Over half a century ago, Peter Wolf first documented the presence of subcellular-sized particles in plasma and serum. This phospholipid-rich “dust” originated from activated platelets and its ability to drive coagulation demonstrated biological activity.1 Ever since, extracellular vesicles (EVs) have been a topic of constant study and controversy. Today, we understand that EVs are small, membrane-bound structures that are universally released by all eukaryotic cells and found throughout the body’s tissues and fluids. These particles are loaded with bioactive microRNAs (miRs), mRNAs, proteins, and lipids2, and cells preferentially and actively select cargoes for packaging – EV contents are thus reminiscent of, but distinct from, those of their originating cells. EV biogenesis takes two actively-regulated forms: smaller exosomes (~50–150 nm in diameter) are released by the fusion of multivesicular bodies with the plasma membrane, while larger microvesicles (~100–500 nm in diameter) are the result of direct budding and fission. EVs play clear roles in cell-cell communication. They are most studied in cancer where they modulate tumorigenesis and metastasis; as such, they hold substantial promise as biomarkers and treatment targets.3 More recently, considerable evidence of their involvement in cardiovascular disease has emerged, as plasma levels of microvesicles are increased in patients with coronary artery disease (CAD) and are predictive of future cardiovascular events.4 Lately, circulating EV-associated miRs have become the focus of biomarker discovery. The EV lipid bilayer protects miRs against extracellular ribonuclease degradation and substantially increases their plasma half-life. Indeed, expression levels of multiple EV-packaged miRs are associated with an lower risk of major adverse cardiovascular events in patients with stable CAD, implying the presence of a protective EV phenotype.5 However, until now it was largely unclear whether atherosclerosis induced alterations in EV cargoes, and whether such modifications might be mechanistically relevant to disease progression.
In this issue of Circulation Research, Liu et al.6 provide exciting clinical and experimental evidence that atherosclerotic conditions drive selective packaging of noncoding miRs into circulating EV populations. Using TaqMan arrays, the authors first screened a small set of patients with stable CAD or acute coronary syndrome (ACS) for differential enrichment of miRs in plasma-derived, ultracentrifugation-isolated microvesicles. Subsequently, nine hits from the array were quantified by RT-PCR in a larger cohort of 180 patients with CAD, ACS, or non-CAD controls. They identified significantly increases of EV-incorporated miR-92a-3p in those with CAD or ACS, and went on to show that circulating CD31+/CD42b- EVs (which they ascribe to endothelial origins) contained the highest level of this miR. Expression and EV packaging of miR-92a-3p was responsive to inflammatory stimuli in cultured endothelial cells (ECs), and delivery of miR-92a-3p to cultured ECs or vascular smooth muscle cells (SMCs) drove cellular migration and proliferation. These effects were, however, not present in the monocytic THP1 cell line due to an absence of EV uptake. Downregulation of thrombospondin 1 (THBS1) appears to be responsible for the angiogenic effect of miR-92a-3p in EV-treated ECs, though it is important to note that the underlying mechanism of action in SMCs was not addressed. Regardless, increased EV-associated miR-92a-3p with CAD/ACS indicates that EVs may harbor a harmful atherogenic role in addition to those putatively vasculoprotective EV-miR combinations.5
It is notable that the use of miR arrays and RT-PCR on a subset of nine miRs limited the depth to which the authors could probe the circulating EV miRNAome – hundreds of miRs are known to be present in human plasma.7 The more widespread use of next-generation sequencing (NGS) approaches will enable a much more complete survey of EV miR contents, and provides an opportunity to identify novel miRs, miR isoforms, and point mutations that may act as both biomarkers and potentiators/inhibitors of disease. In addition, while miR cargoes are undeniably important to the biological activity, intracellular communication functionality, and biomarker potential of EVs, they are only one part of the puzzle – protein and lipid contents of EVs are also bioactive and dynamically regulated. Though historically underappreciated, these non-miR components of EV cargo are also transferred between cells and contribute to cell-cell signaling. For example, during the osteogenic transition of vascular SMCs the multiligand sorting receptor sortilin regulates loading of pro-calcifying enzymes into EVs,8 and serum sortilin levels are associated with the severity of aortic calcification in patients.9 The recent development of less costly, low-input, and high-throughput proteomics and lipidomics techniques will be essential to furthering our understanding of whether EV-associated protein and lipid pools are similarly regulated by pro-atherogenic factors, and how such changes contribute to pathophysiology and clinical outcomes.
Although the data presented by Liu et al. provides convincing evidence of altered miR cargoes in circulating endothelial-derived EVs, these vesicles were not taken up by macrophages and the importance of enhanced migration/proliferation after uptake in ECs and vascular SMCs to lesion progression in vivo must still be explored. Circulating EVs represent a rich source of detectable and stable diagnostic biomarkers, however EVs produced by other cell types present in atherosclerotic plaques (e.g. SMCs10 and macrophages11) also promote CAD. Their proximity to and direct action on the cellular components of the plaque make tissue-resident EVs a rich source for novel biological insights, mechanisms of homeostasis/disease, and drug target discovery. Besides mediating intercellular signaling within the plaque, tissue-entrapped EVs also act as early nucleation sites of microcalcification12 and actively drive plaque calcification through elevated loading of annexins and phosphatidylserine as well as reduction of EV-incorporated inhibitors of calcification such as fetuin-A or matrix Gla protein.10
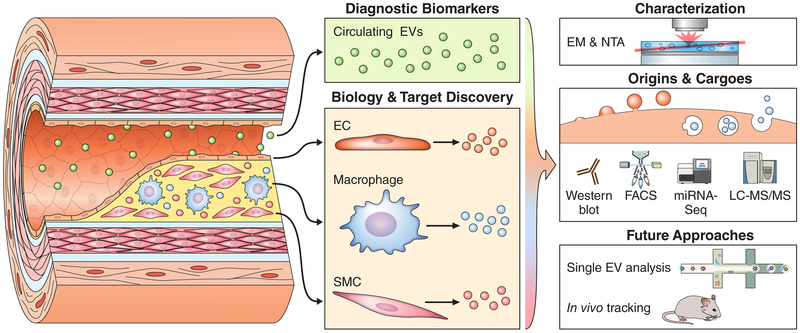
Since cells can produce both exosomal and microvesicular forms of EVs simultaneously, the intracellular origins of EV populations are of the utmost importance to both understanding their biological activity and, more importantly, to be able to leverage their cargoes as drug targets and/or to alter vesicle synthesis/release for therapeutic purposes. Liu et al. used electron microscopy (EM) to identify their EV population as microvesicles on the basis of size. The use of nanoparticle tracking analysis (NTA) to quantitatively assess EV size distributions and western blotting, FACS, or mass spectrometry to identify markers of exosomal or microvesicular origins has also become standardized.13, 14 In short, both circulating and plaque-resident EV pools are likely composed of multiple populations, with multiple cell types of origin, routes of biogenesis, and functionalities. In the future, the advent of technologies that enable resolution of the miRNAome, proteome, and lipidome at the single-vesicle level (much like single-cell RNA-seq has done at the cellular level) have the potential to revolutionize our understanding of this complex interplay.15 New high-resolution imaging modalities that allow vesicle populations to be tracked in vivo in both mice and humans are also sorely needed in order to prove pathogenic causation, identify localization, confirm therapeutic benefit, and clarify the mechanistic basis of EV contributions to CAD (Figure).
Figure 1. Blood- and Tissue-Derived Extracellular Vesicles (EVs) in Atherosclerosis.
Blood-derived circulating populations of EVs (green) are a key component of the “liquid biopsy” and may act with endocrine-like function. These EVs carry a host of detectable and stable putative diagnostic biomarkers in many cardiovascular diseases. In contrast, EVs ensnared in vascular tissues originate from all the constitutive cell types, such as endothelial cells (ECs), macrophages, or vascular smooth muscle cells (SMCs). These tissue-resident EV populations act as mediators of paracrine signaling and cell-cell cross talk, and contribute directly to calcification of atherosclerotic plaques. Their proximity to and direct action on the tissue of interest make them a rich source for novel biological insights, mechanisms of homeostasis/disease, and drug target discovery. Because EVs have context/cargo-dependent protective or harmful effects, careful characterization of the size, intracellular origins (exosomes through multi-vesicular bodies or microvesicles via budding), and RNA/protein cargoes of these EVs is essential to understanding their form and function. This is frequently achieved by the application of well-established optical techniques, including electron microscopy (EM) and nanoparticle tracking analysis (NTA), as well as molecular approaches such as Western blotting, fluorescence-activated cell sorting (FACS), transcriptomics, or liquid chromatography tandem mass spectrometry (LC-MS/MS). Upcoming technologies could allow both examination of EV composition on a vesicle-by-vesicle basis and tracking of individual EV populations within humans or animal models of disease.
Ultimately, the current study by Liu et al. represents an important and substantial step forward in our understanding of the roles that circulating EV miRs play in modulating endothelial and vascular SMC phenotypes under atherosclerotic conditions, and identifies a novel circulating biomarker. Clinical utility remains to be determined: it will be intriguing to see what prognostic/predictive/diagnostic power miR-92a-3p has. Pathobiological understanding and therapeutic avenues may be more directly attainable by studying the origins, cargoes, and function of complex subpopulations of tissue-entrapped EVs. Moving forward, the pairing of cutting-edge omics assays and upcoming single-EV technologies with these difficult-to-access vesicle populations holds the promise of carefully teasing apart the foundations of EV-mediated cell-cell cross talk in the atherosclerotic milieu and clarifying the harmful or protective nature of these particles.
Acknowledgments
Sources of Funding
This work is supported by National Institutes of Health (NIH) grants R01HL114805, R01HL136431 and R01HL109889.
Non-Standard Abbreviations and Acronyms
- ACS
Acute Coronary Syndrome
- CAD
Coronary Artery Disease
- EC
Endothelial Cell
- EM
Electron Microscopy
- EV
Extracellular Vesicle
- LC-MS/MS
Liquid Chromatography Tandem Mass Spectrometry
- miR
microRNA
- NGS
Next Generation Sequencing
- NTA
Nanoparticle Tracking Analysis
- SMC
Smooth Muscle Cell
- THBS1
Thrombospondin 1
Footnotes
Disclosures
None.
References
- 1.Wolf P The nature and significance of platelet products in human plasma. British journal of haematology. 1967;13:269–288 [DOI] [PubMed] [Google Scholar]
- 2.Blaser MC, Aikawa E. Roles and regulation of extracellular vesicles in cardiovascular mineral metabolism. Frontiers in Cardiovascular Medicine. 2018;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinning JM, Losch J, Walenta K, Bohm M, Nickenig G, Werner N. Circulating cd31+/annexin v+ microparticles correlate with cardiovascular outcomes. European heart journal. 2011;32:2034–2041 [DOI] [PubMed] [Google Scholar]
- 5.Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS, Sinning JM, Vasa-Nicotera M, Nickenig G, Werner N. Microrna expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. Journal of the American Heart Association. 2014;3:e001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Li Q, Hosen MR, Zietzer A, Flender A, Levermann P, Schmitz T, Fruhwald D, Goody P, Nickenig G, Werner N, Jansen F. Atherosclerotic conditions promote the packaging of functional microrna-92a-3p into endothelial microvesicles. Circ Res. 2019 [DOI] [PubMed] [Google Scholar]
- 7.Tonge DP, Gant TW. What is normal? Next generation sequencing-driven analysis of the human circulating mirnaome. BMC molecular biology. 2016;17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goettsch C, Hutcheson JD, Aikawa M, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. The Journal of clinical investigation. 2016; 126:1323–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goettsch C, Iwata H, Hutcheson JD, O’Donnell CJ, Chapurlat R, Cook NR, Aikawa M, Szulc P, Aikawa E. Serum sortilin associates with aortic calcification and cardiovascular risk in men. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:1005–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109:e1–12 [DOI] [PubMed] [Google Scholar]
- 11.New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutcheson JD, Goettsch C, Bertazzo S, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nature materials. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632–1648 [DOI] [PubMed] [Google Scholar]
- 14.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. Journal of Extracellular Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS nano. 2015;9:2321–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]