Abstract
Background:
Patients who have diabetes mellitus (DM) are at an increased risk of postoperative complications following total hip arthroplasty (THA). Therefore, much interest has been paid to perioperative glycemic control. However, no prior studies have evaluated the patient variation of HbA1c levels on costs. Therefore, the purpose of this study was to evaluate the impact of obtaining preoperative HbA1c levels on (1) day of surgery (DOS) cost; (2) subsequent 89-day costs; and (3) global 90-day cost.
Methods:
A retrospective query of the Humana insurance claims database was performed from 2007 to 2015 for all DM patients undergoing THA. Only patients with HbA1c (%) levels within 3 months before or after the THA were included. Patients were stratified into 6 groups based on HbA1c starting at 5.5% and increasing by 1% increments to 11.5%; one additional group (11.5%−20%) for extreme cases was analyzed. Correlations between HbA1c level and reimbursements for DOS, subsequent 89-day, and global 90-day period were performed.
Results:
HbA1c level demonstrated a significant correlation to DOS (correlation coefficient = 0.664), subsequent 89-day (correlation coefficient = 0.789), and global 90-day period (correlation coefficient = 0.747) costs. DOS, 89-day, and global 90-day costs significantly increased with increasing HbA1c levels (P < .0001).
Conclusion:
Higher perioperative HbA1c levels increase the DOS, subsequent 89-day, and global 90-day costs of THA. This was expected as these patients require multidisciplinary care, have longer LOS, and develop more complications. Further investigation into postoperative complications based on glycemic control is warranted.
Keywords: total hip arthroplasty, hemoglobin A1c, diabetes mellitus, HbA1c, reimbursements, 90-day cost of care
The Centers for Disease Control and Prevention estimated that 310,800 total hip arthroplasties (THAs) were performed in 2010 among inpatients aged 45 years and older, increasing from 138,700 procedures in 2000 [1]. The Centers for Medicare and Medicaid Services (CMS) has in the recent years created alternative payment models (APM) that reimburse providers for the procedures and the care during a brief follow-up period (most commonly 90 days after surgery) aiming to increase value by improving outcomes and decreasing costs [2]. Since then, various articles have aimed at identifying independent risk factors for increased complications and costs after THA as to not risk stratify patients whom may develop more complications and should not be included in the APM pathway [3–5]. Furthermore, by identifying conditions that can increase complications and costs, surgeons, hospitals and healthcare policy makers can better plan and risk stratify patients into a proper APM amount that accounts for their comorbidities and presurgical status.
One of the most prevalent risk factors for increased complications is diabetes mellitus (DM) [6,7]. Previous research has demonstrated that patients who undergo THA are at an increased risk of superficial site infections, periprosthetic joint infections, aseptic loosening, increased length of stay, and increased total hospital costs [8–11]. This has increased awareness regarding patient optimization before surgery and some sites have established cutoff limits for glycosylated hemoglobin A1c (HbA1C) [12]. However, cutoff thresholds for HbA1c are often unachievable by patients and many with an HbA1c over an optimal threshold will still undergo the THA [13]. Therefore, it is important to estimate the effects of higher HbA1c levels on costs. By understanding the effects of increasing HbA1c levels on costs, APM reimbursements can be customized to better fit patient’s needs. Therefore, the purpose of this study was to evaluate the impact of preoperative HbA1c levels on (1) day of surgery (DOS) costs; (2) subsequent 89-day costs; and (3) global 90-day costs.
Methods
A retrospective query of the Humana insurance claims database via the PearlDiver Supercomputer (Warsaw, IN) was performed from 2007 to 2015. PearlDiver is a de-identified, Health Insurance Portability and Accountability Act-compliant medical records medium for Medicare Standard Analytic Files and Humana private-payer insurance. Medical records can be searched using international classification of disease 9th and 10th revision codes and current procedural terminology codes to investigate diagnosis and procedural volume, patient demographics, length of stay, and hospital reimbursement for over 22 million lives within the Humana system. Additionally, Humana is the only insurance claims database that contains accessibility to laboratory results and thus was selected for this study.
All patients with DM undergoing primary THA were identified based on the codes provided in Table 1. Only patients with HbA1c (%) laboratory values within 3 months before or after the THA were included. Patients without both an HbA1c value and a diagnosis of DM were excluded from this study. Patients were stratified into 6 groups based on HbA1c starting at 5.5% and increasing by 1% increments to 11.5%; one additional group (11.5%−20%) for extreme cases was analyzed. Reimbursements were used as a marker for cost in accordance with previous studies [3,14,15]. For each HbA1c subgroup, reimbursements were analyzed and compared for the DOS, subsequent 89 days of surgery, and global 90-day costs in accordance with the Comprehensive Care for Joint Replacement 90-day model.
Table 1.
Identification Codes.
| Unit | Code | ||
|---|---|---|---|
| Procedure | ICD-9 | ICD-10 | CPT |
| Total hip arthroplasty | 81.51 | 27130 | 0SR90J9/A/Z 0SRB0J9/A/Z |
| Disease | ICD-9 | ICD-10 | |
| Diabetes mellitus | 249.00, 249.01, 249.1, 249.11, 249.2, 249.21, 249.3, 249.4, 249.41, 249.5, 249.51, 249.6, 249.61, 249.7, 249.71, 249.8, 249.81, 249.9, 249.91, 250.00, 250.01, 250.02, 250.03, 250.1, 250.11, 250.12, 250.13, 250.2, 250.21, 250.01, 250.02, 250.03, 250.1, 250.11, 250.12, 250.13, 250.2, 250.21 | E08.01, E08.10, E08.11, E08.21, E08.311, E08.319, E08.36, E08.39, E08.40, E08.41, E08.42, E08.43, E08.44, E08.49, E08.51, E08.610, E08.618, E08.620, E08.621, E08.622, E08.628, E08.630, E08.638, E08.641, E08.65, E08.69, E08.80, E08.90, E09.01, E09.10, E09.21, E09.311, E09.319, E09.36, E09.39, E09.40, E09.41 to E09.44, E09.49, E09.51, E09.610, E09.618, E09.620, E09.621, E09.622, E09.628, E09.630, E09.641, E09.649, E09.65, E09.69, E09.8, E09.90, E10.10, E10.65, E10.69, E11.00, E11.01, E11.65, E11.69, E11.9, E13.00, E13.10, E13.11, E13.39, E13.40 to E13.44, E13.49, E13.59, E13.620, E13.621, E13.622, E13.628, E13.638, E13.641, E13.649, E13.65, E13.69, E13.8, E13.9 | |
ICD-9, International Classification of Disease, 9th revision; ICD-10, International Classification of Disease, 10th revision; CPT, Current Procedural Terminology.
Statistical Analysis
Data analysis was conducted with SPSS, version 20 (IBM; Armonk, NY). Reimbursements were compared between HbA1c cohorts via 1-way analysis of variance. Correlations between HbA1c level and DOS, subsequent 89-day, and global 90-day period were performed via Spearman’s rho correlation. An alpha value of <0.05 was considered statistically significant.
Results
A total of 102,505 patients with DM underwent THA during this time period, with 51,697 patients having HbA1c laboratory results available. There was a significant increase in the number of THA performed in DM patients with 5389 procedures in 2007 to 15,789 in 2015 (P = .033, Fig. 1). Patient demographics across HbA1c subgroups was similar for gender, age, and Charlson Comorbidity Index (see Table 2).
Fig. 1.
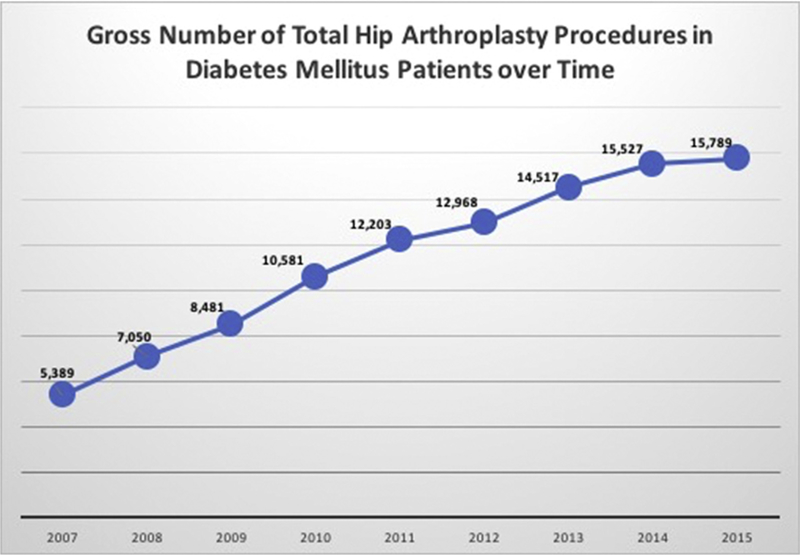
Gross number of total hip arthroplasty procedures in diabetes mellitus patients over time.
Table 2.
Patient Demographics Stratified by Hemoglobin A1c level (%).
| Demographic Variable | 5.5%−6.4% | 6.5%−7.4% | 7.5%−8.4% | 8.5%−9.4% | 9.5%−10.4% | 10.5%−11.4% | 11.5%−20% |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 42% | 45% | 48% | 49% | 50% | 45% | 49% |
| Female | 58% | 55% | 52% | 51% | 50% | 55% | 51% |
| Age | |||||||
| ≤64 y | 20% | 20% | 21% | 25% | 31% | 33% | 35% |
| 65–79 y | 67% | 69% | 68% | 65% | 59% | 59% | 56% |
| ≥80 y | 24% | 22% | 19% | 17% | 14% | 13% | 12% |
| Mean CCI | 4 (SD 3.51) | 4 (SD 3.54) | 5 (SD 3.58) | 5 (SD 3.77) | 5 (SD 3.85) | 5 (SD 3.79) | 5 (SD 3.71) |
CCI, Charlson comorbidity index; SD, standard deviation.
Evaluation of the DOS reimbursements demonstrated that there was a significant difference among reimbursement for THA when stratified by HbA1c level (P < .0001). The lowest reimbursements were seen in patients with an HbA1c level of 5.5% to 5.4% ($6916.40; SD, $1018) or 6.5% to 7.4% ($6871.92; SD, $1294). Patients with HbA1c of 10.5% to 11.4% incurred the greatest costs at $13,702.66 (SD, $7851.98) for the DOS (Table 3). Spearman’s rho correlation analysis revealed a significant correlation between the DOS and cost (correlation coefficient = 0.664).
Table 3.
Mean Costs Stratified by Hemoglobin A1c Level (%).
| Timeframe | 5.5%−6.4% | 6.5%−7.4% | 7.5%−8.4% | 8.5%−9.4% | 9.5%−10.4% | 10.5%−11.4% | 11.5%−20% |
|---|---|---|---|---|---|---|---|
| N | 21,452 | 17,031 | 7220 | 3209 | 1511 | 695 | 579 |
| Day of surgery | $6916.40 (SD $1018) | $6871.92 (SD $1294) | $8441.71 (SD $2263) | $10,711.88 (SD $4201) | $12,237.15 (SD $4758) | $13,702.66 (SD $4940) | $12,653.31 (SD $3307) |
| 89 d | $3979.97 (SD $280) | $3965.83 (SD $334) | $4944.93 (SD $653) | $6850.94 (SD $2115) | $8105.41 (SD $2968) | $7851.98 (SD $1648) | $7349.17 (SD $1484) |
| Global 90 d | $10,896.38 (SD $1109) | $10,837.75 (SD $1557) | $13,386.64 (SD $2812) | $17,562.82 (SD $6247) | $20,342.56 (SD $7613) | $21,554.64 (SD $6213) | $20,002.48 (SD $4254) |
The subsequent 89 days following surgery demonstrated a significant difference when comparing reimbursements stratified by HbA1c level (P < .0001). An HbA1c value of 6.5% to 7.4% represented the lowest reimbursement during the interval 89 days following THA ($3965.83; SD, $334) compared to HbA1c of 9.5% to 10.4% which correlated to the highest reimbursement cost ($8105.41; SD, $2968; Table 3). During the subsequent 89 days of surgery, there was a significant correlation between cost of surgery and rising HbA1c level (correlation coefficient = 0.789).
Reimbursements for the entire episode of care demonstrated significant differences comparing reimbursements after primary THA in DM patients by HbA1c level (P < .0001, Table 3). A significant correlation between cost and HbA1c levels was demonstrated (correlation coefficient = 0.747). The HbA1c level of 10.5% to 11.4% incurred the highest costs for the entire 90-day period at $21, 554.64 (SD, $6213) compared to $10,837.75 (SD, $1557) for patients with HbA1c between 6.5% and 7.4%.
Discussion
THA is one of the fastest growing and successful sectors of healthcare today [16,17]. With the current healthcare climate, the development of APM pathways have focused on decreasing the cost of procedures while increasing the overall value [2]. DM has been identified as one of the most prevalent risk factors for increased complications and is estimated that greater than 22% of arthroplasty patients carry the diagnosis [9–11]. As such, the purpose of this study is to investigate the effect of various HbA1c levels on reimbursement payments following primary THA. Our study demonstrated that there were increased costs in reimbursement for the DOS, subsequent 89 days, and global 90 days with increases in HbA1c levels.
The cost of THA is multifactorial with surgical implants, length of stay, discharge disposition, rehabilitation, and comorbidity all playing a role [15,18]. Complications following THA have been identified as one of the leading cost drivers [19]. It has been well studied that patients with DM undergoing THA are at an increased risk of complications such as superficial site infections, periprosthetic joint infections, aseptic loosening, increased length of stay, and increased total hospital costs [8,11,12,20]. A positive correlation between rising HbA1c levels and increased risk of infection and cost has been shown [21]. As the need for THA in DM patients continues to rise, practitioners have sought to find ways to identify DM patients at the greatest risk.
The development of a cutoff threshold based on HbA1c level as a determinant of patients who are candidates for THA surgery has become a great interest. An HbA1c level of 8 mg/dL has been shown to be associated with an increased risk of deep postoperative infections following THA [8,20]. Furthermore, HbA1c greater than 8 mg/dL correlates with an increased mortality [9]. Nussenbaum et al [22] reported the results of 475 THA veteran patients before and after the implementation of cutoff criteria including HbA1c ≤ 7, hemoglobin ≥ 11, body mass index ≤ 35, and albumin ≥ 3.5. Following implementation of these new guidelines, there was a significant decrease in surgical site infection from 3.8% to 1.2% of patients and total complications dropped by 15.1%. The present study is in agreeance, demonstrating that uncontrolled DM patients incur a substantially higher cost at all time periods throughout the 90-day postoperative phase.
However, despite evidence that increased HbA1c levels are associated with greater risk of complications following THA, it is not always plausible to avoid surgery in these patients. Thus, Lavernia et al [23] sought to investigate well-controlled DM patients with a preoperative HbA1c level < 7% vs uncontrolled DM patients with an HbA1c > 7% undergoing total joint arthroplasty. The study showed no significant differences between length of stay, hospital costs, or short-term postoperative complications. Conversely, Marchant et al [21] found that patients with uncontrolled DM defined as HbA1c > 7% had a significantly greater length of stay (5.1 vs 4.2 days, P < .0001) and incurred greater hospital costs ($29,687 vs $29,054, P < .0001).
This study is not without limitations. The use of a large administrative database relies on the accuracy of data input and coding practices. As such, some patient data may have been missed or input incorrectly. A retrospective bias is inherent; however, data are recorded prospectively to minimize this risk. DM commonly coexists with other comorbidities and while Charlson Comorbidity Index was reported for each HbA1c cohort, it was not controlled for in this analysis. As such, this may contribute to increases in 90-day cost of care. Lastly, patients were stratified based on diagnosis of DM and HbA1c level. Some patients may have had multiple laboratory drawings within the perioperative window and thus may underestimate the cost of THA for such patients. Additionally, it has been reported that up to 33% of patients may have HbA1c values within the prediabetic range [12]. Because patients in this study had to have both an HbA1c value and a diagnosis of DM, the influence of HbA1c on cost may only apply to the diabetic population. Nonetheless, the major strength of our study is the sample size, which is the largest study to date to report on HbA1c level impact on reimbursements.
Perioperative HbA1c levels impact the DOS, subsequent 89-day, and global 90-day cost of THA. As HbA1c levels increase, a corresponding increase in cost can be expected. Further investigation into postoperative complications based on glycemic control is warranted.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.arth.2018.01.062.
References
- [1].Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000–2010. NCHS Data Brief 2015:1–8. [PubMed]
- [2].Services CfMaM. MACRA-MIPS-APM. In: CMS description of APM; 2017.
- [3].Rosas S, Sabeh KG, Buller LT, Law TY, Roche MW, Hernandez VH. Medical comorbidities impact the episode-of-care reimbursements of total hip arthroplasty. J Arthroplasty 2017;32:2082–7. [DOI] [PubMed] [Google Scholar]
- [4].Rozell JC, Courtney PM, Dattilo JR, Wu CH, Lee GC. Should all patients be included in alternative payment models for primary total hip arthroplasty and total knee arthroplasty? J Arthroplasty 2016;31(9 Suppl):45–9. [DOI] [PubMed] [Google Scholar]
- [5].Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty 2016;31:1862–5. [DOI] [PubMed] [Google Scholar]
- [6].Loth FL, Giesinger JM, Giesinger K, MacDonald DJ, Simpson A, Howie CR, et al. Impact of comorbidities on outcome after total hip arthroplasty. J Arthroplasty 2017;32:2755–61. [DOI] [PubMed] [Google Scholar]
- [7].Tsang ST, Gaston P. Adverse peri-operative outcomes following elective total hip replacement in diabetes mellitus: a systematic review and meta-analysis of cohort studies. Bone Joint J 2013;95-b:1474–9. [DOI] [PubMed] [Google Scholar]
- [8].Cancienne JM, Werner BC, Browne JA. Is there a threshold value of hemoglobin A1c that predicts risk of infection following primary total hip arthroplasty? J Arthroplasty 2017;32:S236–40. [DOI] [PubMed] [Google Scholar]
- [9].Chrastil J, Anderson MB, Stevens V, Anand R, Peters CL, Pelt CE. Is hemoglobin A1c or perioperative hyperglycemia predictive of periprosthetic joint infection or death following primary total joint arthroplasty? J Arthroplasty 2015;30: 1197–202. [DOI] [PubMed] [Google Scholar]
- [10].Cunningham DJ, Kavolus JJ 2nd, Bolognesi MP, Wellman SS, Seyler TM. Common medical comorbidities correlated with poor outcomes in hip periprosthetic infection. J Arthroplasty 2017;32:S241–245.e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Khatod M, Cafri G, Namba RS, Inacio MC, Paxton EW. Risk factors for total hip arthroplasty aseptic revision. J Arthroplasty 2014;29:1412–7. [DOI] [PubMed] [Google Scholar]
- [12].Capozzi JD, Lepkowsky ER, Callari MM, Jordan ET, Koenig JA, Sirounian GH. The prevalence of diabetes mellitus and routine hemoglobin A1c screening in elective total joint arthroplasty patients. J Arthroplasty 2017;32:304–8. [DOI] [PubMed] [Google Scholar]
- [13].Giori NJ, Ellerbe LS, Bowe T, Gupta S, Harris AH. Many diabetic total joint arthroplasty candidates are unable to achieve a preoperative hemoglobin A1c goal of 7% or less. J Bone Joint Surg Am 2014;96:500–4. [DOI] [PubMed] [Google Scholar]
- [14].Sabeh KG, Rosas S, Buller LT, Roche MW, Hernandez VH. The impact of discharge disposition on episode-of-care reimbursement after primary total hip arthroplasty. J Arthroplasty 2017;32:2969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rosas S, Sabeh KG, Buller LT, Law TY, Kalandiak SP, Levy JC. Comorbidity effects on shoulder arthroplasty costs analysis of a nationwide private payer insurance data set. J Shoulder Elbow Surg 2017;26:e216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- [17].Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am 2015;97:1386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cary MP Jr, Baernholdt M, Merwin EI. Changes in payment regulation and acute care use for total hip replacement: trends in length of stay, costs, and discharge, 1997–2012. Rehabil Nurs 2016;41:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gonzalez-Velez AE, Romero-Martin M, Villanueva-Orbaiz R, Diaz-Agero-Perez C, Robustillo-Rodela A, Monge-Jodra V. The cost of infection in hip arthroplasty: a matched case-control study. Rev Esp Cir Ortop Traumatol 2016;60:227–33. [DOI] [PubMed] [Google Scholar]
- [20].Cancienne JM, Werner BC, Browne JA. Is there an association between hemoglobin A1C and deep postoperative infection after TKA? Clin Orthop Relat Res 2017;475:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am 2009;91:1621–9. [DOI] [PubMed] [Google Scholar]
- [22].Nussenbaum FD, Rodriguez-Quintana D, Fish SM, Green DM, Cahill CW. Implementation of preoperative screening criteria lowers infection and complication rates following elective total hip arthroplasty and total knee arthroplasty in a veteran population. J Arthroplasty 2018;33:10–3. [DOI] [PubMed] [Google Scholar]
- [23].Lavernia CJ, Heiner AD, Villa JM, Alcerro JC, Rossi MD. Preoperative glycemic control on total joint arthroplasty patient-perceived outcomes and hospital costs. J Arthroplasty 2017;32:6–10. [DOI] [PubMed] [Google Scholar]


