (i.e., cognitive retest learning) while accommodating the mix of pathologies that underlie late-life dementia and mild cognitive impairment (Kapasi et al., 2017; Abner et al., 2017). We found no evidence that individual differences in common forms of neurodegeneration are contributing to variability in long term cognitive retest learning among older persons.
Age is a leading risk factor for dementia and several studies have examined its relation to long term cognitive retest learning. These studies, which unlike the present study have included middle aged participants, have mostly null results with no age differences in retest effects on most cognitive measures (Ferrer et al., 2004; Rabbitt et al., 2004; Ronnlund et al., 2005; Wilson et al., 2006; Gross et al, 2015; Karlamangla et al., 2009) and where age differences have been observed, retest effects have sometimes been stronger in younger persons (Ferrer et al., 2004) and sometimes in older persons (Rabbitt et al., 2004). Group based designs intended to quantify the impact of prior test experience, including the twice-minus-once-tested method (Salthouse, 2015) and the quasi-longitudinal method (Salthouse, 2016), suggest smaller test experience effects at older ages than younger ages. However, age differences in these analyses were only observed for a subset of cognitive outcomes, and longitudinal data collected in a measurement burst design suggested that older individuals benefitted more from prior test experience than younger individuals on some cognitive outcomes with no age difference on others (Salthouse, 2012). In the present analyses, we did not find an association of age with retest learning. Thus, age was not related to the portion of the cognitive trajectory most impacted by prior test experience. Older age was associated with more rapid decline in the second part of the cognitive trajectory, but this component is assumed to be less impacted by testing experience and this association was eliminated after the postmortem markers of neurodegeneration were added to the model.
There was clear evidence of retest learning in the present analyses but no evidence that it was differentially related to the pathologies traditionally linked with late-life dementia. That is, although retest effects impact cognitive trajectory shape, they do not appear to affect the correlation of the underlying pathologic processes with the trajectories. These data complement a recent report that retest effects do not substantially impact estimates of the association of risk factors with cognitive change (Vivot et al., 2016). These observations suggest that adjustment for retest is likely to be most important when attempting to describe cognitive trajectories and less important when attempting to identify trajectory antecedents and consequences.
Study strengths and limitations should be noted. There was a mean of 11 years of observation with a high rate of participation among survivors, enhancing our ability to reliably characterize individual differences in cognitive trajectories. The rate of participation in autopsy exceeded 90%, making it unlikely that selective attrition biased results. The availability of postmortem markers of diverse neurodegenerative processes allowed us to capture the heterogeneity characteristic of late-life dementia (Kapasi et al., 2017). The main limitation is that analyses are based on a selected group. In addition, we did not directly measure retest effects. However, dividing cognitive trajectories into components differentially sensitive to retest provided a straightforward way to test hypotheses about the bases of cognitive retest effects. A limitation of our cognitive trajectory model is that the component following retest learning includes both preterminal and terminal cognitive decline though we have previously shown that common neurodegenerative markers are related to both preterminal and terminal cognitive decline (Wilson et al., 2010; Boyle et al., 2013).
5. CONCLUSION
We used data from a longitudinal clinical-pathologic cohort study to test the hypothesis that common dementia related forms of neurodegeneration impair cognitive retest learning. We found no support for the hypothesis. Despite the impact of retest learning on the shape of cognitive aging trajectories, the present results imply that retest effects do not systematically bias estimates of the association of neurodegeneration with cognitive trajectories or of potential modifiers of that association.
Highlights.
Cognition tested annually; 5 neurodegenerative pathologies assessed at death
Cognitive trajectories decomposed into parts more vs less related to retest learning
Pathologic markers unrelated to retest sensitive part of cognitive trajectory
Pathologic markers related to retest insensitive part of cognitive trajectory
Varying the outcome, participants, and retest duration yielded similar results.
Acknowledgments
We thank the many Catholic nuns, priests, and monks who have participated in the Religious Orders Study and the many Illinois residents who have participated in the Rush Memory and Aging project; Traci Colvin, MPH, for coordination of the clinical data collection; Karen Skish, MS, for coordination of pathological data collection; Woojeong Bang, MS, for statistical programming; and John Gibbons, MS, and Greg Klein, MS, for data management.
Study funding: This research was supported by the National Institute on Aging (R01AG17917, P30AG10161, R01AG15819, R01AG34374) and the Illinois Department of Public Health. The funding sources had no role in study design; collection, analysis, or interpretation of the data; writing the report or the decision to submit for publication.
Footnotes
Declaration of interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abner EL, Kryscio RJ, Schmitt FA, Fardo DW, Moga DC, Ighodaro ET, Jicha GA, Dodge HH, Xiong C, Woltjer RL, Schneider JA, Cairns NJ, Bennett DA, Nelson PT. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol 2017;81:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M, Smith L, Scherr P, Taylor J, Evans D, Funkenstein H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 1991;57:167–178. [DOI] [PubMed] [Google Scholar]
- Baltes PB. Longitudinal and cross-sectional sequences in the study of age and generation effects. Human Development 1968; 11:145–171. [DOI] [PubMed] [Google Scholar]
- Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci 2010; 11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiol 2006a;27:169–176. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006b;66:1837–1844. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res 2012a;9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012b:9;646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiol 2005;25:163–175. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid loan with clinical Alzheimer disease and level of cognition function. Arch Neurol 2004;61:378–384. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment ed2 New York: Oxford University Press; 1994. [Google Scholar]
- Benzecri JP. L’Analyse des Donnes Volume II L’Analyse des Correspondences. Paris, France: Dunod, 1973. [Google Scholar]
- Benzecri JP. Correspondence Analysis Handbook. New York: Marcel Dekker, 1992. [Google Scholar]
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 2013; 74: 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collie A, Maruff P, Darby DG, McStephen M: The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-intervals. J Int Neuropsychol Soc 2003, 9:419–428. [DOI] [PubMed] [Google Scholar]
- Cooper DB, Epker M, Lacritz L, Weiner MF, Rosenberg RN, Honig L, Cullum CM. Effects of practice on category fluency in Alzheimer’s disease. Clin Neuropsychol 2001;15:125–128. [DOI] [PubMed] [Google Scholar]
- Cooper DB, Lacritz LH, Weiner MF, Rosenberg RN, Cullum CM. Category fluency in mild cognitive impairment: reduced effect of practice in test-retest conditions. Alzheimer Dis Assoc Disord 2004;18:120–122. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sager HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early untreated Parkinson’s disease and its relationship to motor disability. Brain 1991; 114:2095–2122. [DOI] [PubMed] [Google Scholar]
- Duff K, Chelune G, Dennett K. Within-session practice effects in patients referred for suspected dementia. Dement Geriatr Cogn Disord 2012;33(4):245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Foster NL, Hoffman JM. Practice effects and amyloid deposition: preliminary data on a method for enriching samples in clinical trials. Alzheimer Dis Assoc Disord 2014;28:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Lyketsos CG, Beglinger LJ, Chelune G, Moser DJ, Arndt S, Schultz SK, Paulsen JS, Petersen RC, McCaffrey RJ. Practice effects predict cognitive outcome in amnestic mild cognitive impairment. Am J Geriatric Psychiatry 2011;19:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstron RB, French JW, Harmen HH, Kermen D. Manual for factor-referenced cognitive tests. Princeton: Educational Testing Service; 1976. [Google Scholar]
- Ferrer E, Salthouse TA, Stewart WF, Schwartz BS. Modeling age and retest processes in longitudinal studies of cognitive abilities. Psychol Aging 2004;19:243–249. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Powlishta KK, Wilkins K, McKeel DW, Xiong C, Grant E, Storandt M, Morris JC. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol 2005;62:758–765. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carli JB, Stern HS, Rubin DB. Bayesian Data Analysis. New York: Chapman and Hall; 2004. [Google Scholar]
- Gross AL, Benitez A, Shih R, Bangen KJ, Glymour MM, Sachs B, Sisco S, Skinner J, Schneider BC, Manly JJ. Predictors of retest effects in a longitudinal study of cognitive aging in a diverse community-based sample. J Int Neuropsychol Soc 2015;21:506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Ying J, Kuo L, Sliwinski M, Buschke H, Katz M, Lipton RB. Estimation of bivariate measurements having different change points, with application to cognitive aging. Stat Med 2001;20:3695–3714. [DOI] [PubMed] [Google Scholar]
- Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. Absence of practice effects in preclinical Alzheimer’s disease. Neuropsychol 2015;29:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausknecht JP, Halpert JA, Di Paolo NT, Gerrard MOM: Retesting in selection: A meta-analysis of coaching and practice effects for tests of cognitive ability. J Appl Psychol 2007, 92:373–385. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Hofer SM, Sliwinski MJ. On the confounds among retest gains and age-cohort differences in the estimation of within-person change in longitudinal studies: a simulation study. Psychol Aging 2011;26:778–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivnik RJ, Smith GE, Petersen RC, Boeve BF, Kokmen E, Tangalos EG. Diagnostic accuracy of four approaches to interpreting neuropsychological test data. Neuropsychol 2000;14:163–177. [DOI] [PubMed] [Google Scholar]
- Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 2017;134:171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Ed2 Philidelphia: Lea & Febiger; 1983. [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wright RG, Chodosh J. Trajectories of cognitive function in late life in the United States demographic and socioeconomic predictors. Am J Epidemiol 2009;170:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird N, Ware J. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions (with discussion). Stat Med 2009;28:3049–3082. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s Disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer’s disease. Ann Neurol 2015;77:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology 2017;88:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Act Neuropathol 2009;117:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Holland F, Mclnnes L. Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. J Gerontol Psychol Sci Soc Sci 2004; 59:84–87. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lunn M, Wong D, Cobain M. Age and ability affect practice gains in longitudinal studies of cognitive change. J Gerontol B Psychol Sci Soc Sci 2008;63:235–240. [DOI] [PubMed] [Google Scholar]
- Rapport LJ, Brines DB, Axelrod BN, Theisen ME: Full scale IQ as mediator of practice effects: The rich get richer. Clin Neuropsychol 1997, 11:375–380. [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary: Standard Progressive Matrices. Oxford, England: Oxford Psychologist’s Press; 1992. [Google Scholar]
- Ronnlund M, Nyberg L, Backman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol Aging 2005;20:3–8. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging cognition unconfounded by prior test experience. J Gerontol B Psychol Sci Soc Sci 2016;71:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Robust cognitive change. J Int Neuropsychol Soc 2012;18:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Test experience effects in longitudinal comparisons of adult cognitive functioning. Dev Psychol 2015;51:1262–1270. [DOI] [PubMed] [Google Scholar]
- Schaie KW. A general model for the study of developmental problems. Psychological Bulletin. 1965; 64:92:107. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009;66:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Li J-L, Li Y, Wilson RS, Kordower JH, Bennett DA. Neurofibrillary tangles in the substantia nigra are related to gait impairment in older persons. Ann Neurol 2006; 59:166–173. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test Manual, revised. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Theisen ME, Rapport LJ, Axelrod BN, Brines DB: Effects of practice in repeated administrations of the Wechsler memory-scale revised in normal adults. Assessment 1998, 5:85–92. [DOI] [PubMed] [Google Scholar]
- Thorndike EL. Practice Effects in Intelligence Tests. Exp Psychol 1922; 5(2):101–107. 10.1037/h0074568. [DOI] [Google Scholar]
- Thorvaldsson V, Hofer SM, Berg S, Skoog I, Sacuiu S, Johansson B. Onset of terminal decline in cognitive abilities in persons without dementia. Neurology 2008; 71:882–887. [DOI] [PubMed] [Google Scholar]
- Vivot A, Power MC, Glymour MM, Mayeda ER, Benitez A, Spiro A, Manly JJ, Proust-Lima C, Dofouil, Gross AL. Jump, hop, or skip: modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol 2016;183;302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio; Psychological Corp.; 1987. [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol 2003; 25:634–642. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 2005; 11:400–407. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002; 17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: Overview and change in cognitive and motor speed. Aging Neuropsychol Cogn 2004; 11:280–303. [Google Scholar]
- Wilson RS, Capuano AW, Marquez DX, Amofa P, Barnes LL, Bennett DA. Change in Cognitive Abilities in Older Latinos. J Int Neuropsychol Soc 2016;22:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Capuano AW, Sytsma J, Bennett DA, Barnes LL. Cognitive aging in older Black and White persons. Psychol Aging 2015;30:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: separating retest effects from the effects of growing older. Psychol Aging 2006; 21:774–789. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgan SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age related cognitive decline. Neurology 2010; 75:1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer’s disease. Psychol Aging 2012;27:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Hizel LP, Boyle PA, Bennett DA. Terminal dedifferentiation of cognitive abilities. Neurology 2012; 78:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurology 2013;70:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]