Abstract
Background/Purpose
Many survivors of childhood cancer will experience premature gonadal insufficiency or infertility as a consequence of their medical treatments. Ovarian tissue cryopreservation (OTC) remains an experimental means of fertility preservation with few reports focused on the surgical technique and postoperative outcomes for OTC in children.
Methods
This is a single institution, retrospective review of OTC cases from January 2011 to December 2017. Children were eligible for OTC if they had a greater than 80% risk of premature ovarian insufficiency or infertility owing to their anticipated gonadotoxic medical treatment.
Results
OTC was performed in 64 patients. Median age was 12 years old (range: 5 months-23 years). Nearly half (48%) of the patients were premenarchal. Laparoscopic unilateral oophorectomy was performed in 84% of patients. There were no surgical complications. In 76% of patients, OTC was performed in conjunction with an ancillary procedure. The majority (96%) of patients were discharged within 24 hours. Median time from operation to medical therapy was six days, with no unanticipated treatments delays attributable to OTC.
Conclusions
Laparoscopic unilateral oophorectomy for OTC can be performed safely, in combination with other ancillary procedures, as an outpatient procedure without delaying medical therapy for children facing a fertility-threatening diagnosis or treatment.
Level of Evidence
IV.
Keywords: Ovarian tissue cryopreservation, Children, Laparoscopy, Oophorectomy, Fertility preservation
Survival rates for pediatric cancer have remarkably improved in recent decades, with mortality decreasing 50% between 1975 and 2010. Today, children who receive a new cancer diagnosis can expect an 80% five-year survival rate [1]. As mortality rates have decreased, emphasis has turned to improving the quality of life for survivors. Many survivors of childhood cancer and/or children with chronic medical conditions experience premature gonadal insufficiency or infertility as a consequence of their medical treatments and may be candidates for fertility preservation (FP) as part of their comprehensive care [2, 3].
Multiple medical societies have recognized FP as an important quality of life issue for adult survivors of childhood cancer [4–6]. Unfortunately, established, non-experimental FP methods, such as embryo and oocyte cryopreservation, have many limitations in both prepubertal and postpubertal pediatric patients. Currently, ovarian tissue cryopreservation (OTC) is the only pretreatment FP option for prepubertal children who require gonadotoxic treatment but is considered experimental by the American Society of Reproductive Medicine and the American Academy of Pediatrics. OTC requires institutional review board (IRB) approval before offering it as ameans of FP [4–6]. Unlike embryo/oocyte cryopreservation, it does not require hormone stimulation prior to tissue removal and can be performed without significantly delaying medical therapy. Current literature reports more than 130 live-births from orthotopic transplantation of ovarian cortical tissue that was harvested in adulthood [7]. Recently, two live-births have been reported after transplantation of thawed cortical tissue that was frozen in prepubertal children [8, 9].
To date, there has been no standard operative technique described for obtaining ovarian cortical tissue for cryopreservation. Reported methods for FP include ovarian cortical biopsies, unilateral hemi-oophorectomy, bilateral hemi-oophorectomy, and unilateral salpingo-oophorectomy [10]. Few publications are dedicated to the operative technique and outcomes of pediatric patients undergoing FP surgery. Therefore, our goal is to review our institution’s experience with OTC and report on our surgical outcomes for laparoscopic unilateral oophorectomy for FP.
1. Methods
1.1. Study protocol
A retrospective review of the Ann and Robert H. Lurie Children’s Hospital of Chicago’s (Lurie Children’s) Fertility and Hormone Preservation and Restoration Program’s surgical cohort was conducted. (IRB # 2016-609) All OTC cases were reviewed between January 2011 and December 2017. The current IRB-approved prepubertal and postpubertal OTC protocols (IRB # 2014–15,534, #2011–14,420) were adopted from the Oncofertility Consortium’s OTC protocol for adult women. Our institution is a member of The National Physicians Cooperative’s Pediatric Initiative Network, which is a nationwide network of pediatric centers dedicated to providing access to the most up-to-date FP treatments and clinical research for children.
To qualify for the study, a patient must have a planned treatment that places her at greater than 80% risk of premature ovarian insufficiency (Table 1). Patients who meet inclusion criteria receive a comprehensive FP consultation prior to OTC. Operative consent is signed for the surgical procedure and research consent is obtained for the cryopreservation of the patient’s ovarian tissue. We also ask for a signed research assent from children 12 to 17 years of age.
Table 1.
Inclusion criteria for IRB-approved ovarian tissue cryopreservation protocol.
General Criteria:
|
|
Oncology/Medical Therapies: Radiation Therapy
|
Alkylating agent dose (AAD): a score of 1, 2, or 3 is given depending on the tertile the alkylating agent falls within.
Cyclophosphamide equivalent dose (CED): risk-stratification calculation according to dosages of alkylating agents received.
1.2. Preoperative preparation
Currently, we collect preoperative serum reproductive biomarkers (anti-mullerian hormone, luteinizing hormone, follicle stimulating hormone, and estradiol) in all candidates receiving OTC. In compliance with Food and Drug Administration (FDA) regulations, an infectious disease panel is also collected for long-term reproductive tissue storage purposes. No imaging studies specific to OTC are required before surgery.
Efforts are made to coordinate other invasive procedures with the oophorectomy in order to limit the child’s exposure to repeated anesthetics. Central venous catheters are placed by the pediatric surgeon performing the oophorectomy, while bone marrow aspirations/biopsies and lumbar punctures are performed by the oncology staff.
1.3. Operative procedure
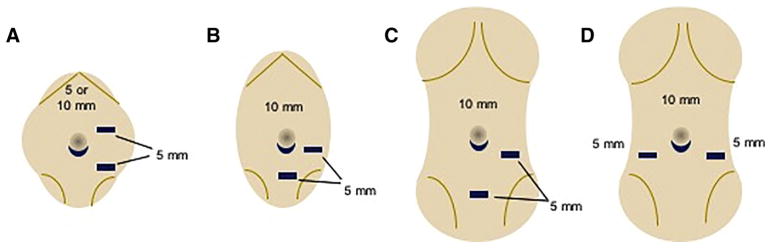
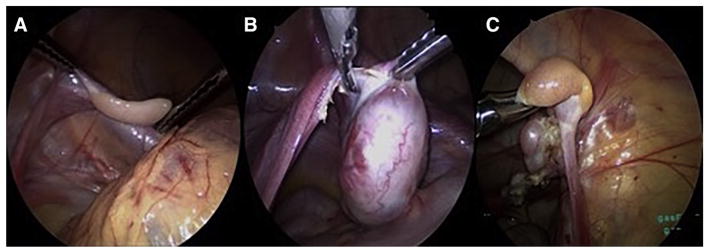
The patient is placed in the supine position in the operating room. Infra-umbilical access is obtained using a Veress needle and pneumoperitoneum achieved. A 10-mm trocar is placed through the inferior umbilical fold followed by a 5-mm 30° scope (Karl Storz Endoscopy-America) to inspect the abdomen and pelvis. Two additional 5-mm working ports are placed under direct visualization in one of the depicted configurations (Fig. 1). Blunt graspers (5-mm, Karl Storz Endoscopy-America) are then used to expose and inspect the uterus and ovaries for masses or cysts. If the patient will receive pelvic radiation therapy, the ovary that will be exposed to the greatest radiation dose may be chosen for removal; otherwise, the right ovary is preferred since it is further away from the sigmoid colon. Once the appropriate ovary is identified, the dissection begins by opening the mesovarium medially, adjacent to the uterus, using an ultrasonic energy device (Harmonic ACE+Shear, Ethicon, Cincinnati, Ohio) (Fig. 2). The dissection continues laterally across the mesovarium taking care to maintain a rim of tissue 2–3 mm from the ovarian capsule. During the dissection, care is taken to preserve the ipsilateral fallopian tube and to use a ‘no-touch’ technique with minimal manipulation of the ovarian capsule, since the majority of the primordial follicle reserve is located just deep to the capsule. This requires maintaining a rim of tissue on the ovarian capsule so that the capsule itself is not grasped. The ovarian artery within the infundibulopelvic ligament is divided as the last step of the oophorectomy to minimize the warm ischemia time of the ovary (Fig. 2). Once the vasculature is divided, the specimen is placed in an extraction bag and withdrawn carefully through the umbilical trocar site. The fascial incision is extended, as needed, to prevent crush injury to the ovary as it is being extracted. A small 3–5 mm piece of ovary is sent for routine pathologic evaluation, while the remaining tissue is immediately placed in holding media (Sage/Origio ART-8040) and sent to the andrology lab at Northwestern Medicine for cryopreservation.
Fig. 1.
Suggested port placement for laparoscopic unilateral oophorectomy for ovarian tissue cryopreservation by age category a Infant, b prepubertal, c/d adolescent/young adult.
Fig. 2.
Intraoperative images during laparoscopic unilateral oophorectomy for ovarian tissue cryopreservation a Display of a prepubertal ovary, b division of the mesovarium from medial to lateral in a post-pubertal patient, c division of the vascular pedicle in a prepubertal patient.
1.4. Cryopreservation/pathologic evaluation
The ovarian cortex is located just deep to the capsule and is where the majority of the primordial follicles reside. These primordial follicles represent the child’s ovarian reserve. The tissue cryopreservation technique is detailed on oncofertility.northwestern.edu using cryopreservation media (Sage/Origio ART-8050). Briefly, the cortex is dissected from the medulla and cut into cortical strips, washed to remove blood cells, and then passed through a series of cryopreservation media. The processed strips are placed in cryovials and undergo a slow-freeze process to −40 °C. The tissues are then transported to a long-term reproductive tissue storage facility in liquid nitrogen. The intraoperative biopsy undergoes pathology evaluation for the presence and stage of ovarian follicles, and the presence or absence of malignancy. If evidence of gross solid tumor is found within the ovary, the cryopreserved tissue is recalled for further pathologic staging and is not stored for the patient’s use. For patients with acute lymphoblastic leukemia or lymphoma, there is a chance that the ovarian stroma may contain malignant cells [11]. Since the ovarian tissue is not required for further pathologic staging, the tissue may be cryopreserved for the patient’s use with the understanding that it cannot be transplanted back into the patient. However, future fertility restoration may be possible through follicle isolation, in vitro follicle maturation, and in vitro fertilization.
1.5. Postoperative course
Children that undergo laparoscopy are expected to have a same-day discharge from the hospital. Chemotherapy typically begins three days after surgery, while radiation therapy is recommended to start seven to fourteen days after the operation. Initiation of radiation therapy depends on the size and location of the radiation field. If there are no postoperative complications, medical therapy proceeds as described above. All cryopreserved tissue is held at a long-term reproductive tissue storage facility. In the event that a child dies before the age of 18 or is able to achieve spontaneous puberty and/or pregnancy without utilizing the cryopreserved cortical tissue, her tissue can be donated to research or discarded. The tissue cannot be designated for use by another individual.
2. Results
2.1. Patient demographics/characteristics
Between January 2011 and December 2017, 64 patients underwent OTC. Median age was 12 years old (range: 5 months-23 years). Thirty-one (48%) patients were premenarchal.
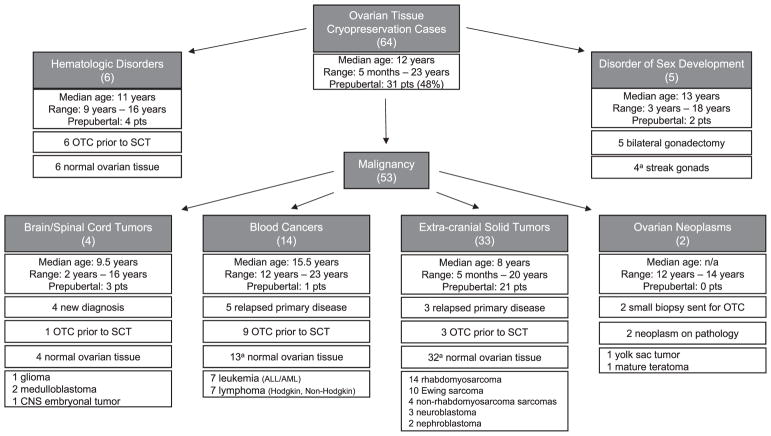
There were 53 children with a primary malignancy, six with a benign hematologic disorder requiring stem cell transplant (SCT), and five with a disorder of sex development (DSD) (Fig. 3).
Fig. 3.
Diagnosis, treatment, and pathology of 64 children who underwent ovarian tissue cryopreservation from 1/2011 to 12/2017 a Details can be found in the body of the text.
Eight patients underwent OTC at the time of disease relapse rather than at the time of initial diagnosis. They did not qualify for OTC initially but became candidates for FP at the time of relapse owing to their cumulative gonadotoxic therapy or need for SCT. Of the newly diagnosed patients, 24 of the 56 had received one or two rounds of their planned chemotherapy prior to surgery. Nineteen patients were referred for OTC prior to anticipated SCT.
Twenty-five percent of patients were referred to our institution for FP; otherwise, these patients receive their medical therapy and are followed at outside institutions.
Two patients had ovarian tissue cryopreserved at the time of planned oophorectomy for ovarian masses. A twelve-year-old patient who had a previous oophorectomy for an immature teratoma required surgery for a new, large cystic-solid mass on the remaining ovary. Ovarian-sparing surgery was performed. A small section of the excised mature teratoma had normal appearing ovarian tissue, and this portion was sent for cryopreservation. The second was a fourteen-year-old patient with bilateral immature teratomas with yolk sac components, who had a small rim of normal appearing ovarian cortical tissue sent for cryopreservation at the time of her bilateral oophorectomy and partial omentectomy.
2.2. Surgical procedures
All primary procedures were performed by four general pediatric surgeons. Fifty-four patients underwent a laparoscopic unilateral oophorectomy, of which 35% also had an ipsilateral salpingectomy and two had a contralateral oophoropexy. Five patients underwent oophorectomy at the time of open primary mass excision. Five DSD patients underwent laparoscopic bilateral gonadectomy.
The majority (76%) of patients had an ancillary procedure performed during their general anesthetic for OTC. Procedures included central venous port placement (30), bone marrow biopsy/aspiration (10), lumbar puncture (6), oophoropexy (2), gastrostomy tube placement (2), and/or MRI (1). On average, patients underwent one additional procedure during their scheduled oophorectomy; 19% of patients underwent two or more additional procedures.
2.3. Intraoperative/postoperative course
No intraoperative complications related to the laparoscopic oophorectomy occurred. The median estimated blood loss of patients undergoing OTC, without primary mass excision, was three milliliters. For patients who underwent laparoscopy, 39 (66%) had their OTC performed as same-day surgery, while 18 (30%) were observational admissions (less than 24 h). Early on, patients were routinely admitted for overnight observation until it was recognized that same-day discharge was safe and feasible. Since 2015, 98% of cases have been performed as same-day surgery. There were no reported 30-day postoperative complications. The median time from operation to initiation of medical therapy was six days, with no unanticipated delays in treatment initiation.
2.4. Follow-up
Six patients enrolled in this study died owing to their underlying diagnosis. The median age of our cohort at the time of this review is 12 (IQR 7–15) years old. There are currently 41 patients who are one year or greater from the time of their OTC. Sixteen are prepubertal and 25 are post-pubertal. Fourteen post-pubertal survivors are followed at our institution, of which 11 (79%) are seen by endocrinology for documented amenorrhea or premature ovarian insufficiency. Nine of these patients have received hormone replacement therapy to resume menses. The remaining two patients resumed menses spontaneously. There have been no documented attempts at pregnancy in the survivors followed at our institution. No patients have utilized their cryopreserved tissue at the time of this review.
2.5. Pathologic evaluation
Fifty-seven of the 59 patients with a cancer or hematologic diagnosis had normal ovarian tissue with identifiable follicles on pathologic evaluation, regardless of their treatment history. Two patients had abnormal specimens. A four-year-old patient initially diagnosed with stage IV neuroblastoma was noted to have a grossly normal ovary at the time of laparoscopy, but the ovarian tissue was found to be infiltrated by B-lymphoblastic lymphoma/leukemia on pathologic evaluation. Prior to OTC, she had received two cycles of non-gonadotoxic chemotherapy. The patient did not have a significant family history of malignancy and no known genetic predisposition to cancer. The lymphoma was incidentally found and diagnosed after OTC, and the patient’s treatment regimen was appropriately modified. The family was informed of the pathology findings and given the option to proceed with tissue freezing in anticipation of a future in vitro option to restore fertility. The family decided to proceed with tissue cryopreservation. A fifteen-year-old patient diagnosed with acute lymphoblastic leukemia who received multiple rounds of non-gonadotoxic chemotherapy prior to OTC was noted to have rare follicles on histologic examination. Again, the patient and her family proceeded with tissue cryopreservation. For children with DSD, at least one streak gonad was identified without ovarian follicles in each patient. One patient with 45, XO/46, XY mosaic Turner syndrome was found to have a unilateral focus of dysgerminoma within a gonadoblastoma. Another patient with 45, XO/46, XY mosaic Turner syndrome had a dysgenetic testis with spermatogonia.
3. Discussion
OTC remains an experimental method of FP but is currently the only pre-treatment option available to prepubertal children and many postpubertal pediatric patients. Elective surgery must be safe with minimal risk to the patient and must not impact treatment for the primary disease. In our experience, we report no complications associated with laparoscopic unilateral oophorectomy for OTC. The majority of cases can be carried out as a same-day surgery and patients can proceed with treatment of their primary disease as planned. Central venous port placement and other ancillary procedures can be combined with the oophorectomy to reduce a child’s exposure to general anesthesia. The majority of patients were found to have normal ovarian follicles on their pathologic specimens, even in those patients who had received previous chemotherapy. Children with a variety of primary medical conditions who are at high-risk for fertility loss because of their treatment may be considered appropriate candidates for an IRB-approved cryopreservation protocol.
OTC is a more widely accepted and performed procedure in Europe for patients at risk for future infertility. Jensen et al. report one of the largest pediatric-specific cohorts published to date discussing the pubertal development of 176 Danish girls under the age of 18 who had previously undergone laparoscopic unilateral oophorectomy for OTC [11]. They report performing laparoscopy in the majority of patients but utilize a mini-laparotomy for children less than two years of age. They do not report on their specific surgical technique or outcomes. Unfortunately, literature on pediatric-specific operative outcomes for OTC remains limited. Of those published, multiple approaches have been reported in children ranging from 7 months to 20 years old, including partial oophorectomy, unilateral/bilateral ovarian biopsy, as well as unilateral oophorectomy for ovarian cortical tissue harvest [12–19]. The majority of these case reports describe a laparoscopic approach with no postoperative complications. Lima et al. report one episode of intraoperative hemorrhage requiring blood transfusion after sharply performing a laparoscopic partial oophorectomy [16]. A major operative complication or non-minimally invasive approach could delay initiation of potentially life-saving medical therapy for a child.
Compared to alternative techniques, laparoscopic unilateral oophorectomy can reduce the risk of bleeding complications associated with a cut tissue surface and may limit the need for cauterization and potential burn damage to the remaining ovarian tissue. The use of bipolar electrocautery and forceful manipulation of the ovarian capsule have been shown to negatively affect the remaining ovarian reserve and can increase the likelihood for future pelvic adhesions [20, 21]. Although laparoscopic unilateral oophorectomy is an established procedure for some benign and malignant ovarian diseases, the technical nuances of the procedure for OTC are unique because the ovary itself is an organ for future transplantation. Based on our institution’s experience, we recommend that a laparoscopic unilateral oophorectomy for OTC be carried out with minimal manipulation to the ovarian capsule, preservation of the ipsilateral fallopian tube, and division of the ovarian artery as the last step of the procedure in attempt to maintain the integrity of the ovary for cryopreservation and adnexal structures for future transplantation. Extra care must be taken when performing the laparoscopic oophorectomy in prepubertal patients to preserve the primordial follicles that reside just deep to the ovarian capsule [22]. The average size of a prepubertal ovary is only one cubic centimeter, which not only makes ovarian biopsy or partial oophorectomy technically challenging, but may also result in inadequate tissue for cryopreservation [23]. With these considerations in mind, the technical skills of a surgeon trained in pediatric advanced minimally invasive surgery are critical for FP procedures in infants and young children [24].
In our early operative experience with OTC, we performed a unilateral salpingo-oophorectomy in patients for whom we were concerned about damaging the ovarian capsule and underlying cortex, especially in prepubertal patients who inherently have a narrow mesovarium. In our current practice, we make a significant attempt to save the ipsilateral fallopian tube to preserve it for future ovarian transplantation options as we anticipate that the technology of fertility and hormone restoration will continue to evolve as our cohort ages. New advances in animal models, such as the development of a bioprosthetic ovary, have persuaded us to preserve as much of the patient’s adnexal anatomy as possible [25]. No differences in surgical outcomes were observed for patients undergoing laparoscopic unilateral oophorectomy versus unilateral salpingo-oophorectomy in our cohort.
The patients who meet our current inclusion criteria for OTC are anticipated to have a greater than 80% risk of future infertility based on their treatment regimen. A unilateral oophorectomy for FP in these children allows for a greater amount of ovarian cortical tissue to be harvested for cryopreservation purposes than an ovarian wedge biopsy or partial oophorectomy. Removal of a whole ovary for FP purposes may raise the question of whether the technique itself could cause premature ovarian failure. Khan et al. suggest that there is a compensatory mechanism that occurs within the remaining ovary following unilateral oophorectomy. Women with a single ovary, who underwent in vitro fertilization, had a higher number of oocytes retrieved as compared to the ipsilateral ovary in women with bilateral ovaries [26]. Additionally, multiple large cohort studies conclude that removal of one ovary hastens menopause onset only by one year [27, 28]. The generalizability of these findings to our patient population is limited given that the majority of long-term outcomes after unilateral oophorectomy were studied in women who did not receive gonadotoxic medical therapy. Alternatively, performing a hemi-oophorectomy or cortical biopsies will preserve more in situ ovarian tissue, but the remaining cortex will be exposed to the gonadotoxic treatments of concern and there will be less cortical tissue for preservation purposes than if removal of a whole ovary is performed. We acknowledge that longitudinal study of all children undergoing laparoscopic oophorectomy for OTC is needed to understand hormone production and reproductive potential as these children mature into adulthood.
Current options for restoration of reproductive hormone function and fertility potential remain experimental for both prepubertal and post-pubertal women and include orthotopic and heterotopic transplantation of ovarian cortical tissue and in vitro maturation of oocytes [29–31]. Outcomes after heterotopic transplantation of thawed ovarian cortex into the subcutaneous tissues are limited and in vitro maturation of immature ovarian follicles remains in its infancy [30, 31]. Conversely, orthotopic transplantation has resulted in over 130 live births and successful hormone restoration in patients who had their ovarian cortical tissue preserved post-menarche. Orthotopic transplantation can be achieved by grafting thawed cortical strips onto the remaining contralateral ovary or within a peritoneal pocket [7, 32, 33]. To date, two live-births have been reported from ovarian tissue cryopreserved in childhood; one in the lay press and one in the medical literature. Demeestere et al. report a patient who underwent a right-sided oophorectomy for OTC at the age of 13 prior to SCT for sickle-cell anemia. Ten years after completion of therapy, she underwent a two-stage robotic procedure with grafting of thawed cortical strips onto the contralateral ovary, within the peritoneal bursa, and in the subcutaneous tissue of a trocar incision. She achieved a spontaneous pregnancy and live-birth two years after surgery [8]. In addition, a woman in London gave birth to a child after undergoing a right oophorectomy for OTC at the age of 9 prior to bone marrow transplant for beta thalassemia. At the age of 23, she underwent ovarian tissue transplantation of cortical strips onto her contralateral failed ovary and uterus. She then underwent successful in vitro fertilization and delivered a healthy child [9]. Ernst et al. were able to successfully induce puberty in a 13-year-old patient with Ewing sarcoma who remained prepubertal with postmenopausal levels of FSH after gonadotoxic treatment. She had undergone an oophorectomy for OTC at 9 years of age. Puberty was induced by transplanting two out of ten preserved cortical strips onto her remaining ovary [34]. In this case, the patient had multiple strips of ovarian cortical tissue preserved which allowed her to use two strips for pubertal transition and continue to store the remainder, if needed, for future fertility restoration. Currently, the average duration of ovarian cortical graft survival has been reported between two and five years [7, 35, 36]. Again, we believe that a unilateral oophorectomy for FP provides the greatest amount of cortical tissue for the patient’s future use as compared to alternative FP techniques.
There are many unique challenges when offering OTC to children at high-risk for premature ovarian insufficiency and infertility, especially given its experimental status and limited live birth and hormone restoration outcomes in patients who had ovarian tissue cryopreserved as a prepubertal child. Our OTC protocol’s inclusion criteria are reserved for those who are at highest risk of developing ovarian insufficiency and are based on what is currently known about the gonadotoxic nature of the patient’s anticipated treatment regimen. There is the possibility that children who qualify for OTC may be able to go through spontaneous puberty and become pregnant without utilizing their stored ovarian cortical tissue and therefore OTC was ultimately unnecessary. There is also the possibility that we may not fully understand the full effects of some of the new antineoplastic medications on future fertility and may be under-referring patients for fertility preservation consultation. Also, patients who may have been considered to be at a low risk for treatment gonadotoxicity at the time of their initial diagnosis may qualify for OTC if they experience relapsed disease or require escalation of medical treatment. The presence of primordial follicles in pediatric ovarian tissue after OTC has been demonstrated regardless of the patient’s diagnosis or history of exposure to gonadotoxic treatment regimens [37]. Future use of the ovarian tissue also remains experimental and will require additional surgery for autotransplantation and/or invasive procedures such as in vitro fertilization which have their inherent risks and additional costs. Additionally, children with a history of pelvic irradiation have been shown to experience an increased risk of pregnancy complications, including spontaneous abortion and preterm labor and may require a surrogate to successfully carry a pregnancy to term [38]. Because of these uncertainties we feel that it is only appropriate to offer OTC under an IRB-approved protocol and with full research consent.
We acknowledge several limitations to the generalizability of this study. First, our institution is one of only a few to have an open IRB-approved OTC protocol for pediatric patients and the resources to carry out all steps required for implementation. These include FP consultation specialists and research coordinators, along with pediatric surgeons and laboratory facilities capable of cryopreserving ovarian tissue. Secondly, longitudinal follow up of patients is challenging as nearly 25% were referred to our institution for FP only and receive medical treatment elsewhere. In addition, many survivors will transition to adult care as our cohort ages. The rate of efficacy of our approach will not be fully understood until a significant portion of our young cohort attempts restoration of their fertility and hormone function. While OTC remains the only FP option available to prepubertal children, we acknowledge that post-pubertal patients physiologically can undergo the hormone stimulation required for oocyte or embryo cryopreservation. Unfortunately, these established, nonexperimental FP methods have their own limitations in pediatric patients. Many postpubertal children with a new cancer diagnosis cannot delay life-saving therapy for the two to four weeks needed for hormone stimulation for oocyte retrieval. Also, oocyte harvesting often requires the use of invasive ultrasound, with possible need for sedation in children. While this technique preserves oocytes, it does not preserve gonadal supporting tissue that may be transplanted and contribute to hormone production and fertility. Additionally, sperm from a partner or donor is required for embryo cryopreservation. Lastly, we recognize that the operative technique described and surgical outcomes are limited to a single-institution’s experience with laparoscopic unilateral oophorectomy for OTC and that direct comparative studies may be challenging. Therefore, animal studies to evaluate the effects of technique on ovarian tissue health are needed. Therefore, our group has developed a prepubertal animal model to evaluate the effects of surgical technique and tissue processing on pediatric ovarian tissue health.
4. Conclusion
OTC remains an experimental FP method, yet laparoscopic unilateral oophorectomy is a safe operative technique for ovarian cortical tissue removal for cryopreservation in children of all ages. OTC can be effectively paired with other necessary procedures to minimize the patient’s exposure to general anesthesia and patients can proceed with treatment of their primary disease as planned. As pediatric patients and their families increasingly opt for FP, we advocate for continued monitoring of surgical, fertility, and hormone outcomes in children to ensure safe surgery while maximizing the quality and quantity of ovarian follicles for future re-implantation and restoration of fertility and hormone function.
Acknowledgments
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) National Center for Translational Research in Reproduction and Infertility (NCTRI) grant [P50HD076188].
Abbreviations
- FP
fertility preservation
- OTC
ovarian tissue cryopreservation
- DSD
disorder of sex development
- FDA
Food and Drug Administration
- SCT
stem cell transplant
References
- 1.Armenian SH, Landier W, Hudson MM, et al. Children’s Oncology Group’s 2013 blueprint for research: survivorship and outcomes. Pediatr Blood Cancer. 2012;60:1063–8. doi: 10.1002/pbc.24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bresters D, Emons JAM, Nuri N, et al. Ovarian insufficiency and pubertal development after hematopoietic stem cell transplantation in childhood. Pediatr Blood Cancer. 2014;61:2048–53. doi: 10.1002/pbc.25162. [DOI] [PubMed] [Google Scholar]
- 3.Raciborska A, Bilska K, Filipp E, et al. Ovarian function in female survivors after multimodal Ewing sarcoma therapy. Pediatr Blood Cancer. 2014;62:341–5. doi: 10.1002/pbc.25304. [DOI] [PubMed] [Google Scholar]
- 4.Fallat ME, Hutter J. The Committee on Bioethics, Section on Hematology/Oncology, and Section on Surgery. Preservation of fertility in pediatric and adolescent patients with cancer Pediatrics. 2008;121:e1461–9. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]
- 5.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. JCO. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Practice Committee of American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013;100:1214–23. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Donnez J, Dolmans M-M. Fertility preservation in women. N Engl J Med. 2017;377:1657–65. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 8.Demeestere I, Simon P, Dedeken L, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30:2107–9. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly L. Woman gives birth to baby using ovary frozen in her childhood in “world first”. The Telegraph. 2016 [Google Scholar]
- 10.Corkum KS, Laronda MM, Rowell EE. A review of reported surgical techniques in fertility preservation for prepubertal and adolescent females facing a fertility threatening diagnosis or treatment. Am J Surg. 2017;214:695–700. doi: 10.1016/j.amjsurg.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen AK, Rechnitzer C, Macklon KT, et al. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: focus on pubertal development. Hum Reprod. 2016:1–11. doi: 10.1093/humrep/dew273. [DOI] [PubMed]
- 12.Biasin E, Salvagno F, Berger M, et al. Ovarian tissue cryopreservation in girls undergoing haematopoietic stem cell transplant: experience of a single Centre. Bone Marrow Transplant. 2015;50:1206–11. doi: 10.1038/bmt.2015.111. [DOI] [PubMed] [Google Scholar]
- 13.Birgit B, Julius H, Carsten R, et al. Fertility preservation in girls with Turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metabol. 2009;94:74–80. doi: 10.1210/jc.2008-0708. [DOI] [PubMed] [Google Scholar]
- 14.Feigin E, Abir R, Fisch B, et al. Laparoscopic ovarian tissue preservation in young patients at risk for ovarian failure as a result of chemotherapy/irradiation for primary malignancy. J Pediatr Surg. 2007;42:862–4. doi: 10.1016/j.jpedsurg.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16:617–30. doi: 10.1093/humupd/dmq010. [DOI] [PubMed] [Google Scholar]
- 16.Lima M, Gargano T, Fabbri R, et al. Ovarian tissue collection for cryopreservation in pediatric age: laparoscopic technical tips. J Pediatr Adolesc Gynecol. 2014;27:95–7. doi: 10.1016/j.jpag.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Poirot CJ, Martelli H, Genestie C, et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr Blood Cancer. 2007;49:74–8. doi: 10.1002/pbc.21027. [DOI] [PubMed] [Google Scholar]
- 18.Revel A, Revel-Vilk S, Aizenman E, et al. At what age can human oocytes be obtained? Fertil Steril. 2009;92:458–63. doi: 10.1016/j.fertnstert.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Wallace WHB, Smith AG, Kelsey TW, et al. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. 2014;15:1129–36. doi: 10.1016/S1470-2045(14)70334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biacchiardi CP, Piane LD, Camanni M, et al. Laparoscopic stripping of endometriomas negatively affects ovarian follicular reserve even if performed by experienced surgeons. Reprod Biomed Online. 2011;23:740–6. doi: 10.1016/j.rbmo.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94:343–9. doi: 10.1016/j.fertnstert.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Lawrenz B, Rothmund R, Neunhoeffer E, et al. Fertility preservation in prepubertal girls prior to chemotherapy and radiotherapy—review of the literature. J Pediatr Adolesc Gynecol. 2012;25:284–8. doi: 10.1016/j.jpag.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Asăvoaie C, Fufezan O, Coşarcă M. Ovarian and uterine ultrasonography in pediatric patients. Pictorial essay Med Ultrason. 2014;16:160–7. doi: 10.11152/mu.201.3.2066.162.ca1of2. [DOI] [PubMed] [Google Scholar]
- 24.Surgical Advisory Panel, American Academy of Pediatrics. Klein MD. Referral to pediatric surgical specialists. Pediatrics. 2014;133:350–6. doi: 10.1542/peds.2013-3820. [DOI] [PubMed] [Google Scholar]
- 25.Laronda MM, Rutz AL, Xiao S, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. doi: 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan Z, Gada RP, Tabbaa ZM, et al. Unilateral oophorectomy results in compensatory follicular recruitment in the remaining ovary at time of ovarian stimulation for in vitro fertilization. Fertil Steril. 2014;101:722–7. doi: 10.1016/j.fertnstert.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Bjelland EK, Wilkosz P, Tanbo TG, et al. Is unilateral oophorectomy associated with age at menopause? A population study (the HUNT2 survey) Hum Reprod. 2014;29:835–41. doi: 10.1093/humrep/deu026. [DOI] [PubMed] [Google Scholar]
- 28.Yasui T, Hayashi K, Mizunuma H, et al. Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas. 2012;72:249–55. doi: 10.1016/j.maturitas.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Donnez J, Dolmans M-M. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32:1167–70. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern CJ, Gook D, Hale LG, et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Hum Reprod. 2013;28:2996–9. doi: 10.1093/humrep/det360. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin M, Albertini DF, Wallace WHB, et al. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod. 2018;24:135–42. doi: 10.1093/molehr/gay002. [DOI] [PubMed] [Google Scholar]
- 32.Donnez J, Dolmans MM, Pellicer A, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31:2031–41. doi: 10.1093/humrep/dew165. [DOI] [PubMed] [Google Scholar]
- 34.Ernst E, Kjaersgaard M, Birkebaek NH, et al. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 2013;49:911–4. doi: 10.1016/j.ejca.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci. 2017;24:1111–20. doi: 10.1177/1933719117702251. [DOI] [PubMed] [Google Scholar]
- 36.Silber S, Kagawa N, Kuwayama M, et al. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–6. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 37.Duncan FE, Pavone ME, Gunn AH, et al. Pediatric and teen ovarian tissue removed for cryopreservation contains follicles irrespective of age, disease diagnosis, treatment history, and specimen processing methods. J Adolesc Young Adult Oncol. 2015;4:174–83. doi: 10.1089/jayao.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzger ML, Meacham LR, Patterson B, et al. Female reproductive health after childhood, adolescent, and young adult cancers: guidelines for the assessment and management of female reproductive complications. J Clin Oncol. 2013;31:1239–47. doi: 10.1200/JCO.2012.43.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]