Abstract
Gene products or pathways that are aberrantly activated in cancer but not in normal tissue hold great promises for being effective and safe anticancer therapeutic targets. Many targeted drugs have entered clinical trials but so far showed limited efficacy mostly due to variability in treatment responses and often rapidly emerging resistance. Toward more effective treatment options, we will need multi-targeted drugs or drug combinations, which selectively inhibit the viability and growth of cancer cells and block distinct escape mechanisms for the cells to become resistant. Functional profiling of drug combinations requires careful experimental design and robust data analysis approaches. At the Institute for Molecular Medicine Finland (FIMM), we have developed an experimental-computational pipeline for high-throughput screening of drug combination effects in cancer cells. The integration of automated screening techniques with advanced synergy scoring tools allows for efficient and reliable detection of synergistic drug interactions within a specific window of concentrations, hence accelerating the identification of potential drug combinations for further confirmatory studies.
Keywords: Drug combinations, High-throughput screening, Experimental design, Synergy scoring, Computational modeling
1. Introduction
A pressing challenge in the development of personalized cancer medicine is to understand how to make the most out of genomic information from a patient when evaluating treatment options. Over the past decade, there has been an extensive effort to sequence cancer genomes in large patient cohorts, sparking expectations to identify novel targets for more effective and selective treatment opportunities. These sequencing efforts have revealed a remarkable degree of genetic heterogeneity between and within tumors, which partly explains why the traditional “one-size-fits-all” anticancer treatment strategies have often produced disappointing outcomes in clinical trials [1]. On the other hand, functional studies using high-throughput drug screening allowed linking cancer genomic vulnerabilities to targeted drug responses [2–4]. However, complex genetic and epigenetic changes may lead to re-activation of multiple compensatory pathways and to emergence of treatment-resistant subpopulations (so-called cancer clonal evolution).
Therefore, to reach effective and sustained clinical responses, one often needs multi-targeted drugs or drug combinations, which selectively inhibit multiple pathways in cancer cells [5, 6]. To facilitate discovery of effective drug combinations, preclinical studies often rely on drug combination screening in cancer cell models. Those serve as a starting point to prioritize the most promising hits for further experimental investigation and therapy optimization. Many of the existing drug combination studies, however, focus on conventional chemotherapeutic drugs tested in a panel of cell lines, for which the drug combination effects might not easily translate into treatment options in the clinic (see (7)). In contrast, primary cell cultures that are derived from patients have shown tremendous potential that could enable the rapid assessment of novel drugs or drug combinations at the individual level [8]. To facilitate clinical translation, we have established at FIMM an Individualized Systems Medicine (ISM) drug combination platform. The ISM platform combines genomics, drug testing, and computational tools to predict drug responses for individual cancer patients. The ISM platform has successfully been used to functionally profile primary leukemia, ovarian cancer, and prostate cancer patient samples ex vivo so that the drug responses can be translated to the in vivo setting [9–12].
The advances in high-throughput drug combination screening have enabled the assaying of a large collection of chemical compounds, generating dynamic dose-response profiles that allow us to quantify the effect of drug combinations at an unprecedented level. A drug combination is usually classified as synergistic, antagonistic, or non-interactive. This classification is based on the deviation of the observed drug combination response from the expected effect of non-interaction (the null hypothesis). To quantify the degree of drug synergy, several models have been proposed, such as those based on the Highest single agent model (HSA) [13], the Loewe additivity model (Loewe) [14], and the Bliss independence model (Bliss) [15]. These existing drug synergy scoring models, together with their software implementations, were initially proposed for low-throughput experiments. In those experiments a limited number of drugs were combined with a fixed level of response, e.g., at their IC50 concentrations. For example, CompuSyn has become a popular tool to calculate a combination index (CI) using the Loewe additivity model [16]. However, CompuSyn allows only for manual input of one drug combination at a time, which makes it less efficient for analyzing multiple drug combinations, particularly when the drug combinations are tested under various concentrations, in a so-called dose-response matrix design.
To facilitate the data analysis of high-throughput drug combination screens, more recent tools have been made available as R implementations (https://www.R-project.org). For example, mix-low is an R package which utilizes a nonlinear mixed-effects model to calculate the CI [17]. However, mixlow works only for an experimental design where the ratio of two drugs in a combination is fixed over all tested concentrations. Therefore, it may not be directly applicable for a dose-response matrix design, where the ratios of two drugs vary. Another R package, called drc, provides an URSA (universal response surface approach) model, which is more suitable for dose-response matrix data [18]. URSA extends the Loewe model by considering the response surfaces over all the tested concentrations. In contrast to the CI, which is defined at a fixed response level, the URSA model provides a summarized drug interaction score from the whole dose-response matrix. However, the URSA implementation in the drc package often leads to fitting errors when the dose responses fail to comply with the model assumptions. To evaluate the appropriateness of URSA, one needs to trace back to its underlying theoretical paper [19]. The Bliss model has also been extended recently by incorporating the response surface concept, similar as in the URSA model, based on which a contour plot of a Bliss interaction index can be constructed [20]. We have recently developed a response surface model, called Zero Interaction Potency (ZIP), which combines the Loewe and the Bliss models, and proposed a delta score to characterize the synergy landscape over the full dose-response matrix [21].
Here, we describe an experimental-computational drug combination analysis pipeline that has been widely used in Finland and elsewhere to test and score effects of drug combinations in cancer cells [22–24]. The pipeline includes both an experimental protocol for dose-response matrix drug combination assays, as well as computational tools to facilitate the plate design and synergy modeling. The pipeline is applicable not only to cancer cell lines but also to patient-derived cancer samples for individualized drug combination optimization. With the increasing size of our compound library, including compounds that target all the known cancer survival pathways, the drug combination discovery can now be targeted toward more personalized anticancer treatment. We first describe the experimental protocol including a computer program, called FIMMcherry, which enables efficient production and visualization of combination assay plates, the output of which can be directly exported to the robotic system for automated dispensing. To address the lack of tailored software tools for high-throughput drug combination scoring, we here report a new R-package, SynergyFinder, which provides efficient implementations for all the popular synergy scoring models, including HSA, Loewe, Bliss, and ZIP. This implementation provides the lab users with more flexibility to explore their drug combination data. We expect that the use of SynergyFinder will greatly improve the interpretation of the drug combination results and may eventually lead to the standardization of preclinical drug combination studies.
2. Materials
2.1. Cell Culture
Established cancer cell lines can be purchased from multiple vendors (see Note 1).
Patient-derived samples are obtained with permission from Finnish biobanks, hospitals, and clinical collaborators [2].
Cell media, serum and supplements recommended by cell line providers.
Trypsin-EDTA.
HyQTase.
CellTox Green Cytotoxicity reagent (Promega).
CellTiter-Glo or CellTiter-Glo 2.0 reagent (Promega).
384-well tissue culture treated sterile assay plates.
MicroClime Environmental Lids.
Beckman Coulter Biomek FXP for dispensing primary cells, which tend to grow as aggregates.
Plate reader.
2.2. Drug Combination Plate Design
FIMMcherry software (see Note 2).
Source plate file in text format.
Drug combination file in text format.
Labcyte Echo 550 acoustic dispenser for dispensing compounds in precise volume with high accuracy (2.5 nL).
Storage pods.
Fig. 1.
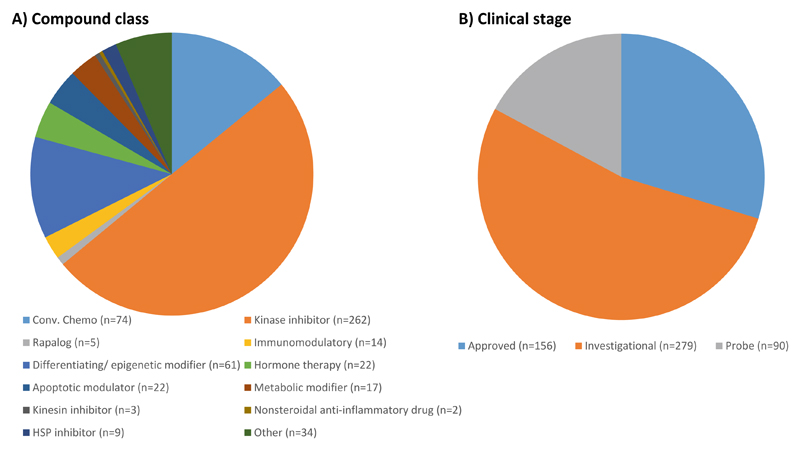
An overview of the FIMM oncology compound collection. The drug combination platform enables the testing of pairwise drug combinations from 525 small-molecular anticancer compounds that cover mainly kinase inhibitors and other signal transduction modulators. About half of the compounds comprised in the library are either FDA-approved or being evaluated in clinical trials at different stages
2.3. Phenotypic Readouts
CellTox Green Cytotoxicity Assay.
CellTiter-Glo or CellTiter-Glo 2.0 Assay.
MultiFlo FX Multi-Mode Dispenser with RAD module or Multidrop Combi Reagent Dispenser for dispensing growth media, CellTiter-Glo reagents and seeding cells.
Plate shaker.
PHERAstar FS or Cytation 5 Cell Imaging Multi-Mode plate readers for CellTox Green (fluorescence) and CellTiter-Glo (luminescence) detection on 384-well plates.
2.4. Software Tools for Data Analysis
3. Methods
The drug combination analysis pipeline starts from sample preparation and compound selection, based on which an automated plate design program called FIMMCherry is utilized. The drug sensitivity and resistance is then profiled in the plate by cell viability, cytotoxicity, and other readouts. The resulting dose-response matrix data is analyzed with the SynergyFinder R package for the detection of synergistic drug combinations (see Fig. 2).
Fig. 2.
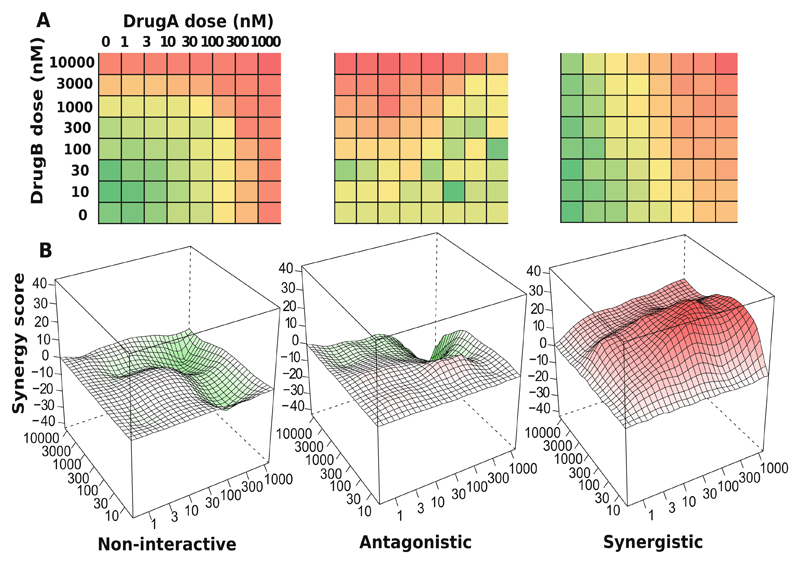
An overview of the drug combination data analysis. (a) A typical high-throughput drug combination screen utilizes a dose-response matrix design where all possible dose combinations for a drug pair can be tested. Colors in the dose-response matrices show different levels of phenotypic responses of the cancer cell with red indicating stronger inhibition and green indicating lower inhibition. (b) Depending on the interaction pattern models derived from the dose-response matrices, a drug combination can be classified as non-interactive, antagonistic, or synergistic
3.1. Cell Culture
Dissociate cells by adding 0.05% trypsin-EDTA or HyQTase to achieve a single-cell suspension.
Titrate cells to define optimal density within exponential growth (log phase). Seed cells in twofold serial dilution starting from 16,000 cells/well on 384-well plates. For most cell lines, the optimal cell number is in the range of 500–2000 cells/well.
Cell toxicity and viability detection after 72 h of incubation using CellTox Green and CellTiter-Glo reagents. Add 5 μL of culture medium to pre-drugged 384-well assay plates using MultiFlo FX Multi-Mode Dispenser with RAD module or Multidrop Combi Reagent Dispenser and shake the plates for 15 min. If toxicity measurement is performed, include 1:2000 dilution of CellTox Green reagent. Seed cells at optimal density to pre-drugged assay plates using MultiFlo FX Multi-Mode Dispenser with RAD module or Multidrop Combi Reagent Dispenser in 20 μL of culture medium. Culture cells for 72 h at 37 °C in the presence of 5% CO2. Measure the amount of dead cells, stained by the CellTox Green reagent, using a plate reader with fluorescence mode. For viability measurement, add 25 μL of CellTiter-Glo reagent to assay plates using MultiFlo FX Multi-Mode Dispenser with RAD module or Multidrop Combi Reagent Dispenser. Shake the plates for 5 min and subsequently spin the plates at 218 × g for 5 min. Measure the CTG signal in the assay wells using a plate reader with luminescence mode.
MicroClime Environmental Lids are used to minimize edge effect and to keep concentrations of solutions constant.
3.2. Drug Combination Plate Design
We utilize a combination plate layout where six compound pairs can be accommodated on one 384-well plate. A given pair of drugs is combined in a series of one blank and seven half-log dilution concentrations, resulting in an 8 × 8 dose matrix. To be able to transfer the compounds according to this matrix format, a pick list defining the source and destination plate locations and transfer volumes for the compounds is needed. An in-house program, called FIMMCherry, has been developed to automatically generate these rather complex pick lists effortlessly (see Note 7).
Two tab-delimited text files are needed as input:
A source plate file provides information of the compound stocks (compound identification, available concentration ranges, source plate identification, and well identification).
A drug combination file containing the selected compound pairs.
After loading the input files, FIMMCherry will show the layout of the plates accordingly (Fig. 3). A pick list that is compatible with the Labcyte Echo dispenser is then created by the program for compound dispensing. The Labcyte Echo 550 acoustic dispenser transfers liquid from source wells to destination wells in a non-contact fashion in 2.5 nL droplets. The pick list generated above is compatible with the Echo Cherry Pick software without further modifications to produce the pre-drugged assay plates [10].
Fig. 3.
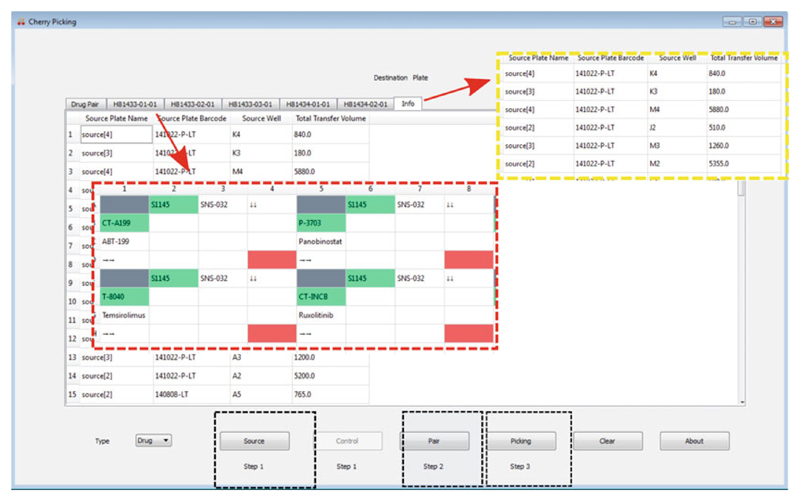
Drug combination plate design using FIMMCherry. The graphical user interface contains a virtual plate enabling an interactive way of designing the plate. After loading the input files including the source, the control, and drug pair information (the black inset boxes), the selected drug combinations and their dose ranges will be listed in the “Drug Pair” tab, for which an echo file will be generated for acoustic dispensing. Each plate can be visualized in a separate tab and will be named by its plate identifier (the red inset box). The “Info” tab shows the liquids consumption in the source plates (the yellow inset box)
The compounds are dissolved in DMSO except for 19 drugs (e.g., platinum drugs) with poor DMSO solubility or stability that are instead dissolved in water. All 525 compounds are transferred in five doses on eight 384-well plates.
The pre-dosed plates are stored in Storage Pods under nitrogen gas at room temperature for up to 1 month.
For quality control, a regular quality check-up of our compound library is performed which includes the testing of the compounds with four assay-ready cell lines (DU4475, HDQ-P1, IGROV-1, and MOLM-13) every 2 months. Following the time-dependent reproducibility of the drug responses allows us to precisely detect any changes in the compound stability and activity.
3.3. Viability Readouts
Transfer 5 μL of media with CellTox Green Cytotoxicity reagent into a 384-well containing the pre-diluted compound library (see Note 8).
Shake the plate on the plate shaker at 450 rpm for 5 min for proper drug dissolving.
Transfer a single-cell suspension in 20 μL of media to a 384-well plate. Final dilution of CellTox Green reagent should be 1:2000 in 25 μL.
Incubate the cells in the plates for 72 h.
Shake the plates on the plate shaker at 500 rpm for 30 s. Read fluorescence in the plates using a plate reader for CellTox Green Cytotoxicity detection.
Transfer 25 μL of CellTiter-Glo reagent to the plate.
Shake the plates on the plate shaker at 450 rpm for 5 min and spin the plate at 218 × g for 5 min.
Read luminescence in the plates for detecting cell viability using a plate reader.
3.4. Synergy Scoring: Installation of the SynergyFinder R-package
Download and install R (https://www.R-project.org).
Download and install Bioconductor (https://www.bioconductor.org/).
- Install the SynergyFinder package by typing in the R console as below:
> source(“https://www.bioconductor.org/biocLite.R”)> biocLite(“synergyfinder”) - Load the package:
> library(synergyfinder)
3.5. Synergy Scoring: Input Data
- A single csv file that describes a drug combination dataset is provided as input. The csv file is in a list format and must contain the following columns:
- BlockID: the identifier for a drug combination. If multiple drug combinations are present, e.g., in the standard 384-well plate where six drug combinations are fitted, then the identifiers for each of them must be unique.
- Row and Col: the row and column indexes for each well in the plate.
- DrugCol: the name of the drug on the columns in a dose-response matrix.
- DrugRow: the name of the drug on the rows in a dose-response matrix.
- ConcCol and ConcRow: the concentrations of the column drugs and row drugs in combination.
- ConcUnit: the unit of concentrations. It is typically nM or μM.
- Response: the effect of drug combinations at the concentrations specified by ConcCol and ConcRow. The effect must be normalized to %inhibition of cell viability or proliferation based on the positive and negative controls. For a well-controlled experiment, the range of the response values is expected from 0 to 100. However, missing values or extreme values are allowed. For input data where the drug effect is represented as %viability, the program will internally convert it to %inhibition value by 100-%viability.
- We provide example input data in the R package, which is extracted from a recent drug combination screen for treatment of diffuse large B-cell lymphoma (DLBCL) [7]. The example input data contains two representative drug combinations (ibrutinib and ispinesib and ibrutinib and canertinib) for which the %viability of a cell line TMD8 was assayed using a 6 by 6 dose matrix design. The example data in the required list format can be loaded and reshaped to a dose-response matrix format for further analysis by typing:
> data(“mathews_screening_data”)> dose.response.mat <- ReshapeData(mathews_screening_data, data.type = “viability”) - The “data.type” parameter specifies the type of drug response, which can be either “viability” or “inhibition.” We will use these example data to illustrate the main functions of SynergyFinder below. More documentation of the input and output parameters for each function can be accessed by typing:
> help(‘ReshapeData’)
3.6. Synergy Scoring: Input Data Visualization
- The input data can be visualized using the function PlotDoseResponse by typing:
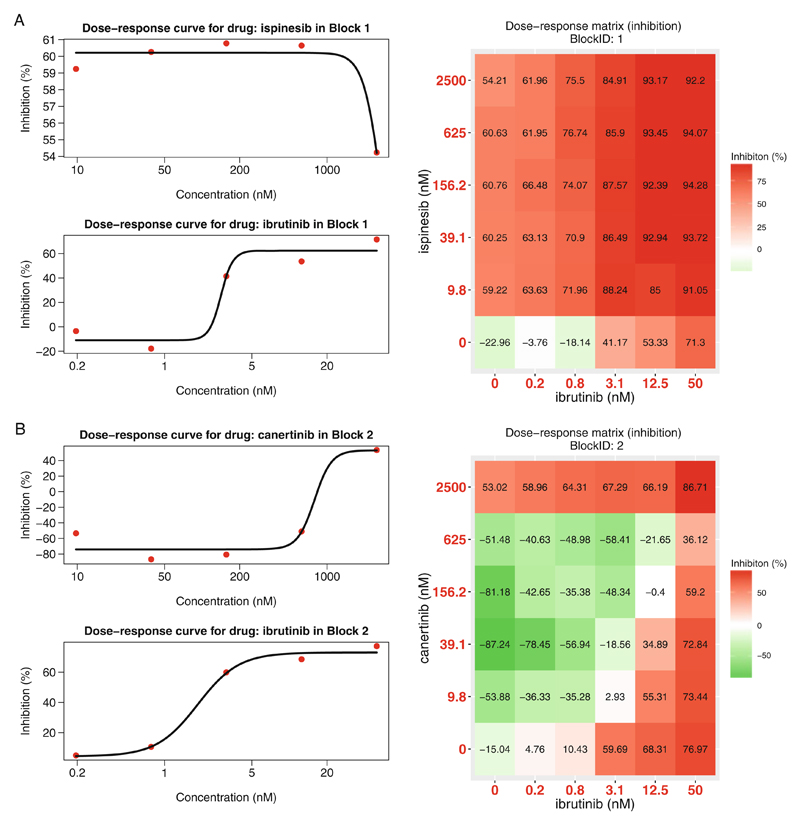
> PlotDoseResponse(dose.response.mat) - The function fits a four-parameter log-logistic model to generate the dose-response curves for the single drugs based on the first row and first column of the dose-response matrix. The drug combination responses are also plotted as heatmaps. From those, one can assess the therapeutic significance of the combination, e.g., by identifying the concentrations at which the drug combination can lead to a maximal effect on the inhibition of cancer cell survival/proliferation (see Fig. 4). The PlotDoseResponse function also provides a high-resolution pdf file by adding the “save.file” parameter:
> PlotDoseResponse(dose.response.mat, save.file = TRUE) The pdf file will be saved under the current work directory with the syntax: “drug1.drug2.dose.response.blockID.pdf.”
Fig. 4.
Plots for single-drug dose-response curves and drug combination dose-response matrices. (a) The ibrutinib and ispinesib combination. (b) The ibrutinib and canertinib combination. Left panel: single drug dose-response curves fitted with the commonly-used 4-parameter log-logistic (4PL) function. Right panel: the raw dose-response matrix data is visualized as a heatmap
3.7. Synergy Scoring: Drug Synergy Scoring (See Notes 9 and 10)
-
The current SynergyFinder package provides the synergy scores of four major reference models, including HSA, Loewe, Bliss, and ZIP. In a drug combination experiment where drug 1 at dose x1 is combined with drug 2 at dose x2, the effect of such a combination is yc as compared to the monotherapy effect y1(x1) and y2(x2). To be able to quantify the degree of drug interactions, one needs to determine the deviation of yc from the expected effect ye of non-interaction, which is calculated in different ways with the individual reference models.
HSA: ye is the effect of the highest monotherapy effect, i.e., ye = max(y1, y2).
Loewe: ye is the effect that would be achieved if a drug was combined with itself, i.e., ye = y1(x1 + x2) = y2(x1 + x2).
Bliss: ye is the effect that would be achieved if the two drugs are acting independently of the phenotype, i.e., ye = y1 + y2 – y1y2.
ZIP: ye is the effect that would be achieved if the two drugs do not potentiate each other, i.e., both the assumptions of the Loewe model and the Bliss model are met.
Once ye can be determined, the synergy score can be calculated as the difference between the observed effect yc and the expected effect ye. Depending on whether yc > ye or yc < ye the drug combination can be classified as synergistic or antagonist, respectively. Furthermore, as the input data has been normalized as %inhibition, the synergy score can be directly interpreted as the proportion of cellular responses that can be attributed to the drug interactions.
- For a given dose-response matrix, one needs to first choose which reference model to use and then apply the CalculateSynergy function to calculate the corresponding synergy score at each dose combination. For example, the ZIP-based synergy score for the example data can be obtained by typing:
> synergy.score <- CalculateSynergy(data = dose.response. mat, method = “ZIP”, correction = TRUE) For assessing the synergy scores with the other reference models, one needs to change the “method” parameter to “HSA,” “Loewe,” or “Bliss.” The “correction” parameter specifies if a baseline correction is applied on the raw dose-response data or not. The baseline correction utilizes the average of the minimum responses of the two single drugs as a baseline response to correct the negative response values. The output “synergy. score” contains a score matrix of the same size to facilitate a dose-level evaluation of drug synergy as well as a direct comparison of the synergy scores between two reference models.
3.8. Synergy Scoring: The Drug Interaction Landscape
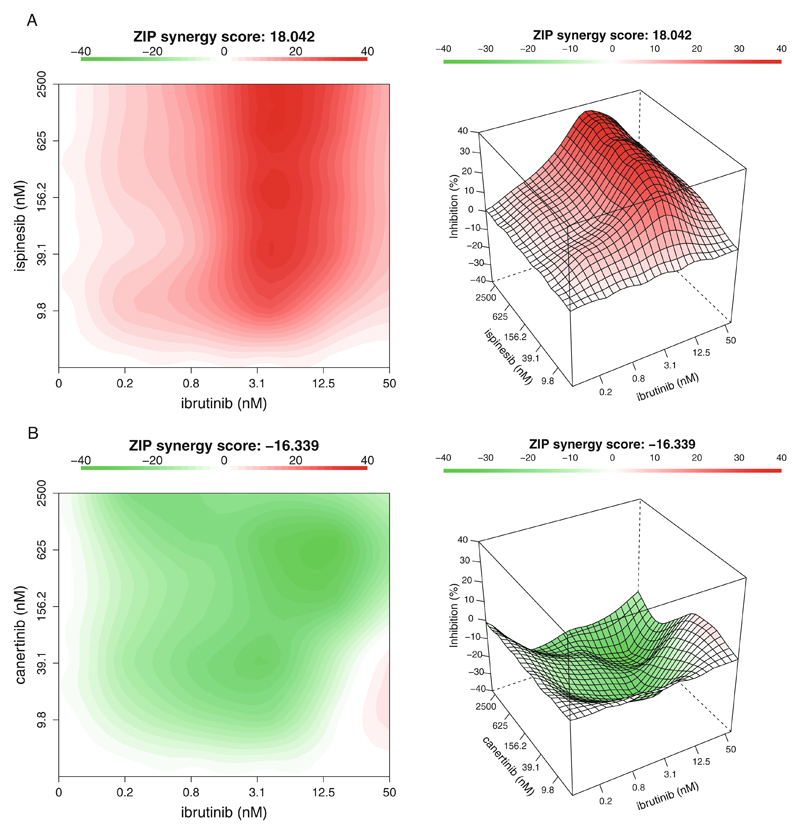
- The synergy scores are calculated across all the tested concentration combinations, which can be visualized as either a two-dimensional or a three-dimensional interaction surface over the dose matrix. The landscape of such a drug interaction scoring is very informative when identifying the specific dose regions where a synergistic or antagonistic drug interaction occurs. The height of the 3D drug interaction landscape is normalized as the % inhibition effect to facilitate a direct comparison of the degrees of interaction among multiple drug combinations. In addition, a summarized synergy score is provided by averaging over the whole dose-response matrix. To visualize the drug interaction landscape, one can utilize the PlotSynergy function as below (see Fig. 5):
> PlotSynergy(synergy.score, type = “all”, save.file = TRUE) The “type” parameter specifies the visualization type of the interaction surface as 2D, 3D, or both.
Fig. 5.
The drug interaction landscapes based on the ZIP model. (a) The ibrutinib and ispinesib combination. (b) The ibrutinib and canertinib combination
4. Notes
Examples of cell lines include four cell lines that are used for quality check of the compound library: DU4475 (breast cancer), HDQ-P1 (breast cancer), IGROV-1 (ovarian cancer), and MOLM-13 (acute monocytic leukemia).
Specific software tools are needed in the experimental design stage and in the data analysis stage. For the 384-well plate design, once the drugs and the concentration ranges are selected, we use the in-house cherry-picking program, FIMM-cherry, to automatically generate the echo files needed for the Labcyte Access system.
The FIMM oncology collection contains both FDA/EMA-approved drugs and investigational compounds (see Fig. 1). The collection is constantly evolving and the current FO4B version contains 525 compounds with concentrations ranging typically between 1 and 10,000 nM. For some compounds, the concentration range is adjusted upward (e.g., platinum drugs, 100,000 nM) or downward (e.g., rapalogs, 100 nM) to better match their relevant concentrations of bioactivity. The full list of the FIMM oncology compounds can be found in Supplementary Table 1.
When the drug combination dose-response matrix data is ready, we then use the SynergyFinder R-package to score and visualize the drug interactions. The SynergyFinder is also available as a web-application without the need to install the R environment.
The SynergyFinder package will be continuously updated for including more rigorous analyses such as statistical significance, effect size, and noise detection.
Availability: The source code for the FIMMCherry program is available at github (https://github.com/hly89/FIMM-Cherry). The SynergyFinder R package for drug combination data analysis is available at CRAN and Bioconductor.
FIMMCherry is a desktop GUI application, which is developed using Python (https://www.python.org/) and Qt application development framework (https://www.qt.io/). The integration of Python and Qt allows FIMMCherry to run on all the major computer platforms including Windows, Linux, and Mac OS X.
We have not seen problems in cell proliferation rate or other major effects when using the reagent. The reagent is stable at least 72 h in the cell culture and the cells dying at the beginning of the 72 h incubation are still stained after 72 h.
We provide an R-package SynergyFinder to calculate the drug synergy scores using four different reference models, acknowledging the fact that the optimal method for standardization of drug combination data analysis remains an open question (28). The users are therefore advised to apply all the models for their data and report a drug combination that can show a detectable level of synergy scores irrespective of the model in selection.
A strong synergy in a drug combination, as revealed using the synergy landscape analysis, might not be sufficient to warrant the next level confirmatory analysis if the synergy does not lead to sufficient overall responses. Therefore, the synergy scoring is always advised to be combined with the raw dose-response matrix data visualized in Fig. 4 to provide an overview of the extra benefits of drug combinations compared to single drugs.
Supplementary Material
Acknowledgments
This work was supported by the Academy of Finland (grants 272437, 269862, 279163, 295504, and 292611 for TA, 272577 and 277293 for KW); the Integrative Life Science Doctoral Program at the University of Helsinki (LH), the Sigrid Juse´lius Foundation (KW) and the Cancer Society of Finland (JT, TA, and KW). This project has received funding from the European Union’s Horizon 2020 research and innovation program 2014–2020 under Grant Agreement No 634143 (MedBioinformatics).
References
- 1.Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pemovska T, Kontro M, Yadav B, et al. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia. Cancer Discov. 2013;3:1416–1429. doi: 10.1158/2159-8290.CD-13-0350. [DOI] [PubMed] [Google Scholar]
- 3.Yang W, Soares J, Greninger P, et al. Genomics of drug sensitivity in cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;D41:D955–D961. doi: 10.1093/nar/gks1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seashore-Ludlow B, Rees MG, Cheah JH, et al. Harnessing connectivity in a large-scale small-molecule sensitivity dataset. Cancer Discov. 2015;5:1210–1223. doi: 10.1158/2159-8290.CD-15-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang J, Aittokallio T. Network pharmacology strategies toward multi-target anticancer therapies: from computational models to experimental design principles. Curr Pharm Des. 2014;20:20–36. doi: 10.2174/13816128113199990470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathews Griner LA, Guha R, Shinn P, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A. 2014;111:2349–2354. doi: 10.1073/pnas.1311846111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crystal AS, Shaw TA, Sequist VL, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346:1480–1486. doi: 10.1126/science.1254721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pemovska T, Johnson E, Kontro M, et al. Axitinib effectively inhibits BCR-ABL1 (T315I) with a distinct binding conformation. Nature. 2015;519:102–105. doi: 10.1038/nature14119. [DOI] [PubMed] [Google Scholar]
- 10.Kulesskiy E, Saarela J, Turunen L, et al. Precision cancer medicine in the acoustic dispensing era: ex vivo primary cell drug sensitivity testing. J Lab Autom. 2016;21:27–36. doi: 10.1177/2211068215618869. [DOI] [PubMed] [Google Scholar]
- 11.Haltia UM, Andersson N, Yadav B, et al. Systematic drug sensitivity testing reveals synergistic growth inhibition by dasatinib or mTOR inhibitors with paclitaxel in ovarian granulosa cell tumor cells. Gynecol Oncol. 2017;144:621. doi: 10.1016/j.ygyno.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Saeed K, Rahkama V, Eldfors S, et al. Comprehensive drug testing of patient-derived conditionally reprogrammed cells from castration-resistant prostate cancer. Eur Urol. 2017;71:319. doi: 10.1016/j.eururo.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Berenbaum MC. What is synergy. Pharmacol Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- 14.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittel-forschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 15.Bliss CI. The toxicity of poisons applied jointly. Ann Appl Biol. 1939;26:585–615. [Google Scholar]
- 16.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 17.Boik JC, Narasimhan B. An R package for assessing drug synergism/antagonism. J Stat Softw. 2010;34:6. [Google Scholar]
- 18.Ritz C, Baty F, Streibig JC. Bioassay analysis using R. J Stat Softw. 2005;12:5. [Google Scholar]
- 19.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 20.Zhao W, Sachsenmeier K, Zhang L, et al. A new bliss independence model to analyze drug combination data. J Biomol Screen. 2014;19:817–821. doi: 10.1177/1087057114521867. [DOI] [PubMed] [Google Scholar]
- 21.Yadav B, Wennerberg K, Aittokallio T, et al. Searching for drug synergy in complex dose-response landscapes using an interaction potency model. Comput Struct Biotechnol J. 2015;13:504–513. doi: 10.1016/j.csbj.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szwajda A, Gautam P, Karhinen L, et al. Systematic mapping of kinase addiction combinations in breast cancer cells by integrating drug sensitivity and selectivity profiles. Chem Biol. 2015;22:1144–1155. doi: 10.1016/j.chembiol.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Gautam P, Karhinen L, Szwajda A, et al. Identification of selective cytotoxic and synthetic lethal drug responses in triple negative breast cancer cells. Mol Cancer. 2016;15:34. doi: 10.1186/s12943-016-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karjalainen R, Pemovska T, Majumder M, et al. JAK1/2 and BCL2 inhibitors synergize to counteract bone marrow stromal cell-induced protection of AML. Blood. 2017;130:789. doi: 10.1182/blood-2016-02-699363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.