Abstract
Background
The Cardiothoracic Surgical Trials Network (CTSN) recently reported no difference in left ventricular end-systolic volume index or in survival at 2-years between patients with severe ischemic mitral regurgitation (MR) randomized to mitral-valve repair or replacement. However, replacement provided more durable correction of MR and fewer cardiovascular readmissions. Yet, cost-effectiveness outcomes have not been addressed.
Methods and Results
We conducted a cost-effectiveness analysis of the surgical treatment of ischemic MR based on the CTSN trial (n=126 for repair; n=125 for replacement). Patient-level data on readmissions, survival, quality-of-life, and U.S. hospital costs were used to estimate costs and quality-adjusted life years (QALYs) per patient over the trial duration and a 10-year time horizon. We performed microsimulation for extrapolation of outcomes beyond the 2-years of trial data. Bootstrap and deterministic sensitivity analyses were done to address parameter uncertainty. In-hospital cost estimates were $78,216 for replacement vs $72,761 for repair (difference: $5,455; 95% uncertainty interval (UI): −7,784–21,193), while 2-year costs were: $97,427 vs $96,261 (difference: $1,166; 95% UI: −16,253–17,172), respectively. QALYs at 2-year were 1.18 for replacement vs 1.23 for repair (difference: −0.05; 95% UI: −0.17–0.07). Over 5- and 10-years, the benefits of reduction in cardiovascular readmission rates with replacement increased, and survival minimally improved compared to repair. At 5-years, cumulative costs and QALYs showed no difference on average, but by 10-year there was a small, uncertain benefit for replacement: $118,023 vs $119,837 (difference: -$1,814; 95% UI: −27,144–22,602) and QALYs: 4.06 vs 3.97 (difference: 0.09; 95% UI: −0.87–1.08). After 10 years, the incremental cost-effectiveness of replacement continued to improve.
Conclusions
Our cost-effectiveness analysis predicts potential savings in cost and gains in quality-adjusted survival at 10 years when mitral-valve replacement is compared to repair for severe ischemic MR. These projected benefits, however, were small and subject to variability. Efforts to further delineate predictors of long-term outcomes in patients with severe ischemic MR are needed to optimize surgical decisions for individual patients, which should yield more cost-effective care.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00807040.
Introduction
Ischemic mitral regurgitation (MR) affects up to 60% of patients with myocardial infarction.1–6 This disorder is typically caused by a change in the geometry of the left ventricle related to myocardial injury, while the valve leaflets and chordae themselves are unaffected.7, 8 Patients who develop severe ischemic MR have a significantly higher incidence of heart failure and mortality.1–4, 6, 9 While both mitral-valve repair and replacement may improve outcomes, repair has been associated with better short-term outcomes and replacement with a more durable correction of the MR.10
The Cardiothoracic Surgical Trials Network (CTSN) has recently published the results of a trial that compared mitral-valve repair to replacement in patients with chronic, severe ischemic MR.11, 12 Two hundred fifty one patients were randomized and followed for two years and the primary endpoint was left ventricular end-systolic volume index (LVESVI). Secondary end points included mortality, major adverse cardiovascular events, hospitalization, recurrent MR, and quality-of-life. No difference between treatment groups was observed in the change of LVESVI at 1 and 2 years compared to baseline. Thirty-day mortality was 1.6% in the repair group and 4.0% in the replacement group (P = 0.26), and no significant differences between treatment arms were observed at 1 and 2 years. While quality-of-life scores also did not differ, the 2-year risk of recurrent MR was significantly higher following repair (58.8% vs 3.8%; P < 0.001). Moreover, repair led to a higher risk of serious adverse events related to heart failure (P = 0.05) and higher risk of cardiovascular readmissions (P = 0.01).
A less durable correction of MR with repair is likely to give rise to higher long-term costs due to greater risk of readmissions and repeat surgery as well as reduction in long-term survival. On the other hand, replacement may be more costly to perform and associated with worse short-term outcomes.10 Therefore, in the present study, a comprehensive cost-effectiveness analysis (CEA) comparing mitral-valve repair and replacement, accounting for the full spectrum of benefits, harms and costs, was performed to determine the economic and health outcomes of the two strategies.
Methods
Trial Design and Population
The severe ischemic MR trial11, 12 was conducted by the CTSN and funded by the National Institutes of Health (NIH) and the Canadian Institutes of Health Research (CIHR). In summary, 251 patients, admitted to 22 clinical centers for severe ischemic MR between December 2008 and April 2012, were randomized to undergo either mitral-valve repair (n=126) or replacement (n=125). Patients were eligible for enrollment, regardless of whether they required concomitant revascularization of underlying coronary disease. In agreement with guidelines,13–15 mitral-valve replacement was performed using a complete chordal-sparing approach and either a biological or mechanical valve was implanted at the surgeon’s discretion. Mitral-valve repair was performed using an undersized complete annuloplasty ring. Conversion to mitral-valve replacement was used when MR was not adequately corrected at the surgeon’s discretion. When indicated, coronary artery bypass grafting (CABG) was performed according to standard procedures. Causes of death and adverse events were adjudicated by an independent committee of experts. An NIH-appointed data and safety monitoring board oversaw trial progress. The institutional review board at each study center approved the trial. All trial participants provided written informed consent. The trial data used in the analyses are available at BioLINCC.16
Cost and Quality-of-Life Data
We estimated costs from a U.S. healthcare perspective using uniform billing (UB) medical claims associated with index hospitalizations (N=172) and readmissions (N=101) at U.S. study sites and obtained this data from Vizient, a healthcare improvement company,17 or directly from study sites themselves. Costs were calculated per hospitalization, by converting charges using departmental cost-to-charge ratios matching reported revenue codes. Departmental cost-to-charge ratios were derived from corresponding Centers for Medicare & Medicaid Services annual hospital cost reports. All costs were expressed into 2015 U.S. dollars using the Personal Health Care index for hospital care.18 Generic health status was converted into utility scores using the SF-6D health utility index (0=death, 1=optimal quality-of-life),19 which was derived from patient-level Short Form (SF)-12 questionnaire trial data collected at baseline, 30 days, 6, 12 and 24 months. We performed multiple imputation for missing costs and SF-6D utility scores (for details see Supplemental Methods). All analyses were done using the intention-to-treat principle.
Within-Trial CEA
We initially performed a within-trial CEA. Cumulative costs were calculated by totaling hospitalization costs for each patient during the trial follow-up period. Quality-adjusted life years (QALYs) were calculated from longitudinal SF-6D utility scores assuming a linear pattern between visits. For the reference case, when an interval death occurred, we assumed that the SF-6D utility score would follow a sudden drop to zero at the moment of death.20 Year 2 costs and QALYs were discounted using a rate of 3%.21 We then calculated the difference in the average costs and QALYs between treatment groups. An incremental cost-effectiveness ratio (ICER) was calculated when the more expensive strategy would also provide more effectiveness. For details see Supplemental Methods.
Long-Term CEA
For predicting costs and QALYs over a 5 and 10-year time horizon we developed an individual-level state-transition (‘microsimulation’) model with a fixed one-month cycle length (Supplemental Figure I). We designed the model to make patient-specific forecasts of mortality and readmissions (for heart failure, other CVD, non-CVD, and reoperations), in addition to tracking the expected costs and loss of quality-of-life related to these adverse events. To increase the precision of event rates, we combined CTSN severe and moderate ischemic MR trial data11, 12, 22, 23 and included an interaction term for trial and treatment assignment to model the trial-specific treatment effect. We individualized event rates by baseline age and gender, and included time-dependent covariates to allow for an increase in subsequent readmission and mortality event rates following a first readmission. Hazard ratios of these covariates were estimated by Andersen-Gill models (Supplemental Table I).24 For the reference case, we modeled baseline readmission rates using cubic spline functions assuming a Weibull distribution for extrapolations beyond the trial follow-up period. Competing mortality rates were assumed to follow an exponential survival distribution in concordance with survival curves from several studies that included ischemic MR patients with a complete follow-up for death.25–32 Parametric baseline hazard rate functions for extrapolation were estimated based on data from 9 months through 2-years post-randomization to account for hazard rates that leveled off over time and to ensure a sufficient amount of failure times (Supplemental Figure II). Model validity was assessed by comparing model-based predictions with empirically calculated 2-year event rates, cumulative costs and QALYs. SF-6D utility scores were assumed to remain stable beyond trial duration.33–35 Cost and quality-of-life penalties were conditioned on readmissions and estimated by prediction models that included age at admission, gender, study arm, and reason for admission. Bleeding events were assumed to occur during hospitalizations. The vast majority of bleeding events observed in the trial occurred during the index hospitalization (17 out of 18) and the one bleeding event that occurred post-discharge happened during a non-CVD readmission. Additional late bleeding events were not modeled, as the use of oral anticoagulation and antiplatelet medication was similar for both treatment arms throughout the trial (Supplemental Table II). We discounted costs and QALYs with a 3% annual rate.21 For details see the Supplemental Methods.
Sensitivity Analyses
We performed probabilistic sensitivity analysis by bootstrapping the trial dataset 1,000 times. Analysis steps performed for both the within-trial and long-term CEA were repeated in each bootstrap replicate to account for correlation among model parameters. Results were summarized as: 1) 95% uncertainty intervals (UIs) using a bias-corrected and accelerated method; 2) as a scatter plot of the 1,000 pairs of difference in average costs and QALYs; and 3) as cost-effectiveness acceptability curves. In the latter, the percentage of bootstraps in which replacement was deemed to be cost-effective was plotted according to a range of different time horizons and cost-effectiveness thresholds including commonly used thresholds from $50K/QALY to 200K/QALY.21
To further evaluate robustness and heterogeneity of our findings, we conducted a number of deterministic sensitivity and scenario analyses. For the within-trial CEA, we assumed that patients who died would have a gradual decline in quality-of-life from the last value measured until death. For the long-term CEA, we varied the annual discount rate from 0 to 5% and used different distribution assumptions for extrapolating readmission and mortality rates. We used the same reoperation risk for the period beyond the 2-year follow-up for the two treatment arms and 36 and based this value on a more recently published trial.37 Because mortality rates are high, the likelihood of reoperations is expected to be low for the severe ischemic MR patient population.38 Nevertheless, we evaluated the potential impact of reoperations for late failure of replacement valves in patients with a long life expectancy using a 20-year time horizon. Based on a recent cohort study,39 we assumed that the increase in reoperation risk occurred after 5-year39 and that the maximum 20-year cumulative incidence would be 15%. While in the reference case we assumed that hazard ratios estimated based on the intention-to-treat principle, reflect treatment cross-over effects, here we accounted for changes in hazard ratios that occurred when patients in the mitral-valve repair arm underwent late reoperation to receive a replacement. Thus, when such cross-overs occurred, we effectively reassigned the patient from repair to replacement and reset the time clock to zero for both the hazard functions of readmissions and death to account for higher hazards in the early post-surgical phase. Moreover, for mortality and readmission rates, we varied the treatment assignment hazard ratio, as well as the hazard ratio associated with previous readmission, over the 95% UI interval. Lastly, to explore heterogeneity in cost-effectiveness, we varied the baseline age of the entire trial cohort from 50 to 85. For details see Supplemental Methods.
Results
Study Population
The mean age was 68±10 years and most patients (62%) were male. The mean baseline LVESVI was 63.4±26.8. The vast majority of patients had a prior history of myocardial infarction and revascularization. Nearly a quarter had a history of heart failure and a third had atrial fibrillation. Concomitant CABG was performed in 75% of patients (Supplemental Table III). Eleven of the 126 patients assigned to repair received replacement before leaving the operating room, one patient in the replacement group underwent repair. One patient who underwent repair had a reoperation with replacement before hospital discharge and three had a reoperation with replacement later on. One patient in the replacement group who initially received a bioprosthesis had a late reoperation and received a mechanical valve.
Within-Trial CEA
During the index hospitalization, there were small differences in resource use between repair and replacement (Supplemental Table IV). For example, cardiopulmonary bypass time and ICU stay were longer in replacement: 151 vs 139 mins (P = 0.04) and 6.5 vs 5.7 days (P = 0.06), respectively. Index hospitalization costs were on average higher in replacement than in repair: $78,216 vs $72,761 (difference: $5,455; 95% UI: −7,784 to 21,193). Yet, this difference decreased over time (Figure 1) and cumulative costs at the 2-year follow-up were: $97,427 vs $96,261 (difference: $1,166; 95% UI: −16,253 to 17,172). A breakdown of resource use and costs by admission type is included in Supplemental Table V. There were no relevant differences in SF-6D utility scores during the trial follow-up period (Figure 2). However, reflecting the marginally lower survival rate, cumulative QALYs were on average slightly lower in the replacement arm. At 2-year, QALYs were 1.18 for replacement vs 1.23 for repair (difference: −0.05; 95% UI: −0.17 to 0.07) (Table 1 and Figure 3).
Figure 1. Within-trial cumulative average cost by study arm.
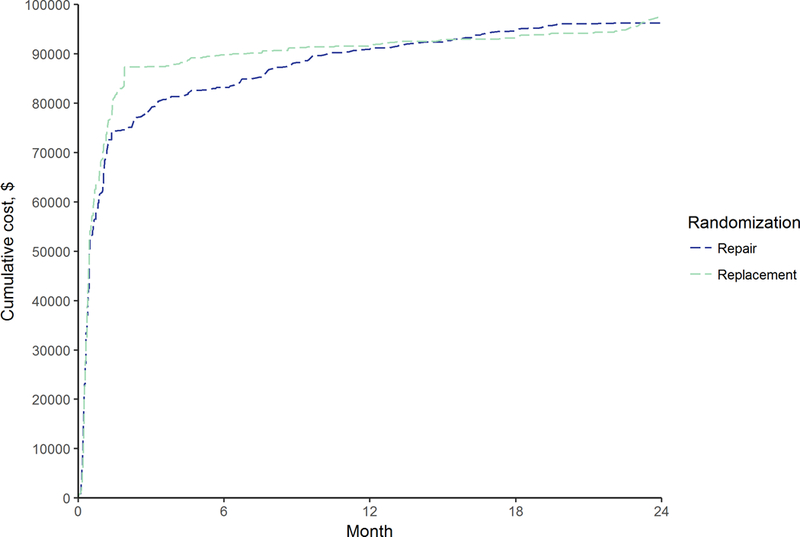
Shown are cumulative costs averaged across N=126 for repair and N=125 for replacement.
Figure 2. Average SF-6D utility index by study arm.
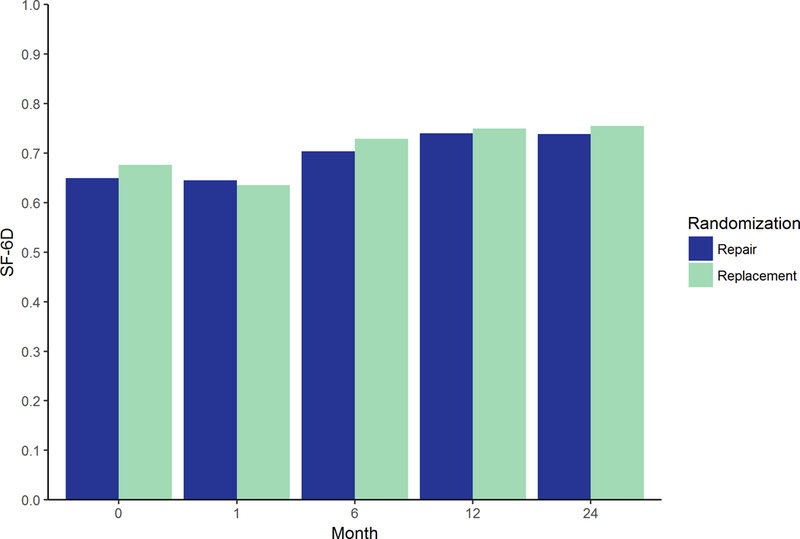
Shown are mean SF-6D utility index scores in N=126 for repair and N=125 for replacement.
Table 1.
Reference case cost-effectiveness outcomes (95% UI)
| Outcome | Repair N=126 |
Replacement N=125 |
|---|---|---|
| Costs, $ | ||
| 1-year | 90,914 (79,089 to 106,107) | 91,762 (81,106 to 107,530) |
| Δ | - | +848 (−16,160 to 16,995) |
| 2-year | 96,261 (83,950 to 111,619) | 97,427 (85,575 to 113,263) |
| Δ | - | +1,166 (−16,253 to 17,172) |
| 5-year | 109,460 (92,212 to 129,023) | 108,667 (92,316 to 130,881) |
| Δ | - | −792 (−20,154 to 18,199) |
| 10-year | 119,837 (96,974 to 147,200) | 118,023 (96,832 to 148,282) |
| Δ | - | −1,814 (−27,144 to 22,602) |
| QALYs | ||
| 1-year | 0.63 (0.59 to 0.66) | 0.60 (0.55 to 0.64) |
| Δ | - | −0.03 (−0.09 to 0.02) |
| 2-year | 1.23 (1.14 to 1.30) | 1.18 (1.07 to 1.25) |
| Δ | - | −0.05 (−0.17 to 0.07) |
| 5-year | 2.59 (2.26 to 2.84) | 2.58 (2.27 to 2.86) |
| Δ | - | −0.02 (−0.40 to 0.39) |
| 10-year | 3.97 (3.14 to 4.59) | 4.06 (3.34 to 4.75) |
| Δ | - | +0.09 (−0.87 to 1.08) |
| ICER, $/QALY | ||
| 1-year | Dominant | - |
| 2-year | Dominant | - |
| 5-year | 48,270 | - |
| 10-year | - | Dominant |
| Probability CE at $50K; $100K; $200K per QALY threshold, % | ||
| 1-year | 64; 69; 76 | 36; 31;24 |
| 2-year | 68; 74; 77 | 32; 26; 23 |
| 5-year | 57; 57; 57 | 43; 43; 43 |
| 10-year | 48; 48; 47 | 52; 52; 53 |
| Probability dominant, % | ||
| 1-year | 53 | 8 |
| 2-year | 51 | 12 |
| 5-year | 33 | 24 |
| 10-year | 24 | 26 |
Abbreviations: ICER, incremental cost-effectiveness ratio; CE, cost-effective; QALY, quality-adjusted life year.
Figure 3. Within-trial cost-effectiveness analysis bootstrap results comparing replacement vs repair.
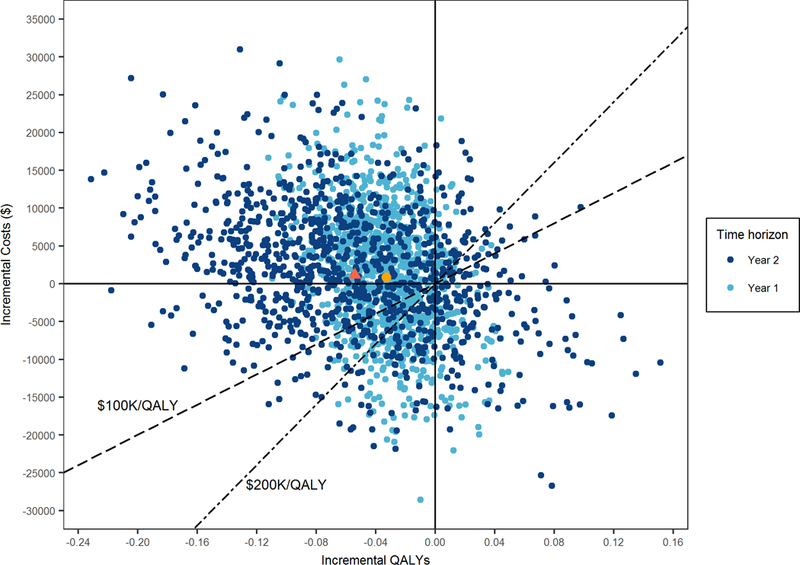
Shown are Δs in average costs and average QALYs as measured in each bootstrap replicate of the trial data with repair as the reference strategy. The yellow and red figures represent the point estimates (Δcosts, ΔQALYs) at 1-year ($848; −0.03) and 2-year ($1,166; −0.05) respectively. The two diagonals represent commonly used cost-effectiveness thresholds of $100K/QALY and $200K/QALY. The proportion of iterations below or to the right of the selected diagonal equals the likelihood of the replacement strategy being cost-effective as compared with repair given the applicable cost-effectiveness threshold.
Long-Term CEA
Predictions beyond trial follow-up demonstrated that mortality would slightly improve with replacement as compared to repair, with an uncertain decrease of 2.4% (95% UI −18.3 to 23.2) in 10-year mortality favoring replacement (Figure 4). Rates of reoperation and cardiovascular readmissions were, however, more substantially reduced in the replacement arm over time, with differences in the 10-year total event counts of −4 (95% UI −13 to 2) and −79 (95% UI −200 to 13) respectively (Figure 5). Predictions of cumulative costs and QALYs were commensurate with these findings. At 5-years, cumulative costs and QALYs were similar between the study arms, although on average repair was still cost-effective with an ICER of $48,270/QALY. However, by 10-years, replacement became the dominant strategy when considering average outcomes: $118,023 vs $119,837 (difference: -$1,814; 95% UI: −27,144 to 22,602) and 4.06 vs 3.97 QALYs (difference: 0.09; 95% UI: −0.87 to 1.08) for replacement and repair, respectively (Supplemental Table I and Figure VI).
Figure 4. Observed and simulated all-cause mortality estimates by study arm.
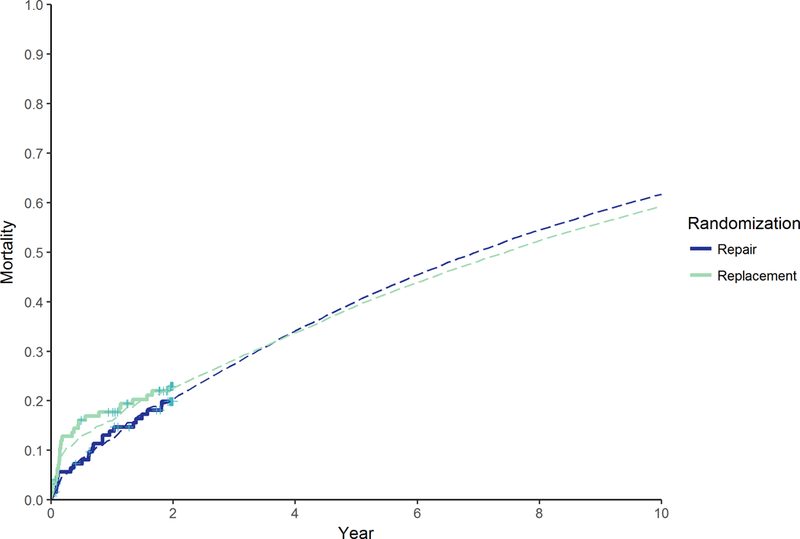
Shown are all-cause mortality estimates based on Kaplan-Meier curves of trial data with censoring at 2-year (solid lines) and simulated mortality estimates within the reference case analysis (dashed lines).
Figure 5. Number of hospital admissions and reoperations during 1-, 2-, 5-, and 10-year follow-up.
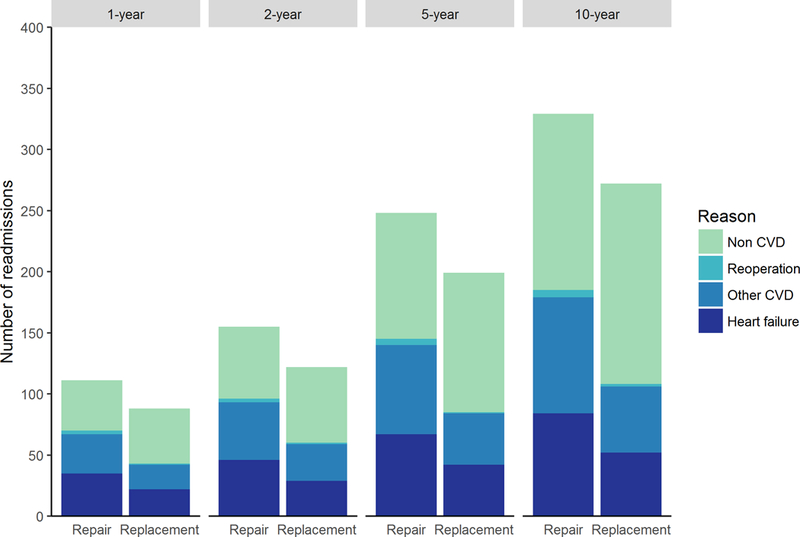
Outcomes at 1- and 2-year are based on trial data; outcomes at 5- and 10-year are based on adding simulated outcomes occurring within 2–5 and 2–10 year time intervals within the reference case analysis.
Outcome Uncertainty and Variation
Results of the within-trial CEA did not change when quality-of-life was assumed to decline gradually prior to death (Supplemental Figure VII). The likelihood that replacement is cost-effective compared to repair increased as the duration of follow-up increased: 25% at 2-year to 53% at 10-year and this trend was seen over a range of cost-effectiveness thresholds from $50K/QALY to 200K/QALY (Table 1). Extending the follow-up time to 20 years further increased the likelihood that replacement is more cost-effective than repair to 56% (Figure 6). Reducing the cost of heart failure readmissions and hazard ratios of repair vs replacement for heart failure, other and non-cardiovascular readmissions favored repair where the 10-year costs were lower. However, replacement remained the more cost effective strategy even when these parameter inputs were set to their lower 95% UI limits (ICERs of replacement fell well below $50,000/QALY). Furthermore, conclusions about cost-effectiveness were stable across different discount rates and distribution types for extrapolating readmission and survival rates, and also did not change when explicitly modeling treatment cross-over effects following reoperation in the repair arm. Increasing the long-term reoperation rate following repair, further increased the cost reduction with replacement to approximately $3,800 (Table 2). When using a longer time horizon of 20 years and modeling higher rates of reoperations for late failures of replacement valves, replacement remained the more cost-effective option (Supplemental Table VI). Only when a significant improvement in late mortality rates with repair was assumed, i.e., a hazard ratio of repair vs replacement ≤ 0.92, would repair become the more effective strategy at 10 years (QALYs ≥ 4.07) and potentially more cost-effective; when assuming a hazard ratio at the lower 95% UI limit its ICER was $16,618/QALY. Cost-effectiveness varied with the age of the patient at the time of surgery: cost savings and health benefits following replacement diminished as age increased (Table 2).
Figure 6. Cost-effectiveness acceptability curves for replacement according to time horizon.
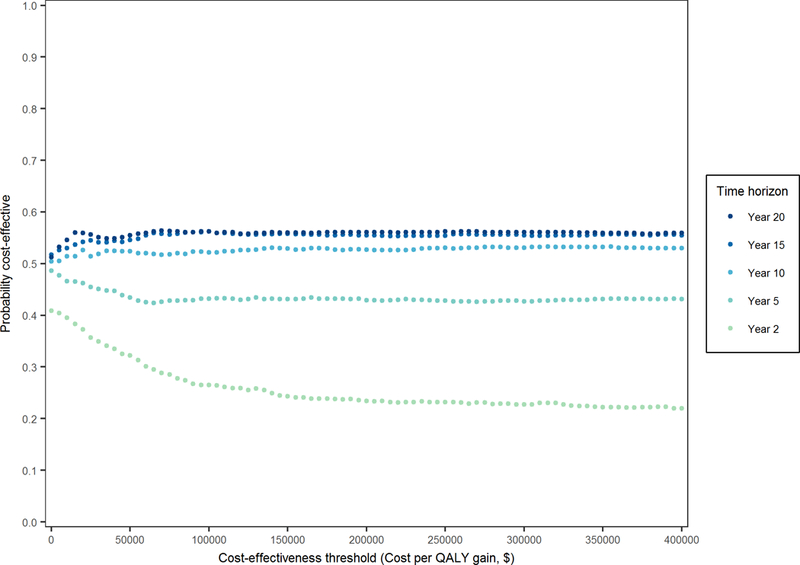
These curves indicate the probability of replacement being cost-effective as compared with repair using different time horizons. Each curve equals the proportion of iterations below or to the right of the diagonal (as shown in Figure 3 for time horizons of 1- and 2-year) by changing the slope of the diagonal from 0 to infinity, i.e. increasing the cost-effectiveness threshold. The probability of repair being cost-effective equals 100% minus the depicted probability of replacement being cost-effective.
Table 2.
Sensitivity analyses using a 10-year time horizon
| Variable | Costs repair, $ | Costs replacement, $ | Δ costs replacement vs repair, $ | QALYs repair | QALYs replacement | Δ QALYs replacement vs repair | ICER, $/QALY replacement vs repair |
|---|---|---|---|---|---|---|---|
| Discount rate | |||||||
| 0% | 123,641 | 121,405 | −2,236 | 4.44 | 4.57 | +0.12 | Dominant |
| 5% | 117,701 | 116,127 | −1,574 | 3.70 | 3.78 | +0.08 | Dominant |
| Distribution for extrapolation of baseline mortality rates | |||||||
| Spline-Weibull | 125,579 | 122,604 | −2,975 | 4.54 | 4.59 | +0.06 | Dominant |
| Weibull | 125,181 | 122,222 | −2,959 | 4.48 | 4.56 | +0.08 | Dominant |
| Log-logistic | 124,937 | 122,424 | −2,514 | 4.47 | 4.56 | +0.08 | Dominant |
| Distribution for extrapolation of baseline readmission rates | |||||||
| Weibull | 120,528 | 116,090 | −4,438 | 3.98 | 4.08 | +0.10 | Dominant |
| Log-logistic | 118,312 | 114,445 | −3,867 | 3.98 | 4.09 | +0.10 | Dominant |
| Hazard ratio of repair vs replacement for heart failure readmissions >2-year follow-up | |||||||
| Lower limit (0.899) | 117,488 | 118,023 | +6,146 | 3.97 | 4.06 | +0.09 | 6,146 |
| Upper limit (2.610) | 124,467 | 118,023 | −6,443 | 3.95 | 4.06 | +0.11 | Dominant |
| Hazard ratio of repair vs replacement for other cardiovascular readmissions >2-year follow-up | |||||||
| Lower limit (0.790) | 116,082 | 118,023 | +1,941 | 3.98 | 4.06 | +0.08 | 23,247 |
| Upper limit (3.227) | 128,024 | 118,023 | −10,000 | 3.95 | 4.06 | +0.11 | Dominant |
| Hazard ratio of repair vs replacement for non-cardiovascular readmissions >2-year follow-up | |||||||
| Lower limit (0.569) | 115,538 | 118,023 | +2,486 | 4.00 | 4.06 | +0.06 | 38,376 |
| Upper limit (1.220) | 126,406 | 118,023 | −8,382 | 3.94 | 4.06 | +0.12 | Dominant |
| Hazard ratio of repair vs replacement for mortality >2-year follow-up | |||||||
| Lower limit (0.494) | 124,915 | 118,023 | −6,891 | 4.48 | 4.06 | −0.41 | 16,618* |
| Upper limit (2.087) | 114,247 | 118,023 | +3,777 | 3.41 | 4.06 | +0.65 | 5,797 |
| Hazard ratio of hospital readmission for mortality >2-year follow-up | |||||||
| Lower limit (2.517) | 122,340 | 119,887 | −2,453 | 4.04 | 4.08 | +0.04 | Dominant |
| Upper limit (6.972) | 113,814 | 112,667 | −1,147 | 3.08 | 3.14 | +0.06 | Dominant |
| Reoperation risk in replacement ≈ repair >2-year follow-up | 119,837 | 118,741 | −1,096 | 3.97 | 4.06 | +0.09 | Dominant |
| 100% higher reoperation risk in repair >2-year follow-up | 120,595 | 118,023 | −2,572 | 3.97 | 4.06 | +0.09 | Dominant |
| 300% higher reoperation risk in repair >2-year follow-up | 121,845 | 118,023 | −3,822 | 3.97 | 4.06 | +0.09 | Dominant |
| Clock set back to zero following reoperation, cross-over to replacement following reoperation in repair | 120,750 | 118,326 | −2,423 | 3.97 | 4.06 | +0.09 | Dominant |
| Costs heart failure readmissions | |||||||
| Lower limit (11,400) | 115,732 | 117,078 | +1,346 | 3.97 | 4.06 | +0.09 | 14,593 |
| Upper limit (24,855) | 124,008 | 122,311 | −1,697 | 3.97 | 4.06 | +0.09 | Dominant |
| Costs other cardiovascular readmissions | |||||||
| Lower limit (12,630) | 114,834 | 114,116 | −718 | 3.97 | 4.06 | +0.09 | Dominant |
| Upper limit (33,690) | 129,305 | 122,463 | −6,842 | 3.97 | 4.06 | +0.09 | Dominant |
| Costs non-cardiovascular readmissions | |||||||
| Lower limit (14,635) | 114,237 | 110,380 | −3,856 | 3.97 | 4.06 | +0.09 | Dominant |
| Upper limit (28,610) | 128,867 | 127,074 | −1,793 | 3.97 | 4.06 | +0.09 | Dominant |
| Age-dependent analyses | |||||||
| Age 50 | 133,207 | 121,057 | −12,149 | 4.86 | 4.97 | +0.11 | Dominant |
| Age 55 | 129,526 | 120,379 | −9,146 | 4.78 | 4.68 | +0.11 | Dominant |
| Age 60 | 126,431 | 119,988 | −6,444 | 4.49 | 4.61 | +0.12 | Dominant |
| Age 65 | 122,835 | 118,980 | −3,855 | 4.27 | 4.31 | +0.04 | Dominant |
| Age 70 | 120,138 | 117,933 | −2,205 | 4.00 | 4.01 | +0.01 | Dominant |
| Age 75 | 118,091 | 117,141 | −950 | 3.67 | 3.65 | −0.03 | 33,764* |
| Age 80 | 116,093 | 115,185 | −908 | 3.36 | 3.29 | −0.07 | 12,726* |
| Age 85 | 113,267 | 114,307 | +1,040 | 2.99 | 2.88 | −0.11 | Dominated |
*Incremental cost-effectiveness ratios (ICERs) are here calculated with replacement as reference. Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Discussion
Our analysis, when restricted to empirical data from the CTSN trial, demonstrated that at 2-years, differences in costs and quality-adjusted survival were small and uncertain for mitral-valve repair and replacement, despite the fact that initially costs for replacement are higher. Yet the real benefit of replacement is in reducing the rate of MR recurrence, which was even apparent by the 2-year mark in the trial. Given that this benefit is expected to continue over time, it is very plausible that the time horizon for the trial itself was not long enough to fully realize the expected down-stream health and cost benefits of the more durable correction of the mitral insufficiency.12 After extrapolating outcomes it appeared that on average, the upfront costs and risks of replacement were offset by the downstream reductions in cardiovascular readmission and reoperation rates. By 10-years, the post-trial reduction in hospitalizations associated with replacement seemed to translate into marginal net cost savings and QALY gains, although these benefits remained uncertain. Yet, conclusions about cost-effectiveness did not change using a range of potential readmission costs and rates for reoperation, survival and readmissions, as well as when varying the treatment effect of repair vs replacement on readmissions and reoperations and varying the impact of a hospital readmission on subsequent mortality. Beyond 10 years, the cost reductions and QALY gains with replacement became more certain.
An important driver of replacement being slightly more effective than repair on average, and, therefore, the dominant strategy, was the absence of a mortality benefit for repair as reflected in the mortality hazard ratio point estimate. In our microsimulation model, long-term survival was further influenced in favor of replacement by the lower likelihood of hospital readmissions. These readmissions lead to a nearly fourfold higher subsequent mortality rate (see the hazard ratio in Supplemental Table I). Conclusions about the cost-effectiveness of replacement, however, were not sensitive to varying this hazard ratio. Reasons are that a) when this hazard ratio increases, the beneficial effect of averting cardiovascular readmissions in replacement patients decreases, because life expectancy and thus time to avoid these readmissions also decreases, and b) when this hazard ratio decreases, life expectancy improves overall, but the difference in survival between replacement and repair patients decreases.
Age of the patient at the time of the procedure had a major impact on the variation in cost-effectiveness outcome. The average age of patients in the CTSN severe ischemic MR trial was approximately 68 years and the median life expectancy for these patients was expected to exceed 7 years (see follow-up time at 50% survival probability in Figure 2). When considering average outcomes only, this was a sufficient period of time for the long-term benefits of replacement to accrue and offset its upfront incremental risks and costs. However, as Table 2 demonstrates, in older ages, this benefit is no longer the case. Thus, in octogenarians and in younger patients with comorbidities that substantially reduce their life expectancy, repair may be the more cost-effective surgical approach.40
Two recently published meta-analyses comparing repair to replacement for ischemic MR showed results consistent with the CTSN trial findings that repair offers a less durable correction of MR.36, 38 Moreover, long-term survival rates of replacement vs repair predicted by our model, seem consistent with the summary hazard ratio of 0.95 (95% CI: 0.84 to 1.09) from propensity-score adjusted studies.38 However, these meta-analyses included studies involving both moderate and severe ischemic MR patients, studies in which chordal sparing and non-chordal sparing mitral-valve replacement approaches were used and studies that did not report on readmission rates. It is therefore difficult to draw inferences about cost-effectiveness from the existing literature. The model reported here conditioned cost and quality-of-life outcomes on survival and readmission rates, which were fully based on detailed patient-level trial data. This enabled us to individualize cumulative costs and QALYs, while taking into account the correlation among these outcomes when analyzing uncertainty by the bootstrap procedure and increases in mortality rates with higher incidence of readmissions. These important elements are difficult to include in a CEA based on aggregated data from the literature, which may subsequently lead to biased outcomes and conclusions about cost-effectiveness.
Our results should be viewed in the context of a number of limitations. First, the microsimulation model used in our long-term CEA was based on two trials,11, 12, 22, 23 which were not designed to primarily evaluate long-term mortality rates. Nonetheless, 5 and 10 year survival, predicted by our model, was generally consistent with publications that were largely based on the experience of patients with severe ischemic MR.25, 27, 29, 30 A recently published trial conducted in Italy showed more favorable 5-year overall survival for repair (71.8%) than was predicted by our model, although this was a small trial with younger patients.37 However, the use of alternative distributions to extrapolate mortality resulted in a similar 5-year survival and, importantly, conclusions about long-term cost-effectiveness did not change. A second limitation to consider is that we did not include costs associated with outpatient services, rehabilitation programs, nursing facilities, medications, productivity loss, and informal care.21 Yet, because differences in long-term survival were small between repair and replacement, the importance of these additional costs would be minimal when assuming no major differences per unit of time across study arms. Third, hospitalization costs showed substantial variation, which led to considerable uncertainty in the cumulative cost estimates. Given these limitations, our findings may be perceived by some as being too uncertain for making recommendations about which procedure is best. However, when policymakers need to make a decision about which intervention should be selected for a given budget, and this decision cannot be deferred, the decision making should be done based on cost-effectiveness point estimates, irrespective of statistical significance.41, 42 Moreover, the sensitivity analyses showed that conclusions based on the point estimates did not change for most of the uncertain parameter inputs with the exception of assuming significantly lower long-term mortality rates with repair. Unfortunately, larger trials are not expected for severe ischemic MR and observational studies have their limitations for extracting unbiased effect estimates.
Recently, the American College of Cardiology/American Heart Association and American Association for Thoracic Surgery updated their guidelines and now recommend it is reasonable to choose mitral-valve replacement over repair in severe ischemic MR (level IIa evidence).13, 15 Our results bring an economic dimension to the choice between repair and replacement and indicate that replacement is potentially a marginally more cost-effective strategy. However, as shown by the sensitivity analysis that varies patient age, the group-level predictions from our CEA should be interpreted with caution when making decisions for patients with clinical and demographic characteristics that are far from the trial norm. Moreover, for an individual patient with severe ischemic MR, it can be expected that the optimal decision regarding repair or replacement depends on the patient’s risk of MR recurrence. In the repair arm of the CTSN severe ischemic MR trial, approximately 40% of the patients remained free from MR recurrence after two years of follow-up, and these patients had on average a greater degree of reverse remodeling than those with recurrence after repair and patients who underwent replacement.12 Clinical decision making must include perioperative findings that are associated with MR recurrence including expected LV-mitral-valve ring mismatch,43 as well as findings of basal aneurysm/dyskinesis, significant leaflet tethering, and severe LV enlargement.44 An evaluation of the cost-effectiveness of an approach using perioperative information was however beyond the scope of this study. The value of a more personalized approach integrating individualized predictions of MR recurrence, adverse outcomes, and life expectancy, together with patient preferences and costs should be evaluated in further research.
Conclusions
Our cost-effectiveness analysis predicts potential savings in cost and gains in quality-adjusted survival at 10 years when mitral-valve replacement is compared to repair for severe ischemic MR. The projected benefits following replacement, however, were small and subject to variability, with cost savings being mediated by expected improvements in long-term cardiovascular readmission and reoperation rates following replacement, and QALY gains being dependent on the absence of a significant long-term survival benefit with repair. Efforts to further delineate predictors of long-term outcomes in patients with severe ischemic MR are needed to potentially optimize surgical decisions for individual patients, which should yield more cost-effective care.Acknowledgements
Study Concept and Design: Ferket, Chang, Bagiella, Gelijns, Moskowitz. Acquisition, Analysis, or Interpretation of Data: All authors. Critical Revision of the Manuscript for Important Intellectual Content: All authors. Statistical Analysis: Ferket and Chang. Obtained Funding: Gelijns and Ferket. Administrative, Technical, or Material Support: Cardiothoracic Surgical Trials Network (CTSN).
Supplementary Material
What is known
A recently reported RCT by the Cardiothoracic Surgical Trials Network, which compared mitral valve repair and replacement for severe ischemic mitral regurgitation, reported no difference in left ventricular end-systolic volume index or in survival at 2-years.
Mitral-valve replacement was found to provide a more durable correction of MR and fewer cardiovascular readmissions.
What the Study Adds
Based on CTSN trial results alone, 2-year costs and quality-adjusted survival were similar for repair and replacement, although replacement was associated with higher index hospitalization costs.
Extrapolating outcomes beyond 2 years of the CTSN empirical data using microsimulation, the high upfront costs of replacement were found to be further offset by downstream reductions in cardiovascular readmission rates, potentially resulting in cost savings and gains in quality-adjusted survival with replacement at 10 years.
Utilization of predictors of long-term clinical and economic outcomes could potentially optimize surgical decisions for more cost-effective care in patients with severe ischemic MR.
Sources of Funding
This work was supported by a cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke of the NIH and the Canadian Institutes of Health Research. Dr. Ferket was supported by American Heart Association Grant #16MCPRP31030016 (Ferket).
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services.
Appendix: Cardiothoracic Surgical Trials Network (CTSN)
The members of the Cardiothoracic Surgical Trials Network (CTSN) involved in this study are as follows:
National Heart, Lung and Blood Institute:Marissa A. Miller, Wendy C. Taddei-Peters, Dennis Buxton, Nancy L. Geller, David Gordon, Neal O. Jeffries, Albert Lee;
National Institute of Neurological Disorders and Stroke: Claudia S. Moy;
Canadian Institutes of Health Research:Ilana Kogan Gombos, Jennifer Ralph;
Network Chairs:Toronto General Hospital, Richard D. Weisel, (Chair); Christiana Care Health System Timothy J. Gardner, (Chair Emeritus); Brigham and Women’s Hospital, Patrick T. O’Gara, (Co-Chair); Icahn School of Medicine, Eric A. Rose (Vice-Chair);
Data Coordinating Center:International Center for Health Outcomes and Innovation Research (InCHOIR), Department of Population Health Science and Policy at the Icahn School of Medicine at Mount Sinai, Annetine C. Gelijns, Michael K. Parides, Deborah D. Ascheim, Alan J. Moskowitz, Ellen Moquete, Emilia Bagiella, Helena Chang, Melissa Chase, James Foo, Lopa Gupta, Katherine Kirkwood, Edlira Dobrev, Ron Levitan, Karen O’Sullivan, Jessica Overbey, Milerva Santos, Deborah Williams, Paula Williams, Xia Ye;
Clinical Site Investigators:Baylor Research Institute : Michael Mack (PI), Tracine Adame, Natalie Settele, Jenny Adams, William Ryan, Robert L. Smith, Paul Grayburn; Brigham and Women’s Hospital : Frederick Y. Chen (PI), Anju Nohria, Lawrence Cohn, Prem Shekar, Sary Aranki, Gregory Couper, Michael Davidson, R. Morton Bolman III, Rita Lawrence; Cleveland Clinic Foundation, Eugene H. Blackstone (PI), A. Marc Gillinov, Carrie Geither, Leoma Berroteran, Diana Dolney, Kristen Doud, Suzanne Fleming, Roberta Palumbo, Christine Whitman, Kathy Sankovic, Denise Kosty Sweeney; NHLBI Clinical Research Scholars: Gregory Pattakos, Pamela A. Clarke; Columbia University, Michael Argenziano (PI), Mathew Williams, Lyn Goldsmith, Craig R. Smith, Yoshifumi Naka, Allan Stewart, Allan Schwartz; Daniel Bell, Danielle Van Patten, Sowmyashree Sreekanth; Duke University, Peter K. Smith (PI), John H. Alexander, Carmelo A. Milano, Donald D. Glower, Joseph P. Mathew, J. Kevin Harrison, Stacey Welsh; NHLBI Clinical Research Scholars: Mark F. Berry, Cyrus J. Parsa, Betty C. Tong, Judson B. Williams; East Carolina Heart Institute, T. Bruce Ferguson (PI), Alan P. Kypson, Evelio Rodriguez, Malissa Harris, Brenda Akers, Allison O’Neal; Emory University, John D. Puskas (PI), Vinod H. Thourani, Robert Guyton, Jefferson Baer, Kim Baio, Alexis A. Neill; Hôpital Laval : Pierre Voisine (PI), Mario Senechal, François Dagenais, Kim O’Connor, Gladys Dussault, Tatiana Ballivian, Suzanne Keilani; Inova Heart & Vascular Institute : Alan M. Speir (PI), Patrick Magee, Niv Ad, Sally Keyte, Minh Dang; Jewish Hospital : Mark Slaughter (PI), Marsha Headlee, Heather Moody, Naresh Solankhi, Emma Birks; Mission Hospital : Mark A. Groh (PI), Leslie E. Shell, Stephanie A. Shepard, Benjamin H. Trichon, Tracy Nanney, Lynne C. Hampton, Ralph Mangusan; Montefiore-Einstein Heart Center, New York, NY, Robert E. Michler (PI), David A. D’Alessandro, Joseph J. DeRose, Jr., Daniel J. Goldstein, Ricardo Bello, William Jakobleff, Mario Garcia, Cynthia Taub, Daniel Spevak, Roger Swayze, Nadia Sookraj; Montreal Heart Institute, Louis P. Perrault (PI), Arsène-Joseph Basmadjian, Denis Bouchard, Michel Carrier, Raymond Cartier, Michel Pellerin, Jean François Tanguay, Ismael El-Hamamsy, André Denault, Jonathan Lacharité, Sophie Robichaud; NIH Heart Center at Suburban Hospital, Keith A. Horvath (PI), Philip C. Corcoran, Michael P. Siegenthaler, Mandy Murphy, Margaret Iraola, Ann Greenberg; Ohio State University Medical Center: Chittoor Sai-Sudhakar (PI), Ayseha Hasan, Asia McDavid, Bradley Kinn; Sacre-Cœur de Montreal : Pierre Pagé (PI), Carole Sirois; University of Maryland : James S. Gammie (PI), Cindi A. Young, Dana Beach, Robert Villanueva; University of Pennsylvania, Michael A. Acker (PI), Y. Joseph Woo, Mary Lou Mayer; University of Southern California : Michael Bowdish (PI), Vaughn A. Starnes, David Shavalle, Ray Matthews, Shadi Javadifar, Linda Romar; University of Virginia, Irving L. Kron (PI), Gorav Ailawadi, Karen Johnston, John M. Dent, John Kern, Jessica Keim, Sandra Burks, Kim Gahring;
Protocol Review Committee:David A. Bull (Chair); Patrice Desvigne-Nickens, Executive Secretary; Dennis O. Dixon, Mark Haigney, Richard Holubkov, Alice Jacobs, Frank Miller, John M. Murkin, John Spertus, Andrew S. Wechsler;
Data and Safety Monitoring Board:Frank Sellke (Chair); Cheryl L. McDonald, Executive Secretary; Robert Byington, Neal Dickert, Dennis O. Dixon, John S. Ikonomidis, David O. Williams, Clyde W. Yancy;
Medical Monitors:James C. Fang, Nadia Giannetti, Wayne Richenbacher;
Overall Event Adjudication Committee:Vivek Rao (Chair); Karen L. Furie, Rachel Miller, Sean Pinney, William C. Roberts, Mary N. Walsh;
Echocardiography Core Lab:Judy Hung (PI), Xin Zeng, Niamh Kilcullen, David Hung;
Cardiopulmonary Testing Core Lab:Steve Keteyian (PI), Heather Aldred, Clinton Brawner;
Neurocognitive Core Lab:Joseph Mathew (PI), Jeffrey Browndyke, Yanne Toulgoat-Dubois.
Footnotes
Disclosures
Bart S. Ferket: None. Gorav Ailawadi: None. Annetine C. Gelijns: None. Michael A. Acker: Consultant/Advisory Board; Modest; Thoratec. Samuel F. Hohmann: None. Helena L. Chang: None. Denis Bouchard: None. David O. Meltzer: None. Robert E. Michler: None. Ellen G. Moquete: None. Pierre Voisine: None. John C. Mullen: None. Anuradha Lala: None. Michael J. Mack: None. A. Marc. Gillinov: Other Research Support; Modest; St. Jude Medical, Abbott. Consultant/Advisory Board; Modest; St. Jude Medical, Edwards Lifesciences. Consultant/Advisory Board; Significant; Abbott, Medtronic, CryoLife, AtriCure. Vinod H. Thourani: Research Grant; Modest; Edwards Lifesciences, Medtronic Corp, Abbott Medical. Consultant/Advisory Board; Modest; Edwards Lifesciences, Abbott Medical. Marissa A. Miller: None. James S. Gammie: None. Michael K. Parides: None. Emilia Bagiella: None. Robert L. Smith: Research Grant; Significant; Edwards LifeSciences. Speakers Bureau; Significant; Abbott Medical. Consultant/Advisory Board; Significant; Abbott Medical, Edwards LifeSciences. Other; Significant; Abbott Medical, Edwards LifeSciences. Peter K. Smith: None. Judy W. Hung: None. Lopa N. Gupta: None. Eric A. Rose: None. Patrick T. O’Gara: None. Alan J. Moskowitz: None.
Contributor Information
Bart S. Ferket, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Gorav Ailawadi, Division of Thoracic and Cardiovascular Surgery, University of Virginia School of Medicine, Charlottesville, VA
Annetine C. Gelijns, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Michael A. Acker, Department of Surgery, Division of Cardiovascular Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA
Samuel F. Hohmann, Center for Advanced Analytics, Vizient, Chicago, IL
Helena L. Chang, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Denis Bouchard, Montréal Heart Institute, University of Montréal, Montréal, QC, Canada
David O. Meltzer, Department of Medicine, University of Chicago, Chicago, IL
Robert E. Michler, Department of Cardiovascular and Thoracic Surgery, Department of Surgery, Montefiore Medical Center/Albert Einstein College of Medicine, New York, NY
Ellen G. Moquete, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Pierre Voisine, Institut Universitaire de Cardiologie de Québec, Hôpital Laval, Quebec, QC, Canada
John C. Mullen, Division of Cardiac Surgery, University of Alberta, Edmonton, AB, Canada
Anuradha Lala, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Michael J. Mack, Department of Cardiothoracic Surgery, Baylor Research Institute, Baylor Scott & White Health, Plano, TX
A. Marc Gillinov, Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Cleveland, OH
Vinod H. Thourani, Clinical Research Unit, Division of Cardiothoracic Surgery, Emory University School of Medicine, Atlanta, GA and Department of Cardiac Surgery, MedStar Heart & Vascular Institute, Washington, D.C
Marissa A. Miller, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, MD
James S. Gammie, Department of Surgery, Division of Cardiac Surgery, University of Maryland Medical Center, Baltimore, MD
Michael K. Parides, Department of Surgery, Division of Cardiovascular and Thoracic Surgery, Duke University Medical Center, Durham, NC International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY.
Emilia Bagiella, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Robert L. Smith, Department of Cardiothoracic Surgery, Baylor Research Institute, Baylor Scott & White Health, Plano, TX
Peter K. Smith, Department of Surgery, Division of Cardiovascular and Thoracic Surgery, Duke University Medical Center, Durham, NC
Judy W. Hung, Division of Cardiology, Massachusetts General Hospital, Boston, MA
Eric A. Rose, Department of Cardiac Surgery, Mount Sinai Health System, New York, NY
Patrick T. O’Gara, Cardiovascular Division, Brigham and Women’s Hospital, Boston, MA
Alan J. Moskowitz, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
References
- 1.Hillis GS, Moller JE, Pellikka PA, Bell MR, Casaclang-Verzosa GC and Oh JK. Prognostic significance of echocardiographically defined mitral regurgitation early after acute myocardial infarction. Am Heart J. 2005;150:1268–75. [DOI] [PubMed] [Google Scholar]
- 2.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Gersh BJ Jr, Basta L, Moye L, Braunwald E and Pfeffer MA. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997;96:827–33. [DOI] [PubMed] [Google Scholar]
- 3.Tcheng JE, Jackman JD, Nelson CL Jr, Gardner LH, Smith LR, Rankin JS, Califf RM and Stack RS. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992;117:18–24. [DOI] [PubMed] [Google Scholar]
- 4.Schroder JN, Williams ML, Hata JA, Muhlbaier LH, Swaminathan M, Mathew JP, Glower DD, O’Connor CM, Smith PK and Milano CA. Impact of mitral valve regurgitation evaluated by intraoperative transesophageal echocardiography on long-term outcomes after coronary artery bypass grafting. Circulation. 2005;112:I293–8. [DOI] [PubMed] [Google Scholar]
- 5.Barzilai B, Gessler C, Perez JE Jr, Schaab CandJaffe AS Significance of Doppler-detected mitral regurgitation in acute myocardial infarction. Am J Cardiol. 1988;61:220–3. [DOI] [PubMed] [Google Scholar]
- 6.Ellis SG, Whitlow PL, Raymond RE and Schneider JP. Impact of mitral regurgitation on long-term survival after percutaneous coronary intervention. Am J Cardiol. 2002;89:315–8. [DOI] [PubMed] [Google Scholar]
- 7.Asgar AW, Mack MJ and Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. Journal of the American College of Cardiology. 2015;65:1231–48. [DOI] [PubMed] [Google Scholar]
- 8.Pierard LA and Carabello BA. Ischaemic mitral regurgitation: pathophysiology, outcomes and the conundrum of treatment. European heart journal. 2010;31:2996–3005. [DOI] [PubMed] [Google Scholar]
- 9.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR and Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. [DOI] [PubMed] [Google Scholar]
- 10.Badiwala MV, Verma S and Rao V. Surgical management of ischemic mitral regurgitation. Circulation. 2009;120:1287–93. [DOI] [PubMed] [Google Scholar]
- 11.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, Argenziano M, Gammie JS, Mack M, Ascheim DD, Bagiella E, Moquete EG, Ferguson TB, Horvath KA, Geller NL, Miller MA, Woo YJ, D’Alessandro DA, Ailawadi G, Dagenais F, Gardner TJ, O’Gara PT, Michler RE, Kron IL and Cardiothoracic Surgical Trials Network (CTSN) Investigators. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. The New England journal of medicine. 2014;370:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, Thourani V, Argenziano M, Gammie JS, Mack M, Demers P, Atluri P, Rose EA, O’Sullivan K, Williams DL, Bagiella E, Michler RE, Weisel RD, Miller MA, Geller NL, Taddei-Peters WC, Smith PK, Moquete E, Overbey JR, Kron IL, O’Gara PT, Acker MA and Cardiothoracic Surgical Trials Network (CTSN) Investigators. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. The New England journal of medicine. 2015;374:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA 3rd, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd and Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 14.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology, European Association for Cardio-Thoracic Surgery, Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL and Zembala M. Guidelines on the management of valvular heart disease (version 2012). European heart journal. 2012;33:2451–96.22922415 [Google Scholar]
- 15.American Association For Thoracic Surgery Ischemic Mitral Regurgitation Consensus Guidelines Writing Committee, Kron IL, LaPar DJ, Acker MA, Adams DH, Ailawadi G, Bolling SF, Hung JW, Lim DS, Mack MJ, O’Gara PT, Parides MK and Puskas JD. 2016 update to The American Association for Thoracic Surgery consensus guidelines: Ischemic mitral valve regurgitation. The Journal of thoracic and cardiovascular surgery. 2017;153:1076–1079. [DOI] [PubMed] [Google Scholar]
- 16.BioLINCC. The National Heart, Lung and Blood Institute (NHLBI) Biologic Specimen and Data Repository. 2018. https://biolincc.nhlbi.nih.gov/. Accessed May 2, 2018.
- 17.Vizient. 2018. https://www.vizientinc.com/. Accessed May 2, 2018.
- 18.Dunn A, Grosse SD and Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health services research. 2016; 53:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brazier JE and Roberts J. The estimation of a preference-based measure of health from the SF-12. Medical care. 2004;42:851–9. [DOI] [PubMed] [Google Scholar]
- 20.Glasziou PP, Cole BF, Gelber RD, Hilden J and Simes RJ. Quality adjusted survival analysis with repeated quality of life measures. Statistics in medicine. 1998;17:1215–29. [DOI] [PubMed] [Google Scholar]
- 21.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE and Ganiats TG. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316:1093–103. [DOI] [PubMed] [Google Scholar]
- 22.Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, Hung JW, Parides MK, Ailawadi G, Perrault LP, Acker MA, Argenziano M, Thourani V, Gammie JS, Miller MA, Page P, Overbey JR, Bagiella E, Dagenais F, Blackstone EH, Kron IL, Goldstein DJ, Rose EA, Moquete EG, Jeffries N, Gardner TJ, O’Gara PT, Alexander JH, Michler RE and Cardiothoracic Surgical Trials Network (CTSN) Investigators. Surgical treatment of moderate ischemic mitral regurgitation. The New England journal of medicine. 2014;371:2178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, Acker MA, Hung JW, Chang HL, Perrault LP, Gillinov AM, Argenziano M, Bagiella E, Overbey JR, Moquete EG, Gupta LN, Miller MA, Taddei-Peters WC, Jeffries N, Weisel RD, Rose EA, Gammie JS, DeRose JJ, Jr., Puskas JD, Dagenais F, Burks SG, El-Hamamsy I, Milano CA, Atluri P, Voisine P, O’Gara PT, Gelijns AC and Cardiothoracic Surgical Trials Network (CTSN) Investigators. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. The New England journal of medicine. 2016;374:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen PK and Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Statist. 1982;10:1100–1120. [Google Scholar]
- 25.Magne J, Girerd N, Senechal M, Mathieu P, Dagenais F, Dumesnil JG, Charbonneau E, Voisine P and Pibarot P. Mitral repair versus replacement for ischemic mitral regurgitation: comparison of short-term and long-term survival. Circulation. 2009;120:S104–11. [DOI] [PubMed] [Google Scholar]
- 26.Milano CA, Daneshmand MA, Rankin JS, Honeycutt E, Williams ML, Swaminathan M, Linblad L, Shaw LK, Glower DD and Smith PK. Survival prognosis and surgical management of ischemic mitral regurgitation. The Annals of thoracic surgery. 2008;86:735–44. [DOI] [PubMed] [Google Scholar]
- 27.Gillinov AM, Wierup PN, Blackstone EH, Bishay ES, Cosgrove DM, White J, Lytle BW and McCarthy PM. Is repair preferable to replacement for ischemic mitral regurgitation? The Journal of thoracic and cardiovascular surgery. 2001;122:1125–41. [DOI] [PubMed] [Google Scholar]
- 28.Mihaljevic T, Lam BK, Rajeswaran J, Takagaki M, Lauer MS, Gillinov AM, Blackstone EH and Lytle BW. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. Journal of the American College of Cardiology. 2007;49:2191–201. [DOI] [PubMed] [Google Scholar]
- 29.Maltais S, Schaff HV, Daly RC, Suri RM, Dearani JA, Sundt TM, Enriquez-Sarano M 3rd, Topilsky Y and Park SJ Mitral regurgitation surgery in patients with ischemic cardiomyopathy and ischemic mitral regurgitation: factors that influence survival. The Journal of thoracic and cardiovascular surgery. 2011;142:995–1001. [DOI] [PubMed] [Google Scholar]
- 30.Crabtree TD, Bailey MS, Moon MR, Munfakh N, Pasque MK, Lawton JS, Moazami N, Aubuchon KA, Al-Dadah AS and Damiano RJ, Jr. Recurrent mitral regurgitation and risk factors for early and late mortality after mitral valve repair for functional ischemic mitral regurgitation. The Annals of thoracic surgery. 2008;85:1537–42; discussion 1542–3. [DOI] [PubMed] [Google Scholar]
- 31.Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, Jain AR, Leng Chua Y, Daly R, Senni M, Mokrzycki K, Menicanti L, Oh JK, Michler R, Wrobel K, Lamy A, Velazquez EJ, Lee KL and Jones RH. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silberman S, Oren A, Klutstein MW, Deeb M, Asher E, Merin O, Fink D and Bitran D. Does mitral valve intervention have an impact on late survival in ischemic cardiomyopathy? The Israel Medical Association journal : IMAJ. 2006;8:17–20. [PubMed] [Google Scholar]
- 33.Abdallah MS, Wang K, Magnuson EA, Spertus JA, Farkouh ME, Fuster V, Cohen DJ and Investigators FT. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310:1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grady KL, Lee R, Subacius H, Malaisrie SC, McGee EC, Jr., Kruse J, Goldberger JJ and McCarthy PM. Improvements in health-related quality of life before and after isolated cardiac operations. The Annals of thoracic surgery. 2011;91:777–83. [DOI] [PubMed] [Google Scholar]
- 35.Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, Herrington SA, Hays RD, Kaplan RM, Ganiats TG, Feeny D and Kind P. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Medical care. 2007;45:1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virk SA, Sriravindrarajah A, Dunn D, Liou K, Wolfenden H, Tan G and Cao C. A meta-analysis of mitral valve repair versus replacement for ischemic mitral regurgitation. Annals of cardiothoracic surgery. 2015;4:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nappi F, Lusini M, Spadaccio C, Nenna A, Covino E, Acar C and Chello M. Papillary Muscle Approximation Versus Restrictive Annuloplasty Alone for Severe Ischemic Mitral Regurgitation. Journal of the American College of Cardiology. 2016;67:2334–46. [DOI] [PubMed] [Google Scholar]
- 38.Dayan V, Soca G, Cura L and Mestres CA. Similar survival after mitral valve replacement or repair for ischemic mitral regurgitation: a meta-analysis. The Annals of thoracic surgery. 2014;97:758–65. [DOI] [PubMed] [Google Scholar]
- 39.Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Fischbein MP and Woo YJ. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. The New England journal of medicine. 2017;377:1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nloga J, Henaine R, Vergnat M, Wautot F, Desebbe O, Robin J, Ninet J and Obadia JF. Mitral valve surgery in octogenarians: should we fight for repair? A survival and quality-of-life assessment. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011;39:875–80. [DOI] [PubMed] [Google Scholar]
- 41.Claxton K The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. Journal of health economics. 1999;18:341–64. [DOI] [PubMed] [Google Scholar]
- 42.Claxton K Exploring uncertainty in cost-effectiveness analysis. PharmacoEconomics. 2008;26:781–98. [DOI] [PubMed] [Google Scholar]
- 43.Capoulade R, Zeng X, Overbey JR, Ailawadi G, Alexander JH, Ascheim D, Bowdish M, Gelijns AC, Grayburn P, Kron IL, Levine RA, Mack MJ, Melnitchouk S, Michler RE, Mullen JC, O’Gara P, Parides MK, Smith P, Voisine P, Hung J and Cardiothoracic Surgical Trials Network I. Impact of Left Ventricular to Mitral Valve Ring Mismatch on Recurrent Ischemic Mitral Regurgitation After Ring Annuloplasty. Circulation. 2016;134:1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kron IL, Hung J, Overbey JR, Bouchard D, Gelijns AC, Moskowitz AJ, Voisine P, O’Gara PT, Argenziano M, Michler RE, Gillinov M, Puskas JD, Gammie JS, Mack MJ, Smith PK, Sai-Sudhakar C, Gardner TJ, Ailawadi G, Zeng X, O’Sullivan K, Parides MK, Swayze R, Thourani V, Rose EA, Perrault LP, Acker MA and Investigators C. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. The Journal of thoracic and cardiovascular surgery. 2015;149:752–61 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


