Abstract
Tuft cells were first discovered in epithelial barriers decades ago, but their function remained unclear until recently. In the last two years, a series of studies has provided important advances that link tuft cells to infectious diseases and the host immune responses. Broadly, a model has emerged in which tuft cells use chemosensing to monitor their surroundings and translate environmental signals into effector functions that regulate immune responses in the underlying tissue. Here we review the current understanding of tuft cell immune function in the intestines, airways, and thymus. In particular, we discuss the role of tuft cells in type 2 immunity, norovirus infection, and thymocyte development. Despite recent advances, many fundamental questions about the function of tuft cells in immunity remain to be answered.
INTRODUCTION
Epithelial cells (and indeed many other non-hematopoietic cells) are perhaps underappreciated by immunologists who focus on cells of the hematopoietic system, yet they make crucial contributions to immunity. Most notably, epithelia form the body’s barrier between self and non-self, and are therefore often the site of first encounter between the host and a foreign microbe or irritant. Although not as diverse as the hematopoietic compartment, epithelial barriers are comprised of multiple cell lineages with both overlapping and distinct functions. Goblet cells, for example, are professional mucus-producing cells, while Paneth cells secrete high levels of antimicrobial peptides, and enteroendocrine cells secrete hormones and communicate with the nervous system. The role of tuft cells, on the other hand, remained enigmatic for more than 60 years until a series of recent discoveries definitively linked tuft cells to immunity. In this review, we will focus on the immune function of tuft cells after a brief discussion of their development and markers.
CHARACTERISTICS & DISTRIBUTION
Tuft cells were first discovered in rat trachea (1) and mouse glandular stomach (2) in 1956, and in human trachea in 1959 (3). The advent of electron microscopy had allowed for visualization of cellular morphology in unprecedented detail, and several investigators quickly noted the presence of a rare but distinctive lineage of epithelial cells, which they termed tuft, brush, caveolated, multivesicular, or fibrillovesicular cells(1, 4). As these cells appear to be very closely related across tissues, we will refer to them collectively as tuft cells. Morphologically, tuft cells are characterized by 1) a “tuft” of long, blunt apical microvilli; 2) prominent actin, villin, and fimbrin rootlets that extend basally from the tips of the microvilli; and 3) abundant apical vesicles that form a tubulovesicular system. They are radiation-resistant epithelial cells (5) with a turnover rate equivalent to their surrounding epithelial cells, which is 3–5 days in the intestine (6–8) and 168–267 days in the trachea (9–11). With the exception of nascent tuft cells in intestinal crypts (12), tuft cells do not express the proliferation marker Ki67, indicating post-mitotic status in both the steady state (7, 12, 13) and during helminth infection (14).
In rodents, tuft cells have been identified in the digestive system [salivary glands (15), stomach (2), gall bladder and bile duct (16, 17), pancreatic duct (18), small intestine (19), cecum (20), and colon (21)]; the respiratory system [nasal cavity (22), auditory tube (23), and trachea (1)]; the urethra (24); and even in the thymus (25), a primary lymphoid organ. In rats, cells with tuft-like morphology have also been observed in alveolar epithelium (26), but in mice they have not been seen below the bronchial branch point. In humans, cell with tuft-like morphology were reported in the trachea (3), small intestine (27, 28), stomach (29, 30), gallbladder (31), and in the alveoli of a 4-month-old patient with pneumonitis (32). As a rule, tuft cells are found in hollow organs or tubes lined by a non-squamous epithelium, but the thymus is a notable exception and there are non-squamous mucosal barriers where tuft cells have as yet not been described, such as the female reproductive tract.
LINEAGE SPECIFICATION
Although tuft cells are found in many tissues, their development and lineage specification has only been studied in detail in the small intestine, likely because the stem cells of the intestinal epithelium are among the best characterized and most prolific in the body (33). In homeostasis, these cells reside at the base of intestinal crypts, express the marker LGR52, and produce enough progeny to replace the entire intestinal epithelium in just 3–5 days (6). Lineage tracing has demonstrated that intestinal tuft cells are indeed derived from LGR5+ stem cells (7), but unlike all other epithelial cells, differentiated intestinal tuft cells continue to express Lgr5 (34, 35).
Immediately above the LGR5+ stem cell compartment is the transit amplifying zone, where uncommitted epithelial progenitors replicate and adopt their terminal fate. The first lineage branch point is regulated by a classical lateral inhibition model in which cells receiving a Notch signal upregulate Hairy and enhancer of split-1 (Hes1) and become enterocytes (36), while those providing a Notch ligand (i.e. Delta-like-ligand 1 (DLL1)-expressing progenitors) retain potential to become all non-enterocyte lineages (goblet, enteroendocrine, Paneth, and tuft). Loss of Notch signaling induces the transcription factor Atoh13, which goblet, enteroendocrine, and Paneth cells all constitutively express. Accordingly, these cells are absent when Atoh1 is deleted from epithelial stem cells (7, 12, 37). Mature tuft cells, on the other hand, do not express Atoh1, and studies that deleted Atoh1 from all intestinal epithelial cells reported conflicting results about the requirement of Atoh1 in intestinal tuft cell development. While tuft cells were absent in the small intestine of Villin-CreErt2 X Atoh1f/f mice (7), their numbers were normal or even increased in Rosa26-CreErt2 X Atoh1f/f (12), Lgr5-CreErt2 x Atoh1f/f (38), and Lrig-CreErt2 X Atoh1f/f (37) mice. Interestingly, colonic tuft cells, which were only studied in Lrig-CreErt2 X Atoh1f/f mice, did require Atoh1, suggesting distinct mechanisms of lineage specification in the small intestine and colon. Although some uncertainty remains, on balance these studies suggest that a binary HES1/ATOH1 model does not fully explain the differentiation of intestinal epithelium.
Intestinal tuft cell development is not affected in the absence of the transcription factors neurogenin 3 (NEUROG3), SAM pointed domain containing ETS transcription factor (SPDEF), and Sex-determining region Y-box 9 (SOX9), which are the lineage transcription factors that define enteroendocrine cells, goblet cells and Paneth cells (7, 12). Instead, POU2F34 and GFI1B5 have been suggested as tuft cell-specific master regulators. All tuft cells express both markers constitutively (12, 39, 40), and tuft cells are entirely absent in Pou2f3−/− mice, while all other epithelial lineages appear normal, at least in the intestine (39). The status of tuft cells in the absence of Gfi1b has not been reported and it is unknown if either POU2F3 or GFI1B is sufficient to drive tuft cell differentiation. In the airway, tuft cells were shown to be derived from basal cells with rapid kinetics in lineage tracing experiments, but the precise signals that specify the tuft cell lineage remain uncertain here as well(41).
The timing of tuft cell emergence during development also remains unclear. One study found that tuft cells appeared by mouse embryonic day 18.5(E18.5) in developing intestine and stomach antrum (30); in other studies, tuft cells did not appear until after birth (42, 43). These studies all demonstrated that both gastric and intestinal tuft cell frequency remained very low before reaching adult-equivalent density after weaning (30, 42, 43), with similar frequency between small intestine and colon in unmanipulated mice (35). In contrast, tracheal tuft cells are present at adult frequency by at least day 5 post-birth (11). Overall there is much more to be learned about tuft cell lineage specification across diverse tissues, particularly how it can be regulated by immune signals in homeostasis and diseases. IL-13 in small intestinal stem cells, for example, can induce tuft cell hyperplasia (14, 44 and discussed in detail below), but it is unclear if this occurs in any other tissues.
TUFT CELL HETEROGENEITY
Although morphologically very similar, the developmental and functional equivalence of tuft cells in different tissues and even in different regions of the same tissue remains unclear. A list of tuft cell markers is included in Table 1 and we recently used bulk RNA-sequencing of Epithelial cell adhesion molecule (EPCAM)+ IL-25+ tuft cells from five different tissues to identify a core transcriptional signature that is shared by all tuft cells (45). In addition to Il25, this signature includes many of the markers listed in Table 1, such as Dclk16, Trpm57, Prostaglandin endoperoxide synthase-1 (Ptgs1), Pou2f3, Gfi1b, and Sialic acid binding Ig-like lectin F (Siglecf). Despite these shared features, RNA sequencing as well as immunostaining have also revealed significant inter- and intra-tissue diversity of tuft cells. For example, a recent study used multiplex immunofluorescence to demonstrate that individual DCLK1+ tuft cells in the intestine express differential levels of markers such as acetylated tubulin (acTUB), SOX9, PTGS1 (COX1) and PTGS2 (COX2) (35). Tuft cell heterogeneity was also identified using single-cell sequencing, which led to the classification of Tuft-1 and Tuft-2 subsets in both the airway and intestine (46, 47). In both tissues, expression of eicosanoid biosynthesis genes and Ptprc (a.k.a. Cd45) is enriched in Tuft-2 cells. Intestinal Tuft-1 cells express a neuronal signature, while the tracheal Tuft-1 subset is associated with a taste transduction signature. In terms of cytokines, Il25 is constitutively expressed in all tuft cells, while Tslp is detectable in both Tuft-1 and Tuft-2 cells of the trachea but only in Tuft-2 cells of the small intestine. In the trachea there is also subset-specific skewing of transcription factors, with Tuft-1 cells enriched for Pou2f3 and Tuft-2 cells for Gfi1b, but both genes remain detectable in all tuft cells. By bulk RNA sequencing (45), one key distinction between tuft cells from distinct tissues was the differential expression of surface receptors, suggesting that tuft cells have evolved to sense different ligands depending on their microenvironment. Whether the effector functions of tuft cells are also tissue-specific requires further investigation.
Table 1:
Tuft cell biomarkers. Markers were grouped by their functional role in tuft cells (ex: structural, chemosensing..etc)
| Structural Markers | ||
|---|---|---|
| Marker | Description | Comment |
| DCLK1 | Doublecortin like kinase 1 is a microtubule-associated kinase first described in neurons that regulates polarization (103). |
The most widely used tuft cell marker. Tuft cells in all tissues express DCLK1 and >95% of DCLK1+ epithelial cells in the murine intestine are tuft cells |
| VIL1 | Villin1 is an actin-binding protein that is abundant in microvilli and therefore concentrated at apical tip of tuft cells (104) (105, 106). |
Apical concentration of VIL1 is unique to tuft cells, but all intestinal epithelial cells express Vil1. Since Vil1-Cre is widely used to target the intestinal epithelium, it is worth noting that tuft cells in respiratory tract also express Vil1 (25). |
| acTUB | Acetylated-alpha-tubulin is required to form microtubule bundles, which are abundant in tuft cells (6, 29). |
Highly specific marker for tuft cells but not widely used. |
| CK18 | Cytokeratin 18 colocalizes with villin in tuft cells (25, 107). |
Some evidence of non-tuft CK18lo epithelial cells (11, 107). |
| UEA-1 | Ulex europaeus agglutinin type 1 is an abundant lectin on the apical surface of intestinal tuft cells (11, 108). |
Although relatively selective for tuft cells in the proximal small intestine, in the distal intestine UEA-1 is widespread on all epithelial cells. |
| Chemosensing Markers | ||
| Marker | Description | Comment |
| TRPM5 | Transient receptor potential cation channel subfamily M member 5 is a calcium-gated cation channel thought to regulate depolarization upon chemosensory stimuli (109) |
Based on TRPM5-GFP reporter, all tuft cells in intestine (64, 69) and nasal cavity (76, 81) express TRPM5. |
| GNAT3 | Alpha-gustducin is a specialized G protein that couples to canonical taste receptors and perhaps other 7 pass transmembrane receptors as well. |
GNAT3 has been detected in non-tuft epithelial cells in the airway (110, 111), and genetic experiments have demonstrated that unlike TRPM5, not all tuft cell sensing is GNAT3-dependent(42) |
| PLCB2 | Phospholipase C beta 2 is activated downstream of GNAT3. |
PLCB2 has been detected in non-tuft epithelial cells in the airway (110, 111). |
| CHAT | Choline acetyltransferase catalyzes the formation of the neurotransmitter acetylcholine |
Based on Chat-GFP reporter and antibody staining, tuft cells in airways(77), gastro- intestinal tract(64, 65, 98), urethral tract(23, 97), and thymus(24) express Chat, but some Chat negative tuft cells have also been observed(65). |
| Transcription Factors | ||
| Marker | Description | Comment |
| POU2F3 | POU class 2 homeobox 3 was first identified as being required for differentiation of taste receptor cells (112), but Pou2f3−/− mice are also completely tuft cell-deficient |
POU2F3 constitutively expressed in all tuft cells, including early tuft cells in intestinal crypts (38, 39). |
| GFI1B | Growth factor independent 1B is a transcriptional repressor. |
GFI1B is constitutively expressed in all tuft cells, including early tuft cells in intestinal crypts (11, 13, 38). It may also expressed in M cells(113). Its function in tuft cells is unknown. |
| Other | ||
| Marker | Description | Comment |
| IL-25 | Interleukin 25 is associated with type 2 inflammation and is required for intestinal clearance of helminths. |
Tuft cells in all tissues analyzed constitutively express IL-25 and all DCLK1+ cells in the intestinal epithelium are also IL- 25+ (13) |
| PTGS1 | Prostaglandin-endoperoxide synthase 1 (a.k.a. COX-1) is required for synthesis of cyclooxygenases |
All tuft cells appear to express PTGS1 and tuft cells are the only epithelial cells in the intestine that express this enzyme (11, 13, 38, 64) |
| SIGLECF | Sialic acid binding Ig-like lectin F encodes immunoreceptor tyrosine- based inhibnitory motifs and is normally associated with hematopoietic lineages (e.g. eosinophils). |
Cell surface marker that can be used in flow cytometry(63). Its function in tuft cells is unknown |
| p-EGFR | EGFR phosphorylated on tyrosine 1068 (34). |
Only tested in intestine |
IMMUNE FUNCTION OF TUFT CELLS IN THE INTESTINE
Tuft-ILC2 Immune Circuit
The initiation of type 1 immune responses, from innate immune sensing to priming of adaptive cells, is relatively well understood. By contrast, much less is known about how helminths, protists, and allergens trigger a type 2 response. Group 2 innate lymphoid cells (ILC2s) are the dominant early source of IL-5, IL-9, and IL-13 in numerous models of type 2 inflammation (48–52), and understanding how ILC2s are activated has therefore been of great interest. ILC2s lack an antigen receptor and there is little evidence that they sense type 2 agonists directly. Instead, ILC2s integrate numerous host-derived activating signals, including cytokines (e.g., IL-33, IL-25) (48, 53, 54), lipids (e.g., leukotrienes) (55–57), and neuronal peptides (e.g., Vasoactive intestinal peptide (VIP), Neuromedin U (NMU)) (49, 58–61). Current models propose that ILC2s use these signals to monitor the status of their surrounding tissue and become activated by disruptions in homeostasis (62).
In the intestine, the link between IL-25 and helminth-induced type 2 responses is well-established: IL-25 (63, 64, 54) and its downstream adaptor Act1 mediate type 2 immunity to promote worm expulsion (65). Furthermore, IL-25 is sufficient to activate ILC2s and promote worm expulsion independently of adaptive Th2 function (48, 64). But the physiologic cellular source of IL-25 remained elusive until recently. Using Il25-RFP reporter mice and immunohistochemistry, recent studies identified tuft cells as the dominant source of IL-25 in the small intestine both at homeostasis and during helminth infection (14, 39). Tuft cell-derived IL-25 helps to drive a feed-forward tuft-ILC2 signaling circuit in which ILC2s are activated to produce IL-5, −9, and −13, thereby promoting type 2 inflammation. IL-13 also signals in undifferentiated epithelial cells, skewing their lineage commitment towards tuft and goblet cells (14, 39, 44). Due to the rapid turnover of the intestinal epithelium, activation of the tuft-ILC2 circuit quickly results in pronounced tuft and goblet cell hyperplasia. The frequency of tuft cells, in particular, can increase more than 10-fold during helminth infection. This circuit can be activated exogenously by stimulating ILC2s with recombinant IL-25 or IL-33, or by giving recombinant IL-13 to drive tuft cell hyperplasia directly in the intestinal epithelium. Removing components of the tuft-ILC2 circuit (e.g Pou2f3−/−, Il25−/−, and Il4Rα−/−) disrupts the intestinal type 2 response and leads to delayed clearance of the roundworm Nippostrongylus brasiliensis (14, 39, 66). Conversely, deleting the innate immune signaling inhibitor TNF alpha induced protein 3 (Tnfaip3, encoding A20) from ILC2s leads to chronic activation of the small intestinal tuft-ILC2 circuit driven by the constitutive expression of IL-25 in tuft cells (43)
Tuft cells are also found constitutively in the gall bladder, pancreatic ducts, cecum, and colon, where they express many of the same markers (e.g. DCLK1, CHAT8, TRPM5) as in the small intestine. An immune function has not, however, been reported for any of these cells. In fact, all evidence so far suggests that the tuft-ILC2 circuit does not operate in these tissues. For example, deleting A20 from ILC2s spontaneously activates the tuft-ILC2 circuit in the small intestine, but there is no evidence of type 2 inflammation in any other intestinal tissues (43). Further, systemic delivery of recombinant IL-4 drives tuft cell hyperplasia only in the small intestine (von Moltke & Locksley, unpublished). It may be that tuft cells and ILC2s still communicate outside the small intestine, but that IL-4/13 signaling in epithelial stem cells at these sites does not induce tuft cell hyperplasia. Small and transient changes in tuft cell frequency have been noted in the colon when germ-free mice are colonized with bacteria (35), but the mechanism for these fluctuations remains unknown.
In young, unmanipulated mice, ILC2s are the dominant IL-25 receptor-expressing and IL-13-producing tissue-resident cells, and are therefore critical for rapid (7–10 days) clearance of the rodent roundworm N. brasiliensis. In chronic infection settings (e.g. with the helminth Heligmosomoides polygyrus) or once immune memory is established, other sources of IL-13 are activated and can likely substitute for ILC2s in the circuit. In fact, the connections between tuft cells and adaptive immunity remain completely unexplored. In addition, several details of the innate tuft-ILC2 circuit require further examination. In particular, how is the circuit regulated if IL-25 expression is constitutive, and what do tuft cells do besides secrete IL-25? Besides cytokines, tuft cells also express enzymes for eicosanoid biosynthesis, such as Cox-1, Cox-2, 5-lipoxygenase (Alox5), and hematopoietic prostaglandin-D synthase (Hpgd) (67, 7, 68, 69, 14, 46). How eicosanoid biosynthesis is regulated in tuft cells and the physiologic function of tuft cell-derived eicosanoids remain unknown.
Tuft Cell Chemosensing
Soon after the link between tuft cells and helminth infection was established, another landmark study revealed that the tuft-ILC2 circuit is also activated by intestinal colonization with Tritrichomonas, a genus of protists found in the commensal flora of many mouse vivariums (44). This study also provided the first functional evidence of a link between chemosensing by tuft cells and type 2 immunity.
Immune cells and intestinal epithelial cells are known to sense microbially-derived molecular patterns with pattern recognition receptor (PRRs) such as Toll-like receptors to initiate type 1 immunity, but the molecular stimuli and the cell type(s) that drive type 2 responses are still elusive. A sensing function has long been hypothesized for tuft cells, and immunostaining provided the first clues that a chemosensing pathway previously characterized in taste transduction might also be active in tuft cells (70–72, 68). In taste receptor cells, signaling through canonical G protein-coupled taste receptors activates a specialized G alpha subunit known as alpha-gustducin (GNAT3), which in turn initiates intracellular calcium flux via phospholipase C beta 2 (PLCB2) (73). The rise in Ca2+ opens the cell surface cation channel TRPM5, leading to depolarization of taste cells. When the first complete transcriptome analysis of intestinal tuft cells was completed in 2008, it confirmed that all components of the pathway, except canonical taste receptors, are indeed highly and selectively expressed in tuft cells (67).
Tuft cells are ideally positioned to act as immune sentinels by monitoring the intestinal lumen and transmitting signals to immune cells in the underlying tissue. Howitt et al. provided the first direct evidence for such a function, by demonstrating that Trpm5−/− and Gnat3−/− mice fail to induce tuft cell hyperplasia when colonized with Tritrichomonas (44). Immune responses to the helminths N. brasiliensis and H. polygyrus are also impaired in Trpm5−/− mice(44), but tuft cell hyperplasia occurs normally in Gnat3−/− mice colonized with N. brasiliensis, suggesting distinct sensing mechanisms for helminths and protists (45).
The lack of canonical taste receptor expression in intestinal tuft cells suggested the hypothesis that other G-protein coupled receptor(s) (GPCR) may be specifically enriched on tuft cells to ‘sense’ protists and helminths. Indeed, the extracellular succinate receptor 1 (SUCNR1) was recently identified to be selectively expressed in both TRPM5+ and IL-25+ small intestinal tuft cells (45, 67, 74). Remarkably, providing succinate in the drinking water of mice is sufficient to drive tuft cell hyperplasia in a Sucnr1-, Il25- and Trpm5-dependent manner (45, 74). Succinate treatment also induces other hallmarks of type 2 responses, such as goblet cell hyperplasia, eosinophilia, and IL-13 production by ILC2s (43, 45). Further, the activation of ILC2s by succinate is Il25-, Trpm5-, and Pou2f3-dependent (43, 45, 74). Succinate is therefore the first ligand identified for intestinal tuft cells and one of the only known innate immune ligands that is sufficient to activate type 2 inflammation.
Succinate is an intermediate of the citric acid cycle and is normally sequestered inside host cells. Many microbial pathogens and commensals, on the other hand, have evolved diverse fermentative metabolic pathways to thrive in the nutrient-rich but oxygen-poor intestinal lumen, and these pathways frequently result in production and secretion of succinate (75). Succinate is detectable in the supernatants of in vitro cultured N. brasiliensis and Tritrichomonas and in the cecum of mice monocolonized with Tritrichomonas. Accordingly, the detection of Tritrichomonas by tuft cells is entirely SUCNR1-dependent (43, 45). By contrast, the immune response to N. brasiliensis is intact in Sucnr1−/− mice (45, 74), demonstrating that SUCNR1 signaling is absent or redundant during helminth infection and underscoring the differences between sensing of protists and helminths that was suggested by experiments using Gnat3−/− mice. There is also evidence that bacterial dysbiosis leads to SUCNR1-dependent tuft cell hyperplasia, although it is not clear whether this occurs physiologically (74). Together, these studies identify a specific metabolite that selectively activates the tuft-ILC2 circuit and define a paradigm in which the intestinal type 2 immune system monitors microbial metabolism. Tuft cells also express another potential metabolite sensor –the short chain fatty acid receptor Ffar3 (45, 46)— but a function for this receptor has remained elusive.
The seemingly intact immune response to N. brasiliensis in Gnat3−/− and Sucnr1−/− mice suggests that there is at least one other sensor upstream of TRPM5 that detects helminth infection. There are also questions remaining about the mechanisms of chemosensing by tuft cells in other tissues. The detection of succinate also warrants further investigation. In particular, the benefits of sensing Tritrichomonas-derived succinate are not clear, since these protists are not eliminated or even reduced in number by the type 2 immune response(45). Given that most protists and helminths have evolved to establish chronic colonization, their sensing by the immune system may therefore be linked principally to host adaptation and tolerance. In support of this idea, activation of the tuft-ILC2 circuit was recently shown to drive small intestinal lengthening (43). Since tuft and goblet cell hyperplasia lead to a decreased frequency of absorptive enterocytes, this intestinal lengthening may help to maintain the absorptive capacity of the intestine. Indeed, there is no overt loss of fitness or decrease in caloric uptake associated with chronic activation of the tuft-ILC2 circuit (43).
Tuft Cells and Norovirus
Human norovirus is the leading cause of gastroenteritis outbreaks worldwide and the acute phase of disease can be followed by weeks or months of viral shedding in the stool (76), suggesting a site of viral persistence in the host. Murine norovirus (MNoV) is even more persistent, with some strains establishing chronic infection, but the cellular tropism in vivo remained unclear until recently. In 2017, immunostaining of non-structural norovirus proteins demonstrated that a rare population of EPCAM+ cells in the small intestine and colon serve as the exclusive viral reservoir in mice infected with MNoVCR6 (77). These cells were soon identified to be tuft cells, which express high levels of the MNoV receptor CD300LF (5). Accordingly, mice were resistant to infection with MNoVCR6 when tuft cells were absent or decreased, while viral titers were enhanced in any context where tuft cell numbers were increased, such as helminth infection or treatment with recombinant IL-25. In contrast, the non-persistent strain MNoVCW3 was unable to infect intestinal epithelial cells (5, 77). Whether human norovirus and/or other enteric viruses also infect tuft cells remains to be determined. It also remains unclear why norovirus would target tuft cells for infections. Perhaps the unique cell biology of tuft cells is important for viral replication, or tuft cells represent an immune-privileged site.
IMMUNE FUNCTION BEYOND THE INTESTINE
Airway Tuft Cells
Although often referred to as brush cells, a population of airway epithelial cells has been identified in the murine and human trachea that share the unique morphology and transcriptional signature of intestinal tuft cells(10, 41, 45, 78). Another very closely related cellular lineage termed solitary chemosensory cells (SCC) has also been identified in the nasal epithelium of mice and humans(22, 72, 79, 80), but its precise relationship to tuft cells has not yet been established. Unlike intestinal tuft cells, both tuft cells and SCCs of the airways express type II taste receptors (T2Rs, also known as bitter taste receptors) in humans (79) and mice (22, 81), and bitter taste receptor polymorphisms correlate with gram-negative bacterial infection in humans (82). T2Rs have been linked to regulation of both tissue physiology and immune responses. For example, denatonium—a potent bitter taste receptor ligand—can act on tuft cells to regulate respiration rate (83), nasal neurogenic inflammation (84), and allergic asthma induced by ovalbumin (OVA) and house dust mite (HDM) in mice (85). The bitter receptors on SCCs are reported to detect acyl-homoserine lactones (AHLs), which are produced by gram-negative bacteria (e.g. Pseudomonas aeruginosa) (86) to indicate population density (80, 87). Moreover, bitter taste receptors activate calcium flux in nasal SCCs to stimulate anti-microbial peptide secretion from surrounding epithelial cells and promote killing of P. aeruginosa, methicillin-resistant S. aureus (MRSA), K. pneumonia, and S. epidermis in human sinonasal tissue (80, 88). SCCs also express canonical sweet taste receptors (T1R2/3), but their activation suppresses calcium flux and bitter taste receptor-induced antimicrobial responses(88). T1R2/3 mediate sensing of glucose and bacterial D-amino acids in SCCs, leading to reduced antimicrobial peptide secretion (β-defensin) (88, 89). Clinically, there are elevated glucose and amino acid concentrations in chronic rhinosinositis patients and colonized fibrosis patients, respectively (88, 90). Together, these important studies suggest that SCCs, and perhaps airway tuft cells, utilize chemosensory machinery to ‘taste’ the upper respiratory tract environment and regulate innate immunity.
Whether airway tuft cells and SCCs are also integrated into tuft-ILC2 circuits and how this might alter type 2 immune responses remains unknown. Manipulation of IL-25 by intranasal administration, systemic blockade, or genetic deficiency all regulates lung type 2 airway inflammation (91, 64, 92, 93), but the expression of IL-17RB (a subunit of the IL-25 receptor) is much lower on lung ILC2s than in the intestine, even with IL-25 injection (94). Furthermore, the restriction of tuft cells to the upper airways in mice is confounding when considering inflammatory responses in the distal lung (10). In humans, SCCs are the major source of IL-25 in patients with chronic rhinosinusitis (95, 96), and ILC2 numbers were elevated in nasal polyps from chronic rhinosinusitis patients (96). These results support the existence of a tuft-ILC2 circuit in the human nasal cavity. There were also human case reports suggesting that brush (tuft) cell numbers are altered in immotile cilia syndrome (97) and interstitial pneumonitis (32). Together, future studies may further investigate the expression, detection mechanism, and corresponding immune function of taste receptors and other novel G-protein receptors on airway tuft cells, especially in the upper versus lower respiratory tract.
Thymic Tuft Cells
In all of the examples discussed so far, tuft cells were found in the non-squamous epithelium of hollow tissues. Therefore, it was surprising when cells with tuft-like morphology and expression of Gnat3, phospholipase C beta 2 (Plcb2), and Chat were identified in the thymic medulla, although unlike tuft cells in upper airways, no contact of these cholinergic chemosensory cells with nerve fibers was observed (25). More recently, RNA sequencing and careful phenotyping confirmed that these cells are indeed bona fide tuft cells and that they comprise 3~10% of medullary thymic epithelial (mTEC) cells in murine thymus and ~3.5% of mTEC in human thymus (98, 99). Based on single cell sequencing, tuft cells comprise mTEC group IV, which is molecularly distinct from other mTECs but closely related to intestinal tuft cells, with Dclk1, Sox9, Trpm5, Il25 and Pou2f3 all being expressed (99, 100). There are, however, also important differences between thymic tuft cells and those of other tissues; most notably, IL-25+ thymic tuft cells express MHC-II, suggesting an antigen presenting function (99). Thymic tuft cells also express a diversity of canonical taste receptors, which have been described in airway tuft cells but appear to be largely absent in intestinal tuft cells (45). As in other tissues, thymic tuft cell development requires Pou2f3 (99, 100).
Functionally, thymic tuft cells support TCRβint CD1d+ IL-4+ invariant NKT2 thymocytes and EOMES+ CD8 thymocytes in an Il25-, Pou2f3-, and Trpm5-dependent manner, although why chemosensing would be required for this function is completely unclear (99). The frequency of thymic Lin− TCR− CD127+ GATA3+ ILC2s is increased in the absence of tuft cells, but the functional significance of this remains unknown (100). Neither Pou2f3 nor Trpm5 deficiency impacted CD4−CD8−, CD4+CD8+, CD4 single positive T cells (CD4SP), or CD8SP numbers in the thymus (98, 99). In sum, thymic tuft cells are a distinct population of mTECs that regulate the frequency of certain thymocyte subsets. Their ‘sensing’ mechanism by taste receptors, their ontogeny, their relationship to antigen presentation, and their function in immune tolerance remain enigmatic.
TUFT CELLS AND NEURONS?
In addition to the outstanding questions already highlighted throughout this review, there is significant interest in the possibility that tuft cells might communicate with neurons, a finding which could provide mechanistic insight for numerous recent studies that have broadly linked the nervous and immune systems and specifically implicated neuronal signaling in type 2 inflammation (58, 101). In this context, it is notable that tuft cells in all tissues express CHAT (83, 68, 84, 45), the enzyme required for synthesis of the neurotransmitter acetylcholine. To date, a link between tuft cells and neurons has been best characterized in the airway. Although tuft cells and neurons do not form synaptic connections, they have been imaged in close proximity in the airway (102, 83, 84), and some of those neurons express acetylcholine receptors (84, 103). Accordingly, the airway inflammation induced by stimulating SCCs requires Trpm5 and acetylcholine (84). For the most part, however, tuft-neuron interactions have not been linked directly to immunity. For example, changes in respiratory rate induced by bitter substances are absent in Trpm5−/− mice and in mice where the airway epithelium has been abraded, suggesting that chemosensing by tuft cells regulates smooth muscle activity (80, 83), presumably via neuronal signaling. Similarly, bitter substances induce acetylcholine release from urethral tuft cells and cause contraction of the bladder detrusor muscle when delivered in vivo (104). Whether these tuft-neuron interactions in the airway and urethra represent mechanisms of avoidance and/or flushing that provide immune protection has not been tested.
The link between tuft cells and neurons in the intestine remains much less clear given different findings regarding tuft-neuron proximity (68, 71, 105). Intriguingly, intestinal tuft cells are positive for both CHAT and the neuropeptide β-endorphin (7, 68), and co-culture of neurons and intestinal organoids supports the differentiation of tuft cells (38). Interactions between tuft cells and the enteric nervous system, if verified, might serve to expand the sensing capacity of the nervous system while also broadly distributing signals initiated in tuft cells.
CONCLUSIONS
After decades of pioneering work provided the first hints of a chemosensing pathway in tuft cells and suggested a role for tuft cells in response to bacterial colonization in the airways, the last two years brought a series of breakthroughs that definitively implicated tuft cells in immune sensing and regulation. It is now clear that tuft cells are a critical component of the type 2 immune response, provide a reservoir for chronic norovirus infection, and contribute to thymic function. While it has been exciting to find an immune role for the previously enigmatic tuft cell lineage, we speculate that this does not represent their most evolutionarily ancient function. Undoubtedly, tuft cells have been critically shaped by co-evolution with helminths, protists, norovirus, and perhaps other microbes, but just as goblet cells produce mucus at homeostasis to support epithelial function and can be hyper-activated to promote helminth expulsion, we propose that tuft cells first evolved epithelial effector functions that were later useful for immunity and/or pathogenic exploitation. In this context, it is intriguing that tuft cells have been implicated in airway contraction, epithelial regeneration, DNA damage repair (18, 69, 106), and tumorigenesis (38, 107–109). If correct, our hypothesis suggests that understanding the unique cell biology of tuft cells and their role in the absence of infection will also advance our understanding of tuft cells in immunity.
Figure 1: Chemosensing by intestinal tuft cells regulates the tuft-ILC2 circuit.
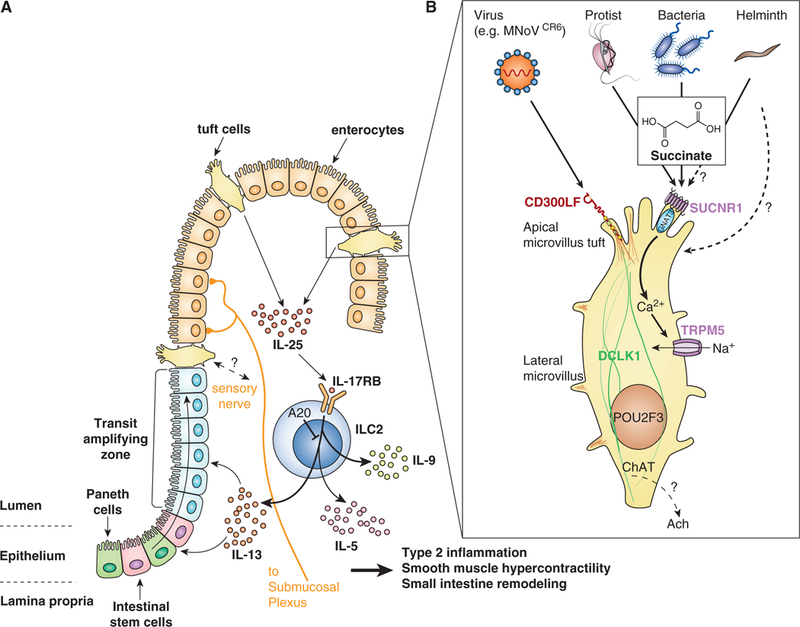
(A) Intestinal Tuft-ILC2 circuit. Tuft cells constitutively express Il25, which acts on group 2 innate lymphoid cells (ILC2s) in the lamina propria to induce production of canonical type 2 cytokines IL-5, −9, and −13, which collectively drive all aspects of innate type 2 inflammation, including eosinophilia and intestinal remodeling. IL-13 in particular signals in undifferentiated epithelial cells to bias their lineage commitment towards tuft and goblet cells, leading to hyperplasia of both cell types and driving the feed-forward tuft-ILC2 circuit. The circuit is amplified but yet unknown mechanisms when helminths or protists are sensed by tuft cells. Deletion of the signaling repressor A20 from ILC2s also amplifies the circuit and leads to chronic type 2 inflammation in the small intestine.
(B) Chemosensing. Tuft cells sense succinate secreted from Tritrichomonas protists and perhaps also bacteria and helminths. Signaling through the G protein coupled succinate receptor SUCNR1 induces an intracellular Ca2+ flux that opens the cation channel TRPM5, leading to influx of Na+ and depolarization of the cells. How cellular depolarization regulates tuft cell effector functions remains unknown, but may include release of neurotransmitter (e.g. acetylcholine (ACh)) that acts on nearby neurons. Tuft cells also express the murine nororvirus receptor CD300LF and are the host reservoir for chronic infection by the CR6 strain of norovirus. It is not clear if and how tuft cells sense this infection.
ACKNOWLEDGEMENTS
We thank T. Billipp, J. McGinty, and M. Fontana for reading the manuscript and for helpful discussions. JVM is a Damon Runyon–Dale Frey Breakthrough Scientist and a Searle Scholar.
Footnotes
This work was supported by NIH 1DP2 OD024087 (JVM) and the University of Washington.
Lgr5: Leucine-rich repeat-containing G-protein coupled receptor 5
Atoh1: Atonal bHLH Transcription Factor 1
Pou2f3: POU class 2 homeobox 3
Gfi1B: Growth factor independent 1B
Dclk1: Doublecortin-like kinase 1
Trpm5: Transient receptor potential cation channel subfamily M member 5c
CHAT: choline acetyltransferase
REFERENCES
- 1.Rhodin J, and Dalhamn T. 1956. Electron microscopy of the tracheal ciliated mucosa in rat. Z. Zellforsch. Mikrosk. Anat. Vienna Austria 1948 44: 345–412. [DOI] [PubMed] [Google Scholar]
- 2.Jarvi O, and Keyrilainen O. 1956. On the cellular structures of the epithelial invasions in the glandular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol. Microbiol. Scand. Suppl 39: 72–73. [PubMed] [Google Scholar]
- 3.Rhodin J 1959. LXVII Ultrastructure of the Tracheal Ciliated Mucosa in Rat and Man. Ann. Otol. Rhinol. Laryngol 68: 964–974. [Google Scholar]
- 4.Reid L, Meyrick B, Antony VB, Chang L-Y, Crapo JD, and Reynolds HY. 2005. The Mysterious Pulmonary Brush Cell. Am. J. Respir. Crit. Care Med 172: 136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilen CB, Lee S, Hsieh LL, Orchard RC, Desai C, Hykes BL, McAllaster MR, Balce DR, Feehley T, Brestoff JR, Hickey CA, Yokoyama CC, Wang Y-T, MacDuff DA, Kreamalmayer D, Howitt MR, Neil JA, Cadwell K, Allen PM, Handley SA, van Lookeren Campagne M, Baldridge MT, and Virgin HW. 2018. Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science 360: 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker N 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol 15: 19–33. [DOI] [PubMed] [Google Scholar]
- 7.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux J-F, Pignodel C, Clevers H, and Jay P. 2011. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol 192: 767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsubouchi S, and Leblond CP. 1979. Migration and turnover of entero-endocrine and caveolated cells in the epithelium of the descending colon, as shown by radioautography after continuous infusion of 3H-thymidine into mice. Am. J. Anat 156: 431–451. [DOI] [PubMed] [Google Scholar]
- 9.Basbaum C, and Jany B. 1990. Plasticity in the airway epithelium. Am. J. Physiol 259: L38–46. [DOI] [PubMed] [Google Scholar]
- 10.Krasteva G, and Kummer W. 2012. “Tasting” the airway lining fluid. Histochem. Cell Biol 138: 365–383. [DOI] [PubMed] [Google Scholar]
- 11.Saunders CJ, Reynolds SD, and Finger TE. 2013. Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am. J. Respir. Cell Mol. Biol 49: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjerknes M, Khandanpour C, Möröy T, Fujiyama T, Hoshino M, Klisch TJ, Ding Q, Gan L, Wang J, Martín MG, and Cheng H. 2012. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev. Biol 362: 194–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, Isomura A, Kawada K, Sakai Y, Yanagita M, Kageyama R, Kawaguchi Y, Taketo MM, Yonehara S, and Chiba T. 2013. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat. Genet 45: 98–103. [DOI] [PubMed] [Google Scholar]
- 14.von Moltke J, Ji M, Liang H-E, and Locksley RM. 2016. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato A, and Miyoshi S. 1988. Ultrastructure of the main excretory duct epithelia of the rat parotid and submandibular glands with a review of the literature. Anat. Rec 220: 239–251. [DOI] [PubMed] [Google Scholar]
- 16.Luciano L, Castellucci M, and Reale E. 1981. The brush cells of the common bile duct of the rat. This section, freeze-fracture and scanning electron microscopy. Cell Tissue Res 218: 403–420. [DOI] [PubMed] [Google Scholar]
- 17.Nevalainen TJ 1977. Ultrastructural characteristics of tuft cells in mouse gallbladder epithelium. Acta Anat. (Basel) 98: 210–220. [DOI] [PubMed] [Google Scholar]
- 18.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu Y-Y, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, Maitra A, and Leach SD. 2014. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology 146: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isomaki A 1962. Electron Microscopic Observations On A Special Cell Type In Gastro-Intestinal Epithelium Of Some Laboratory Animals. ACTA Pathol. Microbiol. Scand 115. [Google Scholar]
- 20.Okamoto K, Hanazaki K, Akimori T, Okabayashi T, Okada T, Kobayashi M, and Ogata T. 2008. Immunohistochemical and electron microscopic characterization of brush cells of the rat cecum. Med. Mol. Morphol 41: 145–150. [DOI] [PubMed] [Google Scholar]
- 21.Silva DG 1966. The fine structure of multivesicular cells with large microvilli in the epithelium of the mouse colon. J. Ultrastruct. Res 16: 693–705. [DOI] [PubMed] [Google Scholar]
- 22.Finger TE, Böttger B, Hansen A, Anderson KT, Alimohammadi H, and Silver WL. 2003. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc. Natl. Acad. Sci. U. S. A 100: 8981–8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krasteva G, Hartmann P, Papadakis T, Bodenbenner M, Wessels L, Weihe E, Schütz B, Langheinrich AC, Chubanov V, Gudermann T, Ibanez-Tallon I, and Kummer W. 2012. Cholinergic chemosensory cells in the auditory tube. Histochem. Cell Biol 137: 483–497. [DOI] [PubMed] [Google Scholar]
- 24.Deckmann K, Krasteva-Christ G, Rafiq A, Herden C, Wichmann J, Knauf S, Nassenstein C, Grevelding CG, Dorresteijn A, Chubanov V, Gudermann T, Bschleipfer T, and Kummer W. 2015. Cholinergic urethral brush cells are widespread throughout placental mammals. Int. Immunopharmacol 29: 51–56. [DOI] [PubMed] [Google Scholar]
- 25.Panneck AR, Rafiq A, Schütz B, Soultanova A, Deckmann K, Chubanov V, Gudermann T, Weihe E, Krasteva-Christ G, Grau V, del Rey A, and Kummer W. 2014. Cholinergic epithelial cell with chemosensory traits in murine thymic medulla. Cell Tissue Res 358: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasper M, Höfer D, Woodcock-Mitchell J, Migheli A, Attanasio A, Rudolf T, Müller M, and Drenckhahn D. 1994. Colocalization of cytokeratin 18 and villin in type III alveolar cells (brush cells) of the rat lung. Histochemistry 101: 57–62. [DOI] [PubMed] [Google Scholar]
- 27.Morroni M, Cangiotti AM, and Cinti S. 2007. Brush cells in the human duodenojejunal junction: an ultrastructural study. J. Anat 211: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moxey PC, and Trier JS. 1978. Specialized cell types in the human fetal small intestine. Anat. Rec 191: 269–285. [DOI] [PubMed] [Google Scholar]
- 29.Johnson FR, and Young BA. 1968. Undifferentiated cells in gastric mucosa. J. Anat 102: 541–551. [PMC free article] [PubMed] [Google Scholar]
- 30.Saqui-Salces M, Keeley TM, Grosse AS, Qiao XT, El-Zaatari M, Gumucio DL, Samuelson LC, and Merchant JL. 2011. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem. Cell Biol 136: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilloteaux J, Pomerants B, and Kelly TR. 1989. Human gallbladder mucosa ultrastructure: evidence of intraepithelial nerve structures. Am. J. Anat 184: 321–333. [DOI] [PubMed] [Google Scholar]
- 32.DiMaio MF, Dische R, Gordon RE, and Kattan M. 1988. Alveolar brush cells in an infant with desquamative interstitial pneumonitis. Pediatr. Pulmonol 4: 185–191. [DOI] [PubMed] [Google Scholar]
- 33.Clevers H 2013. The intestinal crypt, a prototype stem cell compartment. Cell 154: 274–284. [DOI] [PubMed] [Google Scholar]
- 34.Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, and van Oudenaarden A. 2011. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat. Cell Biol 14: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinley ET, Sui Y, Al-Kofahi Y, Millis BA, Tyska MJ, Roland JT, Santamaria-Pang A, Ohland CL, Jobin C, Franklin JL, Lau KS, Gerdes MJ, and Coffey RJ. 2017. Optimized multiplex immunofluorescence single-cell analysis reveals tuft cell heterogeneity. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, and Madsen OD. 2000. Control of endodermal endocrine development by Hes-1. Nat. Genet 24: 36–44. [DOI] [PubMed] [Google Scholar]
- 37.Herring CA, Banerjee A, McKinley ET, Simmons AJ, Ping J, Roland JT, Franklin JL, Liu Q, Gerdes MJ, Coffey RJ, and Lau KS. 2018. Unsupervised Trajectory Analysis of Single-Cell RNA-Seq and Imaging Data Reveals Alternative Tuft Cell Origins in the Gut. Cell Syst 6: 37–51.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, Chen X, May R, Houchen CW, Fox JG, Gershon MD, Quante M, and Wang TC. 2014. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Invest 124: 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, and Jay P. 2016. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita J, Ohmoto M, Yamaguchi T, Matsumoto I, and Hirota J. 2017. Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PloS One 12: e0189340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, Rogel N, Burgin G, Tsankov AM, Waghray A, Slyper M, Waldman J, Nguyen L, Dionne D, Rozenblatt-Rosen O, Tata PR, Mou H, Shivaraju M, Bihler H, Mense M, Tearney GJ, Rowe SM, Engelhardt JF, Regev A, and Rajagopal J. 2018. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerbe F, Legraverend C, and Jay P. 2012. The intestinal epithelium tuft cells: specification and function. Cell. Mol. Life Sci. CMLS 69: 2907–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider C, O’Leary CE, von Moltke J, Liang H-E, Ang QY, Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, and Locksley RM. 2018. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 174: 271–284.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, Artis D, and Garrett WS. 2016. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351: 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, Miller CN, Pollack JL, Nagana Gowda GA, Fontana MF, Erle DJ, Anderson MS, Locksley RM, Raftery D, and von Moltke J. 2018. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 49: 33–41.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, and Regev A. 2017. A single-cell survey of the small intestinal epithelium. Nature 551: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, Rogel N, Burgin G, Tsankov AM, Waghray A, Slyper M, Waldman J, Nguyen L, Dionne D, Rozenblatt-Rosen O, Tata PR, Mou H, Shivaraju M, Bihler H, Mense M, Tearney GJ, Rowe SM, Engelhardt JF, Regev A, and Rajagopal J. 2018. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, and Locksley RM. 2010. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U. S. A 107: 11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, and Locksley RM. 2013. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502: 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang H-E, and Locksley RM. 2016. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol 9: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuki A, Takatori H, Makita S, Yokota M, Tamachi T, Suto A, Suzuki K, Hirose K, and Nakajima H. 2017. T-bet inhibits innate lymphoid cell-mediated eosinophilic airway inflammation by suppressing IL-9 production. J. Allergy Clin. Immunol 139: 1355–1367.e6. [DOI] [PubMed] [Google Scholar]
- 52.Halim TYF 2016. Group 2 innate lymphoid cells in disease. Int. Immunol 28: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J-I, Ohtani M, Fujii H, and Koyasu S. 2010. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463: 540–544. [DOI] [PubMed] [Google Scholar]
- 54.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, and McKenzie ANJ. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, and Broide DH. 2013. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol 132: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Rückerl D, Seddon B, MacDonald AS, McKenzie A, and Wilson MS. 2016. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol 9: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, and Locksley RM. 2017. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J. Exp. Med 214: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Talbot S, Abdulnour R-EE, Burkett PR, Lee S, Cronin SJF, Pascal MA, Laedermann C, Foster SL, Tran JV, Lai N, Chiu IM, Ghasemlou N, DiBiase M, Roberson D, Von Hehn C, Agac B, Haworth O, Seki H, Penninger JM, Kuchroo VK, Bean BP, Levy BD, and Woolf CJ. 2015. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron 87: 341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar A-L, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, Shen X, Kostenis E, König GM, Senda T, Carpenter D, Farber DL, and Artis D. 2017. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549: 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour R-EE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, Haas BJ, Tickle TL, Trombetta JJ, Baral P, Klose CSN, Mahlakõiv T, Artis D, Rozenblatt-Rosen O, Chiu IM, Levy BD, Kowalczyk MS, Regev A, and Kuchroo VK. 2017. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cardoso V, Chesné J, Ribeiro H, García-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, and Veiga-Fernandes H. 2017. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549: 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Moltke J, and Locksley RM. 2014. I-L-C-2 it: type 2 immunity and group 2 innate lymphoid cells in homeostasis. Curr. Opin. Immunol 0: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HRP, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, and Artis D. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med 203: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, and McKenzie ANJ. 2006. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med 203: 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang Z, Swaidani S, Yin W, Wang C, Barlow JL, Gulen MF, Bulek K, Do J, Aronica M, McKenzie ANJ, Min B, and Li X. 2012. Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of Lin(−)c-Kit(+) innate cell population. Immunity 36: 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urban JF, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, and Finkelman FD. 1998. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8: 255–264. [DOI] [PubMed] [Google Scholar]
- 67.Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, and Damak S. 2008. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J. Comp. Neurol 509: 514–525. [DOI] [PubMed] [Google Scholar]
- 68.Schütz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, Chubanov V, Gudermann T, Diener M, Kummer W, Krasteva-Christ G, and Weihe E. 2015. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front. Physiol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chandrakesan P, May R, Weygant N, Qu D, Berry WL, Sureban SM, Ali N, Rao C, Huycke M, Bronze MS, and Houchen CW. 2016. Intestinal tuft cells regulate the ATM mediated DNA Damage response via Dclk1 dependent mechanism for crypt restitution following radiation injury. Sci. Rep 6: 37667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Höfer D, Püschel B, and Drenckhahn D. 1996. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc. Natl. Acad. Sci. U. S. A 93: 6631–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bezençon C, le Coutre J, and Damak S. 2007. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses 32: 41–49. [DOI] [PubMed] [Google Scholar]
- 72.Kaske S, Krasteva G, König P, Kummer W, Hofmann T, Gudermann T, and Chubanov V. 2007. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu P, Zhang C-H, Lifshitz LM, and ZhuGe R. 2017. Extraoral bitter taste receptors in health and disease. J. Gen. Physiol 149: 181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei W, Ren W, Ohmoto M, Urban JF, Matsumoto I, Margolskee RF, and Jiang P. 2018. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. U. S. A 115: 5552–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Müller M, Mentel M, van Hellemond JJ, Henze K, Woehle C, Gould SB, Yu R-Y, van der Giezen M, Tielens AGM, and Martin WF. 2012. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. MMBR 76: 444–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teunis PFM, Sukhrie FHA, Vennema H, Bogerman J, Beersma MFC, and Koopmans MPG. 2015. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol. Infect 143: 1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee S, Wilen CB, Orvedahl A, McCune BT, Kim K-W, Orchard RC, Peterson ST, Nice TJ, Baldridge MT, and Virgin HW. 2017. Norovirus Cell Tropism Is Determined by Combinatorial Action of a Viral Non-structural Protein and Host Cytokine. Cell Host Microbe 22: 449–459.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, Klein AM, and Jaffe AB. 2018. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barham HP, Cooper SE, Anderson CB, Tizzano M, Kingdom TT, Finger TE, Kinnamon SC, and Ramakrishnan VR. 2013. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int. Forum Allergy Rhinol 3: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill MEA, Silver WL, Kinnamon SC, and Finger TE. 2010. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc. Natl. Acad. Sci. U. S. A 107: 3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin W, Ogura T, Margolskee RF, Finger TE, and Restrepo D. 2008. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J. Neurophysiol 99: 1451–1460. [DOI] [PubMed] [Google Scholar]
- 82.Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Beauchamp GK, Doulias P-T, Ischiropoulos H, Kreindler JL, Reed DR, and Cohen NA. 2012. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Invest 122: 4145–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schütz B, Kotlikoff M, Ibanez-Tallon I, and Kummer W. 2011. Cholinergic chemosensory cells in the trachea regulate breathing. Proc. Natl. Acad. Sci. U. S. A 108: 9478–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saunders CJ, Christensen M, Finger TE, and Tizzano M. 2014. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc. Natl. Acad. Sci. U. S. A 111: 6075–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma P, Yi R, Nayak AP, Wang N, Tang F, Knight MJ, Pan S, Oliver B, and Deshpande DA. 2017. Bitter Taste Receptor Agonists Mitigate Features of Allergic Asthma in Mice. Sci. Rep 7: 46166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sbarbati A, Tizzano M, Merigo F, Benati D, Nicolato E, Boschi F, Cecchini MP, Scambi I, and Osculati F. 2009.. Acyl homoserine lactones induce early response in the airway. Anat. Rec. Hoboken NJ 2007 292: 439–448. [DOI] [PubMed] [Google Scholar]
- 87.Smith RS, Harris SG, Phipps R, and Iglewski B. 2002. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol 184: 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, Kreindler JL, Margolskee RF, and Cohen NA. 2014. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Invest 124: 1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee RJ, Hariri BM, McMahon DB, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Jiang P, Margolskee RF, and Cohen NA. 2017. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci. Signal 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barth AL, and Pitt TL. 1996. The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J. Med. Microbiol 45: 110–119. [DOI] [PubMed] [Google Scholar]
- 91.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, and Rennick DM. 2001. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15: 985–995. [DOI] [PubMed] [Google Scholar]
- 92.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, and McKenzie ANJ. 2012. Innate IL-13–producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol 129: 191–198.e4. [DOI] [PubMed] [Google Scholar]
- 93.Reynolds JM, Lee Y-H, Shi Y, Wang X, Angkasekwinai P, Nallaparaju KC, Flaherty S, Chang SH, Watarai H, and Dong C. 2015. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity 42: 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang Y, Mao K, Chen X, Sun M-A, Kawabe T, Li W, Usher N, Zhu J, Urban JF, Paul WE, and Germain RN. 2018. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kohanski MA, Workman AD, Patel NN, Hung L-Y, Shtraks JP, Chen B, Blasetti M, Doghramji L, Kennedy DW, Adappa ND, Palmer JN, Herbert DR, and Cohen NA. 2018. Solitary Chemosensory Cells are a Primary Epithelial Source of Interleukin-25 in Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol [DOI] [PMC free article] [PubMed]
- 96.Patel NN, Kohanski MA, Maina IW, Triantafillou V, Workman AD, Tong CCL, Kuan EC, Bosso JV, Adappa ND, Palmer JN, Herbert DR, and Cohen NA. 2018. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol [DOI] [PMC free article] [PubMed]
- 97.Gordon RE, and Kattan M. 1984. Absence of cilia and basal bodies with predominance of brush cells in the respiratory mucosa from a patient with immotile cilia syndrome. Ultrastruct. Pathol 6: 45–49. [DOI] [PubMed] [Google Scholar]
- 98.Bornstein C, Nevo S, Giladi A, Kadouri N, Pouzolles M, Gerbe F, David E, Machado A, Chuprin A, Tóth B, Goldberg O, Itzkovitz S, Taylor N, Jay P, Zimmermann VS, Abramson J, and Amit I. 2018. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature [DOI] [PubMed]
- 99.Miller CN, Proekt I, von Moltke J, Wells KL, Rajpurkar AR, Wang H, Rattay K, Khan IS, Metzger TC, Pollack JL, Fries AC, Lwin WW, Wigton EJ, Parent AV, Kyewski B, Erle DJ, Hogquist KA, Steinmetz LM, Locksley RM, and Anderson MS. 2018. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature [DOI] [PMC free article] [PubMed]
- 100.Bornstein C, Nevo S, Giladi A, Kadouri N, Pouzolles M, Gerbe F, David E, Machado A, Chuprin A, Tóth B, Goldberg O, Itzkovitz S, Taylor N, Jay P, Zimmermann VS, Abramson J, and Amit I. 2018. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature [DOI] [PubMed]
- 101.Voisin T, Bouvier A, and Chiu IM. 2017. Neuro-immune interactions in allergic diseases: novel targets for therapeutics. Int. Immunol 29: 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sato A 2007. Tuft cells. Anat. Sci. Int 82: 187–199. [DOI] [PubMed] [Google Scholar]
- 103.Alimohammadi H, and Silver WL. 2000. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem. Senses 25: 61–66. [DOI] [PubMed] [Google Scholar]
- 104.Deckmann K, Filipski K, Krasteva-Christ G, Fronius M, Althaus M, Rafiq A, Papadakis T, Renno L, Jurastow I, Wessels L, Wolff M, Schütz B, Weihe E, Chubanov V, Gudermann T, Klein J, Bschleipfer T, and Kummer W. 2014. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc. Natl. Acad. Sci. U. S. A 111: 8287–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gautron L, Rutkowski JM, Burton MD, Wei W, Wan Y, and Elmquist JK. 2013. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. J. Comp. Neurol 521: 3741–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chandrakesan P, May R, Qu D, Weygant N, Taylor VE, Li JD, Ali N, Sureban SM, Qante M, Wang TC, Bronze MS, and Houchen CW. 2015. Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self-renewal. Oncotarget 6: 30876–30886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Delgiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, Olive KP, Spence JR, Sipos B, Wright CVE, Wells JM, and Crawford HC. 2014. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology 146: 233–244.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, Jiang Z, Tanaka T, Dubeykovskaya ZA, Kim W, Chen X, Urbanska AM, Nagar K, Westphalen CB, Quante M, Lin C-S, Gershon MD, Hara A, Zhao C-M, Chen D, Worthley DL, Koike K, and Wang TC. 2017. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 31: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, Chen X, Ormanns S, Nagar K, Tailor Y, May R, Cho Y, Asfaha S, Worthley DL, Hayakawa Y, Urbanska AM, Quante M, Reichert M, Broyde J, Subramaniam PS, Remotti H, Su GH, Rustgi AK, Friedman RA, Honig B, Califano A, Houchen CW, Olive KP, and Wang TC. 2016. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell 18: 441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]


