Abstract
Objective.
To synthesize the available evidence on the use of wearable cardioverter-defibrillator (WCD).
Background.
Observational WCD studies for the prevention of sudden cardiac death (SCD) have provided conflicting data. The VEST trial was the first randomized controlled trial (RCT) showing no reduction in SCD as compared to medical therapy only.
Methods.
We searched PubMed, EMBASE, and Google Scholar for studies reporting on the outcomes of patients wearing WCD from 1/1/2001 through 03/20/2018. Rates of appropriate and inappropriate WCD therapies were pooled. Estimates were derived using DerSimonian and Laird’s method.
Results.
Twenty-eight studies were included (32,426 patients, 27 observational, 1 RCT-WCD arm). The incidence of appropriate WCD therapy was 5 per 100 persons over 3 months (95% CI 3.0, 6.0, I2= 93%). In studies on ischemic cardiomyopathy, the appropriate WCD therapy incidence was lower in VEST trial (1 per 100 persons over 3 months, 95% CI 1.0, 2.0) as compared with observational studies (11 per 100 persons over 3 months, 95% CI 11.0, 20.0, I2= 93%). The incidence of inappropriate therapy was 2 per 100 persons over 3 months (95% CI 1.0, 3.0, I2= 93%). Mortality while wearing WCD was rare; 0.7 per 100 persons over 3 months (95% CI 0.3, 1.7, I2= 94%).
Conclusion.
The rate of appropriately treated WCD patients over 3 months of follow-up was substantial; higher in observational studies as compared with the VEST trial. There was significant heterogeneity. More RCTs are needed to justify continued use of WCD in primary prevention.
Keywords: Wearable cardioverter-defibrillator, shock, death, meta-analysis, systematic review
Condensed abstract.
We conducted a systematic review and meta-analysis on the outcomes of patients prescribed wearable cardioverter-defibrillators (WCDs). We included 28 studies (32,426 patients). The pooled incidence of appropriate WCD therapy was 5 per 100 persons over 3 months. Mortality while wearing the WCD was uncommon (0.7 per 100 persons over 3 months). There was significant heterogeneity. The rate of appropriately treated WCD patients over 3 months of follow-up was substantial, much higher in observational study than the WCD-arm of the VEST trial. More RCTs are needed to justify continued use of WCD in primary prevention.
Introduction
In 2001, the United States Food and Drug Administration (FDA) approved the first wearable cardioverter-defibrillator (WCD)(1). However, until recently, there have been no randomized clinical trials (RCTs) to assess the effectiveness of this technology. ZOLL® (Pittsburgh, PA), the sole manufacturer of WCD (LifeVest®) worldwide, maintains a registry that includes patients who are prescribed the LifeVest. Most published studies are derived from this database. Current guidelines give IIa recommendation for the use of WCD in secondary prevention when ICD removal is required and IIb recommendation for all other scenarios, including primary prevention(2).
Studies on WCD have reported mixed findings(3–29). Except for the VEST (Vest Prevention of Early Sudden Death) trial(30), all these studies were observational using data provided by ZOLL(5,7,9–14,17–21,23,24,27–29). Most of the studies combined patients with different indications, making interpretation of the evidence difficult and leading to significant practice variation in prescribing WCDs. More recently, the VEST trial was the first RCT investigating the benefit of WCD in patients suffering a myocardial infarction and EF ≤ 35% as compared to medical therapy only, reporting no difference in mortality secondary to SCD(30). We performed a systematic review and meta-analysis of all published WCD literature to synthesize the available evidence on the topic in a consistent manner accounting for variation in SCD risk in different diseases.
Methods
We performed a systematic review and meta-analysis of all studies reporting on the rate of shocks delivered by a WCD, following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines(31). We searched PubMed, EMBASE, Google Scholar, and references of the included articles for relevant studies from 1/1/2001 through 3/20/2018. Two investigators (AM and AMA) performed the search independently. The search included the following key terms: “wearable cardioverter defibri llator” or “LifeVest”. We reviewed abstracts of potential studies and full texts of included studies. The WCD-arm of the VEST trial was included initially as a conference abstract presentation and later the published manuscript was reviewed(30).
All studies reporting on patients using WCD were included regardless of indication. All studies had to report on appropriate shock rates for inclusion. If two or more studies reported on the same cohort, the study with the most comprehensive evaluation was included. We included all studies that analyzed data provided by ZOLL, given that we were not able to determine which ones had overlapping data. Wherever possible, studies were subdivided into more homogenous subgroups based on indication for WCD (e.g., ischemic cardiomyopathy (ICM), non-ischemic cardiomyopathy (NICM), ICD explant).
Two investigators (AMA and MAZ) extracted the relevant data. Any disagreement in reporting was further reviewed and validated by a third investigator (AM). Data collected included study characteristics, data source (ZOLL vs. hospital-level data), financial disclosures involving ZOLL (defined as any author declaring any financial conflict of interest related to ZOLL), age, gender, left ventricular ejection fraction (LVEF), percentage of patients with ICM and NICM (NICM subdivided into idiopathic and dilated, myocarditis, peripartum, tachycardia-mediated, and other/unclassified), average daily utilization of WCD (hours/day), and follow up duration. Primary outcome was the pooled incidence of patients treated appropriately and inappropriately by WCD (i.e if a single patient received 2 shocks, this was counted as a single event under the assigned outcome of WCD therapy – a s compared to shock rate which is the actual number of shocks delivered). Secondary outcomes included the pooled incidence of death while wearing WCD, and the pooled incidence of number of shocks delivered appropriately and inappropriately by WCD.
Statistical Analysis
Continuous variables are expressed as means or medians depending upon normal or non-normal distribution, respectively. Categorical variables are expressed as percentages. The average time of follow up was taken to be the mean. When the mean was not available, the median was used. The follow-up time (person-months) was calculated by multiplying the total number of patients within each study multiplied by the mean follow up time in days and divided by 30.4. The rate of events was calculated as events/person-months and multiplied by 3 to provide the rate of events per 3 person-months. Subsequently, the rate was multiplied by a 100 for ease of interpretation. We used the term rate of events per person over 3 months interchangeably with the rate of events per 3 person-months. The standard error of the logarithm transformation of the incidence rate (r) was calculated by using the following formula: SE = Sqrt ((1-r)/c)) – where SE is standard error of the inci dence rate, r is the incidence rate, and c is the number events in the category.
We pooled the study-specific incidence rates using random-effects model meta-analysis using the DerSimonian and Laird’s method to provide a single summary estimate(32). We provided pooled estimates along with corresponding 95% confidence intervals (CIs). Event rates could not be estimated for studies with zero events, thus, these studies did not contribute to the pooled estimate within each outcome. We sub-grouped the studies based on type of cardiomyopathy, initial indication for ICD placement, whether the data came from the ZOLL database, and whether authors had financial disclosures involving ZOLL, based on the a priori hypothesis that these factors may affect reported outcomes.
We assessed heterogeneity between studies using Q and I2 statistics. The I2 statistic(33). In general, I2 values of 25% or less, 50%, and 75% or more represent low, moderate, and high level of heterogeneity, respectively. We explored sources of heterogeneity based on the apriori defined subgroups of cardiomyopathy type, ICD indications, data source, financial disclosures involving ZOLL, study design and study size. Heterogeneity was further explored by performing meta-regression of study-specific incidence estimate on average age of participants, average ejection fraction, proportion of female participants, and average daily use of WCD (in hours per day). Subsequently, we performed single study influence analysis. This was achieved by computing pooled estimates serially by omitting one study at a time and displaying the results graphically. We assessed publication bias using Egger regression test P value for funnel-plot asymmetry(34). We further assessed for presence of publication bias by subgrouping the studies based on the study-size (using arbitrary cut-off of 500 participants) and comparing the pooled estimates for the various outcomes between the larger- and the smaller-sized studies (larger studies are thought to be less susceptible to publication bias). Statistical tests were two-sided and used a significance level of p < 0.05. Analyses were conducted with Stata 13 (Stata Corp LP, College Station, Texas, USA).
Results
Study Selection
The search yielded 367 abstracts. After excluding duplicates and manuscripts that did not meet inclusion criteria, 27 studies were selected, and with the addition of the WCD arm from the VEST trial (targeting this subgroup as a prospective observational study rather than studying how it compared to the controlled arm, which was not included), a total of 28 studies were included in the final analysis (Figure S1, online supplement). The studies by Singh et al(26), Beiert et al(5), Saltzberg et al(24), and Barsheshet et al(4) were each separated into 2 indication- specific sub-groups.
Qualitative Analysis
The 28 studies enrolled 32,426 patients. All were observational in nature (21 retrospective, 6 prospective) except the WCD arm of VEST. As shown in Table 1, most studies had median age > 50 years, predominantly comprised males, included patients with LVEF <35%, had variable compliance with WCD use, and included patients with short duration of WCD use (typically < 120 days). There were 18 studies in which one or more authors declared disclosures involving ZOLL. Twenty studies either used data from the ZOLL database ZOLL or were sponsored by ZOLL (fully or partially). Only 6 studies, all published between 2015 and 2017, included independent data and had no disclosures involving ZOLL, collectively reporting on a total of 710 patients (Table 1). The VEST trial was funded by both the National Heart, Lung, and Blood Institute (NHLBI) and ZOLL. The specific indications for WCD in each study are summarized in Table 2. Few studies reported the outcomes of WCD by subgroups of ICD indication, such as primary vs secondary prevention or ischemic vs non-ischemic cardiomyopathy. Most studies reported on the incidence of referral for ICD after the WCD wear period, which ranged from 4.4% to 59% (Table S1).
Table 1.
Patients demographics and study characteristics
| Study name | Study duration |
Number of patients |
Study design | Country | Females (%) | Age (median) |
LVEF (%) | Daily use (hours/day) |
Duration of use (days) |
financial disclosures involving ZOLL |
Sponsorship/ database from ZOLL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Olgin 2018 | 2008–2017 | 1524 | Prospective | USA + Germany + Poland + Hungary |
27 | 60.9* | 28 | 14.1 | 84.3 | Yes | Yes |
| Wäßnig 2016 | 2010–2013 | 6043 | Retrospective | Germany | 21 | 57 | NR | 23.1 | 59 | Yes | Yes |
| Zishiri 2012 | 2002–2009 | 809 | Retrospective | USA | 19 | 67.6 | 25 | NR | 67 | Yes | Yes |
| Epstein 2013 | 2005–2011 | 8453 | Retrospective | USA | 27 | 62.6* | 24* | 21.8 | 57 | Yes | Yes |
| Chung 2010 | 2002–2006 | 3569 | Retrospective | USA | 26 | 59.3* | NR | 21.7 | 52.6* | No | Yes |
| Ellenbogen 2017 | 2002–2014 | 8058 | Retrospective | USA | 25 | 62* | NR | NR | 53 | Yes | Yes |
| Barraud 2017 | 2015–2016 | 24 | Prospective | France | 17 | 56* | 27* | 23.5 | 90* | No | No |
| Leyton-Mange 2017 | 2012–2013 | 147 | Retrospective | USA | 20 | 59* | 33* | 21 | 50 | No | Yes |
| Kao 2012 | 2007–2010 | 82 | Retrospective | USA | 28 | 61* | 24* | 21.8 | 79 | Yes | No |
| Lamichhane 2016 | 2007–2012 | 220 | Retrospective | USA | 33 | 55.3 | 15 | 20.4 | 394 | No | Yes |
| Bhaskaran 2015 | 2013+ | 8 | Retrospective | Australia | NR | NR | 28 | 23.4* | 77 | No | No |
| Barsheshet 2017 | NR | 75 | Prospective | USA | 31 | 51.4* | 22* | 18 | 59 | Yes | No |
| Beiert 2017 | 2012–2015 | 114 | Retrospective | Germany | 26 | 57.5 | 33 | 23.1 | 52 | No | Yes |
| Naniwadekar 2017 | 2002–2015 | 140 | Retrospective | USA | 38 | 58.2* | 28* | 17.3* | 43 | No | Yes |
| Kondo 2015 | 2010–2014 | 24 | Retrospective | Germany | 8 | 69* | 30 | 23.1 | 33 | No | No |
| Erath 2017 | NR | 130 | Prospective | Germany | 22 | 58* | 28* | 23 | 42 | Yes | Yes |
| Feldman 2003 | NR | 289 | Retrospective | USA + Germany |
18 | 55* | 23* | NR | 93* | Yes | Yes |
| Tanawuttiwat 2014 | 2005–2009 | 97 | Retrospective | USA | 20 | 65* | NR | 20 | 21 | Yes | Yes |
| Quast 2017 | 2009–2016 | 79 | Retrospective | Netherland | 23 | 54 | 25 | 23.3 | 79 | No | No |
| Duncker 2017 | 2012–2016 | 117 | Retrospective | Germany | 44 | 51* | 23* | 21* | 101* | Yes | Yes |
| Singh 2015 | 2004–2015 | 525 | Retrospective | USA | 29 | 61* | 25* | 61 | 22 | No | No |
| Sasaki 2016 | 2014–2015 | 50 | Prospective | Japan | 8 | 56 | 52 | 23.7 | 16 | No | No |
| Salehi 2016 | 2005–2012 | 127 | Retrospective | USA | 12 | 53* | 20* | 18 | 51 | Yes | Yes |
| Erath 2017 | 2012–2015 | 102 | Prospective | Germany | 28 | 59* | 30* | 23 | 54 | Yes | Yes |
| Opreanu 2015 | 2004–2011 | 121 | Retrospective | USA | 31 | 45* | 25* | 20 | 39 | Yes | Yes |
| Saltzberg 2012 | 2003–2009 | 266 | Retrospective | USA | 100 | 32* | 21.5* | 20 | 81 | Yes | Yes |
| Kutyifa 2015 | 2011–2014 | 2000 | Prospective | USA | 30 | 62 | 25 | 22.5 | 90 | Yes | Yes |
| Duncker 2017 | 2011–2016 | 49 | Retrospective | Germany | 100 | 33* | 21* | 21.4* | 120* | Yes | No |
Mean values. NR: not reported
Table 2.
Indications for WCD usage across the included articles
| Study name | Number of patients |
Secondary prevention |
ICD Explant | ICM | NICM | NICM Subgroups | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Idiopathic /DCM | PPCM | Myocarditis | TCM | Others | ||||||
| Olgin 2018 | 1524 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wäßnig 2016 | 6043 | NR | 11.9% | 26.9% | 61.2% | 79.8% | 0.0% | 16.1% | 0.0% | 4.1% |
| Zishiri 2012 | 809 | NR | 0.0% | 100% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Epstein 2013 | 8453 | NR | 0.0% | 100% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Chung 2010 | 2731 | 16.0% | 23.4% | 25.2% | 35.4% | 79.5% | NR | NR | NR | 20.5% |
| Ellenbogen 2017 | 8058 | NR | 100% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Barraud 2017 | 24 | NR | 0.0% | 100% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Leyton-Mange 2017 | 147 | 21.8% | 23.1% | 22.4% | 32.7 | 77.1% | NR | 2.1% | 0.0% | 20.8% |
| Kao 2012 | 80 | NR | 0.0% | 36.3% | 63.7% | 60.8% | 2.0% | 4.0% | NR | 33.2% |
| Lamichhane 2016 | 220 | NR | 0.0% | 35.5% | 64.5% | 95.8% | NR | NR | NR | 4.2% |
| Bhaskaran 2015 | 8 | 0.0% | 37.5% | 0.0% | 62.5% | 40.0% | 20.0% | 20.0% | 0.0% | 20.0% |
| Barsheshet 2017 | 75 | NR | NR | 33.3% | 66.7% | NR | NR | NR | NR | NR |
| Beiert 2017 | 114 | NR | 11.4% | 31.6% | 57.0% | 80.0% | NR | NR | NR | 20.0% |
| Naniwadekar 2017 | 140 | 9.0% | 8.0% | 32.0% | 51.0% | 90.1% | NR | NR | NR | 9.9% |
| Kondo 2015 | 24 | 54.2% | 0.0% | 45.8% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Erath 2017 | 130 | NR | 0.0% | 35.4% | 64.6% | 54.8% | NR | 11.9% | 22.6% | 10.7% |
| Feldman 2003 | 289 | NR | NR | 39% | Unclear† | NR | NR | NR | NR | NR |
| Tanawuttiwat 2014 | 97 | NR | 100% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Quast 2017 | 79 | NR | 41.8% | 15.2% | 43.0% | NR | NR | NR | NR | NR |
| Duncker 2017 | 117 | NR | 0.0% | 0.0% | 100.0% | 77.8% | 17.1% | 5.1% | 0.0% | 0.0% |
| Singh 2015 | 525 | NR | 0.0% | 51.6% | 48.4% | 81.4% | 0.4% | 0.0% | 15.4% | 2.8% |
| Sasaki 2016 | 50 | 76.0% | 0.0% | 12.0% | 12.0% | 0.0% | 0.0% | 0.0% | 0.0% | 100.0% |
| Salehi 2016 | 127 | NR | 0.0% | 0.0% | 100% | 100% | 0.0% | 0.0% | 0.0% | 0.0% |
| Erath 2017 | 102 | NR | 24.5% | 26.5% | 49.0% | 66.0% | 4.0% | 18.0% | 0.0% | 12.0% |
| Opreanu 2015 | 121 | NR | 0.0% | 17.4% | 82.6% | 67.0% | 0.0% | 0.0% | 0.0% | 33.0% |
| Saltzberg 2012 | 266 | 0.0% | 0.0% | 0.0% | 100% | 59.8% | 40.2% | 0.0% | 0.0% | 0.0% |
| Kutyifa 2015 | 2000 | NR | NR | 40.0% | 60.0% | 77.6% | NR | NR | NR | 22.4% |
| Duncker 2017 | 49 | 0.0% | 0.0% | 0.0% | 100% | 0.0% | 100% | 0.0% | 0.0% | 0.0% |
NICM: Non-ischemic cardiomyopathy; ICM: Ischemic cardiomyopathy; PPCM: Peripartum cardiomyopathy; DCM: dilated cardiomyopathy; TCM: tachycardia-induced cardiomyopathy; ICD: implantable cardioverter defibrillator; NR: not reported
It is unclear if the 61% of subjects with heart failure in the WEARIT sub-study had non-ischemic cardiomyopathy.
Quantitative Analysis
Overall analysis.
The overall pooled incidence of appropriate WCD treatment was 5 per 100 persons over 3 months (95% CI 3.0, 6.0; I2=93.1%, p<0.001), Figure 1. The pooled incidence rate for appropriate WCD shock was 7 per 100 persons over 3 months (95% CI 5.0, 9.0; I2=95.9%, p<0.001), for inappropriately treated patients was 2 per 100 persons over 3 months (95% CI 2.0, 4.0; I2=90.3%, p<0.001), and for inappropriate WCD shock was 2 per 100 persons over 3 months (95% CI 1.0, 4.0, I2=93.7%, p<0.001) Figures S2, S3 and S4 (Online Supplement), respectively. Death while wearing the WCD was relatively low; the pooled incidence was 0.7 per 100 persons over 3 months (95% CI 0.3, 1.7, I2=94%, p<0.001). There was significant qualitative and quantitative heterogeneity in all the analyses.
Figure 1.
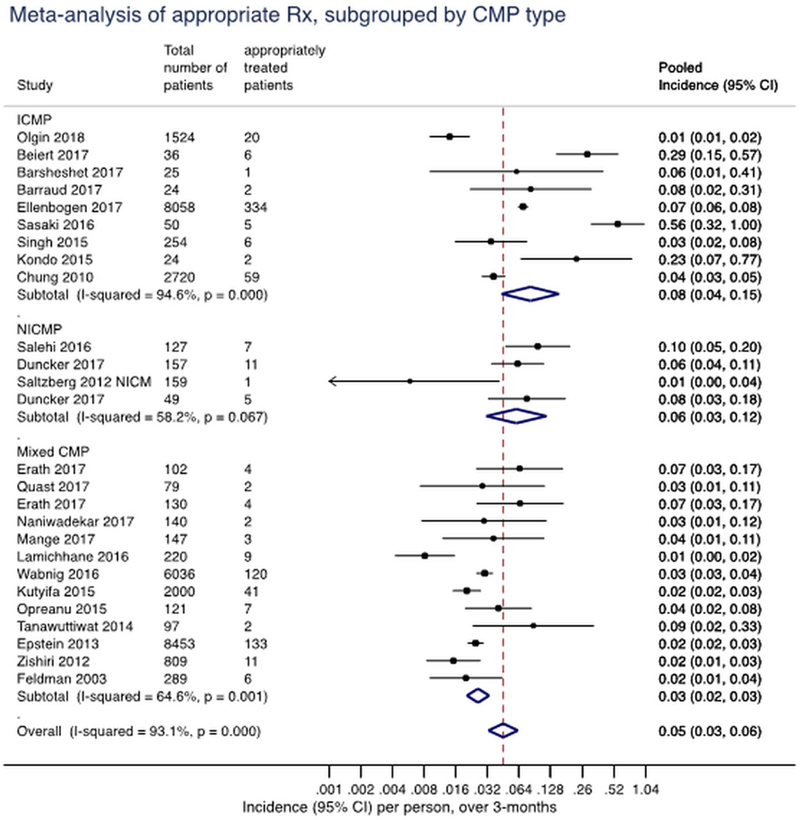
Pooled incidence rate per 1 person over 3 months of appropriately treated patients while wearing the wearable cardioverter-defibrillator. The vertical line represents the summary pooled estimate across all studies shown. Multiply rate by a 100 to get incidence rate per 100 persons over 3 months. ICMP: ischemic cardiomyopathy; NICMP: non-ischemic cardiomyopathy; mixed: mixed indication for the wearable cardioverter-defibrillator; CI: confidence interval. Event rates could not be estimated for studies with zero events (Bhaskaran 2015, Kao 2012, Saltzberg 2012 PPCMP), thus, these studies did not contribute to the pooled estimate within each outcome.
Exploring heterogeneity
Etiology of cardiomyopathy
The studies were sub-grouped into ICM, NICM, or mixed based on the etiology of cardiomyopathy provided in the reports (Figure 2). There was significant difference in the pooled incidence rates of appropriately treated patients with ICM (8 per 100 persons over 3 months, 95% CI 4.0, 15.0), NICM (6 per 100 persons over 3 months, 95% CI 3.0, 12.0), or mixed indication (3 per 100 persons over 3 months, 95% CI 3.0, 4.0; p=0.05). The interaction between cardiomyopathy sub-group and incidence rate of inappropriately treated patients, failed shock, and death while wearing WCD are presented in Figure 2. As compared to observational studies, the WCD arm of VEST reported lower average daily compliance (wear-time) of the WCD and had the lowest incidence of appropriately treated patients compared to the pooled ICM cohorts (1 per 100 persons over 3 months as compared to 11 per 100 persons over 3 months; Figure 1).
Figure 2.
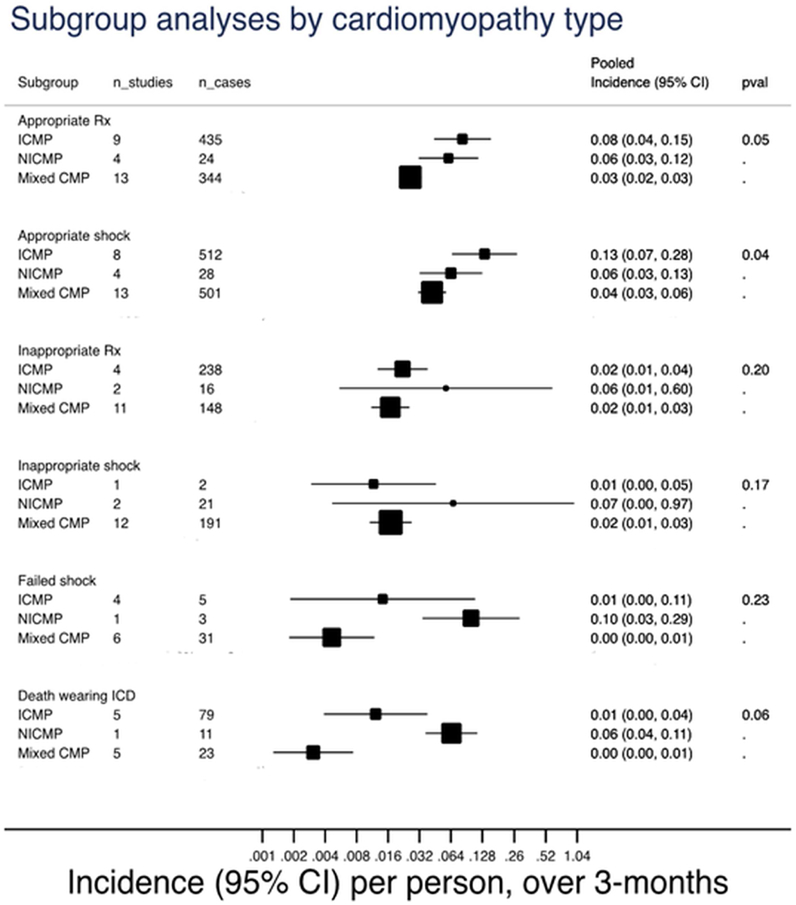
Pooled incidence rate per 1 person over 3 months of appropriately and inappropriately treated patients, appropriate and inappropriate shock, failed shock, and death while wearing the wearable cardioverter-defibrillator, stratified by type of cardiomyopathy into ischemic cardiomyopathy, non-ischemic cardiomyopathy, and mixed indications. Multiply rate by a 100 to get incidence rate per 100 persons over 3 months. Rx: treatment; ICMP: ischemic cardiomyopathy; NICMP: non-ischemic cardiomyopathy; mixed: mixed indication for the wearable cardioverter-defibrillator; WCD: wearable cardioverter-defibrillator; CI: confidence interval; n_studies: number of studies included; n_cases: total number of events in the included studies.
Primary vs. secondary prevention
Most studies had mixed indications without explicit reporting of event rates based on primary vs secondary prevention strategies. Thus, we stratified studies based on the indication for a WCD into studies that included patients (irrespective of the percentage of patients with that indication) referred for primary prevention, secondary prevention, or ICD explants. The pooled incidence rates did not vary significantly by ICD indication (Figure 3).
Figure 3.
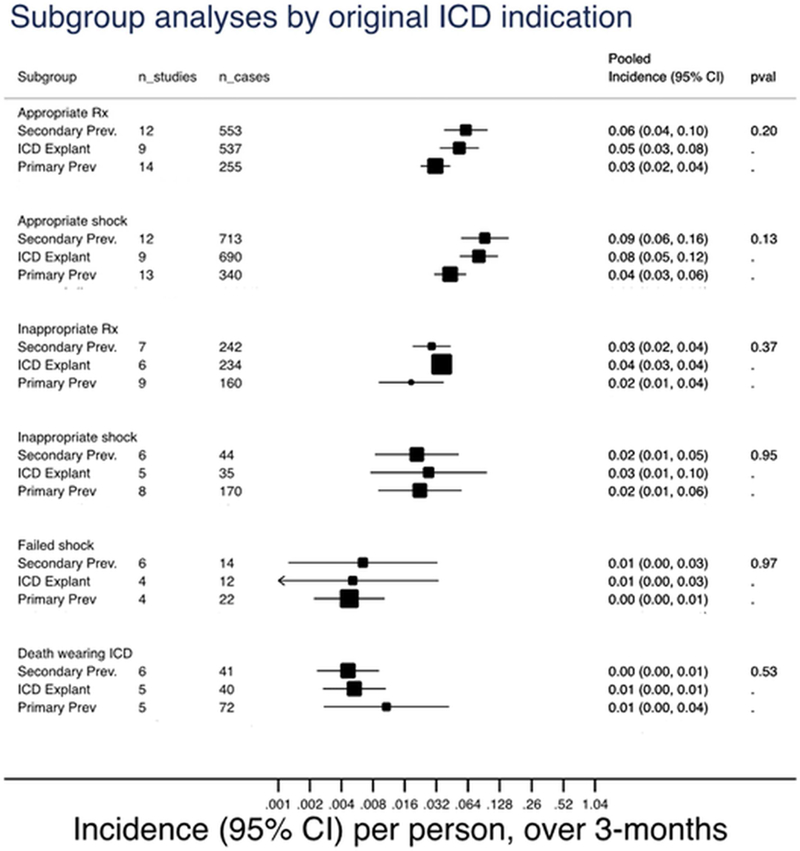
Pooled incidence rate per 1 person over 3 months of appropriately and inappropriately treated patients, appropriate and inappropriate shock, failed shock, and death while wearing the wearable cardioverter-defibrillator, stratified by primary prevention, secondary prevention, and internal cardioverter-defibrillator explant. Multiply rate by a 100 to get incidence rate per 100 persons over 3 months. Rx: treatment; prev: prevention; ICD: internal cardioverter-defibrillator; WCD: wearable cardioverter-defibrillator; CI: confidence interval; n_studies: number of studies included; n_cases: total number of events in the included studies.
Further heterogeneity analyses
Subgrouping studies based on the use of ZOLL vs institutional data, authors’ financial disclosures involving ZOLL vs. none, and prospective vs. retrospective study design did not show different results of all prespecified outcomes; summary rates are presented in Figures S5–7 (Online Supplement), respectively. Meta-regression analysis showed that the incidence of appropriately treated patients varied only by LVEF (continuous variable) of the included studies, in that higher LVEF was associated with greater incidence of appropriately treated patients. LVEF explained 54% of the variability in the incidence of appropriately treated patients (R2 = 0.54, p-value <0.01; Figure S8-A, Online Supplement). However, when the outlier study with an LVEF of 52% (75% of patients were for secondary prevention) was excluded(25), the association between higher LVEF and greater incidence of appropriately treated patients persisted but was weakened (R2 = 0.19, p-value=0.04; Figure S8-B, Online Supplement). Treatment effect did not vary by the other tested factors, such as age, female sex, and average daily use. In influence analysis, we did not identify any single study which significantly influenced overall pooled estimate (Figures S9 A-C, Online Supplement).
Publication bias.
There was no evidence of publication bias in studies evaluating the incidence rate of appropriately treated patients (p-value=0.777, Figure S10, Online Supplement). Similarly, there was no evidence of publication bias when the outcomes of appropriate shocks, inappropriately treated patients, or inappropriate shocks were evaluated. Furthermore, larger and smaller studies generally yielded comparable pooled estimates, indicating lower likelihood of publication bias.
Discussion
In this systematic review and meta-analysis of studies reporting on the use of WCD, we found that the rate of appropriately treated patients was 5 per 100 persons over 3 months, while the rate of inappropriately treated patients was 2 per 100 persons over 3 months for all indications. Restricting analysis to studies reporting only on ICM showed a higher rate of appropriately treated patients as compared with NICM (8 vs 6 per 100 persons over 3 months, respectively). Findings were similar if studies were separated based on primary vs secondary prevention indication, financial disclosures involving ZOLL, and the source database used. Only 6 studies had no author financial disclosures involving ZOLL and were not sponsored or used data provided by ZOLL(3,6,16,22,25,26). Those 6 studies totaled 710 patients, of which 74% were from a single center study. However, on subgroup analyses, there was no difference in the outcome based on data being provided by ZOLL or having financial disclosures involving ZOLL. In the NICM sub-group, the pooled event rate is likely an over-estimation, given that 4 out of 8 studies had a zero-event rate and thus were not calculated into the pooled estimate(4,5,24,26).
Our study puts into perspective the overall published evidence evaluating WCD use. All studies were observational except VEST, in which the included WCD group was part of an interventional RCT(30). Qualitative analysis shows that most studies were not indication-specific, thus diluting our knowledge on the indication-specific utility of WCD and in which patients it should be best used. Selection bias and including mixed indications in observational studies was likely the major determinant of the higher rate of appropriate treatment in patients prescribed a WCD as compared with the WCD arm of the VEST trial(30). This was also evidenced by the results of meta-regression showing a higher incidence rate of appropriate WCD therapy in patients with higher LVEF.
In 2001, the FDA approved the first WCD manufactured by Lifecor (later acquired by ZOLL) based on 2 multi-center prospective observational studies (WEARIT and BIROAD), which enrolled 289 patients(35). Both studies were grouped into one analysis based on FDA request, with each study treated as a subgroup. Over 901 patient-months, 6 out of 8 episodes of VT/VF were successfully treated by the WCD(35). This was compared to historical controls who suffered SCD at home and called emergency services, in whom successful SCD resuscitation was 25%(35). FDA concluded that the WCD device had greater efficacy than bystander resuscitation in the historical control group(1). Besides the flaws of the design and the use of historical controls; only 27% of patients in WEARIT were taking a beta-adrenergic antagonist, 34% were on anti-arrhythmic medications, and 45% were on inotropes. As such, the patients included in those studies do not represent the patients who are currently being prescribed WCD while on optimal medical therapy during the mandated waiting period prior to ICD consideration(2).
The recently published NHLBI and ZOLL-sponsored randomized VEST (Vest Prevention of Early Sudden Death) Trial would be the first RCT in 17 years testing WCD efficacy by randomizing patients post myocardial infarction with LVEF ≤35% to WCD or usual care. However, there was slow enrollment into the trial (2008 – 2017), leading to a change in the primary end-point from all-cause mortality to the less clinically relevant endpoint of SCD, which allowed for a decrease in sample size(30).The primary endpoint that the study was powered for (i.e. SCD) was not different between WCD + medical therapy arm (1.6%) vs. medical therapy only arm (2.4%), p=0.18. The secondary endpoint of all-cause mortality was advertised during the trial presentation to be lower in the WCD group as compared with no-WCD (3.1% vs 4.9%, p=0.04) which appeared to be driven partly by lower stroke rate in the WCD (monitored for atrial fibrillation in an open label study) group. However, the trial was not powered for all-cause mortality and multiple-comparisons correction, such as Bonferroni correction, was not presented(30).
The WCD is one example in which evidence-based practice falls short. In certain practices, the WCD has become the de facto standard of care for patients post MI with an EF ≤ 35% during the mandated 3 months waiting period for an ICD implantation for primary prevention. An online report in 2015 stated that >200,000 WCD have been prescribed(36). This practice pattern is likely driven by the finality of SCD and partly by fear of litigation, despite the absence of data to support it. IRIS and DINAMIT both showed no overall mortality benefit to early ICD implantation(37,38) and DANISH showed no benefit of ICD on overall mortality over 5.6 years follow-up in NICM(39). The primary finding of our study is that the available evidence from observational studies is fraught with poor methodology, selection bias, and confounding concerns. The available evidence from the VEST trial shows that the rate of appropriate treatment by WCD was low (1 in 100 persons over 3 months) and that WCD was not associated with a decreased risk of SCD(30). These findings suggest that WCD should not be used in primary prevention until further RCT data support its use.
Our study has several limitations. First, this was a systematic review and meta-analysis of study-level data, and the authors did not have access to patient-level data. Second, studies were heterogenous with variable inclusion criteria and mostly mixed indications. Third, studies that used the database sponsored by ZOLL had overlapping data that made it impossible to identify which patients or cohort were reported on repeatedly. Fourth, we did not have data on the specific financial disclosures involving ZOLL. Fifth, the event rate of appropriate shock (i.e. aborted SCD) was low, rendering point estimates imprecise. Sixth, given that the rates of WCD therapy in observational studies were higher than the only RCT (VEST); casual inference based on these data is not possible.
Conclusion.
In this systematic review and meta-analysis of studies on the use of WCD, the rate of appropriately treated patients over 3 months of follow-up was substantial and higher in observational studies as compared with the WCD arm of the VEST trial. There was significant heterogeneity across the studies, and most of the studies included data provided by the WCD manufacturer or had financial disclosures involving ZOLL. Our analyses highlight the limitations of the published data that justified the continued use of WCD for years. More RCT data, including cost analyses, are needed to justify the continued use of WCD in primary prevention.
Supplementary Material
Acknowledgments
Funding: Ahmad Masri is supported by a research training grant from the National institute of Health (T32HL129964–02).
Abbreviations List
- FDA
Food and Drug Administration
- WCD
Wearable cardioverter-defibrillator
- RCTs
Randomized clinical trials
- ICD
Implantable cardioverter-defibrillator
- SCD
Sudden cardiac death
- LVEF
Left ventricular ejection fraction
- ICM
Ischemic cardiomyopathy
- NICM
Non-ischemic cardiomyopathy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Perspectives.
Competency in Patient Care. Observational studies on WCDs included mixed indications and high-risk patients. There was a high rate of appropriate and inappropriate WCD shock over 3 months, while mortality was uncommon. The rate was much higher in observational studies as compared to the only randomized trial to date.
Translational Outlook. More randomized, indication-specific clinical trials are needed to address the efficacy and cost-effectiveness of WCD and to justify its continued use.
Conflict of interest: Samir Saba has research support from Medtronic and Boston Scientific. Sandeep Jain and Evan Adelstein have research support from Medtronic. Other co-authors have no conflict of interest to declare.
References
- 1.https://www.accessdata.fda.gov/cdrh_docs/pdf/p010030b.Pdf. Accessed Nov 13th, 2018.
- 2.Al-Khatib SM, Stevenson WG, Ackerman MJ et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017. [DOI] [PubMed]
- 3.Barraud J, Pinon P, Laine M et al. Ventricular Arrhythmia Occurrence and Compliance in Patients Treated With the Wearable Cardioverter Defibrillator Following Percutaneous Coronary Intervention. Heart Lung Circ 2017. [DOI] [PubMed]
- 4.Barsheshet A, Kutyifa V, Vamvouris T et al. Study of the wearable cardioverter defibrillator in advanced heart-failure patients (SWIFT). J Cardiovasc Electrophysiol 2017;28:778–784. [DOI] [PubMed] [Google Scholar]
- 5.Beiert T, Malotki R, Kraemer N et al. A real world wearable cardioverter defibrillator experience - Very high appropriate shock rate in ischemic cardiomyopathy patients at a European single-center. J Electrocardiol 2017;50:603–609. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskaran A, Bartlett M, Kovoor P, Davis LM. The Wearable Cardioverter Defibrillator: an Early Single Centre Australian Experience. Some Pitfalls and Caveats for Use. Heart Lung Circ 2016;25:155–9. [DOI] [PubMed] [Google Scholar]
- 7.Chung MK, Szymkiewicz SJ, Shao M et al. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol 2010;56:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncker D, Konig T, Hohmann S, Bauersachs J, Veltmann C. Avoiding Untimely Implantable Cardioverter/Defibrillator Implantation by Intensified Heart Failure Therapy Optimization Supported by the Wearable Cardioverter/Defibrillator-The PROLONG Study. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed]
- 9.Duncker D, Konig T, Hohmann S, Bauersachs J, Veltmann C. Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study. Clin Cardiol 2017;40:586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellenbogen KA, Koneru JN, Sharma PS, Deshpande S, Wan C, Szymkiewicz SJ. Benefit of the Wearable Cardioverter-Defibrillator in Protecting Patients After Implantable-Cardioverter Defibrillator Explant. JACC: Clinical Electrophysiology 2017;3:243–250. [DOI] [PubMed] [Google Scholar]
- 11.Epstein AE, Abraham WT, Bianco NR et al. Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J Am Coll Cardiol 2013;62:2000–2007. [DOI] [PubMed] [Google Scholar]
- 12.Erath JW, Vamos M, Benz AP, Hohnloser SH. Usefulness of the WCD in patients with suspected tachymyopathy. Clin Res Cardiol 2017. [DOI] [PubMed]
- 13.Erath JW, Vamos M, Sirat AS, Hohnloser SH. The wearable cardioverter-defibrillator in a real-world clinical setting: experience in 102 consecutive patients. Clin Res Cardiol 2017;106:300–306. [DOI] [PubMed] [Google Scholar]
- 14.Feldman AM, Klein H, Tchou P et al. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol 2004;27:4–9. [DOI] [PubMed] [Google Scholar]
- 15.Kao AC, Krause SW, Handa R et al. Wearable defibrillator use in heart failure (WIF): results of a prospective registry. BMC cardiovascular disorders 2012;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo Y, Linhart M, Andrie RP, Schwab JO. Usefulness of the wearable cardioverter defibrillator in patients in the early post-myocardial infarction phase with high risk of sudden cardiac death: A single-center European experience. J Arrhythm 2015;31:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutyifa V, Moss AJ, Klein H et al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation 2015;132:1613–9. [DOI] [PubMed] [Google Scholar]
- 18.Lamichhane M, Gardiner JC, Bianco NR, Szymkiewicz SJ, Thakur RK. National experience with long-term use of the wearable cardioverter defibrillator in patients with cardiomyopathy. J Interv Card Electrophysiol 2017;48:11–19. [DOI] [PubMed] [Google Scholar]
- 19.Leyton-Mange JS, Hucker WJ, Mihatov N et al. Experience With Wearable Cardioverter-Defibrillators at 2 Academic Medical Centers. JACC: Clinical Electrophysiology 2017. [DOI] [PubMed]
- 20.Naniwadekar A, Alnabelsi T, Joshi K, Obasare E, Greenspan A, Mainigi S. Real world utilization and impact of the wearable cardioverter-defibrillator in a community setting. Indian Pacing Electrophysiol J 2017;17:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opreanu M, Wan C, Singh V et al. Wearable cardioverter-defibrillator as a bridge to cardiac transplantation: A national database analysis. J Heart Lung Transplant 2015;34:1305–9. [DOI] [PubMed] [Google Scholar]
- 22.Quast A, van Dijk VF, Wilde AAM, Knops RE, Boersma LVA. Outpatient treatment with the wearable cardioverter defibrillator: clinical experience in two Dutch centres. Neth Heart J 2017;25:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi N, Nasiri M, Bianco NR et al. The Wearable Cardioverter Defibrillator in Nonischemic Cardiomyopathy: A US National Database Analysis. Can J Cardiol 2016;32:1247 e1–1247 e6. [DOI] [PubMed] [Google Scholar]
- 24.Saltzberg MT, Szymkiewicz S, Bianco NR. Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J Card Fail 2012;18:21–7. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki S, Shoji Y, Ishida Y et al. Potential roles of the wearable cardioverter-defibrillator in acute phase care of patients at high risk of sudden cardiac death: A single-center Japanese experience. J Cardiol 2017;69:359–363. [DOI] [PubMed] [Google Scholar]
- 26.Singh M, Wang NC, Jain S, Voigt AH, Saba S, Adelstein EC. Utility of the Wearable Cardioverter-Defibrillator in Patients With Newly Diagnosed Cardiomyopathy: A Decade-Long Single-Center Experience. J Am Coll Cardiol 2015;66:2607–2613. [DOI] [PubMed] [Google Scholar]
- 27.Tanawuttiwat T, Garisto JD, Salow A et al. Protection from outpatient sudden cardiac death following ICD removal using a wearable cardioverter defibrillator. Pacing Clin Electrophysiol 2014;37:562–8. [DOI] [PubMed] [Google Scholar]
- 28.Wassnig NK, Gunther M, Quick S et al. Experience With the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation 2016;134:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zishiri ET, Williams S, Cronin EM et al. Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ Arrhythm Electrophysiol 2013;6:117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olgin JE, Pletcher MJ, Vittinghoff E et al. Wearable Cardioverter-Defibrillator after Myocardial Infarction. The New England journal of medicine 2018;379:1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT GSe. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from http://handbook-5-1.cochrane.org. Accessed Nov 13th, 2018. [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldman AM, Klein H, Tchou P et al. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol 2004;27:4–9. [DOI] [PubMed] [Google Scholar]
- 36.https://www.asahi-kasei.co.jp/asahi/jp/ir/library/business/pdf/150311.pdf. Accessed Nov 13th, 2018
- 37.Steinbeck G, Andresen D, Seidl K et al. Defibrillator implantation early after myocardial infarction. The New England journal of medicine 2009;361:1427–36. [DOI] [PubMed] [Google Scholar]
- 38.Hohnloser SH, Kuck KH, Dorian P et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. The New England journal of medicine 2004;351:2481–8. [DOI] [PubMed] [Google Scholar]
- 39.Kober L, Thune JJ, Nielsen JC et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. The New England journal of medicine 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


