Abstract
Background:
Currently as many as half of women with suspected myocardial ischemia have no obstructive CAD, and abnormal coronary reactivity (CR) is commonly found.
Objectives:
We prospectively investigated CR and longer-term adverse cardiovascular outcomes in women with and with no obstructive CAD in the National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE).
Methods:
Women (n=224) with signs and symptoms of ischemia underwent CR testing. Coronary flow reserve (CFR) and coronary blood flow (CBF) were obtained to test microvascular function, while epicardial CR was tested by coronary dilation response to intracoronary (IC) acetylcholine and IC-nitroglycerine. All-cause mortality, major adverse cardiovascular events (MACE) [cardiovascular death, myocardial infarction, stroke, and heart failure] and angina hospitalizations served as clinical outcomes over a median follow-up of 9.7 years.
Results:
We identified 129 events during the follow-up period. Low CFR was predictor of increased MACE rate (HR 1.06; 95% CI 1.01–1.12, p=0.021), while low CBF was associated with increased risk of mortality (HR=1.12; 95% CI 1.01–1.24, p=0.038) and MACE (HR= 1.11; 95% CI 1.03–1.20, p=0.006) after adjusting for cardiovascular risk factors. In addition, a decrease in cross-sectional area in response to IC-acetylcholine was associated with higher hazard of angina hospitalization (HR=1.05; 95% CI 1.02–1.07, p<0.0001). There was no association between epicardial IC-nitroglycerine dilation and outcomes.
Conclusions:
On longer-term follow-up, impaired microvascular function predicts adverse cardiovascular outcomes in women with signs and symptoms of ischemia. Evaluation of CR abnormality can identify those at higher risk of adverse outcomes in the absence of significant CAD.
Condensed abstract:
Currently as many as half of women with suspected myocardial ischemia undergoing clinically indicated coronary angiography have no obstructive CAD, yet abnormal epicardial and microvascular coronary reactivity is commonly found. In this study, we investigated the relationship between microvascular, epicardial coronary reactivity and clinical cardiovascular outcome. We found impaired coronary microvascular function predicts adverse cardiovascular outcomes in women with signs and symptoms of ischemia. Evaluation of coronary reactivity can identify those at higher risk of adverse outcomes in the absence of significant CAD.
Keywords: coronary reactivity, endothelial function, microvasculature, coronary flow reserve, cardiovascular outcome
Introduction
Prior work that included predominantly men with obstructive coronary artery disease (CAD), abnormal coronary reactivity testing in a non-obstructed epicardial coronary artery predicted adverse outcomes (1). Currently as many as half of women with suspected myocardial ischemia undergoing clinically indicated coronary angiography have no angiographic evidence of obstructive epicardial CAD (2). We have demonstrated that women with no obstructive CAD are at increased risk of major cardiovascular events (e.g. death, myocardial infarction [MI], stroke, or heart failure [HF] hospitalization) during follow-up (3–5). These findings have been confirmed by others and have been extended to apply to men (2,6,7).
We have further documented that abnormal coronary endothelium and non-endothelium dependent reactivity is common in women with signs and symptoms of ischemia but no obstructive CAD (8–11). We also noted that women with reduced coronary flow reserve (CFR) and abnormal epicardial coronary endothelium dilation are at higher risk of relatively short-term (3–5 year) adverse outcomes, predominantly angina hospitalization, compared to those with normal response (9,11). The presence of coronary microvascular dysfunction, ischemia on stress testing, and persistent angina > 1 year, identified women at higher risk for adverse outcomes (4,12). Cost analyses demonstrated WISE women with signs and symptoms of ischemia but no obstructive CAD incurred similar short-term health care costs compared to patients with obstructive CAD (13).
The original WISE study includes up to 10-yr follow-up, therefore we prospectively investigated epicardial and microvascular coronary reactivity and longer-term adverse cardiovascular outcomes in women with signs and symptoms of ischemia including those with no obstructive CAD in the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE).
Methods
We studied women with signs and symptoms of ischemia who were enrolled in the National Heart, Lung, and Blood Institute (NHLBI) - Women’s Ischemia Syndrome Evaluation (WISE) from 1996 to 2000, as previously described(14). The WISE protocol, approved by the relevant institutional review boards, has been described in detail previously(14), and all subjects gave written informed consent before study participation.
Coronary Reactivity Testing
Women were asked to hold vasoactive medications for 48 hours before the procedure. Coronary reactivity testing was performed in an epicardial coronary artery free of obstructive CAD (<50% diameter stenosis). The left anterior descending (LAD) coronary artery was the preferred vessel, followed by the left circumflex coronary artery if anatomic issues prohibited safe access to the LAD. To assess blood flow velocity, a Doppler-tipped guidewire (0.014-inch FloWire, Volcano Corporation, California, USA) was advanced through the diagnostic catheter. Recordings were made once a stable Doppler signal in the proximal or mid vessel was obtained. During coronary reactivity testing the following pathways were evaluated:
Non-endothelium dependent microvascular reactivity was assessed using intracoronary bolus injections of incremental doses of adenosine (18 mcg and 36 mcg). CFR was calculated as a ratio of average peak velocity to average baseline (rest) velocity. CFR in response to each bolus was recorded and a CFR of ≥2.32 was considered normal (9).
Endothelium-dependent reactivity was assessed using intracoronary acetylcholine infusion (IC-Ach) 0.182 µg/ml (10–6 mol/L) at 2mL/min for 3 minutes. This was followed by infusion of 18.2 µg/ml (10–4mol/L) over 3 min and then blood flow and pressure recordings were made and angiography was repeated to assess coronary vessel diameter. The 0.182 µg/ml IC-Ach was used for safety purposes to make sure no significant coronary spasm in response to IC-Ach occurs. Therefore, only the 18.2 µg/ml IC-Ach data are reported. Coronary angiography was obtained after the infusion completed. Endothelium-dependent epicardial coronary reactivity was assessed by calculating the percentage in coronary cross-sectional area change in response to IC-Ach (change in epicardial cross-sectional area >0% is considered normal), while endothelium-dependent microvascular reactivity was evaluated by calculating the change in coronary blood flow (CBF) (change in CBF ≥50% is considered normal).
Non-endothelium-dependent epicardial coronary reactivity was assessed using intra-coronary nitroglycerin (IC-NTG) injection. Coronary angiography was obtained after each injection to measure coronary artery diameter and calculate coronary cross-sectional area (CSA) (change in CSA >20% is considered normal) (15).
Quantitative Coronary Angiography and Blood Flow Recordings
All angiography was done in a standardized oblique projection to minimize overlapping and better visualization of coronary vessels. Angiograms and average blood flow velocity (AV) recordings were made at baseline and after administration of each vasoactive drug. CSA was calculated from coronary diameter measured 5 mm distal to the tip of the Doppler wire. CBF was calculated using the equation CBF = CSAxAVx0.5 (16) at rest and in response to IC-Ach. Change in CBF was calculated as: (CBFpeak-CBFrest/CBFrest) x100. Epicardial coronary artery responses to IC-Ach were assessed by measuring coronary diameter at baseline and after IC-Ach infusion by quantitative coronary angiography. For quantitative coronary angiography, angiograms were analyzed by one investigator masked to all other patient data at the WISE angiographic core laboratory (Rhode Island Hospital, Providence, RI) as described previously (17).
Follow-up, Mortality and Adverse Outcomes
A standardized protocol-directed follow-up was conducted by experienced site nurses or physicians through direct, telephone, and/ or mail contact at 6 weeks, 1 year, and annually thereafter. Women were provided with questionnaires asking about their symptoms, medication use, hospitalizations, and diagnostic or revascularization procedures since last contact. All the reports were reviewed by the study committee and classified as death, cardiovascular death; nonfatal MI; nonfatal stroke and HF hospitalization up to December 2005. In addition, a National Death Index (NDI) search was conducted for all women thought to be alive up to December 2007. Furthermore, because persistent angina predicts cardiovascular events in women with no obstructive CAD (3), we monitored hospitalization due to angina as well. We evaluated two composite outcomes for major adverse cardiovascular events (MACE): (1) a 4-component MACE of cardiovascular death, nonfatal MI, nonfatal stroke, or HF hospitalization; and (2) a 3-component MACE of cardiovascular death, nonfatal MI and nonfatal stroke. We analyzed angina hospitalization separately from MACE outcome; specifically, we did not combine angina and cardiovascular composite outcome in any model.
Statistical Analysis
Clinical variables were summarized using mean ± standard deviation or using count (%) for categorical variables. Follow up time was summarized using medians and ranges. Kaplan Meier analysis was used for stratified analysis of time-to-event for three event types: (1) overall mortality, (2) 4-component MACE, (3) 3-component MACE, and hospitalization due to angina. Cox proportional hazards regression was used to analyze hazard for these events with each of the continuous coronary reactivity testing variables alone, as well as separate models to adjust the association of coronary reactivity variables for clinical factors of age, and binary indicators for history of hypertension, dyslipidemia, smoking status, diabetes, and CAD status. A significance level of 0.05 was used for all analyses. Analysis was carried out using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics and Adverse Outcome
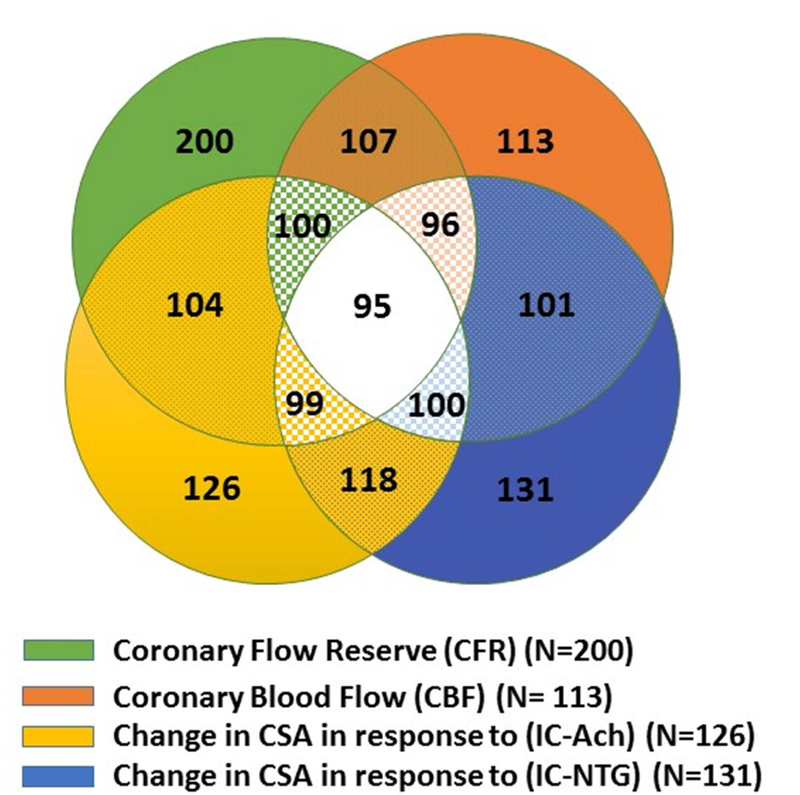
The baseline characteristics of the 224 subjects are summarized in Table 1. Mean age was 55±11 years with median follow-up duration of 9.7 years. Two-hundred (89%) subjects underwent CFR evaluation, 126 (56%) underwent evaluation of endothelium-dependent epicardial coronary reactivity testing using coronary cross-sectional area change in response to IC-Ach, 113 (50%) subjects underwent endothelium-dependent microvascular reactivity testing using CBF change in response to IC-Ach and 131 (58%) subjects received IC-NTG for evaluation of non-endothelium-dependent epicardial coronary reactivity. Most women underwent evaluation of multiple coronary reactivity pathways (Figure 1), related to testing feasibility, vasoactive medication tolerance and safety. We identified 77 (38%) women with abnormal CFR, 55 (49%) with abnormal CBF, while 69 (55%) with abnormal change in CSA in response to IC-Ach and 75 (57%) with abnormal change in CSA in response to IC-NTG. In this cohort, 51% had no CAD (defined as <20% stenosis); 30% had minimal CAD (defined as 20%−50%); and 19% had significant CAD (defined as >50%). During follow-up, there were 32 deaths, eighteen (56%) of them were related to cardiovascular cause. There were 57 4-component MACE events, 37 3-component MACE events, and 58 hospitalizations related to angina (Table 2).
Table 1:
Baseline Characteristics of the Women (N=224)
| Age, years (mean±SD) | 55±11 |
|---|---|
| Race | |
| White, n (%) | 181 (81%) |
| African-American, n (%) | 40 (18%) |
| Others, n (%) | 3 (1%) |
| History of Diabetes, n (%) | 49 (22%) |
| History of Hypertension, n (%) | 125 (56%) |
| Family History Coronary Disease, n (%) | 142 (66%) |
| Dyslipidemia, n (%) | 105 (52%) |
| CAD minimal or none, n (%) | 179 (81%) |
| Current smoking, n (%) | 44 (20%) |
| Coronary Hemodynamics | |
| CFR, mean±SD (N=200) | 2.56 0.76 |
| ΔCBF (%) (N=113) | 79 ± 107% |
| ΔCSA (IC-Ach) (%) (N=126) | 2.68 ± 24.45% |
| ΔCSA (IC-NTG) (%) (N=131) | 20.13 ± 24.51% |
| Baseline Medications | |
| ACE-I/ARB | 54 (24%) |
| Beta Blockers | 65 (29%) |
| CCD | 58 (26%) |
| Nitrates | 47 (21%) |
| Statins | 38 (17%) |
| Aspirin | 110 (50%) |
| Vasodilators | 15 (7%) |
CAD: coronary artery disease, CFR: coronary flow reserve, CBF: coronary blood flow, CSA: coronary cross-sectional area, IC-Ach: intra-coronary acetylcholine infusion, IC-NTG: intra-coronary nitroglycerine, ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, CCB: Calcium channel blockers.
Figure 1. Distribution of selected coronary reactivity testing in 224 women with signs and symptoms of ischemia.
Most women underwent evaluation of more than one coronary reactivity pathway. Green color represents women who underwent evaluation of non-endothelium dependent microvascular reactivity using coronary flow reserve (CFR); orange color represents women who underwent evaluation of endothelium dependent microvascular reactivity using coronary blood flow (CBF); yellow color represents women who underwent evaluation of endothelium dependent epicardial coronary reactivity using change in coronary artery cross sectional area in response to intracoronary acetylcholine (IC-Ach); while blue color represents women who underwent evaluation of non-endothelium dependent epicardial coronary reactivity using change in coronary artery cross sectional area in response to intracoronary nitroglycerine (IC-NTG).
Table 2:
Total Adverse Events during Median Follow-Up of 9.7 years
| Events | Number of Events (n=224) | Number of Events in women with no obstructive CAD (n=179) |
|---|---|---|
| Death | 32 | 22 |
| Cardiovascular Death | 18 | 11 |
| Myocardial infarction | 8 | 6 |
| Stroke | 11 | 9 |
| Heart failure hospitalization | 20 | 13 |
| Angina hospitalization | 58 | 41 |
Non-endothelium Dependent Coronary Microvascular Reactivity in Response to Intracoronary Adenosine
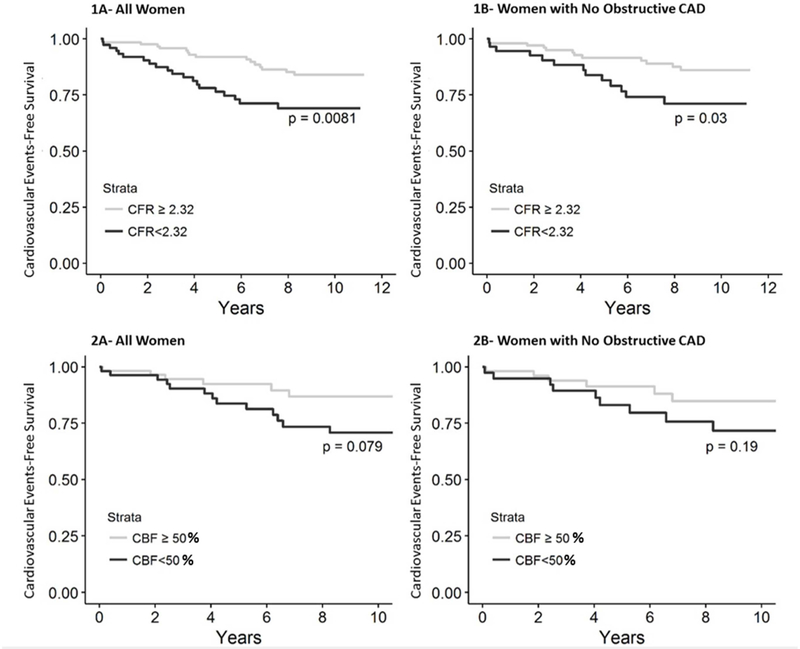
Mean CFR was 2.56 ± 0.76 and 123 out of 200 (62%) subjects had normal CFR (≥2.32). There was no difference in medication regimen between women with CFR≥2.32; and those with CFR<2.32 (S1. supplemental table). There was no difference in all-cause mortality rate between normal and abnormal CFR (13% versus 16%; p=0.48). Both the 4-component and 3-component MACE rates were higher in the group of women with CFR<2.32 compared to those with CFR≥2.32 (26% vs 13%, p=0.008, 19% vs 10%, p=0.028, respectively) (Figure 2). Low CFR was an independent predictor of the 4-component MACE rate (HR 1.06; 95% CI 1.01–1.12, p=0.021). This association remained significant after adjusting for age, history of hypertension, dyslipidemia, smoking status, diabetes, and CAD status (HR=1.06; 95% CI 1.004–1.12, p=0.035). Following adjustment, low CFR was not significantly associated with the 3-component MACE (HR 1.06; 95% CI 0.99–1.14, p=0.064).
Figure 2: Relationship between coronary microvascular function and cardiovascular events.
(1A) Kaplan-Meier analysis showing percentage of women surviving free from the 4-component major adverse cardiovascular event (MACE) including cardiovascular death, nonfatal MI, nonfatal stroke and HF during long-term follow-up stratified by coronary flow reserve (CFR). (1B) Kaplan-Meier analysis showing percentage of women with no obstructive coronary artery disease stratified by CFR surviving free from the 4-component MACE during long-term follow-up. (2A) Kaplan-Meier analysis showing percentage of women stratified by coronary blood flow (CBF) surviving free from the 4-component MACE during long-term follow-up. (2B) Kaplan-Meier analysis showing percentage of women with no obstructive coronary artery disease stratified by CBF surviving free from the 4-component MACE during long-term follow-up.
Women with no obstructive CAD (defined as epicardial coronary artery stenosis<50%) and abnormal CFR (57 [36%]) had higher rates of 4-component MACE compared to those with normal CFR (23% versus 12%; p=0.03). After adjusting for traditional cardiovascular risk factors including age, history of hypertension, dyslipidemia, smoking status, and diabetes, a decrease of 0.1 in CFR was associated with an 8% increase in hazard of adverse cardiovascular events (HR=1.08; 95% CI 1.01–1.16, p=0.036).
Endothelium-dependent Coronary Microvascular Reactivity in Response to Intracoronary Acetylcholine
Mean CBF change was 79±107%. Compared to resting CBF, change in CBF was normal (i.e. increase by ≥50%) in response to IC-Ach in 58 women (51%) and abnormal in 55 women (49%). Medication regimen at baseline was not different in women with abnormal CBF response versus women with normal response (S1. supplemental table). In unadjusted models, there was no association between CBF change and all-cause mortality. However, there was a trend toward higher rates of MACE in the abnormal group compared to those with normal CBF (24% versus 10% events; P=0.078) (Figure 2). Similarly, there was trend toward higher rates of the 3-component MACE in women with low CBF response versus those with normal response (20% versus 7%; p=0.055). After adjusting for age, history of hypertension, dyslipidemia, smoking status, diabetes, and CAD status, for each 10% reduction in CBF there was a 12% significant increased risk of all-cause mortality (HR=1.12; 95% CI 1.01–1.24, p=0.038), 11% increase in the 4-component MACE (HR= 1.11; 95% CI 1.03–1.20, p=0.006), and 12% increase in the 3-component MACE (HR= 1.12; 95% CI 1.03–1.22, p<0.01).
When evaluating women with no obstructive disease, every 10% decrease in CBF was associated with a 23% increase in risk of all-cause mortality (HR=1.23; 95% CI 1.04–1.45, p=0.015), a 16% excess risk for both the 4-component MACE (HR = 1.16; 95% CI 1.06–1.27, p=0.001), and 3-component MACE (HR = 1.16; 95% CI 1.05–1.28, p=0.003).
Endothelium-dependent Epicardial Coronary Reactivity in Response to Intracoronary Acetylcholine
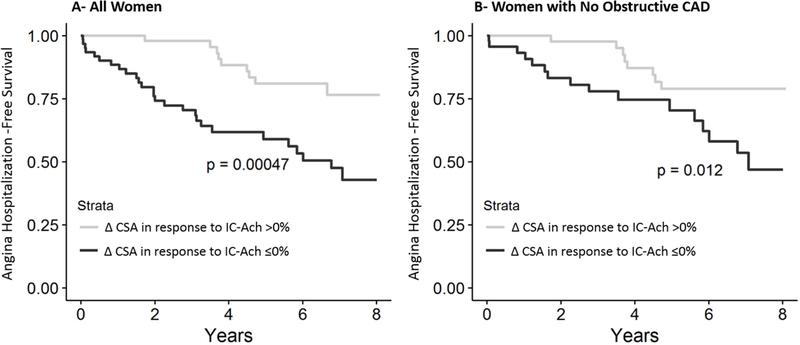
Mean coronary artery cross-sectional area change in response to IC-Ach was 2.68±24.5%. Fifty-seven women (45%) had normal response (i.e. epicardial vasodilatation), while 69 women (55%) had abnormal response (i.e. epicardial vasoconstriction) in response to IC-Ach. Although there was no association between change in cross-sectional area in response to IC-Ach and mortality or MACE, abnormal response (change in cross-sectional area <0%) was associated with an increased rate of hospitalizations due to angina [for each 1% decrease in cross-sectional area in response to IC-Ach, there is an estimated 5% higher hazard of angina hospitalization (HR=1.05; 95% CI 1.02–1.07, p<0.0001). Similarly, epicardial vasoconstriction in response to IC-Ach in women with no obstructive CAD was associated with higher rates of hospitalization due to angina (HR=1.05; 95% CI 1.02–1.07, p=0.0002) (Figure 3).
Figure 3. Relationship between endothelium-dependent epicardial coronary function stratified by change in coronary artery cross-sectional area (CSA) in response to intra-coronary acetylcholine (IC-Ach) and cardiovascular events.
(A) Kaplan-Meier analysis showing percentage of women surviving free from angina hospitalization during long-term follow-up. (B) Kaplan-Meier analysis showing percentage of women with no obstructive coronary artery disease surviving free from angina hospitalization during long-term follow-up.
Non-Endothelium Dependent Epicardial Coronary Reactivity in Response to Intracoronary Nitroglycerine
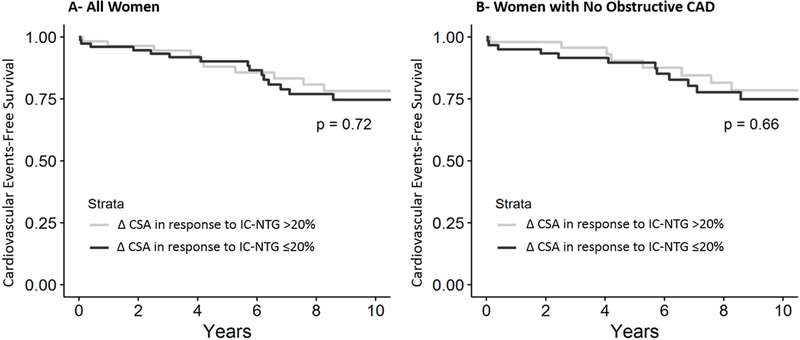
Mean coronary artery cross-sectional area change in response to IC-NTG was 20.13±24.51%. In 43% (56 out of 131 subjects) of the women who received IC-NTG, coronary artery cross-sectional area increased >20%. Changes in coronary artery cross-sectional area in response to nitroglycerine was not predictive of death, MACE or angina hospitalization (Figure 4).
Figure 4. Relationship between endothelium-independent epicardial coronary function stratified by change in coronary artery cross-sectional area (CSA) in response to intra-coronary nitroglycerin (IC-NTG) and cardiovascular events.
(A) Kaplan-Meier analysis showing percentage of women surviving free from the 4-component MACE during long-term follow-up. (B) Kaplan-Meier analysis showing percentage of women with no obstructive coronary artery disease surviving free from the 4-component MACE during long-term follow-up.
Discussion
On longer-term follow-up among women with signs and symptoms of ischemia, we found that impaired endothelium and non-endothelium dependent coronary microvascular reactivity predicts adverse cardiovascular events. Abnormal response to endothelium-dependent pathways in the microvasculature also predicts increased mortality on long term follow up when adjusted for various cardiovascular risk factors. In addition, impaired epicardial endothelium-dependent coronary reactivity was associated with increased angina hospitalizations. Lastly, we noted no relationship between response to IC-NTG and outcomes.
These data highlight the prognostic importance of impaired coronary reactivity on long-term outcomes. Given the relatively young age of this cohort, identifying patients who are at higher risk due to impaired compared to normal reactivity becomes of paramount importance, specifically in women with no obstructive CAD (Central Illustration). Our study extends the findings in previous publications that coronary blood flow regulation might contribute to development and progression of CAD (1,11,18) and that impaired endothelium and non-endothelium dependent coronary reactivity predicts MACE beyond angiographic CAD severity and traditional cardiovascular risk factors (9,11). We add to the existing knowledge base by showing an association between coronary endothelium-dependent microvascular reactivity as assessed by CBF and long-term MACE and its robust association among women with no evidence of obstructive CAD.
Central Illustration. Women with signs and symptoms of ischemia with no obstructive coronary artery disease and the potential role of coronary reactivity testing.
women with no obstructive coronary artery disease (CAD) and potential role of coronary reactivity testing to identify those at higher risk for adverse events. CFR: coronary flow reserve, CBF: coronary blood flow.
The relationship between impaired endothelium-dependent coronary reactivity and adverse cardiovascular events has been demonstrated previously, including in patients with no obstructive CAD (1,11,18–20). The mechanism of this relationship is complex. Based on in-vitro and animal models of atherosclerosis, impaired endothelium-dependent coronary reactivity is associated with rapid progression of atherosclerosis, increased plaque burden and rupture (19,21–24). This may be in part due to decrease in bioavailability of nitric oxide (NO) and increase in endothelial adhesion molecules for monocytes which could lead to inflammation of the vasculature wall, loss of vascular function, progression of atherosclerosis and plaque rupture (25–27). In our cohort of women, after adjusting for traditional cardiovascular risk factors, we found impaired endothelium-dependent microvascular reactivity predicts all-cause mortality and MACE. Our results are similar to Suwaidi et al. (18) which suggested impaired coronary blood flow in response to IC-Ach as a measure of endothelium-dependent microvascular reactivity is associated with development of adverse cardiovascular events.
Several studies have assessed the impact of abnormal CFR on prognosis in both obstructive and non-obstructive CAD patients (28–31). From the WISE study, we reported that among women undergoing invasive assessment of CFR, a value of <2.32 is associated with increased risk of cardiovascular death, MI, stroke and HF hospitalization over an average of 5.4 years of follow-up (9). Our current analysis shows a persistent relationship between abnormal CFR and major adverse cardiovascular events over longer-term follow-up.
Hospitalizations for recurrent angina carry morbidity and are associated with significant health care costs (13). We previously found that 20% of 883 WISE participants, with no obstructive CAD and 38%−55% with 1-vessel to 3-vessel CAD were hospitalized for chest pain through 5 years of follow-up. Angina hospitalization was around 2-fold higher in women with no obstructive CAD compared to those with 1-vessel disease after 1-year follow-up (13). In the current study, we showed a significant association between impaired endothelium-dependent epicardial coronary reactivity in response to IC-Ach and hospitalization due to angina. These findings highlight the need for identifying patients with an objective cause for symptoms of angina such as abnormal coronary reactivity and to appropriately follow and treat these patients to avoid recurrent hospitalizations.
The predominant effect of nitroglycerine is on the smooth muscles in the epicardial coronary arteries (32), likely due to lack of sulfhydryl groups in small coronary arterioles (sulfhydryl groups are required for conversion of nitroglycerine to its active metabolite) (33). We did not find an association between endothelium-independent epicardial coronary reactivity response to nitroglycerine and cardiovascular adverse events. This is in line with multiple previous reports which also failed to show an association (11,20).
Recently, Ford et al. showed that pathway specific medical therapy guided by invasive coronary reactivity testing led to reduction in angina severity and significant improvement in quality of life compared to patients received standard care (34). The main clinical implication of our findings is to endorse efforts towards reliably identifying patients who have angina due to impaired coronary reactivity. In current practice, efforts towards routine invasive assessment of coronary reactivity are hampered by time, expertise and cost. Though these procedures are relatively safe, they require experienced operators familiar with the protocols and techniques. Validation of simpler invasive protocols and non-invasive tools would be needed to appropriately diagnose the majority of these patients.
Our study should be interpreted in the context of strengths and limitations. The strength of this study is the long-term follow up in relatively low risk patients compared to previous studies which included higher-risk patients with significant CAD (1,30). In addition, previous studies included few women (18,35). The current study is an extension of previous studies addressing the relationship between coronary reactivity and adverse outcomes in low risk patients with no significant CAD. Furthermore, despite the relative small sample size for the number of co-variates required in the adjustment model, we performed comprehensive adjustments in our comparisons, which add confidence to our findings. There are several limitations which should be noted. First, the cohort is limited to patients pre-determined to undergo coronary angiography based on inclusion and exclusion criteria. Second, coronary reactivity testing results were from a single testing period, which may not necessarily reflect the status of the coronary artery over time. In addition, most of the women in our cohort underwent testing for multiple reactivity pathways. In women with >1 impaired pathways, it is unknown which testing modality is best predictor of adverse cardiovascular events. In this study, among the women with non-obstructive CAD, only a minority were treated, the women with events conversely had higher use of calcium channel blockers, nitrates and statin, but not ACE/ARB, aspirin or beta blocker, perhaps related to therapeutic uncertainty in the group (S2 and S3. supplemental tables). Medication use in observational study is always confounded, challenging to present concisely, and difficult to interpret. Recently, a stratified medical therapy guided by diagnostic procedure showed improvement in angina and quality of life in patients with non-obstructive CAD (34). Additionally, we were limited by the absence of advanced intra-coronary imaging techniques to assess extent of atherosclerosis. And lastly, coronary reactivity testing is an invasive procedure that requires experienced operators. It is usually performed in centers specialized in vascular function testing.
In conclusion, when evaluating longer-term outcomes, impaired coronary microvascular reactivity predicts adverse cardiovascular outcomes. While abnormal endothelium-dependent epicardial coronary reactivity was associated with increase rate of angina hospitalizations in women with signs and symptoms of ischemia, including those with no angiographic evidence of obstructive coronary artery disease. Evaluation of coronary reactivity can identify those at higher risk of adverse outcomes in the absence of significant CAD. Future research should evaluate feasible, preferably non-invasive, and cost-effective strategies to assess coronary reactivity in clinical practice.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
In women with symptoms and signs of myocardial ischemia, impaired coronary microvascular function is associated with adverse cardiovascular outcomes.
Translational Outlook:
Further studies are needed to better understand the therapeutic implications of abnormal coronary reactivity in women with myocardial ischemia.
Acknowledgments
Source of Funding: Ahmed AlBadri is supported by Ruth L. Kirschstein Institutional National Research Service Award Training Grant (T32HL007745). This work was also supported by contracts from the National Heart, Lung, and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant M01-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR001427, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, D.C., The Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California.
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health
Abbreviations:
- CAD
Coronary artery disease
- CFR
Coronary flow reserve
- CBF
Coronary blood flow
- IC
Intra-coronary
- MACE
Major adverse cardiovascular events
- WISE
Women’s Ischemia Syndrome Evaluation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000;101:1899–906. [DOI] [PubMed] [Google Scholar]
- 2.Jespersen L, Hvelplund A, Abildstrom SZ et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–44. [DOI] [PubMed] [Google Scholar]
- 3.Gulati M, Cooper-DeHoff RM, McClure C et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Archives of internal medicine 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson BD, Shaw LJ, Pepine CJ et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J 2006;27:1408–15. [DOI] [PubMed] [Google Scholar]
- 5.Pepine CJ, Anderson RD, Sharaf BL et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin FY, Shaw LJ, Dunning AM et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol 2011;58:510–9. [DOI] [PubMed] [Google Scholar]
- 7.Murthy VL, Naya M, Taqueti VR et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchthal SD, den Hollander JA, Merz CN et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 2000;342:829–35. [DOI] [PubMed] [Google Scholar]
- 9.Khuddus MA, Pepine CJ, Handberg EM et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol 2010;23:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis SE, Holubkov R, Conrad Smith AJ et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. American heart journal 2001;141:735–41. [DOI] [PubMed] [Google Scholar]
- 11.von Mering GO, Arant CB, Wessel TR et al. Abnormal Coronary Vasomotion as a Prognostic Indicator of Cardiovascular Events in Women. Circulation 2004;109:722. [DOI] [PubMed] [Google Scholar]
- 12.Johnson BD, Shaw LJ, Buchthal SD et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993–9. [DOI] [PubMed] [Google Scholar]
- 13.Shaw LJ, Merz CN, Pepine CJ et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 14.Merz CN, Kelsey SF, Pepine CJ et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol 1999;33:1453–61. [DOI] [PubMed] [Google Scholar]
- 15.Harrison DG, Bates JN. The nitrovasodilators. New ideas about old drugs. Circulation 1993;87:1461–7. [DOI] [PubMed] [Google Scholar]
- 16.Doucette JW, Corl PD, Payne HM et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation 1992;85:1899–911. [DOI] [PubMed] [Google Scholar]
- 17.Sharaf BL, Pepine CJ, Kerensky RA et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). The American journal of cardiology 2001;87:937–41; A3. [DOI] [PubMed] [Google Scholar]
- 18.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948–54. [DOI] [PubMed] [Google Scholar]
- 19.Choi BJ, Prasad A, Gulati R et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. Eur Heart J 2013;34:2047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halcox JP, Schenke WH, Zalos G et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–8. [DOI] [PubMed] [Google Scholar]
- 21.Crauwels HM, Van Hove CE, Holvoet P, Herman AG, Bult H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovascular research 2003;59:189–99. [DOI] [PubMed] [Google Scholar]
- 22.Faint RW, Mackie IJ, Machin SJ. Platelet aggregation is inhibited by a nitric oxide-like factor released from human neutrophils in vitro. British journal of haematology 1991;77:539–45. [DOI] [PubMed] [Google Scholar]
- 23.Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovascular research 1999;43:580–94. [DOI] [PubMed] [Google Scholar]
- 24.Qiang L, Tsuchiya K, Kim-Muller JY, Lin HV, Welch C, Accili D. Increased atherosclerosis and endothelial dysfunction in mice bearing constitutively deacetylated alleles of Foxo1 gene. The Journal of biological chemistry 2012;287:13944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Caterina R, Libby P, Peng HB et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. Journal of Clinical Investigation 1995;96:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89:36–44. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clinical medicine & research 2006;4:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukushima K, Javadi MS, Higuchi T et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2011;52:726–32. [DOI] [PubMed] [Google Scholar]
- 29.Murthy VL, Naya M, Foster CR et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serruys PW, di Mario C, Piek J et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation 1997;96:3369–77. [DOI] [PubMed] [Google Scholar]
- 31.Taqueti VR, Hachamovitch R, Murthy VL et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudhir K, MacGregor JS, Barbant SD et al. Assessment of coronary conductance and resistance vessel reactivity in response to nitroglycerin, ergonovine and adenosine: in vivo studies with simultaneous intravascular two-dimensional and Doppler ultrasound. J Am Coll Cardiol 1993;21:1261–8. [DOI] [PubMed] [Google Scholar]
- 33.Kurz MA, Lamping KG, Bates JN, Eastham CL, Marcus ML, Harrison DG. Mechanisms responsible for the heterogeneous coronary microvascular response to nitroglycerin. Circulation research 1991;68:847–55. [DOI] [PubMed] [Google Scholar]
- 34.Ford TJ, Stanley B, Good R et al. Stratified Medical Therapy Using Invasive Coronary Function Testing In Angina: CorMicA Trial. Journal of the American College of Cardiology 2018. [DOI] [PubMed]
- 35.Widlansky ME, Gokce N, Keaney JF Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 2003;42:1149–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.