Abstract
This review summarizes available evidence on the relationship between red blood cell transfusion, anemia and necrotizing enterocolitis (NEC). We review recent studies that highlight the uncertainty of the effect of red blood cell transfusion on NEC and the potential role of anemia. We also discuss potential pathophysiologic effects of both red blood cell transfusion and anemia and highlight strategies to prevent anemia and red blood cell transfusion. We also discuss ongoing randomized trials that are likely to provide important new evidence to guide red blood cell transfusion practices.
Keywords: Infant, neonate, preterm, blood, oxygenation, morbidity
Introduction
Necrotizing enterocolitis (NEC) is major contributor to morbidity and mortality in infants, accounting for 10% of deaths in the NICU 1,2. Recent data suggest the incidence of NEC is decreasing in the US 3. However, the reported incidence of NEC is highly variable among high-income countries 4. The pathogenesis of NEC is multifactorial and includes innate, maternal and postnatal risk factors. Multiple observational studies have demonstrated an association between NEC and the potentially modifiable risk factors of anemia and red blood cell (RBC) transfusion. Some authors have proposed that the occurrence of NEC in temporal association with an RBC transfusion is a distinct clinical entity, separate from non-transfusion associated NEC 5. This review will appraise data on the epidemiology of NEC with a focus on the potential role of RBC transfusion and anemia.
Overview of transfusion practices
Neonatal hemoglobin (Hb) levels decline in the days and weeks after birth 6. Preterm infants, in comparison to term infants, have a relatively lower Hb level at birth 7 and experience a greater Hb decline during the neonatal period 8. This decline in Hb often leads to treatment with a RBC transfusion. The ideal threshold for administering an RBC transfusion is currently not known. Clinicians often consider the Hb level along with the postnatal age of the infant and need for cardiorespiratory support to guide their decision for when to administer an RBC transfusion. Studies of direct comparisons of restrictive (low Hb threshold) versus liberal (high Hb threshold) strategies are limited to 3 randomized control trials 9–11 and two ongoing trials 12,13. The Hb transfusion thresholds in the restrictive and liberal arms of these trials are summarized in Table 1.
Table 1.
Hemoglobin Transfusion Thresholds Used in Clinical Trials
| Study | Postnatal age (d) | Clinical status | Restrictive Hb threshold (g/dLa) | Liberal Hb threshold (g/dLa) |
|---|---|---|---|---|
| PINT Trial, 200510 | 1-7d | Any respiratory support (Capillary/central sample) | 11.5/10.4 | 13.5/12.2 |
| No respiratory support (Capillary/central sample) | 10.0/9.0 | 12.0/10.9 | ||
| 8-14 d | Any respiratory support (Capillary/central sample) | 10.0/9.0 | 12.0/10.9 | |
| No respiratory support (Capillary/central sample) | 8.5/7.7 | 10.0/9.0 | ||
| ≥ 15 | Any respiratory support (Capillary/central sample) | 8.5/7.7 | 10.0/9.0 | |
| No respiratory support (Capillary/central sample) | 7.5/6.8 | 8.5/7.7 | ||
| Bell, 2005 9 | Ventilated | 11.3 | 15.3 | |
| CPAP or Oxygen | 9.3 | 12.7 | ||
| No respiratory support | 7.3 | 10.0 | ||
| Chen, 2009 11 | Ventilated | 11.7 | 15.0 | |
| CPAP | 10.0 | 13.3 | ||
| Spontaneous breathing | 7.3 | 10.0 | ||
| TOP Trial 12 | Week 1 | Any respiratory support | 11.0 | 13.0 |
| No respiratory support | 10.0 | 12.0 | ||
| Week 2 | Any respiratory support | 10.0 | 12.5 | |
| No respiratory support | 8.5 | 11.0 | ||
| Week 3 | Any respiratory support | 8.5 | 11.0 | |
| No respiratory support | 7.0 | 10.0 | ||
| ETTNO Trial 13 | 3-7 d | Criticalb | 11.3 | 13.6 |
| Non critical | 9.3 | 11.7 | ||
| 8-21d | Criticalb | 10.0 | 12.3 | |
| Non critical | 8.0 | 10.3 | ||
| >21 d | Criticalb | 9.0 | 11.3 | |
| Non critical | 7.0 | 9.3 |
If hematocrit was reported, the Hb threshold was approximated by dividing by 3.
Critical defined as any of the following: (1) requirement of mechanical ventilation (2) requirement of CPAP with FiO2 >0.25 for >12 h per 24 h, (3) patent ductus arteriosus requiring therapy, (4) more than 6 apneas that require stimulation per 24 h, or more than 4 desaturations to SpO2 <60% per 24 h despite methylxanthines and CPAP and (5) acute sepsis or acute NEC requiring inotropic or vasopressor support.
Abbreviations: Hb, Hemoglobin; CPAP, continuous positive airway pressure; PINT, Premature Infants in Need of Transfusion; TOP Trial, Transfusion of Prematures Trial, ETTNO, Effects of Transfusion Thresholds on Neurocognitive Outcome of Extremely Low Birth Weight Infants
Temporal trends suggest increasingly restrictive RBC transfusion practices, with acceptance of a lower concentration of Hb before a RBC transfusion 14–16. In the absence of a clear advantage of either approach, the optimal Hb transfusion threshold remains uncertain with a recent Cochrane review justifying clinical equipoise 17.
Data on RBC transfusion and NEC
Since the publication of several initial reports of a temporal association between RBC transfusion and NEC 18–20, multiple subsequent observational studies 21–36 have reported on the association between RBC transfusion and NEC. Most of these observational studies report on NEC occurring within 48 hours of an RBC transfusion, although the 48-hour cut-off is arbitrary.
Several systematic reviews and meta-analyses have summarized and evaluated the potential association between RBC transfusion and NEC reported in the studies (Table 2). Two meta-analysis of observational studies 37,38 were published in 2017. A meta- analysis by Garg et. al. of 17 observational studies reported no evidence of an association between exposure to RBC transfusion and the risk of NEC (OR 0.96, 95% CI: 0.53-1.71, P=0.88) with high study heterogeneity (I2 = 93%) 37. In addition, the authors performed subgroup analyses and found heterogeneity in results by study type (cohort studies and case-control studies). Analysis of data from 4 cohort studies showed a significant association between RBC transfusion and a lower risk of NEC (OR: 0.51, 95% CI: 0.34-0.75, P = <0.01) with low statistical heterogeneity (I2 = 28%). By comparison, subgroup analysis of 13 case-control studies showed no difference in odds for NEC with RBC transfusion (OR: 1.20, 95% CI: 0.58-2.47, P = 0.63) with high heterogeneity (I2 = 93%). Another meta-analysis by Hay et al. of 13 observational studies found no evidence of an association between RBC transfusion and NEC occurring within 48 hours of transfusion (OR = 1.13, 95% CI: 0.99-1.29) with high statistical heterogeneity among studies (I2 = 93%) 38. The authors concluded that there was a very low confidence of a true relationship between RBC transfusion and NEC, based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
Table 2.
Meta-analyses of observational studies reporting on RBC transfusion and NEC
| Study author, year | Number of studies | Study types | NEC Events related to RBC transfusion / Total | NEC Events unrelated to RBC transfusion / Total | I2 | Summary odds ratio (95% CI) |
|---|---|---|---|---|---|---|
| Kirpalani, 2012 40 | 6 | Cohort studies | 150/2940 | 192/19215 | 98% | 7.48 (5.87-9.53) |
| 4 | Case Control studies | 129/186 | 129/381 | 92% | 2.19 (1.52-3.17) | |
| Mohamed, 2012 39 | 5 | All observational studies reporting unadjusted estimates | N/A | N/A | 58% | 3.91 (2.97-5.14) |
| 4 | All observational studies reporting adjusted estimates | N/A | N/A | 91% | 2.01 (1.61-2.50) | |
| Garg, 2017 37 | 17 | All observational studies | N/A | N/A | 93% | 0.96 (0.53-1.71) |
| 4 | Cohort studies | N/A | N/A | 28% | 0.51 (0.34-0.75) | |
| 13 | Case control studies (3 unmatched, 10 matched) | N/A | N/A | 93% | 1.20 (0.58-2.47) | |
| Hay, 2017 38 | 13 | All observational studies reporting on NEC within 48 hours of transfusion | 479/4498 | 1242/7104 | 93% | 1.13 (0.99-1.29) |
| 9 | All observational studies reporting on NEC at anytime after transfusion | 334/2380 | 256/2541 | 86% | 1.95 (1.60-2.38) |
These results from more-recent meta-analyses are in contrast from results of two previous meta-analyses of observational studies in 2012 by Mohamed and Shah 39 and by Kirpalani and Zupancic 40 that reported an increased risk of NEC within 48 hours after receiving an RBC transfusion. The meta-analysis by Mohamed and Shah of 4 studies reported a pooled adjusted OR for NEC of 2.01 (95% CI 1.61-2.50, I2= 91%) among RBC exposed infants. Kirpalani and Zupancic included only full length publications and excluded data from abstracts and reported an increased risk for NEC with blood transfusion in 6 cohort studies (unadjusted OR 7.48, CI: 5.87-9.53) and in 4 case control studies (OR 2.19, CI: 1.52-3.17).
The differences in the results of meta-analyses from 2012 and 2017 may be from publication bias, as noted by Hay et al. 38, with earlier studies predominantly reporting positive associations between RBC transfusion and NEC and more recent studies reporting no association and some suggesting RBC transfusion may be protective towards NEC. In addition, a meta-analysis of randomized trials 40 comparing restrictive and liberal transfusion strategies in preterm infants found no effect of more restrictive thresholds (leading to fewer RBC transfusions), compared to liberal RBC transfusion thresholds (leading to more RBC transfusions) on the risk of NEC (OR = 1.67, 95% CI: 0.82-3.38). Notably, the estimates are heavily weighted by a single trial (PINT trial) 10 with weight of 89% (Table 3). In addition, these trials did not report on a temporal relationship between RBC transfusion and NEC. In the absence of higher quality data, the question of does RBC transfusion cause NEC remains unresolved.
Table 3.
Meta-analyses of randomized trials reporting on RBC transfusion and NEC
| Study author, year | Number of trials | NEC events with restrictive RBC transfusion threshold | NEC events with liberal RBC transfusion threshold | I2 | Odd ratio of NEC (95% CI), restrictive vs. liberal RBC transfusion threshold |
|---|---|---|---|---|---|
| Kirpalani, 2012 40 | 3 | 21/292 | 13/298 | 0% | 1.67 (0.82-3.38) |
Note: Estimates similar to meta-analyses by Whyte and Kirpalani. Cochrane Database Syst Rev. 2011 Nov 9;(11):CD000512 and, therefore, not repeated.
Data on anemia and NEC
Several observational studies that reported on an association between RBC transfusion and NEC did not report a significant effect of anemia as an independent risk for NEC 23,24,29,41. However, in a case-control study, Singh et al. identified 111 preterm (≤ 32 weeks) infants with NEC and 222 matched controls and reported that, after controlling for other factors, each one point decrease in the nadir hematocrit was associated with a 10% increase in odds for NEC (OR 1.10, 95% CI: 1.02-1.18). Patel et al 42, in a prospective multicenter study, reported that in a given week, severe anemia, defined as Hb level of 8.0 g/dL or less, was associated with a higher adjusted risk for NEC (adjusted cause-specific hazard ratio: 5.99, 95% CI: 2.00-18.0, P = 0.001). However, the study did not evaluate the interaction between severe anemia and RBC transfusion. A recent case-crossover study by Le et al. 43, designed to identify an association of NEC with RBC transfusion, feed advances or fortification, found no evidence of an association between RBC transfusion and NEC (OR = 1.80, 95% CI: 0.60-5.37). A subgroup analysis showed that among anemic infants (Hb ≤ 9.3 g/dL), the risk of RBC transfusion on NEC was higher (OR: 6; 95% CI: 0.72-49.8), compared to those without anemia (OR: 1, 95% CI: 0.20-4.95), but the difference in effect estimates among subgroups was not statistically significant.
It is plausible that the occurrence of NEC after an RBC transfusion is the result of interaction between the effect of anemia and the effect of RBC transfusion. Evaluating such an interplay between the contribution of anemia and RBC transfusion is challenging in clinical studies, as assessing interaction between two exposures (anemia and RBC transfusion) typically requires a much larger sample size than assessing the effect of a single exposure (anemia or RBC transfusion). Additionally, lower Hb oxygen saturation targeting, an important determinant of oxygenation that increases the risk of NEC 44, has not been measured, controlled or reported in observational studies of RBC transfusion-associated NEC, limiting the understanding of the interaction between Hb saturation and anemia. Pre-clinical studies offer an opportunity to assess the biologic plausibility of such an interaction and may provide data on the plausibility of such an interaction that is challenging to assess without very large, adequately powered randomized trials. Two ongoing, large randomized trials 12,13 comparing liberal and restrictive transfusion thresholds are designed to assess the effect of high vs. low transfusion thresholds on survival and long-term neurocognitive outcomes. However, with NEC as a secondary outcome measure, these trials may contribute important data on the effect of both RBC transfusion and anemia (by comparing high and low Hb transfusion thresholds) on NEC when these results are considered alongside those of prior trials.
Potential mechanisms underlying the associations
The development of NEC in an infant is considered the final common end-point of a multitude of etiologic pathways 45 that result in disruption of mucosal integrity and inflammation from responses to intraluminal pathogenic organisms. Several mechanisms for intestinal injury in response to RBC transfusion, with or without the presence of anemia, have been proposed (Figure 1) and are discussed in additional detail below.
Figure 1.
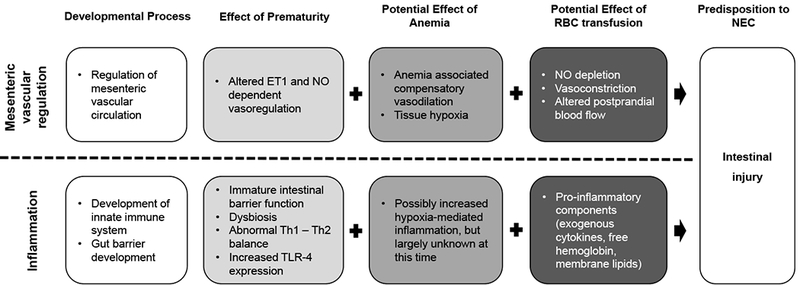
Potential mechanisms for the pathogenesis of intestinal injury from anemia and RBC transfusion.
Abbreviations: NEC, Necrotizing Enterocolitis; ET1, Endothelin 1; NO, Nitric Oxide; TLR-4, Toll-like receptor 4; Th, T helper cell
Hypoxemia and dysregulation of mesenteric blood flow
Reversible binding of oxygen to Hb accounts for more than 98% of oxygen carriage by blood. At physiologic partial pressure of oxygen, 100 ml of plasma contains 0.3 ml of oxygen. In comparison, each gram of Hb can combine with 1.34 ml of oxygen 46. A decrease in blood Hb concentration leads to circulatory adjustments such as increases in capillary perfusion and increased oxygen extraction by tissues 46,47. However, worsening anemia may overwhelm the compensatory mechanisms and significantly impair the ability of blood to meet oxygen demands of the tissues causing hypoxia 48, which may be worsened in the setting of lower oxygen saturations targets 44. Molecular compensatory mechanisms exist to maintain the gut barrier in the setting of hypoxia 49,50. However, it has been proposed that progressive hypoxia may reach a critical imbalance in oxygen delivery as compared to consumption leading to mucosal barrier injury 51 and poor mucosal healing 52, predisposing the neonate to development of NEC 5.
In a growing neonate, the intestines proliferate as the gut elongates and the mucosa grows. Active expression of angiogenic factors in the metabolically active and rapidly proliferating gut mucosa ensures concomitant development of vascular structures in the intestine 53. It has been proposed that the developing thin arterioles may be structurally weak, and upon exposure to an RBC transfusion and associated alterations in oxygen availability, blood pressure, flow or viscosity, these arterioles are prone to injury precipitating ischemic injury to the gut mucosa 5,54. However, experimental evidence is needed to confirm the proposed mechanisms.
Mesenteric or splanchnic blood flow is determined by a dynamic balance between vasoconstrictive and vasodilatory inputs by mediators such as endothelin-1 (ET-1) and nitric oxide (NO) 54. Ontogeny of the regulatory mechanisms of the mesenteric vascular tone during the neonatal period demonstrates distinct responses to autonomic, humoral and paracrine factors as compared to a mature infant 55,56. In the newborn, the balance is favored towards NO-mediated vasodilation 55,57. In addition, as compared to older subjects, NO inhibition leads to a greater increase in vascular resistance in newborn animal models 58–60. RBC transfusion leads to an alteration in post-prandial response to mesenteric blood flow as evident from a clinical study by Krimmel et al. 61. Analysis of pre- and post-prandial mesenteric blood flow in 22 infants (mean gestational age, 27.3 weeks, mean postmenstrual age, 31.8 weeks) demonstrated that anemia was associated with increased flow in the superior mesenteric artery following feeding, which was evident pre-transfusion and absent in the immediate post-transfusion state. One potential mechanism for this decreased blood flow is by RBC transfusion-associated depletion of intravascular NO 62. This may be secondary to depletion of NO in RBCs during storage, consumption of NO through binding to free Hb released from hemolysis or from release of arginase from RBCs, which depletes the NO precursor arginine 62. The transient anemia-associated hypoxia followed by reperfusion after RBC transfusion and associated dysregulation of blood flow may have a cumulative and/or interactive role in pathogenesis of disease.63
Role of inflammation
The occurrence of NEC in response to RBC transfusion or anemia has been proposed to be the outcome of a two-hit mechanism 5, similar to the proposed mechanisms for transfusion-related acute lung injury (TRALI), a condition that can occur following transfusion of any blood component. In TRALI, underlying clinical conditions lead to endothelial activation in the host (first hit), which in the presence of a blood product transfusion and associated exposure to mediators such as donor HLA antibodies, biologically active lipids, free Hb, red cell membrane fragments, and inflammatory cytokines (second hit) lead to a severe inflammatory response and associated lung injury.
It has been proposed that the immature neonatal gut is in a heightened state of immune activation and prone to inflammation. Multiple factors such as mucosal exposure to substrates, hypoxia, changes in the gut microbiome associated with the use of antibiotics and formula feeds 5,16,64–66 have been proposed to contribute to inflammation and associated phenotypic shift from TH2 to TH1 67. Upregulation of Toll Like Receptors (TLR), particularly TLR-4, 68 is a significant contributor to intestinal inflammation 68,69.
In such a background, RBC transfusion can potentially introduce biological response modifiers such as donor antibodies 70, cytokines in stored blood 71 free Hb 72, lipids from RBC membranes and white cells generating an exaggerated systemic immune response that may cause gut mucosal inflammation and injury. Dani et al 73 demonstrated serum cytokine changes after an RBC transfusion event in 20 infants less than 32 weeks gestational age. The study identified significant increases in interferon-gamma, monocyte chemoattractant protein-1, intracellular adhesion molecule-1 (ICAM-1), and interleukins (IL) IL-1β, IL-8 and IL-17 after an RBC transfusion. Ho et al. 74 measured fecal calprotectin (FC) before and after 46 RBC transfusion events in 26 VLBW infants, and showed that FC was higher than baseline after RBC transfusion and was higher in multiply-transfused infants. Notably, FC was the highest in infants with the lowest pre-transfusion hematocrits and in those who received RBCs that had been stored for >21 days.
In a background of constitutive vasodilation and increased reactivity in the neonatal gut vasculature, despite compensatory hemodynamic and molecular changes, progressive anemia may reach a critical level leading to hypoxia. RBC transfusion in such a state may lead to depletion of NO and loss of vasodilation along with abnormal regulation of mesenteric blood flow leading to tissue ischemic injury. Multiple factors associated with prematurity such as dysbiosis and increased TLR-4 expression cause a state of inflammation in the intestinal mucosa that can potentially increase with hypoxia. Introduction of exogenous biological response modifiers in transfused products may lead to heightened immune responses leading to damage to the intestinal mucosa. However, additional data are needed from both human and preclinical studies to better understand the mechanisms that may underlie the possible adverse effects of both RBC transfusion and anemia on the neonatal intestine.
Influence of clinical strategies to prevent anemia and RBC transfusion
Iatrogenic phlebotomy loss, a result of intensive clinical monitoring in critically ill newborns, is a major cause of neonatal anemia and driver of RBC transfusion 75. The common strategies to minimize blood sampling in the neonatal intensive care unit includes the use of noninvasive monitoring and point of care testing 76 and use of umbilical cord blood for admission blood tests for VLBW preterm neonates 77. With a combination of several approaches and ongoing vigilance, studies have shown a significant effect in preventing anemia and decreasing RBC transfusion 78.
Placental transfusion achieved by delayed clamping of the umbilical cord after birth or by milking of the umbilical cord before clamping is now recommended as standard care for neonatal resuscitation of preterm infants 79–81. A 2012 Cochrane review of 15 randomized controlled trials, 5 of which reported NEC as an outcome measure, indicated a decreased risk for NEC in infants receiving delayed cord clamping, compared to immediate cord clamping (n=241, RR = 0.62, 95% CI: 0.43-0.9) 82. However, a more recent meta-analysis, including 12 studies that reported on NEC, found that delayed cord clamping was not associated with a decreased risk for NEC for all infants < 37 weeks’ gestation at birth (n=2397, RR = 0.88, CI 0.65-1.18) and for infants born < 28 weeks’ gestation (4 studies, n = 977, RR = 0.87, CI 0.61-1.24) 83. The quality of evidence was determined as low using the GRADE criteria. Notably, the findings of this recent meta-analysis were weighted heavily by the Australian Placental Transfusion Study (APTS) trial of 1566 infants born < 30 weeks gestation, randomized to placental transfusion by delayed cord clamping or early clamping 84. In this trial 44/712 (6.2%) infants randomized to delayed cord clamping developed NEC, as compared to 41/734 infants (5.6%) randomized to early clamping. Importantly, the effect of delayed cord clamping on Hb nadir and RBC transfusion requirements is likely to depend on postnatal RBC transfusion approaches.
Apart from its role in prompting erythropoiesis and decreasing need for RBC transfusion, Erythropoietin (EPO), which is also present in breast milk 85, may play a role in intestinal development, cellular repair 86 and inhibition of NO formation 87. Ledbetter et al. first reported an association of rEPO administration in infants ≤ 1250 g and a decreased incidence of NEC (4.6% in rEPO group as compared to 10% in controls) 88. A recent Cochrane meta-analysis of RCT of early (< 8d age) administration of erythropoiesis stimulating agents (ESA, EPO or darbepoetin) versus placebo or no intervention included 15 studies (n=2639) 89. The analysis demonstrated a significantly reduced risk for NEC (any stage) in the ESA group compared with the placebo group (RR 0.69, 95% CI 0.52-0.91, I2 = 0%). The quality of the evidence was deemed moderate 89,90. Previous concerns regarding the increased risk for retinopathy of prematurity (all stages) with EPO administration 91 were not demonstrated in this meta-analysis (11 studies, n=2185, RR = 0.92, 95% CI 0.79-1.08; I2 = 0%) 89,91. A Cochrane meta-analysis of late EPO administration (8-28 d) in ELBW infants (6 studies, n= 656) did not demonstrate any difference in the risk for NEC with EPO as compared to placebo or no intervention (RR = 0.88, 95% CI 0.46-1.69, I2 = 0%) 90. Late EPO was associated with a non-significant trend towards an increased risk for ROP (Stage≥ 3, 3 studies, n=442) with a RR 1.73 (95% CI 0.92-3.24, I2 = 18%) and for all ROP stages (3 studies, n=404) with a RR 1.27 (95% CI 0.99-1.64, I2 = 83%). Two ongoing large randomized trials of EPO administration in preterm infants, Preterm Erythropoietin Neuroprotection Trial (PENUT Trial, NCT01378273) 92 and Erythropoietin in Premature Infants to Prevent Encephalopathy (NCT02550054) 92, include NEC as a secondary outcome and will provide additional evidence regarding the effect of EPO on the risk of NEC and the safety of EPO administration in different populations.
Role of feeding during RBC transfusion
With the recognition of a potential association between RBC transfusion and development of NEC, there has been interest in withholding feeding during RBC transfusion. In prospective observational studies, feeding immediately after RBC transfusion has been associated with an attenuation of the postprandial increase in superior mesenteric artery blood flow velocity as compared to the pre-transfusion measurements made using pulse Doppler ultrasound 61,93. However, a prospective observational comparison of infants <33 weeks at birth who were fed (n=9) or not fed (n=8) during RBC transfusion demonstrated that mesenteric tissue oxygenation, as measured by using near-infrared spectroscopy (NIRS), was not influenced by feeding. 94
A systematic review of 7 observational studies reported that withholding feeds during RBC transfusion was associated with lower risk of NEC associated with transfusion (n=7492, RR 0.47, 95% CI 0.28-0.80, I2 = 11%) 95. Although biologically plausible, the results from these observational studies remain vulnerable to bias and confounding. A large randomized controlled trial, Withholding Enteral Feeds Around Transfusion (WHEAT) trial is currently underway 96 and will hopefully provide evidence to answer the clinically relevant question of whether feeding during an RBC transfusion causes NEC.
Role of NIRS
NIRS is a non-invasive technique for monitoring regional tissue oxygenation in real time. NIRS measures the difference between oxyHb and deoxyHb, which reflects oxygen uptake in the specific tissue bed measured 97. This measurement, which is reported as the regional oxygen saturation (rSO2), reflects the balance of oxygen that is delivered minus what is extracted at the tissue level 97. A decreasing NIRS rSO2 reading indicates either increasing oxygen extraction at the tissue level, or, decreasing oxygen delivery to tissues in the region measured.
With its ability to monitor mesenteric tissue oxygenation 98, use of NIRS to monitor intestinal oxygenation in preterm babies has been studied 99. Bailey et al. demonstrated large variability of mesenteric oxygenation during RBC transfusion in preterm neonates 100. Marin et al. compared NIRS measurements in 4 patients with NEC associated with RBC transfusion with 4 controls who received RBC transfusion but did not develop NEC 101. This study demonstrated wide fluctuation and decreases in mesenteric oxygenation patterns that were more pronounced in infants who developed NEC with RBC transfusion as compared to non-NEC infants. In a pilot study, Sood et al. compared the mesenteric and cerebral rSO2 patterns of infants who did not develop NEC within 7 days of RBC transfusion (n=120), infants who developed NEC within 7 days prior to RBC transfusion (n=20) and infants that developed NEC within 7 days after an RBC transfusion (n=8) 102. The study reported decreases in mesenteric rSO2 during and after an RBC transfusion in infants who went on to develop NEC within 7 days as compared to the other two groups who had an increase in rSO2.
While promising, there is currently no evidence to support the use of NIRS monitoring to guide RBC transfusion approaches to prevent NEC. Two prospective trials registered at clinicaltrials.gov, Transfusion of Prematurity (TOP trial) NCT01702805 and Combining Restrictive Guidelines and a NIRS SCORE to Decrease RBC Transfusions (NCT02535208), include arms with NIRS monitoring at different thresholds for RBC transfusion. The data from these trials may provide more insight into the utility of this technique into identifying the need for RBC transfusion and allow for a better understanding of the effects of RBC transfusion and anemia on intestinal oxygenation and NEC.
Summary
Observational studies have provided conflicting evidence regarding the effect of RBC transfusion and anemia on NEC. It is possible that anemia and/or RBC transfusion may lead to tissue hypoxia, dysregulation of mesenteric vascular regulation or inflammation. Such mechanisms may act independently or in combination to lead to intestinal injury and, potentially, the development of NEC. Placental transfusion and the use of ESA have a role in decreasing RBC transfusion and anemia, although it is unclear if these treatments reduce NEC. Ongoing RBC transfusion trials have the potential to provide additional evidence to improve our understanding of transfusion strategies to decrease the risk for NEC.
Key Points.
The optimal hemoglobin thresholds to administer red blood cell (RBC) transfusion are currently uncertain.
Results of ongoing randomized trials are likely to provide important new evidence to guide RBC transfusion.
Until new trial data are available, it is advisable to avoid using routine RBC transfusion thresholds above the liberal arm or below the conservative arm of thresholds studied in trials to date in preterm infants, as the safety of such approaches is uncertain.
Practices to minimize RBC transfusion and anemia, such as placental transfusion by delayed cord clamping, have important benefits but it is unclear if these practices reduce NEC.
Best Practices Box.
What is the current practice?
Currently, there is uncertainty regarding the optimal Hb thresholds to transfuse RBCs into preterm infants.
What changes in current practice are likely to improve outcomes?
The following practices are suggested:
Provide placental transfusion, when feasible.
Minimize unnecessary phlebotomy-related blood losses
Avoid using routine RBC transfusion thresholds above the liberal Hb level or below the conservative Hb level of thresholds studied to date, as the safety of such approaches is uncertain.
Summary Statement
Results of data from two multicenter randomized trials of high- vs. low- transfusion thresholds (TOP, ETTNO) are likely to provide important new evidence to guide RBC transfusion. Until these data are available, no confident conclusions regarding the effects of RBC transfusion or anemia on NEC can currently be provided.
Acknowledgements:
The review was supported, in part, by the National Institutes of Health under award K23 HL128942 (R.M.P.), which had no role in the content of this review.
Abbreviations:
- NEC
necrotizing enterocolitis
- RBC
red blood cell
- Hb
hemoglobin
- OR
odds ratio
- CI
confidence interval
- NIRS
near-infrared spectroscopy
- NO
nitric oxide
- TRALI
transfusion-related acute lung injury
- TLR
Toll-like receptor
- FC
fecal calprotectin
- VLBW
very low birth weight
- EPO
erythropoietin
- rSO2
regional oxygen saturation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Josephson has received oxygenation monitoring equipment (e.g. NIRS and pulse oximetry) from Medtronic, Inc. The company had no role in this review. The other authors have no conflicts of interest to report.
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. Jama. 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135(1):e59–65. [DOI] [PubMed] [Google Scholar]
- 3.Horbar JD, Edwards EM, Greenberg LT, et al. Variation in performance of neonatal intensive care units in the united states. JAMA Pediatrics. 2017;171(3):e164396. [DOI] [PubMed] [Google Scholar]
- 4.Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Archives of disease in childhood Fetal and neonatal edition. 2018;103(2):F182–f189. [DOI] [PubMed] [Google Scholar]
- 5.La Gamma EF, Blau J. Transfusion-related acute gut injury: feeding, flora, flow, and barrier defense. Seminars in perinatology. 2012;36(4):294–305. [DOI] [PubMed] [Google Scholar]
- 6.Lundstrom U, Siimes MA, Dallman PR. At what age does iron supplementation become necessary in low-birth-weight infants? The Journal of pediatrics. 1977;91(6):878–883. [DOI] [PubMed] [Google Scholar]
- 7.Blanchette VS, Zipursky A. Assessment of anemia in newborn infants. Clinics in perinatology. 1984;11(2):489–510. [PubMed] [Google Scholar]
- 8.Widness JA. Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. NeoReviews. 2008;9(11):e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115(6):1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. The Journal of pediatrics. 2006;149(3):301–307. [DOI] [PubMed] [Google Scholar]
- 11.Chen HL, Tseng HI, Lu CC, Yang SN, Fan HC, Yang RC. Effect of blood transfusions on the outcome of very low body weight preterm infants under two different transfusion criteria. Pediatrics and neonatology. 2009;50(3):110–116. [DOI] [PubMed] [Google Scholar]
- 12.Kirpalani H, Bell EF, D’Angio C, Hintz S, Kennedy K, Ohls R. Transfusion of Prematures (TOP) trial: does a liberal red blood cell transfusion strategy improve neurologically-intact survival of extremely-low-birth-weight infants as compared to a restrictive strategy? NICHD Neonatal Research Network. 2016. [Google Scholar]
- 13.Investigators E. The ‘Effects of Transfusion Thresholds on Neurocognitive Outcome of Extremely Low Birth-Weight Infants (ETTNO)’ Study: Background, Aims, and Study Protocol. Neonatology. 2012;101(4):301–305. [DOI] [PubMed] [Google Scholar]
- 14.Maier RF, Sonntag J, Walka MM, Liu G, Metze BC, Obladen M. Changing practices of red blood cell transfusions in infants with birth weights less than 1000 g. The Journal of pediatrics. 2000;136(2):220–224. [DOI] [PubMed] [Google Scholar]
- 15.Ekhaguere OA, Morriss FH Jr, Bell EF, Prakash N, Widness JA. Predictive factors and practice trends in red blood cell transfusions for very-low-birth-weight infants. Pediatric research. 2016;79(5):736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidelines for transfusion of erythrocytes to neonates and premature infants. Fetus and Newborn Committee, Canadian Paediatric Society. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 1992;147(12):1781–1792. [PMC free article] [PubMed] [Google Scholar]
- 17.Whyte R, Kirpalani H. Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. The Cochrane database of systematic reviews. 2011(11):Cd000512. [DOI] [PubMed] [Google Scholar]
- 18.McGrady GA, Rettig PJ, Istre GR, Jason JM, Holman RC, Evatt BL. An outbreak of necrotizing enterocolitis. Association with transfusions of packed red blood cells. American journal of epidemiology. 1987;126(6):1165–1172. [DOI] [PubMed] [Google Scholar]
- 19.Agwu JC, Narchi H. In a preterm infant, does blood transfusion increase the risk of necrotizing enterocolitis? Archives of Disease in Childhood. 2005;90(1):102–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Short A, Gallagher A, Ahmed M. Necrotizing Enterocolitis following blood transfusion. Archives of Diseases in Childhood. 2005. [Google Scholar]
- 21.Mally P, Golombek SG, Mishra R, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. American journal of perinatology. 2006;23(8):451–458. [DOI] [PubMed] [Google Scholar]
- 22.Holder G, Doherty D, Patole S. Elective red cell transfusions for anemia of prematurity and development of necrotizing enterocolitis in previously well preterm neonates: Incidence and difficulties in proving a cause-effect relation. Journal of Neonatal-Perinatal Medicine. 2009;2(3):181–186. [Google Scholar]
- 23.Christensen RD, Lambert DK, Henry E, et al. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion. 2010;50(5):1106–1112. [DOI] [PubMed] [Google Scholar]
- 24.Josephson CD, Wesolowski A, Bao G, et al. Do red cell transfusions increase the risk of necrotizing enterocolitis in premature infants? The Journal of pediatrics. 2010;157(6):972–978. e971–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. The Journal of pediatrics. 2011;158(3):403–409. [DOI] [PubMed] [Google Scholar]
- 26.Couselo M, Aguar M, Ibanez V, Mangas L, Garcia-Sala C. [Relation between packed red blood cell transfusion and severity of necrotizing enterocolitis in premature infants]. Cirugia pediatrica : organo oficial de la Sociedad Espanola de Cirugia Pediatrica. 2011;24(3):137–141. [PubMed] [Google Scholar]
- 27.El-Dib M, Narang S, Lee E, Massaro AN, Aly H. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. Journal of perinatology : official journal of the California Perinatal Association. 2011;31(3):183–187. [DOI] [PubMed] [Google Scholar]
- 28.Ghirardello S, Lonati CA, Dusi E, Pugni L, Mosca F. Necrotizing enterocolitis and red blood cell transfusion. The Journal of pediatrics. 2011;159(2):354–355. [DOI] [PubMed] [Google Scholar]
- 29.Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127(4):635–641. [DOI] [PubMed] [Google Scholar]
- 30.Singh R, Visintainer PF, Frantz ID 3rd, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. Journal of perinatology : official journal of the California Perinatal Association. 2011;31(3):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter BM, Holditch-Davis D, Tanaka D, Schwartz TA. Relationship of neonatal treatments with the development of necrotizing enterocolitis in preterm infants. Nursing research. 2012;61(2):96–102. [DOI] [PubMed] [Google Scholar]
- 32.Stritzke AI, Smyth J, Synnes A, Lee SK, Shah PS. Transfusion-associated necrotising enterocolitis in neonates. Archives of disease in childhood Fetal and neonatal edition. 2013;98(1):F10–14. [DOI] [PubMed] [Google Scholar]
- 33.Wan-Huen P, Bateman D, Shapiro DM, Parravicini E. Packed red blood cell transfusion is an independent risk factor for necrotizing enterocolitis in premature infants. Journal of perinatology : official journal of the California Perinatal Association. 2013;33(10):786–790. [DOI] [PubMed] [Google Scholar]
- 34.AlFaleh K, Al-Jebreen A, Baqays A, et al. Association of packed red blood cell transfusion and necrotizing enterocolitis in very low birth weight infants. J Neonatal Perinatal Med. 2014;7(3):193–198. [DOI] [PubMed] [Google Scholar]
- 35.Baxi AC, Josephson CD, Iannucci GJ, Mahle WT. Necrotizing enterocolitis in infants with congenital heart disease: the role of red blood cell transfusions. Pediatric cardiology. 2014;35(6):1024–1029. [DOI] [PubMed] [Google Scholar]
- 36.Garg PM, Ravisankar S, Bian H, Macgilvray S, Shekhawat PS. Relationship between Packed Red Blood Cell Transfusion and Severe Form of Necrotizing Enterocolitis: A Case Control Study. Indian pediatrics. 2015;52(12):1041–1045. [DOI] [PubMed] [Google Scholar]
- 37.Garg P, Pinotti R, Lal CV, Salas AA. Transfusion-associated necrotizing enterocolitis in preterm infants: an updated meta-analysis of observational data. Journal of perinatal medicine. 2017. [DOI] [PubMed] [Google Scholar]
- 38.Hay S, Zupancic JAF, Flannery DD, Kirpalani H, Dukhovny D. Should we believe in transfusion-associated enterocolitis? Applying a GRADE to the literature. Seminars in perinatology. 2017;41(1):80–91. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129(3):529–540. [DOI] [PubMed] [Google Scholar]
- 40.Kirpalani H, Zupancic JA. Do transfusions cause necrotizing enterocolitis? The complementary role of randomized trials and observational studies. Seminars in perinatology. 2012;36(4):269–276. [DOI] [PubMed] [Google Scholar]
- 41.Wallenstein MB, Arain YH, Birnie KL, et al. Red blood cell transfusion is not associated with necrotizing enterocolitis: a review of consecutive transfusions in a tertiary neonatal intensive care unit. The Journal of pediatrics. 2014;165(4):678–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel RM, Knezevic A, Shenvi N, et al. Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. Jama. 2016;315(9):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le VT, Klebanoff MA, Talavera MM, Slaughter JL. Transient effects of transfusion and feeding advances (volumetric and caloric) on necrotizing enterocolitis development: A case-crossover study. PloS one. 2017;12(6):e0179724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Askie LM, Darlow BA, Finer N, et al. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. Jama. 2018;319(21):2190–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnabl KL, Aerde JEV, Thomson ABR, Clandinin MT. Necrotizing enterocolitis: A multifactorial disease with no cure. World Journal of Gastroenterology : WJG. 2008;14(14):2142–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pittman RN. Regulation of Tissue Oxygenation, Chapter 4, Oxygen Transport. San Rafael (CA): Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 47.Szabo JS, Mayfield SR, Oh W, Stonestreet BS. Postprandial gastrointestinal blood flow and oxygen consumption: effects of hypoxemia in neonatal piglets. Pediatric research. 1987;21(1):93–98. [DOI] [PubMed] [Google Scholar]
- 48.Tsui AKY, Marsden PA, Mazer CD, et al. Differential HIF and NOS responses to acute anemia: defining organspecific hemoglobin thresholds for tissue hypoxia. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2014;307(1):R13–R25. [DOI] [PubMed] [Google Scholar]
- 49.Furuta GT, Turner JR, Taylor CT, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. The Journal of experimental medicine. 2001;193(9):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colgan SP, Curtis VF, Lanis JM, Glover LE. Metabolic regulation of intestinal epithelial barrier during inflammation. Tissue Barriers. 2015;3(1-2):e970936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lauscher PMD, Kertscho HMD, Schmidt O, et al. Determination of Organ-Specific Anemia Tolerance*. Critical Care Medicine. 2013;41(4):1037–1045. [DOI] [PubMed] [Google Scholar]
- 52.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. The British journal of dermatology. 2010;163(2):257–268. [DOI] [PubMed] [Google Scholar]
- 53.Holmes K, Charnock Jones SD, Forhead AJ, et al. Localization and control of expression of VEGF-A and the VEGFR-2 receptor in fetal sheep intestines. Pediatric research. 2008;63(2):143–148. [DOI] [PubMed] [Google Scholar]
- 54.Nowicki PT. Ischemia and necrotizing enterocolitis: Where, when, and how. Seminars in Pediatric Surgery. 2005;14(3):152–158. [DOI] [PubMed] [Google Scholar]
- 55.Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22(2):83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Seminars in perinatology. 2008;32(2):83–91. [DOI] [PubMed] [Google Scholar]
- 57.Nankervis CA, Nowicki PT. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. The American journal of physiology. 1995;268(6 Pt 1):G949–958. [DOI] [PubMed] [Google Scholar]
- 58.Nowicki PT, Nankervis CA, Miller CE. Effects of ischemia and reperfusion on intrinsic vascular regulation in the postnatal intestinal circulation. Pediatric research. 1993;33(4 Pt 1):400–404. [DOI] [PubMed] [Google Scholar]
- 59.Dani C, Pratesi S, Fontanelli G, Barp J, Bertini G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion. 2010;50(6):1220–1226. [DOI] [PubMed] [Google Scholar]
- 60.Bailey SM, Hendricks-Munoz KD, Wells JT, Mally P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. American journal of perinatology. 2010;27(6):445–453. [DOI] [PubMed] [Google Scholar]
- 61.Krimmel GA, Baker R, Yanowitz TD. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. American journal of perinatology. 2009;26(2):99–105. [DOI] [PubMed] [Google Scholar]
- 62.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17063–17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howarth C, Banerjee J, Aladangady N. Red Blood Cell Transfusion in Preterm Infants: Current Evidence and Controversies. Neonatology. 2018;114(1):7–16. [DOI] [PubMed] [Google Scholar]
- 64.Hosny M, Cassir N, La Scola B. Updating on gut microbiota and its relationship with the occurrence of necrotizing enterocolitis. Human Microbiome Journal. 2017;4:14–19. [Google Scholar]
- 65.Pammi M, Cope J, Tarr PI, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maheshwari A, Kelly DR, Nicola T, et al. TGF-β2 Suppresses Macrophage Cytokine Production and Mucosal Inflammatory Responses in the Developing Intestine. Gastroenterology. 2011;140(1):242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang B, Ohtsuka Y, Fujii T, et al. Immunological development of preterm infants in early infancy. Clinical and Experimental Immunology. 2005;140(1):92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu P, Hackam DJ. Toll-like Receptor Regulation of Intestinal Development and Inflammation in the Pathogenesis of Necrotizing Enterocolitis. Pathophysiology : the official journal of the International Society for Pathophysiology / ISP. 2014;21(1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nature reviews Gastroenterology & hepatology. 2016;13(10):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang-Rodriguez J, Fry E, Fiebig E, et al. Immune response to blood transfusion invery-low-birthweight infants. Transfusion. 2000;40(1):25–34. [DOI] [PubMed] [Google Scholar]
- 71.Kristiansson M, Soop M, Saraste L, Sundqvist KG. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta Anaesthesiologica Scandinavica. 1996;40(4):496–501. [DOI] [PubMed] [Google Scholar]
- 72.Sachs UJ. Recent insights into the mechanism of transfusion-related acute lung injury. Current opinion in hematology. 2011;18(6):436–442. [DOI] [PubMed] [Google Scholar]
- 73.Dani C, Poggi C, Gozzini E, et al. Red blood cell transfusions can induce proinflammatory cytokines in preterm infants. Transfusion. 2017;57(5):1304–1310. [DOI] [PubMed] [Google Scholar]
- 74.Ho TT, Groer MW, Luciano AA, et al. Red blood cell transfusions increase fecal calprotectin levels in premature infants. Journal of perinatology : official journal of the California Perinatal Association. 2015;35(10):837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosebraugh MR, Widness JA, Nalbant D, Veng-Pedersen P. A mathematical modeling approach to quantify the role of phlebotomy losses and need for transfusions in neonatal anemia. Transfusion. 2013;53(6):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lemyre B, Sample M, Lacaze-Masmonteil T. Minimizing blood loss and the need for transfusions in very premature infants. Paediatrics & child health. 2015;20(8):451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prescott CA, Center CRDAM, Walter Reed National Military Medical C, et al. Umbilical Cord Blood Use For Admission Blood Tests of Very Low Birth Weight Preterm Neonates: A Multi-center Randomized Clinical Trial. 2018; https://ClinicalTrials.gov/show/NCT02103296. [Google Scholar]
- 78.Rabe H, Alvarez JR, Lawn C, Seddon P, Amess PN. A management guideline to reduce the frequency of blood transfusion in very-low-birth-weight infants. American journal of perinatology. 2009;26(3):179–183. [DOI] [PubMed] [Google Scholar]
- 79.Delayed Umbilical Cord Clamping After Birth. Pediatrics. 2017. [DOI] [PubMed] [Google Scholar]
- 80.Committee Opinion No. 684: Delayed Umbilical Cord Clamping After Birth. Obstetrics and gynecology. 2017;129(1):e5–e10. [DOI] [PubMed] [Google Scholar]
- 81.WHO Guidelines Approved by the Guidelines Review Committee. Guidelines on Basic Newborn Resuscitation. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 82.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. The Cochrane database of systematic reviews. 2012(8):Cd003248. [DOI] [PubMed] [Google Scholar]
- 83.Fogarty M, Osborn DA, Askie L, et al. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. American journal of obstetrics and gynecology. 2018;218(1):1–18. [DOI] [PubMed] [Google Scholar]
- 84.Tarnow-Mordi W, Morris J, Kirby A, et al. Delayed versus Immediate Cord Clamping in Preterm Infants. The New England journal of medicine. 2017;377(25):2445–2455. [DOI] [PubMed] [Google Scholar]
- 85.Juul SE, Joyce AE, Zhao Y, Ledbetter DJ. Why is erythropoietin present in human milk? Studies of erythropoietin receptors on enterocytes of human and rat neonates. Pediatric research. 1999;46(3):263–268. [DOI] [PubMed] [Google Scholar]
- 86.McPherson RJ, Juul SE. High-dose erythropoietin inhibits apoptosis and stimulates proliferation in neonatal rat intestine. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2007;17(5):424–430. [DOI] [PubMed] [Google Scholar]
- 87.Kumral A, Baskin H, Duman N, et al. Erythropoietin protects against necrotizing enterocolitis of newborn rats by the inhibiting nitric oxide formation. Biology of the neonate. 2003;84(4):325–329. [DOI] [PubMed] [Google Scholar]
- 88.Ledbetter DJ, Juul SE. Erythropoietin and the incidence of necrotizing enterocolitis in infants with very low birth weight. Journal of pediatric surgery. 2000;35(2):178–181; discussion 182. [DOI] [PubMed] [Google Scholar]
- 89.Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. The Cochrane database of systematic reviews. 2017;11:Cd004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. The Cochrane database of systematic reviews. 2014(4):Cd004868. [DOI] [PubMed] [Google Scholar]
- 91.Romagnoli C, Zecca E, Gallini F, Girlando P, Zuppa AA. Do recombinant human erythropoietin and iron supplementation increase the risk of retinopathy of prematurity? European journal of pediatrics. 2000;159(8):627–628. [DOI] [PubMed] [Google Scholar]
- 92.National Institute of Neurological D. Preterm Erythropoietin Neuroprotection Trial (PENUT Trial). 2018; https://ClinicalTrials.gov/show/NCT01378273.
- 93.Pitzele A, Rahimi M, Armbrecht E, Havranek T. Packed red blood cell transfusion (PRBC) attenuates intestinal blood flow responses to feedings in pre-term neonates with normalization at 24 hours. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28(15):1770–1773. [DOI] [PubMed] [Google Scholar]
- 94.Marin T, Josephson CD, Kosmetatos N, Higgins M, Moore JE. Feeding preterm infants during red blood cell transfusion is associated with a decline in postprandial mesenteric oxygenation. The Journal of pediatrics. 2014;165(3):464–471. e461. [DOI] [PubMed] [Google Scholar]
- 95.Jasani B, Rao S, Patole S. Withholding Feeds and Transfusion-Associated Necrotizing Enterocolitis in Preterm Infants: A Systematic Review. Advances in Nutrition. 2017;8(5):764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gale C, Hyde MJ, Modi N. Research ethics committee decision-making in relation to an efficient neonatal trial. Archives of disease in childhood Fetal and neonatal edition. 2017;102(4):F291–f298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moore JE. Newer monitoring techniques to determine the risk of necrotizing enterocolitis. Clinics in perinatology. 2013;40(1):125–134. [DOI] [PubMed] [Google Scholar]
- 98.Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive care medicine. 2001;27(8):1401–1407. [DOI] [PubMed] [Google Scholar]
- 99.Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(4):574–582. [DOI] [PubMed] [Google Scholar]
- 100.Bailey SM, Hendricks-Munoz KD, Mally PV. Variability in splanchnic tissue oxygenation during preterm red blood cell transfusion given for symptomatic anaemia may reveal a potential mechanism of transfusion-related acute gut injury. Blood transfusion = Trasfusione del sangue. 2015;13(3):429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marin T, Moore J, Kosmetatos N, et al. Red blood cell transfusion-related necrotizing enterocolitis in very-lowbirthweight infants: a near-infrared spectroscopy investigation. Transfusion. 2013;53(11):2650–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sood BG, Cortez J, McLaughlin K, et al. Near Infrared Spectroscopy as a Biomarker for Necrotising Enterocolitis following Red Blood Cell Transfusion. J Near Infrared Spectrosc. 2014;22(6):375–388. [Google Scholar]


