Abstract
Individualized medicine, or the tailoring of therapeutic interventions to a patient’s unique genetic, biochemical, physiological, exposure and behavioral profile, has been enhanced, if not enabled, by modern biomedical technologies such as high-throughput DNA sequencing platforms, induced pluripotent stem (iPS) cell assays, biomarker discovery protocols, imaging modalities and wireless monitoring devices. Despite successes in the isolated use of these technologies, however, it is arguable that their combined and integrated use in focused studies of individual patients is the best way to not only tailor interventions for those patients, but also shed light on treatment strategies for patients with similar conditions. This is particularly true for individuals with rare diseases since, by definition, they will require study without recourse to other individuals, or at least without recourse to many other individuals. Such integration and focus will require new biomedical scientific paradigms and infrastructure, including the creation of databases harboring study results, the formation of dedicated multidisciplinary research teams and new training programs. We consider the motivation and potential for such integration, point out areas in need of improvement, and argue for greater emphasis on improving patient health via technological innovations, not merely improving the technologies themselves. We also argue that the paradigm described can in theory be extended to the study of individuals with more common diseases.
Keywords: Individualized Medicine, Genomics, DNA Sequencing, Induced Pluripotent Stem Cells, N-of-1 Clinical Trials, Functional Genomics
Introduction
The rapid development of comprehensive yet cost-effective molecular profiling assays, such as DNA sequencing and proteomic assays, has led to the belief that their use can aid in the determination of optimal therapeutic interventions for an individual patient. The intuition behind this belief is that the unique and very specific set of molecular ‘lesions’ causing a patient’s disease can be identified and the pathophysiological consequences of these lesions determined, leading to insights into how best to reverse or prevent them. It is arguable that the precise set of lesions and consequent pathophysiological mechanisms responsible for any particular patient’s disease state, given his or her unique biochemical and environmental exposure profile, may be nuanced and unlikely to match a different patient’s set of disease-causing mechanisms. Thus, the practice of identifying, and subsequently developing a therapeutic intervention for, patient-specific lesions has been variously referred to as ‘individualized,’ ‘personalized,’ or ‘precision’ medicine.[1–3] In fact, medical reference manuals and textbooks have recently been published which describe strategies for enabling and practicing medicine along these lines.[4, 5] Although many paradigmatic examples of individualized medicine and research have been born out of necessity due to a patient of interest having a unique idiopathic and life-threatening condition (see, e.g., the references associated with Tables 1 and 2), the principles behind individualized medicine and research can be generalized and expanded to the study of patients with more common chronic conditions, albeit with appropriate caveats.
Table 1.
Recent Individual Human Genome Sequencing Studies that Identified Causative Disease Variants that Impacted Diagnostic, Intervention or Therapeutic Decisions
| Condition | Sequencing Strategy | Mutant Gene | Resulting Clinical Decisions | Citation |
|---|---|---|---|---|
| IBD-like Condition | Ex-seq | XIAP | Hematopoietic stem cell transplant | [119] |
| Neuromuscular | WG-seq | SPR | Serotonin/dopamine therapy initiated | [120] |
| Neurocognitive | Ex-seq | MAN2B1 | Stem cell transplantation indicated | [121] |
| Neurocognitive/Ataxia | Ex-seq | SPG11 | Termination of Vitamin E therapy | [121] |
| Arterial Calcification | WGG + CG-seq | NT5E | Adenosine treatment indicated | [122] |
| Chloride-losing Diarrhea | Ex-seq | SLC26A3 | NaCl and KCl treatment indicated | [123] |
| Developmental Delay | Ex-seq | Multiple genes | Likely causal variants identified in 6 of 12 cases | [124] |
| Pachydermoperiostosis | Ex-seq | SLCO2A1 | Causal, likely founder, variants in 7 cases | [125] |
| Cantú syndrome | Ex-seq | ABCC9 | 11 causal variants identified; 6 were de novo | [126] |
| Severe Transfusion-Dependent Anemia | Ex-seq | EPB41 | surgical splenectomy resulted in subsequent transfusion independence in the patient | [127] |
| Neurometabolic Disorders | Ex-seq | Multiple genes | diagnosis in 28/41 proband, alternated therapy beyond genetic counseling in 18 patients | [128] |
| Mitochondrial Disorder | Ex-seq | ACAD9 | optimized treatment of mitochondrial dysfunction and supplementation with riboflavin, resulting in clinical improvement | [129] |
| Pediatric Brain Cancer | Target-seq (510 genes) | Multiple genes | Potentially targetable alterations were identified in 19 patients (61%) | [130] |
| Viral Encephalitis | Total RNA and viral sequencing | new astrovirus strain | Novel oral ribavirin and intravenous immunoglobulin intervention initiated | [131] |
| Cardiac Arrhythmia | Ex-seq | DPP6 | Designed a therapeutic regimen incorporating dalfampridine to target Ito and cilostazol to accelerate heart rate | [132] |
| Clear Cell Carcinoma of the Ovary | Target-seq (315 genes) | Multiple genes | Combined targeted therapy with trametinib and metformin resulted in a dramatic disease regression without toxicity | [133] |
Key: IBD = Inflammatory Bowel Disease; Ex-seq = Exome sequencing; WG-seq = Whole genome sequencing; WGG = Whole genome genotyping; CG-seq = Candidate gene sequencing
Table 2.
Studies Investigating the Utility of Induced Pluripotent Stem Cells for Understanding the Pathobiology of a Single Individual or Small Set of Individuals’ Diseases
| Condition | iPS Cell Samples | Compound Activity Across the IPS cells | Citation |
|---|---|---|---|
| LRRK2 mutation-associated PD | Multiple LRRK2(G2019S) mutation-bearing iPS and wild type cell lines | The use of LRRK2-in-1 inhibitor in LRRK2 (G2019S) cells generally restored normal function | [134] |
| Gaucher’s disease (GD) | 2 GD patient vs. 2 wild type iPS cell lines | 2 nojirimycin analogues increased acid-β-glucosidase and enzyme activity in GD cells | [135] |
| ALS | 1 ALS patient vs. 2 healthy controls | Motor Neurons from ALS patient had increased vulnerability to antagonism of PI3K pathway | [136] |
| ALS | 3 ALS patients vs. 5 healthy controls | Anacardiac acid rescued the abnormal ALS motor neuron phenotype | [137] |
| ALS | 5 ALS patients vs. 4 healthy age matched controls | 4-Aminopyridine rescued the abnormal ALS motor neuron phenotype | [138] |
| Alzheimer’s disease | 4 AD patients vs. 2 healthy controls | β-secretase inhibitors reduced levels of phospho-Tau and aGSK-3β levels in AD-derived neurons | [139] |
| Dilated Cardiomyopathy | 4 affected vs. 3 healthy family members | Beta blockers improved patient-derived induced cardiomyocyte function | [140] |
| CINCA | Mutant vs. wild-type cells from 2 patients | Oxidized ATP and other compounds inhibited IL-1β secretion in patient-derived macrophages | [141] |
| Timothy syndrome (TS) | 5 TS patients vs. 5 control individuals | Roscovitine restored the electrical properties of TS patient derived cardiomyocytes | [142] |
| Long QT Syndrome | 1 affected vs. 1 healthy individual | IKR blocker E-4031 prolonged action potential duration in patient cardiomycytes | [143] |
| Long QT Syndrome | 2 affected vs. 2 healthy family members | Isoproterenol shortened of the duration of the action potential in patient-derived cardiomyocytes | [144] |
| Long‐QT syndrome | 4 affected vs. genome edited isogenic controls | LUF7346 slowed IK-r deactivation in patient-derived cardiomyocytes | [61] |
| Rett syndrome (RT) | RS patient fibroblasts vs. control fibroblasts | IGF1 challenge increased glutamatergic synapse number in RT patient-derived neurons | [145] |
| Familial Dysautonomia | 3 affected individuals vs. controls | Kinetin reduced mutant IKBKAP splice forms from FD-iPSC derived neural crest precursors | [146] |
| Spinal Muscular Atrophy | Single child | Valproic acid increases SMN protein in motor neurons derived from an affected child | [147] |
| BH4 metabolism disorders | 1 affected vs. genome edited isogenic controls | Sepiapterin improves the TH protein level and extracellular DA level in patient-derived dopaminergic neuronal cultures | [54] |
| Marfan syndrome | 2 patients vs. 3 healthy controls | Partial rescue; TGF-b inhibition rescued abnormalities in fibrillin-1 accumulation and MMP expression in patient-derived smooth muscle | [148] |
| Friedreich ataxia (FRDA) | 2 patients vs. 2 healthy controls | HDAC inhibitor 109 rescued FRDA-specific neuronal phenotypes associated with deficient iron-sulfur cluster biogenesis, altered iron metabolism, and oxidative stress | [149] |
| OPHN1 syndrome | 2 patients vs. single healthy parental control | Treatment with Fasudil rescued aberrant ROCK levels and neuronal morphology phenotypes in in patient-derived neurons |
[150] |
| Niemann-Pick disease, type C1 (NPC1) | 1 patient vs. 1 healthy control | Calcium modulators curcumin or dantrolene and the WNT signaling modulator BIO rescued early neuronal death phenotype | [151] |
| manic type I bipolar disorder (BD) | 6 patients vs 4 healthy controls | Lithium selectively diminished hyperexcitability only in neurons derived from patients who were responsive to clinical Li administration. | [152] |
| Chorea-Acanthocytosis | 2 patients vs. 2 age matched healthy controls | The F-actin stabilizer phallacidin or the Src kinase inhibitor PP2 rescued the abnormal medium spiny neurons phenotype | [153] |
| Age Related Macular Degeneration | 4 AMD patients vs 3 healthy age matched controls | nicotinamide (NAM) ameliorated disease-related phenotypes in retinal pigment epithelium | [154] |
Key: iPS = Induced pluripotent stem cells; PD = Parkinson’s disease; CINCA = Chronic infantile neurological cutaneous and articular syndrome; ALS = Amyloid Lateral Sclerosis.
Stratified Medicine
Although virtually every disease has been considered as likely to benefit from more individualized approaches to its treatment, research efforts in the study of cancers have led to the belief that patients can be subdivided or ‘stratified’ into treatment categories on the basis of the existence of particular mutations and genomic anomalies in their tumors.[6–8] Although such stratification does not necessarily focus on individual patient tumor characteristics but rather on patterns observed across patients (i.e., identifying subgroups of patients with similar tumor profiles), such activity is consistent with, and a precursor to, true individualized cancer interventions. In fact, notable successes in matching therapeutics to patients with very specific characteristics have been achieved in the treatment of cancers and this success has led to the formation of informal ‘rules’ for the treatment of cancers based on genomic profiles.[8] For example, if the BCR-ABL gene fusion is present in a patient with chronic myelogenous leukemia, then the use of Imatinib (Gleevec), given its ability to combat the deleterious effects of the BCR-ABL fusion, is appropriate; if the HER2 gene is overexpressed in breast cancer, then the use of Herceptin is appropriate; or, as a more general example, if the EGFR gene is overexpressed in any of a number of different cancers then the use of an EGFR inhibitor is likely to have beneficial effects.[9]
Individualized Medicine
For many forms of cancer, and especially for the vast majority of rare congenital diseases as well as more common complex conditions such as diabetes, arthritis and heart disease, enabling individualized – or even stratified – medicine is much more complicated than suggested by the current literature. Not only must an underlying set of patient-specific molecular lesions be identified and their deleterious impact on physiological function understood to the point where effective corrective strategies can be framed, but any hypothesized corrective strategy must also be vetted at some level or the attempt to make claims about its utility as an effective ‘individualized’ therapy will be incomplete and not likely to be compelling scientifically. Vetting an appropriate individualized or stratified therapeutic intervention is complicated by a number of factors, not the least of which concern a lack of available and relevant therapeutic interventions, inappropriate ways of monitoring functional improvements, and ultimately an incomplete understanding of an individual’s biochemical and environmental exposure profile. For example, even in the context of current cancer therapeutic strategies that stratify patients into treatment categories based on the genomic profiles of their tumors, many therapeutic agents work remarkably well only to fail later on when tumor resistance mechanisms develop that are likely induced by additional tumorigenic mutations lurking in the background of a primary mutation[10]. This is evidenced, e.g., by studies on colorectal cancer and EGFR inhibition in which EGFR inhibitors showed promise early on, but were much less effective over time.[11, 12] Thus, stratifying cancer patients into what are thought to be homogenous treatment categories based on the presence of a single tumor genomic anomaly often defies the underlying nuanced and very heterogeneous nature of individual tumors, necessitating an even more individualized approach to cancer therapeutic intervention than current ‘stratified’ medicine approaches offer.[6–8, 13]
Individual Patient-Oriented Research
Ultimately, identifying fundamental lesions causing an individual’s disease (whether DNA sequence mutations or other molecular perturbations), understanding the pathophysiological consequences of those lesions, developing appropriate corrective interventions, and appropriately testing the efficacy of those interventions, all in the context of a focused research setting, are not trivial. Such comprehensive individual patient-oriented research is not the norm for drawing inferences about disease mechanisms and disease treatments in the biomedical sciences, where emphasis is often on the statistical analysis-based identification of common lesions and therapeutics with robust treatment responses across large numbers of patients. However, statistical techniques that are similar to those used to assess commonalities and differences across or among groups of diseased and non-disease individuals can be used to assess common patterns and variations within single individuals in order to draw valid inferences about pathogenic mechanisms and treatment responsiveness. In addition, individual patient-oriented research can be further enhanced though the application of the integrated use of multiple contemporary biomedical technologies, innovative study designs, more appropriate analytical methods and the development of community resources, such as databases. Finally, the development of appropriate basic and clinical research community infrastructure can enhance ways of generalizing relevant studies and their ultimate clinical impact.
INTEGRATED GENOMIC MEDICINE SCIENTIFIC STRATEGIES
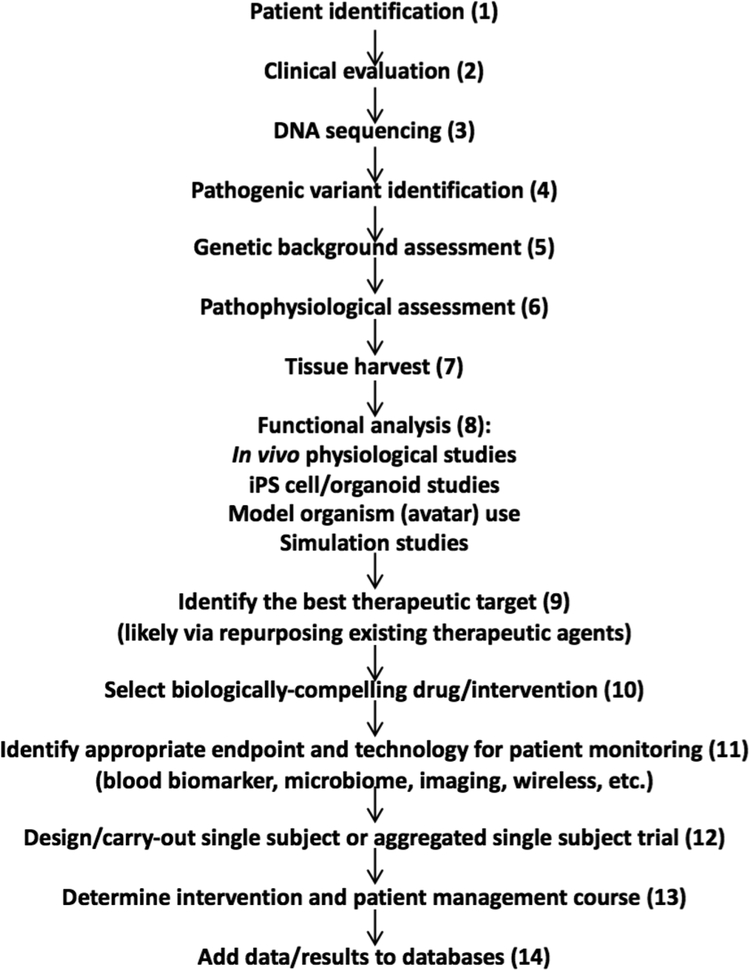
To describe how various assays and technologies can be exploited in research protocols on individual patients, consider Figure 1 which describes a potential ‘workflow’ for determining what might be responsible for an individual patient’s condition, determining how to correct the underlying problem, testing a potential therapeutic intervention, and finally providing the results of the research to the broader research and clinical communities.[14] Each step in the workflow depicted in Figure 1 (numbered for ease of reference) can be considered in isolation and has roots in the current literature. The workflow described here is not meant to be exhaustive and appropriate for all diseases, but rather focuses on the study of patients with rare diseases for which it might be possible to identify highly penetrant pathogenic mutations contributing to their condition. We do believe, however, that aspects of this workflow apply to the study of patients with more common diseases.
Figure 1.
A potential ‘workflow,’ with different components, for determining what might be responsible for an individual patient’s condition, determining how to correct the underlying problem, testing a potential therapeutic intervention, and finally providing the results of the research to the broader research and clinical communities. The numbers in parentheses are used to match statements in the text to the item listed.
Molecular and Genomic Profiling
After a patient has been identified (Steps 1 and 2 of Figure 1), his or her condition may not permit easy diagnosis nor may his or her optimal intervention strategy be obvious. Molecular assays can be applied to determine the lesions likely to either be responsible for the condition or affect the success of a therapeutic intervention. DNA sequencing and genotyping assays have been used routinely to make and confirm diagnoses for many common and rare diseases in this context (Step 3 of Figure 1). Importantly, recent applications of genomic assays have also involved cases in which the assays were pursued because no leads as to what might be causing patients’ unique and likely idiopathic disease were available. Table 1 lists a few recent examples, as well as how the results of the genomic assays impacted clinical decision-making and the choice of a therapeutic intervention.
As noted, in the context of cancer, the stratification of patients into categories based on their tumor genomic profile has led to a number of insights that bear on therapeutic choice.[9] However, the therapeutic choices for patients in these categories have not always resulted in optimal clinical outcomes due to complexities surrounding individual tumor biology (such as passenger vs driver mutations, intra- and inter-tumor heterogeneity, the involvement of stromal and cancer stem cells, etc.), host-related factors, unavailable treatments and a lack of insight into how the available treatments may act in a given patient.[11, 12] For many congenital conditions, particularly rare conditions, such complexities also arise. For example, a recent study exploring the genomic profiles of patients with generalized idiopathic epilepsy suggested that the unique combinations of sequence variants in ion channel genes possessed by a patient complicate the determination of optimal therapeutic strategies for those patients.[15] Essentially, this study suggested that simple prediction of gains and losses in channel activity on the basis of the independent presence of one or another mutation is not possible, such that “even if two mutations (or ‘hits’) are present in relevant genes possessed by a patient, the combinatorial effects on [neuronal] firing behavior are dramatically more complex, indicating that the pattern of genetic variation (functional valence of each allele) overrides its individual impact even at the single cell level. The addition of a third hit can also suppress or aggravate spontaneous rhythmic bursting, an important cellular determinant of neural network behavior” (page 1041 of reference [15]). The phenomenon in which the primary effects of a pathogenic variant are modified or influenced by the presence of other genetic factors is well-documented in the experimental [16–18] and human genetics literature.[19–22] This suggests that a sensitivity to ‘genetic background’ influences over-and-above a single genetic variant must be considered for obtaining appropriate insight into the genetically-mediated pathobiology of a disease, such as those related to ion channel function (step 5 of Figure 1). This is especially true if the goal is to not just determine a single contributor to an individual’s disease, but also craft a therapeutic intervention tailored to that specific patient.
Pathogenic Lesion Identification via Bioinformatics Analysis
The application of modern molecular genetic assays, such as DNA sequencing, to the identification of the root cause of an individual’s disease requires very sophisticated data analysis and bioinformatics techniques given the massive amount of data these assays generate. Thus, even if a pathogenic variant (or set of variants) exists, the identification of that variant with available data analysis tools is challenging (step 6 in Figure 1). The recognition that merely generating data from modern molecular assays is only a fraction of what it takes to harness those assays is well accepted in the biomedical sciences community.[23–25] For the application of DNA sequencing assays exploited in the studies described in Table 1, virtually all of the analysis tools that led to the identification of a likely pathogenic mutation not only relied on very sophisticated algorithms for identifying likely causative variants, but curated literature searches to aid inference-making as well.
Computational tools for general use in the identification of likely disease causative DNA sequence variants, for both congenital diseases and cancers, have been developed, but their success rates in identifying true pathogenic mutations has yet to be determined in large-scale applications.[26–30] In addition, accommodating the complexity of most diseases in such analyses, since most diseases are often influenced by a combination of multiple genes, environmental factors, general genetic background and epigenetic phenomena, as in the case of generalized idiopathic epilepsy[15] and cancer[6, 31], is essential for insights that could lead to effective therapies. This is true for not only diseases known to have multiple genetic determinants, as suggested by the sobering results of population-based genome-wide association studies and genome-wide prediction analyses of common chronic conditions like diabetes and cardiovascular disease,[21, 32, 33] but also overtly monogenic or idiopathic conditions where the success rate for uncovering a causative variant is currently only between 25–50%, with the range in time to diagnosis spanning 1 week to four years.[34]
One additional reason why the success rate of the identification of pathogenic variants has been low is that the analysis of any one assay, such as DNA sequencing, may be inadequate to pin down a truly actionable molecular mechanism responsible for an individual’s condition. This has proven to be the case in the identification of genomic alterations that ‘drive’ particular tumors, as the greatest successes to date have made use of a combination of tumor and germline genomic assays, transcriptomic assays, as well as epigenomic and other assays to identify the most compelling sets of contributing pathogenic alterations for many tumor types (see, e.g., [35, 36]). In addition, many analyses focusing on tumor genomic profiles to identify appropriate treatment strategies largely ignore the heritable factors contributing to tumor formation and growth [6, 37]. Obviously, more sophisticated ways of computationally assessing integrated molecular assay data in the identification of pathogenic alterations are needed, as are ways of interrogating the literature and making accessible new information that could aid in future searches for pathogenic alterations. A recent study exploring the emergence and onset of diabetes in a single patient through the integrated use of a number of genomic, physiological and biochemical assays, offers a reasonable paradigm for individual patient oriented research of the type discussed here.[38] Finally, the inherent difficulty in predicting the effects of non-coding mutations or aberrant epigenetic mechanisms has led many researchers to focus on exome sequencing in patient oriented research, despite the fact that non-coding DNA harbors critical regulatory elements that function as key drivers of human development and specialized cellular function. Massive amounts of data have been generated by dedicated consortia [39] [40] to map functional elements (promoters, bivalent domains, enhancers, transcription factor binding sites, cell type-restricted patterns of DNA methylation, etc.) across the regions harboring non-coding variants. These resources could be leveraged to obtain a better understanding how highly penetrant non-coding mutations and aberrant epigenetic modifications could contribute to disease via disruption of cis-regulatory elements and perhaps could support the selection of drugs to treat specific patients.
Pathophysiologic Assessment and Functional Verification
Once a potential pathogenic genomic variant or mutation has been identified, understanding of its impact on molecular and organismal physiologic function is needed (steps 7, 8 in Figure 1). Although the actual identification of a potential lesion in a particular tissue or set of tissues caused by this variant or mutation will likely be based on an assessment of the consequences of that variant or mutation at some level, further functional characterization of the lesion and its impact on higher-level physiologic function will also be needed in order to determine an appropriate pharmacological intervention. This may involve direct studies on a patient, studies that leverage tissue samples from the patient, computer modeling, or a combination of these approaches. An important point about these strategies is that even though they consider biomaterial and data on a single individual, they can be pursued in as objective a way as studies involving multiple individuals. The issues of generalizability, control for confounding factors, and accommodating covariates in the context of single subject studies parallel issues in studies involving more than one individual. Consider that whereas in studies involving multiple individuals primary interest is in sources of variation across those individuals, for single subject studies the focus is on sources of intra-individual variation across different cell types or tissues harvest from that individual or time points at which those cells or tissues have been stimulated, all of which should be amenable to analysis with appropriate study designs, assays, and statistical techniques.
In Vivo Pathophysiological Studies
To characterize the ‘functional’ or physiological effects of a putative molecular lesion, one would ideally study the patient in question directly in an experimental clinical research setting with appropriate controls, safety precautions and technologies. Although many strategies and technologies might be exploited, for example those involving hemodynamic manipulations[41] or those making use of imaging[42] or wireless monitoring devices[43], extensive invasive studies and certain experimental manipulations necessary to probe dysfunction more thoroughly – especially those that require access to tissues not easy to harvest, like brain tissue – are not likely to be feasible. Ex vivo and in vitro studies involving cells or excised tissues could be exploited to assess molecular dysfunction (see below). However, characterizing broader organismal and intermediate physiologic dysfunction appropriately is, and will continue to be for some time, even more of a challenge. Therefore, a real need exists for the development of appropriate, and hopefully largely non-invasive, technologies to assess dysfunction at the intermediate physiologic level in a way that would allow one to attribute that dysfunction to a specific molecular perturbation or set of perturbations.
Induced Pluripotent Stem Cells (iPSCs)
Given the challenges associated with invasive studies of humans, sophisticated in vitro studies can provide an alternative for characterizing at least the molecular consequences or underpinnings of a particular genetically-mediated disease process. iPSC technologies have potential, and are not likely to suffer from many of the problems associated with stability and biological relevance that historically and currently used substrates for in vitro analyses such as patient-specific transformed lymphoblastoid cell lines are known to suffer from.[44–46] In fact, a number of studies exploiting iPSCs have been pursued for the express purpose of characterizing patient-specific molecular perturbations amenable to pharmacologic manipulation. Table 2 describes a few recent studies, but an excellent and broad review of the application of iPSCs in biomedical research is provided by Belmonte and colleagues. [47] In most cases, iPSCs were used to study the effect of a known pathogenic mutation. However, in a recent study of bipolar disorder, the observation that lithium selectively diminished hyperexcitability only in neurons derived from patients who were lithium responders provides an example wherein iPSCs were used to study disease-relevant cell types and the identification of biomarkers for broader patient stratification that goes beyond those associated with specific mutations.
The use of patient-derived iPSCs could be greatly enhanced in the context of individual patient-oriented research by coupling it with, e.g., DNA sequencing and other genomic technologies, genome editing[48, 49], and drug screening[50, 51]. Correction of a pathogenic mutation using editing technologies in patient-specific iPSCs would allow for modelling of the patient’s disease, providing genotype matched controls to characterize the effect of a mutation in disease-relevant cell types and, possibly, pre and post administration of a therapeutic compound. In fact, a number of isogenic iPSC models have been reported in recent literature, including the generation of genome edited cell lines from patients diagnosed with Alzheimer’s disease, [52] [53] BH4 metabolism disorders, [54] Brugada syndrome, [55] Dravet syndrome, [56] dystrophic epidermolysis bullosa, [57] [58] frontotemporal dementia, [59] ICF syndrome, [60] long QT syndrome, [61] Sickle Cell Disease, [62] spinocerebellar ataxia type 2 [63] and Wiskott-Aldrich syndrome. [64] Obviously, the choice of a cell type to study, the assays used to probe the dysfunction of that cell type, and the use and assessment of a therapeutic compound are crucial for the success of iPS technologies in identifying patient-specific pathophysiologic mechanisms amenable to therapeutic intervention.
Model Organism ‘Avatars’
As an alternative to studying a patient directly, or exploiting iPS cell technologies, a model of an individual patient could be constructed by implanting patient specific lesions (for example, a particular DNA sequence associated with a defective or hypothesized causal gene) in a model organism, such as a mouse. In this light, the pathophysiological consequences of many congenital conditions has been studied by creating, e.g., BAC-transgenic mice, in which a sequence harboring a mutation identified from a patient or set of patients is introduced into a mouse and the consequences of that mutation explored.[65, 66] For studies of cancers, the creation of tumorgraft models, in which patient-derived tumor material is implanted into a mouse, has proven particularly effective in modeling tumor-specific pathobiology and therapeutic response.[67, 68] The limitations of the study of model organism ‘avatars,’ such as BAC transgenic mice and tumorgraft models, for a particular patient are obvious, as there are many differences between, e.g., the mouse and human species, that could confound understanding of individual human patient-specific pathophysiology.
In Silico Modeling and Simulation Studies
One area that is receiving a great deal of attention and is relevant to integrated genomic medicine and individual patient-oriented research is the development of computational models of human molecular and gross physiologic function.[69–72] Systems modeling of everything from basic metabolic networks[73] to cardiac [70] and lung[72] function are being developed for the study of specific perturbations to those systems and possibly lead to therapeutic insights for diseases caused by those perturbations. The clear limitation of computational models of the functional consequences of perturbations in human physiology is that the available models are only as good as the data and knowledge on which they are based. Clearly the biomedical research community has a long way to go before a more complete understanding of, e.g., biochemical networks and neural systems, will be obtained that would facilitate routine, purely computational pathophysiological assessment of patient-specific conditions, except in a few settings.
Therapeutic Choice
Choosing an appropriate therapeutic compound based on an assessment of the pathobiological mechanisms underlying a patient’s condition is not trivial (steps 9 and 10 in Figure 1). There are a number of resources that might aid in the identification of an appropriate compound based on a patient’s molecular genetic profile, such as PubChem,[74] DrugBank[75] and, for cancer, the connectivity map and related databases[76, 77]. However, the use of these resources is problematic if the compound or compounds indicated by them has not been previously assessed for use in humans. Thus, outside of compassionate use settings, therapeutic choices that may result from individual integrated genomic medicine and patient-oriented research of the type described here may require repurposing a particular drug or compound rather than attempting to use a drug that has not been approved for use in humans.[78–80] Other barriers to the use of a particular compound may involve simple access to the compound (i.e., through the group that created it) as well as costs. In this light, partnerships between research groups pursuing studies of individual patients for optimizing therapeutic interventions and the pharmaceutical industry may be of mutual benefit, as focused therapeutic assessment studies and repurposing efforts could help justify new markets for a compound.
Phenotypic Monitoring for Clinical Studies
In the event that a pathophysiologic mechanism is identified that might be amenable to therapeutic intervention, a way of monitoring the influence of that intervention is necessary (step 11 in Figure 1). Such phenotypic monitoring might be obvious (e.g., monitoring blood pressure level if hypertension is the disease of interest, tumor regression or shrinkage if cancer is the disease of interest), but the method of monitoring that phenotype might not be. Many emerging technologies involving blood and other accessible tissue-based biomarkers,[81, 82], including rare circulating cell types,[83, 84] molecular and general neuroimaging assessments,[85–87] and wireless monitoring devices of a wide variety,[43] all have tremendous potential in this context. The recent study of an individual patient who was comprehensively monitored during a diabetogenic episode showcases the potential of comprehensive phenotypic monitoring for diagnostic and therapeutic purposes.[38]
Clinical Trial Design
Merely finding a putative mechanism for a patient’s condition, as well as suggesting an appropriate therapeutic intervention and phenotypic monitoring strategy, would not have value unless the suggested therapeutic intervention was objectively assessed for its utility (steps 12, 13 in Figure 1). Thus, in order to achieve appropriate scientific rigor and assess the utility of an anticipated therapeutic intervention, the design of a study to assess a patient’s response to a chosen therapeutic is a crucial step in comprehensive individual patient-oriented research. Single subject or ‘N-of-1’ studies have been pursued in many domains, but have not been given comprehensive attention by the general biomedical community.[88] The same strategies and technologies exploited for ensuring validity in standard population-based clinical trials, such as the use of randomization to control confounding, blinding, the use of washout periods, accommodating carry over effects and serial correlation among measures obtained, the assessment of multivariate observations and the use of adaptive and sequential designs, can be exploited in studies investigating the utility of a particular intervention for a single patient.[88] Thus, much like studies designed to assess the molecular pathophysiology associated with an individual patient’s disease, single subject trials can be crafted to assess sources of variation across different time frames or intervention periods measured on a single individual.
There are, however, at least four important issues surrounding the design and implementation of a single subject therapeutic intervention trial that deserve attention. First, relevant phenotypic endpoint monitoring is crucial to the trial and could potentially be achieved with a device of some sort. However, the endpoint must be amenable to assessment with great frequency over the course of the trial. This ensures an appropriate number of data points are obtained to allow sufficient power to assess the efficacy of an intervention. Second, not all design elements in a standard trial might be appropriate for a given single-subject trial. For example, it may be unethical to use a placebo comparator or washout periods if the patient’s condition requires constant intervention. Third, as noted, unless one is considering the conduct of a phase I study or a compassionate therapeutic use trial for a particular unapproved compound – which typically come with recommended or accepted guidelines – the design of a therapeutic intervention trial for an individual patient will probably have to be pursued in the context of repurposing an approved drug.[78] Fourth, there is precedent for clinical trials involving a focused study on a unique patient population in the investigation of treatments for rare and orphan diseases that can easily be extended, or at least motivate, studies of individual patients with those diseases.[89–91]
Data Dissemination and Query Capabilities
The information and results of a trial on an individual patient could shed enormous light on disease pathogenesis as well as provide leads on the diagnosis and treatment of related conditions, even if rare or idiopathic. In this vein, the dissemination of the results of individual patient-oriented research protocols is crucial, not just via publication, but by making available raw data from, e.g., appropriate assays or those data associated with a single subject clinical trial (step 14 in Figure 1). Databases such as SRA, [92] GEO,[93, 94] OMIM,[95] and related resources[96] would be excellent repositories for relevant data and outcomes. In addition, it is also possible to combine results of multiple single subject (or ‘N-of-1’) trials to make general claims about the effectiveness of a particular therapeutic intervention.[88, 97, 98] In addition, long term outcomes associated with the administration of a therapeutic intervention would benefit the medical and research communities in assessing the ultimate value or utility of that intervention. Thus, comprehensive integrated genomic medicine and individual patient-oriented research, when pursued on many different patients with similar features, could provide more insightful information on those patients than normally collected in traditional clinical phenotyping studies. The data resulting from these aggregated studies could then be mined for patterns which could further lead to important generalizations and hypothesis-generation. In this light, there is widespread appreciation that machine learning will have profound impact on individualized and stratified medicine if large and appropriate data sets are constructed. [99, 100] Thus, it is almost certain that as bioinformatic strategies continue to advance [101–107], and as the volume of quality data supporting relevant computational modeling grows, the ability to match interventions to patients will steadily increase in power.
INFRASTRUCTURE AND RESOURCES
In order to encourage and facilitate the pursuit of integrated genomic medicine and comprehensive individual patient-oriented research, there are some obvious infrastructure items and resources that need to be developed. Some of the most salient of the resources are briefly described below.
Establishing Research Teams
The different research domains that would be necessary to consider in developing an objectively-determined, optimized therapeutic intervention for an individual patient are extremely varied and not likely to fall within the expertise of researchers within a single academic unit or company division (think of the totality of expertise reflected in the components of Figure 1). Achieving input from relevant researchers and clinicians may thus require the creation of research teams devoted to individual patient research that cut across boundaries associated with, e.g., traditional academic divisions. There are efforts to create such interdisciplinary teams, such as efforts associated with the National Institutes of Health (NIH) Clinical Translational Science Award initiative (CTSA),[108–110] and broad personalized medicine initiatives [14, 111]. However, these initiatives are not focused on issues associated with the study of a single patient – a focus that might be an even harder sell to academic departments for various reasons (e.g., diminished potential for funding, little potential for many individual author-led publications, etc.) despite the high likelihood for breakthroughs in individualized medicine.
Training Programs
The conduct of individual patient-oriented research is an ideal setting for training physician-scientists, physicians generally, and biomedical researchers interested in translational research. The exposure to different disciplines and perspectives, recognition of the value of integrated approaches to medicine and clinical practice, requisite sensitivity to an individual patient’s needs, and a focus on the objective determination of a therapeutic intervention for a patient, would all come about from clinician or clinical researcher involvement in an integrated and comprehensive study of an individual patient. These themes accepted as crucial for advancing clinical practice and research.
Databases and Delivery Systems
The study of a single patient can benefit assessments of other patients only if the data and results on that patient are made available to the broader scientific and clinical communities. Appropriate databases and vehicles for publication, such as the “clinical problem solving” and the “clinical case studies” sections of the New England Journal of Medicine, could be paradigmatic publication vehicles for such efforts, but may not go far enough or be able to handle the complexities of integrated and comprehensive individual patient-oriented studies. Dealing with the very thorny issues of patient privacy however could also be complex, although some lay public initiatives, such as the Personal Genome Project (PGP)[112] and Patients-Like-Me[113] (http://www.patientslikeme.com/) suggest that, in many instances, patients are willing to sacrifice anonymity and privacy for the sake of benefitting the community at large, as well as benefitting from the collective insights of the scientific community. Such ‘citizen science’ initiatives have already provided preliminary validation to the public that individual patient-oriented research at some level can be successful in diagnosing and treating diseases.[114]
DISCUSSION
The debate about how best to enable individualized medicine will continue for some time, most likely because the biomedical research community is still struggling with: 1. the best way to both reliably match available therapeutic interventions to an individual patient’s unique genetic, biochemical, physiological, exposure and behavioral profile; and 2. The development of efficient technologies for the de novo, rapid creation of therapeutics tailored to an individual patient. It is arguable that until such matching or efficient tailored therapeutic intervention strategies are developed, the best way to objectively determine a course of treatment for an individual patient is to research that patient and his or her response to scientifically-backed therapeutic options. Although there are many impediments to the routine pursuit of such studies, motivation for overcoming them exists. For example, in the context of making treatment decisions for a given patient, current medical practice requires substantially less information about that patient’s condition and his or her likely response to a therapeutic intervention than the integrated and comprehensive single patient-oriented research studies envisioned here is likely to yield, despite the fact that it is recognized that current practices do not result in optimal treatment decisions.
The actual design and general pursuit of integrated and comprehensive individual patient-oriented research protocols will most likely defy simple recipes, but the strategies described herein should be seen as good starting points. Obviously, more comprehensive functional assays for assessing the effects of genetic variants, inclusion of systems-level data (epigenetics, gene expression, protein expression), development of more efficient and biologically meaningful iPS cell-based assays, vetting of physiological monitoring devices, identification of clinical outcome measures, better single patient clinical trial designs, and better data analysis methods, would all enhance integrated individual patient-oriented research.
Ultimately, though, since it is unlikely – and probably unjustified both scientifically and economically – that every patient will be treated as a research subject in the future,[115] decisions about which patients should be intensely studied will have to be made. Of the considerations one must take into account for making these decisions, the potential scientific achievements of the study as well as their generalizability and the benefit to the patient will obviously be important. In this context, it is likely that patients with overtly anatomical defects, such as irreversible brain atrophy or limb malformations, are not necessarily good candidates for studies of the type envisioned since the chance that a metabolic therapeutic intervention would actually correct the relevant dysfunction to the same degree a mechanical manipulation would (e.g., artificial limbs), is small, although such patients might be candidates for studies investigating different approaches to anticipatory counseling, patient management, palliative care and the avoidance of disease sequelae. In addition, the study of patients with rare and idiopathic, largely metabolic or neurologic conditions for which current treatments are either limited or not available, are good candidates for the proposed research (Tables 1 and 2) and their study could act as paradigms for integrated comprehensive individual patient-oriented research. This is also true given the emphasis among rare disease researchers on the study of the temporal, long-term or natural history of rare diseases and their clinical courses, the need for the objective determination of the efficacy of specific therapeutic interventions to treat them, as well as the need for regulatory changes in accommodating assessments of novel uses of preexisting therapeutic compounds that might be useful.[34, 116–118] In this sense the goal of integrated genomic medicine and comprehensive individual patient-oriented research is not necessarily to promote the belief that future clinical practice will require the pursuit of comprehensive studies on each patient in order to determine an optimal course of therapy, but rather to initiate comprehensive individual patient-oriented research on enough patients to determine what it is that might be generalizable to future patients.
Acknowledgements
NJS is supported in part by NIH grants: U19 AG023122–09, UL1TR001442–01 and U24AG051129–01. Please note that the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors would like to thank Dr. Laura Goetz for commenting on the manuscript.
Contributor Information
Nicholas J. Schork, The Translational Genomics Research Institute, 445 North Fifth Street, Phoenix, AZ 85004, nschork@tgen.org, 858-794-4054
Kristopher Nazor, MYi Diagnostics and Discovery, 5310 Eastgate Mall, San Diego, CA 92121, kristopher.nazor@myidiagnostics.com, 858-458-9305
References
- 1.Willard HF, Angrist M, Ginsburg GS. Genomic medicine: genetic variation and its impact on the future of health care. Philos Trans R Soc Lond B Biol Sci 360(1460), 1543–1550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feero WG, Guttmacher AE, Collins FS. Genomic medicine--an updated primer. N Engl J Med 362(21), 2001–2011 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Green ED, Guyer MS. Charting a course for genomic medicine from base pairs to bedside. Nature 470(7333), 204–213 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Genomic and Personalized Medicine. Willard HF, Ginsburg GS (Ed.^(Eds). Academic Press, (2009). [DOI] [PubMed] [Google Scholar]
- 5.Jain KK. Textbook of Personalized Medicine. Springer, New York: (2009). [Google Scholar]
- 6.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW. Cancer genome landscapes. Science 339(6127), 1546–1558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garraway LA, Lander ES. Lessons from the cancer genome. Cell 153(1), 17–37 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov 6(4), 287–293 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos N, Kinzler KW, Vogelstein B. The role of companion diagnostics in the development and use of mutation-targeted cancer therapies. Nat Biotechnol 24(8), 985–995 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Martini M, Vecchione L, Siena S, Tejpar S, Bardelli A. Targeted therapies: how personal should we go? Nat Rev Clin Oncol 9(2), 87–97 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Diaz LA Jr., Williams RT, Wu J et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486(7404), 537–540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misale S, Yaeger R, Hobor S et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486(7404), 532–536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence MS, Stojanov P, Polak P et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature doi: 10.1038/nature12213 nature12213 [pii] (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitcomb DC. What is personalized medicine and what should it replace? Nat Rev Gastroenterol Hepatol 9(7), 418–424 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klassen T, Davis C, Goldman A et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 145(7), 1036–1048 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doetschman T Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol 530 423–433 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel WN. Taking stock of complex trait genetics in mice. Trends Genet 11(12), 471–477 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Torkamani A, Schork NJ. Background gene expression networks significantly enhance drug response prediction by transcriptional profiling. Pharmacogenomics J doi: 10.1038/tpj.2011.35 tpj201135 [pii] (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashley EA, Butte AJ, Wheeler MT et al. Clinical assessment incorporating a personal genome. Lancet 375(9725), 1525–1535 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shriver MD. Ethnic variation as a key to the biology of human disease. Ann Intern Med 127(5), 401–403 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Manolio TA, Collins FS, Cox NJ et al. Finding the missing heritability of complex diseases. Nature 461(7265), 747–753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lander ES, Schork NJ. Genetic dissection of complex traits. Science 265(5181), 2037–2048 (1994). [DOI] [PubMed] [Google Scholar]
- 23.Mardis ER. The $1,000 genome, the $100,000 analysis? Genome Med 2(11), 84(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Davies K. $1,000 Genome: The Revolution in DNA Sequencing and the New Era of Personalized Medicine Free Press, (2010). [Google Scholar]
- 25.Torkamani A, Scott-Van Zeeland AA, Topol EJ, Schork NJ. Annotating individual human genomes. Genomics 98(4), 233–241 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rope AF, Wang K, Evjenth R et al. Using VAAST to identify an X-linked disorder resulting in lethality in male infants due to N-terminal acetyltransferase deficiency. Am J Hum Genet 89(1), 28–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yandell M, Huff C, Hu H et al. A probabilistic disease-gene finder for personal genomes. Genome Res 21(9), 1529–1542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dees ND, Zhang Q, Kandoth C et al. MuSiC: Identifying mutational significance in cancer genomes. Genome Res doi:gr.134635.111 [pii] 10.1101/gr.134635.111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams DR, Sincan M, Fuentes Fajardo K et al. Analysis of DNA sequence variants detected by high-throughput sequencing. Hum Mutat 33(4), 599–608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sincan M, Simeonov DR, Adams D et al. VAR-MD: a tool to analyze whole exome-genome variants in small human pedigrees with mendelian inheritance. Hum Mutat 33(4), 593–598 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Solimini NL, Xu Q, Mermel CH et al. Recurrent hemizygous deletions in cancers may optimize proliferative potential. Science 337(6090), 104–109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts NJ, Vogelstein JT, Parmigiani G, Kinzler KW, Vogelstein B, Velculescu VE. The predictive capacity of personal genome sequencing. Sci Transl Med 4(133), 133ra158(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JH, Wacholder S, Gail MH et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet 42(7), 570–575 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gahl WA, Markello TC, Toro C et al. The National Institutes of Health Undiagnosed Diseases Program: insights into rare diseases. Genet Med 14(1), 51–59 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones S, Zhang X, Parsons DW et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321(5897), 1801–1806 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Baran J, Cros A et al. International Cancer Genome Consortium Data Portal--a one-stop shop for cancer genomics data. Database (Oxford) 2011 bar026 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilpivaara O, Aaltonen LA. Diagnostic cancer genome sequencing and the contribution of germline variants. Science 339(6127), 1559–1562 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Chen R, Mias GI, Li-Pook-Than J et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell 148(6), 1293–1307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernstein BE, Stamatoyannopoulos JA, Costello JF et al. The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol 28(10), 1045–1048 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature 489(7414), 57–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flack JM. Noninvasive hemodynamic measurements: an important advance in individualizing drug therapies for hypertensive patients. Hypertension 47(4), 646–647 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Mcnitt-Gray M, Kinahan P, Jackson E. MO-A-BRA-01: State of the Art in Quantitative Imaging in CT, PET and MRI. Med Phys 39(6), 3862–3863 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Topol EJ. Transforming medicine via digital innovation. Sci Transl Med 2(16), 16cm14(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shirley MD, Baugher JD, Stevens EL et al. Chromosomal variation in lymphoblastoid cell lines. Hum Mutat 33(7), 1075–1086 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shukla SJ, Dolan ME. Use of CEPH and non-CEPH lymphoblast cell lines in pharmacogenetic studies. Pharmacogenomics 6(3), 303–310 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Saferali A, Grundberg E, Berlivet S et al. Cell culture-induced aberrant methylation of the imprinted IG DMR in human lymphoblastoid cell lines. Epigenetics 5(1), 50–60 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Tiscornia G, Vivas EL, Belmonte JC. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nat Med 17(12), 1570–1576 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Hockemeyer D, Jaenisch R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell 18(5), 573–586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng LT, Sun LT, Tada T. Genome editing in induced pluripotent stem cells. Genes Cells 17(6), 431–438 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Song M, Paul S, Lim H, Dayem AA, Cho SG. Induced pluripotent stem cell research: a revolutionary approach to face the challenges in drug screening. Arch Pharm Res 35(2), 245–260 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Atkinson SP, Lako M, Armstrong L. Potential for Pharmacological Manipulation of Human Embryonic Stem Cells. Br J Pharmacol doi: 10.1111/j.1476-5381.2012.01978.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poon A, Schmid B, Pires C et al. Generation of a gene-corrected isogenic control hiPSC line derived from a familial Alzheimer’s disease patient carrying a L150P mutation in presenilin 1. Stem Cell Res 17(3), 466–469 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Pires C, Schmid B, Petraeus C et al. Generation of a gene-corrected isogenic control cell line from an Alzheimer’s disease patient iPSC line carrying a A79V mutation in PSEN1. Stem Cell Res 17(2), 285–288 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Ishikawa T, Imamura K, Kondo T et al. Genetic and pharmacological correction of aberrant dopamine synthesis using patient iPSCs with BH4 metabolism disorders. Hum Mol Genet doi: 10.1093/hmg/ddw339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang P, Sallam K, Wu H et al. Patient-Specific and Genome-Edited Induced Pluripotent Stem Cell-Derived Cardiomyocytes Elucidate Single-Cell Phenotype of Brugada Syndrome. J Am Coll Cardiol 68(19), 2086–2096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maeda H, Chiyonobu T, Yoshida M et al. Establishment of isogenic iPSCs from an individual with SCN1A mutation mosaicism as a model for investigating neurocognitive impairment in Dravet syndrome. J Hum Genet 61(6), 565–569 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Shinkuma S, Guo Z, Christiano AM. Site-specific genome editing for correction of induced pluripotent stem cells derived from dominant dystrophic epidermolysis bullosa. Proc Natl Acad Sci U S A 113(20), 5676–5681 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sebastiano V, Zhen HH, Haddad B et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med 6(264), 264ra163(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nimsanor N, Poulsen U, Rasmussen MA et al. Generation of an isogenic, gene-corrected iPSC line from a symptomatic 59-year-old female patient with frontotemporal dementia caused by an R406W mutation in the microtubule associated protein tau (MAPT) gene. Stem Cell Res 17(3), 576–579 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Horii T, Tamura D, Morita S, Kimura M, Hatada I. Generation of an ICF syndrome model by efficient genome editing of human induced pluripotent stem cells using the CRISPR system. Int J Mol Sci 14(10), 19774–19781 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sala L, Yu Z, Ward-Van Oostwaard D et al. A new hERG allosteric modulator rescues genetic and drug-induced long-QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol Med 8(9), 1065–1081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang X, Wang Y, Yan W et al. Production of Gene-Corrected Adult Beta Globin Protein in Human Erythrocytes Differentiated from Patient iPSCs After Genome Editing of the Sickle Point Mutation. Stem Cells 33(5), 1470–1479 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marthaler AG, Tubsuwan A, Schmid B et al. Generation of an isogenic, gene-corrected control cell line of the spinocerebellar ataxia type 2 patient-derived iPSC line H266. Stem Cell Res 16(1), 202–205 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Laskowski TJ, Van Caeneghem Y, Pourebrahim R et al. Gene Correction of iPSCs from a Wiskott-Aldrich Syndrome Patient Normalizes the Lymphoid Developmental and Functional Defects. Stem Cell Reports 7(2), 139–148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang XW, Gong S. An overview on the generation of BAC transgenic mice for neuroscience research. Curr Protoc Neurosci Chapter 5 Unit 5 20 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Devoy A, Bunton-Stasyshyn RK, Tybulewicz VL, Smith AJ, Fisher EM. Genomically humanized mice: technologies and promises. Nat Rev Genet 13(1), 14–20 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov 5(9), 741–754 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Monsma DJ, Monks NR, Cherba DM et al. Genomic characterization of explant tumorgraft models derived from fresh patient tumor tissue. J Transl Med 10(1), 125(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghosh S, Matsuoka Y, Asai Y, Hsin KY, Kitano H. Software for systems biology: from tools to integrated platforms. Nat Rev Genet 12(12), 821–832 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Kohl P, Viceconti M. The virtual physiological human: computer simulation for integrative biomedicine II. Philos Transact A Math Phys Eng Sci 368(1921), 2837–2839 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Winslow RL, Trayanova N, Geman D, Miller MI. Computational medicine: translating models to clinical care. Sci Transl Med 4(158), 158rv111(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huh D, Leslie DC, Matthews BD et al. A Human Disease Model of Drug Toxicity-Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci Transl Med 4(159), 159ra147(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jamshidi N, Vo TD, Palsson BO. In silico analysis of SNPs and other high-throughput data. Methods Mol Biol 366 267–285 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Li Q, Cheng T, Wang Y, Bryant SH. PubChem as a public resource for drug discovery. Drug Discov Today 15(23–24), 1052–1057 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knox C, Law V, Jewison T et al. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res 39(Database issue), D1035–1041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barretina J, Caponigro G, Stransky N et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483(7391), 603–607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garnett MJ, Edelman EJ, Heidorn SJ et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483(7391), 570–575 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li YY, Jones SJ. Drug repositioning for personalized medicine. Genome Med 4(3), 27(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boguski MS, Mandl KD, Sukhatme VP. Drug discovery. Repurposing with a difference. Science 324(5933), 1394–1395 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Ross E Unapproved drug use: compassionate or cause for concern? Lancet Neurol 8(2), 136–137 (2009). [DOI] [PubMed] [Google Scholar]
- 81.Ostroff RM, Bigbee WL, Franklin W et al. Unlocking biomarker discovery: large scale application of aptamer proteomic technology for early detection of lung cancer. PLoS One 5(12), e15003(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanash SM, Baik CS, Kallioniemi O. Emerging molecular biomarkers--blood-based strategies to detect and monitor cancer. Nat Rev Clin Oncol 8(3), 142–150 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Kuhn P, Bethel K. A fluid biopsy as investigating technology for the fluid phase of solid tumors. Phys Biol 9(1), 010301(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Damani S, Bacconi A, Libiger O et al. Characterization of circulating endothelial cells in acute myocardial infarction. Sci Transl Med 4(126), 126ra133(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Opportunities Morgan B. and pitfalls of cancer imaging in clinical trials. Nat Rev Clin Oncol 8(9), 517–527 (2011). [DOI] [PubMed] [Google Scholar]
- 86.Symms M, Jager HR, Schmierer K, Yousry TA. A review of structural magnetic resonance neuroimaging. J Neurol Neurosurg Psychiatry 75(9), 1235–1244 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rombouts SaRB, Barkhof F, Sheltens P. Clinical applications of functional brain MRI. Oxford University Press, (2007). [Google Scholar]
- 88.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med 8(2), 161–173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strauss KA, Brumbaugh J, Duffy A et al. Safety, efficacy and physiological actions of a lysine-free, arginine-rich formula to treat glutaryl-CoA dehydrogenase deficiency: focus on cerebral amino acid influx. Mol Genet Metab 104(1–2), 93–106 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Ekins S, Williams AJ, Krasowski MD, Freundlich JS. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov Today 16(7–8), 298–310 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Vanderver A, Tonduti D, Auerbach S et al. Neurotransmitter abnormalities and response to supplementation in SPG11. Mol Genet Metab doi:S1096–7192(12)00209–0 [pii] 10.1016/j.ymgme.2012.05.020 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leinonen R, Sugawara H, Shumway M, International Nucleotide Sequence Database C. The sequence read archive. Nucleic Acids Res 39(Database issue), D19–21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barrett T, Edgar R. Gene expression omnibus: microarray data storage, submission, retrieval, and analysis. Methods Enzymol 411 352–369 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baker M Gene data to hit milestone. Nature 487(7407), 282–283 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Amberger J, Bocchini CA, Scott AF, Hamosh A. McKusick’s Online Mendelian Inheritance in Man (OMIM). Nucleic Acids Res 37(Database issue), D793–796 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuntzer J, Eggle D, Klostermann S, Burtscher H. Human variation databases. Database (Oxford) 2010 baq015(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zucker DR, Ruthazer R, Schmid CH. Individual (N-of-1) trials can be combined to give population comparative treatment effect estimates: methodologic considerations. J Clin Epidemiol 63(12), 1312–1323 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zucker DR, Schmid CH, Mcintosh MW, D’agostino RB, Selker HP, Lau J. Combining single patient (N-of-1) trials to estimate population treatment effects and to evaluate individual patient responses to treatment. J Clin Epidemiol 50(4), 401–410 (1997). [DOI] [PubMed] [Google Scholar]
- 99.Bibault JE, Giraud P, Burgun A. Big Data and machine learning in radiation oncology: State of the art and future prospects. Cancer Lett 382(1), 110–117 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Obermeyer Z, Emanuel EJ. Predicting the Future - Big Data, Machine Learning, and Clinical Medicine. N Engl J Med 375(13), 1216–1219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong C, Guo Y, Yang H, He Z, Liu X, Wang K. iCAGES: integrated CAncer GEnome Score for comprehensively prioritizing driver genes in personal cancer genomes. Genome Med 8(1), 135(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eyal-Altman N, Last M, Rubin E. PCM-SABRE: a platform for benchmarking and comparing outcome prediction methods in precision cancer medicine. BMC Bioinformatics 18(1), 40(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ross EG, Shah NH, Dalman RL, Nead KT, Cooke JP, Leeper NJ. The use of machine learning for the identification of peripheral artery disease and future mortality risk. J Vasc Surg 64(5), 1515–1522 e1513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sengupta PP, Huang YM, Bansal M et al. Cognitive Machine-Learning Algorithm for Cardiac Imaging: A Pilot Study for Differentiating Constrictive Pericarditis From Restrictive Cardiomyopathy. Circ Cardiovasc Imaging 9(6), (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singhal A, Simmons M, Lu Z. Text Mining Genotype-Phenotype Relationships from Biomedical Literature for Database Curation and Precision Medicine. PLoS Comput Biol 12(11), e1005017(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stanfield Z, Coskun M, Koyuturk M. Drug Response Prediction as a Link Prediction Problem. Sci Rep 7 40321(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valdes G, Luna JM, Eaton E, Simone CB 2nd, Ungar LH, Solberg TD. MediBoost: a Patient Stratification Tool for Interpretable Decision Making in the Era of Precision Medicine. Sci Rep 6 37854(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shuster JJ. US Government mandates for clinical and translational research. Clin Transl Sci 5(1), 83–84 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dilts DM, Rosenblum D, Trochim WM. A virtual national laboratory for reengineering clinical translational science. Sci Transl Med 4(118), 118cm112(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maxmen A Translational research: The American way. Nature 478(7368), S16–18 (2011). [DOI] [PubMed] [Google Scholar]
- 111.Whitcomb DC. Going MAD: development of a “matrix academic division” to facilitate translating research to personalized medicine. Acad Med 86(11), 1353–1359 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ball MP, Thakuria JV, Zaranek AW et al. A public resource facilitating clinical use of genomes. Proc Natl Acad Sci U S A 109(30), 11920–11927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alemi F, Erdman H, Griva I, Evans CH. Improved Statistical Methods are Needed to Advance Personalized Medicine. Open Transl Med J 1 16–20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marcus AD. Patients as Partners: An online network for sufferers of inflammatory bowel disease provides some clues to the power of collaboration. Wall Street Journal (2012). [Google Scholar]
- 115.Scuffham PA, Nikles J, Mitchell GK et al. Using N-of-1 trials to improve patient management and save costs. J Gen Intern Med 25(9), 906–913 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bamshad MJ, Shendure JA, Valle D et al. The Centers for Mendelian Genomics: A new large-scale initiative to identify the genes underlying rare Mendelian conditions. Am J Med Genet A 158A(7), 1523–1525 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miyamoto BE, Kakkis ED. The potential investment impact of improved access to accelerated approval on the development of treatments for low prevalence rare diseases. Orphanet J Rare Dis 6 49(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nih. Workshop Summary: Workshop on Natural History Studies of Rare Diseases: Meeting the Needs of Drug Development and Research (https://events-support.com/Documents/Summary-NHS.pdf). (2012).
- 119.Worthey EA, Mayer AN, Syverson GD et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med 13(3), 255–262 (2011). [DOI] [PubMed] [Google Scholar]
- 120.Bainbridge MN, Wiszniewski W, Murdock DR et al. Whole-genome sequencing for optimized patient management. Sci Transl Med 3(87), 87re83(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dixon-Salazar TJ, Silhavy JL, Udpa N et al. Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med 4(138), 138ra178(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.St Hilaire C, Ziegler SG, Markello TC et al. NT5E mutations and arterial calcifications. N Engl J Med 364(5), 432–442 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi M, Scholl UI, Ji W et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A 106(45), 19096–19101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Need AC, Shashi V, Hitomi Y et al. Clinical application of exome sequencing in undiagnosed genetic conditions. J Med Genet 49(6), 353–361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sasaki T, Niizeki H, Shimizu A et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci 68(1), 36–44 (2012). [DOI] [PubMed] [Google Scholar]
- 126.Van Bon BW, Gilissen C, Grange DK et al. Cantu syndrome is caused by mutations in ABCC9. Am J Hum Genet 90(6), 1094–1101 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lacy JN, Ulirsch JC, Grace RF et al. Exome sequencing results in successful diagnosis and treatment of a severe congenital anemia. Cold Spring Harb Mol Case Stud 2(4), a000885(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tarailo-Graovac M, Shyr C, Ross CJ et al. Exome Sequencing and the Management of Neurometabolic Disorders. N Engl J Med 374(23), 2246–2255 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aintablian HK, Narayanan V, Belnap N, Ramsey K, Grebe TA. An atypical presentation of ACAD9 deficiency: Diagnosis by whole exome sequencing broadens the phenotypic spectrum and alters treatment approach. Mol Genet Metab Rep 10 38–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kline CN, Joseph NM, Grenert JP et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol doi: 10.1093/neuonc/now254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fremond ML, Perot P, Muth E et al. Next-Generation Sequencing for Diagnosis and Tailored Therapy: A Case Report of Astrovirus-Associated Progressive Encephalitis. J Pediatric Infect Dis Soc 4(3), e53–57 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Sturm AC, Kline CF, Glynn P et al. Use of whole exome sequencing for the identification of Ito-based arrhythmia mechanism and therapy. J Am Heart Assoc 4(5), (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Castro MP, Whitcomb BP, Zajchowski DA, Coleman RL. Successful use of next generation genomic sequencing (NGS)-directed therapy of clear cell carcinoma of the ovary (CCCO) with trametinib and metformin in a patient with chemotherapy-refractory disease. Gynecol Oncol Res Pract 2 4(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu GH, Qu J, Suzuki K et al. Progressive degeneration of human neural stem cells caused by pathogenic LRRK2. Nature 491(7425), 603–607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tiscornia G, Lorenzo Vivas E, Matalonga L et al. Neuronopathic Gaucher’s disease: induced pluripotent stem cells for disease modelling and testing chaperone activity of small compounds. Hum Mol Genet doi:dds471 [pii] 10.1093/hmg/dds471 (2012). [DOI] [PubMed] [Google Scholar]
- 136.Bilican B, Serio A, Barmada SJ et al. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc Natl Acad Sci U S A 109(15), 5803–5808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Egawa N, Kitaoka S, Tsukita K et al. Drug Screening for ALS Using Patient-Specific Induced Pluripotent Stem Cells. Sci Transl Med 4(145), 108(2012). [DOI] [PubMed] [Google Scholar]
- 138.Naujock M, Stanslowsky N, Bufler S et al. 4-Aminopyridine Induced Activity Rescues Hypoexcitable Motor Neurons from Amyotrophic Lateral Sclerosis Patient-Derived Induced Pluripotent Stem Cells. Stem Cells 34(6), 1563–1575 (2016). [DOI] [PubMed] [Google Scholar]
- 139.Israel MA, Yuan SH, Bardy C et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482(7384), 216–220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun N, Yazawa M, Liu J et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 4(130), 130ra147(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tanaka T, Takahashi K, Yamane M et al. Induced pluripotent stem cells from CINCA syndrome patients as a model for dissecting somatic mosaicism and drug discovery. Blood doi:blood-2012-03-417881 [pii] 10.1182/blood-2012-03-417881 (2012). [DOI] [PubMed] [Google Scholar]
- 142.Yazawa M, Hsueh B, Jia X et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471(7337), 230–234 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Itzhaki I, Maizels L, Huber I et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471(7337), 225–229 (2011). [DOI] [PubMed] [Google Scholar]
- 144.Moretti A, Bellin M, Welling A et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 363(15), 1397–1409 (2010). [DOI] [PubMed] [Google Scholar]
- 145.Marchetto MC, Carromeu C, Acab A et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143(4), 527–539 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee G, Papapetrou EP, Kim H et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461(7262), 402–406 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ebert AD, Yu J, Rose FF Jr. et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457(7227), 277–280 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Granata A, Serrano F, Bernard WG et al. An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nat Genet 49(1), 97–109 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Codazzi F, Hu A, Rai M et al. Friedreich ataxia induced pluripotent stem cell-derived neurons show a cellular phenotype that is corrected by a benzamide HDAC inhibitor. Hum Mol Genet doi: 10.1093/hmg/ddw308 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Compagnucci C, Barresi S, Petrini S et al. Rho Kinase Inhibition Is Essential During In Vitro Neurogenesis and Promotes Phenotypic Rescue of Human Induced Pluripotent Stem Cell-Derived Neurons With Oligophrenin-1 Loss of Function. Stem Cells Transl Med 5(7), 860–869 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Efthymiou AG, Steiner J, Pavan WJ et al. Rescue of an in vitro neuron phenotype identified in Niemann-Pick disease, type C1 induced pluripotent stem cell-derived neurons by modulating the WNT pathway and calcium signaling. Stem Cells Transl Med 4(3), 230–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mertens J, Wang QW, Kim Y et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 527(7576), 95–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stanslowsky N, Reinhardt P, Glass H et al. Neuronal Dysfunction in iPSC-Derived Medium Spiny Neurons from Chorea-Acanthocytosis Patients Is Reversed by Src Kinase Inhibition and F-Actin Stabilization. J Neurosci 36(47), 12027–12043 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saini JS, Corneo B, Miller JD et al. Nicotinamide Ameliorates Disease Phenotypes in a Human iPSC Model of Age-Related Macular Degeneration. Cell Stem Cell doi: 10.1016/j.stem.2016.12.015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]