Abstract
Background:
Mutations in the KCNQ1-encoded Kv7.1 potassium channel cause type 1 long QT syndrome (LQT1). It has been suggested that ~10-20% of rare LQTS case-derived variants in the literature may have been published erroneously as LQT1-causative mutations and may be “false positives.”
Objective:
To determine which previously published KCNQ1 case variants are likely false positives.
Methods:
A list of all published, case-derived KCNQ1 missense variants (MVs) was compiled. The occurrence of each MV within the Genome Aggregation Database (gnomAD) was assessed. Eight in silico tools were used to predict each variant’s pathogenicity. Case-derived variants that either i) were too frequently found in gnomAD or ii) were absent in gnomAD but predicted to be pathogenic by ≤ 2 tools were considered potential false positives. Three of these variants were characterized functionally using whole cell patch clamp technique.
Results:
Overall, there were 244 KCNQ1 case-derived MVs. Of these, 29 (12%) were seen in ≥ 10 individuals in gnomAD and are demotable. However, 157/244 (64%) MVs were absent in gnomAD. Of these, 7 (4%) were predicted to be pathogenic by ≤ 2 tools, 3 of which we characterized functionally. There was no significant difference in current density between heterozygous KCNQ1-F127L, -P477L, or -L619M variant-containing channels compared to KCNQ1-WT.
Conclusion:
Here, we offer preliminary evidence for the demotion of 32 (13%) previously published LQT1 MVs. Of these, 29 MVs were demoted because of their frequent sighting in gnomAD. Additionally, in silico analysis and in vitro functional studies have facilitated the demotion of three ultra-rare MVs (F127L, P477L, and L619M).
Keywords: Arrhythmia, Genetics, Long QT Syndrome, KCNQ1, Pediatrics, Heart Arrest
INTRODUCTION
Long QT Syndrome (LQTS) is an inherited cardiac arrhythmia/electrical channelopathy that is characterized by delayed repolarization of the ventricular myocardium and a prolonged cardiac action potential that is often evidenced by QT interval prolongation on a 12-lead surface electrocardiogram.1, 2 LQTS may manifest as torsades de pointes (TdP)-mediated syncope, - seizures or -sudden cardiac arrest (SCA) typically following a precipitating event such as exertion, extreme emotion, auditory stimuli, or even at rest in an otherwise healthy individual with a structurally normal heart. The prevalence of LQTS in the general population is approximately 1:2000 individuals.3 Extreme variable expressivity, ranging from a life-long asymptomatic course to sudden death during infancy, and incomplete penetrance are hallmark features of the disorder.2 Therefore, it is critical to promptly and accurately diagnose and treat patients after clinical and genetic evaluation.
LQTS is usually inherited as an autosomal dominant trait. Mutations in three genes (KCNQ1, KCNH2, and SCN5A) that encode for ion channel alpha subunits (Kv7.1, Kv11.1, and NaV1.5 respectively) account for 75-80% of all cases of LQTS. Loss of function mutations in the KCNQ1-encoded Kv7.1 pore-forming voltage- gated potassium channel alpha subunit, that is responsible for the slow activation late repolarizing potassium current (IKs), cause type 1 LQTS (LQT1); the most common form of LQTS that accounts for 35-40% of cases.1, 2 Thus, the estimated incidence of LQT1 in the general population is around 1:5000 individuals.
Between 2000 and 2009, hundreds of KCNQ1 missense variants (MVs) were reported and published in several large compendia as “putatively pathogenic.”4-6 In retrospect, most of these MVs were published as “putative pathogenic” based in part on their absence in only a small number of controls (i.e. absent in 50 to 200 “healthy” controls).4-6 However, since these large compendia were published, studies have revealed a 1% background rate of rare KCNQ1 MVs within the general population, and it has been suggested recently that up to 10-20% of rare LQTS case-derived variants that have been published in the literature may have been published prematurely and erroneously as pathogenic, LQTS-causative variants when they may be so-called “false positives.”7-9
Here, we use ~140,000 publically available exome/genome sequences from unrelated individuals in the Genome Aggregation Database (gnomAD), computational variant prediction tools, and in vitro functional studies to assess the pathogenicity of all KCNQ1 case-derived MVs from 6 previously published large compendia of LQTS-associated “putative pathogenic” mutations.9-13
METHODS
Compilation of KCNQ1 Case-Derived Missense Variants (MVs)
A list of all “pathogenic” KCNQ1 case-derived MVs was compiled from 6 previously published large compendia [Splawski compendium10, Tester 54111, Napolitano compendium12, FAMILION13, Kapa 3889, and AMC (personal email communication from Dr. Arthur Wilde)].
Population-Based Rarity Assessment
Each unique KCNQ1 case-derived MV was assessed for rarity using gnomAD (n = 141,352 individuals, http://gnomad.broadinstitute.org/).14 Variants were considered “ultra-rare” if completely absent (allele count = 0), “rare” if seen 1 to 9 times, or “common” if seen in ≤ 10 individuals within gnomAD. Based on our current understanding of the prevalence of LQTS (1:2000 individuals in general, 1:5000 for LQT1 in particular), MVs occurring in ≥ 10 individuals (1:14,000) were considered too common to be self-sufficient, LQT1-causative variants and were therefore demoted statistically to “non-pathogenic” and considered to be false-positive calls in the published compendia of case-derived KCNQ1 variants.
Computational Pathogenicity Prediction Tools
To assess the predicted pathogenicity of all KCNQ1 case-derived MVs, regardless of rarity, analyses were conducted using eight publically available computational pathogenicity prediction tools (paralog conservation ortholog conservation, Grantham values, SIFT, PolyPhen2, Mutation Assesor, KvSNP, and MutPred) as described previously.15 Each variant was given a tool-based composite score between 0 and 8 according to how many of the tools predicted that variant to be pathogenic. The variants were placed in one of three categories: ≤ 2 tools predicting pathogenicity (“likely benign”), 3-5 tools (“uncertain significance”), or ≥ 6 tools (“likely pathogenic”).
KCNQ1 and KCNE1 Mammalian Expression Vectors and Mutagenesis
Three case-derived KCNQ1 MVs (F127L, P477L, and L619M) that were considered “ultra-rare” (i.e. absent in > 140,000 individuals) but had a “likely benign” composite in silico tool score were selected for further heterologous expression studies to determine if they represent true pathogenic variants or benign, false-positive calls. Wild-type KCNQ1 and KCNE1 cDNA were subcloned into pIRES2-EGFP (Clontech, Mountain View, CA) and pIRES2-dsRed2 (Clontech, Mountain View, CA) respectively to produce pIRES2-KCNQ1-WT-EGFP and pIRES2-KCNE1-WT-dsRed2. The Quickchange XL Site-Directed Mutagenesis Kit was used to engineer the F127L, P477L, and L619M variants into pIRES2-KCNQ1-WT-EGFP. DNA sequencing was used to confirm the integrity of all vectors.
TSA 201 Cell Culture and Transfection
TSA 201 Cells were cultured in Dulbecco’s Modification of Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 1.0% L-glutamine, and 1.2% penicillin/streptomycin solution in a 5% CO2 incubator at 37°C. Heterologous expression of the IKs channel was accomplished by using 5 μl of Lipofectamine (Invitrogen) to co-transfect 0.5 μg of both pIRES2-KCNQ1-WT-EGFP and pIRES2-KCNQ1-Mutant-EGFP, with 1 μg of pIRES2-KCNE1-WT-dsRed2. The transfection media was replaced with fresh OPTI-MEM after 4-6 hours. Transfected TSA 201 cells were cultured in OPTI-MEM and incubated for 48 hours before electrophysiological experiments.
Electrophysiological Measurements
Standard whole-cell patch clamp technique was used to measure KCNQ1-WT and –variant-containing IKs currents at room temperature (22-24°C) using an Axopatch 200B amplifier, Digidata 1440A, and pclamp version 10.2 software (Axon Instruments, Sunnyvale, CA). The extracellular (bath) solution contained (mmol/L): 150 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 1 Na-Pyruvate, and 15 HEPES. pH was adjusted to 7.4 with NaOH. The intracellular (pipette) solution contained (mmol/L): 20 KCl, 125 K-Aspartate, 1 MgCl2, 10 EGTA, 5 MgATP, 5 HEPES, 2 Na2-Phosphocreatine, and 2 Na2-GTP. pH was adjusted to 7.2 with KOH.16 Microelectrodes were fire polished to a final resistance of 1-2 MΩ after being pulled using a P-97 puller (Sutter Instruments, Novato, CA). Series resistance was compensated by 80-85%. Currents were filtered at 1 kHz and digitized at 5 kHz with an eight-pole Bessel filter. The voltage dependence of activation was determined using voltage-clamp protocols described in the figure legend. Data were analyzed using Clampfit (Axon Instruments, Sunnyvale, CA), Excel (Microsoft, Redmond, WA) and fitted with Origin 2016 (OriginLab Corporation, Northampton, MA) software.
Statistical Analysis
Student’s t-tests were performed to determine statistical significance between two groups. A Kruskal-Wallis test followed by Dunn’s multiple comparisons test were used to determine statistical significance between three groups. A p<0.05 was considered to be significant.
RESULTS
Overall, 244 unique case-derived KCNQ1 MVs were identified in the review of 6 large compendia of putative LQT1-causative mutations. Of these, 14 (6%) were localized to the N-terminus, 135 (55%) to the transmembrane-spanning domains, and 95 (39%) to the C-terminus of the protein. The MVs localizing to the transmembrane-spanning domains had a higher average composite in silico score (6.13 ± 1.43) compared to the C-terminus (4.92 ± 2.43; p=0.002) and N-terminus (4.07 ± 2.33; p=0.007) localizing variants (Table 1).
Table 1.
Location and Average in silico Scores of all Case-Derived KCNQ1 Variants Based on Three Categories of Rarity
| Ultra-Rare | Rare | Common | Total | Average in silico Score | Range of in silico Scores | |
|---|---|---|---|---|---|---|
| N-Terminal Variants | 8 (5%) | 4 (7%) | 2 (7%) | 14 | 4.07±2.33 | 1 - 8 |
| Transmembrane Variants | 96 (61%) | 30 (52%) | 9 (31%) | 135 | 6.13±1.43 | 0 - 8 |
| C-Terminal Variants | 53 (34%) | 24 (41%) | 18 (62%) | 95 | 4.92±2.43 | 0 - 8 |
| Total | 157 | 58 | 29 | 244 | ||
| Average in silico Score | 6.05±1.61 | 5.41±2.07 | 3.00±2.25 | |||
| Range of in silico Scores | 0 - 8 | 0 - 8 | 0 - 7 |
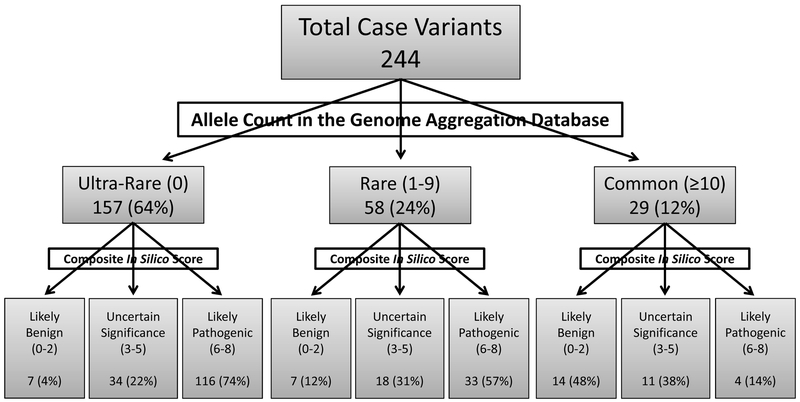
Of the 244 KCNQ1 case-derived MVs, 29 (12%) were present in ≥ 10 individuals in gnomAD. These 29 variants are considered statistically too common to be LQT1-causative mutations based on the following reasoning. If LQTS is truly a 1:2000 disease3, then ~70 individuals within gnomAD should have LQTS (2000:141,352). It is well established that LQT1, being the most common subtype, accounts for 35-40% of all cases of LQTS.1, 2 Accordingly, this would mean that ~24 out of these 70 individuals will have LQT1. Considering that only ~20-25% of variants within the published compendia are seen more than once in unrelated subjects11, 13, it is highly unlikely that any of these variants could account for ≥ 10 of the ~24 individuals in gnomAD with LQT1. Therefore, unless LQTS is far more common and far less penetrant than ever before considered, these 29 MVs are much too common to be disease causing and can be demoted readily to “non-pathogenic” status. Moreover, 13 of these 29 (44.8%) variants were each seen in more than 24 unrelated exomes/genomes in gnomAD; a prevalence of 1 in 2000 individuals (Table 2, Figure 1). The majority of these “common” variants localized to the C-terminus (18/29, 62%), followed by the transmembrane domain (9/29, 31%), and the N-terminus (2/29, 7%). Overall, the average composite pathogenicity in silico score was only 3.00 ± 2.25 (Table 1). Only 4/29 (14%, R195Q, P197L, K362R, and R397W) were predicted to be “likely pathogenic” (composite score ≥ 6 tools predicting pathogenic), while 11/29 (38%) were of “uncertain significance” (3-5 tools), and 14/29 (48%) predicted to be “likely benign” (≤ 2 tools, Table 2, Figure 1).
Table 2.
Previously Reported “Putative Pathogenic” KCNQ1 Missense Variants Observed in ≥ 10 GnomAD Exomes/Genomes - Candidates for Variant Demotion
| Variant | Region | Number of Cases |
gnomAD Count |
gnomAD Alleles | gnomAD Minor Allele Frequency |
In Silico Composite Score |
|---|---|---|---|---|---|---|
| P73T | N-Terminal | 5 | 26 | 184800 | 1.41E-04 | 2 |
| V110I | N-Terminal | 1 | 16 | 256662 | 6.23E-05 | 1 |
| T153M | Transmembrane | 1 | 47 | 282484 | 1.66E-04 | 3 |
| V172M | Transmembrane | 2 | 15 | 281308 | 5.33E-05 | 3 |
| R195Q | Transmembrane | 3 | 11 | 280744 | 3.92E-05 | 6 |
| P197L | Transmembrane | 1 | 12 | 250424 | 4.79E-05 | 7 |
| I274V | Transmembrane | 1 | 50 | 282172 | 1.77E-04 | 5 |
| A287E | Transmembrane | 2 | 14 | 251832 | 5.56E-05 | 4 |
| G292D | Transmembrane | 3 | 12 | 282052 | 4.25E-05 | 4 |
| R293C | Transmembrane | 5 | 11 | 282024 | 3.90E-05 | 5 |
| A300T | Transmembrane | 1 | 12 | 251490 | 4.77E-05 | 5 |
| K362R | C-Terminal | 14 | 10 | 252376 | 3.96E-05 | 7 |
| A370V | C-Terminal | 1 | 41 | 282496 | 1.54E-04 | 5 |
| K393N | C-Terminal | 8 | 295 | 282632 | 1.04E-03 | 3 |
| R397W | C-Terminal | 3 | 52 | 282622 | 4.84E-04 | 6 |
| A399S | C-Terminal | 1 | 21 | 252344 | 8.32E-05 | 1 |
| P408A | C-Terminal | 4 | 455 | 282646 | 1.61E-03 | 1 |
| D446E | C-Terminal | 2 | 19 | 251590 | 7.55E-05 | 0 |
| P448R | C-Terminal | 11 | 1980 | 281966 | 7.02E-03 | 1 |
| R452Q | C-Terminal | 1 | 29 | 281386 | 1.03E-04 | 2 |
| R452W | C-Terminal | 1 | 29 | 281402 | 1.03E-04 | 5 |
| G460S | C-Terminal | 1 | 12 | 250486 | 4.79E-05 | 2 |
| V576I | C-Terminal | 3 | 16 | 252148 | 6.35E-05 | 0 |
| G589D | C-Terminal | 2 | 13 | 282250 | 4.61E-05 | 5 |
| T600M | C-Terminal | 3 | 47 | 213756 | 2.20E-04 | 2 |
| D611N | C-Terminal | 1 | 20 | 210372 | 9.51E-05 | 2 |
| G629S | C-Terminal | 1 | 11 | 212754 | 5.17E-05 | 0 |
| G643S | C-Terminal | 11 | 1407 | 223544 | 6.29E-03 | 0 |
| V648I | C-Terminal | 7 | 525 | 214146 | 2.45E-03 | 0 |
Figure 1. Breakdown of KCNQ1 case-derived missense variants in gnomAD and after in silico pathogenicity analysis.
Each of 244 unique KCNQ1 case-derived MVs were assessed for rarity using gnomAD. Variants were considered “ultra-rare” if completely absent (allele count = 0), “rare” if seen 1 to 9 times, or “common” if seen in ≥10 individuals within gnomAD. In silico analysis was conducted using eight publically available tools to assess the predicted pathogenicity of all KCNQ1 case-derived MVs. Each variant was placed into one of three categories based on how many tools predicted that variant to be pathogenic: likely benign (≤ 2 tools), uncertain significance (3-5 tools), or likely pathogenic (≥ 6 tools).
Considered “rare,” 58 (24%) MVs were present in 1 to 9 individuals in gnomAD (Figure 1). The majority localized to the transmembrane domain (30/58, 52%) followed by the C-terminus (24/58, 41%), and the N-terminus (4/58, 7%). The average composite in silico score was 5.41 ± 2.07 (Table 1). Of these 58 variants, 33 (57%) were predicted pathogenic (≥ 6 tools), 18 (31%) were of uncertain significance (3-5 tools), and only 7 (12%) were predicted to be likely benign (≤ 2 tools) (Figure 1).
Of the 244 unique case-derived KCNQ1 MVs, 157 (64%) were completely absent in gnomAD (n = 141,352 unrelated individuals) and considered to be “ultra-rare” (Figure 1). Like the “rare” variants, the majority of “ultra-rare” variants localized to the transmembrane domain (96/157, 61%) followed by the C-terminus (53/157, 34%), and the N-terminus (8/157, 5%). Compared to the “rare” and “common” MVs, the average composite in silico score was greatest at 6.05 ± 1.61 for these “ultra-rare” MVs (Table 1). The vast majority, 116 (74%), were predicted to be likely pathogenic (≥ 6 tools) and 34 (22%) were of uncertain significance (3-5 tools). Despite being “ultra-rare,” 7 (4%) variants (A2V, F127L, S389P, K398R, P477L, F479L, and L619M) were likely benign based on ≤ 2 in silico tools predicting a pathogenic effect (Figure 1). The A2V variant localizes to the N-terminus, F127L to the S1 transmembrane spanning domain, and S389P, K398R, P477L, F479L, and L619M localize to the C-terminus. The L619M variant localized to the Subunit Assembly Domain within the C-terminus.
According to the literature, only 41/157 (26%) ultra-rare case-derived KCNQ1 MVs, have been characterized functionally. Interestingly, all 32 previously characterized variants with a composite score of ≥ 6 (“likely pathogenic”) were identified as having an abnormal electrophysiological phenotype, whereas 1 of the 9 “uncertain significance” variants with a composite score of 3 to 5 exhibited a wild-type phenotype. Here, we characterized functionally 3 ultra-rare, case-derived KCNQ1 MVs (F127L, P477L, and L619M) that had a composite in silico score ≤ 2 (i.e. “likely benign”).
Functional Characterization of KCNQ1 Variants
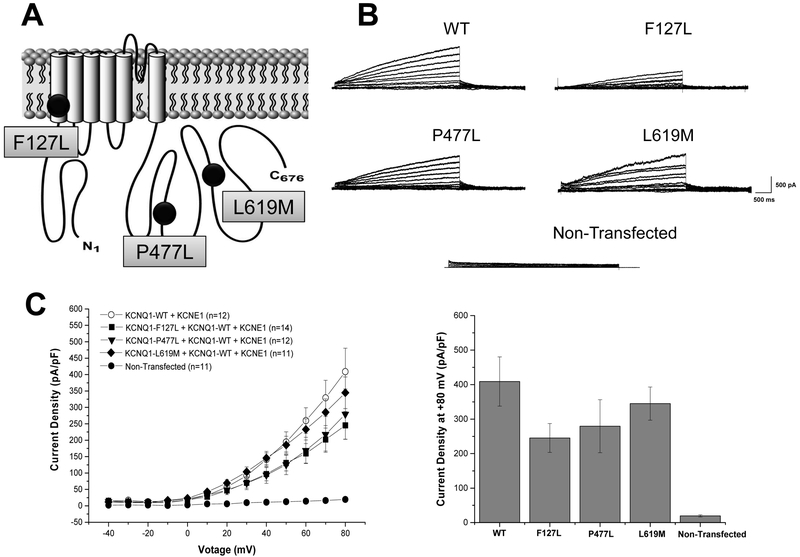
When variants were co-expressed with KCNQ1-WT, analysis of the current-voltage relationship between voltages of −40 mV and +80 mV revealed no significant differences in current density between KCNQ1-F127 (245.1 ± 41.9 pA/pF; n=14), KCNQ1-P477L (279.4 ± 76.7 pA/pF; n=12), or KCNQ1-L619M (344.8 ± 48.2 pA/pF; n=11) when compared to KCNQ1-WT (408.9 ± 71.4 pA/pF; n=12; p=NS) (Figure 2A-C). There was no significant difference in any of the properties of activation or deactivation between any mutant channel and KCNQ1-WT (Supplementary Figure 1). All values represent mean ± standard error of the mean.
Figure 2. KCNQ1-F127L, -P477L, and -L619M missense variants did not significantly alter IKs current density in heterologous TSA 201 cells.
(A) Depicted is a schematic representation of the KCNQ1-encoded potassium channel alpha subunit (Kv7.1) with KCNQ1-F127L, -P477L, and -L619M variants. (B) Whole cell IKs current representative tracings from non-transfected TSA201 cells and TSA201 cells expressing KCNQ1-WT, -F127L, -P477L, and -L619M determined from a holding potential of −80 mV and testing potentials from −40 mV to +80 mV in 10 mV increments with 4s duration. (C) Current-voltage relationship for IKs KCNQ1-WT, -F127L, -P477L, and -L619M missense variants and for non-transfected cells. All values represent mean ± SEM. A p<0.05 was considered to be significant.
DISCUSSION
Since the dawn of precision medicine, the genetic test has become an increasingly important tool for the evaluation of patients and families suspected to have an inheritable cardiac channelopathy. Not only does clinical genetic testing play an integral role in the diagnosis and treatment of the proband in whom a variant is found, but also for that individual’s immediate family members. However, this useful tool comes with risks, and extreme caution must be used when interpreting a genetic test result.
In the early 2000s when research-based genetic interrogation and the early phases of transformation to clinical genetic testing for LQTS began, there was an underlying assumption that if a genetic variant identified within a known disease-causing gene was rare (i.e. absent in 100 control alleles), then it likely constitutes “the disease-causative mutation.” This was based on the subsequently proven, erroneous belief that these disease-causing genes could not host rare but innocuous variants.17 Unfortunately, some genetic testing companies still cite the mere presence of a specific variant within an early manuscript as enough evidence for pathogenicity, despite the lack of 1) adequate number of healthy controls, 2) functional characterization of the variant, or 3) illustration of proper co-segregation with the disease phenotype within a multi-generational pedigree that would lend support for the designation of a pathogenic variant.
In 2003, we performed the first study to comprehensively analyze and determine the prevalence of rare non-synonymous variants in the major LQTS-causing genes (KCNQ1, KCNH2, and SCN5A) in a cohort of nearly 800 ostensibly healthy individuals.7, 8 In 2009, we expanded this analysis to include over 1300 healthy individuals and 388 unrelated definite cases of LQTS in order to attempt to distinguish pathogenic mutations from benign variants by comparing the localization of case-associated variants and healthy control variants.9 These studies, and others like it, determined that there is a substantial rate of background “genetic noise” in the 3 major LQTS-susceptibility genes (1% in KCNQ1, 2% in KCNH2, and 2.6% in SCN5A). The prevalence of rare amino acid altering variants may be even higher in non-Caucasian individuals, where for example, 4.5% of blacks had a rare SCN5A variant.7-9
This background genetic noise has led to a high level of uncertainty as to how rare variants should be interpreted, with many being labeled as variants of uncertain significance (VUS) and falling into what we have termed “genetic purgatory.”17 These initial experiments highlighted the need for proper and careful rare variant adjudication before assigning them with a label of “pathogenic” variant.
Major advancements in next-generation sequencing technology have provided the scientific community with an unprecedented number of publically available exome/genome sequences from approximately 140,000 unrelated individuals in the Genome Aggregation Database (gnomAD). This database allows the user to search for their variant of interest for any gene in the human genome and to determine the approximate allele frequency of that variant within the general population and within specific ethnicities. This valuable resource allows for the quick re-evaluation of the rarity or commonness of a variant that was previously reported as “pathogenic.” In fact, utilizing allele frequencies in gnomAD, we were able to demote 12% of the previously published 244 LQTS case-derived KCNQ1 MVs from “putative pathogenic” to “non-pathogenic” simply based on the prevalence of these variants within this database.
The greater challenge resides with the identification of the erroneous LQT1 classifications when the MV of interest is completely absent from gnomAD. Here, the use of multiple in silico mutation prediction tools may help to polarize those ultra-rare variants that are most likely “non-pathogenic” from those that may indeed cause disease. We considered that the ultra-rare (are absent in gnomAD) KCNQ1 case-derived MVs that are most likely to be false positives are those that are predicted to be pathogenic by ≤ 2 out of the 8 in silico tools that were used. Out of the 157 ultra-rare MVs that have been previously published as “putative pathogenic,” 7 were considered to be “false-positive” calls based on this criteria. To illustrate this, we functionally characterized 3 of these rare potential false positives (F127L, P477L, and L619M) using patch clamp heterologous expression studies and found all to have a wild type electrophysiological KCNQ1 phenotype, thus suggesting that these previous “pathogenic” mutations, albeit ultra-rare, can also be demoted to “non-pathogenic” status.
While the purpose of this study was not to validate the use of multiple in silico tools for variant interpretation, some conclusions can be made as to their ability to predict the pathogenicity of variants. While they are not perfect, multiple in silico algorithms in aggregate do hold some predictive value when attempting to differentiate between case and control variants in aggregate.15, 18, 19 Our study is focused solely on alleged case-associated variants, but when all KCNQ1 case-derived MVs are categorized by rarity within gnomAD, we found that the average composite in silico score for ultra-rare MVs was two-fold higher (6.05) than it was for common variants (3.00). This suggests that the synergistic use of 8 in silico tools can be a useful strategy to differentiate pathogenic KCNQ1 variants from those that are most likely “non-pathogenic.”
In addition, there is a correlation between predicted in silico phenotypes and those observed through in vitro electrophysiological studies15, and we found the same to be true here. Of the 116 ultra-rare KCNQ1 case-derived MVs that are predicted as likely pathogenic, 32 have previously been studied in vitro, and all 32 exhibited an abnormal phenotype. On the other hand, of the 7 ultra-rare KCNQ1 MVs predicted to be benign, 3 have been studied in vitro (all in this study) with each exhibiting a wild type phenotype in their functional validation assay. This data supports the previous finding, and gives support for the use of multiple in silico tools for variant interpretation when used in conjunction with additional lines of evidence.
Despite their potential ability to differentiate between case and control variants and their correlation with in vitro studies, computational pathogenicity prediction tools can still be inaccurate, even when used in aggregate.15, 19 For this reason the ACMG guidelines for variant interpretation classify computational predictive programs as having only “supporting” evidence when interpreting the pathogenicity of the variant.20 While the guidelines recommend the use of multiple of in silico tools when performing variant interpretation, they also caution that these predictions should not be used as the only source of evidence when attempting to determine the pathogenicity of a patient’s variant in a clinical setting.20 Additional strong pieces of evidence are needed to make that determination.20
It is important for physicians to remember in the midst of this new and exciting era of precision medicine that the phenotype still matters the most.21 While genetic testing is an exciting tool that should be utilized in a clinical setting, it should always be viewed as a probabilistic test rather than a binary one and should be considered by a physician as one piece of evidence along with phenotype, predicted pathogenicity, and functional studies. Failure to do so can lead to disastrous results.21
CONCLUSION
Using a combination of gnomAD, in silico tools, and a functional validation assay, we offer evidence for the demotion of 32 previously published “putative” pathogenic LQT1-associated KCNQ1 MVs. Our data supports previous estimates that 10-15% of KCNQ1 variants that were reported originally during the era of research-based, pseudo-clinical genetic testing, may indeed represent “false-positive” calls. Physicians with patients hosting variants identified during this era should consider a thorough re-evaluation of the current evidence for pathogenicity of those variants.
Supplementary Material
ACKNOWLEGEMENTS
None
Funding Sources:
This work was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program. JDK is supported by the NIH grant GM72474-08. JDK thanks the Mayo Clinic Medical Scientist Training Program for fostering an outstanding environment for physician-scientist training.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
MJA is a consultant for Audentes Therapeutics, Boston Scientific, Gilead Sciences, Invitae, Medtronic, MyoKardia, and St. Jude Medical. MJA, DJT, and Mayo Clinic have received sales-based royalties in the past from Transgenomic for their FAMILION-LQTS and FAMILION-CPVT genetic tests. MJA and Mayo Clinic have an equity/royalty relationship with AliveCor, Blue Ox Health, and StemoniX. However, none of these entities have contributed to this study in any manner.
REFERENCES
- 1.Bezzina CR, Lahrouchi N, Priori SG. Genetics of sudden cardiac death. Circulation Research 2015;116:1919–1936. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz PJ, Ackerman MJ, George AL, Wilde AAM. Impact of genetics on the clinical management of channelopathies. Journal of the American College of Cardiology 2013;62:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalence of the congenital long-QT syndrome. Circulation 2009;120:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rook MB, Alshinawi CB, Groenewegen WA, Van Gelder IC, Van Ginneken AC, Jongsma HJ, Mannens MM, Wilde AA. Human SCN5A gene mutations alter cardiac sodium channel kinetics and are associated with the Brugada syndrome. Cardiovascular Research 1999;44:507–517. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima-Taniguchi C, Matsui H, Fujio Y, Nagata S, Kishimoto T, Yamauchi-Takihara K. Novel missense mutation in Cardiac Troponin T gene found in Japanese patient with hypertrophic cardiomyopathy. Journal of Molecular and Cellular Cardiology 1997;29:839–843. [DOI] [PubMed] [Google Scholar]
- 6.Erdmann J, Daehmlow S, Wischke S, Senyuva M, Werner U, Raible J, Tanis N, Dyachenko S, Hummel M, Hetzer R, Regitz-Zagrosek V. Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clinical Genetics 2003;64:339–349. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman MJ, Splawski I, Makielski JC, et al. Spectrum and prevalence of cardiac sodium channel variants among Black, White, Asian, and Hispanic individuals: Implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm 2004; 1: 600–607. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman MJ, Tester DJ, Jones G, Will MK, Burrow CR, Curran M. Ethnic differences in cardiac potassium channel variants: Implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clinic Proceedings 2003;78:1479–1487. [DOI] [PubMed] [Google Scholar]
- 9.Kapa S, Tester DJ, Salisbury BA, Harris-Kerr C, Pungliya MS, Alders M, Wilde AA, Ackerman MJ. Genetic testing for long-QT syndrome: Distinguishing pathogenic mutations from benign variants. Circulation 2009;120:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 2000;102:1178–1185. [DOI] [PubMed] [Google Scholar]
- 11.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2005;2:507–517. [DOI] [PubMed] [Google Scholar]
- 12.Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, Nastoli J, Bottelli G, Cerrone M, Leonardi S. Genetic testing in the long QT syndrome: Development and validation of an efficient approach to genotyping in clinical practice. Journal of the American Medical Association 2005;294:2975–2980. [DOI] [PubMed] [Google Scholar]
- 13.Kapplinger JD, Tester DJ, Salisbury BA, Carr JL, Harris-Kerr C, Pollevick GD, Wilde AA, Ackerman MJ. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm 2009;6:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapplinger JD, Giudicessi JR, Ye D, Tester DJ, Callis TE, Valdivia CR, Makielski JC, Wilde AA, Ackerman MJ. Enhanced classification of Brugada syndrome- and long QT syndrome-associated genetic variants in the SCN5A-encoded Nav1.5 cardiac sodium channel. Circulation: Cardiovascular Genetics 2015;8(4)582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. American Journal of Physiology Heart and Circulatory Physiology 2011;301:H2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackerman MJ. Genetic Purgatory and the cardiac channelopathies: Exposing the variants of uncertain/unknown significance (VUS) issue. Heart rhythm 2015;12(11):2325–2331. [DOI] [PubMed] [Google Scholar]
- 18.Kapplinger JD, Tseng AS, Salisbury BA, Tester DJ, Callis TE, Alders M, Wilde AA, Ackerman MJ. Enhancing the predictive power of mutations in the C-terminus of the KCNQ1-encoded Kv7.1 voltage-gated potassium channel. Journal of Cardiovascular Translational Research 2015;8:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong IU, Stuckey A, Lai D, Skinner JR, Love DR. Assessment of the predictive accuracy of five in silico prediction tools, alone or in combination, and two metaservers to classify long QT syndrome gene mutations. BMC Medical Genetics 2015;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackerman JP, Bartos DC, Kapplinger JD, Tester DJ, Delisle BP, Ackerman MJ. The promise and peril of precision medicine: Phenotyping still matters most. Mayo Clinic Proceedings 2016;91:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.