Abstract
Necrotizing enterocolitis (NEC) remains a leading cause of preterm infant mortality. NEC is multifactorial and thought to be a consequence of intestinal immaturity, microbial dysbiosis and an exuberant inflammatory response. Over the last decade, exaggerated TLR4 activity in the immature intestine of preterm neonates emerged as an inciting event preceding NEC. High TLR4 activity in epithelial cells results in the initiation of an uncontrolled immune response and destruction of the mucosal barrier. We will discuss the state of the science of the molecular mechanisms involved in TLR4-mediated inflammation during NEC and the development of new therapeutic strategies to prevent NEC.
Keywords: Necrotizing enterocolitis, TLR4, inflammation, epithelial cells
Introduction
Since the emergence of early eukaryotic organisms, a plethora of sophisticated defense mechanisms have evolved in order to protect the host from invading pathogens and to ensure species survival1,2. A key feature of an optimal protective mechanism is the effective discrimination of harmful threats from innocuous and beneficial commensal entities3. Despite the high efficacy of the immune system in preventing deadly infections in the adults, the morbidity inherent to infectious diseases is substantially higher in human neonates4,5. Furthermore, the prevalence of infections is significantly greater in preterm infants when compared to term newborns6. Shortly after birth, the neonates experience a massive microbial colonization of their mucosal surfaces7.The early crosstalk between these microbial communities and the host is critical for the optimal development of host immunity8. This was evidenced by the fact that antibiotic treatment during infancy as well as the lack of microbial colonization in mice models lead to increased susceptibility to autoimmune and infectious diseases9–12. On the other hand, the bacterial colonization of the immature intestine of preterm infants is associated with the induction of a detrimental inflammatory response leading to the development of Necrotizing Enterocolitis (NEC)13. NEC is a devastating gastrointestinal disease affecting up to 10% of premature infants with the highest incidence among those with birth weight < 1500 g and post-menstrual age of 32 weeks14–16. Although significant progress has been made in our understanding of the molecular mechanisms behind the development of NEC, the optimal therapeutic strategies remain elusive17. Therefore, a better understanding of the developmental changes of the immune system during the neonatal period is required to implement new treatments to prevent the development of this devastating disease.
The immune surveillance of the host environment is carried out by pattern recognition receptors (PRRs) that play a central role in the first line of host defense. These receptors, which include Toll-like receptors (TLRs), retinoic acid-inducible gene-I-like (RIG-I-like) receptors, nucleotide-binding oligomerization domain (NOD)-like receptors, and C-type lectin receptors have the ability to recognize a wide range of intrinsic and extrinsic danger signals namely pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs)2. In this chapter, we will describe how the innate TLR response during early life correlates with the susceptibility of infants to inflammatory disorders with a particular emphasis on the role of epithelial TLR4 in the initiation of the gastrointestinal disease NEC.
TLRs function in the hematopoietic cell compartment during early life
TLRs are expressed by various types of hematopoietic and non-hematopoietic cells where they play a critical role in the induction of protective host immune responses as well as the development of detrimental inflammation2. In the hematopoietic cell compartment of neonates, TLRs and their downstream signaling molecules seem to be expressed at comparable levels to their adult counterparts18. Moreover, the peripheral blood monocytes can enhance the expression of TLRs in response to newborn sepsis indicating that they are adequately expressed during infection19. Despite the normal expression levels of TLRs on newborn leukocytes, the cytokines produced upon their activation differ drastically from those observed in adults. Intriguingly, TLR-mediated cytokine production is also distinct between preterm and term infants. TLR stimulation of preterm human blood leukocytes is characterized by high levels of IL-10, while IL-6 and IL-23 are the main landmarks of the term neonates’ immune cells 20–22. These patterns tend to change over the course of life toward tumor necrosis factor alpha (TNFA) and interleukin 1 beta (IL1-B) production21. Furthermore, the poor TLR-mediated interferon response observed in the newborns evolve quickly during the first weeks of life to reach similar levels to those observed in adults21. The molecular basis of these differences is still not well understood. The weak interferon response during early life was attributed to the impaired nuclear translocation of Interferon Response Factor 723. Furthermore, the newborn blood contains high amounts of adenosine when compared to adults’ plasma 24. Adenosine signals through adenosine receptors (AR) to generate cAMP, which in turn can inhibit Th1 differentiation while promoting IL10, IL6 and IL23 production24–26. This pattern has a direct implication on vaccine responsiveness in neonates. In line with the anti-inflammatory cytokine production in preterm infants, several studies have highlighted the weak immune response induced by vaccines that were administered to preterm infants around birth while later vaccinations result in effective protection27,28. On the other hand, the exaggerated immune response following TLR stimulation in early life has been associated with a large panel of inflammatory disorders including NEC, neonatal chronic lung disease and periventricular white matter injury29,30. This seems to be in contradiction with the aforementioned regulatory cytokine profile in preterm immune cells, suggesting that the susceptibility of premature neonates to TLR-driven inflammation is at least partly initiated in the non-hematopoietic cell compartment.
Epithelial TLR4 signaling: a key factor in NEC development.
Over the last several years, considerable attention has been devoted to investigate the role of TLR4 signaling in the pathogenesis of NEC. This particular interest was motivated by early studies demonstrating that TLR2 and TLR4 are constitutively expressed by fetal enterocytes and that lipopolysaccharide (LPS) as well as IL1-B can modulate their expression31. Furthermore TLR2, TLR4 and their related signaling molecules were shown to be highly expressed in fetal enterocytes when compared to their mature adult counterparts. This differential expression was further evidenced in small intestinal cells during NEC, which are characterized by significantly higher expression of TLR4 than the healthy enterocytes32. Strikingly, it was shown that the cytosolic internalization of TLR4 is significantly higher in the mature intestinal cells suggesting that the excessive inflammatory response observed during NEC is at least partly due to the over-expression and the exaggerated activity of TLR4 in the preterm intestine32. These findings were further supported by the fact that LPS activation of TLR4 in intestinal cells is significantly higher in preterm mice compared to naturally delivered controls33.Importantly, the expression of TLR4 is substantially elevated during human NEC34. In an attempt to determine the molecular mechanism behind the high expression of TLR4 in preterm infants, a study performed by Soliman et al. suggested that platelet-activating factor (PAF), initially incriminated in the development of NEC, is responsible for the over-expression of TLR4 in the premature intestine, thereby resulting in an exaggerated inflammation during NEC35,36. Other than the well-documented role of TLR4 in the activation of an inflammatory program in epithelial cells, it is also known to contribute to bacterial translocation across the mucosal barrier, which likely contributes to the development of the septic shock picture observed during NEC37. The functional evidence for the implication of TLR4 in the development of NEC came from the use of a murine model of the disease. Jilling et al., as well as Hackam and colleagues have shown that TLR4 is required for development of NEC through the impairment of the epithelial barrier integrity38–40. Mechanistically, TLR4 can affect the homeostasis of the intestinal epithelium through different pathways. Indeed, during intestinal injury, enterocyte migration throughout the crypt-villus axis can be affected by the activation of TLR4. This can be achieved by modulation of the cell-extracellular matrix interaction39,41. Unlike in the adult, TLR4 activation in the premature intestine is associated with the induction of an apoptotic program and the reduction of cell proliferation resulting in the impairment of epithelial cell regeneration39,42,43 (Figure 1). Interestingly, the stimulation of the TLR4 signaling cascade is associated the induction of autophagy in epithelial cells, which negatively impacts their migration during NEC44. Therefore, mice lacking the expression of the autophagy gene ATG7 were found to be protected against NEC via the reduction of RhoA-GTP-ase activation and subsequently, the enhancement of enterocyte migration. In line with these data, the expression levels of autophagy-related genes are relatively high in the premature intestine44. The role of TLR4-mediated inhibition of enterocyte migration was further evidenced in another study where the extracellular high mobility group box-1 released during intestinal inflammation was found to enhance cell-matrix adhesiveness and to reduce enterocyte migration by the activating Ras Homolog Family Member A (RhoA) in a TLR4-dependent manner45. In parallel to the inhibition of cell migration, Sodhi et al. showed that a TLR4-mediated reduction of epithelial cell proliferation is triggered by the activation of Glycogen Synthase Kinase 3β43 and the reduction of β-catenin activity43. The co-expression of TLR4 and LGR5, an intestinal stem cell ISC marker, suggested that TLR4 regulation of the epithelial cell homeostasis could be driven by the activation of NF-κB in ISCs. Thus, TLR4 was found to promote the activation of p53 up-regulated modulator of apoptosis (PUMA), which in turn prevents cell proliferation and initiates a cell death program42. A recent report has uncovered another mechanism by which TLR4 modulates ISC viability via the initiation of an endoplasmic reticulum (ER) stress during NEC46. The study revealed that TLR4 activation triggers ER stress in ISC by activating PERK (protein kinase-related PKR-like ER kinase) and CHOP (C/EBP homologous protein)46. Moreover, genetic perturbation of PERK and CHOP conferred a significant protection against experimental murine NEC46. Apart from the role of TLR4 in the modulation of the epithelial barrier integrity, it was shown to influence the differentiation of epithelial cells as mice deficient in TLR4 specifically in their intestinal epithelium are characterized by a marked increase in goblet cell number and reduced Mucin 2 expression47. The effect of decreased goblet cell number on NEC development in premature infant was evidenced by Sodhi et al. who demonstrated that the inhibition of γ secretase and NOTCH signaling enhance goblet cell development leading to a significant decrease in NEC susceptibility. In light of these findings, it is tempting to speculate that the increased differentiation of goblet cells upon the ablation of the TLR4 gene and the resulting enhancement of antimicrobial peptide protection might explain the attenuated disease severity in these mice compared to their wild-type littermates.
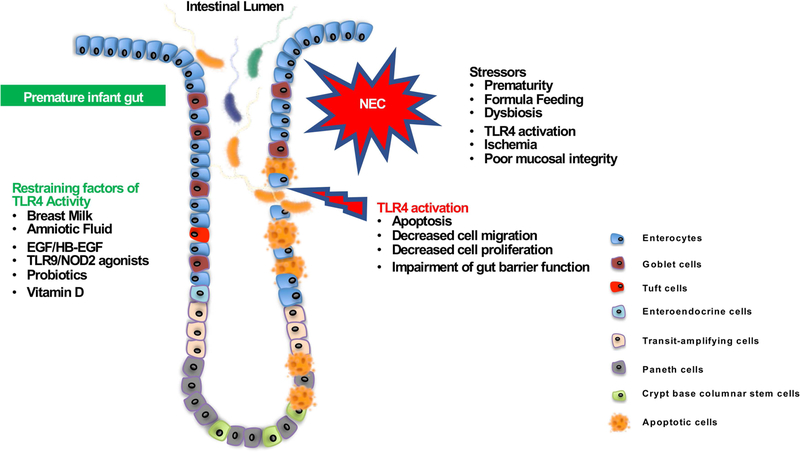
Figure 1. Inhibition of TLR4 signaling as a potential strategy to cure/prevent the development of necrotizing enterocolitis.
During the first days of life, several factors such as formula feeding, high expression of TLR4, microbial colonization and hypoxic stress can trigger an inflammatory process leading to the development of NEC. Exaggerated TLR4 activity in the premature intestine plays a critical role in the pathogenesis of NEC by inducing epithelial cell apoptosis as well as reducing cell proliferation and cell migration throughout the crypt villi-axis. All together, these events lead to the impairment of the epithelial barrier integrity and the translocation of the luminal microorganisms which in turn initiate an exaggerated intestinal inflammatory response. Factors found in the breast milk and/or in amniotic fluid including EGF/HB-EGF and vitamin D have the ability to counteract the TLR4-mediated inflammation. In addition, probiotics can prevent the development of NEC by activating TLR9 and NOD2 and dampen TLR4 signaling.
Besides the critical role of TLR4 in the impairment of the epithelial integrity, a recent report has highlighted the role of TLR4 in the modulation of the CD4 T cell response during NEC. Similarly to inflammatory bowel disease in adults, NEC is associated with an intestinal Th17/Treg imbalance40,48,49. In accordance with this observation, RAG knockout mice, which lack B and T cell repertoires, are protected from NEC40,50. Furthermore, Colliou et al. have identified a commensal Propionibacterium strain named UF1 that has the ability to dampen the intestinal inflammation during NEC via the reduction of Th17 cell expansion in the gut51. Interestingly, Egan et al. demonstrated that TLR4 activation in intestinal epithelial cells was responsible for the Treg/Th17 imbalance through the up-regulation of CCR9/CCL2540. Moreover, the TLR4-mediated Th17 polarization during NEC was associated with the disruption of the epithelial barrier. Hence, the neutralization of IL-17 therefore resulted in reduced NEC severity40.
As mentioned above, TLRs are expressed by different non-hematopoietic cells including endothelial cells where they modulate their function upon pathogen invasion and tissue damage52. It has long been believed that the impairment of the intestinal blood perfusion is a prerequisite for the development of intestinal injury and NEC in preterm infants53. Therefore, it was hypothesized that TLR4 activation specifically on the endothelial cell compartment is an important event preceding NEC development. This hypothesis was tested by Yazji et al. who investigated the impact of a specific deletion of the TLR4 locus in endothelial cells in a mouse model of NEC54. They found that the absence of TLR4 expression in murine endothelial cells is associated with a significant reduction of NEC severity when compared to endothelial TLR4 sufficient animals54. In addition, they showed that the stimulation of TLR4 in the endothelium is accompanied by reduced perfusion of the intestinal microvasculature. This impairment of the microvasculature perfusion was due to MyD-88-dependent reduction of endothelial nitric oxide synthase (eNOS) production54. Importantly, these data further suggest that the absence of nitrate in infant formula might be the cause of impaired intestinal perfusion during NEC. As such, supplementation of infant formula with nitrate restored the intestinal perfusion and lead to a reduced disease severity in a neonatal mouse model of NEC54.
Restraining factors of TLR4 activity in the premature intestine
Despite the experimental evidence attesting the central role of TLR4 in the development of NEC, it is worth noting that only a fraction of premature infants are affected by NEC. This suggests the existence of physiological processes that can counteract the deleterious effects of the high TLR4-mediated activity in the intestine of premature neonates. Several studies have shed light on different molecular mechanisms allowing the premature intestine to prevent the initiation of a harmful TLR4-mediated inflammation in the setting of NEC and will be discussed in further detail.
TLR9 and NOD2
By extending the investigation to the other PRRs, TLR9 and NOD2 were identified as potent inhibitors of TLR4 signaling, thus preventing the development of NEC. Unlike TLR4, which recognizes bacterial lipopolysaccharide, TLR9 has the ability to recognize and to interact with microbial unmethylated CpG DNA motifs55. Interestingly, the apical TLR9 activation prevents the subsequent TLR4-induced inflammation by inhibiting NF-κB signaling56. Furthermore, TLR9 deficient mice are more susceptible to colonic inflammation than their wild littermates56. A time course study revealed that the high TLR4 expression in embryonic murine intestine drastically decreases around delivery, while TLR9 expression follows an opposite pattern suggesting that the intestinal TLR4/TLR9 balance might determine the susceptibility of the premature intestine to NEC57. In agreement with these findings and by contrast to TLR4, TLR9 expression in the intestines of human infants with NEC is significantly lower in comparison with controls57,58. Moreover, the reduced incidence of NEC upon TLR9 stimulation was attributed to the inhibition of TLR4 activity through the localization of IRAK-M in the Golgi apparatus57. In a recent report, we have demonstrated that the protection conferred by Lactobacillus rhamnosus HN001 and its microbial DNA against NEC is achieved by the inhibition of TLR4 in a TLR9-dependent manner59.
In addition to TLR9, it seemed intuitive to explore the implication of Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) in the pathogenesis of NEC given its established role in adult inflammatory bowel disease60. Thus, the genetic analysis performed in a population of infants with a very low birth weight revealed that the accumulation of two or more genetic variants in NOD2 alleles was associated with a high risk of NEC development requiring surgical intervention61. Similarly, Richardson et al. have shown that NOD2 reduces the susceptibility to NEC in mice through the inhibition of TLR4 signaling in enterocytes62. The authors found that the activation of NOD2 resulted in the repression of SMAC (second mitochondria-derived activator of caspases), which in turn leads to reduced enterocyte apoptosis and the enhancement of the mucosal barrier integrity62. Strikingly, it was recently shown that NOD2 could sense the intensity of TLR4 activity in macrophages and can modulate the resulting IL-12 expression63. Under intense TLR4 signaling, NOD2 can repress IL-12 expression while poor TLR4 signaling can switch NOD2 activity toward a synergic stimulation of IL-12 production63. The balance of NOD2 activity in macrophages is regulated by receptor-interacting serine/threonine kinase 2 and the transcriptional regulator CCAAT/enhancer-binding protein α63. However, the existence of such regulatory mechanisms in epithelial cells remains unknown.
Heat shock protein 70 (HSP70)
During heat stress, cells can respond to the deleterious aggregation of unfolded proteins by enhancing the expression of a large panel of protective factors referred to as heat shock proteins64. HSP70, a member of the heat stress protein family, displays a cytoprotective role in intestinal epithelial cells65–67. HSP70 expression in epithelial cells can be triggered by several factors including intestinal microbiota and cytokines. Moreover, HSP70 is induced by breast milk to promote the maintenance of the epithelial barrier in rat immature intestine68. Accordingly, the low expression of HSP70 was associated with the development of NEC69. From the molecular perspective, TLR4 signaling is restrained by HSP70 via a co-chaperone binding domain in the C-terminus of HSP7069. Hence, HSP70 deficiency leads to enhanced disease severity in a murine NEC model, whereas the overexpression or the chemical induction of HSP70 protect against NEC69.
Breast Milk and amniotic fluid
It widely accepted that breast-feeding is critical in shaping the newborn immune system and providing protection against infections and inflammatory disorders later in life70. In the context of NEC, breast milk administration is associated with a lower incidence of the disease suggesting the existence of protective components of breast milk that can potentially be used in new therapeutic approaches71,72. In the same manner, our work revealed that breast milk protects against the development of NEC in murine pups73. This protection was abolished by removing epidermal growth factor (EGF) from the breast milk, indicating that this growth factor is necessary to protect the intestine of premature infant against NEC. Similarly, heparin-binding epidermal growth factor-like growth factor (HB-EGF) was shown to prevent the development of NEC in neonatal rats74,75. These data were further supported the fact that mice lacking the expression of epidermal growth factor receptor (EGFR) in their intestinal epithelium were not protected against NEC with breast milk73. Therefore, genetic polymorphism analysis of the EGFR locus in infants with NEC would be of a great interest. Additional studies revealed that the EGF contained in breast milk prevents NEC development likely via the inhibition of TLR4-induced epithelial cell death and by promoting the regeneration of the mucosal barrier by stimulating enterocyte proliferation73. Moreover, our data showed that the inhibition of TLR4 activation by breast milk is achieved by the suppression of GSK3β activity in enterocytes73.
Similarly to breast milk, amniotic fluid contains several immunomodulatory, and antimicrobial factors, which can improve the mucosal barrier integrity in the setting of intestinal inflammation76. In order explore the effect of amniotic fluid on gut inflammation, newborn rats were injected with amniotic fluid stem (AFS) cells and subjected to experimental NEC77. The animals, which received AFS cells, displayed reduced NEC incidence and enhanced epithelial cell regeneration. The beneficial effect of amniotic fluid was ascribed to an augmented expression of cyclooxygenase 2 in stromal cells77. It was also suggested that the protective function of COX-2 might be due to an indirect activation of EGFR77. In another study, we have shown that the reduction of epithelial EGFR expression was associated increased susceptibility to NEC in mice and humans78. Similarly, to breast milk, we found that amniotic fluid dampens TLR4-mediated inflammation through the activation of EGFR signaling in neonatal intestine78. This was consistent with the absence of amniotic fluid protection in the mice lacking the expression of EGFR in intestinal epithelial cells78. It is worth mentioning that breast milk of mothers giving birth to extremely premature infants is significantly enriched in EGF, suggesting that at least some of the premature infants have defective EGFR signaling79
Vitamin D
Vitamin D is a fat-soluble vitamin with a large spectrum of biological activities80. In the liver, Vitamin D is transformed into 1,25-dihydroxyvitamin D, an active metabolite that signals through vitamin D receptor (VDR)80. Vitamin D plays an important role in regulating both innate and adaptive immune responses81. For example, it was shown that vitamin D– deficiency was positively correlated with the severity of human as well as murine IBD82–84. Consistently, in vivo and in vitro data demonstrated that Vitamin D promotes epithelial cell migration and the enhancement of tight junctions84. In addition, Vitamin D vitiates NF-Κb signaling, resulting in reduced epithelial cell death83. In the same way, a low level of maternal/neonatal 1,25-dihydroxyvitamin D was shown to be a risk for NEC development85,86. In accordance with the protective role of vitamin D in the context of adult IBD, Shi et al. have found that vitamin D treatment significantly attenuates NEC severity in a rat model. Notably, they also and found that Vitamin D significantly repressed TLR4 expression, while promoting the expression of tight junction proteins and dampening epithelial cell apoptosis86.
Summary
Despite the recent advances in our understanding of the pathophysiology of NEC, the translation into an effective therapy has yet to occur, and the mortality caused by this disease in the premature infants remains high. As described above, TLR4 signaling plays a central role in the induction of NEC through the modulation of the epithelial cell barrier and the regulation of the innate and adaptive immune responses. Therefore, TLR4 inhibition seems to be a promising target for new drug development and it is our hope that one day we can prevent this devastating consequence of prematurity.
Key points.
Necrotizing enterocolitis is a devastating gastrointestinal disease associated with microbial colonization of the immature intestine, which can result in an inappropriate immune response.
The inflammatory response is partly due to the high expression of TLR4 in the intestine of premature infants.
Exaggerated TLR4 signaling results in the impairment of the epithelial barrier.
Inhibition of the TLR4-signaling pathway and its downstream targets can lead to reduced NEC severity, and may translate into new drugs to treat and/or to prevent NEC development.
Best Practices Box
What is the current practice?
Necrotizing enterocolitis (NEC) is a common gastrointestinal disease with a high mortality rate that affects premature infants.
Risk factors for NEC include prematurity, microbial colonization and lack of breast milk feedings.
NEC involves an exaggerated immune response in the setting of elevated expression of toll-like receptor 4 (TLR4) leading to necrosis of the epithelial barrier.
Currently, there is no effective treatment of optimal therapeutic strategy to prevent NEC.
What changes in current practice are likely to improve outcomes?
Prioritizing human milk feedings can decrease the risk of NEC in premature infants.
Several potential TLR4 signaling related molecules have been identified as potential targets to identify infants at the highest risk for NEC and to develop novel therapeutics.
Summary Statement
NEC is a devastating consequence of prematurity and microbial imbalance in the intestine. The use of pre-clinical animal models has identified potential new therapeutic strategies which could be translated into clinical trials in the near future.
Acknowledgments
Funding Sources: MG is supported by grants K08DK101608 and R03DK111473 from the National Institutes of Health, March of Dimes Foundation Grant No. 5-FY17–79, and the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose and no conflicts of interest.
References
- 1.Zhang Q, Zmasek CM, Godzik A. Domain architecture evolution of pattern-recognition receptors. Immunogenetics. 2010;62(5):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140(6):805–820. [DOI] [PubMed] [Google Scholar]
- 3.Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol 2013;14(7):668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torow N, Marsland BJ, Hornef MW, Gollwitzer ES. Neonatal mucosal immunology. Mucosal Immunol 2017;10(1):5–17. [DOI] [PubMed] [Google Scholar]
- 5.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017;46(3):350–363. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Z, Ye GY. 1:4 matched case-control study on influential factor of early onset neonatal sepsis. Eur Rev Med Pharmacol Sci 2013;17(18):2460–2466. [PubMed] [Google Scholar]
- 7.Gritz EC, Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr 2015;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 2017;66(4):515–522. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi J, Bobe AM, Miyoshi S, et al. Peripartum Antibiotics Promote Gut Dysbiosis, Loss of Immune Tolerance, and Inflammatory Bowel Disease in Genetically Prone Offspring. Cell Rep 2017;20(2):491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouskra D, Brezillon C, Berard M, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008;456(7221):507–510. [DOI] [PubMed] [Google Scholar]
- 11.Hall JA, Bouladoux N, Sun CM, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 2008;29(4):637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012;130(4):e794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Rosa PS, Warner BB, Zhou Y, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A 2014;111(34):12522–12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yee WH, Soraisham AS, Shah VS, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129(2):e298–304. [DOI] [PubMed] [Google Scholar]
- 15.Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol 2013;40(1):27–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364(3):255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasari P, Zola H, Nicholson IC. Expression of Toll-like receptors by neonatal leukocytes. Pediatr Allergy Immunol 2011;22(2):221–228. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JP, Yang Y, Levy O, Chen C. Human neonatal peripheral blood leukocytes demonstrate pathogen-specific coordinate expression of TLR2, TLR4/MD2, and MyD88 during bacterial infection in vivo. Pediatr Res 2010;68(6):479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavoie PM, Huang Q, Jolette E, et al. Profound lack of interleukin (IL)-12/IL-23p40 in neonates born early in gestation is associated with an increased risk of sepsis. J Infect Dis 2010;202(11):1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbett NP, Blimkie D, Ho KC, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 2010;5(11):e15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009;183(11):7150–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danis B, George TC, Goriely S, et al. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur J Immunol 2008;38(2):507–517. [DOI] [PubMed] [Google Scholar]
- 24.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol 2006;177(3):1956–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drygiannakis I, Ernst PB, Lowe D, Glomski IJ. Immunological alterations mediated by adenosine during host-microbial interactions. Immunol Res 2011;50(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Power Coombs MR, Belderbos ME, Gallington LC, Bont L, Levy O. Adenosine modulates Toll-like receptor function: basic mechanisms and translational opportunities. Expert Rev Anti Infect Ther 2011;9(2):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito S, Serra D, Gualtieri L, Cesati L, Principi N. Vaccines and preterm neonates: why, when, and with what. Early Hum Dev 2009;85(10 Suppl):S43–45. [DOI] [PubMed] [Google Scholar]
- 28.Baxter D Impaired functioning of immune defenses to infection in premature and term infants and their implications for vaccination. Hum Vaccin 2010;6(6):494–505. [DOI] [PubMed] [Google Scholar]
- 29.Volpe JJ. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr 2008;153(2):160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pryhuber GS. Postnatal Infections and Immunology Affecting Chronic Lung Disease of Prematurity. Clin Perinatol 2015;42(4):697–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res 2001;49(4):589–593. [DOI] [PubMed] [Google Scholar]
- 32.Nanthakumar N, Meng D, Goldstein AM, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One 2011;6(3):e17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 2006;203(4):973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Mandat Schultz A, Bonnard A, Barreau F, et al. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PLoS One 2007;2(10):e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caplan MS, Sun XM, Hsueh W. Hypoxia causes ischemic bowel necrosis in rats: the role of platelet-activating factor (PAF-acether). Gastroenterology 1990;99(4):979–986. [DOI] [PubMed] [Google Scholar]
- 36.Soliman A, Michelsen KS, Karahashi H, et al. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis. PLoS One 2010;5(10):e15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neal MD, Leaphart C, Levy R, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 2006;176(5):3070–3079. [DOI] [PubMed] [Google Scholar]
- 38.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 2006;177(5):3273–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leaphart CL, Cavallo J, Gribar SC, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 2007;179(7):4808–4820. [DOI] [PubMed] [Google Scholar]
- 40.Egan CE, Sodhi CP, Good M, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 2016;126(2):495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qureshi FG, Leaphart C, Cetin S, et al. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology 2005;128(4):1012–1022. [DOI] [PubMed] [Google Scholar]
- 42.Neal MD, Sodhi CP, Jia H, et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem 2012;287(44):37296–37308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sodhi CP, Shi XH, Richardson WM, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 2010;138(1):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neal MD, Sodhi CP, Dyer M, et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol 2013;190(7):3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai S, Sodhi C, Cetin S, et al. Extracellular high mobility group box-1 (HMGB1) inhibits enterocyte migration via activation of Toll-like receptor-4 and increased cell-matrix adhesiveness. J Biol Chem 2010;285(7):4995–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afrazi A, Branca MF, Sodhi CP, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem 2014;289(14):9584–9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sodhi CP, Neal MD, Siggers R, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012;143(3):708–718 e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weitkamp JH, Koyama T, Rock MT, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 2013;62(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang Y, Du X, Xu X, Wang M, Li Z. Monocyte activation and inflammation can exacerbate Treg/Th17 imbalance in infants with neonatal necrotizing enterocolitis. Int Immunopharmacol 2018;59:354–360. [DOI] [PubMed] [Google Scholar]
- 50.He YM, Li X, Perego M, et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat Med 2018;24(2):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colliou N, Ge Y, Sahay B, et al. Commensal Propionibacterium strain UF1 mitigates intestinal inflammation via Th17 cell regulation. J Clin Invest 2017;127(11):3970–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salvador B, Arranz A, Francisco S, et al. Modulation of endothelial function by Toll like receptors. Pharmacol Res 2016;108:46–56. [DOI] [PubMed] [Google Scholar]
- 53.Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg 2013;22(2):83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yazji I, Sodhi CP, Lee EK, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A 2013;110(23):9451–9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000;408(6813):740–745. [DOI] [PubMed] [Google Scholar]
- 56.Lee J, Mo JH, Katakura K, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 2006;8(12):1327–1336. [DOI] [PubMed] [Google Scholar]
- 57.Gribar SC, Sodhi CP, Richardson WM, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 2009;182(1):636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin Y, Liu F, Li Y, Tang R, Wang J. mRNA expression of TLR4, TLR9 and NF-kappaB in a neonatal murine model of necrotizing enterocolitis. Mol Med Rep 2016;14(3):1953–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Good M, Sodhi CP, Ozolek JA, et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol 2014;306(11):G1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckmann L, Karin M. NOD2 and Crohn’s disease: loss or gain of function? Immunity 2005;22(6):661–667. [DOI] [PubMed] [Google Scholar]
- 61.Hartel C, Hartz A, Pagel J, et al. NOD2 Loss-of-Function Mutations and Risks of Necrotizing Enterocolitis or Focal Intestinal Perforation in Very Low-birth-weight Infants. Inflamm Bowel Dis 2016;22(2):249–256. [DOI] [PubMed] [Google Scholar]
- 62.Richardson WM, Sodhi CP, Russo A, et al. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology 2010;139(3):904–917, 917 e901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim H, Zhao Q, Zheng H, Li X, Zhang T, Ma X. A novel crosstalk between TLR4-and NOD2-mediated signaling in the regulation of intestinal inflammation. Sci Rep 2015;5:12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell 2010;40(2):253–266. [DOI] [PubMed] [Google Scholar]
- 65.Hu S, Zhu X, Triggs JR, et al. Inflammation-induced, 3’UTR-dependent translational inhibition of Hsp70 mRNA impairs intestinal homeostasis. Am J Physiol Gastrointest Liver Physiol 2009;296(5):G1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musch MW, Sugi K, Straus D, Chang EB. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology 1999;117(1):115–122. [DOI] [PubMed] [Google Scholar]
- 67.Kojima K, Musch MW, Ren H, et al. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology 2003;124(5):1395–1407. [DOI] [PubMed] [Google Scholar]
- 68.Liedel JL, Guo Y, Yu Y, et al. Mother’s milk-induced Hsp70 expression preserves intestinal epithelial barrier function in an immature rat pup model. Pediatr Res 2011;69(5 Pt 1):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Afrazi A, Sodhi CP, Good M, et al. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol 2012;188(9):4543–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 2007;61(1):2–8. [DOI] [PubMed] [Google Scholar]
- 71.Barlow B Letter: Necrotizing enterocolitis: protective factor in breast milk. N Engl J Med 1976;294(15):844–845. [PubMed] [Google Scholar]
- 72.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990;336(8730):1519–1523. [DOI] [PubMed] [Google Scholar]
- 73.Good M, Sodhi CP, Egan CE, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol 2015;8(5):1166–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg 2007;42(1):214–220. [DOI] [PubMed] [Google Scholar]
- 75.Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology 2009;137(1):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dasgupta S, Jain SK. Protective effects of amniotic fluid in the setting of necrotizing enterocolitis. Pediatr Res 2017;82(4):584–595. [DOI] [PubMed] [Google Scholar]
- 77.Zani A, Cananzi M, Fascetti-Leon F, et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 2014;63(2):300–309. [DOI] [PubMed] [Google Scholar]
- 78.Good M, Siggers RH, Sodhi CP, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci U S A 2012;109(28):11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dvorak B, Fituch CC, Williams CS, Hurst NM, Schanler RJ. Increased epidermal growth factor levels in human milk of mothers with extremely premature infants. Pediatr Res 2003;54(1):15–19. [DOI] [PubMed] [Google Scholar]
- 80.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29(6):726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun J Vitamin D and mucosal immune function. Curr Opin Gastroenterol 2010;26(6):591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 2000;130(11):2648–2652. [DOI] [PubMed] [Google Scholar]
- 83.Liu W, Chen Y, Golan MA, et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest 2013;123(9):3983–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008;294(1):G208–216. [DOI] [PubMed] [Google Scholar]
- 85.Cetinkaya M, Erener-Ercan T, Kalayci-Oral T, et al. Maternal/neonatal vitamin D deficiency: a new risk factor for necrotizing enterocolitis in preterm infants? J Perinatol. 2017;37(6):673–678. [DOI] [PubMed] [Google Scholar]
- 86.Shi Y, Liu T, Zhao X, et al. Vitamin D ameliorates neonatal necrotizing enterocolitis via suppressing TLR4 in a murine model. Pediatr Res 2018;83(5):1024–1030. [DOI] [PubMed] [Google Scholar]